Total Synthesis of Putative 11-epi-Lyngbouilloside Aglycon
- 1Laboratoire de Chimie Organique, Institute of Chemistry, Biology and Innovation, ESPCI Paris, Centre National de la Recherche Scientifique (UMR8231), PSL Research University, Paris, France
- 2LGCR-Analytical Sciences, Sanofi, Vitry-sur-Seine, France
We report here the total synthesis of 11-epi-lyngbouilloside aglycon. Our strategy features a Boeckman-type esterification followed by a RCM to form the 14-membered ring macrolactone and a late-stage side chain introduction via a Wittig olefination. Overall, the final product was obtained in 20 steps and 2% overall yield starting from commercially available 3-methyl-but-3-enol. Most importantly, the strategy employed is versatile enough to eventually allow us to complete the synthesis of the natural product and irrevocably confirm its structure.
Introduction
Lyngbouilloside (1) is a glycosidic macrolide isolated by Gerwick et al. (Tan et al., 2002) from the cyanobacteria Lyngbya bouillonii (Hoffmann and Demoulin, 1991), which also produce several other structurally intriguing natural products including the tetrapeptide lyngbyapeptin (Klein et al., 1999a,b), several macrolides such as laingolide, laingolide A, and madangolide (Klein et al., 1996, 1999a,b), and various lyngbouilloside analogs such as lyngbyaloside (2) (Klein et al., 1997), lyngbyaloside B (3) (Luesch et al., 2002; Matthew et al., 2010), and lyngbyaloside C (4) (Matthew et al., 2010; Figure 1). The structure of lyngbouilloside was determined after exhaustive 1D and 2D NMR analysis, HR-FABMS, IR, and UV absorption experiments, which unveiled the presence of the pendant dienyl side chain, the 14-membered ring lactone, the presence of hydroxyl groups, the chair conformation of the tetrahydropyran ring and the relative configuration of the stereogenic centers in the aglycon portion of the natural product. The nature of the sugar, on the other hand, was assigned by correlations in the 1H-1H COSY and HMBC spectral data and comparison with the sugar unit present in auriside A. Interestingly, lyngbouilloside exhibits only a moderate cytotoxic activity (IC50 = 17 μM) toward neuroblastoma cell lines. Nonetheless, its structural resemblance with several biologically active 14-membered macrolides, such as callipeltoside A (5), auriside A (6), or dolastatin 19 (7), encouraged a few groups including ours to complete its synthesis (Gebauer et al., 2008; Webb et al., 2009; ElMarrouni et al., 2012; Sabitha et al., 2014). In this context, we recently reported the total synthesis of nominal lyngbouilloside aglycone via a flexible approach featuring an acyl ketene macrolactonization and a late stage side chain introduction, which led us to suggest a stereochemical reassignment at C11. With this hypothesis in mind, we embarked on the synthesis of putative 11-epi-lyngbouilloside aglycon; we report here the results of our endeavor.
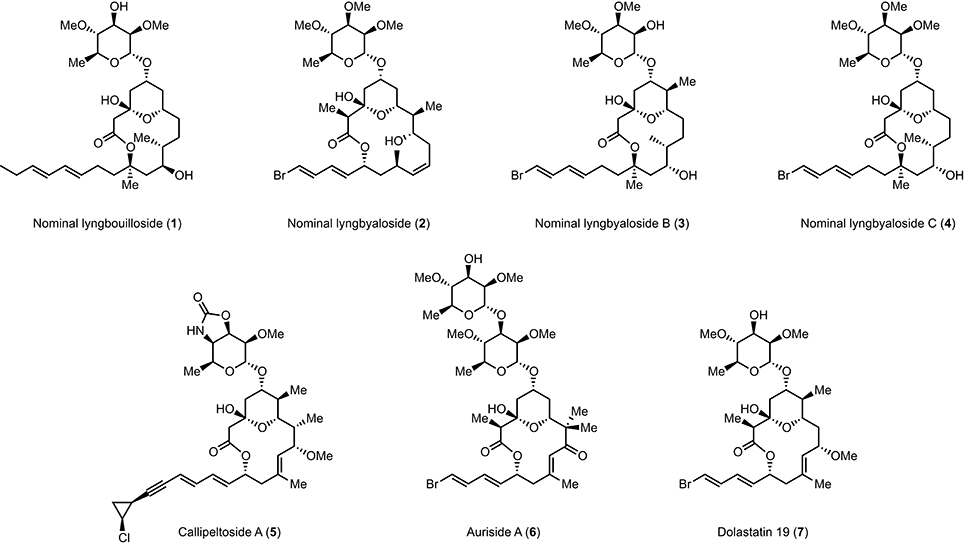
Figure 1. Structures of lyngbouilloside, lyngbyaloside, lyngbyaloside B, lyngbyaloside C, callipeltoside A, auriside A, and dolastatin 19.
Materials and Methods
Experimental procedures and compound characterization data are furnished in the Supplementary Material.
Results and Discussion
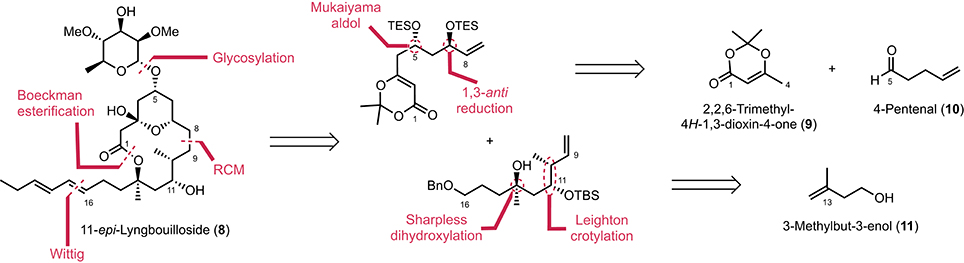
Our initial route to 11-epi-lyngbouilloside 8 relied on the same acyl ketene macrolactonization and Wittig olefination that were previously used to complete the synthesis of the proposed structure of lyngbouilloside aglycone. Unfortunately, the poor yields obtained in the macrolactonization process, combined with the difficulties encountered while trying to selectively reduce the C8–C9 double bond in the presence of the pendant alkyne side chain, led us to reconsider our strategy. We therefore opted for a slightly modified route, which involved a Boeckman-type esterification between an alcohol and an acyl ketene (Boeckman and Pruitt, 1989) and a ring-closing metathesis to form the 14-membered ring macrolactone, while a pendant hydroxyl group was placed instead of an alkynyl group in order to introduce the dienyl side-chain via a stereoselective Wittig reaction (Figure 2). We projected to control the stereogenic centers at C7 and C13 via a Sharpless dihydroxylation (Jacobsen et al., 1988; Kolb et al., 1994) and a 1,3-anti reduction respectively, while the C10 and C11 stereogenic centers were to be controlled through a Leighton type crotylation.
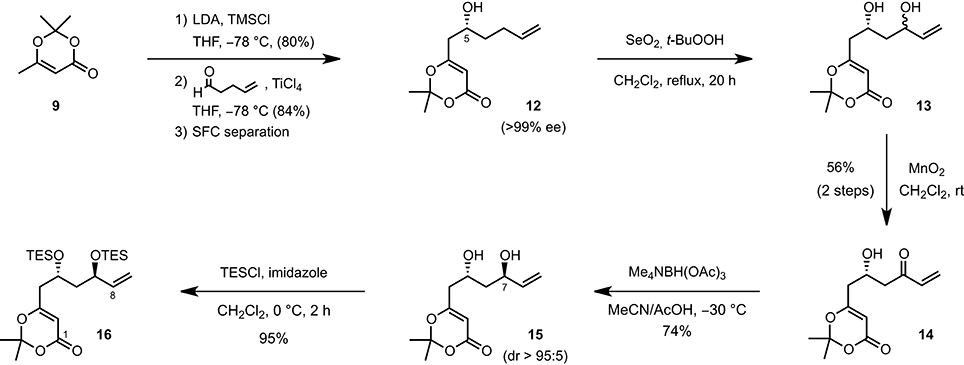
The synthesis of 11-epi-lyngbouilloside 8 began by first converting 2,2,6-trimethyl-4H-1,3-dioxin-4-one 9 to the corresponding silyl dienol ether (LDA, TMSCl, THF, −78°C) and subjecting the latter to 4-pentenal under asymmetric vinylogous aldol conditions (Denmark et al., 2005a,b). Among the various enantioselective catalytic processes developed so far in the field of asymmetric Mukaiyama aldol, the ones reported by Denmark et al. (Denmark et al., 2002, 2005a,b; Denmark and Beutner, 2003), involving the combination of a catalytic amount of chiral bis-phosphoramide and silicon tetrachloride to promote a highly enantio- and diastereoselective addition of silyl ketene acetals to aldehydes (SiCl4, CH2Cl2, −78°C), appeared particularly attractive. Unfortunately, the application of these conditions to our system afforded the desired product 12 in a modest 65% ee. The conditions reported by Sato [Ti(Oi-Pr)4 (20 mol%), (S)-BINOL (20 mol%), THF, −78°C; Sato et al., 1995] and more recently by Scettri [Ti(Oi-Pr)4 (8 mol%), (S)-BINOL (8 mol%), and 2 equiv of silyl dienol ether instead of 1.4 equiv, THF, −78°C; De Rosa et al., 2003] were also tested but afforded compound 12 in moderate yields albeit in up to 86% ee. With these rather disappointing results in hand, we decide to perform the aldol reaction in a racemic fashion (TiCl4, THF, −78°C) and separate the racemate by chiral preparative supercritical fluid chromatography (SFC) (Scheme 1). This preparative separation allowed to readily obtain large quantities of alcohol 12 in optically pure form (>99% ee) and with an acceptable overall yield of 34%. The absolute configuration was secured after hydrogenating the terminal double bond and comparing the optical rotation of the resulting product { −21.0 (c 0.1, CHCl3)} with the one reported in the literature { +19.0 (CHCl3)} (Sato et al., 1995). To complete the synthesis of the C1-C8 fragment, alcohol 12 was eventually treated with SeO2 and t-BuOOH (CH2Cl2, rt) to afford the corresponding diol, which was subsequently engaged in a MnO2-mediated oxidation to provide the desired enone 14 in 56% overall yield. A diastereoselective anti-reduction [Me4NBH(OAc)3, MeCN/AcOH, −30°C; Evans et al., 1988] followed by the protection of the resulting diol as a bis(triethylsilyl) ether (TESCl, imidazole, CH2Cl2, 0°C) finally provided the C1–C8 fragment 16 in six steps and 14% overall yield starting from the inexpensive dioxolenone 9.
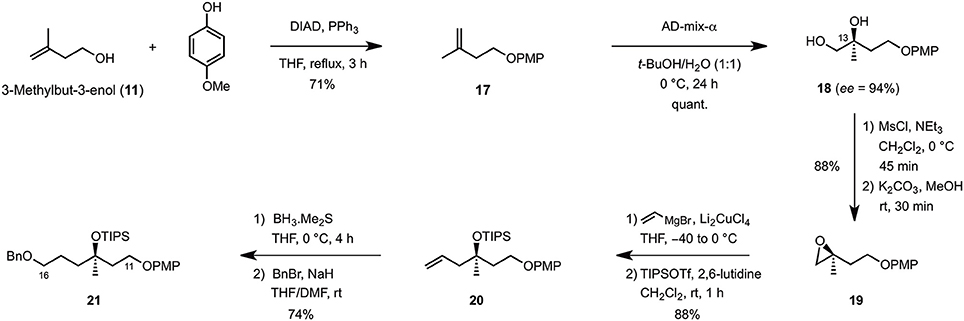
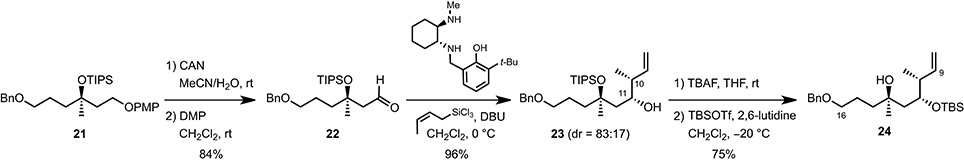
The synthesis of the C9–C16 fragment started off from commercially available 3-methylbuten-3-enol (11), which was first protected as its PMP-ether under Mitsunobu conditions (DIAD, PPh3, THF reflux) (Mitsunobu and Yamada, 1967) before it was engaged in the asymmetric Sharpless dihydroxylation (AD-mix-α, t-BuOH/H2O, 0°C) to quantitatively afford diol 17 in 94% ee (Scheme 2). Mesylation (MsCl, Et3N, CH2Cl2, 0°C) and cyclization under basic conditions (K2CO3, MeOH) then yielded epoxide 19 which, after Cu-catalyzed ring-opening using vinyl magnesium bromide (Li2CuCl4, THF, −40 to 0°C) and TIPS-protection (TIPSOTf, 2,6-lutidine, CH2Cl2, rt), produced the corresponding homoallylic ether 20 in 88% overall yield. Finally, hydroboration of the terminal double bond (BH3·Me2S, THF, 0°C) and benzylation of the primary alcohol obtained upon oxidative workup (BnBr, NaH, THF/DMF, rt) gave rise to the C11–C16 fragment 21 in 74% yield over two steps.
To control the two stereogenic centers at C10 and C12 and complete the synthesis of the C9–C16 fragment, we performed a syn-crotylation of aldehyde 22 obtained upon sequential PMP-deprotection (CAN, MeCN/H2O, rt)/oxidation [DMP, CH2Cl2, rt] using a procedure recently developed by Leighton and co-workers (Kim et al., 2011) (Scheme 3). This almost quantitatively afforded a mixture of the two diastereoisomeric homoallylic alcohols 23 (dr = 83:17), which could be converted to the desired C9–C16 fragment 24 by simple protecting group manipulation (TBAF, THF, rt, then TBSOTf, 2,6-lutidine, CH2Cl2, −40°C) in 75% yield.
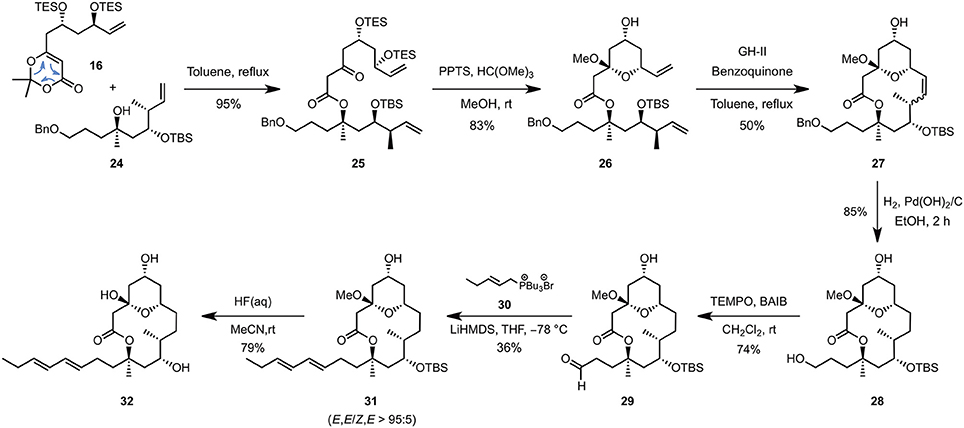
The C1–C8 and C9–C16 fragments were eventually coupled together using the approved intermolecular acyl ketene trapping by mixing the two fragments in refluxing toluene, giving rise to the fully functionalized carbon backbone of the natural product in an excellent yield of 95% (Scheme 4). Hemi-acetal formation (PPTS, MeOH, trimethyl orthoformate), ring-closing metathesis using the Grubbs-Hoveyda 2nd generation catalyst (GH-II) and a final catalytic hydrogenation allowed to isolate the 14-membered macrolactone 27 possessing a hydroxypropyl side-chain appropriate for the elongative olefination (3 steps, 37% overall yield). The latter could be achieved by a selective TEMPO-mediated oxidation (BAIB, CH2Cl2, rt) followed by a Wittig reaction of the resulting aldehyde 29 with tributyl phosphonium bromide 30 (LiHMDS, THF, −78°C), which enabled the E,E-dienyl moiety to be installed in a highly diastereoselective fashion but with a yet unoptimized yield of 27%. Finally, removal of the remaining TBS-protecting group (HF, MeCN, rt) afforded the putative structure of 11-epi-lyngbouilloside aglycone 32 as a single diastereoisomer in 20 steps and 2% overall yield starting from commercially available 3-methyl-but-3-enol (11). Unfortunately, comparison of the NMR chemical shifts of our synthetic aglycon with the ones reported for natural lyngbouilloside, particularly in the C9-C13 region, revealed some disparities suggesting one or more of the stereochemical configurations of the natural product needed to be reassigned.
Conclusion
In summary, we have completed the synthesis of what we believed was the actual structure of lyngbouilloside aglycon. Unfortunately, after careful analysis of the spectroscopic data of our final product with the ones reported for lyngbouilloside, some discrepancies still remained. This observation combined with the recent syntheses of lyngbyaloside B and C by Fuwa (Fuwa et al., 2016) and Taylor (Chang et al., 2015), suggest not only a stereochemical reassignment for C11, but also for C10 and C13. Nonetheless, our strategy featuring a ring-closing metathesis (RCM) to form the 14-membered ring macrolactone, a late stage side chain introduction via a Wittig olefination and a glycosylation to introduce the rhamnose should allow to complete the synthesis of lyngbouilloside and irrevocably confirm its structure.
Author Contributions
SA and JC conceived the project and designed the research. AK, JG, AE, and RL carried out the experimental work. CP and EB were in charge of the preparative HPLC separations. SA and JG wrote the manuscript. All authors commented on the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer ZX and handling Editor declared their shared affiliation, and the handling Editor states that the process nevertheless met the standards of a fair and objective review.
Acknowledgments
We would like to thank Generalitat de Catalunya for financial support to AE.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/article/10.3389/fchem.2016.00034
References
Boeckman, R. K., and Pruitt, J. R. (1989). A new, highly efficient, selective methodology for formation of medium-ring and macrocyclic lactones via intramolecular ketene trapping: an application to a convergent synthesis of (–)-kromycin. J. Am. Chem. Soc. 111, 8286–8288. doi: 10.1021/ja00203a044
Chang, C.-F., Stefan, E., and Taylor, R. E. (2015). Total synthesis and structural reassignment of lyngbyaloside C highlighted by intermolecular ketene esterification. Chem. Eur. J. 21, 10681–10686. doi: 10.1002/chem.201502132
Denmark, S. E., and Beutner, G. L. (2003). Lewis base activation of Lewis acids. Vinylogous aldol reactions. J. Am. Chem. Soc. 125, 7800–7801. doi: 10.1021/ja035448p
Denmark, S. E., Beutner, G. L., Wynn, T., and Eastgate, M. D. (2005b). Lewis base activation of Lewis acids: catalytic, enantioselective addition of silyl ketene acetals to aldehydes. J. Am. Chem. Soc. 127, 3774–3789. doi: 10.1021/ja047339w
Denmark, S. E., Heemstra, J. R., and Beutner, G. L. (2005a). Catalytic, enantioselective, vinylogous aldol reactions. Angew. Chem. Int. Ed. 44, 4682–4698. doi: 10.1002/anie.200462338
Denmark, S. E., Wynn, T., and Beutner, G. L. (2002). Lewis base activation of Lewis acids. Addition of silyl ketene acetals to aldehydes. J. Am. Chem. Soc. 124, 13405–13407. doi: 10.1021/ja0282947
De Rosa, M., Acocella, M. R., Villano, R., Soriente, A., and Scettri, A. (2003). A convenient catalytic procedure for the highly enantioselective aldol condensation of O-silyldienolates. Tetrahedron Asymmetry 14, 2499–2502. doi: 10.1016/S0957-4166(03)00494-4
ElMarrouni, A., Lebeuf, R., Gebauer, J., Heras, M., Arseniyadis, S., and Cossy, J. (2012). Total synthesis of nominal lyngbouilloside aglycon. Org. Lett. 14, 314–317. doi: 10.1021/ol203064r
Evans, D. A., Chapman, K. T., and Carreira, E. M. (1988). Directed reduction of β-hydroxy ketones employing tetramethylammonium triacetoxyborohydride. J. Am. Chem. Soc. 110, 3560–3578. doi: 10.1021/ja00219a035
Fuwa, H., Yamagata, N., Okuaki, Y., Ogata, Y., Saito, A., and Sasaki, M. (2016). Total synthesis and complete stereostructure of a marine macrolide glycoside, (–)-lyngbyaloside B. Chem. Eur. J. 22, 6815–6829. doi: 10.1002/chem.201600341
Gebauer, J., Arseniyadis, S., and Cossy, J. (2008). Facile synthesis of the C1-C13 fragment of lyngbouilloside. Synlett 5, 712–714. doi: 10.1055/s-2008-1042805
Hoffmann, L., and Demoulin, V. (1991). Marine cyanophyceae of Papua New Guinea. II. Lyngbya bouillonii sp. nov., a remarkable tropical reef-inhabiting blue-green alga. Belg. J. Bot. 124, 82–88.
Jacobsen, E. N., Markó, I., Mungall, W. S., Schroeder, G., and Sharpless, K. B. (1988). Asymmetric dihydroxylation via ligand-accelerated catalysis. J. Am. Chem. Soc. 110, 1968–1970. doi: 10.1021/ja00214a053
Kim, H., Ho, S., and Leighton, J. L. (2011). A more comprehensive and highly practical solution to enantioselective aldehyde crotylation. J. Am. Chem. Soc. 133, 6517–6520. doi: 10.1021/ja200712f
Klein, D., Braekman, J. C., Daloze, D., Hoffmann, L., Castillo, G., and Demoulin, V. (1999a). Lyngbyapeptin A, a modified tetrapeptide from Lyngbya bouillonii (Cyanophyceae). Tetrahedron Lett. 40, 695–696. doi: 10.1016/S0040-4039(98)02451-4
Klein, D., Braekman, J. C., Daloze, D., Hoffmann, L., Castillo, G., and Demoulin, V. (1999b). Madangolide and laingolide A, two novel macrolides from Lyngbya bouillonii (Cyanobacteria). J. Nat. Prod. 62, 934–936. doi: 10.1021/np9900324
Klein, D., Braekman, J. C., Daloze, D., Hoffmann, L., and Demoulin, V. (1996). Laingolide, a novel 15-membered macrolide from Lyngbya bouillonii (Cyanophyceae). Tetrahedron Lett. 37, 7519-7520. doi: 10.1016/0040-4039(96)01728-5
Klein, D., Braekman, J. C., Daloze, D., Hoffmann, L., and Demoulin, V. (1997). Lyngbyaloside, a novel 2,3,4-tri-O-methyl-6-deoxy-α-mannopyranoside macrolide from Lyngbya bouillonii (Cyanobacteria). J. Nat. Prod. 60, 1057–1059. doi: 10.1021/np9702751
Kolb, H. C., Van Nieuwenhze, M. S., and Sharpless, K. B. (1994). Catalytic asymmetric dihydroxylation. Chem. Rev. 94, 2483–2547. doi: 10.1021/cr00032a009
Luesch, H., Yoshida, W. Y., Harrigan, G. G., Doom, J. P., Moore, R. E., and Paul, V. J. (2002). Lyngbyaloside B, a new glycoside macrolide from a palauan marine cyanobacterium, Lyngbya sp. J. Nat. Prod. 65, 1945–1948. doi: 10.1021/np0202879
Matthew, S., Salvador, L., Schupp, P. J., Paul, V. J., and Luesch, H. (2010). Cytotoxic halogenated macrolides and modified peptides from the apratoxin-producing marine cyanobacterium Lyngbya bouillonii from Guam. J. Nat. Prod. 73, 1544–1552. doi: 10.1021/np1004032
Mitsunobu, O., and Yamada, Y. (1967). Preparation of esters of carboxylic and phosphoric acid via quaternary phosphonium salts. Bull. Chem. Soc. Jpn. 40, 2380–2382. doi: 10.1246/bcsj.40.2380
Sabitha, G., Rammohan Reddy, T., Yadava, J. S., and Sirisha, K. (2014). Stereoselective synthesis of the C1–C8 and C9–C16 fragments of revised structure of (–)-lyngbouilloside. RSC Adv. 4, 3149–3152. doi: 10.1039/C3RA44354J
Sato, M., Sunami, S., Sugita, Y., and Kaneko, C. (1995). An efficient asymmetric aldol reaction of 4-trimethylsiloxy-6-methylene-1,3-dioxines by chiral binaphthol-titanium complex catalysis. Heterocycles 41, 1435–1444. doi: 10.3987/COM-95-7065
Tan, L. T., Márquez, B. L., and Gerwick, W. H. (2002). Lyngbouilloside, a novel glycosidic macrolide from the marine cyanobacterium Lyngbya bouillonii. J. Nat. Prod. 65, 925–928. doi: 10.1021/np010526c
Keywords: lyngbouilloside, total synthesis, Lyngbya bouillonii, Boeckman esterification, Mukaiyama aldol, asymmetric Sharpless dihydroxylation, ring-closing metathesis
Citation: Kolleth A, Gebauer J, ElMarrouni A, Lebeuf R, Prévost C, Brohan E, Arseniyadis S and Cossy J (2016) Total Synthesis of Putative 11-epi-Lyngbouilloside Aglycon. Front. Chem. 4:34. doi: 10.3389/fchem.2016.00034
Received: 27 May 2016; Accepted: 15 July 2016;
Published: 09 August 2016.
Edited by:
Tao Ye, Peking University, ChinaCopyright © 2016 Kolleth, Gebauer, ElMarrouni, Lebeuf, Prévost, Brohan, Arseniyadis and Cossy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stellios Arseniyadis, s.arseniyadis@qmul.ac.uk
Janine Cossy, janine.cossy@espci.fr
†Present Address: Stellios Arseniyadis, School of Biological and Chemical Sciences, Queen Mary University of London, London, UK
 Amandine Kolleth1
Amandine Kolleth1  Abdelatif ElMarrouni
Abdelatif ElMarrouni Stellios Arseniyadis
Stellios Arseniyadis