Host interferon-γ inducible protein contributes to Brucella survival
- 1Department of Microbiology and Immunology, Louisiana State University Health Sciences Center – Shreveport, Shreveport, LA, USA
- 2Department of Veterinary Microbiology and Preventive Medicine, Iowa State University, Ames, IA, USA
Brucella spp. are highly adapted intracellular pathogens of mammals that cause chronic infections while surving and replicating in host monocytes and macrophages. Although monocytes are normally susceptible to infection, pretreatment with pro-inflammatory cytokine interferon-γ (IFN-γ) activates cellular defense mechanisms that increase intracellular killing of Brucella and prevents bacterial replication. We examined the contribution of the IFN-γ inducible GTPase, LRG-47, to B. abortus 2308 infection in in vitro and in vivo murine models. Infecting non-activated macrophages from LRG-47−/− mice revealed that loss of this host protein negatively effected the intracellular survival and replication of IgG opsonized B. abortus. In contrast, survival and replication of non-opsonized B. abortus was the same in both C57/B6 and LRG-47−/− peritoneal macrophages. Following IFN-γ activation of LRG-47−/− monocytes, IgG opsonized B. abortus survived better than non-opsonized bacteria. The differential fate of opsonized and non-opsonized B. abortus was only observed in macrophages collected from LRG-47−/− mice. Given the specific nature of the relationship between this host protein and the mechanism of Brucella internalization, LRG-47−/− mice were infected with B. abortus to assess whether the loss of the lrg47 protein would affect the ability of the bacteria to colonize or persist within the host. B. abortus were able to establish and maintain similar numbers of bacteria in both C57/B6 mice and LRG-47−/− through 3 weeks post intraperitoneal infection. By 9 weeks p.i. fewer B. abortus were recovered from LRG-47−/− mice than controls, suggesting that the host protein has a positive role in maintaining long term persistence of the bacteria within the host. These observations demonstrating a positive role for a host IFN-γ induced protein defense protein has yet to be reported. These results provide interesting insight into the complex interaction between Brucella and their host.
Introduction
Brucella spp. are highly infectious intracellular pathogens that establish chronic infections by surviving and replicating within host monocytes and macrophages. Production of pro-inflammatory cytokines by the host stimulates phagocytosis and killing of pathogenic microbes. In vivo and in vitro experiments demonstrate that the most potent inducer of anti-Brucella activity in monocytes and macrophages is type II interferon (IFN-γ) (Jiang and Baldwin, 1993a). IFN-γ stimulation of monocytes and macrophages increases production of reactive oxygen and nitrogen species. In the case of Brucella abortus, reactive oxygen intermediate (ROI) production was found to play a larger role in intracellular resistance to the bacteria than nitric oxide (NO) (Jiang et al., 1993), while Brucella suis infected monocytes reduced bacterial load primarily through inducible nitric oxide synthase (iNOS) activity (Gross et al., 1998). Interestingly, pretreatment of monocytes with the cytokine IFN-γ yielded the strongest anti-Brucella activity. This activity was characterized as a bacteriostatic mechanism that prevented intracellular replication of the Brucella spp. rather than simply increasing killing (Jiang and Baldwin, 1993b).
Human T-cells respond to Brucella spp. by stimulating interleukin (IL)-2 production, whereby increasing IFN-γ release (Blay et al., 1992). It was shown by Stevens et al. that intraperitoneal injection of recombinant murine IFN-γ into mice significantly lowered the number of B. abortus isolated from the spleen at day 7 (IFN-γ was delivered 1 day prior and 2 and 4 days post infection). This increase in resistance to B. abortus was amplified by co-administration with the cyclooxygenase inhibitor, indomethacin, which blocks prostaglandin E2 (PGE2), whereby increasing IL-12 and IFN-γ production (Stevens et al., 1992). Murine macrophages infected with B. abortus secrete IFN-γ, and inactive forms of IL-12, while B. abortus lipopolysccharide (LPS) treated monocytes produce IFN-γ and active IL-12 (Fernandez-Lago et al., 1999). Hoover et al. demonstrated that IFN-γ treatment did not stimulate Brucella killing, although intracellular replication of opsonized and non-opsonized B. abortus was significantly reduced in cultures treated with the cytokine (Eze et al., 2000). As confirmed by the literature, the restriction in intracellular replication of Brucella involves more than the NO and ROI generating mechanisms of the host cell.
Recently, a family of Immunity-Related p47 GTPases (IRG) has been identified whose activities are strongly induced from undetectably low resting levels (Martens et al., 2004), by the cytokine IFN-γ (Henry et al., 2009). This 47–48 kDa family of proteins, which was first identified in the mouse, is divided into two groups based on sequence homology of their G1 motif (Martens et al., 2004; Chen and He, 2009). Group I p47s (GMS) consists of interferon gamma-induced GTPase (IGTP) (Irgm3), GTPI (Irgm2) and LRG-47 (Irgm1). Group II (GKS) includes IRG-47 (Irgd), TGTP/Mg21 (Irgb6) and IIGP (Irga6) (Chen et al., 2010). These two groups of proteins do not act independently, but interdependently to control intracellular and extracellular pathogen invasion (Chen and He, 2009). The GMS proteins function as regulators of the GKS proteins' GTPase cycle acting as attenuators by preventing premature activation of the GKS proteins (Hunn et al., 2008; Hunn and Howard, 2010).
These IRG proteins have pathogen specific behavior; each protein is involved in controlling specific pathogens (Martens et al., 2004; Butcher et al., 2005; Chen and He, 2009). The recent discovery and characterization of these IFN-γ inducible proteins reveals that the intracellular environment within monocytes is substantially altered in response to IFN-γ to defend against intracellular pathogens. Recent data indicated that, specifically, LRG-47 is essential in macrophage resistance to a variety of intracellular pathogens such as Toxoplasma cruzi (Santiago et al., 2005), Toxoplasma gondii (Collazo et al., 2001; Butcher et al., 2005), Listeria monocytogenes (Collazo et al., 2001), Mycobacterium avium (Taylor et al., 2004), Mycobacterium tuberculosis (MacMicking et al., 2003) and Chlamydia trachomatis (Coers et al., 2008). LRG-47 is normally associated with the cis- and medial-golgi network inside a macrophage (Martens et al., 2004; Butcher et al., 2005; Zhao et al., 2010a). Following phagocytosis of a particle, LRG-47 is recruited to the maturing phagosome, where it will stay for the full life cycle of the phagosomal compartment (Martens et al., 2004). Since this IRG protein remains associated with the phagosomal membrane upon association with a lysosome, LRG-47 is found to colocalize with the lysosome-associated membrane protein LAMP1 (Martens et al., 2004; Zhao et al., 2010b), and the acidic vesicle stain LysoTracker® inside macrophages (Zhao et al., 2010b). Zhao et al. speculates that the Golgi-localized LRG-47 is the GDP-bound form (inactive protein), whereas, the lysosome-associated LRG-47 is the GTP bound form (active protein). It is noted by Hunn et al. and MacMicking et al. that LRG-47 binds Mycobacterium containing vacuoles where it accelerates fusion with the lysosome, whereby controlling pathogen replication and survival. Pathogen load in the host cell can also be controlled by enhancement of the formation of autophagosomes (Gutierrez et al., 2004; Hunn and Howard, 2010), and by promotion of phagosome acidification by LRG-47. LRG-47 deficient mice infected with M. tuberculosis have an increased bacterial burden and tissue damage within their lung tissue as compared to wild-type mice. The susceptibility was traced to a deficiency in phagosome maturation; stimulated macrophages from LRG-47 knockout mice failed to acidify Mycobacterium containing phagosomes (MacMicking et al., 2003). It has been hypothesized that LRG-47 regulates an alternative pathway for delivery of proteins from the trans-Golgi network (TGN) This pathway may aid the host cell in overcoming attempts by pathogens to inhibit phagosome-lysosome fusion (Martens et al., 2004). To our knowledge, there have been no studies relating the changes in Brucella trafficking to the decrease in intracellular viability induced by IFN-γ stimulation.
Prior work in our laboratory has revealed that the type of intracellular replicative niche that Brucella occupies depends on how the bacteria were internalized. In the absence of opsonin, Brucella are internalized into vesicles through a process that is dependent on lipid raft aggregation at the plasma membrane prior to uptake. In contrast, opsonization of Brucella with specific immunonoglobulin directs internalization through an Fc receptor-dependent process. Opsonized bacteria were found to replicate within modified endosomes that were non-acidic, and indicated a lack of fusion with a lysosome. Since the activity of individual IFN-γ induced proteins is associated with their intracellular localization within the cell, both opsonized and non-opsonized bacteria were examined in the following experiments to determine whether different intracellular niches were uniquely sensitive to specific IFN-γ induced anti-Brucella activities.
Materials and Methods
Bacterial Culture
All chemicals were obtained from Sigma-Aldrich unless otherwise stated. All research with live B. abortus were conducted in University, state and Federally approved BSL3 facilities at either Louisiana State University Health Sciences Center-Shreveport or Iowa State University College of Veterinary Medicine. Virulent B. abortus laboratory strain 2308 cultures were grown on Trypticase Soy agar (Difco) supplemented with 5% bovine blood (BA) (Gemini Bioproducts) under 5% CO2 at 37°C. A GFP-expressing derivative of B. abortus 2308 was constructed by introducing the plasmid pBBR1MCS6-Y encoding GFP expression downstream of the constitutively active promoter for aph3A-derived from pBlueKS+Kan (Stratagene) (Murphy et al., 2002). GFP positive Brucella were maintained in culture using a concentration of 6 μg/ml chloramphenicol. Heat killed B. abortus cells were prepared by incubating cell suspensions at 70°C for 30 min. Loss of viability was confirmed by plating portions of the heated cell suspension on BA and subsequent incubation at 37°C for 4 days.
Culture of Murine Peritoneal, Bone Marrow Derived Monocytes and RAW264.7 Monocytic Cells
Mice were euthanized by halothane overdose prior to removal of the long bones of the legs. Marrow was flushed out using a pre-filled 5 cc syringe with 5 ml DMEM and a 26-G needle. Cells were pelleted from marrow wash solution by centrifugation and resuspended in DMEM + 10% FBS supplemented with 1000 units/ml M-CSF and incubated for 24 h at 37°C with 5% CO2. At which time, nonadherent cells were transferred to a 75 cm2 tissue culture flask with 10 ml DMEM and 1000 units/ml M-CSF. After 7 days of culture cells were plated for experimental use. Isolation of non-elicited peritoneal macrophages were harvested by first introducing 5 ml of replete growth media into the peritoneal cavity of euthanized mice as previously described (Robertson and Roop, 1999). Low passage RAW 264.7 (ATCC #TIB-71) were routinely cultured in RPMI 1640 medium with 2 mM glutamine supplemented with 1.5 g/ml sodium bicarbonate (Mediatech) and 10% fetal bovine serum (Gemini Bioproducts). Cells were seeded into both 24 and 96 well plates at a density of 1 × 105 cells/ml, 24 h prior to infection. Cell culture viability was monitored in suspension by hemocytometer-trypan blue dye exclusion. Transient transfection of RAW264.7 cells employed the Nucleofector device using the manufacturers recommended protocols and solution reagents (Amaxa-Lonza Biologics).
Assessing Intracellular Survival and Replication of B. abortus
Bacterial suspensions were generated by scraping 48 h cultures of the B. abortus strains grown on BA into screw cap microfuge tubes containing PBS. Pellets of bacteria were resuspended by vigorous vortexing and numbers of bacteria present in the suspensions were determined by OD600 measurements. Bacterial suspensions were diluted to desired concentrations in complete RPMI 1640 medium and split into two aliquots where one received nonagglutinating concentrations of 1/2500–1/5000 of anti-Brucella IgG antibody (Difco) to opsonize bacteria (opsonized), and the other received the same volume of PBS to serve as a mock control (non-opsonized). Bacterial cell suspensions and antisera were incubated at room temperature for 30 min followed by brief vortexing. Bacteria were added to monocytes monolayers generally at a multiplicity of infection (bacteria to monocyte ratio) of 20:1 for opsonized and 100:1 for non-opsonized Brucella. Due to anticipated differences for internalization kinetics of opsonized and non-opsonized bacteria, synchronized internalization was achieved by cooling infected cultures to 4°C followed by 10 min centrifugation at 270 × g. Monolayers were washed gently with cold PBS to remove non-adherent bacteria. Phagocytosis of adherent bacteria was initiated by the addition of fresh pre-warmed media after which, cells were incubated for 20 min at 37°C/5% CO2. After incubation, cultures were washed 3 times with cold PBS and fresh media containing 100 μg/ml gentamicin was added to cells for 1 h to kill adherent but not internalized bacteria. After 1 h antibiotic treatment, media was removed and replaced with fresh media containing 10 μg/ml gentamicin. Cells remained in this medium for the duration of the experiment. Viability of intracellular Brucella was determined by lysing monocytes with 0.1% deoxycholate, diluting suspensions in PBS, and plating aliquots in triplicate on BA medium (Robertson and Roop, 1999). Percent survival of bacteria at 24 and 48 h were calculated based on the number of internalized bacteria detected at 1 h post infection which represents 100% of internalized bacteria. Statistical comparisons were made using Student's t-test.
Fluorescence Microscopy
Infected monolayers were prepared for immunofluorescence microscopy at indicated times post infection using methods described previously (Bellaire et al., 2005). Goat anti-LRG-47 primary antibody (clone P-20) was purchased from SantaCruz Biotechnology (Santa Cruz, CA). Coverslips were washed three times with cold BSP and affixed to glass slide with Pro-long with DAPI mounting solution (Molecular Probes/Invitrogen). Secondary antibodies conjugated with fluorochromes indicated in text were purchased from Jackson Immunoresearch (WestGrove, PA). Immunofluorescence microscopy was performed using an Olympus IX-71 epifuorescence microscope with appropriate DAPI/GFP/TRITC filter sets and image processing was performed with ImageJ v1.33g (http://rsb.info.nih.gov/ij/index.html).
Animal Experiments
Animal facilities and protocols were ALAAC approved and all B. abortus experiments were performed using appropriate ABSL3 protocols and procedures. Five- to seven-week-old female BALB/c mice were infected with 1 × 105B. abortus 2308 through the intraperitoneal route using previously reported protocols. At the indicated times post infection, mice were humanely euthanized to harvest spleens and livers. Organs were homogenized in PBS and tissue lysates were serially diluted and then plated onto TSA blood agar to enumerate bacteria (Bellaire et al., 1999). A mouse colony was established and maintained in AALAC accredited facilities for LRG-47−/− mice using breeding pairs generously gifted from Gregory Taylor (Collazo et al., 2001). Averages and standard deviations were calculated from CFU data collected from a minimum of 5 mice for each group at each time point. Statistical significance was determined using a Student's two-way t-test.
Results
Expression and Localization of LRG-47 to Brucella-Containing Vesicles
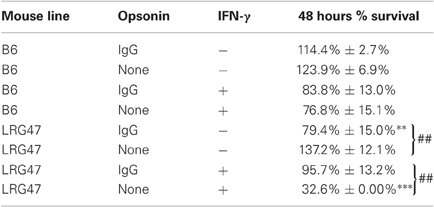
To determine if LRG-47 can be found on Brucella-containing phagosomes in IFN-γ activated monocytes, RAW264.7 cells were pre-treated with IFN-γ, infected with opsonized GFP-expressing-B. abortus, and fixed at 18 h post infection. Immunostaining for LRG-47 revealed that some but not all Brucella colocalized with LRG-47 (Figure 1). Similar results were also obtained with IFN-γ activated peritoneal macrophages (data not shown).
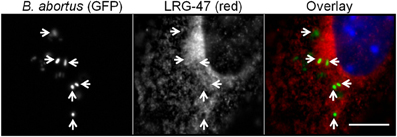
Figure 1. B. abortus containing phagosomes adjacent to membranes containing the IFN-γ inducible protein LRG-47. Monolayers of RAW264.7 cells were stimulated with IFN-γ, infected with opsonized-B. abortus 2308 fixed 18 h p.i. and stained to visualize the spatial relationship of the LRG-47 with the host cell (red). Brucella were observed in phagosomes adjacent to membranes positive for LRG-47 (arrow heads). Panel of images shown are representative of results from three separate experiments (bar = 5 μm).
Constitutive Expression of LRG-47 does not Alter the Course of in vitro Brucella Infection
Since LRG-47 is induced by IFN-γ and can be localized to Brucella-containing phagosomes in these cells, we postulated that LRG-47 had a role in limiting the intracellular growth of the bacteria. This notion was examined by generating stable RAW264.7 cells that constitutively overexpressed a FLAG-tagged LRG-47 fusion protein. Constitutive overexpression was monitored by immunofluorescence microscopy using antibodies directed either toward LRG-47 or the FLAG tag (DNS). The distribution pattern of the LRG-47-Flag protein in non-IFN-γ activated cells was indistinguishable from that of endogenous LRG-47 in non-transformed IFN-γ treated RAW264.7 cells. Overexpressing cells were infected with opsonized Brucella in the absence of exogenous IFN-γ treatment and the intracellular viability of the bacteria was followed over 48 h post infection. CFU's recovered at 0.5, 1, and 24 post infection were equivalent among infected cells either pretreated with IFN-γ or constitutively expressing LRG-47. While the IFN-γ treated monocytes further reduced the number of viable Brucella at 48 h, bacterial replication occurred in the RAW264.7 LRG-47 expressers that was equivalent to the non-IFN-γ treated cultures (Figure 2). The results show that overexpressing of LRG-47 alone does not evoke IFN-γ induced anti-Brucella activity. Although long term intracellular survival was not reduced by LRG-47 overexpression, the level of Brucella in these cells were more similar to IFN-γ treated cells than non-IFN-γ treated controls up to 24 h post infection. These observations suggest a possible role for LRG-47 in opsonized Brucella internalization and early survival; however, LRG-47 by itself does not limit bacterial replication.
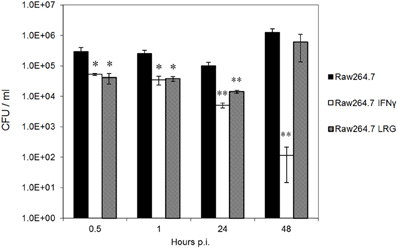
Figure 2. Constitutive expression of LRG-47 aids in bacteria replication and survival. RAW264.7 murine macrophages stabley transfected with Flag-tagged LRG-47 (Raw264.7 LRG) were infected with virulent Brucella abortus. Colony forming units/ml were enumerated at 0.5, 1, 24, and 48 h post infection and compared with the CFU/ml values for non-activated RAW264.7 cells (Raw264.7) and IFN-γ activated RAW264.7 cells (Raw264.7 IFN) both of which are not overexpressing the LRG-47 protein. (Student's T-test p values * <0.05; ** <0.01).
LRG-47 has a Positive Role in Brucella's Residence in Murine Monocytes
Since the overexpression of LRG-47 alone did not affect the viability of Brucella within monocytes, we wanted to examine if the loss of this host protein would upset the ability of IFN-γ to induce an anti-Brucella activity by monocytes. It was anticipated that macrophages from LRG-47−/− mice would be defective to some degree in their IFN-γ induced anti-Brucella activity while non-stimulated macrophages would behave similarly to non-activated cells from normal mice. Intracellular viability of Ig-opsonized Brucella in peritoneal macrophages isolated from C57/B6 and LRG47−/− were compared in the presence or absence of IFN-γ stimulation (Figure 3). Interestingly, significantly fewer viable Brucella were recovered from LRG47−/− cells compared to similarly treated C57/B6 cultures, including non-IFN-γ treated cultures. At 48 h, the number of bacteria recovered from IFN-γ treated LRG47−/− cultures increased from 24 h values, suggesting that some degree of intracellular bacterial replication does occur. By contrast, both Brucella survival (CFU at 24 h) and replication (CFU at 48 h) were significantly reduced in non-stimulated LRG-47−/− cells, suggesting that the IFN-γ inducible protein contributed to intracellular bacterial survival. Repeat experiments with non-stimulated peritoneal macrophages showed similar reductions in replication of opsonized Brucella within monocytes from LRG-47−/− mice (Figure A3).
Figure 3. Intracellular viability of Brucella in peritoneal macrophages harvested from C57/B6 and LRG47 knockout mice. IFN-γ was added 24 h prior to infection with immunoglobulin opsonized B. abortus 2308. Values and standard deviations were calculated from triplicate samples (**p ≤ 0.01 Student's t-Test compared to C57/B6 Ops). Results shown are representative of three independent experiments demonstrating similar significant differences.
Survival and Replication of Brucella within RAW264.7 Cells and IFNγ Activated Cells
Bacteria opsonized by serum components are internalized faster with greater efficiency then non-opsonized bacteria. Cell surface receptors that mediate internalization of opsonized particles influence the intracellular trafficking of the new phagosomes through their cytoplasmic domains (Gyles, 2010). Previous studies determined that immunoglobulin opsonized B. abortus localized to a replicative compartment that lacked ER components (Bellaire et al., 2005). In contrast, non-opsonized B. abortus did localize to an ER positive compartment as others have reported (Pizarro-Cerda et al., 1998). Thus, intracellular fate of Brucella depends on the context by which the bacteria are presented to monocytes. To determine if the mode of Brucella uptake altered the subsequent fate of these bacteria, we performed an intracellular viability experiment with RAW264.7 cells infected with either IgG opsonized or non-opsonized virulent B. abortus 2308 employing increasing MOIs. Numbers of intracellular Brucella recovered at 24 and 48 h were largely in proportion to the number of bacteria internalized at the beginning of the experiment (Figure A1). Comparing the groups receiving opsonized bacteria, an identical number of internalized bacteria were seen with MOI's of 20:1 for opsonized and 100:1 for non-opsonized. Interestingly, by calculating the survival of Brucella at 48 h as a percentage of the number of bacteria initially internalized revealed that the replication of 20:1 opsonized (1236.4% ± 15.6%) was double that of 100:1 non-opsonized (600.0% ± 1.3%). Considering these results, future experiments maintained MOI's of 20:1 for opsonized and 100:1 for non-opsonized to maintain consistency in the number of internalized bacteria.
To determine if IFN-γ treatment was equally effective for opsonized and non-opsonized bacteria, intracellular viability experiments were performed with RAW264.7 cells stimulated 24 h prior to infection with 50 U/ml of recombinant IFN-γ (rIFN-γ). Similar degrees of IFN-γ anti-Brucella activity were observed at 24 h and 48 h post infection and did not differ among opsonized or non-opsonized bacteria (Figure A2). Treatment of monocytes with IFN-γ resulted in, roughly, a 2 log reduction in both opsonized and non-opsonized bacteria by 24 h, and reduced the number of Brucella recovered at 48 h down to the limit of detection. Calculating percent survival did not uncover any additional significant findings. It is of note that pretreatment of monocytes with IFN-γ did increase the number of non-opsonized bacteria internalized 2.5-fold over any other group examined, including the 20:1 opsonized bacteria in IFN-γ treated cells.
Effect of Opsonization on Brucella Survival
An earlier report by our laboratory showed that intracellular localization of Brucella depended on how the bacteria were initially internalized by the host cell. Those results revealed that non-opsonized bacteria replicated in human cells in an intracellular compartment resembling the endoplasmic reticulum. Others have demonstrated Brucella trafficking through autophagosomes before reaching their replicative compartment (Starr et al., 2008). By contrast, Ig opsonized bacteria replicated in modified late-endosomes and do not traffic through an autophagosomal compartment. Since opsonized and non-opsonized bacteria differ in their interactions with autophagosomes, we wanted to explore whether the survival of Brucella varied in LRG-47−/− cells in response to varying the method of bacterial internalization. Comparisons between non-stimulated (resting) and IFN-γ stimulated (activated) cells isolated from either C57/B6 or LRG-47−/− mice were carried out using optimal MOI's of 20:1 for Ig-opsonized and 100:1 non-opsonized (yielding equivalent numbers of internalized bacteria within RAW264.7 cells). Infected peritoneal macrophages from wild-type C57/B6 mice treated with IFN-γ exhibited significant and equal anti-Brucella activity against both opsonized and non-opsonized Brucella. In contrast, Brucella viability in LRG-47−/− cells was dependent on the whether the bacteria were opsonized, and on the presence of IFN-γ activation (Table 1). Resting LRG-47 deficient macrophages harbored significantly more non-opsonized Brucella at 48 h (137.2% ± 12.1%) than Ig-opsonized (79.4% ± 15%). In addition, Ig-opsonized Brucella survival in LRG-47 KO was significantly lower than was seen in resting C57/B6 isolated macrophages (114% ± 2.7%). An inverse relationship was seen between Brucella survival and the affects of opsonization in IFN-γ stimulated-LRG-47 KO macrophages (Figure A3). Percent survival of non-opsonized Brucella dropped dramatically in IFN-γ activated LRG47−/− cells, to 32.6%, while Ig-opsonized Brucella survived as well as, or better than, the opsonized bacteria in the resting LRG-47 KO or C57/B6 macrophages. Post hoc analysis revealed that averaging the opsonized and non-opsonized LRG-47 KO results together based on IFN-γ activated and non-activated status yielded values similar to those recorded from the corresponding IFN-γ treated C57/B6 macrophage cultures.
Brucella Survival in LRG-47−/− Unaffected by Autophagy Inhibitor
Reduced survival of M. tuberculosis in IFN-γ activated macrophages has been attributed to the ability of LRG-47 to redirect resident intracellular bacteria toward lysosomes by repackaging intracellular pathogens within autophagosomes (Gutierrez et al., 2004; Hunn and Howard, 2010). The autophagosomal pathway could provide a safehaven for the intracellular bacteria enabling them to survive and replicate while evading the host immune response, which seems counterintuitive for the host. It is plausible that IFN-γ induction of autophagosome biogenesis could either drive the elimination of Brucella, or accelerate its survival and intracellular replication. To determine which of these mechanisms has the dominant role in Brucella's intracellular viability, we treated infected peritoneal C57/B6 and LRG47−/− macrophages with a 50 μM of 3-methyladenine (3-MA), an inhibitor of autophagosome formation. Treatment of C57/B6 cells with the inhibitor reduced Brucella CFUs (4.6 ± 0.09 log10) compared to non-treated cultures (5.8 ± 0.16 log10). CFUs from 3-MA treated cultures were equivalent to the number of bacteria recovered from C57/B6 IFN-γ activated cultures (4.3 ± 0.56 log10) (Figure 4). Addition of 3-MA acted synergistically with IFN-γ stimulation to further decrease bacterial viability in C57/B6 macrophage cultures (3.2 ± 0.06 log10). For LRG-47 KO macrophages, 3-MA treatment alone did not alter intracellular bacterial viability in non-IFN-γ stimulated cells. In contrast, stimualtion of these monocytes with IFN-γ a dramatic decrease in the ability of intracellular B. abortus to survive within these cells (0.6 ± 0.8 log10). The relative health of infected macrophages was monitored for gross morphological changes by phase microscopy, and enzymatically by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction assays. Incubating cells with 3-MA and IFN-γ at the levels tested was found to have no detrimental effect on macrophage health.
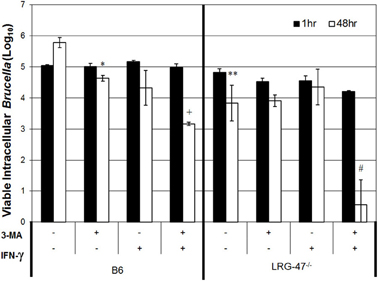
Figure 4. Brucella survival in LRG47−/− macrophages is unaffected by the autophagy inhibitor 3-methyladenine (3-MA). Peritoneal macrophages were collected from C57/B6 and LRG-47−/− mice and infected with Ig-opsonized virulent B. abortus. Cells were stimulated with IFN-γ and treated with 50 μM 3-MA. Colony forming units were enumerated at 1 h and 48 h post infection. Loss of LRG-47 in non-activated cells had a similar effect as treatment with 3-MA or IFN-γ in C57/B6 cells on Brucella survival after 48 h. The inhibition of autophagy plus IFN-γ activation greatly decreased the number of viable Brucella after 48 h in LRG-47−/− cells. Statistical significance of Student's T-test comparing C57/B6 non-stimulated 48 h controls CFUs to indicated groups; p values * < 0.05; * < 0.01, comparison to C57/B6 IFN-γ only p +< 0.01. Statistical comparison to non-stimulated LRG-47−/− control p value #< 0.001.
LRG-47 Contributes to Stability of Chronic Brucellosis in Mice
To determine the overall contribution of LRG-47 to host resistance during chronic brucellosis, the number of B. abortus residing within the spleens of experimentally infected C57/B6 and LRG-47−/− mice were followed for 9 weeks post infection (Silva et al., 2011). The LRG-47 mutation was introduced into the L129 background (Santiago et al., 2005). Both C57/B6 and L129 F-2 hybrids were used as controls for Brucella infection. Time points at 2 and 6 weeks post infection showed no significant difference between any of the mouse lines, including the controls (data not shown). We determined the chronic brucellosis models in C57/B6 and L129 mice were equivalent and subsequent mouse experiments were performed in C57/B6 mice. The splenic colonization study was repeated with C57/B6 and LRG-47 KO mice and no differences in colonization of B. abortus were seen between the two models by 6 weeks post infection. Continuing this second study out to 9 weeks post infection revealed a significant decrease in the amount of bacteria recovered from the spleens of LRG-47−/− mice (Figure 5). A semi-quantitative assessment for the presence of anti-Brucella antibodies revealed that sera from LRG-47−/− mice was less able to agglutinate heat killed bacteria, suggesting a possible deficiency in the production of anti-Brucella antibodies.
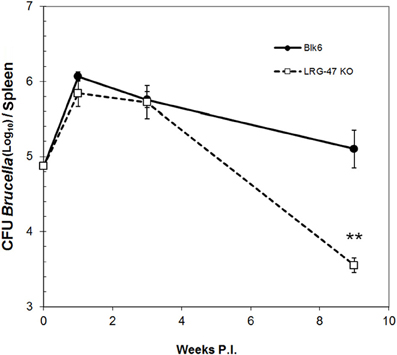
Figure 5. Murine model of chronic brucellosis. Transgenic LRG-47−/− mice and C57/B6 mice were infected intraperitoneally with 1 × 105 CFU of B. abortus 2308. Values represent the average and standard deviation of CFU's enumerated from a minimum of 5 mice per group (**p ≤ 0.01 Student's t-Test). Separate experiments that included 129/B6 F2 mice yielded similar results of no defect spleen colonization before 4 weeks post infection for any group tested, including LRG-47 KO mice.
Discussion
Pro-inflammatory cytokines elicit antimicrobial responses in monocytes and macrophages that serve as a critical defense against intracellular pathogens. Recently, attention has been focused on the ability of IFN-γ to stimulate monocytes to alter normal intracellular vesicle and organelle trafficking to regain control over intracellular pathogens. Many p47 GTPases induced by IFN-γ localize to specific intracellular compartments or structures, and most have been shown to play a role in limiting intracellular parasitism by bacterial or protozoan pathogens. Discovery of the different defense systems of individual intracellular niches led to the idea that each of these proteins may patrol a different intracellular region within the cell thus providing defense against a variety of intracellular pathogens (Collazo et al., 2001). For Brucella, production of ROI and NO was found to play a relatively minor role in the potent antimicrobial response induced by IFN-γ stimulation in macrophages. The most significant effect of IFN-γ activation is the dramatic inhibition of Brucella replication that is found to occur between 24 and 48 h in non-activated monocytes. Given that IFN-γ production by the host is critically important to restriction of Brucella replication in the host, we examined the ability of one of the IFN-γ inducible proteins, LRG-47, to inhibit Brucella's ability to replicate intracellularly.
Peritoneal macrophages collected from LRG-47−/− mice were infected with opsonized Brucella with and without IFN-γ stimulation. We observed a significant reduction in the number of bacteria present at 24 and 48 h post infection in the LRG-47−/− cells without stimulation. This suggests a requirement for LRG-47 for Brucella survival in the early stages after entry into the host cell. Within RAW264.7 cells expressing the Flag-tagged LRG-47 protein, the overexpressed LRG-47 colocalized with Brucella phagosomes in an identical pattern to native LRG-47 using IFN-γ activated primary and RAW264.7 macrophages. Intracellular replication of bacteria in these cells was only inhibited when monocytes were stimulated with IFN-γ. Overexpression experiments demonstrate that LRG-47 alone is not sufficient to fully support intracellular replication of these bacteria. It is likely that additional cellular factors stimulated by IFN-γ inhibit Brucella replication; however, these data show that LRG-47 is, in fact, required by Brucella for replication and/or survival inside a host macrophage.
Intracellular viability of Brucella requires the biogenesis of autophagosomes for replication and/or survival (Starr et al., 2008). A plausible explanation is that Brucella must orchestrate autophagosome biogenesis to maintain an intracellular niche. Recently, Starr et al. described that the activity of autophagy-initiation proteins (ULK1, Beclin 1, ATG14L and PI3-kinase), but not autophagy-elongating proteins (ATG5, ATG4B, ATG16L1 and LC3B) are required for the autophagic B. abortus-containing vacuole (aBCV) formation in non-stimulated monocytes (Starr et al., 2012). Treating infected cells with the PI3-kinase inhibitor 3-MA, these authors observed a decrease in aBCV formation. Changes in aBCV formation reported by these authors coincide with the dramatic decrease in intracellular viability that we observed when treating either WT or LRG-47 KO IFN-γ activated monocytes with 3-MA (Figure 4). Given the importance of aBCV formation on the intracellular pathogenesis of Brucella, bacterial control over autophagosome formation becomes even more critical when antimicrobial activity in macrophages is increased by IFN-γ activation (Starr et al., 2012). Loss of LRG-47 was similar in effectiveness to 3-MA in inhibiting Brucella replication in non-stimulated macrophages, it is likely that process of LRG-47 and 3-MA interference in Brucella pathogenesis involve similar disruptions in critical autophagy dependent mechanisms. Moreover, blocking autophagosome formation in LRG-47−/− cells revealed that this host protein likely supports Brucella viability when host cells are activated with IFN-γ. This support of bacterial replication is likely in the form of stimulating autophagosome formation (Starr et al., 2012). It should be noted that Brucella did not survive as well in LRG-47 KO cells grown under non-stimulating conditions suggesting that Brucella possibly requires not only LRG-47, but also a low amount of IFN-γ present in the host cell to effectively replicate and survive.
Brucella are trafficked into different replicative compartments based on whether they were internalized by opsonized or non-opsonized means. Opsonized Brucella replicate in a modified late endosome, whereas, non-opsonized bacteria replicate in a vesicle sharing markers with the endoplasmic reticulum (Bellaire et al., 2005). This difference in bacteria trafficking of opsonized vs. non-opsonized Brucella translates to a difference in a LRG-47 requirement for survival and replication in stimulated and unstimulated RAW264.7 macrophages. We observed a significant decrease in the percent survival of opsonized Brucella in non-stimulated LRG-47 KO cells after 48 h of infection, suggesting a requirement for LRG-47 in the survival and replication of opsonized Brucella in unstimulated macrophages. LRG-47 would be required to provide Brucella with access to a replicative late endosome-like vesicle. A low percentage of non-opsonized Brucella was found to survive after 48 h of infection in a stimulated LRG47 KO cell. These data suggest that LRG-47 plays a protective role for non-opsonized Brucella in an IFN-γ stimulated cell. LRG-47 may “rescue” the non-opsonized Brucella and provide it with a niche protecting it from anti-bacterial actions of the host cell that occur during IFN activation.
It is clear by in vitro experiments that LRG-47 supports replication of Ig-opsonized Brucella in non-activated cells and the survival of non-opsonized Brucella in IFN-γ activated cells. Given that ample anti-Brucella antibody is maintained throughout infection, we would anticipate any change in Brucella tissue survival to appear at times post infection when IFN-γ levels decrease at 4–6 weeks (Baldwin and Goenka, 2006). For our in vivo experiments using LRG-47−/− mice, changes in the ability of the bacteria to persist were not seen until week 9 post infection when IFN-γ levels would be predicted to decrease. This remains speculative as we did not attempt to measure IFN-γ levels within the tissues of these mice during infection.
Survival of Brucella within monocytes is the single most important aspect of pathogenesis contributing to persistence of the bacteria in host tissues. The previous in vitro data suggests that the role LRG-47 plays during Brucella infection is conditional based on IFN-γ stimulation and the opsonization status of the bacteria prior to internalization. High IFN-γ levels during the chronic phase are attributed to limiting the numbers of Brucella within the spleens of infected mice. Anti-Brucella immunoglobulin undergoes rapid class switching to high affinity IgG2a in an IFN-γ dependent manner, coincidently, aiding in the establishment of the chronic plateau phase of Brucella occurring at 2 weeks post infection. Internalization would likely transition from mostly non-opsonized within the first few days toward immunoglobulin-opsonization that would last throughout the chronic phase. It is difficult to predict which set of conditions would predominate within the host at any particular time during infection. Infection of LRG-47 KO mice with B. abortus did not differ in the magnitude or timing of splenic colonization compared to C57/B6 mice. Considering that IFN-γ levels and antibody production increase over this time span, the stable number of Brucella detected in vivo during the chronic phase is likely the product of mixed populations of variably opsonized bacteria together with fluctuating IFN-γ activation of host monocytes. In fact, recalculating percent survival of Brucella in LRG-47 cells (Table 1) by averaging the opsonized and non-opsonized values together for IFN-γ treated and non-treated equal the percent survival of Brucella recovered from similarly treated C57/B6 macrophages.
Past research regarding LRG-47's effect on pathogenic infection has determined a requirement for LRG-47 to reduce the replicative capacity of infectious agents such as Toxoplasma gondii, Mycobacterium tuberculosis (MacMicking et al., 2003) and Listeria monocytogenes (Collazo et al., 2001). Brucella abortus is unique from other intracellular pathogens in that LRG-47 does not inhibit its survival and replication, but aids it. LRG-47 and other members of the p47 GTPase family are pathogen specific with the ability to decipher between two pathogens that share >99% of their open reading frames as in C. trachomatis and C. muridarum with C. trachomatis infection being kept at bay by LRG-47, but not C. muridarum (Coers et al., 2008). In the future it will be important to perform similar experiments using B. melitensis to determine the role of LRG-47 in this related subtype's ability to survive and replicate inside the host. Similarly, examination of changes in trafficking, survival, and replication experiments among Ig-opsonized and non-opsonized B. melitensis in IFN-γ activated and non-activated cells will be important to understand the mechanism host defense proteins have in controlling the fate of Brucella.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to acknowledge the funding sources of the Healthy Livestock Initiative and USDA Formula Fund program and startup funds to Bryan H. Bellaire by the ISU Office of Research and College of Veterinary Medicine.
References
Baldwin, C. L., and Goenka, R. (2006). Host immune responses to the intracellular bacteria Brucella: does the bacteria instruct the host to facilitate chronic infection? Crit. Rev. Immunol. 26, 407–442.
Bellaire, B. H., Elzer, P. H., Baldwin, C. L., and Roop, R. M. 2nd. (1999). The siderophore 2,3-dihydroxybenzoic acid is not required for virulence of Brucella abortus in BALB/c mice. Infect. Immun. 67, 2615–2618.
Bellaire, B. H., Roop, R. M. 2nd, and Cardelli, J. A. (2005). Opsonized virulent Brucella abortus replicates within nonacidic, endoplasmic reticulum-negative, LAMP-1-positive phagosomes in human monocytes. Infect. Immun. 73, 3702–3713.
Blay, R., Hernandez, D., Betts, M., Clerici, M., Lucey, D. R., Hendrix, C., Hoffman, T., and Golding, B. (1992). Brucella abortus stimulates human T cells from uninfected and HIV-infected individuals to secrete IFN gamma: implications for use of Brucella abortus as a carrier in development of human vaccines. AIDS Res. Hum. Retroviruses 8, 479–486.
Butcher, B. A., Greene, R. I., Henry, S. C., Annecharico, K. L., Weinberg, J. B., Denkers, E. Y., Sher, A., and Taylor, G. A. (2005). p47 GTPases regulate Toxoplasma gondii survival in activated macrophages. Infect. Immun. 73, 3278–3286.
Chen, F., and He, Y. (2009). Caspase-2 mediated apoptotic and necrotic murine macrophage cell death induced by rough Brucella abortus. PLoS One 4:e6830. doi: 10.1371/journal.pone.0006830
Chen, X., Du, X., Zhang, M., Zhang, D., Ji, M., and Wu, G. (2010). IFN-inducible p47 GTPases display differential responses to Schistosoma japonicum acute infection. Cell. Mol. Immunol. 7, 69–76.
Coers, J., Bernstein-Hanley, I., Grotsky, D., Parvanova, I., Howard, J. C., Taylor, G. A., Dietrich, W. F., and Starnbach, M. N. (2008). Chlamydia muridarum evades growth restriction by the IFN-gamma-inducible host resistance factor Irgb10. J. Immunol. 180, 6237–6245.
Collazo, C. M., Yap, G. S., Sempowski, G. D., Lusby, K. C., Tessarollo, L., Woude, G. F., Sher, A., and Taylor, G. A. (2001). Inactivation of LRG-47 and IRG-47 reveals a family of interferon gamma-inducible genes with essential, pathogen-specific roles in resistance to infection. J. Exp. Med. 194, 181–188.
Eze, M. O., Yuan, L., Crawford, R. M., Paranavitana, C. M., Hadfield, T. L., Bhattacharjee, A. K., Warren, R. L., and Hoover, D. L. (2000). Effects of opsonization and gamma interferon on growth of Brucella melitensis 16M in mouse peritoneal macrophages in vitro. Infect. Immun. 68, 257–263.
Fernandez-Lago, L., Rodriguez-Tarazona, E., and Vizcaino, N. (1999). Differential secretion of interleukin-12 (IL-12) subunits and heterodimeric IL-12p70 protein by CD-1 mice and murine macrophages in response to intracellular infection by Brucella abortus. J. Med. Microbiol. 48, 1065–1073.
Gross, A., Spiesser, S., Terraza, A., Rouot, B., Caron, E., and Dornand, J. (1998). Expression and bactericidal activity of nitric oxide synthase in Brucella suis-infected murine macrophages. Infect. Immun. 66, 1309–1316.
Gutierrez, M. G., Master, S. S., Singh, S. B., Taylor, G. A., Colombo, M. I., and Deretic, V. (2004). Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell 119, 753–766.
Henry, S. C., Daniell, X. G., Burroughs, A. R., Indaram, M., Howell, D. N., Coers, J., Starnbach, M. N., Hunn, J. P., Howard, J. C., Feng, C. G., Sher, A., and Taylor, G. A. (2009). Balance of Irgm protein activities determines IFN-gamma-induced host defense. J. Leukoc. Biol. 85, 877–885.
Hunn, J. P., and Howard, J. C. (2010). The mouse resistance protein Irgm1 (LRG-47): a regulator or an effector of pathogen defense? PLoS Pathog. 6:e1001008. doi: 10.1371/journal.ppat.1001008
Hunn, J. P., Koenen-Waisman, S., Papic, N., Schroeder, N., Pawlowski, N., Lange, R., Kaiser, F., Zerrahn, J., Martens, S., and Howard, J. C. (2008). Regulatory interactions between IRG resistance GTPases in the cellular response to Toxoplasma gondii. EMBO J. 27, 2495–2509.
Jiang, X., and Baldwin, C. L. (1993a). Iron augments macrophage-mediated killing of Brucella abortus alone and in conjunction with interferon-gamma. Cell. Immunol. 148, 397–407.
Jiang, X., and Baldwin, C. L. (1993b). Effects of cytokines on intracellular growth of Brucella abortus. Infect. Immun. 61, 124–134.
Jiang, X., Leonard, B., Benson, R., and Baldwin, C. L. (1993). Macrophage control of Brucella abortus: role of reactive oxygen intermediates and nitric oxide. Cell. Immunol. 151, 309–319.
MacMicking, J. D., Taylor, G. A., and McKinney, J. D. (2003). Immune control of tuberculosis by IFN-gamma-inducible LRG-47. Science 302, 654–659.
Martens, S., Sabel, K., Lange, R., Uthaiah, R., Wolf, E., and Howard, J. C. (2004). Mechanisms regulating the positioning of mouse p47 resistance GTPases LRG-47 and IIGP1 on cellular membranes: retargeting to plasma membrane induced by phagocytosis. J. Immunol. 173, 2594–2606.
Murphy, E., Robertson, G. T., Parent, M., Hagius, S. D., Roop, R. M. 2nd, Elzer, P. H., and Baldwin, C. L. (2002). Major histocompatibility complex class I and II expression on macrophages containing a virulent strain of Brucella abortus measured using green fluorescent protein-expressing brucellae and flow cytometry. FEMS Immunol. Med. Microbiol. 33, 191–200.
Pizarro-Cerda, J., Meresse, S., Parton, R. G., van der Goot, G., Sola-Landa, A., Lopez-Goni, I., Moreno, E., and Gorvel, J. P. (1998). Brucella abortus transits through the autophagic pathway and replicates in the endoplasmic reticulum of nonprofessional phagocytes. Infect. Immun. 66, 5711–5724.
Robertson, G. T., and Roop, R. M. Jr. (1999). The Brucella abortus host factor I (HF-I) protein contributes to stress resistance during stationary phase and is a major determinant of virulence in mice. Mol. Microbiol. 34, 690–700.
Santiago, H. C., Feng, C. G., Bafica, A., Roffe, E., Arantes, R. M., Cheever, A., Taylor, G., Vieira, L. Q., Aliberti, J., Gazzinelli, R. T., and Sher, A. (2005). Mice deficient in LRG-47 display enhanced susceptibility to Trypanosoma cruzi infection associated with defective hemopoiesis and intracellular control of parasite growth. J. Immunol. 175, 8165–8172.
Silva, T. M., Costa, E. A., Paixao, T. A., Tsolis, R. M., and Santos, R. L. (2011). Laboratory animal models for brucellosis research. J. Biomed. Biotechnol. 2011, 518323.
Starr, T., Child, R., Wehrly, T. D., Hansen, B., Hwang, S., Lopez-Otin, C., Virgin, H. W., and Celli, J. (2012). Selective subversion of autophagy complexes facilitates completion of the Brucella intracellular cycle. Cell Host Microbe 11, 33–45.
Starr, T., Ng, T. W., Wehrly, T. D., Knodler, L. A., and Celli, J. (2008). Brucella intracellular replication requires trafficking through the late endosomal/lysosomal compartment. Traffic 9, 678–694.
Stevens, M. G., Pugh, G. W. Jr., and Tabatabai, L. B. (1992). Effects of gamma interferon and indomethacin in preventing Brucella abortus infections in mice. Infect. Immun. 60, 4407–4409.
Taylor, G. A., Feng, C. G., and Sher, A. (2004). p47 GTPases: regulators of immunity to intracellular pathogens. Nat. Rev. Immunol. 4, 100–109.
Zhao, X. Y., Li, N., Ding, H. G., and Jiang, F. F. (2010a). [Detection and evaluation of serum GP73, a resident Golgi glycoprotein, as a marker in diagnosis of hepatocellular carcinoma]. Zhonghua Zhong Liu Za Zhi 32, 943–945.
Zhao, Y. O., Konen-Waisman, S., Taylor, G. A., Martens, S., and Howard, J. C. (2010b). Localisation and mislocalisation of the interferon-inducible immunity-related GTPase, Irgm1 (LRG-47) in mouse cells. PLoS One 5:e8648. doi: 10.1371/journal.pone.0008648
Appendix
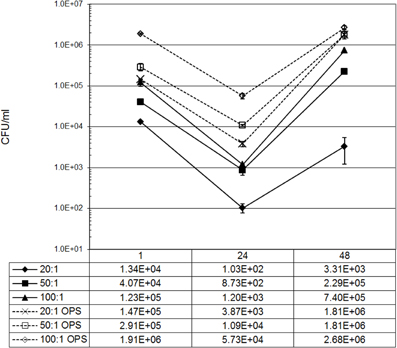
Figure A1. Internalization and intracellular viability of IgG opsonized and non-opsonized B. abortus. Monolayers of Raw264.7 cells were infected with increasing concentrations of IgG-opsonized or non-opsonized B. abortus. The average CFU per time point illustrates the correlation between the amount of Brucella internalized following infection, and the rate of Brucella replication and survival. To compare results from opsonized and non-opsonized Brucella, remaining experiments were normalized to numbers of Brucella taken up by cells using MOI's of 20:1 for ops and 100:1 for non-ops. Results presented were calculated from samples performed in triplicate and confirmed by separate independent experiments.
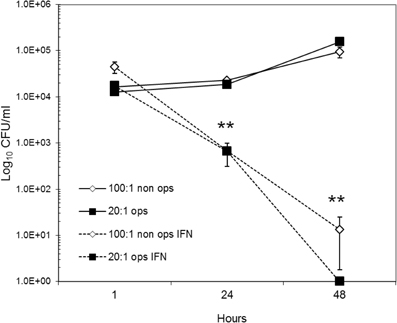
Figure A2. Effect of IFN-γ treatment on Brucella infected Raw264.7 cells. Pretreatment of Raw264.7 cells with 50 ug/ul of IFN-γ 24 h prior to infection with B. abortus induces an anti-bacterial response that increases Brucella killing at 24 h p.i. (T = 1), continuing through 48 h p.i. (T = 2). Magnitude of anti-Brucella effect was equal among opsonized and non-opsonized Brucella. Similarly, bacterial survival at 24 h p.i., and replication detected at 48 h were the same between opsonized and non-opsonized bacteria infected at normalized MOI's (Figure 1). Results represent the averages and standard deviation from six replicate wells for each condition and time point. Statistical analysis was performed using non-paired Student's t-test.
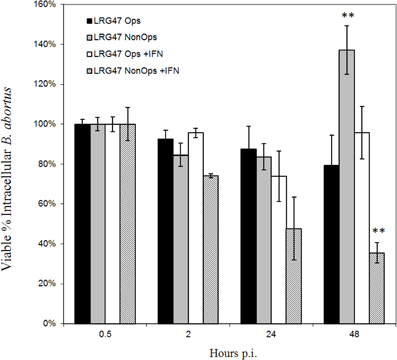
Figure A3. Effects of opsonization on Brucella survival in LRG-47 knockout peritoneal macrophages. Peritoneal macrophages were collected from LRG-47−/− mice and infected with Ig-opsonized or non-opsonized virulent Brucella abortus. Colony forming units (CFU) were enumerate at 30 min, 2 h, 24 h, and 48 h post infection and percent survival was calculated compared to CFU/ml 30 min post infection. Ig-opsonized Brucella have a lower percent survival in the non-activated cells than they do in the activated cells suggesting a requirement for LRG-47 in opsonized Brucella survival in non-activated macrophages. In IFN-γ stimulated cells the percent survival of the Brucella steadily decreases over the duration of 48 h whereas in the non-activated cells there is a sharp increase in percent survival between 24 and 48 h post infection. (**p ≤ 0.01 Student's t-Test vs. opsonized for each stimulation group).
Keywords: Brucella pathogenesis, intracellular survival, LRG-47, interferon gamma, chronic infection, opsonin, macrophage, Brucella abortus
Citation: Ritchie JA, Rupper A, Cardelli JA and Bellaire BH (2012) Host interferon-γ inducible protein contributes to Brucella survival. Front. Cell. Inf. Microbio. 2:55. doi: 10.3389/fcimb.2012.00055
Received: 13 January 2012; Accepted: 07 April 2012;
Published online: 27 April 2012.
Edited by:
Thomas A. Ficht, Texas A&M University, USACopyright: © 2012 Ritchie, Rupper, Cardelli and Bellaire. This is an open-access article distributed under the terms of the Creative Commons Attribution Non Commercial License, which permits non-commercial use, distribution, and reproduction in other forums, provided the original authors and source are credited.
*Correspondence: Bryan H. Bellaire, Department of Veterinary Microbiology and Preventive Medicine, Iowa State University, 1136 Veterinary Medicine Complex, Ames, IA 50011, USA. e-mail: bbella@iastate.edu