PrPC from stem cells to cancer
- 1Toxicology, Pharmacology and Cellular Signaling, INSERM UMR-S1124, Paris, France
- 2Toxicology, Pharmacology and Cellular Signaling, Université Paris Descartes, Sorbonne Paris Cité, UMR-S1124, Paris, France
- 3Université Paris Sud 11, ED419 Biosigne, Orsay, France
- 4U892 Virologie et Immunologie Moléculaires, INRA, Jouy-en-Josas, France
- 5UMR1313 Génétique Animale et Biologie Intégrative, INRA, Jouy-en-Josas, France
- 6AP-HP Service de Biochimie, Fondation FondaMental, INSERM U942 Hôpital Lariboisière, Paris, France
- 7Pharma Research Department, F. Hoffmann-La-Roche Ltd., Basel, Switzerland
The cellular prion protein PrPC was initially discovered as the normal counterpart of the pathological scrapie prion protein PrPSc, the main component of the infectious agent of Transmissible Spongiform Encephalopathies. While clues as to the physiological function of this ubiquitous protein were greatly anticipated from the development of knockout animals, PrP-null mice turned out to be viable and to develop without major phenotypic abnormalities. Notwithstanding, the discovery that hematopoietic stem cells from PrP-null mice have impaired long-term repopulating potential has set the stage for investigating into the role of PrPC in stem cell biology. A wealth of data have now exemplified that PrPC is expressed in distinct types of stem cells and regulates their self-renewal as well as their differentiation potential. A role for PrPC in the fate restriction of embryonic stem cells has further been proposed. Paralleling these observations, an overexpression of PrPC has been documented in various types of tumors. In line with the contribution of PrPC to stemness and to the proliferation of cancer cells, PrPC was recently found to be enriched in subpopulations of tumor-initiating cells. In the present review, we summarize the current knowledge of the role played by PrPC in stem cell biology and discuss how the subversion of its function may contribute to cancer progression.
Introduction
The discovery of the cellular prion protein PrPC dates back to 1985 with the identification that the scrapie prion protein PrPSc, the main component of the infectious agent responsible for Transmissible Spongiform Encephalopathies (TSEs) was encoded by a gene of the host, termed Prnp (Oesch et al., 1985). PrPC has been extensively scrutinized as the endogenous substrate for conversion into its pathogenic PrPSc counterpart (Aguzzi and Calella, 2009), while studies on its physiological function have long been overlooked. At the molecular and cellular levels, it is well established that PrPC is anchored to the outer leaflet of the plasma membrane through a glycosyl-phosphatidylinositol (GPI) moiety (Linden et al., 2008). It may exist under a great diversity of isoforms as a result of heterogeneous glycosylation (Ermonval et al., 2003) and proteolytic cleavage (McDonald et al., 2014). Although it is suspected that the wide repertoire of PrPC species may endow the protein with the capacity to interact with multiple soluble ligands, extracellular matrix components or cell-surface proteins, the specific tissue distribution, and function of each isoform remain elusive (Linden et al., 2008). That research on PrPC function has lagged behind that of TSE pathophysiology may notably be explained by the lack of major abnormalities in PrP-null mice (Steele et al., 2007), whose most obvious phenotype is their resistance to TSE agents (Bueler et al., 1993). Because PrP is ubiquitously expressed and very much conserved in mammals, with Prnp orthologs identified in fish, birds, and reptiles (Premzl and Gamulin, 2007), the apparent normal phenotype of PrP null mice was quite unexpected and proposed to reflect the occurrence of compensatory mechanisms. One major contribution of these mice, however, was the demonstration that PrPC is mandatory for the long-term repopulating activity of hematopoietic stem cells (HSCs) (see below) (Zhang et al., 2006). This seminal report set the stage for investigating into the role exerted by PrPC in stem cell biology. Here, we provide an overview of the recent advances regarding the contribution of PrPC to stem cell biology and their pathophysiological implications.
PrPC Expression and Role During Development
Studies on PrPC have initially focused on the adult central nervous system (CNS), since it is the only target of PrPSc-associated toxicity (Aguzzi and Calella, 2009). Further, PrPC is most abundantly found in neurons (Linden et al., 2008). Notwithstanding, PrPC is highly expressed during embryonic development, as first shown by Manson et al. over two decades ago (Manson et al., 1992). This in situ hybridization analysis revealed widespread expression of Prnp transcripts in the developing central and peripheral nervous system at embryonic days E13.5 and E16.5, as well as in other tissues such as the intestine or the dental lamina (Manson et al., 1992). Prnp mRNA was also detected in extra-embryonic tissues from E6.5, pointing for the first time to a potential role for PrPC in the placenta, which has started to be accurately assessed recently (Alfaidy et al., 2012; Passet et al., 2012). These first data were refined with the detection of Prnp mRNA starting at E8.5–E9 in the differentiating neuroepithelium (Miele et al., 2003). The induction of Prnp expression at this stage in the developing CNS and heart was confirmed in a study using Prnp-LacZ reporter mice (Tremblay et al., 2007).
Based on this developmental pattern of expression, transcriptomic analyses were carried out on early Prnp knockout vs. wild-type (WT) embryos and revealed prominent alterations, with a total number of 263 genes differentially expressed at day E7.5 (Khalife et al., 2011). The array of genes with altered expression notably includes a set of growth factors and growth factor receptors, supporting the notion that PrPC plays an important role in the regulation of cascades associated with embryonic development (Khalife et al., 2011). Interestingly, the pattern of pathways affected overlaps with that obtained after early embryonic gene expression profiling of zebrafish PrP2 morphants (Nourizadeh-Lillabadi et al., 2010).
The zebrafish model actually allowed bringing to light a vital function for PrPC since morpholino-mediated knockdown of the PrP ortholog PrP1 in this species leads to loss of embryonic cell adhesion and gastrulation arrest (Malaga-Trillo et al., 2009). Of note, the defects observed could be rescued with mouse Prnp mRNA, indicating that this function is evolutionary conserved (Malaga-Trillo et al., 2009). Thus, the overall data gained at the animal scale argue that PrPC fulfills an important function during embryogenesis and that its ablation in mice triggers the implementation of yet-to-be-deciphered compensatory mechanisms.
PrPC Regulates the Self-Renewal of Stem/Progenitor Cells
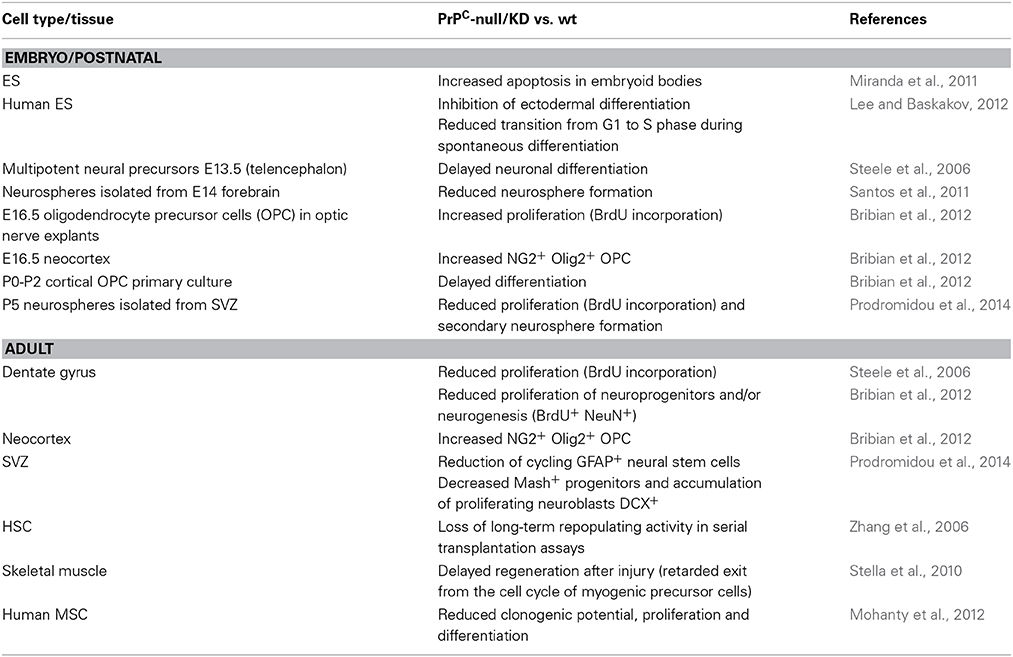
The link between PrPC and stem cell biology was first uncovered in HSCs. Investigations of PrPC in the hematopoietic system were initially prompted by the observation that PrPSc accumulates in lymphoid organs and by the quest to understand the cellular mechanisms sustaining prion propagation in the periphery (Mabbott and MacPherson, 2006). These studies demonstrated that PrPC is highly expressed at the surface of various hematopoietic cells, including B and T lymphocytes, monocytes, dendritic cells, megakaryocytes and platelets, but not erythrocytes or granulocytes (Linden et al., 2008). In the human bone marrow, PrPC was found to be present in the CD34+ stem / progenitor cell population (Dodelet and Cashman, 1998) and to be preferentially expressed on murine CD43+, B220−, IL-7R− cells, enriched in immature progenitors (Liu et al., 2001). A major advance was the discovery by the team of Lodish that PrPC is very abundant at the surface of mouse bone marrow Lin−Sca+Endoglin+ cells, a population comprising immature HSCs (Zhang et al., 2006). These authors then assessed the ability of bone marrow-derived Lin−Sca+Endoglin+ cells from Prnp knockout mice to reconstitute the hematopoietic system of lethally irradiated mice in serial transplantation assays, and demonstrated that HSCs from Prnp null mice lack long-term repopulating activity (see Table 1) (Zhang et al., 2006). These experiments further allowed substantiating that the Lin−Sca+Endoglin+ cell population endowed with long-term repopulating activity is PrPC positive (Zhang et al., 2006).
Much like HSCs, neuroepithelial stem cells have the capacity to proliferate through repeated symmetric divisions and generate radial glial cells, which then undergo asymmetric division and give rise to neurons, oligodendrocytes, and astrocytes (Gotz and Huttner, 2005). The proper proceeding of these expansive and neurogenic phases is crucial for the development of the CNS. By comparing neural stem cells isolated from Prnp knockout, WT or PrP overexpressing mice at embryonic day 13.5, Steele et al. documented that PrPC levels directly increase the differentiation rate of multipotent neural precursor cells (Steele et al., 2006). In the same study, PrPC expression levels were further found to correlate with the proliferation rate in the two adult neurogenic regions, the subventricular zone (SVZ) or the dentate gyrus (DG) (Steele et al., 2006). The latter finding was corroborated by two independent studies showing that the formation of neurospheres from fetal (Santos et al., 2011) or postnatal (Prodromidou et al., 2014) brains is less efficient with Prnp knockout than WT mice.
The notion that PrPC contributes to the proliferation of stem cells extends beyond hematopoietic and neural stem cells. Indeed, the level of PrPC was found to serve as an effective cell surface marker for self-renewing mammary gland stem cells in mice (Liao et al., 2007). More recently, PrPC was further shown to promote the expansion and engraftment of bone marrow-derived human mesenchymal stem cells (MSCs) (Mohanty et al., 2012). Finally, PrPC was shown to exert either an anti- or a pro-proliferative effect in human embryonic stem (ES) cells, depending on whether they are grown under self-renewing or differentiating conditions, respectively (Lee and Baskakov, 2012).
PrPC Influences Stem Cell Fate
The identification of PrPC as a broad cell surface marker for stem / progenitor cells raises the question as to whether the expression of PrPC is a determinant of the stem cell fate. In this respect, we (Mouillet-Richard et al., 1999) and others (Peralta et al., 2011) have provided evidence that PrPC is upregulated following the cell fate restriction of multipotential ES or embryonic carcinoma (EC) cells toward the neuronal lineage. Similarly, PrPC is induced in ES-derived cardiomyogenic progenitors obtained after embryoid body (EB) formation (Hidaka et al., 2010). In line with this, the expression of PrPC was found to be increased during spontaneous differentiation of mouse and human ES cells (Lee and Baskakov, 2010; Miranda et al., 2011) and, reciprocally, induction of PrPC in human ES cells grown under self-renewal conditions was shown to promote their differentiation (Lee and Baskakov, 2012). Intriguingly, exposure of ES cells to recombinant PrP delays their spontaneous differentiation (Lee and Baskakov, 2010). In view of the early expression of PrPC in extra-embryonic tissues, it is tempting to speculate that placenta-derived PrPC may serve as a paracrine signal to maintain the self-renewal of inner mass cells, until their appropriate induction toward either of the three lineages.
Beyond lineage specification, the regulation of PrPC expression also accompanies differentiation toward a given fate. Along the hematopoietic lineage, PrPC appears to be downregulated upon differentiation of CD34+ progenitors toward a granulocytic fate, while its expression is retained in B and T lymphocytes as well as monocytes (Dodelet and Cashman, 1998). In addition, PrPC is absent from erythrocytes (Dodelet and Cashman, 1998) and abundant in megakaryocytes and platelets (Starke et al., 2005), suggesting that the expression of PrPC is switched off with the commitment of megakaryocytic-erythrocytic progenitors toward the erythroid fate, or is decreased along erythroid differentiation, in line with (Panigaj et al., 2011).
As for neural progenitor cells, the expression of PrPC was reported to be increased along neuronal differentiation, while barely detected in astrocytes or oligodendrocytes (Steele et al., 2006). This high neuronal PrPC expression is in line with the well-documented contribution of PrPC to neuronal differentiation, including neurite outgrowth (Chen et al., 2003; Santuccione et al., 2005; Loubet et al., 2012; Santos et al., 2013) or synapse maturation (Kanaani et al., 2005). The lack of PrPC detection in differentiating oligodendrocytes and astrocytes in the study by Steele et al. (2006) is, however, in contrast with several reports documenting an abundant PrPC expression in these two cell types in late embryos or in the postnatal brain (Moser et al., 1995; Lima et al., 2007; Bribian et al., 2012). Interestingly, both oligodendrocytic (Bribian et al., 2012) and astrocytic (Arantes et al., 2009) differentiation kinetics appear to be delayed in Prnp knockout mice. These observations recall the delay in neuronal differentiation, as initially reported by Steele et al. (2006), as well as the slower regeneration of muscle after injury (Stella et al., 2010) in a PrP null context. Whether PrPC expression affects the balance from one fate to another remains, however, to be investigated. In this regard, it is worth noting that prion infection in adult neural stem cells (NSCs) favors the differentiation toward the glial lineage at the expense of neuronal differentiation (Relaño-Ginés et al., 2013).
Stem Cells and Prion Replication
Whether stem cells are susceptible to prion infection may at first seem a question without relevance, since TSEs are neurodegenerative diseases. However, as rightly underlined in the study by Relaño-Ginés (Relaño-Ginés et al., 2013), exploiting the potential of adult NSCs is currently considered as a promising avenue to mitigate neurodegeneration (Bellenchi et al., 2013). While several studies had reported an efficient replication of PrPSc in neurospheres isolated from fetal brain (Milhavet et al., 2006; Herva et al., 2010), the susceptibility of adult NSC toward prion infection has been evaluated only recently. In line with the results obtained with embryonic-derived cultures, neurospheres isolated from the SVZ or the DG of adult mice were shown to support prion replication (Relaño-Ginés et al., 2013). The same study further documented the presence of dense PrPSc deposits in the DG and the SVZ of prion-infected mice, indicating that prions colonize adult NSCs, the brain's endogenous repair machinery (Relaño-Ginés et al., 2013, 2014). Of note, prion replication of adult NSCs was found to impair neuronal differentiation, both in vitro and in vivo (Relaño-Ginés et al., 2013). Thus, in addition to constituting a reservoir of PrPSc, the replication of prions in adult NSCs may also compromise the regeneration of damaged neurons. Finally, because PrPSc is known to deviate the normal function of PrPC (Westergard et al., 2007; Pradines et al., 2013), these observations suggest that studying the impact of prion infection on the self-renewal and fate of NSCs may improve our understanding of the physiological role exerted by PrPC in these processes.
PrPC-Dependent Control of Stem Cell Self-Renewal and Fate: Mechanistic Insight
Notwithstanding the well-established involvement of PrPC in the self-renewal of diverse types of stem /progenitor cells, the molecular mechanisms at play remain obscure. One possible mode of action of PrPC would be through the interaction with one of its ligands. This view is clearly supported by the demonstration that the binding of PrPC with STI-1 is critical for the formation and proliferation of neurospheres cultured from fetal forebrain (Santos et al., 2011). While several signaling cascades elicited by the interaction of STI-1 with PrPC have been described in a neuronal context (Hirsch et al., 2014), the pathways mobilized to sustain neurosphere self-renewal and proliferation have not been analyzed so far (Santos et al., 2011). On another hand, the presence of PrPC on neurospheres was recently shown to be required for NCAM-induced neuronal differentiation (Prodromidou et al., 2014). These two sets of observations raise the question as to the PrPC isoforms that respectively bind STI-1 and NCAM, since these two molecules instruct distinct responses. Another PrPC partner that may have relevance to stem cell biology is the amyloid precursor protein APP, whose functional interaction with PrP in the zebrafish modulates cell adhesion and CNS development (Kaiser et al., 2012). Whether the APP-PrPC interaction is involved in the regulation of E-cadherin-dependent adhesion in zebrafish embryos deserves further investigation (Malaga-Trillo et al., 2009). Interestingly, our own studies on a neuroectodermal stem cell line also substantiate a disruption of cadherin-mediated cell contacts upon PrPC depletion (Martin-Lannerée et al., unpublished observations). It is of note that cell adhesion processes are now recognized as major determinants of stem cell biology in relation with their local microenvironment (stem cell niche) (Marthiens et al., 2010). By affecting adhesion properties of stem cells, the depletion of PrPC may thus in turn impact on their retention, self-renewal or exit from their niche.
A Role for PrPC in Cancer Stem Cells?
The contribution of PrPC to cell proliferation appears to apply to many cell types beyond stem/progenitor cells. These notably include cancer cells, as first demonstrated in gastric tumor cell lines (Liang et al., 2007a). In these cells, PrPC was shown to accelerate the G1 to S phase transition in the cell cycle and to sustain proliferation by inducing the expression of Cyclin D1 through a PI3K/Akt pathway (Liang et al., 2007a). The PrPC-interacting protein(s) involved in this cascade remain(s), however, to be identified. Beyond proliferation, PrPC overexpression in cancer cells was further shown to confer resistance to various cytotoxic agents (Mehrpour and Codogno, 2010) as well as invasive properties (Pan et al., 2006). For instance, PrPC levels were shown to correlate with resistance to TNFα-induced cell death in the MCF-7 breast cancer cell line (Diarra-Mehrpour et al., 2004). Very recently, PrPC was found to interact with the cell surface protein CD44 in adriamycin-resistant breast cancer cells, and to promote their proliferation and migration (Cheng et al., 2013). Interestingly, CD44 has been reported to be enriched at the cell surface of various types of tumor-initiating cells, which bear similarities with embryonic or adult stem cells and are often referred to as cancer stem cells (CSCs) (Medema, 2013). It is also noteworthy that CSCs have been associated with increased resistance to antitumor treatments (Singh and Settleman, 2010). In line with the above-mentioned role of PrPC in the self-renewal of stem cells, Du et al. depicted a population of CD44+PrPC+ cells from primary colorectal tumors endowed with enhanced tumor-initiating and metastatic capacity (Du et al., 2013). At a mechanistic level, PrPC was shown to promote an epithelial to mesenchymal transition (EMT) through the regulation of the Twist transcription factor (Du et al., 2013). These observations are in agreement with the notion that the emergence of CSCs and EMT are intimately connected (Singh and Settleman, 2010).
A still unresolved question concerns the molecular mechanisms sustaining the enhanced expression of PrPC in cancer cells. PrPC expression has been shown to be increased in response to hypoxia in gastric cancer cell lines (Liang et al., 2007b). Other PrPC-inducing signals include oxidative (Sauer et al., 1999) and endoplasmic-reticulum (Dery et al., 2013) stresses. Some deregulation of PrPC function may also arise with aging. Indeed, PrPC was recently shown to accumulate in lipid rafts in the mouse aging brain (Agostini et al., 2013). Whether this change in PrPC distribution also occurs in other tissues with aging is worth considering, since it would potentially impact on the recruitment of downstream signaling cascades. As observed in the context of neurodegeneration (Hirsch et al., 2014), the subversion of PrPC function may over-activate src kinases and further promote alterations in lipid raft-initiated signaling pathways, known to be detrimental in cancer (Patra, 2008). Such changes may in turn have consequences on the cell local environment, and, in the case of stem cells, deregulate the interactions with their niche. This scenario is considered as a potential cause of CSCs emergence (Rezza et al., 2014), and this may have particular relevance with respect to aging.
Open Questions and Therapeutic Prospects
Harnessing the self-renewal and differentiation potential of stem cells represents a major challenge for regenerative medicine. The recent accumulation of data regarding the involvement of PrPC in stem cell biology warrants further studying the molecular and cellular mechanisms sustaining the contribution of this protein to the proliferation of stem cells, their maintenance in an undifferentiated state, their capacity to respond to fate determination inputs and to implement a given differentiation program. Achieving this task is complicated by the multiplicity of PrPC isoforms and partners, which may fulfill promiscuous functions. That PrPC is required for efficient tissue repair after injury is clearly indicated in the context of bone-marrow reconstitution (Zhang et al., 2006) or muscle regeneration (Stella et al., 2010), which suggest that the mobilization of PrPC-dependent cascades via appropriate ligands may provide a fruitful approach to enhance the regeneration of lesionned tissues. As a prerequisite, manipulating conditions would need to be carefully adjusted in order to control activating signals, given the pathological implications that may ensue from PrPC over-activation.
Finally, the emerging roles of PrPC in stemness on the one hand and in various aspects of cancer cell biology on the other hand bring new light on this already fascinating molecule. Given the relationship between stem cells and oncogenesis, advance in the understanding of the role played by PrPC in stem cells is likely to illuminate the issue of its contribution to tumorigenesis and vice-versa. One major remaining challenge is to decipher the mechanisms controlling the expression levels of PrPC in normal and cancer stem cells. While the cues underlying the induction of PrPC during embryonic development are elusive, several cancer-associated conditions, including hypoxia (Liang et al., 2007b), oxidative (Sauer et al., 1999) or endoplasmic reticulum (Dery et al., 2013) stresses have been reported to activate PrPC transcription. More broadly, increasing our knowledge of the regulation of PrP gene expression may help design novel strategies for therapeutic intervention in cancer, beyond directly targeting PrPC through antisense oligonucleotides (Meslin et al., 2007) or monoclonal antibodies (Du et al., 2013).
To conclude, progress in the stem cell and cancer fields should increase our knowledge of how PrPC, as a cell surface receptor or co-receptor, connects cells with their environment to drive adaptive, homeostatic responses and how this function is corrupted in disease-associated states.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We apologize to colleagues in the field whose work we were unable to cite owing to space limitations. We acknowledge financial support from the ARC, INSERM, as well as the Region Ile de France (DIM-Stem Pôle).
References
Agostini, F., Dotti, C. G., Perez-Canamas, A., Ledesma, M. D., Benetti, F., and Legname, G. (2013). Prion protein accumulation in lipid rafts of mouse aging brain. PLoS ONE 8:e74244. doi: 10.1371/journal.pone.0074244
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Aguzzi, A., and Calella, A. M. (2009). Prions: protein aggregation and infectious diseases. Physiol. Rev. 89, 1105–1152. doi: 10.1152/physrev.00006.2009
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Alfaidy, N., Chauvet, S., Andrei, S., Salomon, A., Saoudi, Y., Richaud, P., et al. (2012). Prion protein expression and functional importance in developmental angiogenesis: role in oxidative stress and copper homeostasis. Antioxid. Redox Signal. 18, 400–411. doi: 10.1089/ars.2012.4637
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Arantes, C., Nomizo, R., Lopes, M. H., Hajj, G. N., Lima, F. R., and Martins, V. R. (2009). Prion protein and its ligand stress inducible protein 1 regulate astrocyte development. Glia 57, 1439–1449. doi: 10.1002/glia.20861
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Bellenchi, G. C., Volpicelli, F., Piscopo, V., Perrone-Capano, C., and di Porzio, U. (2013). Adult neural stem cells: an endogenous tool to repair brain injury? J. Neurochem. 124, 159–167. doi: 10.1111/jnc.12084
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Bribian, A., Fontana, X., Llorens, F., Gavin, R., Reina, M., Garcia-Verdugo, J. M., et al. (2012). Role of the cellular prion protein in oligodendrocyte precursor cell proliferation and differentiation in the developing and adult mouse CNS. PLoS ONE 7:e33872. doi: 10.1371/journal.pone.0033872
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Bueler, H., Aguzzi, A., Sailer, A., Greiner, R. A., Autenried, P., Aguet, M., et al. (1993). Mice devoid of PrP are resistant to scrapie. Cell 73, 1339–1347. doi: 10.1016/0092-8674(93)90360-3
Chen, S., Mange, A., Dong, L., Lehmann, S., and Schachner, M. (2003). Prion protein as trans-interacting partner for neurons is involved in neurite outgrowth and neuronal survival. Mol. Cell. Neurosci. 22, 227–233. doi: 10.1016/S1044-7431(02)00014-3
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Cheng, Y., Tao, L., Xu, J., Li, Q., Yu, J., Jin, Y., et al. (2013). CD44/Cellular prion protein interact in multidrug resistant breast cancer cells and correlate with responses to neoadjuvant chemotherapy in breast cancer patients. Mol. Carcinog. 53, 686–697. doi: 10.1002/mc.22021
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Dery, M. A., Jodoin, J., Ursini-Siegel, J., Aleynikova, O., Ferrario, C., Hassan, S., et al. (2013). Endoplasmic reticulum stress induces PRNP prion protein gene expression in breast cancer. Breast Cancer Res. 15, R22. doi: 10.1186/bcr3398
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Diarra-Mehrpour, M., Arrabal, S., Jalil, A., Pinson, X., Gaudin, C., Pietu, G., et al. (2004). Prion protein prevents human breast carcinoma cell line from tumor necrosis factor alpha-induced cell death. Cancer Res. 64, 719–727. doi: 10.1158/0008-5472.CAN-03-1735
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Dodelet, V. C., and Cashman, N. R. (1998). Prion protein expression in human leukocyte differentiation. Blood 91, 1556–1561.
Du, L., Rao, G., Wang, H., Li, B., Tian, W., Cui, J., et al. (2013). CD44-positive cancer stem cells expressing cellular prion protein contribute to metastatic capacity in colorectal cancer. Cancer Res. 73, 2682–2694. doi: 10.1158/0008-5472.CAN-12-3759
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ermonval, M., Mouillet-Richard, S., Codogno, P., Kellermann, O., and Botti, J. (2003). Evolving views in prion glycosylation: functional and pathological implications. Biochimie 85, 33–45. doi: 10.1016/S0300-9084(03)00040-3
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Gotz, M., and Huttner, W. B. (2005). The cell biology of neurogenesis. Nat. Rev. Mol. Cell Biol. 6, 777–788. doi: 10.1038/nrm1739
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Herva, M. E., Relaño-Ginés, A., Villa, A., and Torres, J. M. (2010). Prion infection of differentiated neurospheres. J. Neurosci. Methods 188, 270–275. doi: 10.1016/j.jneumeth.2010.02.022
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Hidaka, K., Shirai, M., Lee, J. K., Wakayama, T., Kodama, I., Schneider, M. D., et al. (2010). The cellular prion protein identifies bipotential cardiomyogenic progenitors. Circ. Res. 106, 111–119. doi: 10.1161/CIRCRESAHA.109.209478
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Hirsch, T. Z., Hernandez-Rapp, J., Martin-Lanneree, S., Launay, J. M., and Mouillet-Richard, S. (2014). PrPC signalling in neurons: from basics to clinical challenges. Biochimie 104C, 2–11. doi: 10.1016/j.biochi.2014.06.009
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kaiser, D. M., Acharya, M., Leighton, P. L., Wang, H., Daude, N., Wohlgemuth, S., et al. (2012). Amyloid beta precursor protein and prion protein have a conserved interaction affecting cell adhesion and CNS development. PLoS ONE 7:e51305. doi: 10.1371/journal.pone.0051305
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kanaani, J., Prusiner, S. B., Diacovo, J., Baekkeskov, S., and Legname, G. (2005). Recombinant prion protein induces rapid polarization and development of synapses in embryonic rat hippocampal neurons in vitro. J. Neurochem. 95, 1373–1386. doi: 10.1111/j.1471-4159.2005.03469.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Khalife, M., Young, R., Passet, B., Halliez, S., Vilotte, M., Jaffrezic, F., et al. (2011). Transcriptomic analysis brings new insight into the biological role of the prion protein during mouse embryogenesis. PLoS ONE 6:e23253. doi: 10.1371/journal.pone.0023253
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Lee, Y. J., and Baskakov, I. V. (2010). Treatment with normal prion protein delays differentiation and helps to maintain high proliferation activity in human embryonic stem cells. J. Neurochem. 114, 362–373. doi: 10.1111/j.1471-4159.2010.06601.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Lee, Y. J., and Baskakov, I. V. (2012). The cellular form of the prion protein is involved in controlling cell cycle dynamics, self-renewal, and the fate of human embryonic stem cell differentiation. J. Neurochem. 124, 310–322. doi: 10.1111/j.1471-4159.2012.07913.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Liang, J., Bai, F., Luo, G., Wang, J., Liu, J., Ge, F., et al. (2007b). Hypoxia induced overexpression of PrP(C) in gastric cancer cell lines. Cancer Biol. Ther. 6, 769–774. doi: 10.4161/cbt.6.5.4001
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Liang, J., Pan, Y., Zhang, D., Guo, C., Shi, Y., Wang, J., et al. (2007a). Cellular prion protein promotes proliferation and G1/S transition of human gastric cancer cells SGC7901 and AGS. FASEB J. 21, 2247–2256. doi: 10.1096/fj.06-7799com
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Liao, M. J., Zhang, C. C., Zhou, B., Zimonjic, D. B., Mani, S. A., Kaba, M., et al. (2007). Enrichment of a population of mammary gland cells that form mammospheres and have in vivo repopulating activity. Cancer Res. 67, 8131–8138. doi: 10.1158/0008-5472.CAN-06-4493
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Lima, F. R., Arantes, C. P., Muras, A. G., Nomizo, R., Brentani, R. R., and Martins, V. R. (2007). Cellular prion protein expression in astrocytes modulates neuronal survival and differentiation. J. Neurochem. 103, 2164–2176. doi: 10.1111/j.1471-4159.2007.04904.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Linden, R., Martins, V. R., Prado, M. A., Cammarota, M., Izquierdo, I., and Brentani, R. R. (2008). Physiology of the prion protein. Physiol. Rev. 88, 673–728. doi: 10.1152/physrev.00007.2007
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Liu, T., Li, R., Wong, B. S., Liu, D., Pan, T., Petersen, R. B., et al. (2001). Normal cellular prion protein is preferentially expressed on subpopulations of murine hemopoietic cells. J. Immunol. 166, 3733–3742. doi: 10.4049/jimmunol.166.6.3733
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Loubet, D., Dakowski, C., Pietri, M., Pradines, E., Bernard, S., Callebert, J., et al. (2012). Neuritogenesis: the prion protein controls beta1 integrin signaling activity. FASEB J. 26, 678–690. doi: 10.1096/fj.11-185579
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Mabbott, N. A., and MacPherson, G. G. (2006). Prions and their lethal journey to the brain. Nat. Rev. Microbiol. 4, 201–211. doi: 10.1038/nrmicro1346
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Malaga-Trillo, E., Solis, G. P., Schrock, Y., Geiss, C., Luncz, L., Thomanetz, V., et al. (2009). Regulation of embryonic cell adhesion by the prion protein. PLoS Biol. 7:e55. doi: 10.1371/journal.pbio.1000055
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Manson, J., West, J. D., Thomson, V., McBride, P., Kaufman, M. H., and Hope, J. (1992). The prion protein gene: a role in mouse embryogenesis? Development 115, 117–122.
Marthiens, V., Kazanis, I., Moss, L., Long, K., and Ffrench-Constant, C. (2010). Adhesion molecules in the stem cell niche–more than just staying in shape? J. Cell Sci. 123, 1613–1622. doi: 10.1242/jcs.054312
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
McDonald, A. J., Dibble, J. P., Evans, E. G., and Millhauser, G. L. (2014). A new paradigm for enzymatic control of alpha-cleavage and beta-cleavage of the prion protein. J. Biol. Chem. 289, 803–813. doi: 10.1074/jbc.M113.502351
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Medema, J. P. (2013). Cancer stem cells: the challenges ahead. Nat. Cell Biol. 15, 338–344. doi: 10.1038/ncb2717
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Mehrpour, M., and Codogno, P. (2010). Prion protein: from physiology to cancer biology. Cancer Lett. 290, 1–23. doi: 10.1016/j.canlet.2009.07.009
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Meslin, F., Hamai, A., Gao, P., Jalil, A., Cahuzac, N., Chouaib, S., et al. (2007). Silencing of prion protein sensitizes breast adriamycin-resistant carcinoma cells to TRAIL-mediated cell death. Cancer Res. 67, 10910–10919. doi: 10.1158/0008-5472.CAN-07-0512
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Miele, G., Alejo Blanco, A. R., Baybutt, H., Horvat, S., Manson, J., and Clinton, M. (2003). Embryonic activation and developmental expression of the murine prion protein gene. Gene Expr. 11, 1–12. doi: 10.3727/000000003783992324
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Milhavet, O., Casanova, D., Chevallier, N., McKay, R. D., and Lehmann, S. (2006). Neural stem cell model for prion propagation. Stem Cells 10, 2284–2291. doi: 10.1634/stemcells.2006-0088
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Miranda, A., Pericuesta, E., Ramirez, M. A., and Gutierrez-Adan, A. (2011). Prion protein expression regulates embryonic stem cell pluripotency and differentiation. PLoS ONE 6:e18422. doi: 10.1371/journal.pone.0018422
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Mohanty, S. T., Cairney, C. J., Chantry, A. D., Madan, S., Fernandes, J. A., Howe, S. J., et al. (2012). A small molecule modulator of prion protein increases human mesenchymal stem cell lifespan, ex vivo expansion, and engraftment to bone marrow in NOD/SCID mice. Stem Cells 30, 1134–1143. doi: 10.1002/stem.1065
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Moser, M., Colello, R. J., Pott, U., and Oesch, B. (1995). Developmental expression of the prion protein gene in glial cells. Neuron 14, 509–517. doi: 10.1016/0896-6273(95)90307-0
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Mouillet-Richard, S., Laurendeau, I., Vidaud, M., Kellermann, O., and Laplanche, J. L. (1999). Prion protein and neuronal differentiation: quantitative analysis of prnp gene expression in a murine inducible neuroectodermal progenitor. Microbes Infect. 1, 969–976. doi: 10.1016/S1286-4579(99)80514-0
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Nourizadeh-Lillabadi, R., Seilo Torgersen, J., Vestrheim, O., Konig, M., Alestrom, P., and Syed, M. (2010). Early embryonic gene expression profiling of zebrafish prion protein (Prp2) morphants. PLoS ONE 5:e13573. doi: 10.1371/journal.pone.0013573
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Oesch, B., Westaway, D., Walchli, M., McKinley, M. P., Kent, S. B., Aebersold, R., et al. (1985). A cellular gene encodes scrapie PrP 27-30 protein. Cell 40, 735–746. doi: 10.1016/0092-8674(85)90333-2
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Pan, Y., Zhao, L., Liang, J., Liu, J., Shi, Y., Liu, N., et al. (2006). Cellular prion protein promotes invasion and metastasis of gastric cancer. FASEB J. 20, 1886–1888. doi: 10.1096/fj.06-6138fje
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Panigaj, M., Glier, H., Wildova, M., and Holada, K. (2011). Expression of prion protein in mouse erythroid progenitors and differentiating murine erythroleukemia cells. PLoS ONE 6:e24599. doi: 10.1371/journal.pone.0024599
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Passet, B., Young, R., Makhzami, S., Vilotte, M., Jaffrezic, F., Halliez, S., et al. (2012). Prion protein and Shadoo are involved in overlapping embryonic pathways and trophoblastic development. PLoS ONE 7:e41959. doi: 10.1371/journal.pone.0041959
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Patra, S. K. (2008). Dissecting lipid raft facilitated cell signaling pathways in cancer. Biochim. Biophys. Acta 1785, 182–206. doi: 10.1016/j.bbcan.2007.11.002
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Peralta, O. A., Huckle, W. R., and Eyestone, W. H. (2011). Expression and knockdown of cellular prion protein (PrPC) in differentiating mouse embryonic stem cells. Differentiation 81, 68–77. doi: 10.1016/j.diff.2010.09.181
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Pradines, E., Hernandez-Rapp, J., Villa-Diaz, A., Dakowski, C., Ardila-Osorio, H., Haik, S., et al. (2013). Pathogenic prions deviate PrP(C) signaling in neuronal cells and impair A-beta clearance. Cell Death Dis. 4, e456. doi: 10.1038/cddis.2012.195
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Premzl, M., and Gamulin, V. (2007). Comparative genomic analysis of prion genes. BMC Genomics 8:1. doi: 10.1186/1471-2164-8-1
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Prodromidou, K., Papastefanaki, F., Sklaviadis, T., and Matsas, R. (2014). Functional cross-talk between the cellular prion protein and the neural cell adhesion molecule is critical for neuronal differentiation of neural stem/precursor cells. Stem Cells 32, 1674–1687. doi: 10.1002/stem.1663
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Relaño-Ginés, A., Gabelle, A., Hamela, C., Belondrade, M., Casanova, D., Mourton-Gilles, C., et al. (2013). Prion replication occurs in endogenous adult neural stem cells and alters their neuronal fate: involvement of endogenous neural stem cells in prion diseases. PLoS Pathog. 9:e1003485. doi: 10.1371/journal.ppat.1003485
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Relaño-Ginés, A., Lehmann, S., and Crozet, C. (2014). Prion diseases and adult neurogenesis: how do prions counteract the brain's endogenous repair machinery? Prion 8, 1–7. doi: 10.4161/pri.29021
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Rezza, A., Sennett, R., and Rendl, M. (2014). Adult stem cell niches: cellular and molecular components. Curr. Top. Dev. Biol. 107, 333–372. doi: 10.1016/B978-0-12-416022-4.00012-3
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Santos, T. G., Beraldo, F. H., Hajj, G. N., Lopes, M. H., Roffe, M., Lupinacci, F. C., et al. (2013). Laminin-gamma1 chain and stress inducible protein 1 synergistically mediate PrPC-dependent axonal growth via Ca2+ mobilization in dorsal root ganglia neurons. J. Neurochem. 124, 210–223. doi: 10.1111/jnc.12091
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Santos, T. G., Silva, I. R., Costa-Silva, B., Lepique, A. P., Martins, V. R., and Lopes, M. H. (2011). Enhanced neural progenitor/stem cells self-renewal via the interaction of stress-inducible protein 1 with the prion protein. Stem Cells 29, 1126–1136. doi: 10.1002/stem.664
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Santuccione, A., Sytnyk, V., Leshchyns'ka, I., and Schachner, M. (2005). Prion protein recruits its neuronal receptor NCAM to lipid rafts to activate p59fyn and to enhance neurite outgrowth. J. Cell Biol. 169, 341–354. doi: 10.1083/jcb.200409127
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Sauer, H., Dagdanova, A., Hescheler, J., and Wartenberg, M. (1999). Redox-regulation of intrinsic prion expression in multicellular prostate tumor spheroids. Free Radic. Biol. Med. 27, 1276–1283. doi: 10.1016/S0891-5849(99)00164-1
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Singh, A., and Settleman, J. (2010). EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene 29, 4741–4751. doi: 10.1038/onc.2010.215
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Starke, R., Harrison, P., Mackie, I., Wang, G., Erusalimsky, J. D., Gale, R., et al. (2005). The expression of prion protein (PrP(C)) in the megakaryocyte lineage. J. Thromb. Haemost. 3, 1266–1273. doi: 10.1111/j.1538-7836.2005.01343.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Steele, A. D., Emsley, J. G., Ozdinler, P. H., Lindquist, S., and Macklis, J. D. (2006). Prion protein (PrPc) positively regulates neural precursor proliferation during developmental and adult mammalian neurogenesis. Proc. Natl. Acad. Sci. U.S.A. 103, 3416–3421. doi: 10.1073/pnas.0511290103
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Steele, A. D., Lindquist, S., and Aguzzi, A. (2007). The prion protein knockout mouse: a phenotype under challenge. Prion 1, 83–93. doi: 10.4161/pri.1.2.4346
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Stella, R., Massimino, M. L., Sandri, M., Sorgato, M. C., and Bertoli, A. (2010). Cellular prion protein promotes regeneration of adult muscle tissue. Mol. Cell. Biol. 30, 4864–4876. doi: 10.1128/MCB.01040-09
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Tremblay, P., Bouzamondo-Bernstein, E., Heinrich, C., Prusiner, S. B., and DeArmond, S. J. (2007). Developmental expression of PrP in the post-implantation embryo. Brain Res. 1139, 60–67. doi: 10.1016/j.brainres.2006.12.055
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Westergard, L., Christensen, H. M., and Harris, D. A. (2007). The cellular prion protein (PrP(C)): its physiological function and role in disease. Biochim. Biophys. Acta 1772, 629–644. doi: 10.1016/j.bbadis.2007.02.011
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Zhang, C. C., Steele, A. D., Lindquist, S., and Lodish, H. F. (2006). Prion protein is expressed on long-term repopulating hematopoietic stem cells and is important for their self-renewal. Proc. Natl. Acad. Sci. U.S.A. 103, 2184–2189. doi: 10.1073/pnas.0510577103
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Keywords: cellular prion protein, stem cell, cancer, self-renewal, cell fate specification, prion infection
Citation: Martin-Lannerée S, Hirsch TZ, Hernandez-Rapp J, Halliez S, Vilotte J-L, Launay J-M and Mouillet-Richard S (2014) PrPC from stem cells to cancer. Front. Cell Dev. Biol. 2:55. doi: 10.3389/fcell.2014.00055
Received: 30 June 2014; Accepted: 11 September 2014;
Published online: 29 September 2014.
Edited by:
Craig Michael Walsh, University of California, Irvine, USAReviewed by:
Mario Cioce, New York University Langone Medical Center, USAHan-Ming Shen, National University of Singapore, Singapore
Copyright © 2014 Martin-Lannerée, Hirsch, Hernandez-Rapp, Halliez, Vilotte, Launay and Mouillet-Richard. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sophie Mouillet-Richard, Toxicology, Pharmacology and Cellular Signaling, INSERM U-1124, Université Paris Descartes, 45, rue des Saints Pères, 75006 Paris, France e-mail: sophie.mouillet-richard@parisdescartes.fr
 Séverine Martin-Lannerée
Séverine Martin-Lannerée Théo Z. Hirsch
Théo Z. Hirsch Julia Hernandez-Rapp
Julia Hernandez-Rapp Sophie Halliez
Sophie Halliez Jean-Luc Vilotte
Jean-Luc Vilotte Jean-Marie Launay
Jean-Marie Launay Sophie Mouillet-Richard
Sophie Mouillet-Richard