Flexibility of short ds-DNA intercalated by a dipyridophenazine ligand
- 1Institut de Physique et Chimie des Matériaux de Strasbourg/Centre National de la Recherche Scientifique, University of Strasbourg, Strasbourg, France
- 2Department of Physics, School of Science, Shangdong University, Zibo, China
We use Förster Resonant Energy Transfer (FRET) in order to measure the increase of flexibility of short ds-DNA induced by the intercalation of dipyridophenazine (dppz) ligand in between DNA base pairs. By using a DNA double strand fluorescently labeled at its extremities, it is shown that the end-to-end length increase of DNA due to the intercalation of one dppz ligand is smaller than the DNA base pair interdistance. This may be explained either by a local bending of the DNA or by an increase of its flexibility. The persistence length of the formed DNA/ligand is evaluated. The described structure may have implications in the photophysical damages induced by the complexation of DNA by organometallic molecules.
1. Introduction
Since the first observation of the molecular “light switch” effect of Ru(bpy)2dppz2+ for DNA their interaction with DNA has been intensively studied (Friedman et al., 1990; Brennaman et al., 2002, 2004; Hu et al., 2009; Lim et al., 2009; Klajner et al., 2010; Sun et al., 2010; Song et al., 2012; Vidimar et al., 2012). Ru(bpy)2dppz2+ is a highly sensitive spectroscopic reporter of double-helical DNA (Very et al., 2012). In aqueous solution, luminescence is detectable when Ru(bpy)2dppz2+ intercalates into the nucleic acid structure (Batista and Martin, 2005). The emission properties (both in terms of intensity and spectral band) furthermore depend on the different helical forms of the polynucleotides, allowing the use of Ru(bpy)2dppz2+ as a sensitive, non-radioactive, luminescent DNA probe in both heterogeneous and homogeneous assays. Ru(phen)2dppz2+ possesses similar properties. Both complexes Ru(bpy)2dppz2+ and Ru(phen)2dppz2+ have served as “molecular light switch” for DNA, luminescing intensely in the presence of DNA but with no photoluminescence in aqueous solution and can be seen as unique reporters of nucleic acid structures (Song et al., 2012). The luminescent enhancement observed upon binding is attributed to the sensitivity of the excited state to quenching by water; the metal complex, upon intercalation into the DNA helix, is protected from the aqueous solvent, thereby preserving the luminescence (Friedman et al., 1990). Correlations between the extent of protection (depending upon the DNA conformation) and the luminescence parameters have been proven. Indeed, the strongest luminescent enhancement is observed for intercalation into DNA conformations which afford the greatest amount of overlap with access from the major groove, such as in triple helices. Differences are observed in the luminescent parameters between the two complexes which also correlate with the level of water protection. In the presence of nucleic acids, these two complexes exhibit biexponential decays in emission (Jenkins et al., 1992). Quenching studies are consistent with two intercalative binding modes for the dppz ligand in the major groove: one in which the metal-phenazine axis lies along the DNA dyad axis and another where the metal-phenazine axis lies almost perpendicular to the DNA dyad axis.
Upon binding to mismatched DNA base pair, Ru(bpy)2dppz2+ exhibits significant luminescent enhancements compared to well-matched DNA (Lim et al., 2009). In the presence of a single base mismatch, large luminescent enhancements are evident when ruthenium binds to an oligonucleotide containing an abasic site. Titrations with hairpin oligonucleotides containing a variable mismatch site have revealed correlation between the level of luminescent enhancement and the thermodynamic destabilization associated with the mismatch (Lim et al., 2009). This correlation is reminiscent of that found earlier for a bulky rhodium complex that binds mismatched DNA sites through metalloinsertion, where the complex binds the DNA from the minor groove side, ejecting the mismatched bases into the major groove (Lim et al., 2009). The smaller size of the dppz ligand also allows the ruthenium complex to bind through classical intercalation between two consecutive well-matched base pairs. Intercalated complexes are also located in the minor groove, stabilized by extensive ancillary interactions (Erkkila et al., 1999). This discrepancy notwithstanding, the crystal structure attests the remarkable structural flexibility of DNA upon high-density ligand binding, and illustrates the nuanced binding geometries sampled by a non-covalently bound small molecule. It highlights the dominance of metalloinsertion as the preferred binding mode to destabilized regions of DNA.
2. Flexibility
Although it is well-known that the length of DNA increases approximately linearly with the number of intercalated Ru(bpy)2dppz2+ molecules into the DNA double strand (Vladescu et al., 2007), the induced dynamical changes of the DNA chain have not been studied. Here, we quantify the variation of double strand flexibility induced by the intercalation of Ru(bpy)2dppz2+ into a short dsDNA (15 base pair long). We use Fluorescence Resonance Energy Transfer (FRET) in order to monitor the average distance between the extremities of a 15 bp dsDNA modified with two fluorophores at its extremities: both ends of dsDNA are modified by two types of fluorophores: Alexa488 and Alexa568. When Alexa488 is excited, it can decay by transferring non-radiative energy to Alexa568, which then de-excites by emitting photons of lower energy than those emitted by Alexa488. The efficiency of this energy transfer can be quantified from the measurement of the intensities emitted at low and high energy. It depends a priori on the coupling efficiency (and therefore the distance) between the two fluorophores. We will show that the increase of the average distance between the DNA extremities is incompatible with the assumption of a rigid and straight DNA/Ru(bpy)2dppz2+ complex. Nevertheless, the observation is made difficult due to the strong quenching of the fluorophores induced by the intercalation of the Ru(bpy)2dppz2+. Thus, in this article, from the analysis of the evolution of the lifetime of the donor on one hand, and that of the acceptor on the other hand, we deduce the evolution of the efficiency of the energy transfer in the concentration of ligand. We analyze the evolution of the emitted intensity of the donor and the lifetime of its excited state, and those of the acceptor, taking into account the photophysical properties of the ruthenium complex. We then obtain the evolution of the dsDNA average length as a function of the Ru(bpy)2dppz2+ complexation.
3. Materials and Methods
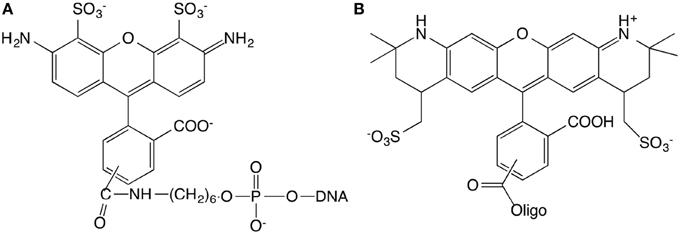
15 base pair dsDNA are used to perform the experiment. The number of DNA base pairs is limited by the range over which the FRET can take place that is approximately 10 nm. Complementary strands were purchased from IBA (Germany company) with sequences GGA GAC CAG AGG CCT and CCT CTG GTC TCC GGA. The length of 15 base pairs is 5.1 nm. The extremity of the short double-stranded DNA (dsDNA) is labeled with different kinds of fluorophores. The first sequence is modified in three different ways. (1) 5′-end is labeled with Alexa488, (2) 3′-end is labeled with Alexa568, (3) 5′-end and 3′-end are labeled with Alexa488 and Alexa568, respectively (Figure 1). The Alexa fluorophore is chemically linked with DNA base. Titrations are performed by the addition of Ru(bpy)2dppz2+ to a solution of DNA at constant DNA concentration, equal to 1μM.
A pulsed laser of 495 nm wavelength with a repetition rate of 1 MHz is used to excite the fluorophore. The photon detector temporal resolution is 0.1 ns. The wavelength collected is at 517 nm and 600 nm with 2 nm spectral slit. The acquisition time is chosen so that the number of photons collected in the first channel is equal to 104. Under these experimental conditions, a measurement lasts between 10 and 120 min. All measurements are performed at constant room temperature (20°C).
The notations used in the analysis of the results are given in Table 1.
4. Analysis of the Emission at 517 nm
We now turn to the analysis of the intensity emitted at 517 nm. The most important factor we care in FRET process is the transfer efficiency, that relies on two assumptions. First, it is assumed that the presence of the acceptor neither changes nor adds any relaxation process except for the FRET process. Then, the number donor molecules excited by the laser is assumed to remain constant when the acceptor is present. The transfer efficiency is defined as the probability that an excited donor fluorophore comes back to its ground state by FRET process. This can be expressed as:
where κT is the FRET transfer rate. κT is directly related to the distance r between the two fluorophores according to: , where R0 is the Förster distance and τD the relaxation time of the donor fluorophore in the absence of the acceptor. τDA is the overall characteristic decay time of the donor molecule in the presence of the acceptor: it is directly measured as the decay time of the fluorophore emission at its maximum wavelength. According to the first assumption, the presence of the acceptor does not change any relaxation process except for the FRET process. We thus have:
where τD is the characteristic decay time in the absence of acceptor, that can also be measured directly. Then the transfer efficiency may be rewritten as:
D* being the number concentration of excited donor molecules, we have:
leading to:
where D* is the number of excited donors at time t, D*02 is the initial population of excited donors in the presence of acceptor, τDA and τD are the lifetimes in the presence and absence of acceptor, respectively. Nevertheless, D* cannot be measured directly: the emitted intensity is measured. But the donor emits photons both at 517 nm and at the maximum emission wavelength of this acceptor, at 600 nm (this is the cross-talk phenomenon).The number of photons emitted by the donor in the presence, NDA517, and in the absence,ND517, of the acceptor, at 517 can be expressed as the probability that their relaxation occurs through the emission of a photon at the corresponding wavelength:
where τD and τDA are the overall decay times in the absence and presence of the acceptor, τD517 and τD600 the characteristic decay times of photon emission at 517 nm and 600 nm by the donor. D*01 and D*02 are the initial number of excited donors by the laser in the absence and presence of acceptor. We thus have:
assuming that the number of donor molecules excited by the laser does not change when the acceptor fluorophore is present. This leads to a new expression of the transfer efficiency:
We first consider the interaction between Ru(bpy)2dppz2+ and the fluorophores. The overall intensities and relaxation times of single labeled DNA molecules is observed to decrease slightly in the presence of Ru(bpy)2dppz2+, due to quenching. The inverse of the intensity emitted by the donor fluorophore as well as its life time are represented in Figure 2. One observes that:
where the subscript indicates that the measurement is performed at null Ru(bpy)2dppz2+ concentration. This relation implies that Ru(bpy)2dppz2+ dynamically quenches the emission of the donor fluorophore and defines the dynamical quenching constant. One finds KD = 0.3 μM−1.
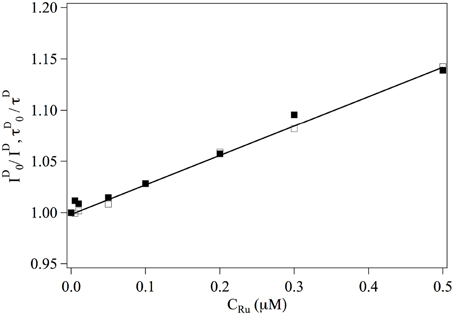
Figure 2. Evolution of ■ and □ as a function of the ruthenium concentration. Continuous line is a adjustment fit of the data from which one gets KD = 0.3 μM.−1.
The transfer rate κT and the transfer efficiency E are separately computed from Equations 2, 9. The evolutions of and E as a function of the ruthenium complex concentration are shown in Figure 3. On the whole, τF increases with the increase of the complex whereas the transfer efficiency decreases with increasing the Ru(bpy)2dppz2+ concentration. In the range of studied concentrations, the evolution of τF and E with the Ru(bpy)2dppz2+ concentration may be well-described by a linear behavior.
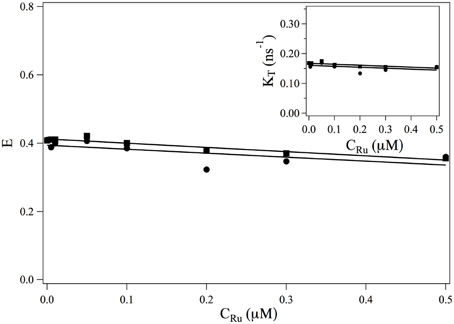
Figure 3. Evolution of the FRET efficiency E and of κT = 1/τF (insert) as a function of the ruthenium concentration. ■: values obtained from the measurements at 517 nm (Equations 9, 2). •: values obtained from the measurements at 600 nm (Equation 19) and κT = E/τDA.
4.1. Dynamical Measurements of the Emission at 600 nm
The acceptor can be excited following two different mechanisms, either by direct excitation by the laser or by energy transfer from the donor fluorophore. τA is the overall relaxation time of the acceptor. The existence of FRET does not change the relaxation path of an excited acceptor, and τA is identical to the relaxation time of acceptor excited by the laser in the absence of donor. From the perspective of the acceptor, the FRET process can be determined by the following equations:
where the first term represents the excitation through FRET and the second one the desexcitation of the acceptor. With the initial condition A*(0) = A*02, the solution is:
where A*02 is the number of acceptor molecules excited by the laser at time 0. This result states that the A*(t) should exhibit a two-time relaxation process, the first one equals to the relaxation time of the donor population in the presence of the acceptor, τDA, and the second one equals to the relaxation time of the acceptor, τA. Moreover, if , the amplitude of the acceptor decay over τDA is negative.
The number of photons emitted by the acceptor, NDA600 can be written as:
Where τA600 is the characteristic time of photon emission at 600 nm by the acceptor, and τA = τAD, since the presence of the donor does not change the relaxation of the acceptor. Finally, using E = κT τDA, we have:
where A*02 is the number of excited acceptor from the lasers excitation, and D*02 E is the number of acceptor molecules excited by the FRET process.
We now consider the emission at 600 nm of double labeled dsDNA bound with ruthenium complex, N600. There are three contributions to this emission. (i) the contribution of the donor fluorophore, NDA600, the intensity emitted at 600 nm by the donor after a pulse, IDA600(t),(ii) the contribution of the acceptor, NAD600, the intensity emitted at 600 nm by the acceptor after a pulse, IAD600(t), and (iii) the emission of Ru(bpy)2dppz2+ that is luminescent when intercalated between DNA base pairs, NRu600
So the total number of photons emitted at 600 nm can be expressed as N600 = NRu600 + NDA600 + NAD600, whose we are going to perform an analysis term by term.
The intensity of emission at 600 nm of bound Ru(bpy)2dppz2+ has been measured when the ruthenium complex bound with non-labeled DNA. The contribution of the ruthenium complex is found to be smaller than 0.5% of the total observed intensity, so that we will neglect NRu600 in the subsequent analysis.
The emission of the donor fluorophore at 600 nm in the presence of the acceptor cannot be measured independently and will be obtained indirectly. The number of photons emitted by the donor at the two studied wavelengths with and without the acceptor are given by the probabilities that the relaxation process occurs by the emission of a photon at the corresponding wavelength. We have (Equation 8):
and similarly at 600 nm:
We finally obtain the number of photons emitted by the donor in the presence of the acceptor, at 600 nm:
The ratio does not depend on the ruthenium complex concentration, and is measured at the null ruthenium concentration. It is equal to 2.74%. NDA517 is then measured directly from which NDA600 can be calculated as a function of the ruthenium concentration. We can then obtain the emission of the acceptor in the presence of the donor fluorophore, at 600 nm: . The efficiency is then computed:
We now determine the three ratios involved:
• : τA is the fluorescence lifetime of single labeled DNA with alexa568, and NAD600 has been obtained in the previous paragraph.
• is obtained from the amplitude of the 2 relaxation modes of the intensity emitted at 600 nm by two labeled DNA. This intensity is I600(t):
with
and
which defines α1 and α2. They are obtained from the measurement of . We thus obtain .
• does not depend on the ruthenium concentration. Its value can not be measured from measurements of I600(t) as it always appears under the form . The evolution of the efficiency E as a function of the ruthenium concentration is thus obtained up to a constant multiplicative factor. In order to compare the evolution of E (CRu) with the efficiency obtained from measurement at 517 nm, we determine this multiplication factor at CRu = 0. The evolution of the FRET efficiency and of the transfer rate κT are plotted in Figure 3•.
5. Discussion
At the concentration under study, the fraction of DNA strand complexed by more than one ruthenium molecule may be neglected. We thus have a mixture of uncomplexed DNA and DNA strands complexed with one ruthenium molecule. Moreover this molecule may be intercalated at one or other of the 14 positions along the DNA double strand The intensities and lifetimes measured are thus average values of the intensities and lifetimes of these different complexes. The measured lifetime may be written as:
where ξ is the fraction of DNA strands complexed with one Ru(bpy)2dppz2+, τ0 is the decay time of non-complexed DNA, τ1, n is the decay time of DNA strand intercalated with Ru(bpy)2dppz2+ at the nth position. We moreover assume that all the intercalation positions are equiprobable. The decay time may be expressed as the consequence of the existence of two processes:
where τD0 is the donor decay time in the absence of the acceptor at concentration CRu, for a non-complexed dsDNA, and τF0 = κ−1T is the inverse of the FRET rate in the absence of complexation. τF0 does not depend on the Ru(bpy)2dppz2+ concentration and is measured at CRu = 0.
with similar notations. We have used the fact the τD1, n(CRu) does not depend on n, as the observed quenching is dynamic. We thus have:
where r1, n is the distance between the two fluorophores when the intercalation occurs at the nth position. It is known that the intercalation of the ruthenium compound induces a length increase of the DNA chain equal to the base pair distance. We moreover assume that the DNA double strand remains linear and rigid (Vladescu et al., 2007) Then, the length of the dsDNA, complexed with one Ru(bpy)2dppz2+ does not depend on the intercalation position and we have r1, n = 16/15 r0 where r0 is the length of the 15 bp dsDNA chain. We now compute r1 from thee experimental measurements. We can use Equation 26, recognizing that:
We obtain r1/r0. The values of r1/r0 are plotted in Figure 4 as a function of ξ. At low ξ, the bound ruthenium fraction is too low and the measurement is not accurate, but for values of ξ ≥ 0.2, r1/r0 saturates and its average value over the three highest complexation ratios is 1.028. This value is smaller than if the intercalation would have led to a length increase of a straight double strand, we would have: r1, n/r0 = 16/15 = 1.067. We thus conclude that the complexation induces a bending in the dsDNA. This bending may be static or dynamic, that is, due to an increase of flexibility of the DNA double strand at the intercalation. We define θ the bent angle induced by the intercalation, θ is of the order of 30°. It has been observed that short ds-DNA molecules are more flexible than worm-like chains of small lengths and whose persistence length would be equal to that of long DNA chains (Cloutier and Widom, 2005; Wiggins et al., 2006; Kahn, 2014; Le and Kim, 2014). This may also contribute to the increase of flexibility observed in our measurements.
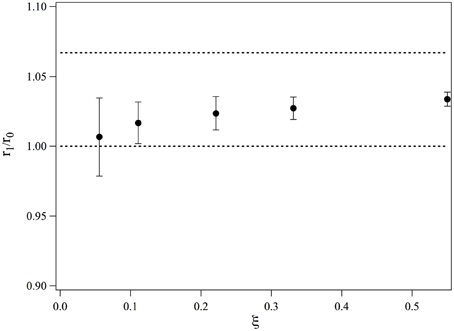
Figure 4. Evolution of the average end-to-end distance of a 15-bp dsDNA bound to one molecule of Ru(bpy)2dppz2+, measured for different ratios of complexation.
As a conclusion, we have observed that the end-to-end distance increase of 15 bp dsDNA complexed with Ru(bpy)2dppz2+ is smaller than that would be increase if the DNA would remain rigid upon complexation. We may thus conclude that DNA bends upon complexation. Nevertheless, our experiments cannot determine whether time average bending is due to a local dynamic flexibility or a static kink induced by the intercalation.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Batista, E. R., and Martin, R. L. (2005). On the excited states involved in the luminescent probe [ru(bpy)2dppz]2+. J. Phys. Chem. A 109, 3128–3133. doi: 10.1021/jp050673+
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Brennaman, M. K., Alstrum-Acevedo, J. H., Fleming, C. N., Jang, P., Meyer, T. J., and Papanikolas, J. M. (2002). Turning the [ru(bpy)2dppz]2+ light-switch on and off with temperature. J. Am. Chem. Soc. 124, 15094–15098. doi: 10.1021/ja0279139
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Brennaman, M. K., Meyer, T. J., and Papanikolas, J. M. (2004). [ru(bpy)2dppz]2+ light-switch mechanism in protic solvents as studied through temperature-dependent lifetime measurements†. J. Phys. Chem. A 108, 9938–9944. doi: 10.1021/jp0479670
Cloutier, T. E., and Widom, J. (2005). DNA twisting flexibility and the formation of sharply looped protein–dna complexes. Proc. Natl. Acad. Sci. U.S.A. 102, 3645–3650. doi: 10.1073/pnas.0409059102
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Erkkila, K. E., Odom, D. T., and Barton, J. K. (1999). Recognition and reaction of metallointercalators with DNA. Chem. Rev. 99, 2777–2796. doi: 10.1021/cr9804341
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Friedman, A. E., Chambron, J. C., Sauvage, J. P., Turro, N. J., and Barton, J. K. (1990). A molecular light switch for DNA: ru(bpy)2(dppz)2+. J. Am. Chem. Soc. 112, 4960–4962. doi: 10.1021/ja00168a052
Hu, L., Bian, Z., Li, H., Han, S., Yuan, Y., Gao, L., et al. (2009). [ru(bpy)2dppz]2+ electrochemiluminescence switch and its applications for dna interaction study and label-free atp aptasensor. Anal. Chem. 81, 9807–9811. doi: 10.1021/ac901925x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Jenkins, Y., Friedman, A. E., Turro, N. J., and Barton, J. K. (1992). Characterization of dipyridophenazine complexes of ruthenium(II): the light switch effect as a function of nucleic acid sequence and conformation. Biochemistry 31, 10809–10816. doi: 10.1021/bi00159a023
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kahn, J. D. (2014). DNA, flexibly flexible. Biophys. J. 107, 282–284. doi: 10.1016/j.bpj.2014.06.007
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Klajner, M., Hébraud, P., Sirlin, C., Gaiddon, C., and Harlepp, S. (2010). DNA binding to an anticancer organo-ruthenium complex. J. Phys. Chem. B 114, 14041–14047. doi: 10.1021/jp1044783
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Le, T. T., and Kim, H. D. (2014). Probing the elastic limit of DNA bending. Nucleic Acids Res. 42, 10786–10794. doi: 10.1093/nar/gku735
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lim, M. H., Song, H., Olmon, E. D., Dervan, E. E., and Barton, J. K. (2009). Sensitivity of ru(bpy)2dppz2+ luminescence to DNA defects. Inorg. Chem. 48, 5392–5397. doi: 10.1021/ic900407n
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Song, H., Kaiser, J. T., and Barton, J. K. (2012). Crystal structure of δ-[ru(bpy)2dppz]2+ bound to mismatched dna reveals side-by-side metalloinsertion and intercalation. Nat. Chem. 4, 615–620. doi: 10.1038/nchem.1375
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sun, Y., Collins, S. N., Joyce, L. E., and Turro, C. (2010). Unusual photophysical properties of a ruthenium(II) complex related to [ru(bpy)2(dppz)]2+. Inorg. Chem. 49, 4257–4262. doi: 10.1021/ic9025365
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Very, T., Despax, S., Hébraud, P., Monari, A., and Assfeld, X. (2012). Spectral properties of polypyridyl ruthenium complexes intercalated in DNA: theoretical insights into the surrounding effects of [ru(dppz)(bpy)2]2+. Phys. Chem. Chem. Phys. 14, 12496–12504. doi: 10.1039/c2cp40935f
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Vidimar, V., Meng, X., Klajner, M., Licona, C., Fetzer, L., Harlepp, S., et al. (2012). Induction of caspase 8 and reactive oxygen species by ruthenium-derived anticancer compounds with improved water solubility and cytotoxicity. Biochem. Pharmacol. 84, 1428–1436. doi: 10.1016/j.bcp.2012.08.022
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Vladescu, I. D., McCauley, M. J., Nuñez, M. E., Rouzina, I., and Williams, M. C. (2007). Quantifying force-dependent and zero-force DNA intercalation by single-molecule stretching. Nat. Methods 4, 517–522. doi: 10.1038/nmeth1044
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Wiggins, P. A., van der Heijden, T., Moreno-Herrero, F., Spakowitz, A., Phillips, R., Widom, J., et al. (2006). High flexibility of DNA on short length scales probed by atomic force microscopy. Nat. Nanotechnol. 1, 137–141. doi: 10.1038/nnano.2006.63
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Keywords: DNA intercalation, DNA flexibility, FRET, ruthenium compounds, dppz intercalator
Citation: Jia F, Despax S, Münch J-P and Hébraud P (2015) Flexibility of short ds-DNA intercalated by a dipyridophenazine ligand. Front. Chem. 3:25. doi: 10.3389/fchem.2015.00025
Received: 22 January 2015; Paper pending published: 02 March 2015;
Accepted: 20 March 2015; Published: 16 April 2015.
Edited by:
Elise Dumont, Ecole Normale Supérieure de Lyon, FranceReviewed by:
Xavier Assfeld, Université de Lorraine, FranceJosé Pedro Cerón-Carrasco, Universidad de Murcia, Spain
Copyright © 2015 Jia, Despax, Münch and Hébraud. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pascal Hébraud, Institut de Physique et Chimie des Matériaux de Strasbourg/Centre National de la Recherche Scientifique, University of Strasbourg, 23 rue du Loess, 67034 Strasbourg, France pascal.hebraud@ipcms.unistra.fr
 Fuchao Jia
Fuchao Jia Stéphane Despax1
Stéphane Despax1  Pascal Hébraud
Pascal Hébraud