Implications of Spatiotemporal Regulation of Shigella flexneri Type Three Secretion Activity on Effector Functions: Think Globally, Act Locally
- 1Department of Chemistry and Biomolecular Sciences, University of Ottawa, Ottawa, ON, Canada
- 2Independent Researcher, Ottawa, ON, Canada
Shigella spp. are Gram-negative bacterial pathogens that infect human colonic epithelia and cause bacterial dysentery. These bacteria express multiple copies of a syringe-like protein complex, the Type Three Secretion apparatus (T3SA), which is instrumental in the etiology of the disease. The T3SA triggers the plasma membrane (PM) engulfment of the bacteria by host cells during the initial entry process. It then enables bacteria to escape the resulting phagocytic-like vacuole. Freed bacteria form actin comets to move in the cytoplasm, which provokes bacterial collision with the inner leaflet of the PM. This phenomenon culminates in T3SA-dependent secondary uptake and vacuolar rupture in neighboring cells in a process akin to what is observed during entry and named cell-to-cell spread. The activity of the T3SA of Shigella flexneri was recently demonstrated to display an on/off regulation during the infection. While the T3SA is active when bacteria are in contact with PM-derived compartments, it switches to an inactive state when bacteria are released within the cytosol. These observations indicate that effector proteins transiting through the T3SA are therefore translocated in a highly time and space constrained fashion, likely impacting on their cellular distribution. Herein, we present what is currently known about the composition, the assembly and the regulation of the T3SA activity and discuss the consequences of the on/off regulation of T3SA on Shigella effector properties and functions during the infection. Specific examples that will be developed include the role of effectors IcsB and VirA in the escape from LC3/ATG8-positive vacuoles formed during cell-to-cell spread and of IpaJ protease activity against N-miristoylated proteins. The conservation of a similar regulation of T3SA activity in other pathogens such as Salmonella or Enteropathogenic Escherichia coli will also be briefly discussed.
Introduction
Shigella spp. (e.g., S. flexneri, S. sonnei, S. dyssenteriae, and S. boydii) are gram negative enteropathogen bacteria that are closely related to commensal Escherichia coli. As such, they are often considered to be E. coli. pathovars. Homo sapiens are the only known natural hosts of Shigella spp. By invading the colonic mucosa, Shigella spp. cause dysentery that is characterized by bloody and mucous rich diarrhea accompanied by abdominal cramps. There are about 200 million infection cases annually and ~1.1 million deaths, among which the majority are children under 5 years (Kotloff et al., 1999). Associated to poor sanitation and water quality control (Kotloff et al., 1999; Phalipon et al., 2008; Johansson et al., 2009), the prevalence of the disease is highly correlated with economic wealth. In addition, the etiology of the disease differs between low- and high-income countries, where S. flexneri and S. sonnei prevail, respectively. Potential reasons for this remarkable phenomenon are discussed in detail elsewhere (Thompson et al., 2015).
Shigella spp. pathogenicity essentially depends on a large virulence plasmid of ~200 kb that is also found in enteroinvasive E. coli (EIEC). This virulence plasmid (Buchrieser et al., 2000; Venkatesan et al., 2001; Zhang et al., 2003; Jiang et al., 2005), and the chromosomes (Lukjancenko et al., 2010; Onodera et al., 2012) of many Shigella spp. have now been sequenced. Still, the majority of what we know concerning the infectious cycle of Shigella spp. and the molecular determinants of their pathogenicity comes from studies on S. flexneri, namely strains M90T (serotype 5a), 2457T (serotype 2a), and YSH6000 (serotype 2a) either in in vitro culture of immortalized intestinal cells, or from the infection of various animal hosts, including primate, rabbit, guinea pig, or mouse (Sansonetti et al., 1983; Sansonetti and Arondel, 1989; Martino et al., 2005; Shim et al., 2007; Arena et al., 2015). While none of these experimental systems constitute a natural Shigella host, they have nevertheless provided many insights about the inflammatory response component of shigellosis. This is particularly true of the rabbit ileal loop model (Sansonetti et al., 1983; Schnupf and Sansonetti, 2012; Puhar et al., 2013).
The infectious cycle of Shigella spp. consists in several consecutive steps. Upon their adhesion to host cells, Shigella spp. use genes expressed from their virulence plasmid to trigger their uptake by otherwise non-phagocytic epithelial cells, access their host cell cytoplasm and then, eventually spread to neighboring cells (reviewed in Valencia-Gallardo et al., 2015). The virulence plasmid also allows the bacteria to survive inside and kill macrophages (Zychlinsky et al., 1992; Fernandez-Prada et al., 2000; Suzuki et al., 2014), and perturb the function of T and B cells (Konradt et al., 2011; Salgado-Pabón et al., 2013; Nothelfer et al., 2014). Protein products of many genes harbored on the virulence plasmid are necessary for the assembly of a nanomolecular machine named the Type Three Secretion Apparatus (T3SA) (Burkinshaw and Strynadka, 2014). Also known as injectisome, this T3SA plays an essential role in most of Shigella invasion steps. The T3SA spans the bacterial inner and outer membranes adopting roughly the shape and function of a syringe. T3SA have a narrow conduit in their center that permits the secretion of proteins. In the initial stage of T3SA activation that takes place after initial contacts with the PM, a first class of protein called translocators are secreted. The translocators assemble to form a pore also called translocon across the host membrane. A second group of proteins called effectors then transit through the T3SA and ultimately through the pore to be delivered in the host cytoplasm. Simultaneously the host PM engulf the bacteria through a process requiring actin microfilaments remodeling, similarly to what is seen is regular phagocytosis (reviewed in Ménard et al., 1996; Carayol and Tran Van Nhieu, 2013; Valencia-Gallardo et al., 2015). The bacterial uptake is completed when bacteria are found in closed vacuoles. The T3SA is also necessary for subsequent rupture of these vacuoles (Blocker et al., 1999; Page et al., 1999; Schuch et al., 1999; Paz et al., 2010). Once in the cytoplasm Shigella spp. use the outer membrane protein IcsA (also known as VirG) to form actin comet tails that enable cytoplasm movement and ultimately, cell-to-cell spreading (Bernardini et al., 1989). The collision of a motile bacterium with the inner leaflet of the PM leads to the formation of a protrusion, which is a double membrane finger-like projection of the PM of the initially infected cells into a neighboring cell. Protrusions resolved into secondary vacuole (Campbell-Valois et al., 2014b; Dragoi and Agaisse, 2014; Kuehl et al., 2015); secretion of translocators and effectors are known to be essential, as well, for the lysis of secondary vacuoles through a process hypothesized to be essentially similar to entry (Page et al., 1999; Schuch et al., 1999). The ensuing release of bacteria into the cytoplasm of secondary infected cells effectively completes cell-to-cell spreading events.
As yet, the translocators and effectors arsenal of S. flexneri is encoded by 32–38 genes (Buchrieser et al., 2000; Ogawa et al., 2008; Parsot, 2009). While the N-terminal region of most of these effectors appears required for their targeting to the T3SA, their level of homology do not allow the identification of any clear consensus targeting sequence (Ramamurthi and Schneewind, 2003; Ghosh, 2004; Lilic et al., 2006). In addition, the structural stability of several of these effectors and their efficient targeting to the T3SA can be dependent on the formation of a complex with their cognate chaperone protein (reviewed in detail elsewhere Burkinshaw and Strynadka, 2014). The nine effectors that binds the chaperone Spa15 were recently shown to harbor a conserved chaperon binding domain required for efficient secretion and conserved across many pathogen species (Costa et al., 2012). However, most of the effectors, whose expression is up regulated when T3SA are active, do not seem to necessitate any chaperone (Parsot, 2009).
In this review, we first focus on the current knowledge concerning the assembly and the structure of the T3SA. We then describe the evidences indicating that Shigella T3SA activity oscillates depending on the adhesion of bacteria to the host PM. In the third part, we discuss the consequences of this dynamic activity of the T3SA on the properties and functions of Shigella effectors. Finally, we relay this model to recent data concerning effector functions and discuss its extension to other T3SA-bearing pathogens.
Expression, Assembly, and Structure of Shigella T3SA
The expression of T3SA is controlled at the transcriptional level. Essential genes for assembly of T3SA are located in two juxtaposed, but inversely oriented operons, located in the center of the virulence plasmid: the mxi/spa operon (approximately 20 kb length and 26 genes) and the ipaABCD operon (~10 kb length and 10 genes) (Buchrieser et al., 2000). Importantly, the transcription of the T3SA is tightly associated and synchronized to those of the effector proteins. Indeed, at temperature above >32°C, inhibition by the nucleoid factor H-NS is relieved (Maurelli and Sansonetti, 1988; Falconi et al., 1998, 2001), triggering a signaling cascade implicating transcription activators VirF and VirB that induces the expression of mxi/spa and ipaABCD operons (Tobe et al., 1991; Kane and Dorman, 2012). The output of this cascade consists in the formation of an intracellular store of translocators and so-called first wave effectors with their cognate chaperones (Ménard et al., 1994b), and the assembly of T3SA (Figure 1). Therefore, bacteria at permissive temperatures display at their surface inactive T3SA that can be switched to the active state upon contact with host cells, allowing almost instantaneous secretion of prestored translocators and effectors (Enninga et al., 2005).
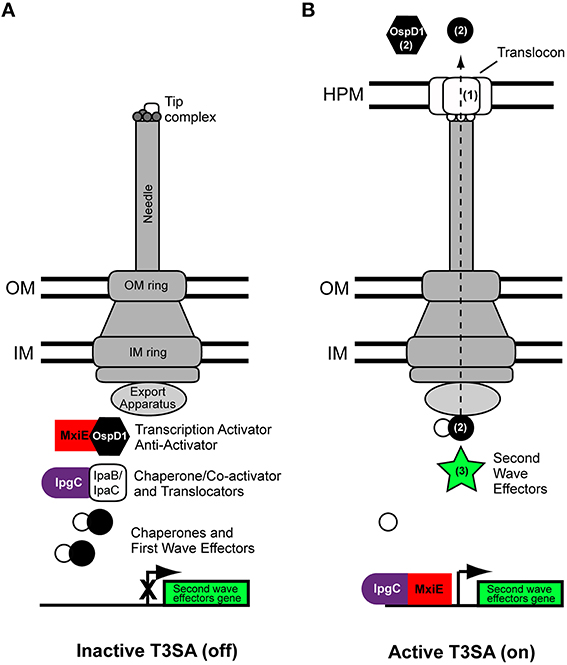
Figure 1. Scheme of the type three secretion apparatus of Shigella flexneri. Basic scheme of Shigella flexneri T3SA at permissive temperatures (e.g., 37°C) in the inactive (A) and active states (B). The tip complex is composed of IpaD (grey circles) and IpaB (white rectangles) adopting a closed conformation and an open conformation in the inactive and active states, respectively. Activation of secretion leads to MxiE-IpgC-dependent expression of second wave effectors. The dashed arrow indicates the route followed by translocators and effectors during secretion. They travel through a conduit located at the center of the T3SA that comprises successively the sorting platform, the inner membrane ring, rod protein (not visible on this scheme), the outer membrane ring, the needle, and translocon. Numbers in parenthesis in the right panel indicate the secretion order of translocators, first wave and second wave effectors. Labelings of bacterial cytoplasmic complex components are indicated from left to right. IM, inner membrane; OM, outer membrane; HPM, host plasma membrane.
The inactive T3SA is hierarchically assembled in the bacterial membranes (reviewed in detail elsewhere Burkinshaw and Strynadka, 2014) (Figure 1). Proteins MxiG/MxiJ and MxiD/MxiM, constituting the inner and outer membrane rings of the basal body of the T3SA, respectively, are assembled first (Hodgkinson et al., 2009). MxiA, Spa13, and Spa47 and the sorting platform, which is composed of Spa33, MxiK, and MxiN (Morita-Ishihara et al., 2006; Lara-Tejero et al., 2011; Hu et al., 2015), associate with the cytoplasmic face of the inner membrane ring where they can recognize proteins targeted to the T3SA. Remarkable high resolution electron microscopy images of the T3SA of Shigella, recently provided compelling evidence about the composition and function of the cytoplasmic components of the T3SA, including the sorting platform (Hu et al., 2015). On the basis of its Salmonella homolog PrgJ, the rod protein MxiI is hypothesized to associate with the socket in the upper part of the inner membrane ring and contribute to regulating secretion of MxiH (Marlovits et al., 2006), which homopolymerizes to form the needle of the syringe (Demers et al., 2013). Interaction of Spa32 with Spa40 is also essential for the formation of needles (Botteaux et al., 2008, 2010). Spa32, the homolog of YscP in Yersinia pestis (Journet et al., 2003), acts as a molecular ruler and is secreted when the needle reaches the correct length (Botteaux et al., 2008). A small fraction of the total cellular pool of IpaB and IpaD is then secreted, but remains associated with the needle, hence forming the tip complex. In the absence of activation signal, the tip complex is closed and composed of one molecule of IpaB and four molecules of IpaD (Veenendaal et al., 2007; Epler et al., 2012; Cheung et al., 2015). The association of the closed conformation of the tip complex with the needle is a hallmark of inactive T3SA. In contrast, T3SA devoid of this normal tip complex, which are obtained by deletion of the ipaB or ipaD locus, are constitutively active (Ménard et al., 1993, 1994a). In the case of the ipaB mutated strain, the open conformation of the tip complex appeared to be formed of five IpaD molecules (Cheung et al., 2015), but it is likely that total absence of a tip complex would also lead to deregulated secretion. In the inactive state of T3SA, the gatekeeper protein MxiC blocks effectors secretion (Botteaux et al., 2009; Martinez-Argudo and Blocker, 2010). It does so by associating with the entrance of the inner conduit of the T3SA, probably through binding with MxiI (Cherradi et al., 2013). MxiC remains at this position until it is itself secreted, an event that is probably induced by the depletion of the intracellular store of translocators occurring in the active state, and that may involve needle conformational changes (Martinez-Argudo and Blocker, 2010).
Regulation of the Activity of Shigella T3SA Inside Infected Cells
The T3SA is activated upon contacting the host cell, likely upon binding of the tip complex to cholesterol and/or sphingolipid molecules composing the host PM (Lafont et al., 2002; van der Goot et al., 2004; Veenendaal et al., 2007; Epler et al., 2009). This activation triggers the secretion of the cytoplasmic fraction of translocators IpaB and IpaC. IpaB and IpaC insert into the host PM to form a pore, or translocon, through which effectors will be transferred into the host cytoplasm (Figure 1) (Edgren et al., 2012). Thus, a first hallmark of an active T3SA state is the adoption of an open conformation by the tip complex or, alternatively, its absence from the needle of active T3SA. Both situations result in the unsealing of the syringe. A second hallmark is the cooperation between the T3SA and the translocon. Importantly, this cooperation is necessary to infect cells, but dispensable for constitutive in vitro activity, as for example in the case of ipaB and ipaD mutant strains.
Upon persistent activation of the T3SA, bacteria intracellular stores of translocators IpaB and IpaC and of the anti-activator OspD1 become depleted (Figure 1). This putatively allows the formation of a complex between the translocator chaperone IpgC and the transcription activator MxiE (Pilonieta and Munson, 2008), which induce the expression of genes harboring a MxiE-box (Mavris et al., 2002a,b; Le Gall et al., 2007; Bongrand et al., 2012). Genes possessing a MxiE-box hence constitute a second wave of effectors that are secreted through the T3SA (Parsot, 2009) (Figure 1).
Transcriptional fusions of MxiE-box containing promoters with β-galactosidase (LacZ) were constructed and used to monitor the T3SA activity of bacteria recovered from infected HeLa epithelial cells (Demers et al., 1998). HeLa cells are not very permissive for cell-to-cell spreading (Tran Van Nhieu et al., 2003), but allow the study of events taking place during the initial uptake and vacuolar rupture. In the absence of secretion activity, such as when bacteria are grown in broth at 37°C, the β-galactosidase activity of MxiE-promoters was nil. In contrast, when bacteria were put in contact with Hela cells, the β-galactosidase activity of MxiE-promoters was induced. The β-galactosidase activity of Shigella recovered from HeLa cells was higher at 60 than at 150 min post-entry. The activity at 150 min had in fact decreased to the background level observed in bacteria cultivated in absence of host cells. These results provided the first indication that following entry into epithelial cells, T3SA were inactivated (Demers et al., 1998).
Use of the green fluorescent protein (GFP) allowed the design of fluorescent Transcription-based Secretion Activity Reporter (TSAR) relying on the MxiE-promoter of ipaH7.8. The TSAR allowed for monitoring the T3SA activity inside infected cells in close to real-time fashion (Campbell-Valois et al., 2014a,b). It confirmed the results obtained with the previous β-galactosidase transcriptional fusion (Demers et al., 1998). Indeed, the secretion proved inactivated in the host cell cytoplasm 30–60 min post-entry. In addition, because these experiences were performed in colonic epithelial cell line TC7 (a clone of Caco-2), the dynamic of the TS3A activity during the spreading of bacteria to neighboring cells could be observed. Interestingly, a significant fraction of bacteria that had escaped the entry vacuole were observed to reactivate their secretion between 60 and 120 min post-entry. Based on several lines of evidence, this phenotype was attributed to the fraction of motile cytoplasmic bacteria that had formed protrusions (Figure 2A). For example, non-motile Shigella obtained by genetic manipulation (e.g., icsA mutant) or treatment with the actin polymerization inhibitor cytochalasin D, both resulted in background level of T3SA secretion activity at 240 min post-entry. In contrast, using a conditional mutant ipaC allele that remained trapped in protrusions or in vacuoles that derived from it, or using the F-actin depolymerizing inhibitor jasplakinolide, which induced host cell retraction that causes random collisions between intracellular bacteria and the PM, we demonstrated that interactions of cytoplasmic bacteria with the PM compartments formed during cell-to-cell spread was critical for reactivation of T3SA. These results demonstrate that interactions of cytoplasmic bacteria with the PM formed during cell-to-cell spread were critical for reactivation of T3SA. Fluorescence Recovery After Photobleaching (FRAP) of the TSAR indicated that the secretion activity was induced when bacteria were trapped in protrusions and in a lesser measure in vacuoles, but not in cytoplasmic bacteria (Campbell-Valois et al., 2014b). This study indicated that intracellular Shigella undergoes cyclical all-or-none activation of its T3SA depending on interactions with the PM during entry or cell-to-cell spreading steps of the infection cycle. In addition, these results also indicate that endomembrane compartments are likely unable to induce T3SA activation. Whether this phenomenon stems from the biochemical composition of the endomembrane compartments itself, which would fail to activate T3SA due to weaker mutual interactions, or from the infrequent docking of Shigella on endomembrane compartments is an open question. It is also possible that T3SA display low level activity or too transient activation in the cytoplasm to be detectable with the TSAR system. Another important question to tackle is the regulatory mechanism of T3SA activity in infected cells and tissue. How T3SA can be activated both during entry and cell-to-cell spread while the bacteria is alternately facing the external face of a single PM and the internal face of a double PM (Figure 2B)? Additionally, what are the mechanisms of inactivation of T3SA in the cytoplasm (Figure 2C)? Concerning the latter question, the most plausible mechanism is the reconstitution of tip complexes composed of newly synthesized IpaB and IpaD capable of plugging T3SA shortly after loss of contacts with the vacuolar membrane. The alternative hypothesis of a partial or complete disassembly of T3SA following bacterial release in the cytoplasm appears less likely, but cannot be completely ruled out yet (Campbell-Valois et al., 2014b).
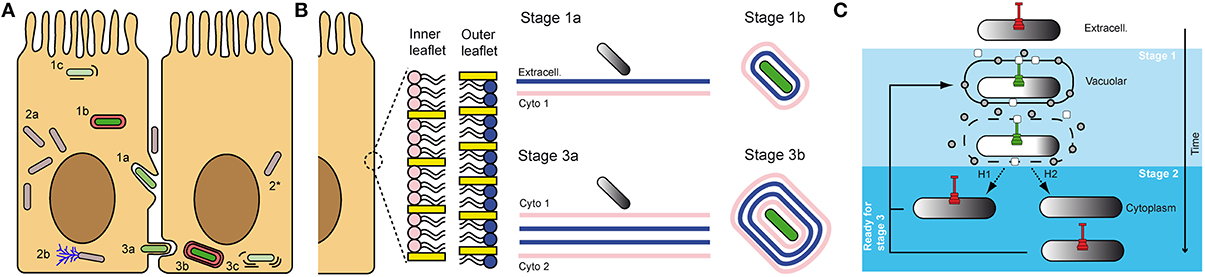
Figure 2. Shigella infectious cycle: interaction of T3SA with membrane compartments is key for the regulation of its activity. The “invade and evade” infectious strategy of S. flexneri can be broken down in two phases: (1) entry, characterized by residence of bacteria in vacuoles derived from the PM (1a,b), which are ultimately ruptured (1c); (2) cytoplasmic residence, where most replication events occur (2a) and motility through actin comet formation is possible (2b). After bacteria have reached the cytoplasm, they are in position to iterate this cycle and progressively invade neighboring cells, before evading once again the secondary vacuole. This process is characterized by the formation of protrusions (3a) and vacuoles (3b) composed of a double membrane derived from the PM in which bacteria reside until their lysis (3c), and escape in the cytoplasm (2*). It was demonstrated that secreting bacteria (green) were systematically associated with entry and cell-to-cell spread vacuoles and protrusions derived from the PM, while cytoplasmic bacteria were not actively secreting (gray) (A). Magnification of the inner and outer leaflet of the PM. Density of cholesterol (yellow rectangles) and overall phospholipids composition (pink vs. blue) is variable in both leaflets. Therefore, bacteria are not facing the same biochemical cues when they are performing entry vs. cell-to-cell spread. As mentioned in panel (A), bacteria also face four membranes during cell-to-cell spread instead of two during entry (B). Proposed mechanisms of inactivation of T3SA in intracellular Shigella. Grey circles and white rectangles represent secreted tip complex proteins, which are incapable of blocking T3SA conduit. H1 and H2 represent alternative hypotheses for inactivation of T3SA in the cytoplasm of host cells, as described in the text. H1: replenishment of functional tip complex; H2: disassembly of T3SA before vacuole escape and replenishment with inactive T3SA in the host cytoplasm (C).
A Model Integrating the Influence of the Oscillating T3SA Activity on the Properties and Functions of Effectors
As delineated above, Shigella infectious cycle can be summed up as an “invade and evade” strategy, where bacteria first “invade” cells by triggering their own uptake in epithelial cells (or by not blocking their uptake by professional phagocytes) and “evade” the vacuole formed around them during phagocytosis using membrane-disrupting translocators and effectors. Once bacteria have evaded their vacuole, they are in position to iterate this cycle and progressively invade neighboring cells, before evading once again the secondary vacuole. The observation that the T3SA oscillates between its active and inactive states between two “invade-evade” cycles (Campbell-Valois et al., 2014b) has likely important consequences on the subcellular distribution of bacterial effectors during infection. This subcellular distribution is influenced by three main parameters: the location of secretion, the regulation of secretion, and the diffusion capacity of the effector within the host cytoplasm, either passively through Brownian movement or actively by binding specific host factors or organelles. The cellular cytoplasm is characterized by a high concentration of biomolecules or macromolecular crowding, which considerably impedes the excluded volume of solvent accessible to diffusing proteins, hence decreasing their diffusion rate (Zhou et al., 2008). The macromolecular crowding is heterogeneous and peaks at the vicinity of the host cell PM (Kühn et al., 2011). In consequence, the protein diffusion rate in this region is decreased (Kühn et al., 2011). The formation of protein complexes and the level of cytoskeleton polymerization participate to the heterogeneity of the macromolecular crowding. Therefore, any perturbation in the density of the cytoskeleton network can potentially further restricts protein diffusion. Interestingly, many pathogens such as Salmonella, E. coli, and Shigella, increase the density of the actin meshwork in their vicinity using T3SA effectors. Specifically, Shigella entry and cell-to-cell spreading is characterized by the formation of actin foci or actin rich structures around actively secreting bacteria (Carayol and Tran Van Nhieu, 2013). Specific Shigella effectors involved in that process will be discussed later.
Hence, if the intrinsic properties of effectors as well as their capacity to interact with host protein targets obviously play a determinant role in their function, the site of their secretion is also crucial (Galán, 2009). Furthermore, the realization that the T3SA activity is maximal in PM-derived compartments such as protrusions and bacteria-containing vacuoles strongly suggests that the effective concentration of effectors upon their secretion should follow a very steep gradient (Figure 3A). This prediction was experimentally corroborated by the apparent retention of translocators and effectors in the vicinity of actively secreting bacteria (Campbell-Valois et al., 2014b, 2015). This high effective concentration of effectors should hence potentiate their binding and enzymatic properties, as long as their host protein targets are as well localized in this region.
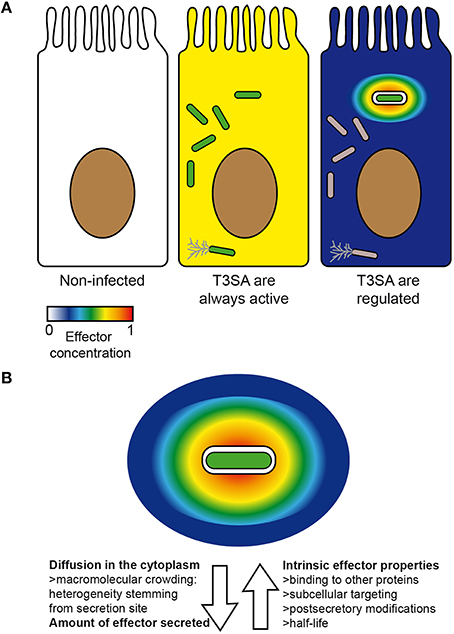
Figure 3. Factors impacting on the concentration and distribution of effectors inside host cells. Theoretical concentration and distribution of bacterial effectors in an uninfected cell (left) vs. in infected cells in the case scenario where all intracellular bacteria (center) or only those in vacuoles are secreting (right) (A). An actively secreting bacterium located in a vacuole is represented with its theoretical gradient of effectors. Arrows oriented away and toward the bacteria represent respectively factors favoring or disfavoring rapid and homogenous diffusion of effectors inside host cells (B). Secreting bacteria, green; non-secreting bacteria, gray. Color scale represents concentration of effectors from 0 (min) to 1 (max) (arbitrary unit).
Despite the importance of the initial local concentration of effectors, experimental observations of their subcellular distribution have demonstrated that many effectors can nevertheless eventually diffuse from their secretion site, when given enough time (Campbell-Valois et al., 2014b, 2015). Again, the diffusion rate and the final subcellular distribution of these effectors will depend on the properties of the cytoplasm of the (infected) cell and the intrinsic properties of effectors (Figure 3B). Therefore, the local delivery and ensuing diffusion of effectors may confer them two sets of functions: one in the vicinity of secreting bacteria (i.e., local functions) and one upon diffusion across the infected cell (i.e., distant functions) (Figure 4). The balance between local vs. distant functions for a given effector would also be modulated by the actual amount of secreted effector, the relationship between its binding affinity and/or catalytic activity toward its host targets and its stability or half-life. Finally, the amount of actively secreting bacteria within a given infected host cell might also impact on the concentration and therefore the distribution of effectors across infected cells. Distant functions might be favored, when the amount of bacteria and secreted effectors cell rise and/or when the amount of actively secreting bacteria decreases in infected host cells (Campbell-Valois et al., 2014b). In conclusion, this local/distant model of effectors function would provide a passive and nevertheless elegant manner for bacteria to adapt their activity in regard of the bacterial load within host cells.
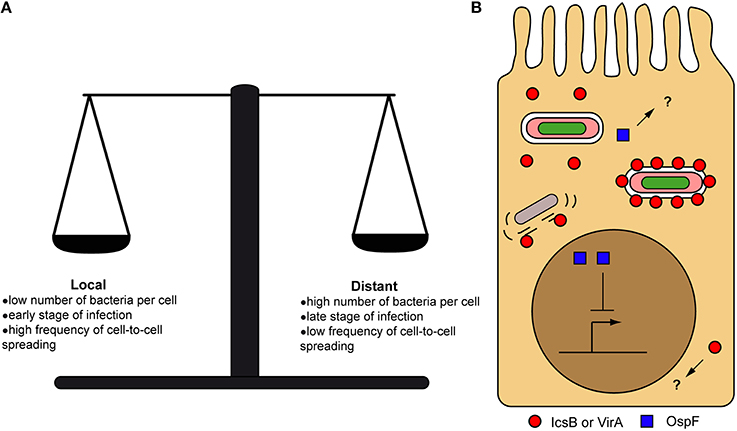
Figure 4. Local vs. distant action of secreted effectors. Factors influencing the balance between local and distant action of effectors from their secretion site inside host cells (A). Examples of local and distant action of effectors inside host cells. OspF is acting in the nucleus (distant action) to block the inflammatory response, while IcsB and VirA were recently proposed to act directly on the cell-to-cell spread vacuole (local action) to favor bacterial escape in the cytoplasm. Question marks indicate the possibility that OspF and IcsB/VirA could also have local and distant functions, respectively (B).
Recent Progresses in the Role of Shigella Effectors During Infection: Evidence for and Against Our Model
Examples of Effector Acting Locally, Near their Secretion Site
Few studies performed on S. flexneri support the existence of local function of effectors. A classical example is the local function of IpaB (in collaboration with IpaC) through its translocon-forming ability to induce bacterial uptake and vacuole rupture (Blocker et al., 1999; Page et al., 1999; Schuch et al., 1999; Carayol and Tran Van Nhieu, 2013), which can be contrasted with IpaB distant function in inducing pyroptosis of macrophages once liberated in their cytoplasm (Zychlinsky et al., 1992, 1994). Other effectors implicated in F-actin manipulation, such as IpaA, IpgB1, and IpgB2 are also known to act near or around the actin foci to enable bacterial uptake (Carayol and Tran Van Nhieu, 2013). Phosphatidylinositol-5-phosphate produced by the inositol phosphate phosphatase IpgD is enriched around entry sites (actin foci), suggesting IpgD is acting in the vicinity of its secretion site (Pendaries et al., 2006). However, the reduction in the concentration of its substrate phosphatidylinositol-4,5-biphosphate is rapidly detected in whole cell extract (Niebuhr et al., 2002), and a range of global to local effects of the enzymatic activity of IpgD has been reported in the literature (Puhar et al., 2013; Mellouk et al., 2014), complicating the portrait. When it comes to events downstream of entry, reports of effectors acting locally have been scarcer, although a few examples have emerged.
Recently, the fragmentation of the golgi apparatus of infected epithelial cells was reported independently by two research groups (Mounier et al., 2012; Burnaevskiy et al., 2013). A newly identified effector named IpaJ has been implicated in this phenotype (Burnaevskiy et al., 2013, 2015; Dobbs et al., 2015). IpaJ does so using its cysteine protease activity to cleave the N-miristoyl modification of ARF1 and ARF2 (Burnaevskiy et al., 2013). In a latter study, the same group revealed that IpaJ demiristoylated a large group of host proteins during in vitro experiments, but that it was highly specific to Golgi apparatus-associated ARF/ARL small GTPases when delivered inside host cells through the T3SA (Burnaevskiy et al., 2015). Specificity was also mediated in part by the capacity of IpaJ to recognize the GTP-bound form of golgi associated ARFs. Other factors impacting on the subcellular localization of IpaJ could be implicated in the selections of its substrates, as it also recognizes the GTP-bound form of the PM-associated ARF6 although the N-miristoyl of this latter protein is not cleaved in vivo. These data support the notion that the specificity of IpaJ enzymatic activity may come from its secretion site, where only a limited number of its potential N-miristoylated substrates are accessible, rather than from the specialization of its catalytic site to bind only a subset of N-miristoylated proteins. It is noteworthy though that the in vivo identification of IpaJ substrates was performed at 6 h post-entry (Burnaevskiy et al., 2015). It would be interesting to check if IpaJ is processing alternative N-miristoylated targets more early during the infection process when both its distribution and concentration should be very different, as one would hypothesize based on our model.
Autophagy is the process that leads to capture, classically in a double membrane compartment, and degradation of cytoplasmic content in response to specific metabolic cues. ATG8/MAPLC3 (LC3) proteins are canonical marker of autophagosomes. A subset of autophagy called xenoautophagy is used as a countermeasure against foreign particles such as viruses and bacteria (Baxt et al., 2013; Huang and Brumell, 2014). S. flexneri had been previously shown to resist xenoautophagy using its effectors IcsB and VirA (Ogawa et al., 2005; Dong et al., 2012). Harnessing the power of the TSAR system to distinguish S. flexneri intracellular sub-populations (Campbell-Valois et al., 2014b), we recently provided evidence that IcsB and VirA are both acting in the vicinity of actively secreting bacteria during cell-to-cell spread (Campbell-Valois et al., 2015). IcsB, VirA, and LC3 relocated around secreting bacteria in this context. A higher proportion of icsB and virA than wild type bacteria were LC3 positive during cell-to-cell spreading. icsB virA double mutant strain was even more attenuated than the single mutants in a plaques formation assay, displaying strongly diminished cell-to-cell spreading capacity. Moreover, icsB virA mutated bacteria were trapped in LAMP2 positive compartments from which they could hardly escape. These results suggested that IcsB and VirA are acting in synergy to allow escape from LC3 positive compartments formed during cell-to-cell. LC3 is also recruited around secreting bacteria during entry, but escape from the LC3 positive compartments in this context seems to be relatively independent of IcsB and VirA, although time of residence in the entry vacuole might be slightly extended for icsB virA bacteria (Campbell-Valois et al., 2015). Taking advantage of LC3 recruitment during entry, we demonstrated that LC3-positive entry vacuoles containing actively secreting bacteria were composed of a single membrane, as in the process of LC3-associated phagocytosis (LAP) previously reported in many bacterial pathogens (Lai and Devenish, 2012; Huang and Brumell, 2014). Therefore, we concluded that: (1) LC3 is recruited directly to existing bacteria-containing vacuoles; (2) vacuolar bacteria concomitantly secrete IcsB and VirA; (3) IcsB and VirA associate transiently with the vacuolar membranes, (4) but they act locally to favor escape from LC3-positive compartments most significantly during cell-to-cell spread (Figure 4B). Observations that wild type bacteria failed to complement in trans the deficiency of icsB virA bacteria support this model (Campbell-Valois et al., 2015). The action of IcsB and VirA in the vicinity of secreting bacteria is also supported by evidence that icsB icsA, virA icsA, and icsB virA icsA strains, which are all confined to the cytoplasm due to cell-to-cell spread deficiency, did not significantly recruit LC3 (Campbell-Valois et al., 2015). Although IcsB and VirA are acting in synergy, they have been shown to act on apparently unrelated targets (Ogawa et al., 2005; Dong et al., 2012). VirA is a Rab GTPase activating Protein (GAP) with Rab1-GTP being the most efficiently catalyzed substrate, although other Rabs (e.g., Rab33 and Rab35) are also processed efficiently (approximately three-fold less than Rab1) (Dong et al., 2012). The recruitment of Rab1 to bacteria containing vacuoles, phagosome, and autophagosome (Ingmundson et al., 2007; Zoppino et al., 2010; Huang et al., 2011; Campbell-Valois et al., 2012) suggests that Rab1 could be recruited as well to vacuoles containing S. flexneri rendering it available to neighboring VirA that would have been freshly delivered through T3SA (Campbell-Valois et al., 2015). IcsB has been suggested to protect S. flexneri from autophagy by shielding IcsA from direct recognition by ATG5, a component of the autophagy pathway (Ogawa et al., 2005). Other results rather suggest that the role of IcsA in LC3 recruitment is indirect through the formation of cell-to-cell spread vacuoles that, as phagosome-like compartments, could be subject to LAP (Campbell-Valois et al., 2015). This alternative model would readily explain LC3 recruitment during entry, but its absence at later stages of infection in icsA strains (Baxt and Goldberg, 2014; Campbell-Valois et al., 2015). A cholesterol-binding domain was also identified in IcsB and showed to be essential for the ability of IcsB to enable autophagy escape (Kayath et al., 2010). Cholesterol being putatively found in abundance in the Golgi apparatus, the PM and in compartments such as early phagosomes derived from it (van Meer et al., 2008), freshly secreted IcsB could act directly using its cholesterol binding domain on the membrane of S. flexneri-containing vacuole. Interestingly, interruption of cholesterol flux inside macrophages have been shown to block fusion of phagosomes with lysosomes (Huynh et al., 2008). As yet, there are still many unknowns concerning the targets and modes of action of IcsB and VirA that enable escape from LC3-positive vacuoles. In particular, how IcsB and VirA activities synergize in that context is completely unknown.
An interesting example of how intrinsic properties of a given effector could impact on its range of action was recently reported for OspG. OspG is endowed with Ser/Thr kinase activity; it binds specifically to E2 ubiquitin conjugating enzyme (e.g., UbCH5, UbCH7) loaded with ubiquitin (E2~Ub) and blocks IκBα degradation induced by tumor necrosis factor-α (TNFα, Kim et al., 2005). It has also been shown to bind free ubiquitin and polyubiquitin chains (Zhou et al., 2013), although E2~Ub seems to bind OspG with more affinity and increases its kinase activity more readily than free ubiquitin (Grishin et al., 2014; Pruneda et al., 2014). OspG is an atypical Ser/Thr kinase with a shorter primary structure than its eukaryotic counterparts (Kim et al., 2005; Grishin et al., 2014; Pruneda et al., 2014). Structures of E2~Ub-OspG complexes were recently reported (Grishin et al., 2014; Pruneda et al., 2014). OspG is binding at the intersection of the ubiquitin C-terminus and the catalytic site of the E2 to which the latter is tethered, hence contacting both proteins constituting the E2~Ub complex. OspG adopts the active conformation of Ser/Thr kinases (Grishin et al., 2014; Pruneda et al., 2014). Both studies showed that disrupting interfaces between OspG and E2~Ub abrogated the capacity of OspG to decrease IkBα degradation (Grishin et al., 2014; Pruneda et al., 2014). What is particularly interesting for the main matter discussed here, is that mutants in the primary structure of OspG disrupting its capacity to interact with E2~Ub have a much shorter half-life than the wild type within host cells (Grishin et al., 2014). This observation suggests that integration of OspG in a ternary complex with E2~Ub stabilizes its structure and/or protect it from proteases. The range of action of OspG upon secretion is therefore likely regulated by its binding affinity to E2~Ub and the fraction of OspG found in the complex with E2~Ub at any given time. Assuming that E2~Ub concentration is relatively homogenous across the cytoplasm, one can assume that the likelihood of forming the tripartite complex will be maximal in the vicinity of secreting bacteria where OspG concentration would be higher. As OspG, either free or in the tripartite complex, diffuses away, its effective concentration will decrease thereby mechanically reducing the fraction found in the stabilizing tripartite complex. In consequence, the concentration of free OspG will be higher further down its diffusion gradient leading to reduced activity and degradation. Therefore, this phenomenon will effectively restrain OspG capacity to act at long distances. Nevertheless, many aspects of the interplay between OspG, its kinase activity, the E2~Ub complex and the degradation of IκBα remain to be understood.
Counterexamples: Effectors Acting at a Long Distance from their Secretion Site
Shigella spp. possess 12 ipaH genes, but due to pseudogenes and gene duplications they give rise to a maximum of 9 distinct proteins across Shigella spp. (Bongrand et al., 2012). IpaHs are E3 ubiquitin ligases (Rohde et al., 2007; Singer et al., 2008; Zhu et al., 2008), and the search for their host targets has attracted considerable interest (Rohde et al., 2007; Ashida et al., 2010, 2013, 2014; Wang et al., 2013; Suzuki et al., 2014; Tanner et al., 2015). The substrates identified so far are molecules implicated in inflammatory pathways converging on NFκb. Most of these validated targets (e.g., NEMO, NFκB p65 etc.) are cytoplasmic proteins that have not been reported in these studies to physically associate or to relocate to S. flexneri-containing protrusions or vacuoles where secretion is actively taking place (Campbell-Valois et al., 2014b). The single exception might be glomulin, which is degraded by the proteasome in an IpaH7.8-dependent manner in macrophages (Suzuki et al., 2014). In this study, glomulin was found in the vicinity of ipaH7.8 bacteria only. It is not clear though if IpaH7.8 is ubiquitylating glomulin specifically around secreting bacteria or away of bacteria, hence preventing its recruitment to cytoplasmic bacteria. Since there have not been many studies on glomulin reported in the literature, further work will help shedding light on its role during bacterial infection.
OspF is arguably the prototypical example of effectors acting at a long distance from their secretion site. Indeed, OspF is a phosphothreonine lyase that specifically removes the O-phosphate group from the threonine of the activation loop of MAP kinases (i.e., Thr183 in Erk1) (Li et al., 2007). This modification irreversibly inactivates the MAPK (e.g., ERK1/2, p38 etc.), blocks the activation of the interleukin-8 promoter by the NFκb pathway and strongly dampens the inflammatory response (Arbibe et al., 2007). These events are taking place in the nucleus, where most phosphorylated ERK1/2 are found. OspF also spontaneously locates to the nucleus upon transfection of tissue culture cells (Arbibe et al., 2007) (Figure 4B). OspF is sufficiently small (~28 kDA) to freely diffuse in the nucleus and it does not display a typical basic nuclear localization sequence within its primary structure. OspF could be anchored to the appropriate sites in the nucleus through binding to Heterochromatin Protein 1 γ (HP1γ) (Harouz et al., 2014), which is historically associated with heterochromatin formation but has also been associated with transcriptionally active loci such as the IL-8 promoter. Supporting the important role of OspF in the nucleus, the ospF mutant modulates the transcription of more genes than other mutant strains tested including mxiE strain, which lacks expression of second-wave effectors (Parsot, 2009). In addition, affected genes are attributed to three distinct pathways: inflammation, apoptosis and stress response, going way beyond its classical role in dampening the inflammatory response (Lippmann et al., 2015). OspB is another effector that is located to the nucleus and that could be implicated in modulating the inflammatory response, potentially coordinating its action with OspF (Zurawski et al., 2009; Ambrosi et al., 2015). Nevertheless, even in the case of effectors shown to be acting at a long distance, it is impossible to discard the possibility that they have also a local function that has not been uncovered yet (Figure 4B).
Are T3SA in Other Bacteria also Spatio-Temporally Regulated?
Due to the “invade and evade” infectious strategy used by S. flexneri (i.e., successive PM- and cytoplasm association), spatio-temporal regulation of its T3SA is a plausible mode of action. Since Burkholderia mallei and Burkhloderia pseudomallei have also adopted a similar infectious strategy (Stevens et al., 2006; Gong et al., 2011), their T3SA is probably regulated similarly to S. flexneri. What about other type of T3SA-expressing pathogens? Two main alternative infectious strategies exist: (i) bacteria residing in vacuole, such as is the case with Salmonella spp. or Chlamydia spp.; (ii) bacteria associating with the extracellular face of the PM in a transient (Yersinia spp.) or stable fashion (enteropathogenic E. coli and Citrobacter rodentium) (Figure 5). Although they are considered paradigmatic vacuolar pathogens, Salmonella spp. are not only found in large vacuoles and tighter tubular compartments, but also in the cytoplasm (LaRock et al., 2015; Liss and Hensel, 2015). These bacteria use T3SA encoded by the Salmonella Pathogenicity Island-1 (SPI-1) to invade epithelial cells. It was shown that acidification of the bacteria-containing vacuole and ensuing sensing of neutral pH of the cytoplasm through its translocon led successively to Salmonella Pathogenicity Island-2 (SPI-2) T3SA assembly and activation (Yu et al., 2010), which is important for shaping the vacuolar niche of this pathogen (LaRock et al., 2015; Liss and Hensel, 2015). Nevertheless, whether bacteria that are located in the middle of large vacuoles and in which the T3SA is not directly contacting the host membrane are actively secreting or not is currently unknown. As infection progresses, the evolution of these vacuoles into tight tubular compartments (LaRock et al., 2015; Liss and Hensel, 2015) might allow membrane-bound Salmonella to maintain lasting SPI-2 T3SA activities. Another possible opportunity for inactivation of SPI-1 and SPI-2 T3SA could happen in cytoplasmic bacteria, which represent between 6 and 51% of intracellular bacteria depending on the stage of infection (Knodler et al., 2014). Enteropathogenic E. coli (EPEC) and C. rodentium associate stably with the PM through the formation of pedestals structure by secreting their own receptor Tir (Kenny et al., 1997; Mundy et al., 2005). As yet, methods developed to measure secretion activity have not shown regulation of T3SA activity following initial activation (Charpentier and Oswald, 2004; Mills et al., 2008, 2013; Yerushalmi et al., 2014). The different stages of adhesion in EPEC (e.g., bundling forming pili-, T3SS/Tir-, and EspA-dependent), leading progressively to more intimate interactions between bacteria and the host PM might nonetheless represent circumstances where the T3SA activity would be modulated (Cleary et al., 2004). Studies about T3SA regulation mechanisms in these bacterial pathogens and others would certainly benefit from the development of secretion activity reporters as well (Campbell-Valois and Sansonetti, 2014).
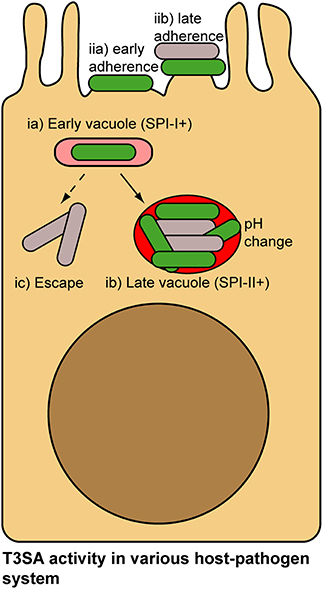
Figure 5. Alternative lifestyles of pathogenic bacteria associated with host cells and potential mechanisms of regulation of their T3SA. Salmonella invade epithelial cells using Salmonella Pathogenicity Island-1 (SPI-1) T3SA (ia). pH change in the vacuole and concomitant sensing of cytosolic pH induce activation of Salmonella Pathogenicity Island-2 (SPI-2) T3SA in Salmonella (ib). As bacteria accumulate in vacuole, they are not in contact with the membrane, which would probably inactivate SPI-1 and SPI-2 T3SA (ib). Occasionally Wt Salmonella escape their vacuole and access the cytoplasm. Loss of contact with vacuolar membrane in this case could also potentially lead to T3SA inactivation (ic). EPEC adhesion to the PM of epithelial cells proceeds in multiple stages (e.g., bundling forming pili-, T3SS/Tir- and EspA-dependent, etc.), culminating in the formation of actin-rich pedestals structure at bacterial adhesion point. Throughout this adhesion process, activity of the T3SA could be modulated (iia,b). In addition, within microcolonies some bacteria will occasionally loose contact with the PM, which similarly to the previous example could inactivate T3SA. Secreting bacteria, green; non-secreting bacteria, gray.
Conclusions
Many studies discussed above addressed how the regulated secretion of bacterial effectors impacts on their subcellular distribution, concentration, and function. Such observations could have important consequences. For example, part of effectors specificity could stem from their location rather than from the evolution of their catalytic site to accommodate a more restrained group of substrates. Historically, experimental approaches employed to determine host protein targets of bacterial effectors have been relying mostly on yeast-two-hybrid screens and overexpression in tissue culture cells. Although the legacy of these approaches in host-pathogen interactions is considerable, they are not optimal to find host targets that are selected on the basis of their location at or around actively secreting bacteria. Novel experimental strategies will have to be developed to tackle these questions.
Author Contributions
FXCV wrote the initial and final version. SP contributed ideas and wrote the final version.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to Laurie Pinaud for suggestions to improve the manuscript. This article was prepared with the financial contribution of the Faculty of Science of the University of Ottawa. FXCV was recently awarded a grant from the John R. Evans Leader Funds from the Canada Foundation for Innovation to pursue is work on intracellular pathogen adaptation to their host.
References
Ambrosi, C., Pompili, M., Scribano, D., Limongi, D., Petrucca, A., Cannavacciuolo, S., et al. (2015). The Shigella flexneri OspB effector: an early immunomodulator. Int. J. Med. Microbiol. 305, 75–84. doi: 10.1016/j.ijmm.2014.11.004
Arbibe, L., Kim, D. W., Batsche, E., Pedron, T., Mateescu, B., Muchardt, C., et al. (2007). An injected bacterial effector targets chromatin access for transcription factor NF-kappaB to alter transcription of host genes involved in immune responses. Nat. Immunol. 8, 47–56. doi: 10.1038/ni1423
Arena, E. T., Campbell-Valois, F.-X., Tinevez, J.-Y., Nigro, G., Sachse, M., Moya-Nilges, M., et al. (2015). Bioimage analysis of Shigella infection reveals targeting of colonic crypts. Proc. Natl. Acad. Sci. U.S.A. 112, E3282–E3290. doi: 10.1073/pnas.1509091112
Ashida, H., Kim, M., and Sasakawa, C. (2014). Exploitation of the host ubiquitin system by human bacterial pathogens. Nat. Rev. Microbiol. 12, 399–413. doi: 10.1038/nrmicro3259
Ashida, H., Kim, M., Schmidt-Supprian, M., Ma, A., Ogawa, M., and Sasakawa, C. (2010). A bacterial E3 ubiquitin ligase IpaH9.8 targets NEMO/IKKgamma to dampen the host NF-kappaB-mediated inflammatory response. Nat. Cell Biol. 12, 66–73; sup pp 1–9. doi: 10.1038/ncb2006
Ashida, H., Nakano, H., and Sasakawa, C. (2013). Shigella IpaH0722 E3 ubiquitin ligase effector targets TRAF2 to inhibit PKC-NF-kappaB activity in invaded epithelial cells. PLoS Pathog. 9:e1003409. doi: 10.1371/journal.ppat.1003409
Baxt, L. A., Garza-Mayers, A. C., and Goldberg, M. B. (2013). Bacterial subversion of host innate immune pathways. Science 340, 697–701. doi: 10.1126/science.1235771
Baxt, L. A., and Goldberg, M. B. (2014). Host and bacterial proteins that repress recruitment of LC3 to Shigella early during infection. PLoS ONE 9:e94653. doi: 10.1371/journal.pone.0094653
Bernardini, M. L., Mounier, J., d'Hauteville, H., Coquis-Rondon, M., and Sansonetti, P. J. (1989). Identification of icsA, a plasmid locus of Shigella flexneri that governs bacterial intra- and intercellular spread through interaction with F-actin. Proc. Natl. Acad. Sci. U.S.A. 86, 3867–3871. doi: 10.1073/pnas.86.10.3867
Blocker, A., Gounon, P., Larquet, E., Niebuhr, K., Cabiaux, V., Parsot, C., et al. (1999). The tripartite type III secreton of Shigella flexneri inserts IpaB and IpaC into host membranes. J. Cell Biol. 147, 683–693. doi: 10.1083/jcb.147.3.683
Bongrand, C., Sansonetti, P. J., and Parsot, C. (2012). Characterization of the promoter, MxiE box and 5' UTR of genes controlled by the activity of the type III secretion apparatus in Shigella flexneri. PLoS ONE 7:e32862. doi: 10.1371/journal.pone.0032862
Botteaux, A., Kayath, C. A., Page, A. L., Jouihri, N., Sani, M., Boekema, E., et al. (2010). The 33 carboxyl-terminal residues of Spa40 orchestrate the multi-step assembly process of the type III secretion needle complex in Shigella flexneri. Microbiology 156, 2807–2817. doi: 10.1099/mic.0.039651-0
Botteaux, A., Sani, M., Kayath, C. A., Boekema, E. J., and Allaoui, A. (2008). Spa32 interaction with the inner-membrane Spa40 component of the type III secretion system of Shigella flexneri is required for the control of the needle length by a molecular tape measure mechanism. Mol. Microbiol. 70, 1515–1528. doi: 10.1111/j.1365-2958.2008.06499.x
Botteaux, A., Sory, M. P., Biskri, L., Parsot, C., and Allaoui, A. (2009). MxiC is secreted by and controls the substrate specificity of the Shigella flexneri type III secretion apparatus. Mol. Microbiol. 71, 449–460. doi: 10.1111/j.1365-2958.2008.06537.x
Buchrieser, C., Glaser, P., Rusniok, C., Nedjari, H., d'Hauteville, H., Kunst, F., et al. (2000). The virulence plasmid pWR100 and the repertoire of proteins secreted by the type III secretion apparatus of Shigella flexneri. Mol. Microbiol. 38, 760–771. doi: 10.1046/j.1365-2958.2000.02179.x
Burkinshaw, B. J., and Strynadka, N. C. J. (2014). Assembly and structure of the T3SS. Biochim. Biophys. Acta 1843, 1649–1663. doi: 10.1016/j.bbamcr.2014.01.035
Burnaevskiy, N., Fox, T. G., Plymire, D. A., Ertelt, J. M., Weigele, B. A., Selyunin, A. S., et al. (2013). Proteolytic elimination of N-myristoyl modifications by the Shigella virulence factor IpaJ. Nature 496, 106–109. doi: 10.1038/nature12004
Burnaevskiy, N., Peng, T., Reddick, L. E., Hang, H. C., and Alto, N. M. (2015). Myristoylome profiling reveals a concerted mechanism of ARF GTPase deacylation by the bacterial protease IpaJ. Mol. Cell 58, 110–122. doi: 10.1016/j.molcel.2015.01.040
Campbell-Valois, F.-X., Sachse, M., Sansonetti, P. J., and Parsot, C. (2015). Escape of actively secreting Shigella flexneri from ATG8/LC3-positive vacuoles formed during cell-to-cell spread is facilitated by IcsB and VirA. mBio 6, e02567-14. doi: 10.1128/mBio.02567-14
Campbell-Valois, F.-X., and Sansonetti, P. J. (2014). Tracking bacterial pathogens with genetically-encoded reporters. FEBS Lett. 588, 2428–2436. doi: 10.1016/j.febslet.2014.05.022
Campbell-Valois, F.-X., Schnupf, P., Nigro, G., Sachse, M., Sansonetti, P. J., and Parsot, C. (2014b). A fluorescent reporter reveals on/off regulation of the Shigella type III secretion apparatus during entry and cell-to-cell spread. Cell Host Microbe. 15, 177–189. doi: 10.1016/j.chom.2014.01.005
Campbell-Valois, F. X., Schnupf, P., and Sansonetti, P. J. (2014a). Design of a transcription-based secretion activity reporter (TSAR) for the Type III secretion apparatus of Shigella flexneri and uses thereof. Bio-Protocol. 4:e1270. Available online at: http://www.bio-protocol.org/e1270
Campbell-Valois, F.-X., Trost, M., Chemali, M., Dill, B. D., Laplante, A., Duclos, S., et al. (2012). Quantitative proteomics reveals that only a subset of the endoplasmic reticulum contributes to the phagosome. Mol. Cell. Proteomics 11, M111.016378. doi: 10.1074/mcp.M111.016378
Carayol, N., and Tran Van Nhieu, G. (2013). Tips and tricks about Shigella invasion of epithelial cells. Curr. Opin. Microbiol. 16, 32–37. doi: 10.1016/j.mib.2012.11.010
Charpentier, X., and Oswald, E. (2004). Identification of the secretion and translocation domain of the enteropathogenic and enterohemorrhagic Escherichia coli effector Cif, using TEM-1 beta-lactamase as a new fluorescence-based reporter. J. Bacteriol. 186, 5486–5495. doi: 10.1128/JB.186.16.5486-5495.2004
Cherradi, Y., Schiavolin, L., Moussa, S., Meghraoui, A., Meksem, A., Biskri, L., et al. (2013). Interplay between predicted inner-rod and gatekeeper in controlling substrate specificity of the type III secretion system. Mol. Microbiol. 87, 1183–1199. doi: 10.1111/mmi.12158
Cheung, M., Shen, D.-K., Makino, F., Kato, T., Roehrich, A. D., Martinez-Argudo, I., et al. (2015). Three-dimensional electron microscopy reconstruction and cysteine-mediated crosslinking provide a model of the type III secretion system needle tip complex. Mol. Microbiol. 95, 31–50. doi: 10.1111/mmi.12843
Cleary, J., Lai, L.-C., Shaw, R. K., Straatman-Iwanowska, A., Donnenberg, M. S., Frankel, G., et al. (2004). Enteropathogenic Escherichia coli (EPEC) adhesion to intestinal epithelial cells: role of bundle-forming pili (BFP), EspA filaments and intimin. Microbiology 150, 527–538. doi: 10.1099/mic.0.26740-0
Costa, S. C. P., Schmitz, A. M., Jahufar, F. F., Boyd, J. D., Cho, M. Y., Glicksman, M. A., et al. (2012). A new means to identify type 3 secreted effectors: functionally interchangeable class IB chaperones recognize a conserved sequence. mBio 3, e00243-e00211. doi: 10.1128/mbio.00243-11
Demers, B., Sansonetti, P. J., and Parsot, C. (1998). Induction of type III secretion in Shigella flexneri is associated with differential control of transcription of genes encoding secreted proteins. EMBO J. 17, 2894–2903. doi: 10.1093/emboj/17.10.2894
Demers, J.-P., Sgourakis, N. G., Gupta, R., Loquet, A., Giller, K., Riedel, D., et al. (2013). The common structural architecture of Shigella flexneri and Salmonella typhimurium type three secretion needles. PLoS Pathog. 9:e1003245. doi: 10.1371/journal.ppat.1003245
Dobbs, N., Burnaevskiy, N., Chen, D., Gonugunta, V. K., Alto, N. M., and Yan, N. (2015). STING activation by translocation from the ER is associated with infection and autoinflammatory disease. Cell Host Microbe 18, 157–168. doi: 10.1016/j.chom.2015.07.001
Dong, N., Zhu, Y., Lu, Q., Hu, L., Zheng, Y., and Shao, F. (2012). Structurally distinct bacterial TBC-like GAPs link Arf GTPase to Rab1 inactivation to counteract host defenses. Cell 150, 1029–1041. doi: 10.1016/j.cell.2012.06.050
Dragoi, A.-M., and Agaisse, H. (2014). The serine/threonine kinase STK11 promotes Shigella flexneri dissemination through establishment of cell-cell contacts competent for tyrosine kinase signaling. Infect. Immun. 82, 4447–4457. doi: 10.1128/IAI.02078-14
Edgren, T., Forsberg, A., Rosqvist, R., and Wolf-Watz, H. (2012). Type III secretion in Yersinia: injectisome or not? PLoS Pathog. 8:e1002669. doi: 10.1371/journal.ppat.1002669
Enninga, J., Mounier, J., Sansonetti, P., and Tran Van Nhieu, G. (2005). Secretion of type III effectors into host cells in real time. Nat. Methods 2, 959–965. doi: 10.1038/nmeth804
Epler, C. R., Dickenson, N. E., Bullitt, E., and Picking, W. L. (2012). Ultrastructural analysis of IpaD at the tip of the nascent MxiH type III secretion apparatus of Shigella flexneri. J. Mol. Biol. 420, 29–39. doi: 10.1016/j.jmb.2012.03.025
Epler, C. R., Dickenson, N. E., Olive, A. J., Picking, W. L., and Picking, W. D. (2009). Liposomes recruit IpaC to the Shigella flexneri type III secretion apparatus needle as a final step in secretion induction. Infect. Immun. 77, 2754–2761. doi: 10.1128/IAI.00190-09
Falconi, M., Colonna, B., Prosseda, G., Micheli, G., and Gualerzi, C. O. (1998). Thermoregulation of Shigella and Escherichia coli EIEC pathogenicity. A temperature-dependent structural transition of DNA modulates accessibility of virF promoter to transcriptional repressor H-NS. EMBO J. 17, 7033–7043. doi: 10.1093/emboj/17.23.7033
Falconi, M., Prosseda, G., Giangrossi, M., Beghetto, E., and Colonna, B. (2001). Involvement of FIS in the H-NS-mediated regulation of virF gene of Shigella and enteroinvasive Escherichia coli. Mol. Microbiol. 42, 439–452. doi: 10.1046/j.1365-2958.2001.02646.x
Fernandez-Prada, C. M., Hoover, D. L., Tall, B. D., Hartman, A. B., Kopelowitz, J., and Venkatesan, M. M. (2000). Shigella flexneri IpaH(7.8) facilitates escape of virulent bacteria from the endocytic vacuoles of mouse and human macrophages. Infect. Immun. 68, 3608–3619. doi: 10.1128/IAI.68.6.3608-3619.2000
Galán, J. E. (2009). Common themes in the design and function of bacterial effectors. Cell Host Microbe 5, 571–579. doi: 10.1016/j.chom.2009.04.008
Ghosh, P. (2004). Process of protein transport by the type III secretion system. Microbiol. Mol. Biol. Rev. 68, 771–795. doi: 10.1128/MMBR.68.4.771-795.2004
Gong, L., Cullinane, M., Treerat, P., Ramm, G., Prescott, M., Adler, B., et al. (2011). The Burkholderia pseudomallei type III secretion system and BopA are required for evasion of LC3-associated phagocytosis. PLoS ONE 6:e17852. doi: 10.1371/journal.pone.0017852
Grishin, A. M., Condos, T. E. C., Barber, K. R., Campbell-Valois, F.-X., Parsot, C., Shaw, G. S., et al. (2014). Structural basis for the inhibition of host protein ubiquitination by Shigella effector kinase OspG. Structure 22, 878–888. doi: 10.1016/j.str.2014.04.010
Harouz, H., Rachez, C., Meijer, B. M., Marteyn, B., Donnadieu, F., Cammas, F., et al. (2014). Shigella flexneri targets the HP1γ subcode through the phosphothreonine lyase OspF. EMBO J. 33, 2606–2622. doi: 10.15252/embj.201489244
Hodgkinson, J. L., Horsley, A., Stabat, D., Simon, M., Johnson, S., da Fonseca, P. C. A., et al. (2009). Three-dimensional reconstruction of the Shigella T3SS transmembrane regions reveals 12-fold symmetry and novel features throughout. Nat. Struct. Mol. Biol. 16, 477–485. doi: 10.1038/nsmb.1599
Hu, B., Morado, D. R., Margolin, W., Rohde, J. R., Arizmendi, O., Picking, W. L., et al. (2015). Visualization of the type III secretion sorting platform of Shigella flexneri. Proc. Natl. Acad. Sci. U.S.A. 112, 1047–1052. doi: 10.1073/pnas.1411610112
Huang, J., and Brumell, J. H. (2014). Bacteria-autophagy interplay: a battle for survival. Nat. Rev. Microbiol. 12, 101–114. doi: 10.1038/nrmicro3160
Huang, J., Birmingham, C. L., Shahnazari, S., Shiu, J., Zheng, Y. T., Smith, A. C., et al. (2011). Antibacterial autophagy occurs at PI(3)P-enriched domains of the endoplasmic reticulum and requires Rab1 GTPase. Autophagy 7, 17–26. doi: 10.4161/auto.7.1.13840
Huynh, K. K., Gershenzon, E., and Grinstein, S. (2008). Cholesterol accumulation by macrophages impairs phagosome maturation. J. Biol. Chem. 283, 35745–35755. doi: 10.1074/jbc.M806232200
Ingmundson, A., Delprato, A., Lambright, D. G., and Roy, C. R. (2007). Legionella pneumophila proteins that regulate Rab1 membrane cycling. Nature 450, 365–369. doi: 10.1038/nature06336
Jiang, Y., Yang, F., Zhang, X., Yang, J., Chen, L., Yan, Y., et al. (2005). The complete sequence and analysis of the large virulence plasmid pSS of Shigella sonnei. Plasmid 54, 149–159. doi: 10.1016/j.plasmid.2005.03.002
Johansson, E. W., Wardlaw, T., Binkin, N., Brocklehurst, C., Dooley, T., Salama, P., et al. (2009). Diarrhoea: Why Children are Still Dying and What can be Done. New York, NY: United Nations International Children's Emergency Fund/World Health Organization.
Journet, L., Agrain, C., Broz, P., and Cornelis, G. R. (2003). The needle length of bacterial injectisomes is determined by a molecular ruler. Science 302, 1757–1760. doi: 10.1126/science.1091422
Kane, K. A., and Dorman, C. J. (2012). VirB-mediated positive feedback control of the virulence gene regulatory cascade of Shigella flexneri. J. Bacteriol. 194, 5264–5273. doi: 10.1128/JB.00800-12
Kayath, C. A., Hussey, S., El hajjami, N., Nagra, K., Philpott, D., and Allaoui, A. (2010). Escape of intracellular Shigella from autophagy requires binding to cholesterol through the type III effector, IcsB. Microbes Infect. 12, 956–966. doi: 10.1016/j.micinf.2010.06.006
Kenny, B., DeVinney, R., Stein, M., Reinscheid, D. J., Frey, E. A., and Finlay, B. B. (1997). Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell 91, 511–520. doi: 10.1016/S0092-8674(00)80437-7
Kim, D. W., Lenzen, G., Page, A.-L., Legrain, P., Sansonetti, P. J., and Parsot, C. (2005). The Shigella flexneri effector OspG interferes with innate immune responses by targeting ubiquitin-conjugating enzymes. Proc. Natl. Acad. Sci. U.S.A. 102, 14046–14051. doi: 10.1073/pnas.0504466102
Knodler, L. A., Nair, V., and Steele-Mortimer, O. (2014). Quantitative assessment of cytosolic Salmonella in epithelial cells. PLoS ONE 9:e84681. doi: 10.1371/journal.pone.0084681
Konradt, C., Frigimelica, E., Nothelfer, K., Puhar, A., Salgado-Pabon, W., di Bartolo, V., et al. (2011). The Shigella flexneri type three secretion system effector IpgD inhibits T cell migration by manipulating host phosphoinositide metabolism. Cell Host Microbe 9, 263–272. doi: 10.1016/j.chom.2011.03.010
Kotloff, K. L., Winickoff, J. P., Ivanoff, B., Clemens, J. D., Swerdlow, D. L., Sansonetti, P. J., et al. (1999). Global burden of Shigella infections: implications for vaccine development and implementation of control strategies. Bull. World Health Organ. 77, 651–666.
Kuehl, C. J., Dragoi, A.-M., Talman, A., and Agaisse, H. (2015). Bacterial spread from cell to cell: beyond actin-based motility. Trends Microbiol. 23, 558–566. doi: 10.1016/j.tim.2015.04.010
Kühn, T., Ihalainen, T. O., Hyväluoma, J., Dross, N., Willman, S. F., Langowski, J., et al. (2011). Protein diffusion in mammalian cell cytoplasm. PLoS ONE 6:e22962. doi: 10.1371/journal.pone.0022962
Lafont, F., Tran Van Nhieu, G., Hanada, K., Sansonetti, P., and van der Goot, F. G. (2002). Initial steps of Shigella infection depend on the cholesterol/sphingolipid raft-mediated CD44-IpaB interaction. EMBO J. 21, 4449–4457. doi: 10.1093/emboj/cdf457
Lai, S. C., and Devenish, R. J. (2012). LC3-Associated Phagocytosis (LAP): connections with host autophagy. Cells 1, 396–408. doi: 10.3390/cells1030396
Lara-Tejero, M., Kato, J., Wagner, S., Liu, X., and Galán, J. E. (2011). A sorting platform determines the order of protein secretion in bacterial type III systems. Science 331, 1188–1191. doi: 10.1126/science.1201476
LaRock, D. L., Chaudhary, A., and Miller, S. I. (2015). Salmonellae interactions with host processes. Nat. Rev. Microbiol. 13, 191–205. doi: 10.1038/nrmicro3420
Le Gall, T., Clermont, O., Gouriou, S., Picard, B., Nassif, X., Denamur, E., et al. (2007). Extraintestinal virulence is a coincidental by-product of commensalism in B2 phylogenetic group Escherichia coli strains. Mol. Biol. Evol. 24, 2373–2384. doi: 10.1093/molbev/msm172
Li, H., Xu, H., Zhou, Y., Zhang, J., Long, C., Li, S., et al. (2007). The phosphothreonine lyase activity of a bacterial type III effector family. Science 315, 1000–1003. doi: 10.1126/science.1138960
Lilic, M., Vujanac, M., and Stebbins, C. E. (2006). A common structural motif in the binding of virulence factors to bacterial secretion chaperones. Mol. Cell 21, 653–664. doi: 10.1016/j.molcel.2006.01.026
Lippmann, J., Gwinner, F., Rey, C., Tamir, U., Law, H. K. W., Schwikowski, B., et al. (2015). Bacterial internalization, localization, and effectors shape the epithelial immune response during Shigella flexneri infection. Infect. Immun. 83, 3624–3637. doi: 10.1128/IAI.00574-15
Liss, V., and Hensel, M. (2015). Take the tube: remodelling of the endosomal system by intracellular Salmonella enterica. Cell. Microbiol. 17, 639–647. doi: 10.1111/cmi.12441
Lukjancenko, O., Wassenaar, T. M., and Ussery, D. W. (2010). Comparison of 61 sequenced Escherichia coli genomes. Microb. Ecol. 60, 708–720. doi: 10.1007/s00248-010-9717-3
Marlovits, T. C., Kubori, T., Lara-Tejero, M., Thomas, D., Unger, V. M., and Galán, J. E. (2006). Assembly of the inner rod determines needle length in the type III secretion injectisome. Nature 441, 637–640. doi: 10.1038/nature04822
Martinez-Argudo, I., and Blocker, A. J. (2010). The Shigella T3SS needle transmits a signal for MxiC release, which controls secretion of effectors. Mol. Microbiol. 78, 1365–1378. doi: 10.1111/j.1365-2958.2010.07413.x
Martino, M. C., Rossi, G., Martini, I., Tattoli, I., Chiavolini, D., Phalipon, A., et al. (2005). Mucosal lymphoid infiltrate dominates colonic pathological changes in murine experimental shigellosis. J. Infect. Dis. 192, 136–148. doi: 10.1086/430740
Maurelli, A. T., and Sansonetti, P. J. (1988). Identification of a chromosomal gene controlling temperature-regulated expression of Shigella virulence. Proc. Natl. Acad. Sci. U.S.A. 85, 2820–2824. doi: 10.1073/pnas.85.8.2820
Mavris, M., Page, A. L., Tournebize, R., Demers, B., Sansonetti, P., and Parsot, C. (2002a). Regulation of transcription by the activity of the Shigella flexneri type III secretion apparatus. Mol. Microbiol. 43, 1543–1553. doi: 10.1046/j.1365-2958.2002.02836.x
Mavris, M., Sansonetti, P. J., and Parsot, C. (2002b). Identification of the cis-acting site involved in activation of promoters regulated by activity of the type III secretion apparatus in Shigella flexneri. J. Bacteriol. 184, 6751–6759. doi: 10.1128/JB.184.24.6751-6759.2002
Mellouk, N., Weiner, A., Aulner, N., Schmitt, C., Elbaum, M., Shorte, S. L., et al. (2014). Shigella subverts the host recycling compartment to rupture its vacuole. Cell Host Microbe 16, 517–530. doi: 10.1016/j.chom.2014.09.005
Ménard, R., Dehio, C., and Sansonetti, P. J. (1996). Bacterial entry into epithelial cells: the paradigm of Shigella. Trends Microbiol. 4, 220–226. doi: 10.1016/0966-842X(96)10039-1
Ménard, R., Sansonetti, P. J., and Parsot, C. (1993). Nonpolar mutagenesis of the ipa genes defines IpaB, IpaC, and IpaD as effectors of Shigella flexneri entry into epithelial cells. J. Bacteriol. 175, 5899–5906.
Ménard, R., Sansonetti, P., and Parsot, C. (1994a). The secretion of the Shigella flexneri Ipa invasins is activated by epithelial cells and controlled by IpaB and IpaD. EMBO J. 13, 5293–5302.
Ménard, R., Sansonetti, P., Parsot, C., and Vasselon, T. (1994b). Extracellular association and cytoplasmic partitioning of the IpaB and IpaC invasins of S. flexneri. Cell 79, 515–525. doi: 10.1016/0092-8674(94)90260-7
Mills, E., Baruch, K., Aviv, G., Nitzan, M., and Rosenshine, I. (2013). Dynamics of the type III secretion system activity of enteropathogenic Escherichia coli. mBio 4, e00303–e00313. doi: 10.1128/mbio.00303-13
Mills, E., Baruch, K., Charpentier, X., Kobi, S., and Rosenshine, I. (2008). Real-time analysis of effector translocation by the type III secretion system of enteropathogenic Escherichia coli. Cell Host Microbe 3, 104–113. doi: 10.1016/j.chom.2007.11.007
Morita-Ishihara, T., Ogawa, M., Sagara, H., Yoshida, M., Katayama, E., and Sasakawa, C. (2006). Shigella Spa33 is an essential C-ring component of type III secretion machinery. J. Biol. Chem. 281, 599–607. doi: 10.1074/jbc.M509644200
Mounier, J., Boncompain, G., Senerovic, L., Lagache, T., Chrétien, F., Perez, F., et al. (2012). Shigella effector IpaB-induced cholesterol relocation disrupts the Golgi complex and recycling network to inhibit host cell secretion. Cell Host Microbe 12, 381–389. doi: 10.1016/j.chom.2012.07.010
Mundy, R., MacDonald, T. T., Dougan, G., Frankel, G., and Wiles, S. (2005). Citrobacter rodentium of mice and man. Cell. Microbiol. 7, 1697–1706. doi: 10.1111/j.1462-5822.2005.00625.x
Niebuhr, K., Giuriato, S., Pedron, T., Philpott, D. J., Gaits, F., Sable, J., et al. (2002). Conversion of PtdIns(4,5)P(2) into PtdIns(5)P by the S.flexneri effector IpgD reorganizes host cell morphology. EMBO J. 21, 5069–5078. doi: 10.1093/emboj/cdf522
Nothelfer, K., Arena, E. T., Pinaud, L., Neunlist, M., Mozeleski, B., Belotserkovsky, I., et al. (2014). B lymphocytes undergo TLR2-dependent apoptosis upon Shigella infection. J. Exp. Med. 211, 1215–1229. doi: 10.1084/jem.20130914
Ogawa, M., Handa, Y., Ashida, H., Suzuki, M., and Sasakawa, C. (2008). The versatility of Shigella effectors. Nat. Rev. Microbiol. 6, 11–16. doi: 10.1038/nrmicro1814
Ogawa, M., Yoshimori, T., Suzuki, T., Sagara, H., Mizushima, N., and Sasakawa, C. (2005). Escape of intracellular Shigella from autophagy. Science 307, 727–731. doi: 10.1126/science.1106036
Onodera, N. T., Ryu, J., Durbic, T., Nislow, C., Archibald, J. M., and Rohde, J. R. (2012). Genome sequence of Shigella flexneri serotype 5a strain M90T Sm. J. Bacteriol. 194, 3022–3022. doi: 10.1128/JB.00393-12
Page, A. L., Ohayon, H., Sansonetti, P. J., and Parsot, C. (1999). The secreted IpaB and IpaC invasins and their cytoplasmic chaperone IpgC are required for intercellular dissemination of Shigella flexneri. Cell. Microbiol. 1, 183–193. doi: 10.1046/j.1462-5822.1999.00019.x
Parsot, C. (2009). Shigella type III secretion effectors: how, where, when, for what purposes? Curr. Opin. Microbiol. 12, 110–116. doi: 10.1016/j.mib.2008.12.002
Paz, I., Sachse, M., Dupont, N., Mounier, J., Cederfur, C., Enninga, J., et al. (2010). Galectin-3, a marker for vacuole lysis by invasive pathogens. Cell. Microbiol. 12, 530–544. doi: 10.1111/j.1462-5822.2009.01415.x
Pendaries, C., Tronchère, H., Arbibe, L., Mounier, J., Gozani, O., Cantley, L., et al. (2006). PtdIns5P activates the host cell PI3-kinase/Akt pathway during Shigella flexneri infection. EMBO J. 25, 1024–1034. doi: 10.1038/sj.emboj.7601001
Phalipon, A., Mulard, L. A., and Sansonetti, P. J. (2008). Vaccination against shigellosis: is it the path that is difficult or is it the difficult that is the path? Microbes Infect. 10, 1057–1062. doi: 10.1016/j.micinf.2008.07.016
Pilonieta, M. C., and Munson, G. P. (2008). The chaperone IpgC copurifies with the virulence regulator MxiE. J. Bacteriol. 190, 2249–2251. doi: 10.1128/JB.01824-07
Pruneda, J. N., Smith, F. D., Daurie, A., Swaney, D. L., Villén, J., Scott, J. D., et al. (2014). E2~Ub conjugates regulate the kinase activity of Shigella effector OspG during pathogenesis. EMBO J. 33, 437–449. doi: 10.1002/embj.201386386
Puhar, A., Tronchère, H., Payrastre, B., Nhieu, G. T. V., and Sansonetti, P. J. (2013). A Shigella effector dampens inflammation by regulating epithelial release of danger signal ATP through production of the lipid mediator PtdIns5P. Immunity 39, 1121–1131. doi: 10.1016/j.immuni.2013.11.013
Ramamurthi, K. S., and Schneewind, O. (2003). Substrate recognition by the Yersinia type III protein secretion machinery. Mol. Microbiol. 50, 1095–1102. doi: 10.1046/j.1365-2958.2003.03777.x
Rohde, J. R., Breitkreutz, A., Chenal, A., Sansonetti, P. J., and Parsot, C. (2007). Type III secretion effectors of the IpaH family are E3 ubiquitin ligases. Cell Host Microbe 1, 77–83. doi: 10.1016/j.chom.2007.02.002
Salgado-Pabón, W., Celli, S., Arena, E. T., Nothelfer, K., Roux, P., Sellge, G., et al. (2013). Shigella impairs T lymphocyte dynamics in vivo. Proc. Natl. Acad. Sci. U.S.A. 110, 4458–4463. doi: 10.1073/pnas.1300981110
Sansonetti, P. J., and Arondel, J. (1989). Construction and evaluation of a double mutant of Shigella flexneri as a candidate for oral vaccination against shigellosis. Vaccine 7, 443–450. doi: 10.1016/0264-410X(89)90160-6
Sansonetti, P. J., Hale, T. L., Dammin, G. J., Kapfer, C., Collins, H. H. J., and Formal, S. B. (1983). Alterations in the pathogenicity of Escherichia coli K-12 after transfer of plasmid and chromosomal genes from Shigella flexneri. Infect. Immun. 39, 1392–1402.
Schnupf, P., and Sansonetti, P. J. (2012). Quantitative RT-PCR profiling of the rabbit immune response: assessment of acute Shigella flexneri infection. PLoS ONE 7:e36446. doi: 10.1371/journal.pone.0036446
Schuch, R., Sandlin, R. C., and Maurelli, A. T. (1999). A system for identifying post-invasion functions of invasion genes: requirements for the Mxi-Spa type III secretion pathway of Shigella flexneri in intercellular dissemination. Mol. Microbiol. 34, 675–689. doi: 10.1046/j.1365-2958.1999.01627.x
Shim, D. H., Suzuki, T., Chang, S. Y., Park, S. M., Sansonetti, P. J., Sasakawa, C., et al. (2007). New animal model of shigellosis in the Guinea pig: its usefulness for protective efficacy studies. J. Immunol. 178, 2476–2482. doi: 10.4049/jimmunol.178.4.2476
Singer, A. U., Rohde, J. R., Lam, R., Skarina, T., Kagan, O., Dileo, R., et al. (2008). Structure of the Shigella T3SS effector IpaH defines a new class of E3 ubiquitin ligases. Nat. Struct. Mol. Biol. 15, 1293–1301. doi: 10.1038/nsmb.1511
Stevens, J. M., Galyov, E. E., and Stevens, M. P. (2006). Actin-dependent movement of bacterial pathogens. Nat. Rev. Microbiol. 4, 91–101. doi: 10.1038/nrmicro1320
Suzuki, S., Mimuro, H., Kim, M., Ogawa, M., Ashida, H., Toyotome, T., et al. (2014). Shigella IpaH7.8 E3 ubiquitin ligase targets glomulin and activates inflammasomes to demolish macrophages. Proc. Natl. Acad. Sci. U.S.A. 111, E4254–E4263. doi: 10.1073/pnas.1324021111
Tanner, K., Brzovic, P., and Rohde, J. R. (2015). The bacterial pathogen-ubiquitin interface: lessons learned from Shigella. Cell. Microbiol. 17, 35–44. doi: 10.1111/cmi.12390
Thompson, C. N., Duy, P. T., and Baker, S. (2015). The Rising Dominance of Shigella sonnei: an intercontinental shift in the etiology of bacillary dysentery. PLoS Negl. Trop. Dis. 9:e0003708. doi: 10.1371/journal.pntd.0003708
Tobe, T., Nagai, S., Okada, N., Adler, B., Yoshikawa, M., and Sasakawa, C. (1991). Temperature-regulated expression of invasion genes in Shigella flexneri is controlled through the transcriptional activation of the virB gene on the large plasmid. Mol. Microbiol. 5, 887–893. doi: 10.1111/j.1365-2958.1991.tb00762.x
Tran Van Nhieu, G., Clair, C., Bruzzone, R., Mesnil, M., Sansonetti, P., and Combettes, L. (2003). Connexin-dependent inter-cellular communication increases invasion and dissemination of Shigella in epithelial cells. Nat. Cell Biol. 5, 720–726. doi: 10.1038/ncb1021
Valencia-Gallardo, C. M., Carayol, N., and Tran Van Nhieu, G. (2015). Cytoskeletal mechanics during Shigella invasion and dissemination in epithelial cells. Cell. Microbiol. 17, 174–182. doi: 10.1111/cmi.12400
van der Goot, F. G., Tran Van Nhieu, G., Allaoui, A., Sansonetti, P., and Lafont, F. (2004). Rafts can trigger contact-mediated secretion of bacterial effectors via a lipid-based mechanism. J. Biol. Chem. 279, 47792–47798. doi: 10.1074/jbc.M406824200
van Meer, G., Voelker, D. R., and Feigenson, G. W. (2008). Membrane lipids: where they are and how they behave. Nat. Rev. Mol. Cell Biol. 9, 112–124. doi: 10.1038/nrm2330
Veenendaal, A. K. J., Hodgkinson, J. L., Schwarzer, L., Stabat, D., Zenk, S. F., and Blocker, A. J. (2007). The type III secretion system needle tip complex mediates host cell sensing and translocon insertion. Mol. Microbiol. 63, 1719–1730. doi: 10.1111/j.1365-2958.2007.05620.x
Venkatesan, M. M., Goldberg, M. B., Rose, D. J., Grotbeck, E. J., Burland, V., and Blattner, F. R. (2001). Complete DNA sequence and analysis of the large virulence plasmid of Shigella flexneri. Infect. Immun. 69, 3271–3285. doi: 10.1128/IAI.69.5.3271-3285.2001
Wang, F., Jiang, Z., Li, Y., He, X., Zhao, J., Yang, X., et al. (2013). Shigella flexneri T3SS effector IpaH4.5 modulates the host inflammatory response via interaction with NF-kappaB p65 protein. Cell. Microbiol. 15, 474–485. doi: 10.1111/cmi.12052
Yerushalmi, G., Litvak, Y., Gur-Arie, L., and Rosenshine, I. (2014). Dynamics of expression and maturation of the type III secretion system of enteropathogenic Escherichia coli. J. Bacteriol. 196, 2798–2806. doi: 10.1128/JB.00069-14
Yu, X.-J., McGourty, K., Liu, M., Unsworth, K. E., and Holden, D. W. (2010). pH sensing by intracellular Salmonella induces effector translocation. Science 328, 1040–1043. doi: 10.1126/science.1189000
Zhang, J., Liu, H., Zhang, X., Yang, J., Yang, F., Yang, G., et al. (2003). Complete DNA sequence and gene analysis of the virulence plasmid pCP301 of Shigella flexneri 2a. Sci. China C Life Sci. 46, 513–521. doi: 10.1360/02yc0060
Zhou, H.-X., Rivas, G., and Minton, A. P. (2008). Macromolecular crowding and confinement: biochemical, biophysical, and potential physiological consequences. Annu. Rev. Biophys. 37, 375–397. doi: 10.1146/annurev.biophys.37.032807.125817
Zhou, Y., Dong, N., Hu, L., and Shao, F. (2013). The Shigella type three secretion system effector OspG directly and specifically binds to host ubiquitin for activation. PLoS ONE 8:e57558. doi: 10.1371/journal.pone.0057558
Zhu, Y., Li, H., Hu, L., Wang, J., Zhou, Y., Pang, Z., et al. (2008). Structure of a Shigella effector reveals a new class of ubiquitin ligases. Nat. Struct. Mol. Biol. 15, 1302–1308. doi: 10.1038/nsmb.1517
Zoppino, F. C., Militello, R. D., Slavin, I., Alvarez, C., and Colombo, M. I. (2010). Autophagosome formation depends on the small GTPase Rab1 and functional ER exit sites. Traffic 11, 1246–1261. doi: 10.1111/j.1600-0854.2010.01086.x
Zurawski, D. V., Mumy, K. L., Faherty, C. S., McCormick, B. A., and Maurelli, A. T. (2009). Shigella flexneri type III secretion system effectors OspB and OspF target the nucleus to downregulate the host inflammatory response via interactions with retinoblastoma protein. Mol. Microbiol. 71, 350–368. doi: 10.1111/j.1365-2958.2008.06524.x
Zychlinsky, A., Kenny, B., Menard, R., Prevost, M. C., Holland, I. B., and Sansonetti, P. J. (1994). IpaB mediates macrophage apoptosis induced by Shigella flexneri. Mol. Microbiol. 11, 619–627. doi: 10.1111/j.1365-2958.1994.tb00341.x
Keywords: Shigella, type three secretion apparatus, type three secretion system, effectors, enteropathogens, host-pathogen interactions, signal transduction
Citation: Campbell-Valois F-X and Pontier SM (2016) Implications of Spatiotemporal Regulation of Shigella flexneri Type Three Secretion Activity on Effector Functions: Think Globally, Act Locally. Front. Cell. Infect. Microbiol. 6:28. doi: 10.3389/fcimb.2016.00028
Received: 03 November 2015; Accepted: 23 February 2016;
Published: 09 March 2016.
Edited by:
Guy T. V. Nhieu, College de France, FranceCopyright © 2016 Campbell-Valois and Pontier. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: F.-X. Campbell-Valois, fcampbel@uottawa.ca
 F.-X. Campbell-Valois
F.-X. Campbell-Valois Stéphanie M. Pontier
Stéphanie M. Pontier