- 1Department of Urology, Faculty of Medicine Carl Gustav Carus, Dresden University of Technology, Dresden, Germany
- 2Department of Physiology, Faculty of Medicine Carl Gustav Carus, Dresden University of Technology, Dresden, Germany
- 3Department of Pharmacology and Toxicology, Julius Maximilian University Würzburg, Würzburg, Germany
- 4Leibniz-Institute für Analytische Wissenschaften-ISAS-e.V., Dortmund, Germany
- 5West German Heart and Vascular Center Essen, University Hospital Essen-Duisburg, Duisburg, Germany
- 6Department of Pharmacology, Johannes Gutenberg University, Mainz, Germany
Background and Objective: In order to characterize the β-adrenoceptor (AR) subtypes involved in agonist-stimulated relaxation of murine urinary bladder we studied the effects of (-)-isoprenaline and CL 316,243 on tonic contraction and spontaneous contractions in detrusor strips of wild-type (WT) and β2-AR knockout (β2-AR KO) mice.
Materials and Methods: Urinary bladders were isolated from male WT and β2-AR KO mice. β-AR subtype expression was determined with quantitative real-time PCR. Intact muscle strips pre-contracted with KCl (40 mM) were exposed to cumulatively increasing concentrations of (-)-isoprenaline or β3-AR agonist CL 316,243 in the presence and absence of the subtype-selective β-AR blockers CGP 20712A (β1-ARs), ICI 118,551 (β2-ARs), and L748,337 (β3-ARs).
Results: Quantitative real-time PCR confirmed lack of β2-AR expression in bladder tissue from β2-AR KO mice. In isolated detrusor strips, pre-contraction with KCl increased basal tone and enhanced spontaneous activity significantly more in β2-AR KO than in WT. (-)-Isoprenaline relaxed tonic tension and attenuated spontaneous activity with similar potency, but the concentrations required were two orders of magnitude higher in β2-AR KO than WT. The concentration-response curves (CRCs) for relaxation were not affected by CGP 20712A (300 nM), but were shifted to the right by ICI 118,551 (50 nM) and L748,337 (10 μM). The -logEC50 values for (-)-isoprenaline in WT and β2-AR KO tissue were 7.98 and 6.00, respectively, suggesting a large receptor reserve of β2-AR. (-)-CL 316,243 relaxed detrusor and attenuated spontaneous contractions from WT and β2-AR KO mice with a potency corresponding to the drug’s affinity for β3-AR. L743,337 shifted the CRCs to the right.
Conclusion: Our findings in β2-AR KO mice suggest that there is a large receptor reserve for β2-AR in WT mice so that this β-AR subtype will mediate relaxation of tone and attenuation of spontaneous activity under physiological conditions. Nevertheless, upon removal of this reserve, β3-AR can also mediate murine detrusor relaxation.
Introduction
Standard therapy of overactive bladder syndrome consists of muscarinic receptor antagonists, but β3-AR agonists have recently been introduced as a promising alternative (Chapple et al., 2014). Experimental studies of β-AR-mediated relaxation in isolated detrusor strips are complicated by species differences. While such relaxation of human detrusor is mediated predominantly if not exclusively by the β3-AR (for reference, see Wuest et al., 2009), most studies in rats have reported an involvement of both β2- and β3-AR (Takeda et al., 2003; Uchida et al., 2005). Subtypes involved in mouse bladder are controversial. While we have found that detrusor relaxation is mediated via β2-AR (Wuest et al., 2009; Propping et al., 2015a), other authors have suggested β3-ARs as the relevant subtype (Deba et al., 2009). Some of this discrepancy may be due to different experimental conditions, but another major issue is that the various drugs employed may actually not exhibit the assumed β-AR subtype selectivity (Cernecka et al., 2014).
Irrespective of the debate on β-AR subtypes involved in detrusor relaxation in various species, it has been questioned whether a direct effect on detrusor smooth muscle cell tone indeed is the underlying cellular mechanism for in vivo inhibition of detrusor overactivity by β-AR agonists (Eastham et al., 2015). This is based on the observation that concentrations of β3-AR agonists as for instance mirabegron to induce human detrusor strip relaxation are considerably higher (EC50 ∼1.7 μM, Svalo et al., 2013) than the plasma concentrations at therapeutic doses (30–75 nM, Krauwinkel et al., 2012). There is some evidence, that modulation of spontaneous contractions could represent an alternative target for the therapeutic effect of β3-AR agonists in overactive bladder syndrome. Pre-contracting isolated detrusor tissue with KCl or muscarinic agonists not only increases tonic tension but also induces irregular force oscillations of variable amplitude and frequency (spontaneous contractions, also referred to as “phasic contractions” or “microcontractions”; Gillespie et al., 2015a) Interestingly, spontaneous contractions of detrusor in rats are more sensitive to suppression by (-)-isoprenaline than nerve-mediated contractions evoked by electric field stimulation, but this may be mediated via β1-AR (Gillespie et al., 2015b). β-AR subtypes mediating inhibition of spontaneous contractions in other species including mice have not been explored in a systematic manner.
Therefore, we have examined which β-AR subtype mediates inhibition of murine detrusor tone and spontaneous contractions. To address this, we have used the general β-AR agonist (-)-isoprenaline and the β3-AR agonist CL 316,243 in KCl-precontracted strips of β2-AR knockout (β2-AR KO) mice and their wild-type (WT) controls with separate analysis of detrusor tone and spontaneous contractions. Our results confirm the importance of β2-ARs for murine detrusor relaxation and attenuation of spontaneous contractions, but also attest contribution of β3-ARs.
Materials and Methods
The control experiments of the present study were performed in FVB/N-WT mice, which match the genetic background of β2-AR KO mice. The mice were bred in the Department of Pharmacology and Toxicology, University of Würzburg, Germany. All experiments were performed in accordance with the local authorities (permission number 24D-9168.24-1/2007-17 of the Regierungspräsidium Dresden and of the Regierung of Unterfranken permission number 55.2-2531.01-60/13, Germany) and comply with the European Commission Directive 86/609/EEC regarding the protection and welfare of animals used for experimental as well as scientific purposes.
Determination of β-AR Subtypes Expression in Mouse Detrusor
Male FVB/N-WT controls and β2-AR KO mice (24–40 weeks) were killed by cervical dislocation under CO2 anesthesia, and urinary bladders and lungs were removed. The bladders were cut open and detrusor tissue and mucosa were dissected with sharp scissors and further processed separately. RNA was isolated from the tissue samples using the RNeasy®-Kit (Qiagen) and total RNA was reverse transcribed using Superscript II reverse transcriptase (Invitrogen). For cDNA amplification of β1-ARs, β2-ARs, β3-ARs and glyceraldehyde-3-phosphate dehydrogenase (GAPDH), a reaction mixture was used containing SsoFast EvaGreen Supermix (BioRad). Quantitative real-time PCR was performed using the C1000 Thermal Cycler CFX96 (BioRad) and the data was analyzed as previously described (Vidal et al., 2012). Amplification conditions for RT-PCR were 15 s at 95°C followed by five cycles of 30 s at 94°C, 30 s at 60°C (for Adrb2 at 64°C) and 30 s at 72°C, five cycles of 30 s at 94°C, 30 s at 62°C and 30 s at 72°C and 25 cycles of 30 s at 94°C, 30 s at 64°C and 30 s at 72°C and an additional cycle of 15 s at 80°C. The following primers were used (Evans et al., 1999; Chernogubova et al., 2005; Wuest et al., 2009; Vidal et al., 2012):
GAPDH forward primer 5′-TGGCAAAGTGGAGATTG TTG-3′;
GAPDH reverse primer 5′-CATTATCGGCCTTGACTG TG-3′;
β1 -AR forward primer 5′-CCGCTGCTACCACGACCC CAAG-3′;
β1 -AR reverse primer 5′-AGCCAGTTGAAGAAGAG CAAGAGGCG-3′;
β2 -AR forward primer 5′-GGTTATCGTCCTGGCCAT CGTGTTTG-3′;
β2 -AR reverse primer 5′-TGGTTCGTGAAGAAGTCA CAGCAAGTCTC-3′;
β3 -AR forward primer 5′-TCTAGTTCCCAGCGGAGTT TTCATCG-3′;
β3 -AR reverse primer 5′-CGCGCACCTTCATAGCCAT CAAACC-3′.
Experimental Procedure for Measureing Detrusors Contractions and Relaxations
Characterization of mice: the WT and β2-AR KO mice had average body weights of 29 ± 3 g for WT (n = 55) and 28 ± 4 g for β2-AR KO mice (n = 49). Strips of mouse urinary bladder were dissected as described previously (Propping et al., 2015a,b). Muscle strips with an intact mucosal layer (mean weight WT mice 2.9 ± 1.6 mg, n = 75 strips; β2-AR KO mice 2.4 ± 1.8 mg, n = 74, P = 0.26) were mounted in an organ bath filled with 5 ml of modified Tyrode solution of the following composition (in mM): NaCl 126.9, KCl 5.4, MgCl2 1.05, CaCl2 1.8, NaH2HPO4 0.45, NaHCO3 22, EDTA 0.04, ascorbic acid 0.2, glucose 5.6. Phentolamine (3 μM) and prazosin (1 μM) were added to block α-ARs. The solution in the bath was maintained at 37°C, and was oxygenated by vigorously bubbling with carbogen (95% O2, 5% CO2). The pH was 7.4. All drugs were obtained from the same sources and dissolved either in distilled water of dimethyl sulfoxide as in our previous study (Propping et al., 2015c). The DMSO concentration in the bath did not exceed 0.3%.
The detrusor strips were connected to an isometric force transducer (GM2; Föhr Medical Instruments, Seeheim/Ober-Beerbach Germany) and preloaded with 5 mN. After 30 min in the organ bath, tension was readjusted to 5 mN. Force of contraction was recorded with Chart 4.0TM (AD Instruments, Sydney, NSW, Australia). Tonic tension was analyzed as the increase of force produced by 40 mM KCl, measured from the lower limit of the “noise” produced by spontaneous activity under baseline conditions and in the presence of KCl. The amplitudes and the time integral of spontaneous contractions were analyzed during the 2-min period before the next concentration increase, using Chart software. Agonist-induced attenuation of spontaneous activity was expressed as integral in percent of control.
The preparations were allowed to equilibrate for at least 60 min. During this period, they were stimulated two consecutive times with KCl (40 mM, without osmotic compensation). After another 45 min of washout, the strips were pre-contracted by depolarization with 40 mM KCl. Relaxation was induced with cumulatively increasing concentrations of (-)-isoprenaline, CL 316,243 or forskolin. Relaxation was measured as the difference between minimum force prior to addition of agonist (steady state force) and force in the presence of the agonist, and was expressed in percent of the response to 10 μM forskolin added at the end of each experiment (= 100%). All β-AR subtype-selective blockers were added 30 min before the start of KCl pre-contraction and remained in the bath solution throughout the remainder of the experiment. The concentrations of antagonists were CGP 20712A 300 nM, ICI 118,551 50 nM (Wuest et al., 2009; Propping et al., 2015a), and L748,337 100 nM to 10 μM (Deba et al., 2009).
Calculation of -logEC50 Values
Concentration-response curves were constructed by non-linear regression for each individual experiment by using Prism 5.0®(GraphPad®Software, Inc., San Diego, CA, USA). The negative logarithm to the base of 10 of the molar concentration producing 50% of the maximum response (-logEC50 [M]) as well as the maximum response (Emax) were calculated and expressed as mean ± SD. Please note that the non-linear regression curves depicted in the figures were fitted to the mean values of the data.
In Schild plots, log(CR-1) was plotted versus log(molar concentration of antagonist), where CR stands for concentration ratio, i.e., the agonist concentration producing 50% of the maximum effect (EC50) in the presence of the antagonist divided by the EC50 of in the absence of antagonist (Schild, 1947).
The pA2 value as a measure of potency of a surmountable antagonist was extrapolated from a straight line with the slope of unity, given by the formula
The same formula was used for calculating the apparent pA2 values by substituting the experimental values for one concentration only.
Statistical Analysis
The results are represented as mean ± standard deviation (mean ± SD). A two-tailed t-test for unequal samples with different variances was used for two-group comparisons and was calculated with Prism 5.0®(GraphPad®Software, Inc., San Diego, CA, USA). Analysis of variance (ANOVA) was used for multiple group comparison, followed by an additional Bonferroni comparison test where appropriate. P < 0.05 was regarded as significant.
Results
Expression of β-AR Subtypes in Intact Murine Detrusor Muscle
Expression of the three β-AR subtypes was determined by RT-PCR and quantitative real-time PCR in intact detrusor tissue from WT and genetically modified animals in order to verify knock-out of β2-ARs and to check for any compensatory changes in expression of β1- and β3-ARs. Figure 1 shows that β2-ARs were only detectable in detrusor tissue from WT but not from β2-AR KO mice, and the same results were obtained in the respective lung tissues, which served as controls. Furthermore, β1- and β3-ARs were expressed in bladder and lung tissue from all animals (Figure 1A). Between WT and β2-AR KO mice there were no differences in expression levels of β1- and β3-ARs (Figure 1B).
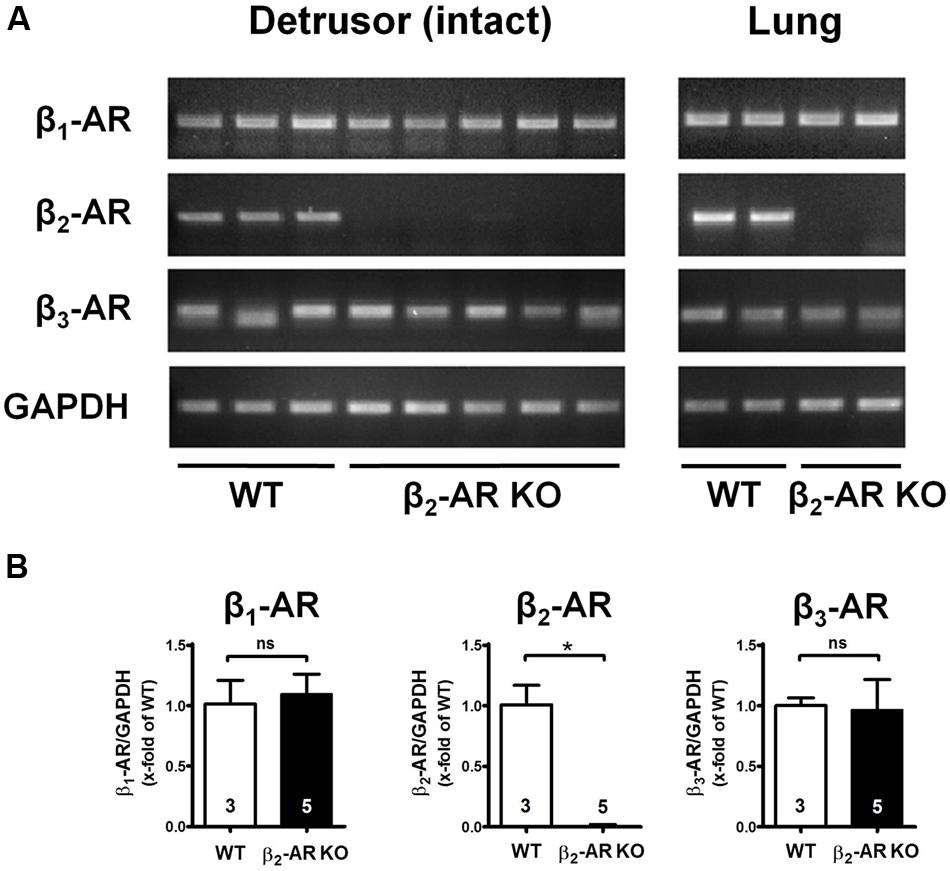
FIGURE 1. Expression of β-AR subtypes in urinary bladders und lungs of WT and β2-AR knockout mice. (A) Agarose gel electrophoresis of qualitative PCR of cDNA obtained from urinary bladder detrusor samples (left panel) and lung samples (right panel) of WT and β2-AR KO mice for β1-AR, β2-AR, β3-AR and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). (B) mRNA expression of β1-AR, β2-AR, and β3-AR normalized to GAPDH in detrusor (WT, n = 3; β2-AR KO, n = 5). n represent numbers of mice measured in triplicates. Mean values ± SD.
Baseline and KCl-induced Detrusor Contractions in WT and β2-AR KO Mice
Depolarization of mouse detrusor strips with 40 mM KCl induced a rapid increase in tone and spontaneous activity, which stabilized within 45 min (Figure 2A). Mean values of peak force (Fpeak) increases were greater in strips from β2-AR KO (2.86 ± 1.34 mN/mg w.w., n = 7/7) than WT mice (1.42 ± 0.97 mN/mg w.w., n = 18/14; P < 0.05); steady-state tone (Fss) was 1.57 ± 0.76 mN/mg w.w. β2-AR KO and 0.76 ± 0.28 mN/mg w.w. in WT (P < 0.05).
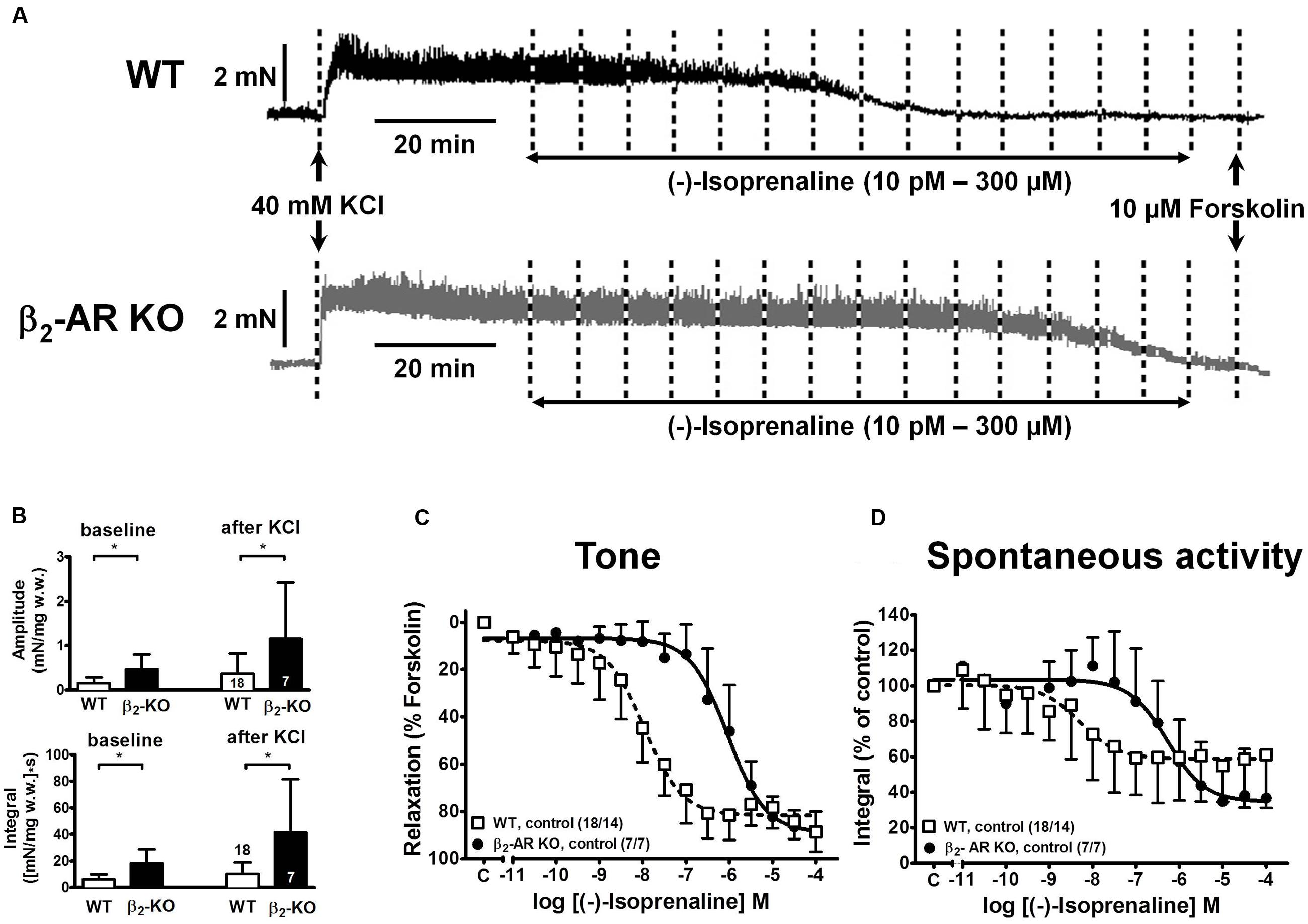
FIGURE 2. Relaxation of KCl-precontracted intact detrusor strips from WT and β2-AR KO mice. (A) Original tracings of force of contraction (in mN) from two intact detrusor strips precontracted with KCl (40 mM) and subsequently relaxed with cumulatively increasing concentrations of (-)-isoprenaline. Forskolin (10 μM) was added at the end of each experiment. (B) Baseline amplitude (upper panel) and baseline integral (lower panel) of spontaneous activity before, and amplitudes and integrals after addition of KCl. (C) CRCs for the relaxing effects of (-)-isoprenaline on KCl-induced tone in detrusor strips from WT (open symbols) and β2-AR KO (closed symbols). Since tonic contraction was completely relaxed with 10 μM forskolin, the CRCs were normalized to the response with forskolin (= 100%). (D) CRCs for spontaneous activity expressed as force-time integral in percent of pre-agonist control for WT (open symbols) and β2-AR KO (closed symbols). A quantitative analysis of the data is shown in Table 1.
In the same detrusor strips the baseline amplitudes of spontaneous contractions and their time integrals were greater in β2-AR KO than in WT mice [amplitudes: 0.46 ± 0.34 mN/mg w.w. and 0.16 ± 0.13 mN/mg w.w., P < 0.05; integral: 18.34 ± 10.51 (mN/mg w.w.)∗s, and 6.26 ± 3.74 (mN/mg w.w.)∗s, P < 0.05]. After addition of KCl the integral for spontaneous detrusor activity increased significantly to 41.60 ± 39.78 (mN/mg w.w.)∗s in β2-AR KO and to 10.30 ± 8.77 (mN/mg w.w.)∗s in WT (P < 0.05; Figure 2B).
The β1-AR blocker CGP 20712A (300 nM) had little effect on KCl-induced Fpeak and Fss in either mouse strain (Figure 3), except for Fss in WT mice (Figure 3B). CGP 20712A did not affect spontaneous contraction integral neither at baseline nor after KCl (Figures 3C,D). Exposure to the β2-AR blocker ICI 118,551 (50 nM) increased Fpeak and baseline spontaneous activity in strips from β2-AR KO mice. Responses to the β3-AR blocker L748,337 (100 nM) exhibited great variability, but appeared to increase Fpeak and Fss rather than spontaneous activity.
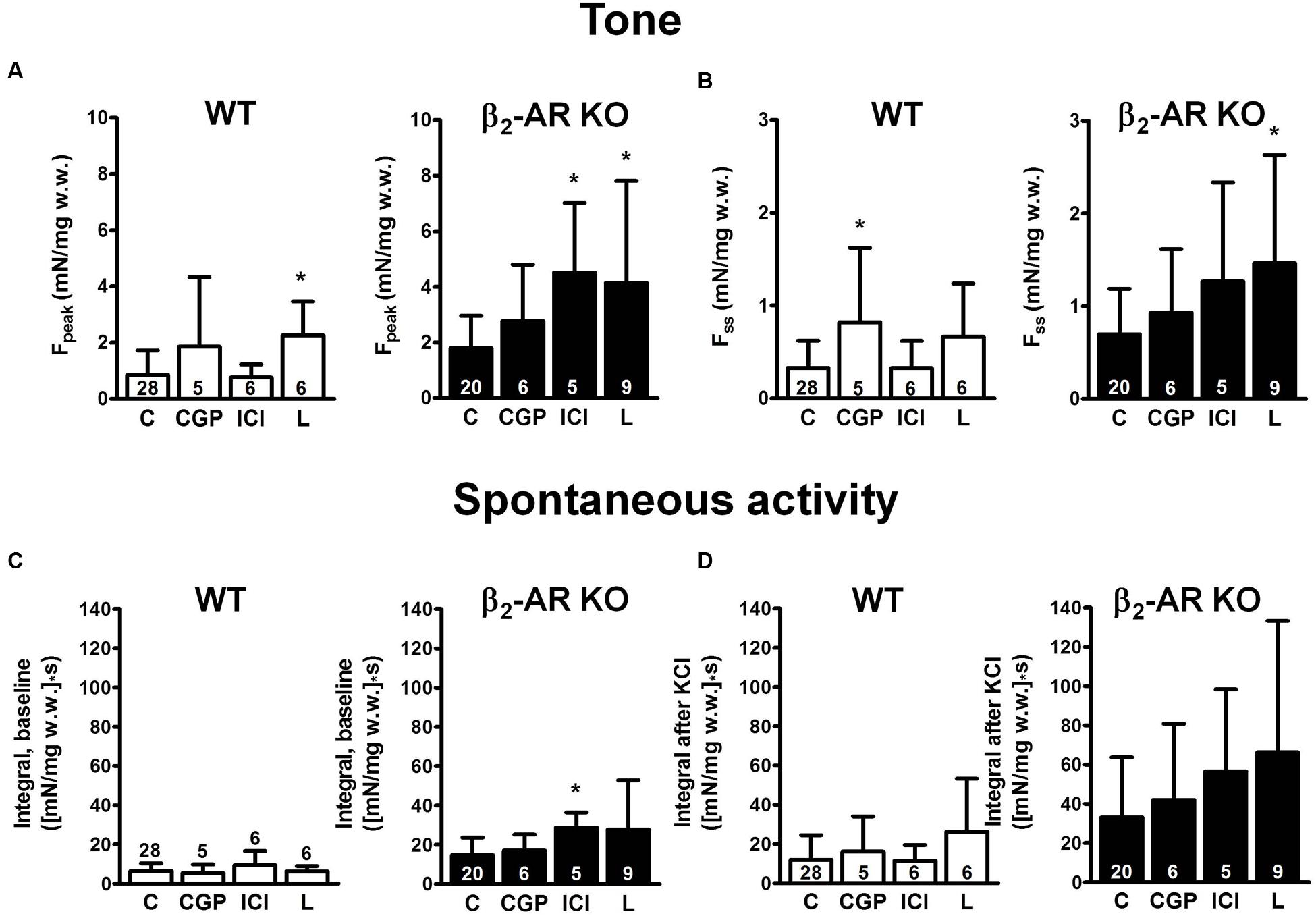
FIGURE 3. Effects β-AR blockers on detrusor tone and time-integral of spontaneous activity after exposure to 40 mM KCl (CGP, 300 nM CGP 20712A; ICI, 50 nM ICI 118,551; L748,337, 100 nM L748,337). (A,B) Peak increase (Fpeak) and steady-state increase in tone (Fss), respectively, after exposure to 40 mM KCl from WT and β2-AR KO preparations. Please note the difference in scale. (C,D) Time-integral of spontaneous contractions from WT and β2-AR KO preparations; baseline time-integral before (C) and time-integral after (D) exposure to 40 mM KCl. Mean ± SD from number of experiments as indicated. ∗denotes P < 0.05 compared with control.
(-)-Isoprenaline-induced Detrusor Relaxation in WT and β2-AR KO Mice
Increasing concentrations of (-)-isoprenaline caused almost complete relaxation of tone, and attenuation of spontaneous activity in intact strips from WT and β2-AR KO mice (Figures 2C,D). However, strips from β2-AR KO mice were significantly less sensitive by almost 2 log units. The -logEC50 values for (-)-isoprenaline-induced relaxation of tone were 7.98 for WT and 6.00 for β2-AR KO mice (see Table 1). The respective values for the (-)-isoprenaline-induced attenuation of integral for spontaneous contractions were 8.39 ± 1.06 and 6.34 ± 0.63, and were not significantly different from the effect on relaxation of tone.
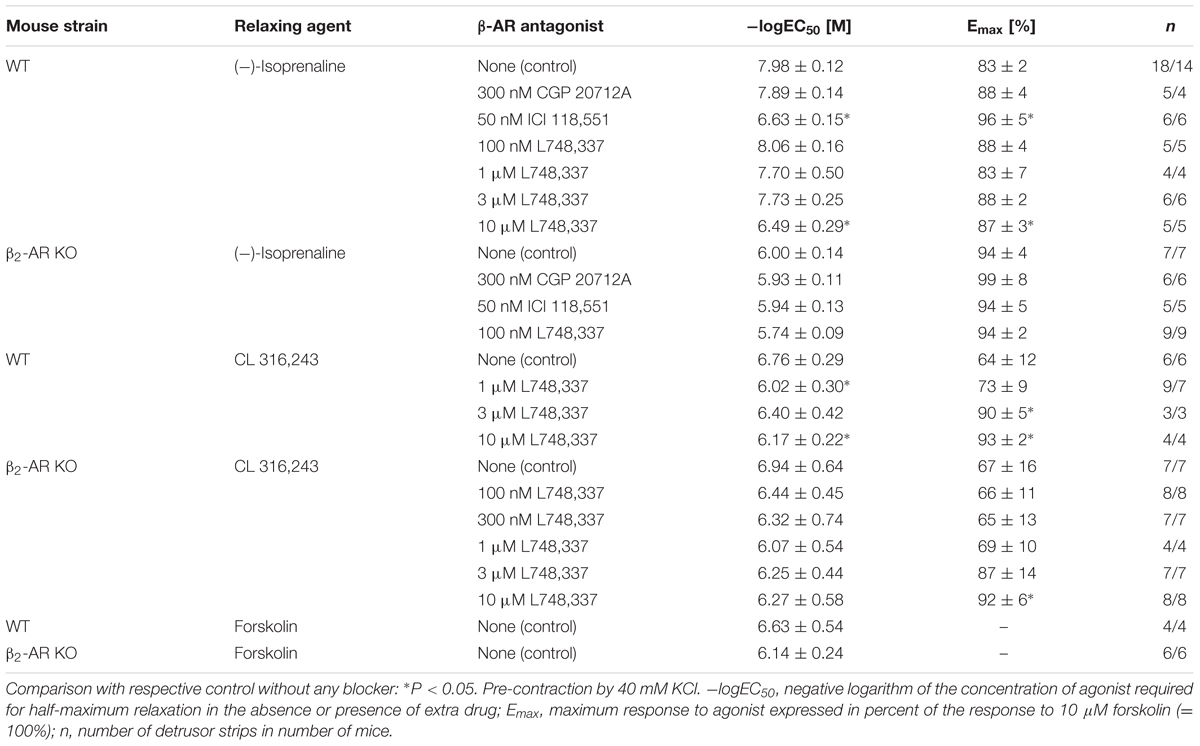
TABLE 1. Relaxing effects of (-)-isoprenaline, CL 316,243 and forskolin in murine detrusor strips and their modulation by selective β-AR antagonists.
In detrusor strips from WT mice, CGP 20712A (300 nM) and L748,337 (100 nM) had little effect on the CRCs of (-)-isoprenaline, whereas the β2-AR blocker ICI 118,551 (50 nM) shifted the CRCs to higher (-)-isoprenaline concentrations by about 1.4 log units (Figure 4, top panels). In β2-AR KO mice, the three β-AR blockers produced little effect on the CRCs of (-)-isoprenaline (Figures 4A–C), and the small shift to the right with 100 nM L748,337 did not reach significance. All -logEC50 and Emax values are summarized in Table 1. Spontaneous activity was attenuated by (-)-isoprenaline to a somewhat larger extent in β2-AR KO than WT strips (Figures 4D–F). The effects of (-)-isoprenaline on spontaneous activity were not influenced by blocking β1-AR with CGP 20712A (Figure 4D). There was a trend for a shift in the CRC by the β2-AR blocker ICI 118,551 in WT strips (Figure 4E) and by the β3-AR blocker L748,337 in β2-AR KO strips (Figure 4F). Again, due to the large variability, the effects on spontaneous contractions were less clear than on tonic contraction.
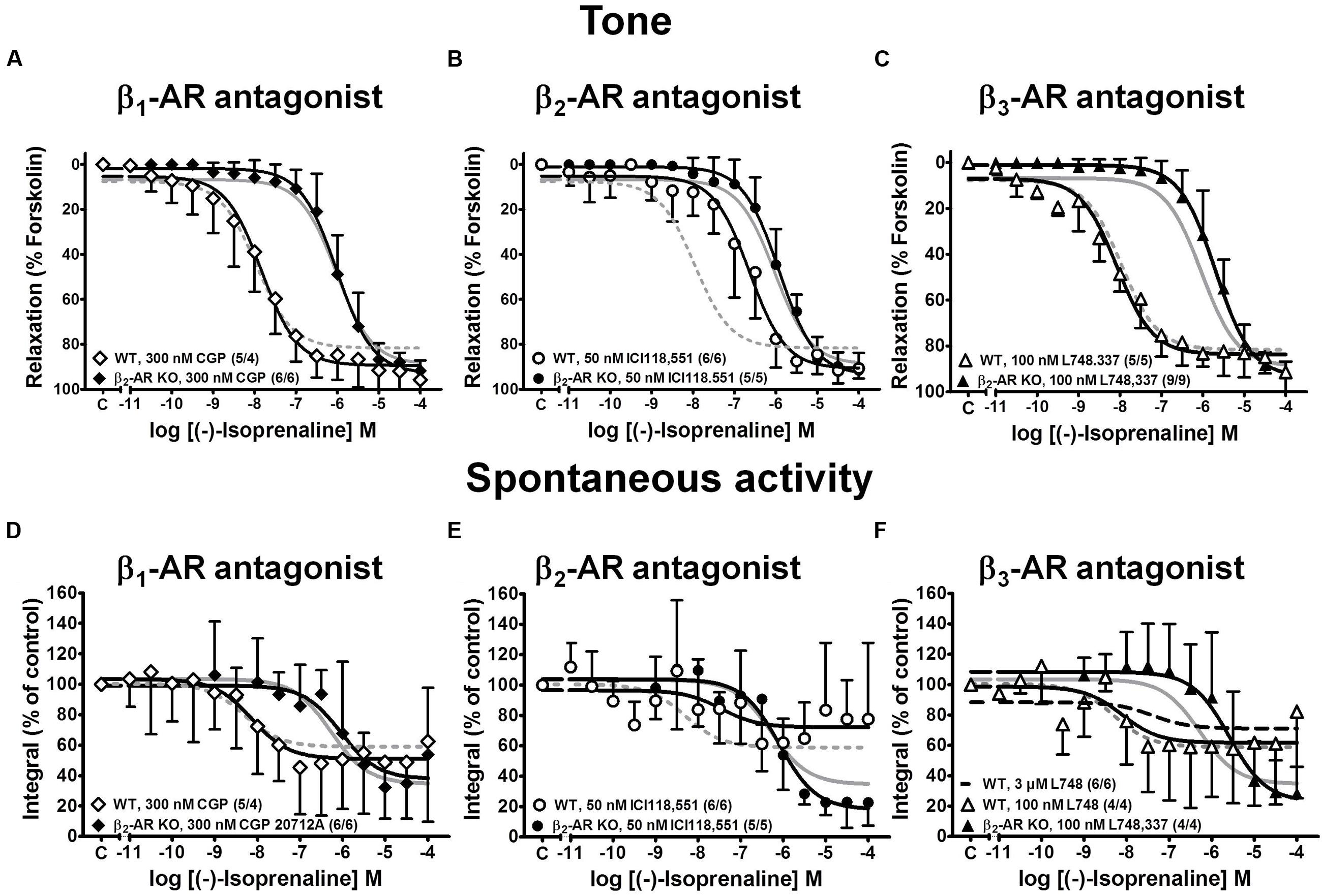
FIGURE 4. Effects of various β-AR subtype selective blockers on CRCs for (-)-isoprenaline-induced relaxation of KCl-induced tone (A–C) and spontaneous contractions (D–F) in detrusor strips from WT (open symbols) and β2-AR KO mice (closed symbols). CRCs were performed in the presence of the β1-AR-selective blocker CGP 20712A (A,D), the β2-AR-selective blocker ICI 118,551 (B,E), the β3-AR-selective blocker L748,337 (C,F). The respective controls (WT, dashed gray lines; β2-AR KO, continuous gray lines) were taken from the same experiments as in Figure 2. Mean values ± SD from n experiments as indicated in parenthesis (number of strips/number of animals).
These findings confirm our previous observations (Wuest et al., 2009; Propping et al., 2013, 2015a) that β2-ARs are the major β-AR subtype mediating relaxation in WT detrusor strips. However, given the low affinity of L748,337 for murine β3-ARs (Cernecka et al., 2014), the concentration of 100 nM L748,337 may not have been sufficient to block murine β3-AR. Therefore, higher L748,337 concentrations were employed to antagonize (-)-isoprenaline-mediated relaxation in WT detrusor (Figure 5). With 10 μM L478,337, the CRC was clearly shifted to the right (Figure 5C). The results with 1 and 3 μM L748,557 were less consistent (Figures 5A,B). Nevertheless, these findings suggest that β3-ARs may be involved to a larger extent than previously anticipated by us (Propping et al., 2015a), but as suggested by Deba et al. (2009). The Schild plot (Figure 5D) clearly deviated from unity suggesting a more complex mechanism than simple competition of (-)-isoprenaline and L748,337 for a single binding site. Fitting a linear regression of slope 1 to the data points, yielded a pA2 value of 6.08 for L748,337 in strips from WT mice.

FIGURE 5. Effects of the β3-AR-selective blocker L748,337, 1 μM (A), 3 μM (B), and 10 μM (C), on CRCs for (-)-isoprenaline-induced relaxation of KCl-precontracted detrusor strips from WT mice. The control is taken from the same experiments as in Figure 2. Mean values ± SD from n experiments as indicated in parenthesis (number of strips/number of animals). (D) Schild plot for determining the pA2 value of L748,337 (see Materials and Methods for further details).
CL 316,243-Induced Detrusor Relaxation in WT and β2-AR KO Mice
In order to resolve these different interpretations, we next investigated the effects of the β3-AR-selective agonist CL 316,243 that was employed by Deba et al. (2009). Increasing concentrations of CL 316,243 relaxed detrusor strips from WT and β2-AR KO with similar potency and efficacy (Figure 6). In comparison with (-)-isoprenaline, CL 316,243 tended to be less potent in strips from WT than β2-AR KO mice (compare Figure 2, Table 1). Again, more vigorous spontaneous activity was observed in β2-AR KO than WT strips (Figure 6B). The complete CRCs revealed that detrusor strips relaxed less completely (Figure 6C, Table 1) and that attenuation of spontaneous activity failed to reach significance both in WT and β2-AR KO strips (Figure 6D). Concentrations of 100–300 nM L748,337 had no significant effect on the CRCs for CL 316,243 under any experimental condition, but concentrations between 1 and 10 μM L748,337 induced complex changes in relaxation (Figures 7A,B). In strips from WT mice, L748,337 did not shift the CRCs of CL 316,243, but with 3 and 10 μM L748,337 relaxation became more complete. In β2-AR KO mice, L748,337 caused a shift of the CRCs to higher concentrations of CL 316,243, in addition to more complete relaxation. Again, the Schild plot (Figure 7C) deviated from unity indicating a complex mechanism of interaction also between CL 316,243 and L748,337. Using a slope factor of 1, the pA2 value was 6.70 for L748,337 in strips from β2-AR KO mice.
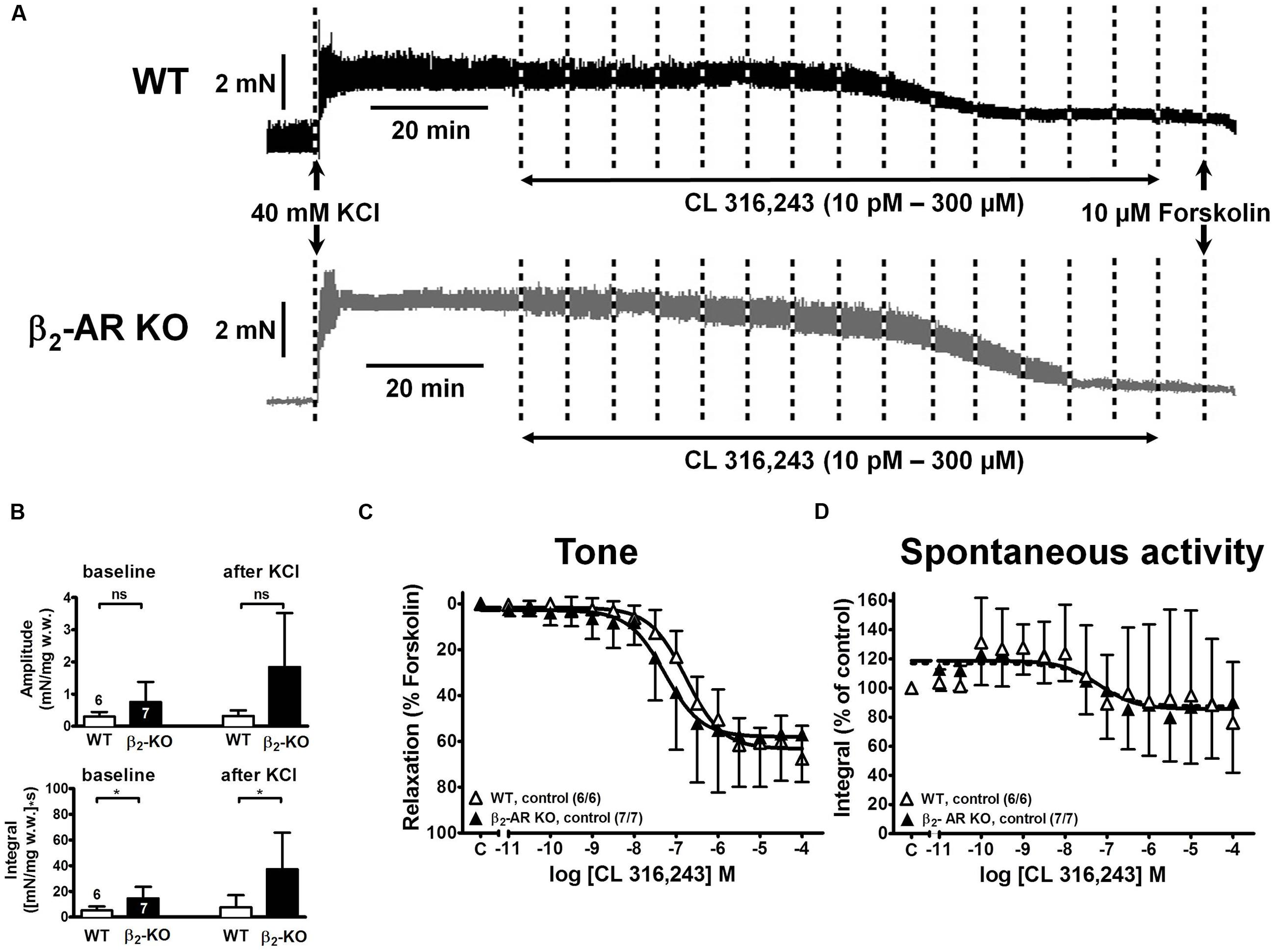
FIGURE 6. Relaxation in response to the β3-AR-selective agonist CL 316.243 of KCl-pre-contracted detrusor strips from wild type (WT) and β2-AR KO mice. (A) Original tracings of force of contraction (in mN) from two detrusor strips precontracted with KCl (40 mM) and subsequently relaxed with cumulatively increasing concentrations of CL 316,243. At the end of each experiment complete relaxation was induced with forskolin (10 μM) as indicated. (B) Amplitude (upper panel) and integral (lower panel) of spontaneous activity before and after addition of KCl. (C,D) CRCs for the CL 316,243 effects on tone and spontaneous activity in WT and β2-AR KO, respectively. Similar lay-out as in Figure 2.
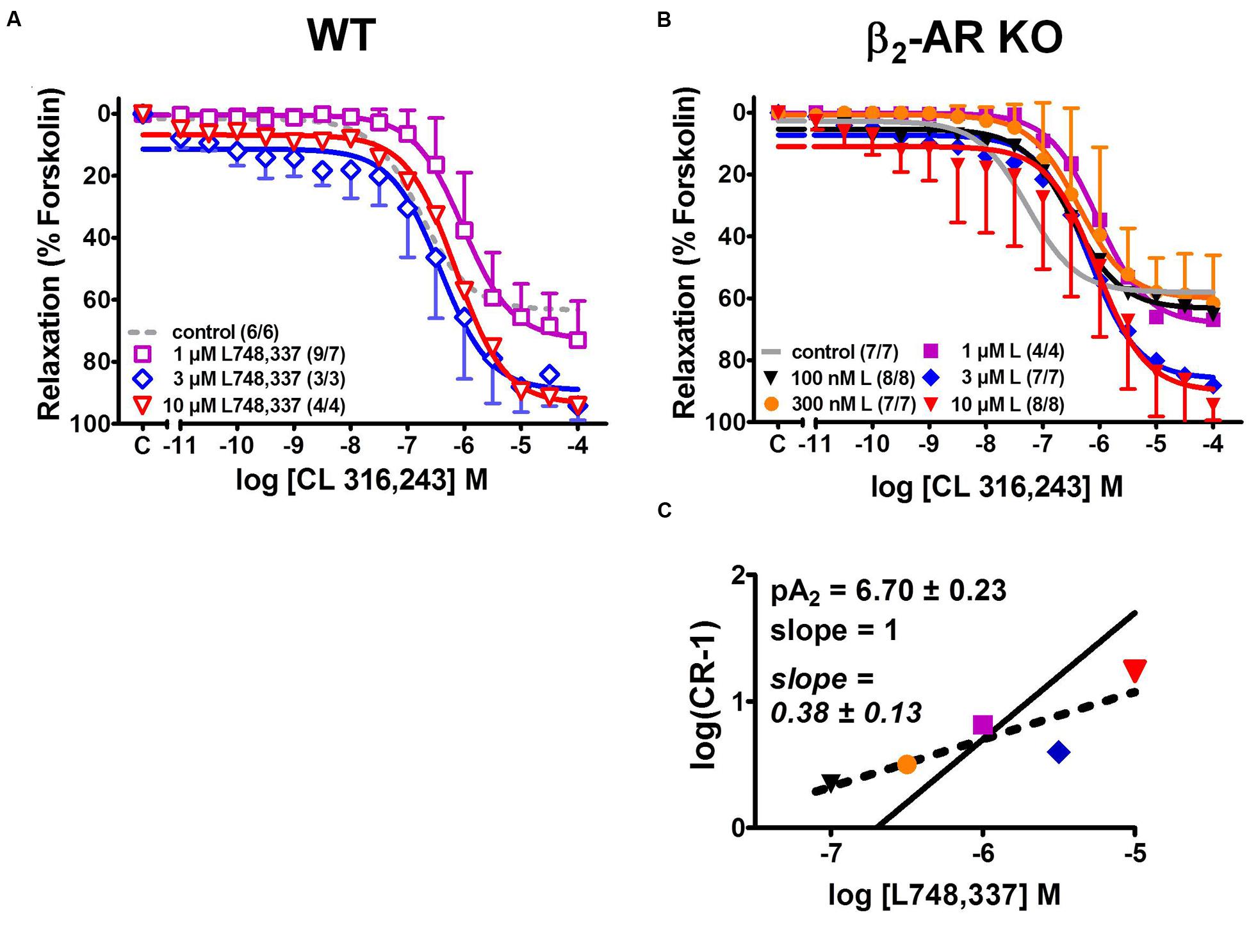
FIGURE 7. Effects of various concentrations of the β3-AR-selective blocker L748,337 on CRCs for the tone relaxing effects of CL 316,243 in KCl-precontracted detrusor strips from WT (A) and β2-AR KO mice (B). Control data (WT, dashed gray lines; β2-AR KO, continuous gray lines) were taken from Figure 2. (C) Schild plot for determining the pA2 value of L748,337 in β2-AR KO (see Materials and Methods for further details). Please note that no Schild plot could be obtained for data from WT. Mean values ± SD from n experiments as indicated in parenthesis (number of strips/number of animals).
β-AR-independent Detrusor Relaxation by Forskolin in WT and β2-AR KO Mice
In order to estimate receptor-independent relaxation we have also studied the responses to adenylyl cyclase activation with forskolin (Morita et al., 1986) in WT and β2-AR KO mice (Figure 8). Forskolin completely relaxed tonic tension (Figure 8A) and attenuated spontaneous contractions (Figure 8B) and there were no differences in sensitivity between strips from WT and β2-AR KO mice (Table 1).
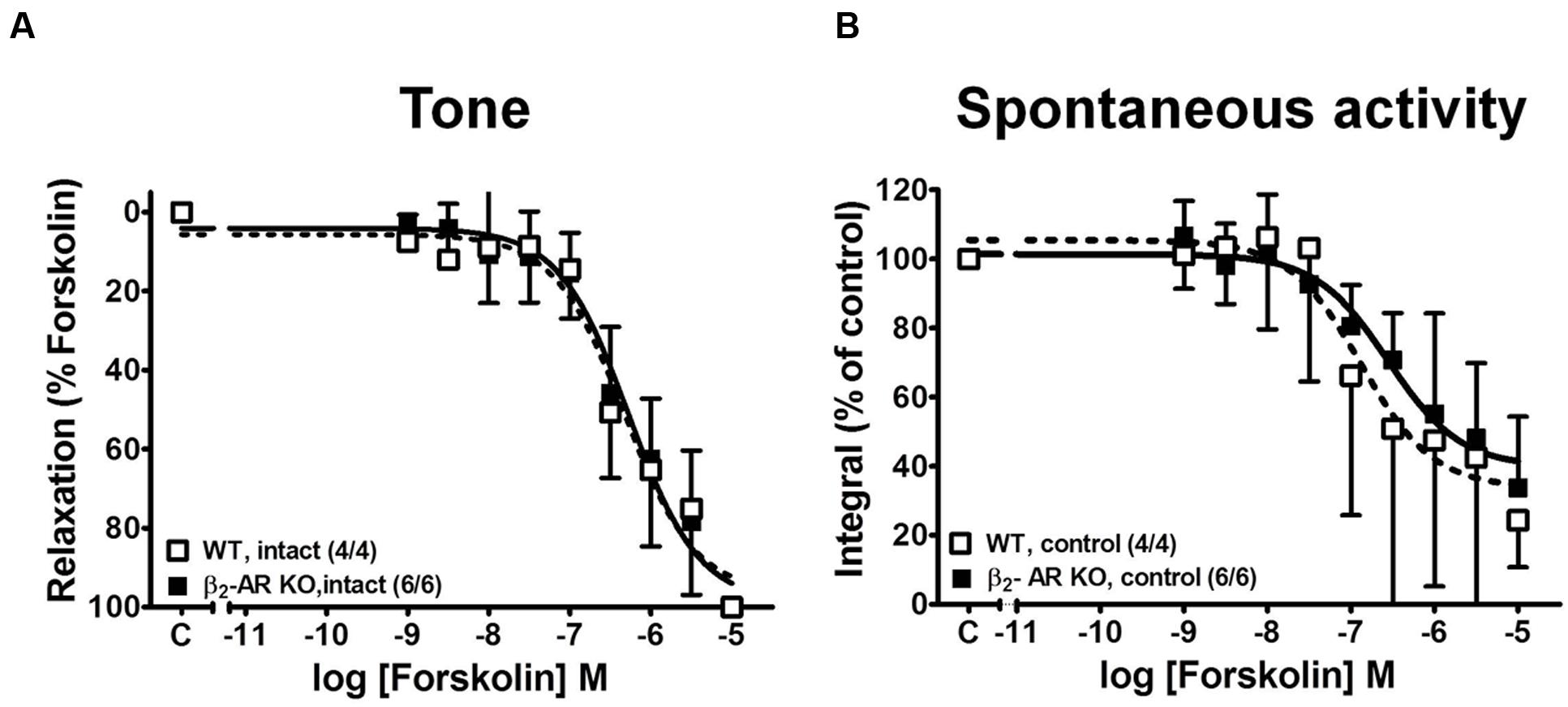
FIGURE 8. Concentration-response curves for the relaxing effects of forskolin on KCl-induced contractions in detrusor strips from WT and β2-AR KO mice. (A) Forskolin effects on tone; effects on spontaneous contractions (B). Mean values ± SD from n experiments as indicated in parenthesis (number of strips/number of animals).
Discussion
The aim of the present study was to investigate the β-AR subtypes involved in attenuating tonic and spontaneous contraction of murine detrusor in order to elucidate the discrepancy between our own results (Wuest et al., 2009; Propping et al., 2015a) and those of others (Deba et al., 2009) by utilizing β2-AR KO mice and β-AR subtype-selective ligands as pharmacological tools (Schmid et al., 2015).
Regulation of detrusor contractility is complex and not fully understood. The central force-developing step in detrusor contraction is the interaction between myosin and actin filaments that occurs upon phosphorylation of myosin light chains (MLC) via Ca2+-calmodulin dependent MLC kinase (MLCK, Andersson and Arner, 2004; Hashitani et al., 2004). Several signal transduction pathways are likely to be involved in β-AR-mediated smooth muscle relaxation. The canonical signaling pathway for β-AR involves stimulation of adenylyl cyclase, elevation of cellular cAMP levels and activation of protein kinase A (PKA). Activated PKA phosphorylates MLCK thereby impairing its Ca2+-calmodulin-dependent activation, which reduces MLC phosphorylation and hence muscle tone (Andersson and Arner, 2004; Hashitani et al., 2004). β-AR-mediated relaxation also involves Ca2+-activated K+ channels of large conductance (BKCa channels; Petkov, 2014). Enhanced BKCa channel activity via PKA-mediated phosphorylation may contribute to relaxation by hyperpolarizing the cell membrane and reducing Ca2+ influx via voltage-dependent Ca2+ channels (Wegener et al., 2004). However, Frazier et al. (2005) questioned the role of cAMP in β-AR-mediated relaxation because inhibitors of adenylyl cyclase or PKA had limited effect on rat detrusor relaxation, suggesting that other unknown pathways are involved as well.
Which β-AR Subtype Mediates (-)-Isoprenaline- or CL 316,243-induced Murine Detrusor Relaxation?
Constitutive systemic knockout of a particular receptor subtype may cause compensatory changes in expression of other receptors or proteins (Michel and Seifert, 2015). Therefore, the expression of all β-AR subtypes in detrusor tissue from WT and transgenic mice was checked using RT- PCR and quantitative real-time PCR. Despite cumulating evidence that GAPDH expression is decreased under conditions of increased sympathetic tone (Michel-Reher and Michel, 2015) we normalized the expression data to GAPDH as a housekeeping gene. In real-time PCR GAPDH remained constant (data not shown) so that we felt safe with this normalization. Our results clearly indicated complete absence of the β2-AR subtype, whilst compensatory expression of β3-(or β1-)ARs is absent in detrusor tissue from β2-AR KO mice.
Since the β2-AR KO mice were bred against a genetic background different from the previously used C57Bl6 mice, new control experiments had to be performed with FVB/N-WT mice. The results were similar as in C57Bl6 mice (Wuest et al., 2009; Propping et al., 2015a), i.e., the β2-AR blocker ICI 118,557 significantly shifted the CRCs for (-)-isoprenaline to the right, confirming that β2-ARs are involved in this relaxing effect. The affinity estimate calculated for ICI 118,557 based on these shifts (apparent pA2 values: 8.63) was in good agreement with its known affinity at β2-AR (for instance 8.92 and 8.8 for human β2-AR expressed in CHO cells, respectively, Tate et al., 1991; Palea et al., 2012).
In the absence of any β2-ARs in the KO animals, the detrusor relaxation response to (-)-isoprenaline could be mediated by β1- or β3-AR. Since β1-ARs are predominant only in guinea-pig urinary bladder (Yamamoto et al., 1998), the relaxant effect of (-)-isoprenaline in the β2-AR KO mice must have been mediated via β3-ARs. The (-)-isoprenaline concentrations required for half-maximum relaxation were about ∼90-fold higher in detrusor from β2-AR KO than WT mice. Given the affinity of murine β3-ARs for (-)-isoprenaline, e.g., -logEC50 5.57 (Blin et al., 1994), this is indeed in the concentration range required for β3-AR activation. Furthermore, the selective β3-AR agonist CL 316,243 clearly produced relaxation in detrusor strips both from WT and β2-AR KO mice with similar -logEC50 values which corresponded to the -logEC50 value of 8.61 for CL 316,243 at rodent β3-AR (Clouse et al., 2007).
The -logEC50 value of (-)-isoprenaline for relaxation of WT mouse detrusor, i.e., 7.98 was about 1.5 orders of magnitude lower than its known affinity at mammalian β2-AR (pKi 6.4; www.guidetopharmacology.org). This finding suggests that there is a large receptor reserve for detrusor relaxation via β2-AR. When β2-AR are blocked with ICI 118,551 or are absent as in β2-AR KO mice, (-)-isoprenaline is able to relax mouse detrusor via β3-AR stimulation, but spare receptors do not appear to play a role in this case.
Although, small molecule inhibitors have been valuable tools to characterize receptor subtypes in pharmacological studies, the interpretation of such results is complicated because the compounds may exhibit non-anticipated receptor-activation patterns or lack of selectivity (Michel and Seifert, 2015). In our previous work, we concluded that the relaxing effect of (-)-isoprenaline in murine detrusor was mediated via β2-ARs, because only ICI 118,551 (50 nM) shifted the CRCs for (-)-isoprenaline to the right, whereas the β1-AR blocker CGP 20712A (300 nM) and the β3-AR blocker L748,337 (100 nM) were without effect (Wuest et al., 2009; Propping et al., 2015a). In contrast, based on experiments with CL 316,243 and with higher concentrations of L748,337 (1–10 μM), other groups reported that murine detrusor relaxes via activation of β3-ARs (Deba et al., 2009). Our previous failure to detect a shift in (-)-isoprenaline CRCs by L748,337 (100 nM) in mouse detrusor may in retrospect be explained by the recent observation that L748,337 has 10-100-fold lower affinity to rodent than human β3-ARs (Palea et al., 2012; van Wieringen et al., 2013). Some of these species differences in potency have been related to differences in the binding pocket for L748,337 between human and rodent β3-AR (Cernecka et al., 2014).
In the present study we employed L748,337 concentrations up to 10 μM and found significant effects on both (-)-isoprenaline- and CL 316,243-induced relaxation of detrusor from WT and β2-AR KO mice. In our previous work, Schild plot analysis revealed a surmountable antagonism between ICI 118,551 and (-)-isoprenaline (or adrenaline) in C57Bl6 murine detrusor and between L748,337 and (-)-isoprenaline (or noradrenaline) in human detrusor (Wuest et al., 2009; Propping et al., 2013). However, the mode of antagonism by L748,337 seems to be more complex in the mouse. As expected, the antagonistic effect of L748,337 was most consistent in CL-316,243-stimulated detrusor from β2-AR KO mice, i.e., under conditions when relaxation was most likely produced by β3-AR activation.
Effects of β-AR Agonists on Spontaneous Activity
Although, spontaneous activity of detrusor muscle is not fully understood, increasing evidence suggests that smooth muscle cells posses intrinsic mechanisms for spontaneous contractions and that these are synchronized and modulated by interstitial cells distributed throughout the bladder wall (Davidson and McCloskey, 2005; Hashitani, 2006; Lagou et al., 2006). Spontaneous activity in smooth muscle cells and interstitial cells is associated with intracellular Ca2+ oscillations but appears to be generated by different mechanism as evidenced by different pharmacological responses (Johnston et al., 2008). Here, we observed larger and more spontaneous c contractions in β2-AR KO than WT strips, and in addition, more spontaneous activity developed in β2-AR KO strips that were exposed to the β2-AR antagonist ICI 118,551. While the former finding could suggest adaptive responses to the chronic absence of β2-AR mediated signaling pathways, we do not have a plausible explanation for the latter puzzling finding, which needs to be verified in future studies in order to exclude random variation as an underlying cause. Attenuation of spontaneous contractions by (-)-isoprenaline occurred in the same concentration ranges in WT and β2-AR KO strips as relaxation of tonic tension, and the CRC was only shifted to the right by ICI118,551 suggesting a dominant role for β2-AR in this process. Nevertheless, the small attenuation of spontaneous activity by CL 316,243 indicates a modulating effect of β3-AR as well. Our findings do not confirm that suppression of phasic contractions by (-)-isoprenaline is most sensitive to β1-AR blockers (Gillespie et al., 2015b), because we did not observe any shift in CRC with CGP 20712A. Taken together, comparison of the effects of subtype-selective β-AR agonists and antagonists suggests that tonic and spontaneous detrusor contractions may be modulated by different pathways but both β2- and β3-AR appear to be involved.
Effects of Forskolin
Relaxation of tonic and phasic detrusor contractions after receptor-independent activation of adenylyl cyclase with forskolin in WT and β2-AR KO mice were similar between the two groups. Furthermore, also after forskolin, spontaneous activity was suppressed less completely than tonic tension.
Conclusion
We have reported an example how false extrapolation of drug affinities for a given receptor subtype from different species can lead to an incomplete picture. Our novel findings in β2-AR KO mice suggest that there is a large receptor reserve for β2-AR so that this β-AR subtype will be activated preferentially by physiological ligands. Nevertheless β3-AR can also mediate relaxation and attenuate spontaneous contractions in the absence of β2-AR, when β2-AR are blocked or when selective β3-AR agonists are used.
Author Contributions
SP: experimental procedure, result analysis, evaluation of results, writing the manuscript; KL: experimental procedure, result analysis, evaluation of results, writing the manuscript; MM: result analysis, evaluation of results, revision of the manuscript; MW: result analysis, evaluation of results, revision of the manuscript; UR: result analysis, evaluation of results, writing and revision of the manuscript.
Funding
The study was funded by budget resources of the Department of Urology and Department of Pharmacology of the Medizinische Fakultät Carl Gustav Carus, Technische Universität Dresden.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Manja Newe, Judith Müller, Nadine Yurdagül-Hemmrich, and Gesine Haller for their excellent technical support.
We acknowledge support by the German Research Foundation and the Open Access Publication Funds of the TU Dresden.
Abbreviations
β-AR, β-adrenoceptor; β2-AR KO, β-2-adrenoceptor knockout; CGP 20712A, 1-[2-((3-carbamoyl-4-hydroxy)phenoxy)ethylamino]-3-[4-(1-methyl-4-trifluoromethyl-2-imidazolyl)phenoxy]-2-propanol; CL 316,243, (R,R)-5-[2-[[2-(3-chlorophenyl)-2-hydroxy-ethyl]-amino]propyl]-1,3-benzodioxole-2,2-dicarboxylate; CRC, concentration response curve; ICI 118,551, (±)-1-[2,3-(dihydro-7-methyl-1H-inden-4-yl)oxy]-3-[(1-methylethyl)amino]-2-butanol; (-)-isoprenaline, 4-[1-hydroxy-2-[(1-methylethyl)amino] ethyl]-1,2-benzenediol hydro-chloride; L748,337, (S)-N-[4-[2-[3-[3-(acetamidomethyl) phenoxy]-2-hydroxypropyl]-amino]-ethyl]-phenylbenzsulfonamide); RT-PCR, reverse transcription polymerase chain reaction.
References
Andersson, K. E., and Arner, A. (2004). Urinary bladder contraction and relaxation: physiology and pathophysiology. Physiol. Rev. 84, 935–986. doi: 10.1152/physrev.00038.2003
Blin, N., Nahmias, C., Drumare, M. F., and Strosberg, A. D. (1994). Mediation of most atypical effects by species homologues of the beta 3-adrenoceptor. Br. J. Pharmacol. 112, 911–919. doi: 10.1111/j.1476-5381.1994.tb13167.x
Cernecka, H., Sand, C., and Michel, M. C. (2014). The odd sibling: features of beta3-adrenoceptor pharmacology. Mol. Pharmacol. 86, 479–484. doi: 10.1124/mol.114.092817
Chapple, C. R., Cardozo, L., Nitti, V. W., Siddiqui, E., and Michel, M. C. (2014). Mirabegron in overactive bladder: a review of efficacy, safety, and tolerability. Neurourol. Urodyn. 33, 17–30. doi: 10.1002/nau.22505
Chernogubova, E., Hutchinson, D. S., Nedergaard, J., and Bengtsson, T. (2005). Alpha1- and beta1-adrenoceptor signaling fully compensates for beta3-adrenoceptor deficiency in brown adipocyte norepinephrine-stimulated glucose uptake. Endocrinology 146, 2271–2284. doi: 10.1210/en.2004-1104
Clouse, A. K., Riedel, E., Hieble, J. P., and Westfall, T. D. (2007). The effects and selectivity of beta-adrenoceptor agonists in rat myometrium and urinary bladder. Eur. J. Pharmacol. 573, 184–189. doi: 10.1016/j.ejphar.2007.06.016
Davidson, R. A., and McCloskey, K. D. (2005). Morphology and localization of interstitial cells in the guinea pig bladder: structural relationships with smooth muscle and neurons. J. Urol. 173, 1385–1390. doi: 10.1097/01.ju.0000146272.80848.37
Deba, A., Palea, S., Rouget, C., Westfall, T. D., and Lluel, P. (2009). Involvement of beta(3)-adrenoceptors in mouse urinary bladder function: role in detrusor muscle relaxation and micturition reflex. Eur. J. Pharmacol. 618, 76–83. doi: 10.1016/j.ejphar.2009.07.012
Eastham, J., Stephenson, C., Korstanje, K., and Gillespie, J. I. (2015). The expression of beta3-adrenoceptor and muscarinic type 3 receptor immuno-reactivity in the major pelvic ganglion of the rat. Naunyn Schmiedebergs Arch. Pharmacol. 388, 695–708. doi: 10.1007/s00210-015-1122-5
Evans, B. A., Papaioannou, M., Hamilton, S., and Summers, R. J. (1999). Alternative splicing generates two isoforms of the beta3-adrenoceptor which are differentially expressed in mouse tissues. Br. J. Pharmacol. 127, 1525–1531. doi: 10.1038/sj.bjp.0702688
Frazier, E. P., Mathy, M. J., Peters, S. L., and Michel, M. C. (2005). Does cyclic AMP mediate rat urinary bladder relaxation by isoproterenol? J. Pharmacol. Exp. Ther. 313, 260–267. doi: 10.1124/jpet.104.077768
Gillespie, J. I., Rouget, C., Palea, S., Granato, C., Birder, L., and Korstanje, C. (2015a). The characteristics of intrinsic complex micro-contractile activity in isolated strips of the rat bladder. Naunyn Schmiedebergs Arch. Pharmacol. 388, 709–718. doi: 10.1007/s00210-015-1131-4
Gillespie, J. I., Rouget, C., Palea, S., Granato, C., and Korstanje, C. (2015b). Beta adrenergic modulation of spontaneous microcontractions and electrical field-stimulated contractions in isolated strips of rat urinary bladder from normal animals and animals with partial bladder outflow obstruction. Naunyn Schmiedebergs Arch. Pharmacol. 388, 719–726. doi: 10.1007/s00210-015-1136-z
Hashitani, H. (2006). Interaction between interstitial cells and smooth muscles in the lower urinary tract and penis. J. Physiol. 576, 707–714. doi: 10.1113/jphysiol.2006.116632
Hashitani, H., Brading, A. F., and Suzuki, H. (2004). Correlation between spontaneous electrical, calcium and mechanical activity in detrusor smooth muscle of the guinea-pig bladder. Br. J. Pharmacol. 141, 183–193. doi: 10.1038/sj.bjp.0705602
Johnston, L., Carson, C., Lyons, A. D., Davidson, R. A., and McCloskey, K. D. (2008). Cholinergic-induced Ca2+ signaling in interstitial cells of Cajal from the guinea pig bladder. Am. J. Physiol. Renal Physiol. 294, F645–F655. doi: 10.1152/ajprenal.00526.2007
Krauwinkel, W., van Dijk, J., Schaddelee, M., Eltink, C., Meijer, J., Strabach, G., et al. (2012). Pharmacokinetic properties of mirabegron, a beta3-adrenoceptor agonist: results from two phase I, randomized, multiple-dose studies in healthy young and elderly men and women. Clin. Ther. 34, 2144–2160. doi: 10.1016/j.clinthera.2012.09.010
Lagou, M., De Vente, J., Kirkwood, T. B., Hedlund, P., Andersson, K. E., Gillespie, J. I., et al. (2006). Location of interstitial cells and neurotransmitters in the mouse bladder. BJU Int. 97, 1332–1337. doi: 10.1111/j.1464-410X.2006.06203.x
Michel, M. C., and Seifert, R. (2015). Selectivity of pharmacological tools: implications for use in cell physiology. A review in the theme: cell signaling: proteins, pathways and mechanisms. Am. J. Physiol. Cell Physiol. 308, C505–C520. doi: 10.1152/ajpcell.00389.2014
Michel-Reher, M. B., and Michel, M. C. (2015). Regulation of GAPDH expression by treatment with the beta-adrenoceptor agonist isoprenaline–is GADPH a suitable loading control in immunoblot experiments? Naunyn Schmiedebergs Arch. Pharmacol. 388, 1119–1120. doi: 10.1007/s00210-015-1166-6
Morita, T., Wheeler, M. A., Miyagawa, I., Kondo, S., and Weiss, R. M. (1986). Effects of forskolin on contractility and cyclic AMP levels in rabbit detrusor muscle. Tohoku J. Exp. Med. 149, 283–285. doi: 10.1620/tjem.149.283
Palea, S., Rekik, M., Rouget, C., Camparo, P., Botto, H., Rischmann, P., et al. (2012). Fenoterol functionally activates the beta(3)-adrenoceptor in human urinary bladder, comparison with rat and mouse: implications for drug discovery. Eur. J. Pharmacol. 690, 202–206. doi: 10.1016/j.ejphar.2012.06.036
Petkov, G. V. (2014). Central role of the BK channel in urinary bladder smooth muscle physiology and pathophysiology. Am. J. Physiol. Regul. Integr. Comp. Physiol. 307, 571–584. doi: 10.1152/ajpregu.00142.2014
Propping, S., Newe, M., Kaumann, A. J., Wirth, M. P., and Ravens, U. (2015a). Mucosa of murine detrusor impairs beta2 -adrenoceptor-mediated relaxation. Neurourol. Urodyn. 34, 592–597. doi: 10.1002/nau.22627
Propping, S., Newe, M., Lorenz, K., Wirth, M. P., and Ravens, U. (2015b). Beta-adrenoceptor-mediated relaxation of carbachol-pre-contracted mouse detrusor. Urol. Int. 95, 92–98. doi: 10.1159/000369075
Propping, S., Roedel, M., Wirth, M. P., and Ravens, U. (2015c). Pharmacological modulation of mucosa-related impairment of beta-adrenoceptor-mediated relaxation in human detrusor. Urol. Int. 95, 300–308. doi: 10.1159/000431260
Propping, S., Wuest, M., Eichhorn, B., Wirth, M. P., Kaumann, A. J., and Ravens, U. (2013). Mucosa of human detrusor impairs contraction and beta-adrenoceptor-mediated relaxation. BJU Int. 112, 1215–1222. doi: 10.1111/bju.12267
Schild, H. O. (1947). pA, a new scale for the measurement of drug antagonism. Br. J. Pharmacol. Chemother. 2, 189–206. doi: 10.1111/j.1476-5381.1947.tb00336.x
Schmid, E., Neef, S., Berlin, C., Tomasovic, A., Kahlert, K., Nordbeck, P., et al. (2015). Cardiac RKIP induces a beneficial beta-adrenoceptor-dependent positive inotropy. Nat. Med. 21, 1298–1306. doi: 10.1038/nm.3972
Svalo, J., Nordling, J., Bouchelouche, K., Andersson, K. E., Korstanje, C., and Bouchelouche, P. (2013). The novel beta3-adrenoceptor agonist mirabegron reduces carbachol-induced contractile activity in detrusor tissue from patients with bladder outflow obstruction with or without detrusor overactivity. Eur. J. Pharmacol. 699, 101–105. doi: 10.1016/j.ejphar.2012.11.060
Takeda, H., Matsuzawa, A., Igawa, Y., Yamazaki, Y., Kaidoh, K., Akahane, S., et al. (2003). Functional characterization of beta-adrenoceptor subtypes in the canine and rat lower urinary tract. J. Urol. 170, 654–658. doi: 10.1097/01.ju.0000074622.50255.a8
Tate, K. M., Briend-Sutren, M. M., Emorine, L. J., Delavier-Klutchko, C., Marullo, S., and Strosberg, A. D. (1991). Expression of three human beta-adrenergic-receptor subtypes in transfected Chinese hamster ovary cells. Eur. J. Biochem. 196, 357–361. doi: 10.1111/j.1432-1033.1991.tb15824.x
Uchida, H., Shishido, K., Nomiya, M., and Yamaguchi, O. (2005). Involvement of cyclic AMP-dependent and -independent mechanisms in the relaxation of rat detrusor muscle via beta-adrenoceptors. Eur. J. Pharmacol. 518, 195–202. doi: 10.1016/j.ejphar.2005.06.029
van Wieringen, J. P., Michel-Reher, M. B., Hatanaka, T., Ueshima, K., and Michel, M. C. (2013). The new radioligand [(3)H]-L 748,337 differentially labels human and rat beta3-adrenoceptors. Eur. J. Pharmacol. 720, 124–130. doi: 10.1016/j.ejphar.2013.10.039
Vidal, M., Wieland, T., Lohse, M. J., and Lorenz, K. (2012). Beta-Adrenergic receptor stimulation causes cardiac hypertrophy via a Gbetagamma/Erk-dependent pathway. Cardiovasc. Res. 96, 255–264. doi: 10.1093/cvr/cvs249
Wegener, J. W., Schulla, V., Lee, T. S., Koller, A., Feil, S., Feil, R., et al. (2004). An essential role of Cav1.2 L-type calcium channel for urinary bladder function. FASEB J. 18, 1159–1161.
Wuest, M., Eichhorn, B., Grimm, M. O., Wirth, M. P., Ravens, U., and Kaumann, A. J. (2009). Catecholamines relax detrusor through beta 2-adrenoceptors in mouse and beta 3-adrenoceptors in man. J. Pharmacol. Exp. Ther. 328, 213–222. doi: 10.1124/jpet.108.142562
Keywords: detrusor muscle, relaxation, mucosa, β2-adrenoceptor knockout, β3-adrenoceptors, isoprenaline, CL 316,243
Citation: Propping S, Lorenz K, Michel MC, Wirth MP and Ravens U (2016) β-Adrenoceptor-mediated Relaxation of Urinary Bladder Muscle in β2-Adrenoceptor Knockout Mice. Front. Pharmacol. 7:118. doi: 10.3389/fphar.2016.00118
Received: 16 January 2016; Accepted: 22 April 2016;
Published: 09 May 2016.
Edited by:
Paulo Correia-de-Sá, Universidade do Porto, PortugalReviewed by:
James Innes Gillespie, Newcastle University, UKEdson Antunes, State University of Campinas, Brazil
Copyright © 2016 Propping, Lorenz, Michel, Wirth and Ravens. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stefan Propping, stefan.propping@uniklinikum-dresden.de
 Stefan Propping
Stefan Propping Kristina Lorenz
Kristina Lorenz Martin C. Michel
Martin C. Michel Manfred P. Wirth1
Manfred P. Wirth1 Ursula Ravens
Ursula Ravens