High-throughput screening of tick-borne pathogens in Europe
- 1UMR BIPAR, Animal Health Laboratory, ANSES, Maisons-Alfort, France
- 2IdentyPath Platform, Food Safety Laboratory, ANSES, Maisons-Alfort, France
- 3Nancy Laboratory for Rabies and Wildlife, Wildlife EcoEPIdemiology and Surveillance Unit, ANSES, Malzéville, France
- 4Department of Bacteriology, National Veterinary Institute (SVA), Uppsala, Sweden
- 5Department of Virology, Immunobiology and Parasitology, National Veterinary Institute (SVA), Uppsala, Sweden
- 6Department of Infection Biology, Central Veterinary Institute, Wageningen UR, Lelystad, Netherlands
- 7Laboratory for Zoonoses and Environmental Microbiology, National Institute for Public Health and Environment (RIVM), Bilthoven, Netherlands
- 8National Veterinary Institute, DTU, Copenhagen, Denmark
Due to increased travel, climatic, and environmental changes, the incidence of tick-borne disease in both humans and animals is increasing throughout Europe. Therefore, extended surveillance tools are desirable. To accurately screen tick-borne pathogens (TBPs), a large scale epidemiological study was conducted on 7050 Ixodes ricinus nymphs collected from France, Denmark, and the Netherlands using a powerful new high-throughput approach. This advanced methodology permitted the simultaneous detection of 25 bacterial, and 12 parasitic species (including; Borrelia, Anaplasma, Ehrlichia, Rickettsia, Bartonella, Candidatus Neoehrlichia, Coxiella, Francisella, Babesia, and Theileria genus) across 94 samples. We successfully determined the prevalence of expected (Borrelia burgdorferi sensu lato, Anaplasma phagocytophilum, Rickettsia helvetica, Candidatus Neoehrlichia mikurensis, Babesia divergens, Babesia venatorum), unexpected (Borrelia miyamotoi), and rare (Bartonella henselae) pathogens in the three European countries. Moreover we detected Borrelia spielmanii, Borrelia miyamotoi, Babesia divergens, and Babesia venatorum for the first time in Danish ticks. This surveillance method represents a major improvement in epidemiological studies, able to facilitate comprehensive testing of TBPs, and which can also be customized to monitor emerging diseases.
Introduction
In Europe, ticks are the most important vectors of human and animal infectious diseases, and transmit more pathogens than any other arthropod (Jongejan and Uilenberg, 2004; Colwell et al., 2011). These diseases are normally maintained in stable natural cycles involving ticks, wildlife, and/or domestic animals, whereas humans are accidental hosts (De La Fuente et al., 2008). Ixodes ricinus is the most widespread and abundant European tick species capable of transmitting several diseases of both medical and veterinary importance (Heyman et al., 2010). Ixodes ricinus has the greatest impact on human public health by transmitting Lyme borreliosis etiological agents, caused by at least four Borrelia genospecies in Europe: Borrelia burgdorferi sensu stricto, Borrelia garinii, Borrelia afzelii, and Borrelia spielmanii. The relapsing fever spirochete, Borrelia miyamotoi, is transmitted by the same Ixodes species and has recently been described in ticks as well as in a human case from the Netherlands (Hovius et al., 2013). In addition to Borrelia transmission, Ixodes ricinus can transmit many other pathogens, including: Anaplasma spp. such as Anaplasma phagocytophilum, Rickettsia spp. from the spotted fever group, Candidatus Neoehrlichia mikurensis, Ehrlichia spp., Bartonella spp., Francisella tularensis, and Coxiella burnetii (Parola and Raoult, 2001; Cotte et al., 2008; Fertner et al., 2012). Ticks can also transmit Babesia genus protozoa, such as Babesia divergens or the newly described Babesia venatorum (sp. EU1) and Theileria spp. (Bishop et al., 2004; Bonnet et al., 2007a).
Increased human travel, animal transport, and environmental changes are responsible for the emergence and/or spread of numerous tick-borne pathogens (TBPs) in Europe (Dantas-Torres et al., 2012). Therefore, effective tick-based surveillance is essential for monitoring human and/or animal disease emergence (Diuk-Wasser et al., 2014). Ticks harbor a variety of pathogens, some of which are obligate intracellular organisms and/or are impossible to artificially culture. Consequently, molecular approaches are thus indispensable for TBP identification. In conventional amplification-based assays, TBP detection occurs for a restricted number of target pathogens known to be transmitted by certain tick species collected at particular sites (Cotte et al., 2010). The main disadvantage of this approach is the limited number of different targets that can be tested, given the quantity of DNA required for one PCR. To improve surveillance of human and animal diseases, new investigative tools are required which perform high-throughput testing of a wider panel of TBPs.
Therefore, the aim of this study was to conduct high-throughput monitoring of tick-borne human and animal pathogens in Europe. Accordingly, we developed a novel high-throughput epidemiological surveillance method to identify both major and neglected European TBPs (bacteria and parasites). This tool utilizes a microfluidic system (BioMark™ dynamic array system, Fluidigm) that is capable of performing parallel real-time PCRs using either 96.96 chips or 48.48 chips resulting in either 9216 or 2304 individual reactions, respectively (Liu et al., 2003). In a single experiment, 94 ticks or pools of ticks can be tested for the presence of 25 bacteria and 12 parasites, as well as confirmation of the tick species. As only a few microliters of sample are required for each test, this system can also be used in conjuction with the typically low-volume DNA extracts prepared from ticks. Then we applied this method to screen 7050 Ixodes ricinus collected from three European countries; France, Denmark, and the Netherlands. We demonstrated increased surveillance efficiency of major and neglected TBPs, and improved monitoring of the emerging diseases important to public and animal health.
Materials and Methods
Study Area and Tick Collection
A total of 7050 Ixodes ricinus nymphs, from six different locations in France, Denmark, and the Netherlands, divided in 47 pools of 25 nymphs per site, were studied. Questing nymphs were collected using the flagging technique (Vassallo et al., 2000). In France, ticks were collected from Murbach (F1) (N 47° 55′, E 7° 9′) and Wasselonne (F2) (N 48° 37′, E 7° 27′) in 2011. In Denmark, ticks were collected from Vestskoven (D1) (N 55° 42′, E 12° 21′) and Grib Skov (D2) (N 56° 02′, E 12° 20′) in 2012. In the Netherlands, ticks were collected from the Duin en Kruidberg area (N1) (N 52° 17′, E 4° 49′) in 2010 and 2011, and from the Austerlitz area (N2) (N 52° 5′, E 5° 18′) over a period from 2008 to 2012.
DNA Extraction
Ticks were morphologically identified to species level (Pérez-Eid, 2007) and preserved at −80°C. After washing once in 70% ethanol for 5 min and twice in distilled water for 5 min, pools of 25 nymphs were crushed in 300 μl of DMEM with 10% fetal calf serum and six steel balls using the homogenizer Precellys®24 Dual (Bertin, France) at 5500 rpm for 20 s.
DNA was then extracted using the Wizard genomic DNA purification kit (Promega, France). Total DNA per sample was eluted in 50 μl of rehydration solution and stored at −20°C until further use.
Primers and Probe Design
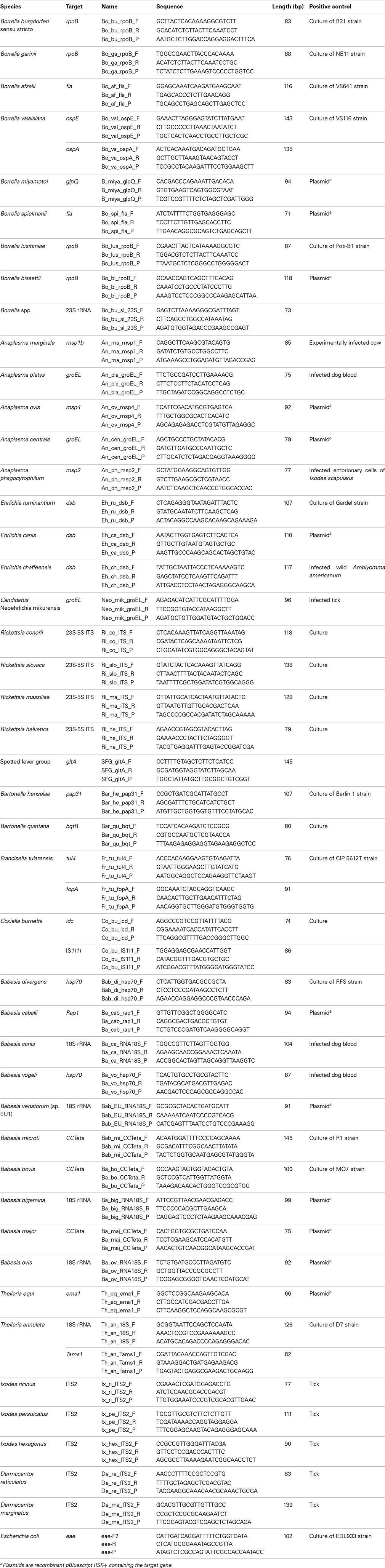
Pathogens, targeted genes and primers/probe sets are listed in Table 1. For each pathogen or tick, primers and probes were specifically designed for this study. Each primer or probe set was validated on dilution range of several positive controls (Table 1) and real-time TaqMan PCRs on a LightCycler® 480 (LC480) (Roche Applied Science, Germany). Real-time PCR assays were performed in a final volume of 12 μl using the LightCycler® 480 Probe Master Mix 1× (Roche Applied Science, Germany), with primers and probes at 200 nM and 2 μl of control DNA. Thermal cycling conditions were as follows: 95°C for 5 min, 45 cycles at 95°C for 10 s and 60°C for 15 s and one final cooling cycle at 40°C for 10 s. Four pathogens (Borrelia valaisiana, Francisella tularensis, Coxiella burnetii, and Theileria annulata) were targeted by real-time PCRs on two different sequences to improve detection.
DNA Pre-Amplification
For DNA pre-amplification, the TaqMan PreAmp Master Mix (Applied Biosystems, France) was used according to the manufacturer's instructions. Primers (except those which target tick DNA) were pooled combining equal volume of primers (200 nM final each). The reaction was performed in a final volume of 5 μ l containing 2.5 μ l TaqMan PreAmp Master Mix, 1.2 μ l pooled primers mix and 1.3 μ l DNA, with one cycle at 95°C for 10 min, 14 cycles at 95°C for 15 s and 4 min at 60°C. At the end of the cycling program the reactions were diluted 1:10. Pre-amplified DNAs were stored at −20°C until needed.
High-Throughput Real-Time PCR System
The BioMark™ real-time PCR system (Fluidigm, USA) was used for high-throughput microfluidic real-time PCR amplification using either the 96.96 or the 48.48 dynamic arrays (Fluidigm). These chips dispense 96 (or 48) PCR mixes and 96 (or 48) samples into individual wells, after which on-chip microfluidics assemble PCR reactions in individual chambers prior to thermal cycling resulting in either 9216 or 2304 individual reactions.
Amplifications were performed using 6-carboxyfluorescein (FAM)- and black hole quencher (BHQ1)-labeled TaqMan probes with TaqMan Gene expression master mix in accordance with manufacturer's instructions (Applied Biosystems, France). A 6 μ l sample mix was prepared per sample, containing 3 μl TaqMan® Gene expression Master Mix (Applied Biosystems, Foster City, CA), 0.3 μl sample Loading Reagent (Fluidigm PN 85000746) and 2.7 μl of diluted pre-amplified DNA. A TaqMan® primer assay was prepared for each target, containing 18 μM of each primer and 4 μM of probe. Three microliters of these primer assays were mixed with equal volumes of Dynamic Array (DA) assay loading reagent (Fluidigm PN 85000736) to make assay mixes (9 μ M primers and 2 μ M probe). Prior to loading the samples and assay mixes into the inlets, the chip was primed in the IFC Controller HX apparatus. Five μl of sample mixes, prepared as described, were then loaded into each sample inlet of the dynamic array chip and 5 μ l of assay mixes were loaded into assay inlets. The chip was then placed on the IFC Controller HX for loading and mixing. After approximately 45 min the chip was ready for thermal cycling and detection of the reaction products on the Biomark. PCR cycling comprised of 2 min at 50°C, 10 min at 95°C, followed by 40 cycles of 2-step amplification of 15 s at 95°C, and 1 min at 60°C. Data were acquired on the BioMark™ Real-Time PCR System and analyzed using the Fluidigm Real-time PCR Analysis software to obtain crossing point (CP) values.
For microfluidic tool evaluation on field samples, the assays were performed in duplicate. Two negative water controls were included per chip. Ixodes ricinus DNA served to confirm the tested tick species and as a DNA extraction control. To determine if factors present in the sample could inhibit the PCR, Escherichia coli strain EDL933 DNA was added to each sample as an internal inhibition control. Primers and probe specific for the E. coli eae gene (Nielsen and Andersen, 2003) were used for an internal control.
Validation of the Results by PCR and Sequencing
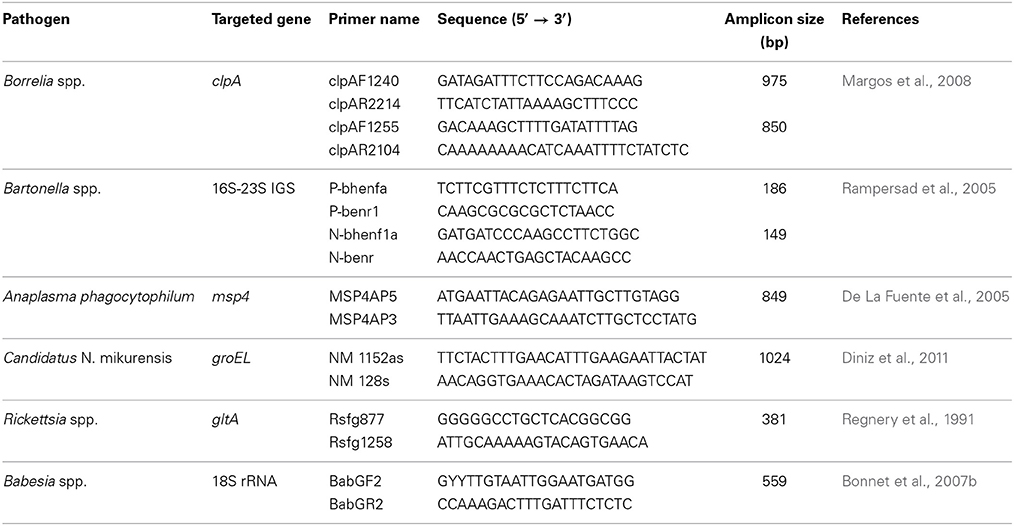
Conventional PCR using primers targeting different genes or regions than those of the BioMark™ system (Table 2), were used to confirm the presence of pathogenic DNA in the field samples. Amplicons were sequenced by Eurofins MWG Operon (Germany), and then assembled using BioEdit software (Ibis Biosciences, Carlsbad). An online BLAST (National Center for Biotechnology Information) was used to compare results with published sequences listed in GenBank sequence databases.
Prevalence Estimation
Prevalences were estimated assuming perfect sensitivity and specificity of pathogen detection using the online statistical program “Pooled prevalence for fixed pool size and perfect test” Method 2 (AusVet Animal Health Service http://epitools.ausvet.com.au/content.php?page=home). Point estimates were based on the maximum likelihood method developed by Kline et al. (1989). Exact 95% confidence intervals were obtained by assuming binomial distribution for the number of positive pools (Cowling et al., 1999). If all pools were positive, prevalence was recorded as >14.3%, as the highest prevalence that can be distinguished from 100% when testing 47 pools of 25 ticks. If all pools were negative, prevalence was recorded as <0.25%, since the 95% probability of sampling n negative ticks from a population with prevalence p is given as (1 − p)n.
Results
Implementation of High-Throughput Real-Time PCR System to Detect TBPs
Primers and probes were specially designed to detect 37 TBPs and 4 tick species (Table 1). Each set of primers and probes specifically identified their corresponding positive control samples via Taqman real-time PCRs on a LightCycler 480 apparatus. Resulting CP values varied from 8 to 40 depending on sample type. Among the 37 TBP DNAs used as positive controls, 10 were not detected by the BioMark™ system. Consequently, an initial step of DNA pre-amplification was added, which enabled detection of all positive controls. Subsequently all tick DNA samples were pre-amplified prior to pathogen detection on the BioMark™ system. The specificity of each primer set was then evaluated using 37 TBPs, and 4 tick species positive controls (Figure 1). Results demonstrated high specificity for each primers/probe set after pre-amplification, using a cut-off of 30 CP (Figure 1). Indeed, 45 assays were only positive for the corresponding positive control. Three assays showed cross-reactivity with other pathogen targets. The assay for B. burgdorferi sensu stricto cross-reacted with B. garinii and B. valaisiana DNA. The assay targeting R. conorii cross-reacted with R. massiliae, as well as with R. slovaca DNA, cross-reactivity was also observed reciprocally.
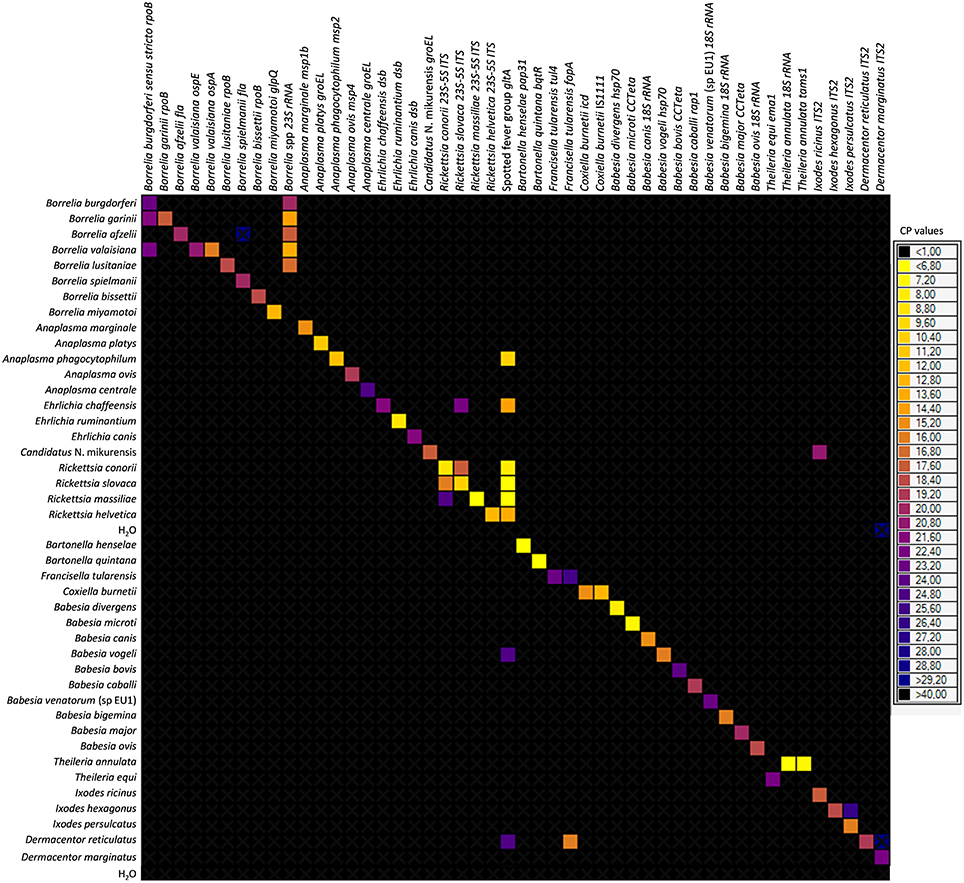
Figure 1. BioMark™ dynamic array system specificity test (48.48 chip). Each square corresponds to a single real-time PCR reaction, where rows indicate the pathogen in the positive control and columns represent the targets of each primers/probe set. CP values for each reaction are indicated by color; the corresponding color scale is presented in the legend on the right. The darkest shade of blue and black squares are considered as negative reactions with CP > 30.
Large Scale Prevalence Study of TBPs
A total of 7050 nymphs, in 47 pools of 25, from six different European sites were tested using the BioMark™ system. Among the targeted pathogens, 15 bacteria (B. lusitaniae, B. bissettii, A. marginale, A. platys, A. ovis, A. centrale, E. ruminantium, E. canis, E. chaffeensis, R. conorii, R. slovaca, R. massiliae, B. quintana, F. tularensis, and C. burnetii) and 10 parasites (B. caballi, B. canis, B. vogeli, B. microti, B. bovis, B. bigemina, B. major, B. ovis, T. equi, and T. annulata) were not detected in any country. The number of positive pools for each pathogen is presented in Table 3 and the prevalence was estimated at each site of collection (Table 4).
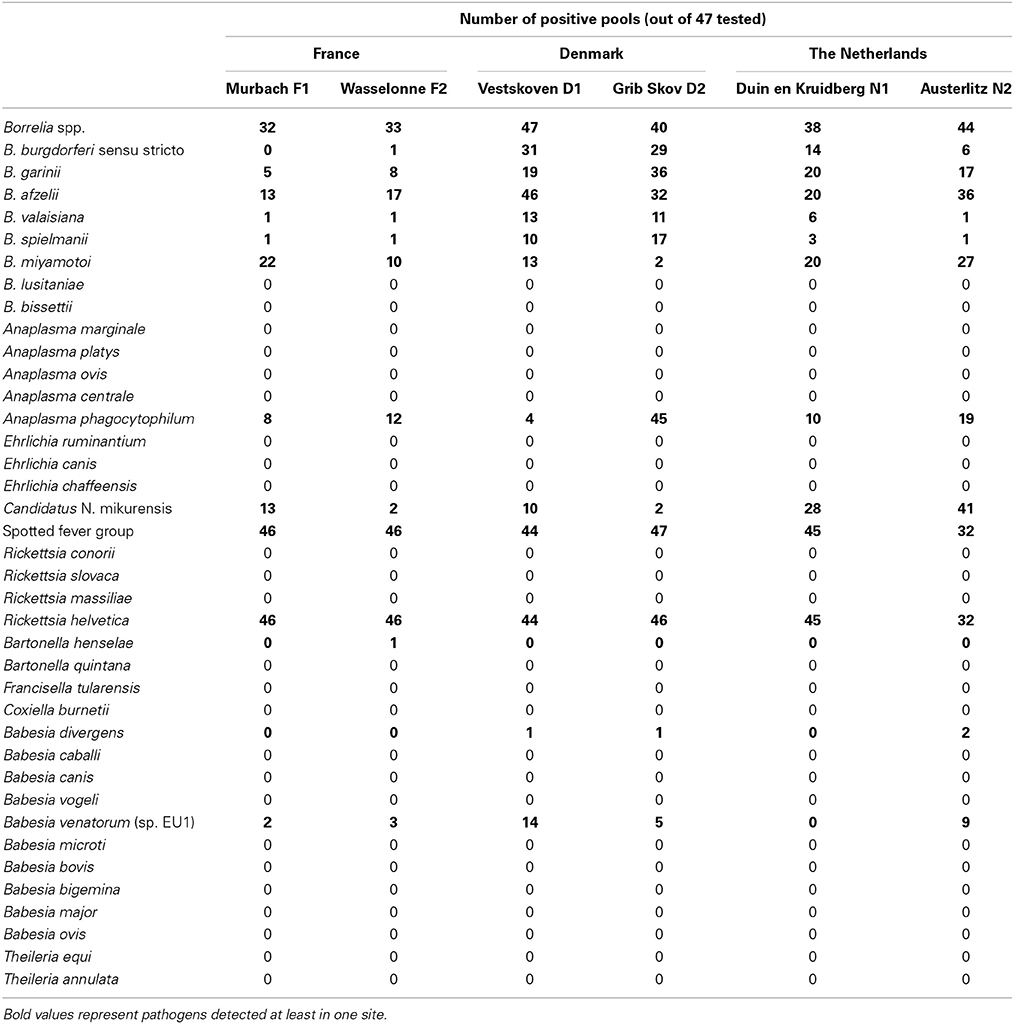
Table 3. Number of positive pools of ticks out of the 47 tested, for two sites in France, Denmark, and the Netherlands using the microfluidic tool (BioMark™ system).
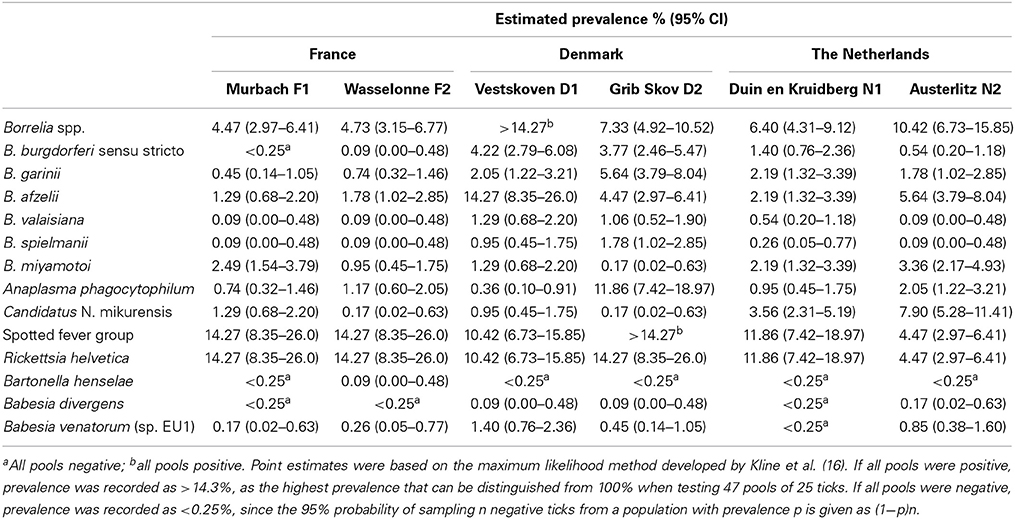
Table 4. Estimated prevalence of pathogens detected in Ixodes ricinus in France, Denmark, and the Netherlands.
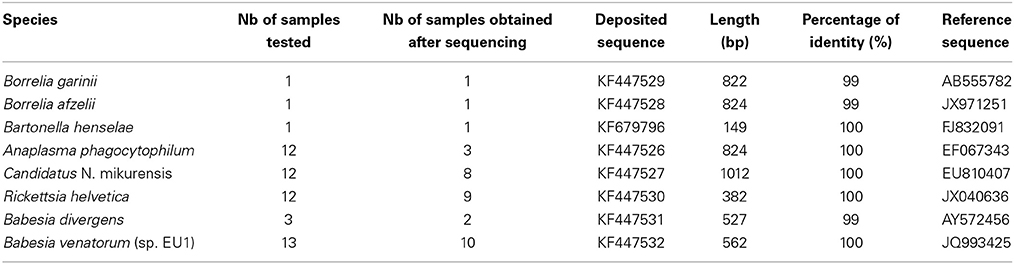
In order to confirm the results obtained on the BioMark™ system and to validate this new method, classical PCR and sequencing were performed on extracted DNA for a subset of field samples. All sequences showed at least 99% identity with reference sequences (Table 5), and have been deposited in GenBank (Accession numbers; KF447526-KF447532, and KF679796). Due to primers which can only detect Borrelia and Babesia at the genus level, only those samples which tested positive for a single species (and not potentially co-infected samples) were confirmed (Table 2).
France
Among the seven genospecies of Borrelia burgdorferi s.l., four were detected in both French sites. Borrelia afzelii is the dominant genospecies with a prevalence of 1.8% in F2, as previously described (Beytout et al., 2007). The other genospecies (B. garinii, B. valaisiana, and B. spielmanii) had prevalence rates of under 1%. B. burgdorferi s.s. was only detected in F2 at low prevalence (0.1%). The relapsing fever spirochete B. miyamotoi was detected in both sites, with very different prevalences (2.5% in F1 and 0.9% in F2) and was the most abundant Borrelia species in F1. This spirochete has already been detected in France, but only in female adult ticks (Reis et al., 2011). Anaplasma phagocytophilum was more abundant in F2 (1.2%) and its estimated prevalence is in accordance with a previous study (Beytout et al., 2007). Candidatus N. mikurensis was more abundant in F1 (1.3%). This pathogen has already been described in bank voles in France (Vayssier-Taussat et al., 2012) but this is the first estimation of its prevalence in French ticks. Rickettsia helvetica was the only Rickettsiaceae identified in this study and was detected in 46/47 pools, showing the highest prevalence (14.3%) of all tested pathogens, much higher than data reported in the literature (1.4–6%) (Cotte et al., 2010). Bartonella henselae was only detected in F2 (0.1%), in a single pool. Among all assessed parasitic species, Babesia venatorum was the only parasite detected in France with a low prevalence (0.2 and 0.3%) as previously described (Reis et al., 2011).
Denmark
Five genospecies of B. burgdorferi s.l. were detected in Danish ticks, four previously described (Skarphedinsson et al., 2007; Vennestrom et al., 2008) and one, B. spielmanii, detected for the first time. In previous studies, B. afzelii was the most prevalent genospecies (Skarphedinsson et al., 2007; Vennestrom et al., 2008). In our study, B. afzelii was the most prevalent (14.3%) in D1, while B. garinii was the most abundant (5.7%) in D2. B. burgdorferi s.s. was detected in both sites with similar prevalences (4.2% and 3.8%), as well as B. valaisiana, and B. spielmanii (approximately 1%). B. lusitaniae was identified in a previous study (Vennestrom et al., 2008), but was not encountered in the present study. Relapsing fever-causing B. miyamotoi was detected for the first time in Danish I. ricinus with variable prevalences between the two sites (1.3% in D1 and 0.2% in D2). The estimated prevalence of A. phagocytophilum was approximately 30 times higher in D2 (11.9%) than in D1 (0.4%) whereas its prevalence was estimated at 15% in a previous study (Skarphedinsson et al., 2007). Candidatus N. mikurensis was detected with a low prevalence in the Danish sites (1% in D1 and 0.2% in D2) in agreement with a previous report (Fertner et al., 2012). Rickettsia helvetica is the only species of Rickettsia spp. reported in Denmark. This bacterium was respectively identified in 44 and 46 pools of the samples, corresponding to high prevalences of 10.4% in D1 and 14.3% in D2. In previous reports, the prevalence of R. helvetica appeared to vary considerably, ranging from 1.4 to 13% (Svendsen et al., 2009; Kantso et al., 2010). Two parasitic species were found for the first time in the Danish samples, B. divergens (0.1% in D1 and D2) and B. venatorum (1.4% in D1 and 0.5% in D2). These parasites have never previously been reported in Danish ticks until now, even if B. divergens is frequently found in cattle.
The Netherlands
Five genospecies of B. burgdorferi s.l. were detected in Dutch ticks. B. garinii and B. afzelii were the more abundant genospecies while the other genospecies (B. burgdorferi, B. valaisiana, and B. spielmanii) were found less frequently, as previously described (Tijsse-Klasen et al., 2011; Sprong et al., 2012a). B. garinii and B. afzelii were detected with equal prevalences in N1 (2.2%) and variable prevalences in N2 (1.8 and 5.6%, respectively). The prevalence of B. burgdorferi s.s. was estimated at 1.4 and 0.5% in N1 and N2, respectively. B. valaisiana and B. spielmanii were identified in a single pool from the N2 site, but their prevalences were estimated at 0.5 and 0.3% in N1. In 2009, B. lusitaniae was described at one location (Sprong et al., 2012a), but was not encountered in the present study. The relapsing fever spirochete, B. miyamotoi, previously identified in the Netherlands in a human case of meningoencephalitis (Hovius et al., 2013), occurred in both Dutch sites and was most prevalent in N2 (3.4%). In N1, B. miyamotoi showed the same prevalence as B. garinii and B. afzelii (2.2%). Anaplasma phagocytophilum and Candidatus N. mikurensis were found in both sites with variable prevalences, both more abundant in N2 (2 and 7.9%, respectively). The estimated prevalence of R. helvetica was highly variable depending on the sites (11.9% in N1 and 4.5% in N2). These three bacteria are well recognized in Dutch ticks and have previously been reported in the Netherlands (Nijhof et al., 2007; Sprong et al., 2009; Tijsse-Klasen et al., 2011). Two parasitic species were found in Dutch ticks but were only observed in N2, B. divergens (0.2%) and B. venatorum (0.8%) with prevalence rates similar to previous reports (0.07 and 0.4% for B. divergens and 0.9 and 1.2% for B. venatorum) (Nijhof et al., 2007; Wielinga et al., 2009).
Discussion
In this study, we implemented a method using multiple primers/probe sets able to perform high-throughput detection of TBPs on an unprecedented scale. This large-scale investigation has (i) enabled the detection of rare pathogens such as Bartonella henselae and (ii) generated prevalence estimations for frequent, rare, or unexpected pathogens, thus creating a comprehensive overview of the epidemiological situation for 37 bacteria and parasites present in I. ricinus, in six European sites (two in France, Denmark, and the Netherlands).
Initial testing of the BioMark™ system showed that some pathogens could not be detected. Indeed, assessment was performed on positive DNA controls extracted from cultures, animal blood, ticks, or plasmids, therefore DNA quality and concentration were highly variable between samples. An initial step of pre-amplification was therefore added to specifically amplify targeted pathogen sequences. Regarding the three non-specific assays, two hypotheses can be made: either lack of specificity or potential co-infection of the DNA samples. As positive controls were isolated from pure bacterial cultures, only non-specific cross-reaction explains the lack of specificity. The set of primers and probe designed against B. burgdorferi s.s. cross-reacted with B. garinii and B. valaisiana. However, this cross-reaction did not occur for every field sample. Cross-reactions were also observed between R. conorii and R. slovaca. There was a difference of approximately 10 cycles between the CP values for the expected Rickettsia species and the cross-reacting species. It will be interesting to test both sets of primers and probe on DNA extracted from ticks uniquely infected with each of the Rickettsia. However, this issue is not likely to arise with field samples, as R. slovaca is transmitted by Dermacentor marginatus and R. conorii by Rhipicephalus sanguineus. In conclusion, the primers and probe sets for B. burgdorferi s.s., R. conorii, and R. slovaca need further optimization, so the current results obtained for these species should be interpreted with care. Several of the targeted pathogens cannot be cultured, or are rare and consequently unavailable from field samples, therefore plasmids containing target sequences were used as positive controls. For these pathogens and associated primers/probe sets, further evaluation of specificity is required. This tool was developed for epidemiologic rather than diagnostic purposes, therefore detection limits and sensitivity have not been experimentally determined. These experiments are somewhat difficult to implement and require a gold standard for each pathogen and consistent positive controls, which are not available for all TBPs.
Two sites per country were studied for the field investigation. The technique permitted the detection of 10 bacterial species; B. burgdorferi s.s., B. garinii, B. afzelii, B. valaisiana, B. spielmanii, B. miyamotoi, A. phagocytophilum, Candidatus N. mikurensis, R. helvetica, B. henselae, and two parasitic species; B. divergens, and B. venatorum, with variable prevalences according to the site of collection. Taken together, the estimated prevalences for all pathogens obtained on pools of 25 nymphs in this study are mostly consistent with European published data. For future studies, it will be fascinating to investigate smaller nymph pools to obtain more accurate estimations of TBP prevalences. The prevalence of B. miyamotoi is reported for the first time in Denmark at two sites and is quite similar between the three European countries in our study. Borrelia miyamotoi is transmitted by the same Ixodes species as the etiologic agents of European Lyme borreliosis, and has been detected in Ixodes ticks in Europe (Richter et al., 2003). Up until now no human cases have been reported in France or Denmark, but our data and the recent case of human infection described in the Netherlands (Hovius et al., 2013) suggest that surveillance needs to be improved. Candidatus N. mikurensis was detected in all three countries, with the highest prevalence in the Netherlands. Several human cases have been reported over the past decade in Europe (Maurer et al., 2013). However, clinical symptoms are not pathognomonic, suggesting the existence of unreported cases due to reduced awareness of symptoms by public health professionals (Jahfari et al., 2012). As this emerging human pathogen is widespread in Europe, it requires careful monitoring. Rickettsia helvetica was described as the most prevalent pathogen in all three countries. Even if its pathogenicity remains unclear, R. helvetica has been implicated in the development of fatal perimyocarditis (Sprong et al., 2009). Isolation of the bacterium from a patient is needed to definitely confirm R. helvetica as a human pathogen; however, R. helvetica already represents an excellent candidate for future emergence (Parola, 2004). Over the last few years, I. ricinus has been identified as a competent vector for Bartonella henselae (Cotte et al., 2008). Little data are available on its prevalence in ticks; and it has been estimated at between 11 and 40% in Europe (Dietrich et al., 2010). Bartonella henselae has never been reported in Danish ticks, but two variant types were detected in cats and mice (Engbaek and Lawson, 2004). Its presence in French ticks could be linked to the presence of wild cats in eastern France compared to the other countries. Babesiosis can be a variable but potentially severe disease, and is best known as an animal affliction. However, increasing numbers of human cases have refocused epidemiological attention on this emerging zoonosis (Hildebrandt et al., 2013). Our study demonstrates that these hemoparasites are widely present in European ticks, and were observed for the first time in Danish ticks. Babesia microti was not encountered in this study but has previously been detected in the Netherlands at a prevalence ranging from 0.1 to 9% (Wielinga et al., 2009; Tijsse-Klasen et al., 2011).
Interestingly, among the targeted pathogens, 15 bacterial species and 10 parasitic species were not detected in any country, leading us to conclude that they are not present in I. ricinus from those European sites. Indeed, these TBPs are either very rare (Parola and Raoult, 2001; Sprong et al., 2012b) or have never been previously detected in the sampled regions, or are transmitted by other stage or other tick species (Parola and Raoult, 2001). Francisella tularensis and Coxiella burnetii are linked to important human and veterinary public health problems that require surveillance (Sprong et al., 2012b; Carvalho et al., 2014); however, the role of ticks in the transmission of these pathogens is nonetheless debated. Their apparent absence across the three European countries in I. ricinus ticks suggests that the risk of acquiring tularemia or Q fever from questing ticks could be negligible.
This new screening approach based on microfluidic systems allowing multiple parallel real-time PCRs, is a powerful tool for TBP surveillance in Europe. This study demonstrates the technique's capacity for large-scale studies utilizing the unique ability to simultaneously analyze large numbers of samples and multiple target pathogens. As demonstrated for babesiosis, vector surveillance could be very useful for monitoring disease emergence (Diuk-Wasser et al., 2014). Compared to an array with fixed panels of probes, this new tool presents the major advantage that it can be easily adapted to new situations, as it is entirely possible to add or remove primers/probe sets in order to modify the panel of targeted pathogens and tick species. Further studies will indeed confirm if this approach heralds the necessary breakthrough in epidemiological surveillance of vector-borne pathogens, broadening the monitoring of human and animal diseases.
In conclusion, our study clearly demonstrates the utility of a fast tool that allows comprehensive testing of high numbers of TBPs in ticks, and can be easily customized to fit regional demands or to screen tick or host samples for new or emerging diseases.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This project was funded by CoVetLab grant (ANSES, SVA, CVI, DTU), ANSES grant and by Animal Health Department grant from INRA. This project was done under the framework of EurNegVec COST Action TD1303. We are grateful to Muriel Vayssier-Taussat and Sarah Bonnet for kindly reviewing this paper and their valuable comments. We also thank all the colleagues who provided DNA positive controls: Nathalie Boulanger and Benoît Jaulhac, National Reference Center for Borrelia, France; Lise Gern, University of Neuchâtel, Switzerland; Jose de la Fuente and Isabel Garcia Fernandez de Mera, IREC, Spain; Snorre Stuen, Norwegian School of Veterinary Science, Norway; Henri-Jean Boulouis, National Veterinary School of Alfort, France; Damien Meyer, CIRAD/INRA, France; Michael L. Levin and Galina Zemtsova, CDC, USA; Lars Råberg, Lund University, Sweden; Pierre-Edouard Fournier and Didier Raoult, National Reference Center for Rickettsia, Aix-Marseilles University, France; Nora Madani, ANSES, France; Elodie Rousset, ANSES, France; Laurence Malandrin, ONIRIS, France; Jeune Equipe “Hémopathogènes vectorisés,” University of Lyon - VetAgro Sup, France; Emmanuel Cornillot, University of Montpellier, France; Huseyin Bilgin Bilgic, University of Adnan Menderes, Turkey; Dirk Dobbelaere and Isabel Hostettler, University of Bern, Switzerland; Anu Jaaskelainen, University of Helsinki, Finland.
References
Beytout, J., George, J. C., Malaval, J., Garnier, M., Beytout, M., Baranton, G., et al. (2007). Lyme borreliosis incidence in two French departments: correlation with infection of Ixodes ricinus ticks by Borrelia burgdorferi sensu lato. Vector Borne Zoonotic Dis. 7, 507–517. doi: 10.1089/vbz.2006.0633
Bishop, R., Musoke, A., Morzaria, S., Gardner, M., and Nene, V. (2004). Theileria: intracellular protozoan parasites of wild and domestic ruminants transmitted by ixodid ticks. Parasitology 129(Suppl.), S271–S283. doi: 10.1017/S0031182003004748
Bonnet, S., Jouglin, M., L'hostis, M., and Chauvin, A. (2007a). Babesia sp. EU1 from roe deer and transmission within Ixodes ricinus. Emerg. Infect. Dis. 13, 1208–1210. doi: 10.3201/eid1308.061560
Bonnet, S., Jouglin, M., Malandrin, L., Becker, C., Agoulon, A., L'hostis, M., et al. (2007b). Transstadial and transovarial persistence of Babesia divergens DNA in Ixodes ricinus ticks fed on infected blood in a new skin-feeding technique. Parasitology 134, 197–207. doi: 10.1017/S0031182006001545
Carvalho, C. L., Lopes De Carvalho, I., Ze-Ze, L., Nuncio, M. S., and Duarte, E. L. (2014). Tularaemia: a challenging zoonosis. Comp. Immunol. Microbiol. Infect. Dis. 37, 85–96. doi: 10.1016/j.cimid.2014.01.002
Colwell, D. D., Dantas-Torres, F., and Otranto, D. (2011). Vector-borne parasitic zoonoses: emerging scenarios and new perspectives. Vet. Parasitol. 182, 14–21. doi: 10.1016/j.vetpar.2011.07.012
Cotte, V., Bonnet, S., Cote, M., and Vayssier-Taussat, M. (2010). Prevalence of five pathogenic agents in questing Ixodes ricinus ticks from western France. Vector Borne Zoonotic Dis. 10, 723–730. doi: 10.1089/vbz.2009.0066
Cotte, V., Bonnet, S., Le Rhun, D., Le Naour, E., Chauvin, A., Boulouis, H. J., et al. (2008). Transmission of Bartonella henselae by Ixodes ricinus. Emerg. Infect. Dis. 14, 1074–1080. doi: 10.3201/eid1407.071110
Cowling, D. W., Gardner, I. A., and Johnson, W. O. (1999). Comparison of methods for estimation of individual-level prevalence based on pooled samples. Prev. Vet. Med. 39, 211–225. doi: 10.1016/S0167-5877(98)00131-7
Dantas-Torres, F., Chomel, B. B., and Otranto, D. (2012). Ticks and tick-borne diseases: a one health perspective. Trends Parasitol. 28, 437–446. doi: 10.1016/j.pt.2012.07.003
De La Fuente, J., Estrada-Pena, A., Venzal, J. M., Kocan, K. M., and Sonenshine, D. E. (2008). Overview: ticks as vectors of pathogens that cause disease in humans and animals. Front. Biosci. 13, 6938–6946. doi: 10.2741/3200
De La Fuente, J., Massung, R. F., Wong, S. J., Chu, F. K., Lutz, H., Meli, M., et al. (2005). Sequence analysis of the msp4 gene of Anaplasma phagocytophilum strains. J. Clin. Microbiol. 43, 1309–1317. doi: 10.1128/JCM.43.3.1309-1317.2005
Dietrich, F., Schmidgen, T., Maggi, R. G., Richter, D., Matuschka, F. R., Vonthein, R., et al. (2010). Prevalence of Bartonella henselae and Borrelia burgdorferi sensu lato DNA in Ixodes ricinus ticks in Europe. Appl. Environ. Microbiol. 76, 1395–1398. doi: 10.1128/AEM.02788-09
Diniz, P. P., Schulz, B. S., Hartmann, K., and Breitschwerdt, E. B. (2011). “Candidatus Neoehrlichia mikurensis” infection in a dog from Germany. J. Clin. Microbiol. 49, 2059–2062. doi: 10.1128/JCM.02327-10
Diuk-Wasser, M. A., Liu, Y., Steeves, T. K., Folsom-O'keefe, C., Dardick, K. R., Lepore, T., et al. (2014). Monitoring human babesiosis emergence through vector surveillance New England, USA. Emerg. Infect. Dis. 20, 225–231. doi: 10.3201/eid1302/130644
Engbaek, K., and Lawson, P. A. (2004). Identification of Bartonella species in rodents, shrews and cats in Denmark: detection of two B. henselae variants, one in cats and the other in the long-tailed field mouse. APMIS 112, 336–341. doi: 10.1111/j.1600-0463.2004.apm1120603.x
Fertner, M. E., Mølbak, L., Boye Pihl, T. P., Fomsgaard, A., and Bødker, R. (2012). First detection of tick-borne “Candidatus Neoehrlichia mikurensis” in Denmark 2011. Euro Surveill. 17. Available online at: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20096
Heyman, P., Cochez, C., Hofhuis, A., Van Der Giessen, J., Sprong, H., Porter, S. R., et al. (2010). A clear and present danger: tick-borne diseases in Europe. Expert. Rev. Anti. Infect. Ther. 8, 33–50. doi: 10.1586/eri.09.118
Hildebrandt, A., Gray, J. S., and Hunfeld, K. P. (2013). Human babesiosis in Europe: what clinicians need to know. Infection 41, 1057–1072. doi: 10.1007/s15010-013-0526-8
Hovius, J. W., De Wever, B., Sohne, M., Brouwer, M. C., Coumou, J., Wagemakers, A., et al. (2013). A case of meningoencephalitis by the relapsing fever spirochaete Borrelia miyamotoi in Europe. Lancet 382, 658. doi: 10.1016/S0140-6736(13)61644-X
Jahfari, S., Fonville, M., Hengeveld, P., Reusken, C., Scholte, E. J., Takken, W., et al. (2012). Prevalence of Neoehrlichia mikurensis in ticks and rodents from North-west Europe. Parasit. Vectors 5:74. doi: 10.1186/1756-3305-5-74
Jongejan, F., and Uilenberg, G. (2004). The global importance of ticks. Parasitology 129(Suppl.), S3–S14. doi: 10.1017/S0031182004005967
Kantso, B., Svendsen, C. B., Jensen, P. M., Vennestrom, J., and Krogfelt, K. A. (2010). Seasonal and habitat variation in the prevalence of Rickettsia helvetica in Ixodes ricinus ticks from Denmark. Ticks Tick Borne Dis. 1, 101–103. doi: 10.1016/j.ttbdis.2010.01.004
Kline, R. L., Brothers, T. A., Brookmeyer, R., Zeger, S., and Quinn, T. C. (1989). Evaluation of human immunodeficiency virus seroprevalence in population surveys using pooled sera. J. Clin. Microbiol. 27, 1449–1452.
Liu, J., Hansen, C., and Quake, S. R. (2003). Solving the “world-to-chip” interface problem with a microfluidic matrix. Anal. Chem. 75, 4718–4723. doi: 10.1021/ac0346407
Margos, G., Gatewood, A. G., Aanensen, D. M., Hanincova, K., Terekhova, D., Vollmer, S. A., et al. (2008). MLST of housekeeping genes captures geographic population structure and suggests a European origin of Borrelia burgdorferi. Proc. Natl. Acad. Sci. U.S.A. 105, 8730–8735. doi: 10.1073/pnas.0800323105
Maurer, F. P., Keller, P. M., Beuret, C., Joha, C., Achermann, Y., Gubler, J., et al. (2013). Close geographic association of human neoehrlichiosis and tick populations carrying “Candidatus Neoehrlichia mikurensis” in eastern Switzerland. J. Clin. Microbiol. 51, 169–176. doi: 10.1128/JCM.01955-12
Nielsen, E. M., and Andersen, M. T. (2003). Detection and characterization of verocytotoxin-producing Escherichia coli by automated 5′ nuclease PCR assay. J. Clin. Microbiol. 41, 2884–2893. doi: 10.1128/JCM.41.7.2884-2893.2003
Nijhof, A. M., Bodaan, C., Postigo, M., Nieuwenhuijs, H., Opsteegh, M., Franssen, L., et al. (2007). Ticks and associated pathogens collected from domestic animals in the Netherlands. Vector Borne Zoonotic Dis. 7, 585–595. doi: 10.1089/vbz.2007.0130
Parola, P. (2004). Tick-borne rickettsial diseases: emerging risks in Europe. Comp. Immunol. Microbiol. Infect. Dis. 27, 297–304. doi: 10.1016/j.cimid.2004.03.006
Parola, P., and Raoult, D. (2001). Ticks and tickborne bacterial diseases in humans: an emerging infectious threat. Clin. Infect. Dis. 32, 897–928. doi: 10.1086/319347
Pérez-Eid, C. (2007). Les Tiques: Identification, Biologie, Importance Médicale et Vétérinaire. Paris: Lavoisier.
Rampersad, J. N., Watkins, J. D., Samlal, M. S., Deonanan, R., Ramsubeik, S., and Ammons, D. R. (2005). A nested-PCR with an Internal Amplification Control for the detection and differentiation of Bartonella henselae and B. clarridgeiae: an examination of cats in Trinidad. BMC Infect. Dis. 5:63. doi: 10.1186/1471-2334-5-63
Regnery, R. L., Spruill, C. L., and Plikaytis, B. D. (1991). Genotypic identification of rickettsiae and estimation of intraspecies sequence divergence for portions of two rickettsial genes. J. Bacteriol. 173, 1576–1589.
Reis, C., Cote, M., Paul, R. E., and Bonnet, S. (2011). Questing ticks in suburban forest are infected by at least six tick-borne pathogens. Vector Borne Zoonotic Dis. 11, 907–916. doi: 10.1089/vbz.2010.0103
Richter, D., Schlee, D. B., and Matuschka, F. R. (2003). Relapsing fever-like spirochetes infecting European vector tick of Lyme disease agent. Emerg. Infect. Dis. 9, 697–701. doi: 10.3201/eid0906.020459
Skarphedinsson, S., Lyholm, B. F., Ljungberg, M., Sogaard, P., Kolmos, H. J., and Nielsen, L. P. (2007). Detection and identification of Anaplasma phagocytophilum, Borrelia burgdorferi, and Rickettsia helvetica in Danish Ixodes ricinus ticks. APMIS 115, 225–230. doi: 10.1111/j.1600-0463.2007.apm_256.x
Sprong, H., Hofhuis, A., Gassner, F., Takken, W., Jacobs, F., Van Vliet, A. J., et al. (2012a). Circumstantial evidence for an increase in the total number and activity of Borrelia-infected Ixodes ricinus in the Netherlands. Parasit. Vectors 5:294. doi: 10.1186/1756-3305-5-294
Sprong, H., Tijsse-Klasen, E., Langelaar, M., De Bruin, A., Fonville, M., Gassner, F., et al. (2012b). Prevalence of Coxiella burnetii in ticks after a large outbreak of Q fever. Zoonoses Public Health 59, 69–75. doi: 10.1111/j.1863-2378.2011.01421.x
Sprong, H., Wielinga, P. R., Fonville, M., Reusken, C., Brandenburg, A. H., Borgsteede, F., et al. (2009). Ixodes ricinus ticks are reservoir hosts for Rickettsia helvetica and potentially carry flea-borne Rickettsia species. Parasit. Vectors 2:41. doi: 10.1186/1756-3305-2-41
Svendsen, C. B., Krogfelt, K. A., and Jensen, P. M. (2009). Detection of Rickettsia spp. in Danish ticks (Acari: Ixodes ricinus) using real-time PCR. Scand. J. Inf. Dis. 41, 70–72. doi: 10.1080/00365540802530653
Tijsse-Klasen, E., Jacobs, J. J., Swart, A., Fonville, M., Reimerink, J. H., Brandenburg, A. H., et al. (2011). Small risk of developing symptomatic tick-borne diseases following a tick bite in The Netherlands. Parasit. Vectors 4:17. doi: 10.1186/1756-3305-4-17
Vassallo, M., Pichon, B., Cabaret, J., Figureau, C., and Perez-Eid, C. (2000). Methodology for sampling questing nymphs of Ixodes ricinus (Acari: Ixodidae), the principal vector of Lyme disease in Europe. J. Med. Entomol. 37, 335–339. doi: 10.1603/0022-2585(2000)037[0335:MFSQNO]2.0.CO;2
Vayssier-Taussat, M., Le Rhun, D., Buffet, J. P., Maaoui, N., Galan, M., Guivier, E., et al. (2012). Candidatus Neoehrlichia mikurensis in bank voles, France. Emerg. Infect. Dis. 18, 2063–2065. doi: 10.3201/eid1812.120846
Vennestrom, J., Egholm, H., and Jensen, P. M. (2008). Occurrence of multiple infections with different Borrelia burgdorferi genospecies in Danish Ixodes ricinus nymphs. Parasitol. Int. 57, 32–37. doi: 10.1016/j.parint.2007.07.004
Wielinga, P. R., Fonville, M., Sprong, H., Gaasenbeek, C., Borgsteede, F., and Van Der Giessen, J. W. (2009). Persistent detection of Babesia EU1 and Babesia microti in Ixodes ricinus in the Netherlands during a 5-year surveillance: 2003-2007. Vector Borne Zoonotic Dis. 9, 119–122. doi: 10.1089/vbz.2008.0047
Keywords: tick borne diseases, molecular epidemiology, surveillance, Europe, microfluidic analyses
Citation: Michelet L, Delannoy S, Devillers E, Umhang G, Aspan A, Juremalm M, Chirico J, van der Wal FJ, Sprong H, Boye Pihl TP, Klitgaard K, Bødker R, Fach P and Moutailler S (2014) High-throughput screening of tick-borne pathogens in Europe. Front. Cell. Infect. Microbiol. 4:103. doi: 10.3389/fcimb.2014.00103
Received: 09 May 2014; Accepted: 10 July 2014;
Published online: 29 July 2014.
Edited by:
Patrick Mavingui, Centre National de la Recherche Scientifique, FranceReviewed by:
Valerio Iebba, ‘Sapienza’ University of Rome, ItalyMax MAURIN, Université Aix-Marseille II, France
Copyright © 2014 Michelet, Delannoy, Devillers, Umhang, Aspan, Juremalm, Chirico, van der Wal, Sprong, Boye Pihl, Klitgaard, Bødker, Fach and Moutailler. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sara Moutailler, UMR BIPAR, Animal Health Laboratory, ANSES, 23 avenue du general de Gaulle, 94706 Maisons-Alfort, France e-mail: sara.moutailler@anses.fr
 Lorraine Michelet
Lorraine Michelet Sabine Delannoy
Sabine Delannoy Elodie Devillers1
Elodie Devillers1  Fimme J. van der Wal
Fimme J. van der Wal Hein Sprong
Hein Sprong Rene Bødker
Rene Bødker Patrick Fach
Patrick Fach Sara Moutailler
Sara Moutailler