Thyroxine transfer from cerebrospinal fluid into choroid plexus and brain is affected by brefeldin A, low sodium, BCH, and phloretin, in ventriculo-cisternal perfused rabbits
- 1ER045, PRASE, Faculty of Sciences-I, Lebanese University, Beirut, Lebanon
- 2Department of Biology, Faculty of Sciences-I, Lebanese University, Beirut, Lebanon
- 3Physics Department, Faculty of Sciences, Lebanese University, Beirut, Lebanon
- 4Department of Family Medicine, University of Toledo, Toledo, OH, USA
- 5Department of Neurosciences, University of Messina, Messina, Italy
- 6Department of Pharmacology and Therapeutics, Institute of Pharmaceutical Science, King's College London, London, UK
Background: Thyroxine (T4) hormone is synthesized by the thyroid gland and then released into the systemic circulation where it binds to a number of proteins. Dysfunction in T4 transport mechanisms has been demonstrated in multiple central nervous system (CNS) diseases including Alzheimer's disease. In the presence of different compounds that inhibit potential T4 transport mechanisms, this study investigated the transfer of T4 from cerebrospinal fluid (CSF) into Choroid Plexus (CP) and other brain tissues. The compounds used were brefeldin A, low sodium artificial CSF (aCSF), BCH, phloretin, and taurocholate (TA).
Methods: Radiolabeled T4 (125I-T4) was perfused continuously into the CSF and was assessed in several brain compartments with reference molecule 14C-mannitol and blue dextran, using the in vivo ventriculo-cisternal perfusion (V-C) technique in the rabbit. The aCSF containing the drug of interest was infused after 1 h of perfusion. Drugs were applied independently to the aCSF after 1 h of control perfusion.
Results: Of interest, in presence of low sodium or BCH, the percentage recovery of 125I-T4, was increased compared to controls, with concomitant increase in T4 clearance. Conversely, brefeldin A, phloretin, and TA did not exert any significant effect on the recovery and clearance of 125I-T4 assessed in aCSF. On the other hand, the uptake of 125I-T4 into CP was raised by 18 fold compared to controls in the presence of brefeldin A. In addition, low sodium, BCH, or phloretin alone, enhanced the uptake of 125I-T4 by almost 3-fold, whereas TA did not show any significant effect. Finally, the uptake and distribution of 125I-T4 into other brain regions including ependymal region (ER) and caudate putamen (CAP) were significantly higher than in controls.
Conclusion: Our study suggests the involvement of different mechanisms for the transfer of 125I-T4 from CSF into CP and other brain regions. This transfer may implicate sodium-dependent mechanisms, amino acid “L” system, or organic anion transporting polypeptide (OATP).
Introduction
Thyroid hormone (TH) is essential for normal growth, development of the central nervous system (CNS) (Goncalves et al., 1990; Koibuchi, 2013), and neuronal regeneration in adults after traumatic brain injury (TBI) (Francon et al., 1989). Thyroxine hormone (T4) is synthesized by the thyroid gland and then released into the systemic circulation where it binds to a number of proteins such as albumin (ALB), transthyretin (TTR), and thyroid binding globulin (TBG) (Baehr et al., 2006). Hypothyroxinemia causes neurological deficit and mental retardation in the fetus (Wei et al., 2013). In order for T4 to reach the CNS, it must first cross the brain barriers, i.e., endothelial cells of the blood-brain barrier (BBB) and epithelial cells of the choroid plexus (CP) of blood-cerebrospinal fluid barrier (BCSF-B). Dysfunction in T4 transport mechanisms has been demonstrated in multiple diseases of the CNS. In fact, low levels of free T4 in the CSF have been demonstrated in patients with multiple sclerosis (Pieragostino et al., 2013) and Alzheimer's Disease (Johansson et al., 2013).
Despite the importance of studying the direction of T4 transport from blood into CNS, the underlying mechanisms responsible for its entry from CSF into CP and brain, has not been fully investigated. Few studies in mice revealed that T4 transport across BBB is primarily directed from brain to blood via carrier-mediated mechanism (Banks et al., 1985). However, T4 injected directly into the CSF compartment in the rat penetrated very poorly into the brain (Dratman et al., 1991; Blay et al., 1993). In the rabbit, our laboratory have demonstrated that T4 transport from CSF into CP's and other brain tissues is carrier-mediated and dependent on the presence of T4 binding protein TTR, synthesized by the CP's (Kassem et al., 2006). Moreover, we have also shown that in isolated perfused sheep CP, T4 transport is partially dependent on sodium (Kassem et al., 2002).
Previous research has concentrated on studying the transport direction of T4 from blood into the brain across the BBB suggesting a role for organic anion transporting polypeptides (OATP) such as OATP 1a4, 1c1, 2, 3, and 14, which are mainly localized at the blood side of the BBB (Abe et al., 1998; Gao and Meier, 2001; Mayerl et al., 2012; Do et al., 2013) and CP (Gao et al., 1999; Gao and Meier, 2001; Mayerl et al., 2012). On the other hand, others have demonstrated a role for OATP2 and multiple drug resistance 1 (MDR1) in the transport of 125I-T4 at the basolateral side of the isolated perfused sheep CPs (Preston and Segal, 1992). Furthermore, a potential role for P-glycoprotein (P-gp) and OATP 1 & 3 was found in T4 transport mechanism (Kassem et al., 2007). Finally, human OATP 1c1 has been reported to have a high specificity for T4 and is considered to play a critical role in its transport across the BBB (Jansen et al., 2005; van der Deure et al., 2008). However, monocarboxylate (MCT8) has been shown to transport T3 and T4 THs (Grijota-Martínez et al., 2011).
The aim of this study is to investigate the effect of several cross-competing drugs on the transport of 125I-T4 from CSF compartment into CP and the brain, using Ventriculo-Cisternal (V-C) perfusion in the rabbit, and to potentially characterize putative transporters contributing to this process. The following drugs/conditions were used: brefeldin A (a blocker of intracellular secretory proteins such as TTR, Klausner et al., 1992), low sodium artificial CSF (aCSF), β-2-aminobicyclo-(2,2.1)-heptane-2-carboxylic acid (BCH) (an amino acid analog inhibitor of L-type transporters, Braun et al., 2011), phloretin (a sugar analog), and taurocholate (TA) (an OATP inhibitor).
Materials and Methods
Animal Handling
All procedures were conducted according to the Home Office legislation of the animal scientific procedures in compliance with the guidelines of the current animal legislation Act, 1986 UK. This study was approved by the Institute Animal Care and Utilization Committee of King's College London.
Ventriculo-cisternal (V-C) Perfusion
This method has been previously described (Davson and Segal, 1970; Kassem et al., 2006, 2007). Briefly, 1.5–4 Kg New Zealand white rabbits of either sex were anesthetized by the intravenous injection of a mixture of sodium Pentobarbital (10 mg/kg−1, Sagatal, Sigma, UK) and Medetomidine hydrochloride (0.5 mg/kg−1, Domitor, Sigma, UK). Bilateral perfusion of the ventricular system was carried out by placing two catheter inflow needles into each lateral ventricle (Kassem et al., 2006). The aCSF was infused into both ventricles by a pump (Harvard 22, UK) at a total rate of 60 μl/min, containing 125I-labeled T4 (0.37 MBq/40 ml), and the extracellular marker mannitol (0.148 MBq/40 ml, New England Nuclear, UK). Artificial CSF contained (in mmol/l): 153 Na+, 2.81 K+, 1.7 Mg2+, 2.81 Ca2+, 131 Cl−, 1.7 , 1.48 , 27.4 , and 5.3 glucose. The solution was then gassed with 95% O2 and 5% CO2. Low sodium aCSF was used by replacing the NaCl and KCl in the perfusate with choline chloride. The sodium content in the aCSF was made up from , which contained ~27 mM Na. Blue dextran, a large marker molecule of 2 × 106 kDa M.wt. (Sigma, UK) was confined to the aCSF and used to determine the secretion rate (Kf) of the newly formed CSF (Davson and Segal, 1970; Kassem et al., 2006). The outflow perfusate was collected continuously, every 10 min, from a single needle positioned in the cisterna magna. Perfusion time lasted for 2 h, in all experiments.
Sampling of Brain Tissues and Isotopes Counting
This was performed as previously described (Kassem et al., 2006, 2007). Briefly, rabbits were sacrificed, their brains rapidly removed, and ventricles opened and flushed with 0.9% saline. The brain hemispheres were split through the midline and cut into series of coronal sections. The CP's were removed, and the ventricular ependymal region (ER) of the frontal cortex was dissected, as 1 mm thick layer (20 mg) from the area next to the midline (Preston and Segal, 1992; Preston et al., 2005). The ER was then gently peeled back from the rest of ventricular wall by a horizontal cut. The uptake of 125I-T4 into ER was compared to tissue samples designated the subependymal region (SER), located immediately below the dissected region (1 mm thick, 20 mg). The rest of the brain was dissected into hippocampus (HC) with or without ER (HC+ER or HC-ER), respectively. The caudate putamen (CAP) was also processed.
Treatments
aCSF inflow and outflow samples (100 μl in triplicate), and tissue samples were dissolved in 0.5 ml Solusol (National diagnostic, UK). A total of 3.5 ml of scintillation liquid was added (Ultima Gold, Packard, UK) and samples were counted with LKB Wallac-1219 Beta liquid scintillation counter. Both 125I and 14C were expressed as disintegration per minute (dpm).
Drugs
After control aCSF perfusion for 1 h, one of the following drugs was separately added to the aCSF for another 1 h: brefeldin A (5.0 μg/ml) (Lei et al., 2003), low sodium (~27 mM), BCH (5.0 mM) (Preston et al., 2005), phloretin (100 μM) (Deane and Segal, 1979), or taurocholate (1.0 mM) (Kitazawa et al., 2000). Drugs were dissolved directly in aCSF. The effect of each drug was compared to its own control experiment. The drugs were applied to separate animals. The control for each experimental animal was done before applying the specific drug, therefore; the effect of each investigated drug was animal specific. Results represents the average effect of each drug on n = 3–4 animals.
Expression of Results
Percent Recovery of 125I-T4 in aCSF
As previously published (Bradbury and Davson, 1964; Kassem et al., 2006), the % of 125I-T4 remaining in outflow aCSF, in each 10 min sample, was calculated from: % recovery = (Cout/Cin) × 100, where the Cout = 125I-T4 dpm.ml−1, Cin = 125I-T4 dpm.ml−1 inflow aCSF. The steady-state ratio of 125I-T4 in the recovered aCSF was calculated from the mean value of % recovery derived from the last four samples of perfusion fluid.
Clearance of 125I-T4 from aCSF
The rate of 125I-T4 loss from the aCSF during V-C perfusion was expressed as clearance, defined as the volume (μl) of aCSF cleared of 125I-T4 per minute. The equation employed has previously been described (Kassem et al., 2006):
Where Cin is the 125I-T4 dpm.ml−1 per unit volume of entering aCSF; Cout is the 125I-T4 dpm.ml−1 of emerging aCSF, Fin is the rate of perfusion (μl. min−1), and RD is the ratio of Dextran in steady-state of Cin/Cout, measured on a Unicam spectrophotometer at 625 nm. The clearance of 125I-T4 from CSF was corrected for CSF secretion rate (μl. min−1), every 10 min throughout each experiment.
Brain Uptake
At the end of 2 h of V-C perfusion, the regional brain uptake of 125I-T4 was expressed as follows:
Where Cin and Cout are 125I-T4 in inflowing and outflowing CSF from the ventricle, respectively. RBr was then corrected for 14C-mannitol content.
Statistics
All statistical calculations were performed using Microsoft Excel and GraphPad Prism version 5.0 (GraphPad Inc). Results are expressed as the mean ± SEM. Statistical comparisons were performed using the Student's t-test in order to determine statistical significance at p < 0.05. Symbols indicate statistical difference: (*) p < 0.05, (**) p < 0.001, (***) p < 0.0001.
Results
Percentage Recovery and Clearance of 125I-T4 in aCSF After 2 hr V-C Perfusion
Results have shown that recovery of blue dextran, used as an indicator dye in this study, and the secretion rate of newly secreted CSF (Figure 1) were consistent with previously published data (Kassem et al., 2007). Indeed, whether or not a drug was added, CSF secretion did not seem to change significantly, after steady state had been reached (p > 0.05, Table 1). The mean curves describing the achievement of steady state during V-C perfusion with aCSF containing 125I-T4 and blue dextran are shown in Figure 1. It's worth noting that 14C-mannitol was used as a reference extracellular marker.

Figure 1. A representative graph showing the recovery of blue dextran, 14C-mannitol and 125I-labeled T4 in outflow aCSF during 2h of V-C perfusion in rabbits. Blue dextran was used as an indicator molecule for the newly secreted CSF under experimental conditions, whereas 14C-mannitol was used as an extracellular marker. Data are presented as mean ± SEM of 13 samples taken at various time points, n = 5 rabbits for each condition.
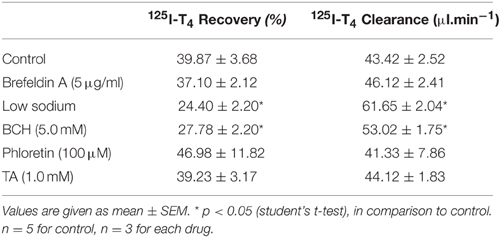
Table 1. Recovery and clearance of 125I-T4 from the aCSF in rabbits, in the presence of various drug treatments.
The effect of brefeldin A, an intracellular secretory protein blocker, or phloretin, a sugar analog, on recovery and clearance of 125I-T4 in aCSF did not reach statistical significance (Table 1). However, when low sodium aCSF was used, the recovery of 125I-T4 in aCSF increased significantly from ~43 to ~62% (p < 0.01). This increase in recovery rate was correlated with a significant reduction in clearance by 47% (p < 0.001, Table 1), suggesting sodium-dependent uptake of T4 from CSF into brain and CP. Moreover, the application of BCH, an amino acid analog, also caused a significant increase in the % recovery of 125I-T4 when compared to controls (p < 0.05, Table 1), which also correlated with a similar reduction in clearance by >50% (Table 1). On the other hand, TA, an OATP inhibitor, did not have any effect on the recovery or clearance of 125I-T4 in aCSF (Table 1).
Uptake into CP
Following the measurement of % recovery and clearance in aCSF, and in order to examine the mechanism of T4 transport from CSF, we have explored the uptake of 125I-T4 into CP. The application of brefeldin A resulted in a significant 18-fold increase of 125I-T4 uptake in CP compared to controls (~26 ml.g−1 vs. 1.4 ml.g−1; respectively, p < 0.001, Figure 2). Moreover, in the presence of low sodium perfusate, there was a significant 2.5 fold accumulation of 125I-T4 into CP as compared to controls (~3.5 ml.g−1 vs. ~1.4 ml.g−1, p < 0.01, Figure 2). Similarly, phloretin induced a significant 2.4 fold increase in the uptake of 125I-T4 into CP (~3.3 ml.g−1 vs. ~1.4 ml.g−1, p < 0.01, Figure 2). Furthermore, BCH significantly enhanced by 3-fold the uptake into CP (p < 0.05) compared to controls. On the other hand, TA did not induce any change in 125I-T4 uptake (Figure 2).
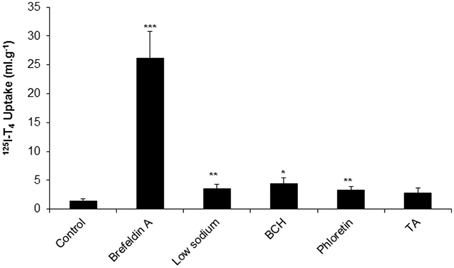
Figure 2. The uptake of 125I-labeled T4 into choroid plexus (CP) of rabbits in the presence of brefeldin A, low sodium, BCH, phloretin, and TA. Data is presented as the mean and error bars represent SEM. Asterisks indicate statistical significance using student's t-test between treatment and control for the indicated brain region, *indicates p < 0.05, **p < 0.01, ***p < 0.001. n = 5 for control, n = 3 for each drug.
Uptake into Other Brain Tissues
Since the uptake of 125I-T4 into CP was significantly raised, we have examined its uptake into specific regions of the brain, namely ER, SER, HC, and CAP. The application of brefeldin A caused more than a 9-fold increase in 125I-T4 uptake throughout various brain regions such as ER, SER, and the CAP. For example, the uptake into ER and SER was increased by ~10-fold as compared to controls (p < 0.05, p < 0.001; respectively, Table 2). Moreover, in presence of low sodium aCSF, the highest brain uptake of 125I-T4 was found into the CAP (Table 2). Brefeldin A treatment also significantly enhanced, but to a lesser extent, the uptake into other brain tissues such as the ER of the frontal cortex and HC (p < 0.05, Table 2). It is worth noting that the latter tissues are very close to the ventricles and have more direct surface contact with CSF. This indicates that 125I-T4 increased uptake into those regions is largely due to a sodium-dependent mechanism. Furthermore, BCH induced the uptake of 125I-T4 into ER and CAP, which are close to the perfusion site, indicating a role for “L” system in the uptake mechanism. Phloretin treatment also caused a similar increase in the 125I-T4 uptake into ER and CAP (Table 2), suggesting a phloretin-dependent mechanism as well. Indeed, phloretin treatment represents the second largest effect on 125I-T4 uptake into brain tissue amongst the drugs tested in this study after that obtained by brefeldin A. Finally, TA treatment resulted in a significant increase in the uptake of 125I-T4 into the ER and CAP brain regions as compared to controls (Table 2).
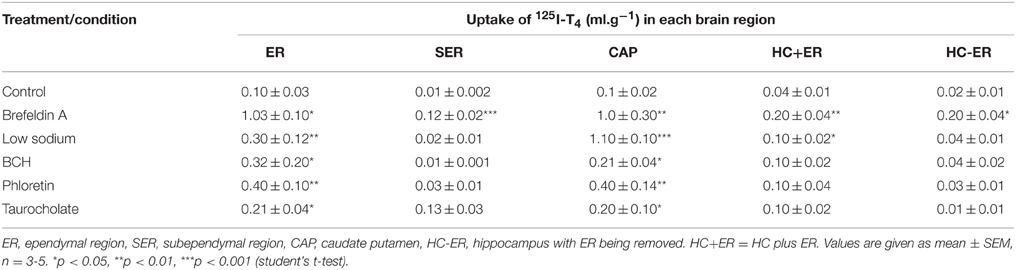
Table 2. Effects of various drug treatments on the uptake of 125I-T4 (ml.g−1) in various brain tissues of rabbits.
Discussion
Our previous studies have shown that T4 transfer from the CSF compartment into lateral CPs and the surrounding brain regions was dependent on TTR and P-gp (Kassem et al., 2006, 2007). The present study reveals that T4 transport is affected by the presence of several conditions/drug inhibitors such as brefeldin A, low sodium, BCH, or phloretin and TA. The following lines of evidence support the above statement: (Baehr et al., 2006) the percentage recovery of 125I-T4, in presence of low sodium or BCH was increased, in comparison to control, compatible with a significant increase in T4 clearance, (Goncalves et al., 1990) the uptake of 125I-T4 into CP was significantly increased in the presence of brefeldin A, low sodium, BCH or phloretin, (Koibuchi, 2013) the uptake of 125I-T4 into other brain regions, especially ER and CAP, was significantly increased in presence of all drugs as compared to controls, (Francon et al., 1989) TA did not have any effect on the uptake of 125I-T4 into CP, but enhanced the uptake into ER and CAP.
Consistent with previously published studies, and using the V-C perfusion method, our data revealed that the baseline characteristics of CSF secretion and blue dextran recovery remained stable (Davson and Segal, 1970; Kassem et al., 2007). On the other hand, treatment with brefeldin A, a fungal toxin that disconnects the secretory, recycling, and degradation pathways for membrane proteins (Farwell et al., 1996), resulted into a considerable 18-fold accumulation of 125I-T4 uptake into the CP. Moreover, it caused a large distribution of 125I-T4 into the surrounding brain regions such as ER, SER, and HC+ER, when compared to controls. However, brefeldin A treatment had little effect on the % recovery of 125I-T4. These data suggest that a membrane transport protein system for T4 is present at the apical side of the CP tissue and on the CSF side of the ER tissue. Therefore, this transport mechanism is involved in the 125I-T4 uptake mechanism. Although there was no direct measurement for the effect of brefeldin A treatment on the uptake of T4 into CP in the presence of TTR, a role for this protein could also be contributing to this transport mechanism. In fact, previous research has demonstrated that incubation of cultured cells with brefeldin A has prevented active secretion of TTR into the culture medium (Patel et al., 2012).
The transport of 125I-T4 from CSF to CP and other regions of the brain was demonstrated to be sodium-dependent. Indeed, not only did the low sodium perfusate significantly inhibit the clearance from CSF, but it also increased 125I-T4 availability in the CSF compartment, and subsequently resulted into a 3 fold accumulation of 125I-T4 uptake into CPs. Our data complement our previous findings which have demonstrated a sodium-dependent mechanism responsible for T4 transport on the apical side of the isolated perfused CP of sheep (Dickson et al., 1987). Previous studies have also shown that CP is an important efflux pathway for T4 (Dickson et al., 1987; Kassem et al., 2001), and other compounds (Strazielle and Ghersi-Egea, 1999). The increase in T4 uptake in the CP in this study could be due to the inhibitory effect of low sodium on sodium-dependent transporters present at the blood side of the CP. In addition, the low extracellular sodium aCSF may also have an inhibitory effect on Na+/H+ exchange with blood side of both BBB and B-CSF barriers. Therefore, a reduction in 125I-T4 efflux across the BBB could subsequently increase its retention within the CSF ventricular compartment. For instance, the increase in 125I-T4 uptake was also observed in CAP which suggests a similar mechanism. Moreover, in hepatic cells, uptake of TH has been induced by the Na+/TA transporting polypeptide (NTCP) and OATP1 (Friesema et al., 1999), which are both found to be expressed at low levels in the rat CP (Choudhuri et al., 2003). Furthermore, the sodium-dependent mechanism of TH has been demonstrated in rat skeletal muscle (Centanni and Robbins, 1987), human glioma cells (Goncalves et al., 1990), rat glial cells (Francon et al., 1989), astrocytes (Beslin et al., 1995), and cerebrocortical neurons (Chantoux et al., 1995). Finally, as a consequence of low sodium effect in this study and results of another study (Kassem et al., 2006), the abundant presence of 125I-T4in CSF suggests a partial rapid diffusion of 125I-T4 into tissue that is driven by a bulk flow of CSF and receptor-mediated endocytosis (Kassem et al., 2006).
Following the addition of BCH, an amino acid analog which functions as a substrate for “L” system transporter (Christensen et al., 1989; Stewart et al., 1989), significant reduction in the clearance of 125I-T4 from CSF was observed. This indicates that 125I-T4 was inhibited from leaving the perfusate and competed with the “L” amino acid transport system. The high accumulation of 125I-T4 uptakeinto CPs, following BCH application, is indicative of an inhibition of the “L” system localized at the basolateral side of the CPs (Christensen et al., 1989; Stewart et al., 1989). It is worth noting that another study employing BCH, using isolated perfused CP of the sheep, has reported a slight increase in the 125I-T3 efflux from CSF to the blood and a significant reduction in the uptake at the blood side (Preston and Segal, 1992). This inhibition of “L” system transport by BCH has been found to contribute significantly to the transport of iodothyronine (T3 and T4) in a human carcinoma cell line (Powell et al., 2000; Hennemann et al., 2001) and brain astrocytes (Braun et al., 2011). Our data on CPs is consistent with others who demonstrated that “L-type” amino acid transporters (LATs) facilitate TH uptake in xenopus oocytes (Jansen et al., 2005). Furthermore, the transport of T3 and T4 appeared to be mediated by L and T amino acid TH transporters in mouse neuroblastoma cultured cells (Hennemann et al., 2001). This transport was decreased by BCH, confirming the involvement of the amino acid “L” system (Lakshmanan et al., 1990). On the other hand, ER appeared to have been affected by the reduction in 125I-T4 clearance from CSF, as a result of “L” system inhibition at the levels of both CPs and BBB. Our data suggests that the “L” system is expressed in ER and CAP and that the T4 uptake mechanism into these brain regions studied is BCH-dependent. This is consistent with previous research which indicated that circulating T4 preferentially crosses the BBB through several amino acid transporters (Koibuchi, 2013). Our data on BCH in 125I-T4 transport from CSF into CPs and the brain, across the “L” system, has not been previously reported and may be of physiological importance.
Phloretin treatment resulted in a 3-fold increase in the uptake of 125I-T4 into CPs, ER and CAP compared to controls. It is well known that this drug has an inhibitory effect on protein binding (McLeese and Eales, 1996), GLUT1 glucose transporter, and the iodothyronine-5′-deiodinase (Movius et al., 1989; Morreale de Escobar et al., 1994). Our data is in agreement with previous studies in the field, which have demonstrated the inhibitory effect of phloretin on the glucose flux from CSF to blood using the isolated perfused CPs of the sheep (Deane and Segal, 1979). It is worth noting that since phloretin binds to the mammalian hexose/sugar transporter (Deane and Segal, 1979), this would involve the energy dependent P-gp. Indeed, the latter has been proven to be involved in the transfer of 125I-T4 between the CSF, CP, and brain (Kassem et al., 2007). In addition, since there is structural similarities between phloretin and T3, more 125I-T4 can diffuse into the cell by lipid partitioning. In fact, phloretin is known to have an inhibitory effect on glucose transporter (GLUT) in human cancer cells (Wu et al., 2009), and therefore may simply compete with sugar transporters or other unknown T4 transporters. Previous studies on rat hepatocarcinoma have reported an inhibitory effect of phloretin on GLUT1 and GLUT2 activity localized on apical and basolateral sides of the CP epithelial cells respectively (Rodríguez-Enríquez et al., 2009; Balmaceda-Aguilera et al., 2012). In addition, it has been proposed that phloretin has competitively inhibited T3 uptake into human hepatocarcinoma cultured cells in a dose-dependent manner (Movius et al., 1989). In contrast, phloretin was shown to reduce the 125I-T4 uptake in isolated trout hepatocytes (Riley and Eales, 1993). Other studies have proven that GLUT1 is localized at the basolateral side of cultured epithelial CP cells (Villalobos et al., 1997) and on both sides of capillary endothelial cells of the BBB (Stewart et al., 1994), hence this transporter may prevent the removal of 125I-T4 from CSF/brain to the blood. Therefore, it can be hypothesized that the retention of 125I-T4 by the brain can be the consequence of a decrease in the efflux of 125I-T4 from the CSF and brain to blood across the brain barriers.
In the presence of TA, the uptake of 125I-T4 into some brain tissues, such as ER and CAP, was increased. This suggests an interaction between TA and OATP 1a4 since TA was proven to be an OATP 1a4 substrate and sensitive transporter (Do et al., 2013). This agrees with a previous study which demonstrated that OATP2 and OATP3 mediate TH transport when their mRNA is injected into xenopus oocytes (Abe et al., 2002). Furthermore, our data suggests that OATP2 of the abluminal membrane is involved in T4 accumulation into those tissues since OATP2 has been identified on the luminal and abluminal membrane of the rat brain capillary endothelial cells (Gao et al., 1999). However, we cannot exclude a role for OATP2 present on the luminal side. Finally, the mediation of T4 uptake by organic anion as well as MCT8 was also found in human hepatocarcinoma cells (Ritchie and Taylor, 2010). MCT8, also known as solute carrier (Slc16a2), has been reported to be involved in the uptake mechanism of 125I-T4 into the brain from the circulation (Kogai et al., 2010; Braun et al., 2011; Horn et al., 2013).
The decrease in TTR synthesis at the blood-CSF-barrier (CP) has been previously shown in aging sheep (Chen et al., 2006). These changes suggested reduced capacity of CP to maintain CSF T4 homeostasis, and could also reduce chelation of beta-amyloid, which may result in an added risk for Alzheimer's disease in patients experiencing similar changes in TTR synthesis. In addition, albumin and TTR have been shown to inhibit T4 uptake from CSF into isolated sheep brain tissue in a dose-dependent manner (Chen et al., 2005). Therefore, those two proteins (albumin and TTR) prevent the loss of T4 from CSF to blood, and enhances the redistribution of T4 around the brain.
The increase in T4 recovery and clearance in presence of low sodium or BCH, was due respectively to the inhibition of Na+/K+-ATPase or L-type amino acid transporters. Both of these transporters are localized at the basolateral side of the CP, which was reflected by the accumulation of T4 in the CP. The results imply that T4 uptake into the CP and brain is largely dependent on the sodium gradient. We suggest that T4 transport into the brain and CP is dependent on the concentration of extracellular sodium and/or OATP2 transport mechanisms. Although, treatment with drugs such as Brefeldin A, Phloretin, and TA resulted in a variable increase in T4 uptake into CP, they failed to cause a significant change in T4 recovery and clearance from CSF. This could be due to either poor penetration of the drugs into the cells, or that the used concentrations were not at optimal levels to produce an effect at the BBB.
In conclusion, the uptake of 125I-T4 from CSF into CPs suggests the involvement of at least two transport mechanisms among the following: sodium-dependent mechanism, amino acid “L-type” transport system, or OATP transporters. Since the uptake into other brain regions was lower than that obtained in the CPs, we hypothesize that the major efflux pathway of 125I-T4 from CSF into the brain tissue toward the blood occurs across the blood-CSF barrier, which could exclude any potential role for the BBB in the process.
Author Contributions
NK and KZ conceived the study and designed experiments. NK performed experiments. AE performed statistical analysis. WJ, ME, and SM reviewed data and manuscript. NK and KZ analyzed data and wrote the manuscript. All authors read and approved the final version of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by a grant from Al-Tajir World of Islam Trust to NK.
Abbreviations
TH, thyroid hormones; T4, thyroxine; T3, triiodothyronine; CSF, cerebrospinal fluid; CP, choroid plexus; V-C, ventriculo-cisternal; BBB, blood-brain barrier; BCSF-B, blood-cerebrospinal fluid barrier; CNS, central nervous system; BCH, β-2-aminobicyclo- (2,2.1)-heptane-2-carboxylic acid; TA, taurocholate.
References
Abe, T., Kakyo, M., Sakagami, H., Tokui, T., Nishio, T., Tanemoto, M., et al. (1998). Molecular characterization and tissue distribution of a new organic anion transporter subtype (Oatp3) that transports thyroid hormones and distribution and taurocholate and comparison with Oatp2. J. Biol. Chem. 273, 22395–22401, doi: 10.1074/jbc.273.35.22395
Abe, T., Suzuki, T., Unno, M., Tokui, T., and Ito, S. (2002). Thyroid hormone transporters: recent advances. Trends Endocrino. Metab. 13, 215–220. doi: 10.1016/S1043-2760(02)00599-4
Baehr, C., Recihel, V., and Fricker, G. (2006). Choroid plexus epithelial monolayers- a cell culture model from porcine brain. CSF Res. 3:13. doi: 10.1186/1743-8454-3-13
Balmaceda-Aguilera, C., Cortés-Campos, C., Cifuentes, M., Peruzzo, B., Mack, L., Tapia, J. C., et al. (2012). Glucose transporter 1 and monocarboxylate transporters 1, 2, and 4 localization within the glial cells of shark blood-brain-barriers. PLoS ONE 7:e32409. doi: 10.1371/journal.pone.0032409
Banks, W. A., Kastin, A. J., and Michals, E. A. (1985). Transport of thyroxine across the blood-brain barrier is directed primarily from brain to blood in the mouse. Life Sci. 37, 2407–2414. doi: 10.1016/0024-3205(85)90108-0
Beslin, A., Chantoux, F., and Blondeau, J. P. (1995). Relationship between the thyroid hormones transport system and the Na+-H+ exchanger in cultured rat brain astrocytes. Endocrinology 136, 5385–5390.
Blay, P., Nilsson, C., Owman, C., Aldred, A., and Schreiber, G. (1993). Transthyretin expression in the rat brain: effect of thyroid functional state and role in thyroxine transport. Brain Res. 632, 114–120, doi: 10.1016/0006-8993(93)91145-I
Bradbury, M. W., and Davson, H. (1964). The transport of urea, creatinine and certain monosaccharides between blood and fluid perfusing the cerebral ventricular system in rabbits. J. Physiol. 170, 195–211. doi: 10.1113/jphysiol.1964.sp007323
Davson, H., and Segal, M. B. (1970). The effects of some inhibitors and accelerators of sodium transport on the turnover of 22Na in the cerebrospinal fluid and the brain. J. Physiol. 209, 131–153. doi: 10.1113/jphysiol.1970.sp009159
Braun, D., Kinne, A., Bräuer, A. U., Sapin, R., Klein, M. O., Köhrle, J., et al. (2011). Developmental and cell type-specific expression of thyroid hormone transporters in the mouse brain and in primary brain cells. Glia 59, 463–471, doi: 10.1002/glia.21116
Centanni, M., and Robbins, J. (1987). Role of sodium in thyroid hormone uptake by rat skeletal muscle. J. Clin. Inest. 80, 1068–1072. doi: 10.1172/JCI113162
Chantoux, F., Blondeau, J. P., and Francon, J. (1995). Characterization of the thyroid hormone transport system of cerebrocortical rat neurons in primary culture. J. Neurochem. 65, 2549–2554. doi: 10.1046/j.1471-4159.1995.65062549.x
Chen, R. L., Athauda, S. B., Kassem, N. A., Zhang, Y., Segal, M. B., and Preston, J. E. (2005). Decrease of Transthyretin synthesis at the blood-Cerebrospinal fluid barrier of old sheep. J. Gerontol A. Biol. Sci. Med. Sci. 60, 852–858. doi: 10.1093/gerona/60.7.852
Chen, R. L., Kassem, N. A., and Preston, J. E. (2006). Dose-dependent transthyretin inhibition of T4 uptake from cerebrospinal fluid in sheep. Neurosci. Lett. 396, 7–11. doi: 10.1016/j.neulet.2005.11.003
Choudhuri, S., Cherrington, N. J., Li, N., and Klaassen, C. D. (2003). Constitutive expression of various xenobiotic and endobiotic transporter mRNA in the choroid plexus of rats. Drug Metab. Dispos. 11, 1337–1345. doi: 10.1124/dmd.31.11.1337
Christensen, D., Sinon, M., and Randley, T. (1989). Anion channels in a leaky epithelium: a patch clamp study of choroid plexus. Pflug. Arch. 415, 37–46. doi: 10.1007/BF00373139
Davson, H., and Segal, M. B. (1970). The Effects of some inhibitors and accelerators of sodium transport on the turnover of 22Na in the cerebrospinal fluid and the brain. J. Physiol. 209, 131–153, doi: 10.1113/jphysiol.1970.sp009159
Deane, R., and Segal, M. B. (1979). The effect of phloridzin, phloretin and theophylline on the transport of sugars by the choroid plexus. J. Physiol. 287, 35–36.
Dickson, P. W., Aldred, A. R., Menting, J. G. T., Marley, P. D., Sawyer, W. H., and Schreiber, G. (1987). Thyroxine transport in choroid plexus. J. Biol. Chem. 262, 13907–13915.
Do, T. M., Bedussi, B., Chasseigneaux, S., Dodacki, A., Yapo, C., Chacun, H., et al. (2013). Oatp1a4 and an L-thyroxine-sensitive transporter mediate the mouse blood-brain barrier transport of Amyloid-β peptide. J. Alzheimers Dis. 36, 555–561. doi: 10.3233/JAD-121891
Dratman, M. B., Crutchfield, F. L., and Schoenhoff, M. B. (1991). Transport of iodothyronines from bloodstream to brain: contributions by blood: brain and choroid plexus: cerebrospinal fluid barriers. Brain Res. 554, 229–236. doi: 10.1016/0006-8993(91)90194-Z
Farwell, A. P., Safran, M., Dubord, S., and Leonard, J. L. (1996). Degradation and recycling of the substrate-binding subunit of type II iodothyronine 5′-deiodinase in astrocytes. J. Biol. Chem. 271, 16369–16374. doi: 10.1074/jbc.271.27.16369
Francon, J., Chantoux, F., and Blondeau, J. P. (1989). Carrier-mediated transport of thyroid hormones into rat glial cells in primary culture. J. Neurochem. 53, 1456–1463. doi: 10.1111/j.1471-4159.1989.tb08538.x
Friesema, E. C. H., Doctera, R., Moeringsa, E. P. C. M., Stiegerb, B., Hagenbuch, B., Meier, P. J., et al. (1999). Identification of thyroid hormone transporters. Biochem. Biophys. Res. Commun. 254, 497–501. doi: 10.1006/bbrc.1998.9974
Gao, B., and Meier, P. J. (2001). Organic anion transport across the choroid plexus. Micro. Res. Tech. 52, 60–64. doi: 10.1002/1097-0029(20010101)52:1<60::AID-JEMT8>3.0.CO;2-C
Gao, B., Stieger, B., Noé, B., Fritschy, J. M., and Meier, P. J. (1999). Localization of the organic anion transporting polypeptide 2 (Oatp2) in capillary endothelium and choroid plexus epithelium of rat brain. J. Histochem. Cytochem. 47, 1255–1264, doi: 10.1177/002215549904701005
Goncalves, E., Lakshmanan, M., Potecovri, A., and Robbins, J. (1990). Thyroid hormone transport in a human glioma cell line. Mol. Cell Endocrinol. 69, 157–165. doi: 10.1016/0303-7207(90)90009-W
Grijota-Martínez, C., Díez, D., Morreale de Escobar, G., Bernal, J., and Morte, B. (2011). Lack of action of exogenously administered T3 on the fetal rat brain despite expression of the monocarboxylate transporter 8. Endocrinology 152, 1713–1721, doi: 10.1210/en.2010-1014
Hennemann, G., Docter, R., Friesema, E. C., de Jong, M., Krenning, E. P., and Visser, T. J. (2001). Plasma membrane transport of thyroid hormones and its role in thyroid hormone metabolism and bioavailability. Endocr. Rev. 22, 451–476. doi: 10.1210/edrv.22.4.0435
Horn, S., Kersseboom, S., Mayerl, S., Müller, J., Groba, C., Trajkovic-Arsic, M., et al. (2013). Tetrac can replace thyroid hormone during brain development in mouse mutants deficient in the thyroid hormone transporter mct8. Endocrinology 154, 968–979. doi: 10.1210/en.2012-1628
Jansen, J., Friesema, E. C., Milici, C., and Viser, T. (2005). Thyroid hormone transport in health and disease. Thyroid 15, 757–768. doi: 10.1089/thy.2005.15.757
Johansson, P., Almqvist, E. G., Johansson, J. O., Mattsson, N., Hansson, O., Wallin, A., et al. (2013). Reduced cerebrospinal fluid level of thyroxine in patients with Alzheimer's disease. Psychoneuroendocrinology 38, 1058–1066, doi: 10.1016/j.psyneuen.2012.10.012
Kassem, N. A., Deane, R., and Segal, M. B. (2001). Efflux of thyroxine and its distribution from the cerebrospinal fluid (abstract). J. Physiol. 536, S157.
Kassem, N. A., Deane, R., Segal, M. B., Chen, R. L., and Preston, J. E. (2007). Thyroxine (T4) transfer from CSF to choroid plexus and ventricular brain regions in rabbit: contributory role of P-glycoprotein and organic anion transporting polypeptides. Brain Res. 1181, 44–50. doi: 10.1016/j.brainres.2007.08.052
Kassem, N. A., Deane, R., Segal, M. B., and Preston, J. E. (2006). Role of transthyretin in thyroxine transfer from cerebrospinal fluid to brain and choroids plexus. Am. J. Physiol. Regul. Integr. Comp. Physiol. 291, R1310–R1315. doi: 10.1152/ajpregu.00789.2005
Kassem, N. A., Segal, M. B., and Deane, R. (2002). A sodium-dependent mechanism responsible for thyroxine transport has been identified on the apical side of the lateral choroid plexus of the sheep. J. Physiol. 544, S129.
Kitazawa, T., Hosoya, K., Tarahashi, T., Sugiyama, Y., and Terasaki, T. (2000). In-vivo and in-vitro evidence of a carrier-mediated efflux transport system for esterone-3-sulphate across the blood-cerebrospinal fluid barrier. J. Pharm. Pharmacol. 52, 281–288. doi: 10.1211/0022357001773968
Klausner, R. D., Donaldson, J. G., and Lippincott-Schwartz, J. (1992). Insights into the control of membrane traffic and organelle structure. J. Cell Biol. 116, 1071–1080, doi: 10.1083/jcb.116.5.1071
Kogai, T., Liu, Y. Y., Richter, L. L., Mody, K., Kagechika, H., and Brent, G. A. (2010). Retinoic acid induces expression of the thyroid hormone transporter monocarboxylate transporter 8 (Mct8). J. Biol. Chem. 285, 27279–27288. doi: 10.1074/jbc.M110.123158
Koibuchi, N. (2013). The role of thyroid hormone on functional organization in the cerebellum. Cerebellum 12, 304–306. doi: 10.1007/s12311-012-0437-8
Lakshmanan, M., Goncalves, E., Lessly, G., Foti, D., and Robbins, J. (1990). The transport of thyroxine into mouse neuroblastoma cells, NB41A3: the effect of L-system amino acids. Endocrinol 126, 3245–3250. doi: 10.1210/endo-126-6-3245
Lei, J., Nowbar, S., Mariash, N. C., and Ingbar, D. H. (2003). Thyroid hormone stimulates Na-K-ATPase activity and its plasma membrane insertion in rat alveolar epithelial cells. Am. J. Physiol. Lun. Cell Mol. Physiol. 285, L762–L772. doi: 10.1152/ajplung.00376.2002
Mayerl, S., Visser, T. J., Darras, V. M., Horn, S., and Heuer, H. (2012). Impact of Oatp1c1 deficiency on thyroid hormone metabolism and action in the mouse brain. Endocrinology 153, 1528–1537. doi: 10.1210/en.2011-1633
McLeese, J. M., and Eales, J. G. (1996). Characteristics of the uptake of 3,5,3'-triiodo-L-thyronine and L-thyroxine into red blood cells of rainbow trout (Oncorhynchus mykiss). Gen. Comp. Endocrinol. 103, 200–208. doi: 10.1006/gcen.1996.0111
Morreale de Escobar, G., Calvo, R., Escobar del Rey, F., and Obregón, M. J. (1994). Thyroid hormones in tissues from fetal and adult rats. Endocrinology 134, 2410–2415.
Movius, E. G., Phyillaier, M. M., and Robbins, J. (1989). Phloretin inhibits cellular uptake and nuclear receptor binding of triiodothyronine in human hep G2 hepatocarcinoma cells. Endocrinol 124, 1988–1997. doi: 10.1210/endo-124-4-1988
Patel, J., Landers, K. A., Mortimer, R. H., and Richard, K. (2012). Expression and uptake of the thyroxine- binding protein transthyretin is regulated by oxygen in primary trophoblast placental cells. J. Endocrinol. 212, 159–167. doi: 10.1530/JOE-11-0348
Pieragostino, D., Del Boccio, P., Di Ioia, M., Pieroni, L., Greco, V., De Luca, G., et al. (2013). Oxidative modifications of cerebral transthyretin are associated with multiple sclerosis. Proteomics 13, 1002–1009. doi: 10.1002/pmic.201200395
Powell, K. A., Mitchell, A. M., Manley, S. W., Mortimer, R. H., and Mortimer, R. H. (2000). Different transporters for tri-iodothyronine (T(3)) and thyroxine (T(4)) in the human choriocarcinoma cell line, JAR. J. Endocrinol. 167, 487–492. doi: 10.1677/joe.0.1670487
Preston, J. E., Kassem, N. A., Segal, M. B., and Deane, R. (2005). Steady state extraction of thyroxine (T4) from blood side of the isolated perfused choroid plexus of the sheep. FASEB 19, A1646.
Preston, J. E., and Segal, M. B. (1992). Saturable uptake of (125I) L–triiodothyronine at the basolateral (blood) and apical (cerebrospinal fluid) sides of the isolated perfused sheep choroid plexus. Brain Res. 592, 84–90, doi: 10.1016/0006-8993(92)91661-W
Riley, W. W. Jr., and Eales, J. G. (1993). Characterization of L-thyroxine transport into hepatocytes isolated from juvenile rainbow trout (Oncorhynchus mykiss). Gen. Comp. Endocrinol. 90, 31–42. doi: 10.1006/gcen.1993.1057
Ritchie, J. W., and Taylor, P. M. (2010). Tryptophan and iodothyronine transport interactions in HepG2 human hepatoma cells. Amino Acids 38, 1361–1367. doi: 10.1007/s00726-009-0344-6
Rodríguez-Enríquez, S., Marín-Hernández, A., Gallardo-Pérez, J. C., and Moreno-Sánchez, R. (2009). Kinetics of transport and phosphorylation of glucose in cancer cells. J. Cell Physiol. 221, 552–559. doi: 10.1002/jcp.21885
Stewart, B. H., Collarini, E. J., Pisoni, R. L., and Christensen, H. N. (1989). Separate and shared lysosomal transport of branched and aromatic dipolar amino acids. Biochim. Biophys. Acta, 987, 145–153, doi: 10.1016/0005-2736(89)90537-3
Stewart, P. A., Hayakawa, K., and Farrell, C. L. (1994). Quantitation of blood-brain barrier ultrastructure. Microsc. Res. Tech. 27, 516–527. doi: 10.1002/jemt.1070270606
Strazielle, N., and Ghersi-Egea, J. F. (1999). Demonstration of a coupled metabolism-efflux process at the choroid plexus as a mechanism of brain protection toward xenobiotics. J. Neurosci. 19, 6275–6289.
van der Deure, W. M., Appelhof, B. C., Peeters, R. P., Wiersinga, W. M., Wekking, E. M., Huyser, J., et al. (2008). Polymorphisms in the brain-specific thyroid hormone transporter OATP1C1 are associated with fatigue and depression in hypothyroid patients. Clin. Endocrinol. 69, 804–811. doi: 10.1111/j.1365-2265.2008.03267.x
Villalobos, A. R., Parmelee, J. T., and Pritchard, J. B. (1997). Functional characterization of choroid plexus epithelial cells in primary culture. J. Pharmacol. Exp. Ther. 282, 1109–1116.
Wei, W., Wang, Y., Wang, Y., Dong, J., Min, H., Song, B., et al. (2013). Developmental hypothyroxinemia induced by maternal mild iodine deficiency delays hippocampal axonal growth in the rat offspring. J. Neuroendocrinol. 25, 852–862. doi: 10.1111/jne.12058
Keywords: thyroid hormone, transport, blood-CSF barrier, blood-brain barrier
Citation: Zibara K, El-Zein A, Joumaa W, El-Sayyad M, Mondello S and Kassem N (2015) Thyroxine transfer from cerebrospinal fluid into choroid plexus and brain is affected by brefeldin A, low sodium, BCH, and phloretin, in ventriculo-cisternal perfused rabbits. Front. Cell Dev. Biol. 3:60. doi: 10.3389/fcell.2015.00060
Received: 16 June 2015; Accepted: 14 September 2015;
Published: 29 September 2015.
Edited by:
Hibah Omar Awwad, University of Oklahoma Health Sciences Center, USACopyright © 2015 Zibara, El-Zein, Joumaa, El-Sayyad, Mondello and Kassem. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kazem Zibara and Nouhad Kassem, ER045, PRASE, Department of Biology, Faculty of Sciences-I, Lebanese University, Museum Street, Beirut, Lebanon, kzibara@ul.edu.lb; nouhad.kassem@hotmail.com
 Kazem Zibara
Kazem Zibara Ali El-Zein1,3
Ali El-Zein1,3  Mohammad El-Sayyad
Mohammad El-Sayyad Stefania Mondello
Stefania Mondello