Age-related deficit in a bimanual joint position matching task is amplitude dependent
- 1Movement Control and Neuroplasticity Research Group, Department of Kinesiology, Biomedical Sciences Group, KU Leuven, Leuven, Belgium
- 2Leuven Research Institute for Neuroscience and Disease (LIND), KU Leuven, Leuven, Belgium
The cognitive load associated with joint position sense increases with age but does not necessarily result in impaired performance in a joint position matching task. It is still unclear which factors interact with age to predict matching performance. To test whether movement amplitude and direction are part of such predictors, young and older adults performed a bimanual wrist joint position matching task. Results revealed an age-related deficit when the target limb was positioned far from (25°) the neutral position, but not when close to (15°, 5°) the neutral joint position, irrespective of the direction. These results suggest that the difficulty associated with the comparison of two musculoskeletal states increases towards extreme joint amplitude and that older adults are more vulnerable to this increased difficulty.
Introduction
Perception is an interpretation of physical reality. Proprioception is the perception of our body state in the absence of vision (Goble et al., 2009; Proske and Gandevia, 2012; Boisgontier and Swinnen, 2014). This state is defined by position, movement, and muscle force or tension. Interpretation of this state is based on the processing of information from peripheral receptors and motor efference copies (Proske and Gandevia, 2012). The proprioception that interprets body segment position is called joint position sense.
Joint position sense has been widely investigated in the context of aging (for a review, see Goble et al., 2009). Some of these investigations used dual-task paradigms to reveal that the cognitive load associated with joint position sense increased with age (Boisgontier et al., 2012; Goble et al., 2012b). However, such increased load does not necessarily result in an impaired performance in a joint position matching task, which is the typical task used to test joint position sense. Indeed, a number of studies reported the absence of an age effect (Jordan, 1978; Stelmach and Sirica, 1986; Batavia et al., 1999; Deshpande et al., 2003; Pickard et al., 2003; Goble et al., 2012a; Wang et al., 2012; Boisgontier and Nougier, 2013a; Schmidt et al., 2013). The factors that determine whether age will impact performance on a matching task are still unclear. These factors can be associated with the individual or with the context and features of the task. The level of physical activity has been shown to be one element in these predictors (Ribeiro and Oliveira, 2007; Adamo et al., 2009), and joint amplitude may be another.
In young adults, matching errors increase with target amplitude (Allen and Proske, 2006; Goble et al., 2006; Goble and Brown, 2008; Rincon-Gonzalez et al., 2011) but are not dependent on target direction (Walsh et al., 2013). The effect of amplitude on position errors could be surprising as joint proprioceptor response to passive movement increases towards the range of motion limits (Burke et al., 1988). However, paradigms of these studies allowed the use of an internal representation of the movement of the target limb (including duration and speed) to perform the matching task (Allen and Proske, 2006; Goble et al., 2006; Goble and Brown, 2008; Rincon-Gonzalez et al., 2011). Therefore, the effect of amplitude on joint position sense reported in these studies could also result from an effect on movement sense and if speed was kept constant across trials, from a tradeoff between accuracy and speed (Fitts, 1954; Fitts and Peterson, 1964).
Here, we propose that the effect of amplitude could be explained by examining the difficulty to match the perceptions of two musculoskeletal states that are different in nature (passive vs. active). To match position of one hand with the other hand in the absence of vision, we rely on proprioceptive signals generated from both limbs (Izumizaki et al., 2010). As described by Walsh et al. (2013), the brain is likely to compare proprioceptive afferent signals from the two limbs and when the difference between the signals is at a minimum, the positions are assumed to match each other. Furthermore, recent studies showed that the brain prioritizes the processing of information from both limbs over information from a single limb, resulting in better performance (Boisgontier and Nougier, 2013b; Savage et al., 2015). Therefore, when proprioceptive information associated to muscle contraction is present only in one limb, this information may not be considered as relevant information to the matching process. In other words, in this case, contraction-related information can be considered as noise. Since the intensity of the contraction increases towards range of motion limits to counter the resistance of passive tissues, contraction-related noise increases concurrently. In other words, amplitude would impact difficulty, i.e., the cognitive load of the task, and may trigger performance decline in older adults as they consistently function at a higher level of processing than young adults (Ward and Frackowiak, 2003; Heuninckx et al., 2008; Goble et al., 2010, 2012b; Boisgontier et al., 2012) and as their cognitive reserve is more limited than in young adults (Boisgontier et al., 2013).
To test whether amplitude and/or direction affect the ability to match two musculoskeletal states, young and older adults performed a bimanual joint position matching task with three amplitudes and two directions. Based on the aforementioned evidence, we hypothesized that the effect of age is dependent on amplitude but not direction.
Materials and Methods
Participants
Thirty young [21.1 ± 1.5 (19–24) years, mean ± SD (range); 14 females] and 28 older [69.4 ± 5.3 (61–82) years; 15 females] healthy volunteers participated in the study. All participants were right-handed according to the Edinburgh Handedness Inventory (Oldfield, 1971). The average lateralization quotient was similar between young and older adults (+91 ± 15 vs. +90 ± 19, respectively, with a +100 score representing extreme right-hand preference and a −100 score representing extreme left-hand preference). All participants had normal or corrected-to-normal vision, and none reported neurological, psychiatric, cardiovascular, or neuromuscular disorders. Older participants were screened for cognitive impairments with the Montreal Cognitive Assessment test using the standard cutoff score of 26 (Nasreddine et al., 2005). All participants gave their written informed consent, and procedures were performed according to guidelines established by the ethics committee for biomedical research at the KU Leuven, and in accordance with the WMA International Code of Medical Ethics (World Medical Association Inc., 1964).
Apparatus
The apparatus used to test wrist joint position sense consisted of two separate, adjustable units (left and right), both equipped with a forearm support and a manipulandum for the palm (Boisgontier et al., 2014). Motion of the right wrist joint (passive limb) was induced by an AC Servo Motor (AMK DV764, Goedhard PMC, Helmond, Netherlands) mounted underneath the right hand unit and coupled to the rotating shaft of the manipulandum via a 1:10 reducer (Alpha LP120 Gearbox). The left hand piece was constructed similarly but allowed free flexion-extension wrist movement (active limb). Shaft encoders (accuracy = 0.088°) were connected to the rotating axis to record angular displacement of the left wrist and the right wrist. Data were sampled at 1000 Hz (Signal software 4.0, Cambridge Electronic Design, Cambridge, UK) and low-pass filtered (second-order Butterworth, cut-off frequency 8 Hz, zero-lag). The angular displacement signals of the two hand pieces were stored for offline analysis.
Procedures
To control for muscle history effects (i.e., thixotropy; Proske et al., 1993), wrist muscle flexors and extensors were conditioned by asking participants to perform isometric contractions for 2 s at approximately half-maximal intensity at the start of the experiment (Allen et al., 2010). To perform the matching task, participants were seated in front of the apparatus with their shoulders in slight abduction (20°), elbows at 90°, forearms supported in neutral prosupination, and wrists in a neutral flexion-extension position. Vision was occluded by opaque goggles. They were instructed to match the right-hand position (target limb) with their left hand (matching limb) as accurately as possible at a self-selected movement speed, with the possibility of final submovements. The task was completed when participants stopped moving the matching limb for more than 1 s. This final position of the limbs was used to compute the dependent variable. The matching task was performed with three amplitudes (5, 15, and 25°) and two directions (flexion and extension). The target limb was positioned through an indirect movement including various flexions and extensions ranging from −25 to 25° to prevent movement-based matching. Each condition was performed three times. Experimental trials were administered in random order.
Electromyographic (EMG) activity from the right flexor carpi radialis and extensor carpi radialis muscles of the wrist was monitored throughout the experiment to control for the absence of muscle activity. EMG signals were amplified (×1000), filtered (4–500 Hz), and sampled at 1000 Hz. When muscle activity was observed in the EMG before the beginning of a trial, participants were instructed to relax their wrist.
Data Analysis
Performance in the matching task was assessed using amplitude total error. The total error, also called total variability, root mean square error, or simply E, is explained equally by the response variability and bias (total error2 = variable error2 + constant error2; Henry, 1975). The total error was therefore preferred over the absolute error, a more complex relationship between the response variability and bias that complicates the determination of the relative contribution of each component (Schutz and Roy, 1973). Total error was defined according to the following formula:
where xi is the score on trial i, t is the target (t = −25, −15, −5, 5, 15, or 25°) and n is the number of trials (n = 3).
Statistical Analyses
To test whether amplitude or direction impacted the effect of aging on joint position sense, total errors were analyzed by a 2 × 3 × 2 analysis of variance (ANOVA) with the factors Age (Young adults, Older adults), Amplitude (5, 15, 25°), and Direction (Flexion, Extension). Level of significance (α) was set at p = 0.05. When the ANOVA revealed significant effects, post hoc tests (Tukey HSD, which corrects for multiple comparisons) were conducted to identify the loci of these effects. Partial eta squared values () were reported to indicate small (≥0.01), medium (≥0.06), and large (≥0.14) effect sizes (Sink and Stroh, 2006).
Results
The three-way ANOVA demonstrated a significant main effect of Amplitude [F(2,112) = 5.06; p = 0.008; = 0.08] with greater total error in the 25-degrees than 5-degrees condition (p = 0.011). Main effects of Age [F(1,56) = 2.75; p = 0.103; = 0.05] and Direction [F(1,56) = 0.90; p = 0.348; = 0.02] were not significant. The Age × Amplitude interaction was significant [F(2,112) = 5.39; p = 0.006; = 0.09; Figure 1] but not the Age × Direction [F(1,56) = 0.28; p = 0.600; < 0.01] and three-way interaction [F(2,112) = 0.37; p = 0.693; < 0.01]. Post hoc analyses revealed an age-related total error increase in the 25-degrees condition (p = 0.049) but not in the other amplitude conditions (p > 0.692; Figure 1).
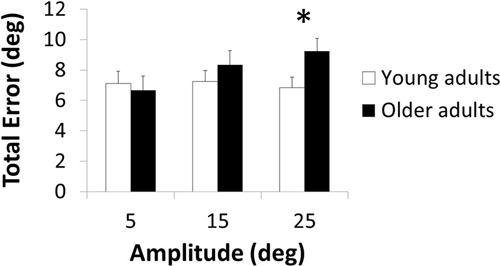
Figure 1. Total error as a function of the amplitude of the target position in young and older adults. *indicates significant difference.
Discussion
Here, we investigated whether the effect of age in a bimanual wrist joint position matching task was dependent on target amplitude and direction. Results revealed an age-related deficit when the target limb was positioned far (25°, p = 0.049) from but not close to (15°, p = 0.692; 5°, p > 0.999) the neutral position, irrespective of direction (p = 0.693).
Sensitivity to an Increase in Task Difficulty in Young and Older Adults
The age-related decline in matching performance observed in the highest amplitude suggested that the cognitive load resulting from the processing of proprioceptive input reached a threshold in this condition, and triggered a decline in matching performance. We believe that such a decline is explained by a combination of the points reported below. Older adults consistently function at a higher level of processing than young adults in order to compensate for a decreased signal-to-noise ratio (Ward and Frackowiak, 2003; Heuninckx et al., 2008; Goble et al., 2010, 2012b; Boisgontier et al., 2012). Furthermore, their cognitive reserve is more limited than in young adults (Boisgontier et al., 2013). Therefore, older adults may be more sensitive to an increase in task difficulty. Such an increase in difficulty can result from the addition of information that is not relevant to the task such as noise at the peripheral and processing levels.
Increased Noise at the Proprioceptor Level
The decrease in signal-to-noise ratio may originate at the proprioceptor level. Due to age-related proprioceptors alteration (Bolognia, 1995; Liu et al., 2005; Aydoğ et al., 2006), the firing rate may show greater variance in older adults, thereby replicating observations made for motor neurons (Laidlaw et al., 2000). Such an age-related increase in the variance of the firing rate would decrease the signal-to-noise ratio. In addition, the standard deviation of motor-neuronal firing has been shown to increase with its mean level (Clamann, 1969; Matthews, 1996). Assuming that standard deviation perception-neuronal firing also increase with its mean level, and as the joint proprioceptor firing rate increases towards range of motion limits (Burke et al., 1988), the age-related decrease in the signal-to-noise ratio would be amplified towards range of motion limits.
Increased Noise at the Processing Level
At the processing level, the hypothesis stating that our findings result from a speed-accuracy tradeoff does not hold, as final submovements were allowed. Additionally, the random flexion-extension movements performed during positioning of the target limb prevented the participant from matching movement features, and only allowed matching of the position per se. We propose that this decline in performance was instead accounted for by an amplitude-dependent amplification of the difficulty to match the perceptions of two musculoskeletal states that are different in nature (passive vs. active). The brain prioritizes the processing of information from both limbs over information from a single limb (Boisgontier and Nougier, 2013b; Savage et al., 2015). In our bimanual matching task, proprioceptive information associated to muscle contraction is only generated in one limb (active matching limb) and may therefore be considered as noise. Since the intensity of the contraction increases towards range of motion limits, matching performance would decline as the target limb moves away from the neutral position, which supports our findings.
Additionally, although not assessed here, range of motion has been shown to be limited in older adults (Chaparro et al., 2000). Therefore, the intensity of the contractions may be higher in older than in young adults, especially when the target limb was positioned far from the neutral position. This increased intensity of the contractions would amplify the noise at both the peripheral and processing level and explain the age-related deficit observed in our study.
No Age-Related Deficit in the Lower Amplitudes
The absence of a difference in matching performance between young and older adult in the lower amplitudes supports studies demonstrating the absence of age-related deficits in joint position sense (Jordan, 1978; Stelmach and Sirica, 1986; Batavia et al., 1999; Deshpande et al., 2003; Pickard et al., 2003; Goble et al., 2012a; Wang et al., 2012; Boisgontier and Nougier, 2013a; Schmidt et al., 2013). These results demonstrate that older adults are able to sense position to the same degree as young adults under certain circumstances. Specifically, it shows that the accuracy of proprioceptor information is either robust against aging or altered by aging but compensated for by central mechanisms. The latter is more likely as age-related changes in muscle (Liu et al., 2005), joint (Aydoğ et al., 2006) and skin (Bolognia, 1995) receptors are thought to reduce the signal-to-noise ratio by decreasing quantity and/or intensity of their output and increasing sensory noise (Speers et al., 2002). Moreover, the greater recruitment of neural resources in older relative to young adults observed in motor tasks supports the idea of a compensation mechanism (Ward and Frackowiak, 2003; Heuninckx et al., 2008; Goble et al., 2010).
Author Contributions
Experimental conception and design: MPB. Experimental conduct: MPB. Data analysis: MPB. Manuscript preparation: MPB, SPS. Both authors approved the final version of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
MPB is supported by a research grant (1504015N) and a post-doctoral fellowship of the Research Foundation—Flanders (FWO). This study was supported by the FWO (G0721.12; G0708.14), the Interuniversity Attraction Poles Programme initiated by the Belgian Science Policy Office (P7/11), and the KU Leuven Research Fund (OT/11/071).
References
Adamo, D. E., Alexander, N. B., and Brown, S. H. (2009). The influence of age and physical activity on upper limb proprioceptive ability. J. Aging Phys. Act. 17, 272–293.
Allen, T. J., Leung, M., and Proske, U. (2010). The effect of fatigue from exercise on human limb position sense. J. Physiol. 588, 1369–1377. doi: 10.1113/jphysiol.2010.187732
Allen, T. J., and Proske, U. (2006). Effect of muscle fatigue on the sense of limb position and movement. Exp. Brain Res. 170, 30–38. doi: 10.1007/s00221-005-0174-z
Aydoğ, S. T., Korkusuz, P., Doral, M. N., Tetik, O., and Demirel, H. A. (2006). Decrease in the numbers of mechanoreceptors in rabbit ACL: the effects of ageing. Knee Surg. Sports Traumatol. Arthrosc. 14, 325–329. doi: 10.1007/s00167-005-0673-2
Batavia, M., Gianutsos, J. G., Ling, W., and Nelson, A. J. (1999). The effects of circumferential wrist pressure on reproduction accuracy of wrist placement in healthy young and elderly adults. J. Gerontol. A Biol. Sci. Med. Sci. 54, M177–M183. doi: 10.1093/gerona/54.4.m177
Boisgontier, M. P., Beets, I. A. M., Duysens, J., Nieuwboer, A., Krampe, R. T., and Swinnen, S. P. (2013). Age-related differences in attentional cost associated with postural dual tasks: increased recruitment of generic cognitive resources in older adults. Neurosci. Biobehav. Rev. 37, 1824–1837. doi: 10.1016/j.neubiorev.2013.07.014
Boisgontier, M. P., and Nougier, V. (2013a). Ageing of internal models: from a continuous to an intermittent proprioceptive control of movement. Age (Dordr.) 35, 1339–1355. doi: 10.1007/s11357-012-9436-4
Boisgontier, M. P., and Nougier, V. (2013b). Proprioception: bilateral inputs first. Neurosci. Lett. 534, 96–100. doi: 10.1016/j.neulet.2012.11.050
Boisgontier, M. P., Olivier, I., Chenu, O., and Nougier, V. (2012). Presbypropria: the effects of physiological ageing on proprioceptive control. Age (Dordr.) 34, 1179–1194. doi: 10.1007/s11357-011-9300-y
Boisgontier, M. P., and Swinnen, S. P. (2014). Proprioception in the cerebellum. Front. Hum. Neurosci. 8:212. doi: 10.3389/fnhum.2014.00212
Boisgontier, M. P., Van Halewyck, F., Corporaal, S. H. A., Willacker, L., Van Den Bergh, V., Beets, I. A. M., et al. (2014). Vision of the active limb impairs bimanual motor tracking in young and older adults. Front. Aging Neurosci. 6:320. doi: 10.3389/fnagi.2014.00320
Burke, D., Gandevia, S. C., and Macefield, G. (1988). Responses to passive movement of receptors in joint, skin and muscle of the human hand. J. Physiol. 402, 347–361. doi: 10.1113/jphysiol.1988.sp017208
Chaparro, A., Rogers, M., Fernandez, J., Bohan, M., Choi, S. D., and Stumpfhauser, L. (2000). Range of motion of the wrist: implications for designing computer input devices for the elderly. Disabil. Rehabil. 22, 633–637. doi: 10.1080/09638280050138313
Clamann, P. H. (1969). Statistical analysis of motor unit firing patterns in human skeletal muscle. Biophys. J. 9, 1233–1251. doi: 10.1016/s0006-3495(69)86448-9
Deshpande, N., Connelly, D. M., Culham, E. G., and Costigan, P. A. (2003). Reliability and validity of ankle proprioceptive measures. Arch. Phys. Med. Rehabil. 84, 883–889. doi: 10.1016/s0003-9993(03)00016-9
Fitts, P. M. (1954). The information capacity of the human motor system in controlling the amplitude of movement. J. Exp. Psychol. 47, 381–391. doi: 10.1037/h0055392
Fitts, P. M., and Peterson, J. R. (1964). Information capacity of discrete motor responses. J. Exp. Psychol. 67, 103–112. doi: 10.1037/h0045689
Goble, D. J., and Brown, S. H. (2008). Upper limb asymmetries in the matching of proprioceptive versus visual targets. J. Neurophysiol. 99, 3063–3074. doi: 10.1152/jn.90259.2008
Goble, D. J., Coxon, J. P., Van Impe, A., De Vos, J., Wenderoth, N., and Swinnen, S. P. (2010). The neural control of bimanual movements in the elderly: brain regions exhibiting age-related increases in activity, frequency-induced neural modulation and task-specific compensatory recruitment. Hum. Brain Mapp. 31, 1281–1295. doi: 10.1002/hbm.20943
Goble, D. J., Coxon, J. P., Van Impe, A., Geurts, M., Van Hecke, W., Sunaert, S., et al. (2012a). The neural basis of central proprioceptive processing in older versus younger adults: an important sensory role for right putamen. Hum. Brain Mapp. 33, 895–908. doi: 10.1002/hbm.21257
Goble, D. J., Mousigian, M. A., and Brown, S. H. (2012b). Compromised encoding of proprioceptively determined joint angles in older adults: the role of working memory and attentional load. Exp. Brain Res. 216, 35–40. doi: 10.1007/s00221-011-2904-8
Goble, D. J., Coxon, J. P., Wenderoth, N., Van Impe, A., and Swinnen, S. P. (2009). Proprioceptive sensibility in the elderly: degeneration, functional consequences and plastic-adaptive processes. Neurosci. Biobehav. Rev. 33, 271–278. doi: 10.1016/j.neubiorev.2008.08.012
Goble, D. J., Lewis, C. A., and Brown, S. H. (2006). Upper limb asymmetries in the utilization of proprioceptive feedback. Exp. Brain Res. 168, 307–311. doi: 10.1007/s00221-005-0280-y
Henry, F. M. (1975). Absolute error vs. “E” in target accuracy. J. Mot. Behav. 7, 227–228. doi: 10.1080/00222895.1975.10735039
Heuninckx, S., Wenderoth, N., and Swinnen, S. P. (2008). Systems neuroplasticity in the aging brain: recruiting additional neural resources for successful motor performance in elderly persons. J. Neurosci. 28, 91–99. doi: 10.1523/jneurosci.3300-07.2008
Izumizaki, M., Tsuge, M., Akai, L., Proske, U., and Homma, I. (2010). The illusion of changed position and movement from vibrating one arm is altered by vision or movement of the other arm. J. Physiol. 588, 2789–2800. doi: 10.1113/jphysiol.2010.192336
Jordan, T. (1978). Age differences in visual and kinesethetic short-term memory. Percept. Mot. Skills 46, 667–674. doi: 10.2466/pms.1978.46.2.667
Laidlaw, D. H., Bilodeau, M., and Enoka, R. M. (2000). Steadiness is reduced and motor unit discharge is more variable in old adults. Muscle Nerve 23, 600–612. doi: 10.1002/(sici)1097-4598(200004)23:4<600::aid-mus20>3.0.co;2-d
Liu, J. X., Eriksson, P. O., Thornell, L. E., and Pedrosa-Domellöf, F. (2005). Fiber content and myosin heavy chain composition of muscle spindles in aged human biceps brachii. J. Histochem. Cytochem. 53, 445–454. doi: 10.1369/jhc.4a6257.2005
Matthews, P. B. C. (1996). Relationship of firing intervals of human motor units to the trajectory of post-spike after-hyperpolarization and synaptic noise. J. Physiol. 492, 597–628. doi: 10.1113/jphysiol.1996.sp021332
Nasreddine, Z. S., Phillips, N. A., Bédirian, V., Charbonneau, S., Whitehead, V., Collin, I., et al. (2005). The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 53, 695–699. doi: 10.1111/j.1532-5415.2005.53221.x
Oldfield, R. C. (1971). The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9, 97–113. doi: 10.1016/0028-3932(71)90067-4
Pickard, C. M., Sullivan, P. E., Allison, G. T., and Singer, K. P. (2003). Is there a difference in hip joint position sense between young and older groups? J. Gerontol. A Biol. Sci. Med. Sci. 58, M631–M635. doi: 10.1093/gerona/58.7.m631
Proske, U., and Gandevia, S. C. (2012). The proprioceptive senses: their roles in signaling body shape, body position and movement and muscle force. Physiol. Rev. 92, 1651–1697. doi: 10.1152/physrev.00048.2011
Proske, U., Morgan, D. L., and Gregory, J. E. (1993). Thixotropy in skeletal muscle and in muscle spindles: a review. Prog. Neurobiol. 41, 705–721. doi: 10.1016/0301-0082(93)90032-n
Ribeiro, F., and Oliveira, J. (2007). Aging effects on joint proprioception: the role of physical activity in proprioception preservation. Eur. Rev. Aging Phys. Act. 4, 71–76. doi: 10.1007/s11556-007-0026-x
Rincon-Gonzalez, L., Buneo, C. A., and Helms Tillery, S. I. (2011). The proprioceptive map of the arm is systematic and stable, but idiosyncratic. PLoS One 6:e25214. doi: 10.1371/journal.pone.0025214
Savage, G., Allen, T. J., and Proske, U. (2015). The senses of active and passive forces at the human ankle joint. Exp. Brain Res. 233, 2167–2180. doi: 10.1007/s00221-015-4287-8
Schmidt, L., Depper, L., and Kerkhoff, G. (2013). Effects of age, sex and arm on the precision of arm position sense-left-arm superiority in healthy right-handers. Front. Hum. Neurosci. 7:915. doi: 10.3389/fnhum.2013.00915
Schutz, R. W., and Roy, E. A. (1973). Absolute error: the devil in disguise. J. Mot. Behav. 5, 141–153. doi: 10.1080/00222895.1973.10734959
Sink, C. A., and Stroh, H. R. (2006). Practical significance: the use of effect sizes in school counseling research. Prof. Sch. Couns. 9, 401–411. doi: 10.5330/prsc.9.4.283746k664204023
Speers, R. A., Kuo, A. D., and Horak, F. B. (2002). Contributions of altered sensation and feedback responses to changes in coordination of postural control due to aging. Gait Posture 16, 20–30. doi: 10.1016/s0966-6362(02)00003-6
Stelmach, G. E., and Sirica, A. (1986). Aging and proprioception. Age 9, 99–103. doi: 10.1007/bf02432281
Walsh, L. D., Proske, U., Allen, T. J., and Gandevia, S. C. (2013). The contribution of motor commands to position sense differs between elbow and wrist. J. Physiol. 591, 6103–6114. doi: 10.1113/jphysiol.2013.259127
Wang, L., Sutter, C., Müsseler, J., Dangel, R. J., and Disselhorst-Klug, C. (2012). Perceiving one’s own limb movements with conflicting densory feedback: the role of mode of movement control and age. Front. Psychol. 3:289. doi: 10.3389/fpsyg.2012.00289
Keywords: aging, joint position sense, proprioception, humans, bimanual matching task
Citation: Boisgontier MP and Swinnen SP (2015) Age-related deficit in a bimanual joint position matching task is amplitude dependent. Front. Aging Neurosci. 7:162. doi: 10.3389/fnagi.2015.00162
Received: 04 May 2015; Accepted: 07 August 2015;
Published: 21 August 2015.
Edited by:
Pranav J. Parikh, University of Houston, USAReviewed by:
Kevin G. Keenan, University of Wisconsin–Milwaukee, USABrach Poston, University of Nevada, Las Vegas, USA
Copyright © 2015 Boisgontier and Swinnen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution and reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Matthieu P. Boisgontier, Movement Control and Neuroplasticity Research Group, Department of Kinesiology, Biomedical Sciences Group, KU Leuven, Tervuursevest 101, B-3000 Leuven, Belgium, matthieu.boisgontier@faber.kuleuven.be
 Matthieu P. Boisgontier
Matthieu P. Boisgontier Stephan P. Swinnen
Stephan P. Swinnen