Glossina spp. gut bacterial flora and their putative role in fly-hosted trypanosome development
- 1UMR 177 InterTryp, IRD-CIRAD, Montpellier, France
- 2Université Aix-Marseille, Université du Sud Toulon-Var, CNRS/INSU, IRD, MIO, UM 110, Marseille, France
- 3Department of Science, University of Yaoundé I, Yaoundé, Cameroon
Human African trypanosomiasis (HAT) is caused by trypanosomes transmitted to humans by the tsetse fly, in which they accomplish their development into their infective metacyclic form. The crucial step in parasite survival occurs when it invades the fly midgut. Insect digestive enzymes and immune defenses may be involved in the modulation of the fly's vector competence, together with bacteria that could be present in the fly's midgut. In fact, in addition to the three bacterial symbionts that have previously been characterized, tsetse flies may harbor additional bacterial inhabitants. This review focuses on the diversity of the bacterial flora in Glossina, with regards to the fly species and their geographical distribution. The rationale was (i) that these newly identified bacteria, associated with tsetse flies, may contribute to vector competence as was shown in other insects and (ii) that differences may exist according to fly species and geographic area. A more complete knowledge of the bacterial microbiota of the tsetse fly and the role these bacteria play in tsetse biology may lead to novel ways of investigation in view of developing alternative anti-vector strategies for fighting human—and possibly animal—trypanosomiasis.
Introduction
A comprehensive understanding of the biology of insects requires investigations on the microbial content of their guts (Steinhaus, 1960). Insects are hosts for a large panel of microorganisms that have developed a variety of interactions ranging from mutualistic to parasitic (Jeyaprakash et al., 2003; Schmitt-Wagner et al., 2003; Campbell et al., 2004; Hongoh et al., 2005). Some of these interactions have been quite well characterized, owing to their ecologic and/or economic importance. However, the exact nature of many of these interactions remains poorly understood and poorly documented.
Human African trypanosomiasis (HAT), or sleeping sickness, caused by trypanosomes transmitted to humans by the tsetse fly (Glossina spp.), belongs to the neglected tropical diseases affecting more than 1 billion people worldwide (Fèvre et al., 2008; Welburn et al., 2009). Regarding sleeping sickness itself, 60 million people are living in HAT-risk areas in the 36 countries that are listed by WHO as being endemic for the disease, among which only 10–15% really undergo epidemiological control (Cattand et al., 2001). This means that the actual number of HAT cases is probably much higher than reported and that HAT remains a serious public health problem even though the prevalence of HAT now seems to be decreasing (Barrett, 2006; WHO, 2010; Simarro et al., 2011). Unless treated the disease is fatal. The drugs currently used to fight the disease are not satisfactory, some are toxic, and all are difficult to administer (Barrett, 2006). Furthermore, trypanosome resistance to some drugs has developed and is increasing (de Koning, 2001). Therefore new strategies to combat the disease need to be developed.
To be transmitted to the mammalian host, trypanosomes must first establish in the insect midgut and, upon their migration to the salivary glands, they have to undergo a maturation process. When the fly feeds on infected mammalian hosts, trypanosomes enter the fly midgut, where they rapidly differentiate into procyclic forms. Then they either die in the midgut of refractory individuals or survive to yield persistent procyclic infections in susceptible insects. Once established, parasites migrate toward the salivary glands where they differentiate into epimastigote forms and, finally, into infectious metacyclic forms (maturation step) that can be transmitted to naïve mammals by the fly when taking another blood meal (Vickerman et al., 1988; Van Den Abbeele et al., 1999). The factors involved in the establishment step are still largely unknown. However, several factors are believed to be involved in this step among which the fly's digestive enzymes and immune defenses and the intestinal microbial flora (Welburn and Maudlin, 1999; MacLeod et al., 2007; Wang et al., 2009, 2012; Weiss and Aksoy, 2011). As reviewed by Dillon and Dillon (2004), insects harbor, mainly in the intestinal organs, diverse communities of microorganisms. The tsetse fly harbors three symbiotic microorganisms (Aksoy, 2000): (i) the obligate primary symbiont, Wigglesworthia glossinidia (Aksoy, 2000), which synthesizes B vitamins (Akman et al., 2002) that the fly is unable to synthesize and which are absent from its blood diet; (ii) Wolbachia (O'Neill et al., 1993), belonging to the Rickettsiaceae family, which infects a broad range of insect species, causing a variety of reproductive abnormalities, and cytoplasmic incompatibility in tsetse flies (Alam et al., 2011); and (iii) Sodalis glossinidius, belonging to the Enterobacteriaceae family, which has been shown to be involved in the fly's vector competence (Dale and Maudlin, 1999). Although most of the studies dedicated to insect gut microbiota focused on the contribution of microbial endosymbionts to the host's nutritional homeostasis (Dillon and Dillon, 2004), others examined the role of gut bacteria in preventing pathogen development (Pumpuni et al., 1993, 1996; Welburn and Maudlin, 1999; Gonzalez-Ceron et al., 2003; Azambuja et al., 2004). Since the trypanosomes have to complete part of their lifecycle within their vector, particularly in its gut, the concomitant presence of diverse bacteria, if any, could affect the parasite's lifecycle and finally the fly's vector competence. Therefore, our knowledge on the composition of the tsetse fly midgut bacterial flora must be improved to gain more detailed insight into the potential interactions between these bacteria and the insect harboring Trypanosoma, and/or even with the parasite itself.
This article reviews the present knowledge on the fly's gut-associated bacteria, other than symbionts, and suggests novel ways of investigation.
Diversity of Microbiota in Tsetse Flies
While the bacterial flora composition of a few of insects [Drosophila and several mosquitoes (Pumpuni et al., 1993, 1996; Broderick and Lemaitre, 2012)] has been investigated for years and is fairly well documented, the bacterial flora composition of the tsetse fly has only recently gained attention. Studies on tsetse flies have been conducted on insectary-reared Glossina palpalis gambiensis flies and on flies belonging to several Glossina species collected in HAT foci in two Africa countries—Angola and Cameroon (Geiger et al., 2009, 2010, 2011)—and on G. fuscipes fuscipes flies from Kenya (Lindh and Lehane, 2011) (Figure 1). It is noteworthy that, using a culture-dependent isolation method and a similar enrichment procedure throughout the studies, the former group evidenced differences in the bacterial flora composition not only with respect to the fly species, but also to their geographical origin. The approach used included dilution series (which ranged from 10−6 to 10−10, depending on the study) of the midgut before bacterial enrichment, in order to ensure the isolation of microorganisms that have actively multiplied in the gut and that can therefore be considered as true gut inhabitants; this process rules out bacteria that are merely transient residents. The isolated bacteria were then identified using molecular phylogeny identification. However, this culture-dependent method does not allow the identification of non-cultivable bacteria. In contrast, the group (Lindh and Lehane, 2011) working on flies collected in East Africa used both culture-dependent and culture-independent approaches that are expected to allow the characterization of not easily cultivated—or even non-cultivable—bacteria, but possibly also of bacteria that are simply in transit in the flies' gut.
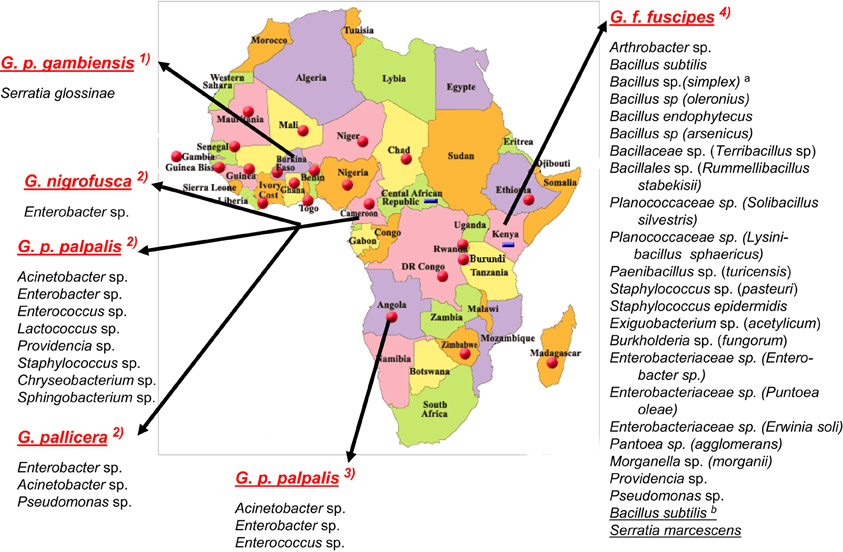
Figure 1. Bacterial species characterized in the midgut of tsetse flies species from sub-Saharan African countries. According, respectively, to: (1) Geiger et al. (2010); (2) Geiger et al. (2011); (3) Geiger et al. (2009); (4) Lindh and Lehane (2011). Bacteria from G. f. fuscipes: athe species between brackets are the closest relatives according to RDPII (Maidak et al., 2001); bthe species underlined were identified with culture-independent methods. No bacteria were identified in G. caliginea.
The Bacterial Flora of Tsetse Flies from Angola, Cameroon, and Kenya
The fly species collected and studied differed from one country to another: Glossina palpalis palpalis in Angola, G. p. palpalis, G. pallicera, G. nigrofusca, and G. caliginea in Cameroon, and G. fuscipes fuscipes in Kenya, which allows limited comparisons only between fly species from different countries (Figure 1). However, one may note the overall relatively high fly infection rates by bacteria for all three countries: 54% in Angola, 53% in Cameroon (Figures 2A and 3A), and 72% in Kenya (42% when discarding the bacteria isolated from the outer cuticle of the mosquitoes), despite the differences observed in the fly species. Similarly, the prevalence of Gram-negative bacteria was much higher than the Gram-positive bacteria. Finally, most often an individual fly harbored only one bacterial species; mixed infections were sometimes observed whatever the fly species studied (Figures 2B and 3B). However, the number of bacterial isolates characterized, three per fly, was low and therefore the prevalence of mixed infection could be underestimated.
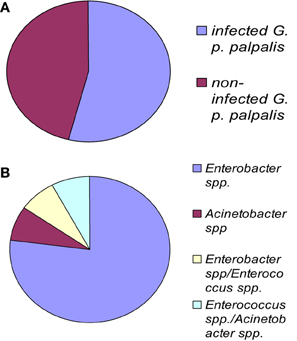
Figure 2. G. palpalis palpalis infections in the Maria Teresa focus in Angola according to Geiger et al. (2009). (A) Prevalence of infection in tsetse flies; (B) frequency of occurrence for each infection type. “Multiple” names indicate the occurrence of a mixed infection.
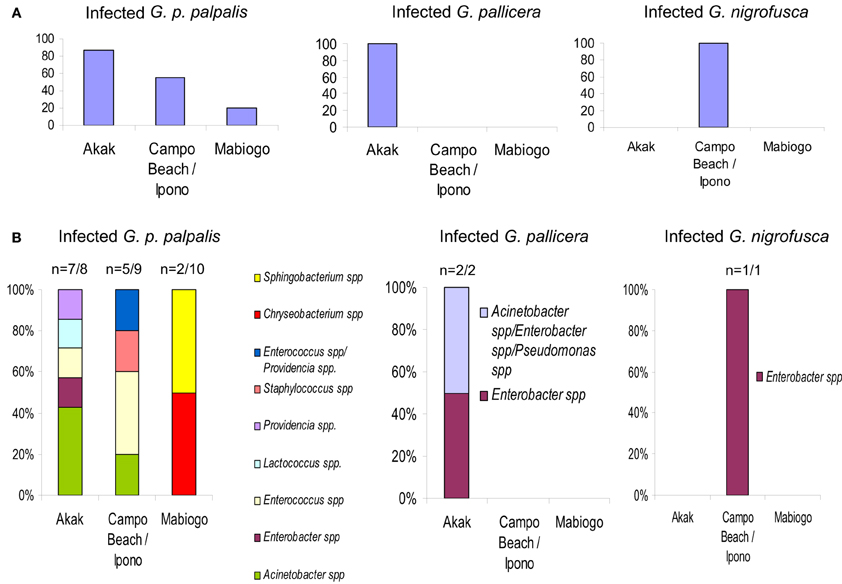
Figure 3. G. palpalis palpalis, G. pallicera and G. nigrofusca fly' bacterial infections occurring in the different villages (Akak, Campo Beach/Ipono and Mabiogo) of the Campo focus in Cameroon, according to Geiger et al. (2011). (A) Prevalence of bacterial infection in the different tsetse fly species; (B) bacterial infection frequency per fly species and per village. “n” indicates the number of bacterial infected flies vs. the number of collected flies. “Multiple” names indicate the occurrence of a mixed infection.
The overall high diversity of bacterial species was also unexpected (Figure 1) with respect to (i) the geographic origin of the flies: 3 bacterial species in flies from Angola, 9 in Cameroon (Figures 2, 3), 22 in Kenya (+2 identified by the culture-independent method), and/or (ii) the fly species: 22 (+2 by molecular approaches) in G. fuscipes fuscipes, 8 in G. p. palpalis, 3 in G. pallicera, 1 in G. nigrofusca, none in G. caliginea. The number of G. pallicera, G. nigrofusca, and G. caliginea flies collected and analyzed was very low making conclusions about the number and types of bacterial species in these flies limited at this time.
Besides these similarities, substantial differences are noted when comparing the results recorded for different countries; in fact, the overall bacterial species are assigned to four different phyla in which they are nevertheless unevenly distributed with reference to the geographic origin of the flies: Actinobacteria, 4% in Kenya, 0% in Angola and Cameroon; Proteobacteria: 36% in Kenya, 66% in Angola, and 44% in Cameroon; Firmicutes: 60% in Kenya, 33% in Angola and Cameroon, and Bacteroidetes: 0% in Kenya and Angola, 22% in Cameroon. In addition, when comparing the overall bacteria species identified in the two West African countries (on G. p. palpalis, G. pallicera, and G. nigrofusca) with those characterized in Kenya (on G. f. fuscipes), only four species were found to be common: Enterobacter spp., Providencia spp., Pseudomonas spp., and Staphylococcus spp. (Figure 1). However, differences in bacterial culture conditions (as opposed to differences in geographic origin) may account for differences in bacterial species. Finally, while a large diversity of bacteria was found in field-collected tsetse flies, only one bacterial species, a novel one pertaining to the Serratia genus, S. glossinae, was isolated from insectary-reared fly midguts of G. p. gambiensis, trapped several years before in Burkina Faso (Geiger et al., 2010) (Figure 1).
Differences in the Bacterial Diversity in Tsetse Flies Collected in Three Areas Belonging to the Same HAT Focus
In contrast to the substantial differences in the diversity of bacterial gut inhabitants recorded according to the geographic origin of the flies, it could be expected that such differences would be much more limited in flies collected in a restricted area. This was not the case, as shown by the results of an investigation carried out in three villages (Akak, Campo Beach/Ipono and Mabiogo) located into the same HAT focus, Campo, in southern Cameroon.
The flies sampled in Campo belonged to four Glossina species, G. p. palpalis, G. pallicera, G. caliginea, and G. nigrofusca; G. p. palpalis accounted for 94% of the fly community. Nine bacterial species were isolated and identified from these sampled flies: Acinetobacter spp., Enterobacter spp., Providencia spp., Pseudomonas spp., Enterococcus spp., Lactococcus spp., and Staphylococcus spp., Chryseobacterium spp., and Sphingobacterium spp.; except for Pseudomonas spp., all of them could be isolated from G. p. palpalis flies (Figures 1, 3).
The large differences in fly infection rates recorded with reference to the collecting sites were surprising. In the most representative species, G. p. palpalis, 87.5% of the flies collected in Akak were infected, in contrast to 55.5% of the flies from Campo Beach/Ipono, and only 20% of those from Mabiogo (Figure 3A). Furthermore, considering G. p. palpalis, the distribution of the different bacteria identified was also very uneven with respect to the origin of the flies. In Mabiogo, the infection rate was the lowest. Two bacterial species were identified: Chryseobacterium spp. and Sphingobacterium spp. These bacteria were not identified in the flies sampled in the two other villages in the performed surveys. Similarly, Enterobacter and Lactococcus spp. infections were restricted to flies collected in Akak (Geiger et al., 2011), and finally, four bacteria species were isolated from flies from Campo Beach/Ipono (Acinetobacter spp., Providencia spp., Enterococcus spp., and Staphylococcus spp.) (Figure 3B). However, since these surveys looked at three bacterial isolates per fly, it is possible that the prevalence of each bacterial species could be underestimated in the different villages tested.
Origin of the Gut Bacteria and their Diversity According to the Fly Species and their Geographic Location
The high prevalence and diversity of bacteria in tsetse flies is unexpected given that these flies are monophagous as they only feed on vertebrate blood throughout their life span. In wild populations of mosquitoes, the origin of the midgut bacteria is unknown (Pumpuni et al., 1996; Straif et al., 1998), as in tsetse flies. However, differences in the environmental conditions and in the food supply may influence the diversity of the bacterial communities harbored. This hypothesis could be acceptable if one considers that the fly may swallow bacteria present in the environment, particularly on the skin of the animals on which it feeds. This possibility cannot be excluded since Poinar et al. (1979) demonstrated that, when applied to the ears of rabbits used as tsetse fly-feeding hosts, the bacterium S. marcescens was ingested during the blood meal and multiplied in the fly's gut. Tsetse flies were shown to feed on a variety of hosts (Simo et al., 2008; Farikou et al., 2010), which probably carry diverse bacteria on their hair and skin, thus implying the possibility of the flies being infected by these bacteria. Nevertheless, the mechanism may be more complex since the G. p. palpalis flies collected in the three villages of the Campo HAT focus differed in their bacterial inhabitants, even though they developed in similar environmental conditions.
Involvement of Midgut Bacteria in the Insect Vector Competence and its Survival
While investigations on the potential effect of gut microbiota on tsetse fly vector competence are nearly non-existent, such studies have been successfully conducted on other insects.
Gonzalez-Ceron et al. (2003) reported that the Plasmodium vivax sporogonic development in field-collected Anopheles albimanus was blocked by bacteria inhabiting the mosquitoes' midgut (Gonzalez-Ceron et al., 2003). When feeding laboratory-reared adult anopheline species with either Gram-negative or Gram-positive bacteria together with Plasmodium falciparum gametocytes, it was shown that Gram-negative, but not Gram-positive, bacteria partially or totally inhibited the formation of oocysts (Pumpuni et al., 1993, 1996). In contrast, working on field-collected mosquitoes, Straif et al. (1998) showed that the presence of Gram-negative bacteria in the midgut did not influence the number of Anopheles funestus infected with P. falciparum sporozoites, while Gram-positive bacteria significantly enhanced the incidence of mosquitoes that contained sporozoites. Furthermore, feeding mosquitoes with gentamicin significantly increased the number of Plasmodium-infected mosquitoes (Beier et al., 1994). In Anopheles albimanus, co-infections with S. marcescens and Plasmodium vivax resulted in only 1% of mosquitoes being infected with parasites, compared to a 71% infection rate in control mosquitoes (Gonzalez-Ceron et al., 2003). Recently, a significant positive correlation was observed between P. falciparum infection and the presence of Enterobacteriaceae in the mosquitoes' midgut (Boissière et al., 2012). In sandflies (Phlebotomus papatasi), microbial infections significantly reduced the rates of infection with Leishmania major (Schlein et al., 1985). In addition, strains of Pseudomonas fluorescens (Mercado and Colon-Whitt, 1982), as well as of S. marcescens (which was isolated from Rhodnius prolixus) (Azambuja et al., 2004) have been reported to be able to lyse Trypanosoma cruzi in vitro. All these examples show potential implication of bacteria isolated from insects in their vector competence.
Some of the bacterial genera/species that were found in at least one species of tsetse fly (Geiger et al., 2009, 2011) have been shown to affect other insects. Stomoxys calcitrans fly larvae require the presence of Acinetobacter spp. for complete development (Lysyk et al., 1999). Conversely, several other bacterial species including Providencia spp. and Pseudomonas spp. are close relatives of known insect bacteria (Jackson et al., 1995; Lacey, 1997). In addition, a number of Gram-negative and Gram-positive bacteria such as S. marcescens, Providencia rettgeri, and several Bacillus spp. induce mortality in G. m. morsitans (Kaaya and Darji, 1989). Furthermore, S. marcescens has been shown to cause increased mortality in Anopheles albimanus mosquitoes and in G. pallidipes flies (Poinar et al., 1979; Gonzalez-Ceron et al., 2003). Other bacteria isolated from field tsetse flies (Geiger et al., 2009, 2011; Lindh and Lehane, 2011) were assigned to the genus Lactobacillus, some members of which are reported to be pathogenic to plants and animals whereas other Lactobacilli are commonly found as members of human microbiota (Hammes and Hertel, 2006).
Symbionts have also been implicated in vector competence and/or tsetse fly survival. Studies from Weiss et al. (2011, 2012) have shown that Wigglesworthia protect against Escherichia coli infection and promote tsetse immune system development. Moreover, interactions between Wigglesworthia and the tsetse peptidoglycan recognition protein (PGRP-LB) may be involved in trypanosome transmission (Wang et al., 2009). Weiss et al. (2013) showed that trypanosome infection in the tsetse fly gut was influenced by microbiota-regulated host immune barriers. Geiger et al. (2007) showed an association between the presence of specific genotypes of Sodalis and G. p. gambiensis midgut infection by Trypanosoma brucei gambiense or Trypanosoma brucei brucei.
Mechanisms Potentially Involved in the Modulation of Parasite Infection by Midgut Microbiota
Several mechanisms may be involved in the modulation of parasite infection by midgut microbiota. One could be the competition for limited resources or the production of antiparasitic molecules by the bacteria inhabiting the vectors' gut. Toxic molecules (Figure 4) with potential antiparasitic activity have been identified. Among them are cytotoxic metalloproteases produced, for example, by S. marcescens and Pseudomonas aeruginosa (Maeda and Morihara, 1995) or hemolysins secreted by Enterobacter spp., E. coli, S. marcescens, and Enterococcus spp. (Hertle et al., 1999; Coburn and Gilmore, 2003). Antibiotics can be produced by Serratia spp.(Thomson et al., 2000); hemagglutinins (Gilboa-Garber, 1972) and siderophore by P. aeruginosa (Schalk et al., 2002). An antitrypanosomal factor has been shown to be produced by P. fluorescens (Mercado and Colon-Whitt, 1982). Pigments such as prodigiosin are produced by the Gram-negative bacteria such as Serratia spp. and Enterobacter spp. (Moss, 2002). They induce the fragmentation of DNA, characterizing an apoptotic action of the toxin (Díaz-Ruiz et al., 2001; Montaner and Perez-Tomas, 2003). Prodigiosin was shown to be toxic for P. falciparum (Lazaro et al., 2002) and T. cruzi (Azambuja et al., 2004). Free hemoglobin, resulting from the hemolysis of the blood meal in the digestive tract of vector insects (Azambuja et al., 2004) has been suggested to be a ready source of iron for bacteria and would contribute to the massive increase in the gut bacteria population, following feeding. However, toxic molecules have not been shown to be constitutively expressed and the production of these may even be indirectly correlated with bacterial density.
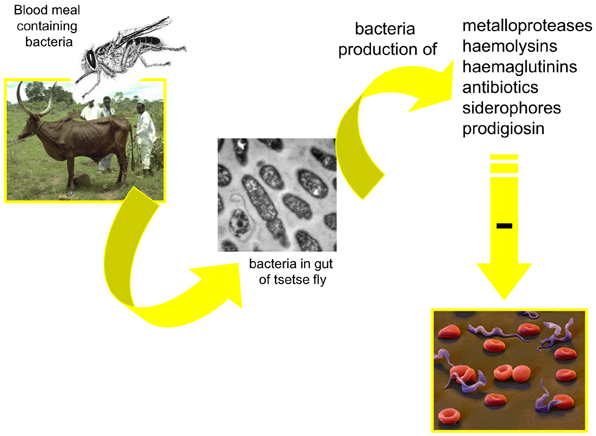
Figure 4. Possible mechanism involved in the modulation, by the midgut microbiota, of the tsetse fly infection by trypanosomes.
Dong et al. (2009) suggested the bacteria-mediated anti-plasmodium effect was due to the mosquito's antimicrobial immune responses, possibly through the activation of basal immunity. Recently, in Zambia, Enterobacter spp. were isolated from wild mosquitoes resistant to infection with P. falciparum. It was suggested the anti-Plasmodium effect was caused by bacterial generation of reactive oxygen species (Cirimotich et al., 2011).
Perspectives
It is crucial to investigate whether any of the recently identified bacteria in tsetse could modulate the fly vector's competence, as do the flies' endosymbionts (Welburn and Maudlin, 1999), and as has already been reported in other insect parasite vectors (Pumpuni et al., 1993, 1996; Straif et al., 1998; Gonzalez-Ceron et al., 2003). Such modulation may occur through direct inhibitory bioactivity, by secreted enzymes or toxins focused on the parasitic trypanosomes. Alternatively, microbiota may constrain pathogen development indirectly by activating or enhancing the host immune system that in turn could clear the parasite; this effect was previously reported for Wigglesworthia affecting PGRP-LB (Wang et al., 2009; Weiss et al., 2013). Investigations on several insect systems indicate that both direct and indirect microbiota-induced phenotypes occur (Dong et al., 2009; Cirimotich et al., 2011). Finally, understanding the mechanisms governing the association between of tsetse flies and the hosted bacteria, and determining how the association is controlled, are important issues. These issues could be addressed by monitoring the diversity and density of bacteria in flies throughout their life cycle and by investigating the possible transmission of these bacteria species by the female fly to its progeny, as occurs for the maternal transmission of the three Glossina endosymbionts.
In wild populations, differences in environmental conditions and in food supply may influence the diversity of the bacterial communities harbored by the flies. This could explain the diversity in the flies' gut bacterial inhabitants and in fly infection rates reported in tsetse fly communities from Angola, Cameroon and Kenya, and therefore point out the need to multiply and diversify the fly collecting areas. Moreover, a greater number of samples has to be collected in order to better assess the occurrence of co-infections and to evidence the possible involvement of the gut-hosted bacteria in the tsetse fly.
All these investigations deserve to be undertaken as they may open novel avenues for tsetse vector competence control through manipulation of gut microbial communities, which in turn may result in novel HAT control strategies.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This study has been supported by the Institut de Recherche pour le Développement (IRD).
References
Akman, L., Yamashita, A., Watanabe, H., Oshima, K., Shiba, T., Hattori, M., and et al. (2002). Genome sequence of the endocellular obligate symbiont of tsetse, Wigglesworthia glossinidia. Nat. Genet. 32, 402–407. doi: 10.1038/ng986
Aksoy, S. (2000). Tsetse-a haven for microorganisms. Parasitol. Today 16, 114–118. doi: 10.1016/S0169-4758(99)01606-3
Alam, U., Medlock, J., Brelsfoard, C., Pais, R., Lohs, C., Balmand, S., and et al. (2011). Wolbachia symbiont infections induce strong cytoplasmic incompatibility in the tsetse fly Glossina morsitans. PLoS Pathog. 7:e1002415. doi: 10.1371/journal.ppat.1002415
Azambuja, P., Feder, D., and Garcia, E. S. (2004). Isolation of Serratia Marcescens in the midgut of Rhodnius prolixus: impact on the establishment of the parasite Trypanosoma cruzi in the vector. Exp. Parasitol. 107, 89–96. doi: 10.1016/j.exppara.2004.04.007
Barrett, M. P. (2006). The rise and fall of sleeping sickness. Lancet 367, 1377–1378. doi: 10.1016/S0140-6736(06)68591-7
Beier, M. S., Pumpuni, C. B., Beier, J. C., and Davis, J. R. (1994). Effects of para-aminobenzoic acid, insulin, and gentamycin on Plasmodium falciparum development in anopheline mosquitoes (Diptera: Culicidae). J. Med. Entomol. 31, 561–565.
Boissière, A., Tchioffo, M. T., Bachar, D., Abate, L., Marie, A., Nsango, S. E., and et al. (2012). Midgut microbiota of the malaria mosquito vector Anopheles gambiae and interactions with Plasmodium falciparum infection. PLoS Pathog. 8:e1002742. doi: 10.1371/journal.ppat.1002742
Broderick, N. A., and Lemaitre, B. (2012). Gut-associated microbes of Drosophila melanogaster. Gut Microbes 3, 307–321. doi: 10.4161/gmic.19896
Campbell, C., Mummey, D., Schmidtmann, E., and Wilson, W. (2004). Culture-independent analysis of midgut microbiota in the arbovirus vector Culicoides sonorensis (Diptera: Ceratopogonidae). J. Med. Entomol. 41, 340–348. doi: 10.1603/0022-2585-41.3.340
Cattand, P., Jannin, J., and Lucas, P. (2001). Sleeping sickness surveillance: an essential step towards elimination. Trop. Med. Int. Health 6, 348–361. doi: 10.1046/j.1365-3156.2001.00669.x
Cirimotich, C. M., Dong, Y., Clayton, A. M., Sandiford, S. L., Souza-Neto, J. A., Mulenga, M., and et al. (2011). Natural microbe-mediated refractoriness to Plasmodium infection in Anopheles gambiae. Science 332, 855–858. doi: 10.1126/science.1201618
Coburn, P. S., and Gilmore, M. S. (2003). The Enterococcus faecalis cytolysin: a novel toxin active against eukaryotic and prokaryotic cells. Cell. Microbiol. 5, 661–669. doi: 10.1046/j.1462-5822.2003.00310.x
Dale, C., and Maudlin, I. (1999). Sodalis gen. nov. and Sodalis glossinidius sp. nov., a microaerophilic secondary endosymbiont of the tsetse fly Glossina morsitans morsitans. Int. J. Syst. Bacteriol. 49, 267–275. doi: 10.1099/00207713-49-1-267
de Koning, H. P. (2001). Transporters in African trypanosomes: role in drug action and resistance. Int. J. Parasitol. 31, 512–522. doi: 10.1016/S0020-7519(01)00167-9
Díaz-Ruiz, C., Montaner, B., and Pérez-Tomás, R. (2001). Prodigiosin induces cell death in gastric cancer cell line. Histol. Histopathol. 16, 415–421.
Dillon, R. J., and Dillon, V. M. (2004). The gut bacteria of insects: nonpathogenic interactions. Annu. Rev. Entomol. 49, 71–92. doi: 10.1146/annurev.ento.49.061802.123416
Dong, Y., Manfredini, F., and Dimopoulos, G. (2009). Implication of the mosquito midgut microbiota in the defense against malaria parasites. PLoS Pathog. 5:e1000423. doi: 10.1371/journal.ppat.1000423
Farikou, O., Njiokou, F., Simo, G., Asonganyi, T., Cuny, G., and Geiger, A. (2010). Tsetse fly blood meal modification and trypanosome identification in two sleeping sickness foci in the forest of southern Cameroon. Acta Trop. 116, 81–88. doi: 10.1016/j.actatropica.2010.06.002
Fèvre, E. M., Wissmann, B. V., Welburn, S. C., and Lutumba, P. (2008). The burden of human African trypanosomiasis. PLoS Negl. Trop. Dis. 2:e333. doi: 10.1371/journal.pntd.0000333
Geiger, A., Fardeau, M. L., Falsen, E., Ollivier, B., and Cuny, G. (2010). Serratia glossinae sp. nov., isolated from the midgut of the tsetse fly Glossina palpalis gambiensis. Int. J. Syst. Evol. Microbiol. 60, 1261–1265. doi: 10.1099/ijs.0.013441-0
Geiger, A., Fardeau, M. L., Grebaut, P., Vatunga, G., Josénando, T., Herder, S., and et al. (2009). First isolation of Enterobacter, Enterococcus, and Acinetobacter spp. as inhabitants of the tsetse fly (Glossina palpalis palpalis) midgut. Infect. Genet. Evol. 9, 1364–1370. doi: 10.1016/j.meegid.2009.09.013
Geiger, A., Fardeau, M. L., Njiokou, F., Joseph, M., Asonganyi, T., Ollivier, B., and et al. (2011). Bacterial diversity associated with populations of Glossina spp. from Cameroon and distribution within the Campo sleeping sickness focus. Microb. Ecol. 62, 632–643. doi: 10.1007/s00248-011-9830-y
Geiger, A., Ravel, S., Mateille, T., Janelle, J., Patrel, D., Cuny, G., and et al. (2007). Vector competence of Glossina palpalis gambiensis for Trypanosoma brucei s.l. and genetic diversity of the symbiont Sodalis glossinidius. Mol. Biol. Evol. 24, 102–109. doi: 10.1093/molbev/msl135
Gilboa-Garber, N. (1972). Purification and properties of haemagglutinin from Pseudomonas aeruginosa and its reaction with human blood cells. Biochim. Biophys. Acta 273, 165–173. doi: 10.1016/0304-4165(72)90204-8
Gonzalez-Ceron, L., Santillan, F., Rodriguez, M. H., Mendez, D., and Hernandez-Avila, J. E. (2003). Bacteria in midguts of field-collected Anopheles albimanus block Plasmodium vivax sporogonic development. J. Med. Entomol. 40, 371–374. doi: 10.1603/0022-2585-40.3.371
Hammes, W., and Hertel, C. (2006). “The genera Lactobacillus and Carnobacterium,” in The Prokaryotes, eds M. Dworkin, S. Falkow, E. Rosenberg, K.-H. Schleifer, and E. Stackebrandt (New York, NY: Springer), 320–403. doi: 10.1007/0-387-30744-3_10
Hertle, R., Hilger, M., Weingardt-Kocher, S., and Walev, I. (1999). Cytotoxic action of Serratia marcescens hemolysin on human epithelial cells. Infect. Immun. 67, 817–825.
Hongoh, Y., Deevong, P., Inoue, T., Moriya, S., Trakulnaleamsai, S., Ohkuma, M., and et al. (2005). Intra- and interspecific comparisons of bacterial diversity and community structure support coevolution of gut microbiota and termite host. Appl. Environ. Microbiol. 71, 6590–6599. doi: 10.1128/AEM.71.11.6590-6599.2005
Jackson, T. J., Wang, H. Y., Nugent, M. J., Griffin, C. T., Burnell, A. M., and Dowds, B. C. A. (1995). Isolation of insect pathogenic bacteria, Providencia rettgeri, from Heterorhabditis spp. J. Appl. Bacteriol. 78, 237–244. doi: 10.1111/j.1365-2672.1995.tb05022.x
Jeyaprakash, A., Hoy, M. A., and Allsopp, M. H. (2003). Bacterial diversity in worker adults of Apis mellifera capensis and Apis mellifera scutellata (Insecta: Hymenoptera) assessed using 16S rRNA sequences. J. Invertebr. Pathol. 84, 96–103. doi: 10.1016/j.jip.2003.08.007
Kaaya, G. P., and Darji, N. (1989). Mortality in adult tsetse, Glossina morsitans morsitans, caused by entomopathogenic bacteria. J. Invertebr. Pathol. 54, 32–38. doi: 10.1016/0022-2011(89)90136-5
Lacey, L. A. (ed.). (1997). Manual of Techniques in Insect Pathology. San Diego, CA: Academic Press.
Lazaro, J. E., Nitcheu, J., Predicala, R. Z., Mangalindan, G. C., Nesslany, F., Marzin, D., and et al. (2002). Heptyl prodigiosin, a bacterial metabolite is antimalarial in vivo and non-mutagenic in vitro. J. Nat. Toxins 11, 367–377.
Lindh, J. M., and Lehane, M. J. (2011). The tsetse fly Glossina fuscipes fuscipes (Diptera: Glossina) harbours a surprising diversity of bacteria other than symbionts. Antonie Van Leeuwenhoek 99, 711–720. doi: 10.1007/s10482-010-9546-x
Lysyk, T. J., Kalischuk-Tymensen, L., Selinger, L. B., Lancaster, R. C., Wever, L., and Cheng, K. J. (1999). Rearing stable fly larvae (Diptera: Muscidae) on an egg yolk medium. J. Med. Entomol. 36, 382–388.
MacLeod, E. T., Maudlin, I., Darby, A. C., and Welburn, S. C. (2007). Antioxidants promote establishment of trypanosome infections in tsetse. Parasitology 134, 827–831. doi: 10.1017/S0031182007002247
Maeda, H., and Morihara, K. (1995). Serralysin and related proteinases. Methods Enzymol. 248, 395–413. doi: 10.1016/0076-6879(95)48026-9
Maidak, B. L., Cole, J. R., Lilburn, T. G., Parker, C. T. Jr. Saxman, P. R., Farris, R. J., and et al. (2001). The RDP-II (Ribosomal Database Project). Nucleic Acids Res. 29, 173–174.
Mercado, T. I., and Colon-Whitt, A. (1982). Lysis of Trypanosoma cruzi by Pseudomonas fluorescens. Antimicrob. Agents Chemother. 22, 1051–1057. doi: 10.1128/AAC.22.6.1051
Montaner, B., and Perez-Tomas, R. (2003). The prodigiosins: a new family of anticancer drugs. Curr. Cancer Drug Targets 3, 57–65. doi: 10.2174/1568009033333772
O'Neill, S. L., Gooding, R. H., and Aksoy, S. (1993). Phylogenetically distant symbiotic microorganisms reside in Glossina midgut and ovary tissues. Med. Vet. Entomol. 7, 377–383.
Poinar, Jr. G. O. Wassink, H. J., Leegwater-van der Linden, M. E., and van der Geest, L. P. (1979). Serratia marcescens as a pathogen of tsetse flies. Acta Trop. 36, 223–227.
Pumpuni, C. B., Beier, M. S., Nataro, J. P., Guers, L. D., and Davis, J. R. (1993). Plasmodium falciparum: inhibition of sporogonic development in Anopheles stephensi by gram-negative bacteria. Exp. Parasitol. 77, 195–199. doi: 10.1006/expr.1993.1076
Pumpuni, C. B., DeMaio, J., Kent, M., Davis, J. R., and Beier, J. C. (1996). Bacterial population dynamics in three anopheline species: the impact on Plasmodium sporogonic development. Am. J. Trop. Med. Hyg. 54, 214–218.
Schalk, I. J., Abdallah, M. A., and Pattus, F. (2002). Recycling of pyoverdin on the FpvA receptor after ferric pyoverdin uptake and dissociation in Pseudomonas aeruginosa. Biochemistry 41, 1663–1671. doi: 10.1021/bi0157767
Schlein, Y., Polacheck, I., and Yuval, B. (1985). Mycoses, bacterial infections and antibacterial activity in sandflies (Psychodidae) and their possible role in the transmission of leishmaniasis. Parasitology 90, 57–66. doi: 10.1017/S0031182000049015
Schmitt-Wagner, D., Friedrich, M. W., Wagner, B., and Brune, A. (2003). Phylogenetic diversity, abundance, and axial distribution of bacteria in the intestinal tract of two soil-feeding termites (Cubitermes spp.). Appl. Environ. Microbiol. 69, 6007–6017. doi: 10.1128/AEM.69.10.6007-6017.2003
Simarro, P. P., Diarra, A., Ruiz Postigo, J. A., Franco, J. R., and Jannin, J. G. (2011). The human African trypanosomiasis control and surveillance programme of the World Health Organization 2000-2009: the way forward. PLoS Negl. Trop. Dis. 5:e1007. doi: 10.1371/journal.pntd.0001007
Simo, G., Njiokou, F., Mbida Mbida, J. A., Njitchouang, G. R., Herder, S., Asonganyi, T., and et al. (2008). Tsetse fly host preference from sleeping sickness foci in Cameroon: epidemiological implications. Infect. Genet. Evol. 8, 34–39. doi: 10.1016/j.meegid.2007.09.005
Steinhaus, E. A. (1960). The importance of environmental factors in the insect-microbe ecosystem. Bacteriol. Rev. 24, 365–373.
Straif, S. C., Mbogo, C. N. M., Toure, A. M., Walker, E. D., Kaufman, M., Toure, Y. T., and et al. (1998). Midgut bacteria in Anopheles gambiae and An. funestus (Diptera: Culicidae) from Kenya and Mali. J. Med. Entomol. 35, 222–226.
Thomson, N. R., Crow, M. A., McGowan, S. J., Cox, A., and Salmond, G. P. C. (2000). Biosynthesis of carbapenem antibiotic and prodigiosin pigment in Serratia is under quorum sensing control. Mol. Microbiol. 36, 539–556. doi: 10.1046/j.1365-2958.2000.01872.x
Van Den Abbeele, J., Claes, Y., Bockstaele, D., Ray, D., and Coosemans, M. (1999). Trypanosoma brucei spp. development in the tsetse fly: characterization of the post-mesocyclic stages in the foregut and proboscis. Parasitology 118, 469–478. doi: 10.1017/S0031182099004217
Vickerman, K., Tetley, L., Hendry, A., and Turner, C. M. (1988). Biology of African trypanosomes in the tsetse fly. Biol. Cell. 64, 109–119. doi: 10.1016/0248-4900(88)90070-6
Wang, J., Brelsfoard, C., Wu, Y., and Aksoy, S. (2012). Intercommunity effects on microbiome and GpSGHV density regulation in tsetse flies. J. Invertebr. Pathol. 112, S32–S39. doi: 10.1016/j.jip.2012.03.028
Wang, J., Wu, Y., Yang, G., and Aksoy, S. (2009). Interactions between mutualist Wigglesworthia and tsetse peptidoglycan recognition protein (PGRP-LB) influence trypanosome transmission. Proc. Natl. Acad. Sci. U.S.A. 106, 12133–12138. doi: 10.1073/pnas.0901226106
Weiss, B., and Aksoy, S. (2011). Microbiome influences on insect host vector competence. Trends Parasitol. 27, 514–522. doi: 10.1016/j.pt.2011.05.001
Weiss, B. L., Maltz, M., and Aksoy, S. (2012). Obligate symbionts activate immune system development in the tsetse fly. J. Immunol. 188, 3395–3403. doi: 10.4049/jimmunol.1103691
Weiss, B. L., Wang, J., and Aksoy, S. (2011). Tsetse immune system maturation requires the presence of obligate symbionts in larvae. PLoS Biol. 9:e1000619. doi: 10.1371/journal.pbio.1000619
Weiss, B. L., Wang, J., Maltz, M. A., Wu, Y., and Aksoy, S. (2013). Trypanosome infection establishment in the tsetse fly gut is influenced by microbiome-regulated host immune barriers. PLoS Pathog. 9:e1003318. doi: 10.1371/journal.ppat.1003318
Welburn, S. C., and Maudlin, I. (1999). Tsetse-trypanosome interactions: rites of passage. Parasitol. Today 15, 399–403. doi: 10.1016/S0169-4758(99)01512-4
Keywords: human African trypanosomiasis, bacteriome, trypanosome, tsetse flies, interactions
Citation: Geiger A, Fardeau M-L, Njiokou F and Ollivier B (2013) Glossina spp. gut bacterial flora and their putative role in fly-hosted trypanosome development. Front. Cell. Infect. Microbiol. 3:34. doi: 10.3389/fcimb.2013.00034
Received: 29 April 2013; Accepted: 08 July 2013;
Published online: 24 July 2013.
Edited by:
Brice Rotureau, Institut Pasteur, FranceReviewed by:
Laura Runyen-Janecky, University of Richmond, USAFrederic S. Landmann, University of California Santa Cruz, USA
Rita Rio, West Virginia University, USA
Copyright © 2013 Geiger, Fardeau, Njiokou and Ollivier. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in other forums, provided the original authors and source are credited and subject to any copyright notices concerning any third-party graphics etc.
*Correspondence: Anne Geiger, UMR 177 InterTryp, IRD-CIRAD, CIRAD TA A-17/G, Campus International de Baillarguet, 34398 Montpellier Cedex 5, France e-mail: anne.geiger@ird.fr