Synchronous Disease Kinetics in a Murine Model for Enterohemorrhagic E. coli Infection Using Food-Borne Inoculation
- 1Molecular Biology and Microbiology, Sackler School of Graduate Biomedical Sciences, Tufts University, Boston, MA, USA
- 2Department of Molecular Biology and Microbiology, Tufts University School of Medicine, Boston, MA, USA
Upon colonization of the intestinal epithelium, the attaching and effacing (AE) pathogen Enterohemorrhagic Escherichia coli (EHEC) effaces microvilli and forms pedestal-like structures beneath the adherent bacterium. The production of one of its virulence factors, the phage-encoded Shiga toxin (Stx) results in systemic disease, including the development of renal failure. Although EHEC does not productively infect conventional mice, EHEC infection can be modeled in mice utilizing a derivative of the natural murine AE pathogen Citrobacter rodentium (CR). Gavage of mice with CR(ΦStx2dact), a C. rodentium lysogenized by a phage encoding an Stx variant with high potency in mice, features AE lesion formation on intestinal epithelium and Stx-mediated systemic disease, including renal damage. This model is somewhat limited by mouse-to-mouse variation in the course of disease, with the time to severe morbidity (and required euthanasia) varying by as many as 5 days, a feature that limits pathological analysis at defined stages of disease. In the current study, we altered and optimized the preparation, dose, and mode of delivery of CR(ΦStx2dact), using food-borne route of infection to generate highly synchronous disease model. We found that food-borne inoculation of as few as 3 × 104 CR(ΦStx2dact) resulted in productive colonization and severe systemic disease. Upon inoculation of 1 × 108 bacteria, the majority of infected animals suffered weight loss beginning 5 days post-infection and all required euthanasia on day 6 or 7. This enhanced murine model for EHEC infection should facilitate characterization of the pathology associated with specific phases of Stx-mediated disease.
Introduction
Enterohemorrhagic E. coli (EHEC) O157:H7 is a Gram-negative bacterium and a causative agent of intestinal and systemic disease (Wadolkowski et al., 1990a; Robinson et al., 2006; Mohawk and O'Brien, 2011; Melton-Celsa et al., 2012; Kaper and O'Brien, 2014). Humans are infected by this organism through contaminated food or water. EHEC is a member of the family of pathogenic bacteria known as attaching and effacing pathogens (AE pathogens), which include enteropathogenic E. coli (EPEC) and Citrobacter rodentium (CR). These organisms are capable of triggering localized actin assembly on epithelial cells beneath bound bacteria, forming pedestal-like structures (Brady et al., 2007; Vingadassalom et al., 2009; Lai et al., 2013). EHEC, however, is unique because it produces a potent phage-encoded cytotoxin termed Shiga-toxin (Stx) that is the major agent that causes the tissue damage during EHEC infection (Wadolkowski et al., 1990a,b; Proulx et al., 2001; Thorpe et al., 2001; Robinson et al., 2006; Obrig, 2010; Melton-Celsa et al., 2012). Upon establishment of intestinal colonization, Stx promotes intestinal damage resulting in bloody diarrhea. The toxin subsequently translocates across the colonic epithelium into the bloodstream, where it targets distal tissues, including the microvasculature of the kidney, which expresses high levels of the Stx receptor, globotriaosylceramide (Gb3) (Obrig et al., 1993; Obata et al., 2008; Obrig, 2010; Melton-Celsa et al., 2012; Bunger et al., 2013). Hence, EHEC infection exhibits distinct clinical phases such as non-bloody diarrhea, followed by bloody diarrhea, and systemic disease, the latter manifested most commonly by hemolytic uremic syndrome (HUS), the triad of hemolytic anemia, thromobocytopenia and renal failure (Keepers et al., 2006; Obrig, 2010; Melton-Celsa et al., 2012; Davis et al., 2014; Melton-Celsa and O'Brien, 2014; Freedman et al., 2016).
Mice are highly sensitive to Stx, which can cause renal tubular damage following IP injection (Keepers et al., 2006; Mohawk and O'Brien, 2011; Mallick et al., 2012). However, EHEC does not efficiently infect conventional mice. Furthermore, although germ-free and antibiotic-treated mice exhibit susceptibility to EHEC infection, these models are not useful for studying EHEC pathogenesis factors, as strains that lack colonization factors required for AE lesion formation are not required for virulence in these models (Wadolkowski et al., 1990a; Eaton et al., 2008; Kamada et al., 2012). To address these limitations, an alternative model, using conventional mice, was developed. The native murine AE pathogen C. rodentium (CR), which like EHEC forms attaching and effacing lesions, was lysogenized with phage Φ1720a-02, isolated from an Stx-producing E. coli strain (Mallick et al., 2012, 2014). This phage encodes and produces Stx variant Stx2dact, which is activated ~18-fold by intestinal mucus (Mallick et al., 2012; Bunger et al., 2013), resulting in high potency in mice (Teel et al., 2002; Bunger et al., 2013). The oral gavage of mice with a high (5 × 109 CFU) dose of an overnight culture of CR(Φ1720a-02), herein referred to as “CR(ΦStx2dact)” for simplicity, recapitulates many of the features of human EHEC infection in an Stx2dact-dependent manner, including colitis, renal damage, weight loss, and systemic injury.
Notably, a major limitation of this model is that disease kinetics are highly variable, with the duration of infection before mice require euthanasia varying from 4 to 9 days (Mallick et al., 2012). Thus, the unpredictable evolution of disease in this model precludes a careful characterization of pathology associated with distinct phases of infection (e.g., intestinal colonization, toxin dissemination, and renal failure). In the current study, we describe a method of preparation and delivery of the inoculum that results in a highly synchronous disease course, which should facilitate investigation of successive stages of Stx-mediated disease, and may more closely model the course of oral infection by an AE pathogen.
Materials and Methods
Strains and Inoculum Preparation
Two days prior to infection, CR(ΦStx2dact) and CR(ΦStx2dact::kanR), described previously (Mallick et al., 2012, 2014), were streaked from frozen glycerol stock onto LB agar containing the appropriate antibiotic (i.e., 10 μg/ml chloramphenicol for CR(ΦStx2dact), and 10 μg/ml chloramphenicol and 25 μg/ml kanamycin CR(ΦStx2dact::kanR) for single colonies. The following day, a single colony was inoculated into 40 ml LB broth (without antibiotic) in a 50 ml conical tube (VWR, catalog number 89039-662) and incubated at 37°C with 5% CO2, without shaking but with the cap loosened to allow for gas exchange. Cultures were incubated typically for 10–12 h, to achieve an OD600 ~ 0.6–0.7. The culture was centrifuged at 10294 × g for 12 min at room temperature. The bacterial pellet was resuspended in 500 μl of Dulbecco's Phosphate-buffered saline DPBS (Life technologies, catalog number 14190-144) and then centrifuged at room temperature at 5226 × g for 2 min. Bacterial pellets were resuspended in 60 μl of DPBS. Six micro liter of a resuspended inoculum, encompassing ~ 1 × 108 colony-forming units (cfu), was carefully pipetted onto a ~35 mg piece of Teklad 2918 irradiated rodent chow, visually ensuring that the inoculum was fully absorbed into the food (~1 min). For smaller inoculums, overnight cultures were appropriately diluted in PBS and into the food as described above. To titer the dose, a sample of the rodent chow inoculated in parallel was resuspended in 1 ml of DPBS, vortexed to disperse chow and bacteria, serially diluted, and plated on LB agar containing the appropriate antibiotic (i.e., 10 μg/ml chloramphenicol for CR(ΦStx2dact), and 10 μg/ml chloramphenicol and 25 μg/ml kanamycin CR(ΦStx2dact::kanR).
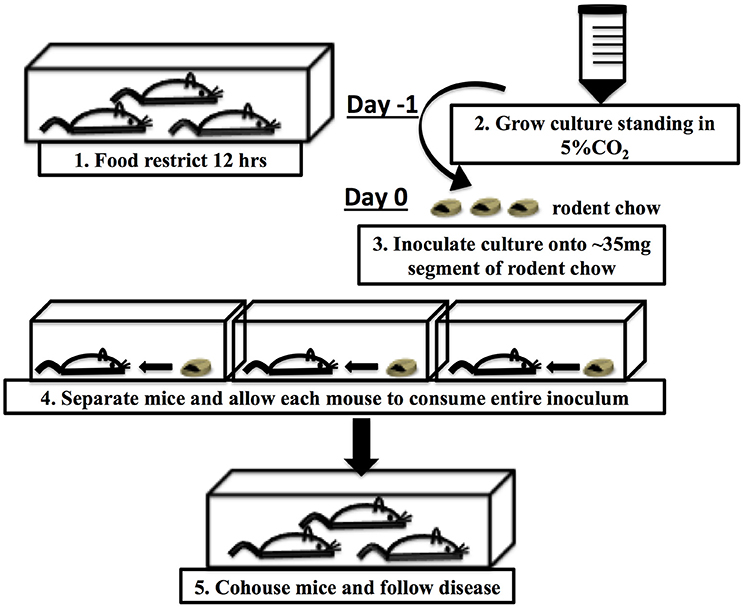
Infection of Mice
All procedures involving live animals were pre-approved by Tufts University Institutional Animal Care and Use Committee (IACUC). Five to six week old C57BL/6 mice were purchased from Jackson Laboratory and housed for 1–2 weeks in the animal facility at Tufts University School of Medicine prior to the start of infection. One day prior to infection, mice were weighed, cohoused on raised wire flooring to prevent coprophagy (Thoren caging systems: CC90F01), and food-restricted for 12 h. After food restriction, each mouse was individually placed into an empty cage and presented with a ~35 mg piece of inoculated rodent chow. After each mouse consumed the entire food inoculum, mice were weighed and returned to the original cage (without raised wire flooring) with access to unlimited food and water. For dose-response studies, mice were returned to caging with raised wire flooring to prevent coprophagy for the duration of the infection. Infection was followed by monitoring of CR(ΦStx2dact) or CR(ΦStx2dact::kanR) titers in the feces, as follows. Fecal samples, were weighed, suspended in DPBS at a volume of 10 μl per 1 mg of stool, and suspensions were serially diluted and plated on agar containing the appropriate antibiotic (10 μg/ml chloramphenicol for CR(ΦStx2dact), and 10 μg/ml chloramphenicol and 25 μg/ml kanamycin CR(ΦStx2dact::kanR). Mice were weighed and observed for clinical distress (ruffled fur, paucity of movement, and shivering) each day. Mice with greater than 12% body weight loss with or without signs of distress, or 10% body weight loss with signs of distress, were euthanized by CO2 inhalation followed by cervical dislocation. In addition, in some experiments, colonic and luminal colonization was measured by sacrificing mice at day 3, 4, or 5 post-infection. Each colon was dissected and stool contents were removed by gently pushing the stool through the colon with the smooth edge of a scalpel. The colonic luminal contents were collected by gently flushing the colons with 1 ml of DPBS. Colonic tissues were homogenized in 500 μl of DPBS using a Fisher Scientific PowerGen 125 Homogenizer. To determine the titer of CR(ΦStx2dact), colonic homogenates and luminal wash were serially diluted and plated on LB agar containing the appropriate antibiotic, as described above. For each weight loss experiment, the data passed the KS normality test.
Results
Inoculation of Mice by Feeding CR(ΦStx2dact) results in Efficient Colonization
The growth conditions of the inoculum are likely to influence the course of infection and disease. For example, host-adapted C. rodentium present in the feces of infected mice are in a hyper-infectious state, i.e., highly transmissible and capable of accelerated colonic colonization, compared to C. rodentium grown in LB (Wiles et al., 2005). EHEC grown in gnotobiotic piglets also exhibit enhanced virulence-related phenotypes (Brady et al., 2011). Thus, in considering ways to develop a consistent and predictable CR(ΦStx2dact) infection model, we first revisited the preparation of the inoculum. The previous described CR(ΦStx2dact) infection model, utilized bacteria that had been grown overnight to stationary phase (Mallick et al., 2012), a growth phase associated with global physiologic changes (Battesti et al., 2011), some of which alter infectivity and/or virulence (Molofsky and Swanson, 2004; Sonenshein, 2005). In addition, the CR(ΦStx2dact) cultures had previously been shaken, which facilitates aerobic growth and is predicted to sensitize C. rodentium to acid killing (Smith and Bhagwat, 2013). Hence, in our current study, we grew CR(ΦStx2dact) in a 5% CO2 atmosphere without shaking, a condition predicted to temper aerobic growth. These cultures were grown to an OD600 = ~0.6–0.7.
In the previously described CR(ΦStx2dact) model (Mallick et al., 2012), bacteria were delivered to the intestinal tract by oral gavage, a method that not only risks aspiration and esophageal and/or gastric puncture, but also induces a host stress response that might alter the course of infection (Hoggatt et al., 2010; Walker et al., 2012). Methods for intestinal delivery of pharmacological agents by feeding have been established that minimize this stress response (Walker et al., 2012). Further, delivery of Listeria monocytogenes in food rather than by gavage altered the fate of bacteria in the host and the virulence factors required for infection. (Bou Ghanem et al., 2012). Hence, 6–8 week old C57BL/6J mice were inoculated by ingestion of 1 × 108 CFU of CR(ΦStx2dact) absorbed into a small (~35 mg) piece of rodent chow (Figure 1 and Materials and Methods).
To determine if inoculation of mice with CR(ΦStx2dact) in this manner resulted in efficient intestinal colonization, we used fecal shedding CR(ΦStx2dact) as a non-terminal indicator of colonic colonization: on successive days following infection, we plated for viable CR(ΦStx2dact) on selective media. High levels of fecal shedding, i.e., ~107 cfu/g of feces, were observed on day 3 post-infection, increasing to >108 cfu/g of feces on days 4 and 5 post-infection (Figure 2A). High levels of colonization were also observed in colonic luminal washes and colonic homogenates following sacrifice. These titers of CR(ΦStx2dact) were roughly equal to the peak fecal shedding, colonic colonization, and luminal washes previously reported (Mallick et al., 2012, 2014) by intra-gastric inoculation. These data indicate that ingestion of food inoculated with mid-log phase bacteria grown without shaking results in efficient intestinal colonization.
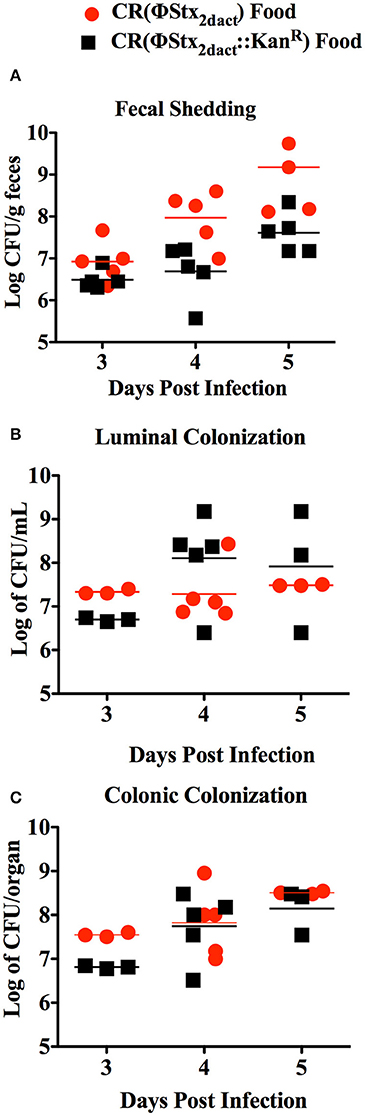
Figure 2. Inoculation of mice by feeding CR(ΦStx2dact) results in efficient colonization. Six-week old female C57/BL6 mice were inoculated with 1 × 108 CR(ΦStx2dact) or CR(ΦStx2dact::kanR) by feeding and viable counts of feces (A), colonic homogenates (B), or luminal washings (C) were determined by plating. Each point represents an individual mouse and each line represents the geometric mean.
The production of Stx by C. rodentium has been associated with slightly higher levels of intestinal colonization following inoculation by oral gavage (Mallick et al., 2012, 2014). Therefore, we also infected mice with CR(ΦStx2dact::kanR), a CR(ΦStx2dact) mutant that does not produce Stx. Fecal shedding of CR(ΦStx2dact::kanR) was indistinguishable from that of CR(ΦStx2dact) (Figure 2A), and CR(ΦStx2dact::kanR) titers were similar to those of CR(ΦStx2dact) in luminal washes and colonic homogenates. Thus, at least for this small sample size (n = 5), Stx did not detectably promote colonization in this model (Figure 2).
Food-Inoculation of Mice with As Few As 3 × 104 CR(ΦStx2dact) results in colonization, Disease, and Mortality
Mice are typically gavaged with 108 C. rodentium to initiate productive infection. To determine if inoculation of C57/BL6J mice with lower doses of CR(ΦStx2dact) by our feeding model would result in productive infection and disease, we fed groups of 10 mice doses of CR(ΦStx2dact) spanning four orders of magnitude, from 3.5 × 108 down to 3 × 104. To avoid coprophagy, which due to the ingestion of bacteria shed in feces would confound accurate quantitation of the oral dose, the mice were housed on elevated wire floors for the duration of the infection. Fecal shedding of CR(ΦStx2dact) revealed that although the kinetics of intestinal colonization appeared to be slightly delayed in mice receiving the lowest dose, all groups of mice were colonized by day 5 post-infection and reached >108/g stool by day 7 (Figure 3A). Similarly, all groups of mice lost weight, the kinetics of which varied with dose: the first indication of significant weight loss occurred on post-infection day 7 for the group of mice infected with 7.5 × 107 or 3.5 × 108 CR(ΦStx2dact), day 8 for the group infected with 3.6 × 106, and day 9 for the group infected with 3.0 × 104 (Figure 3B). Finally, all groups of mice became moribund and required euthanasia (Figure 3C). Consistent with the trends observed for the kinetics of intestinal colonization and weight loss, mice infected with lower doses exhibited a somewhat longer interval between inoculation and required euthanasia: by day 7 post-infection, the group of mice infected with 3.5 × 108 CR(ΦStx2dact) were all euthanized, whereas for groups of mice infected with 7.5 × 107, 3.6 × 105, and 3.0 × 104 bacteria, the day at which 100% were euthanized was day 8, 9, and 10, respectively. We conclude that feeding of C57/BL6J mice with as few as 3.0 × 104 CR(ΦStx2dact) results in productive and lethal infection.
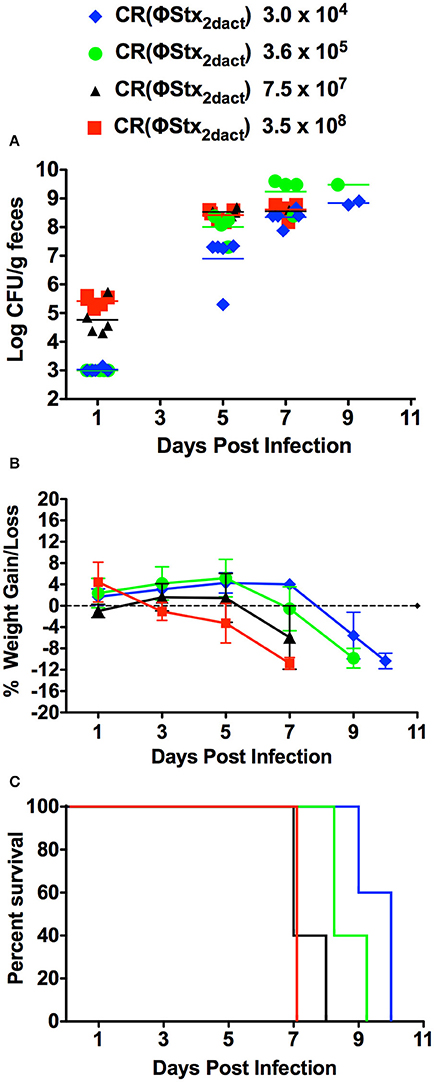
Figure 3. Food-borne inoculation of mice with as few as 3 × 104 CR(ΦStx2dact) results in colonization, disease and mortality. Groups of five mice were food-inoculated with the indicated dose of CR(ΦStx2dact) and fecal shedding (A), body weight as percent change from that prior to infection (B) and percent survival (C) were determined as described in Materials and Methods. In (A), each point represents an individual mouse and each line represents the geometric mean. For all panels, shown is a representative of two independent experiments.
Mice Inoculated with CR(ΦStx2dact) by Feeding Suffer Synchronous Disease and Mortality
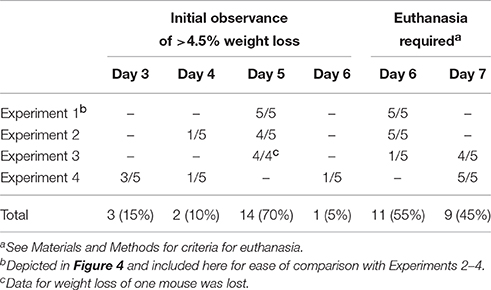
Previous work revealed that the kinetics of disease following gavage inoculation with stationary phase CR(ΦStx2dact) varied widely, e.g., resulting in required euthanasia as early as 4 days and as late as 9 days post-infection within a given experiment (Mallick et al., 2012). In contrast, the trend of delayed colonization and disease kinetics observed for mice given different doses of CR(ΦStx2dact) by feeding suggested that the time course of disease in this model may be more consistent than by oral gavage. To carefully assess the time course of disease, in multiple experiments mice were weighed daily after inoculation with mouse chow containing the relatively high dose of 1 × 108 CR(ΦStx2dact), or, as a control to evaluate Stx-dependent disease, CR(ΦStx2dact::kanR). (For simplicity of the protocol, and in contrast to the dose-response studies described above, mice were not housed on wire floors after inoculation, raising the possibility that the dose may be somewhat higher due to coprophagy.) In our first experiment, all five mice infected with CR(ΦStx2dact) crossed a threshold of 4.5% body weight loss on day 5 post infection, whereas mice infected with CR(ΦStx2dact::kanR) did not suffer weight loss (Figure 4A; Table 1, Experiment 1). In addition and in contrast to mice infected with the Stx-deficient strain, which uniformly survived infection, all mice infected with CR(ΦStx2dact) required euthanasia on day 6 (Figure 4; Table 1). To assess the reproducibility of the course of disease in this model, we repeated the experiment three times for a total of four independent experiments encompassing 20 mice (Table 1). Although the kinetics of weight loss and required euthanasia for Experiments 2 through 4 were not as uniform as in Experiment 1, cumulatively 70% of mice reached the threshold of >4.5% body weight loss on day 5 post-infection, and all mice required euthanasia on day 6 or 7 (Table 1). We conclude that this infection protocol, in contrast to the gavage model using stationary phase CR(ΦStx2dact), results in a highly synchronous course of disease.
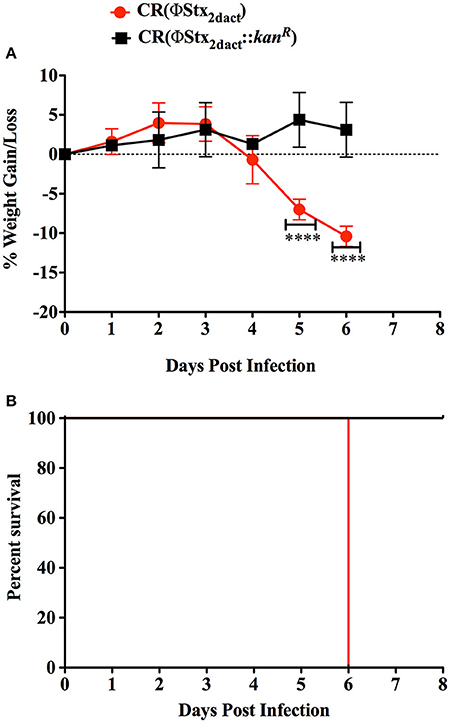
Figure 4. Inoculation with CR(ΦStx2dact) by feeding results in synchronous disease and mortality. Groups of five mice were inoculated with 1 × 108 CR(ΦStx2dact) or CR(ΦStx2dact::kanR), and mean (± SD) body weight as percent change from that prior to infection (A) and percent survival were monitored daily (B). ****Indicates p > 0.0001 by 2-way Anova followed by Bonferroni post-test. Shown is a representative of the four independent experiments that are described in Table 1.
Discussion
Serious life-threatening cases of EHEC infection evolve through successive phases of illness, beginning asymptomatically but followed by the development of non-bloody diarrhea, bloody diarrhea, and, in the most serious cases, culminating in HUS (Thorpe et al., 2001; Keepers et al., 2006; Obrig, 2010; Mohawk and O'Brien, 2011; Melton-Celsa et al., 2012; Davis et al., 2014; Freedman et al., 2016). As the success of a given clinical intervention may depend on the phase of illness, animal models of EHEC infection that permit analysis of specific phases of disease are invaluable to understanding the evolution of disease and developing successful therapeutic strategies. Murine infection by oral gavage with CR(ΦStx2dact) represents a useful EHEC infection model that features AE lesion formation, intestinal epithelial damage, and Stx-mediated renal damage (Davis et al., 2014). However, this model lacks the synchronicity of disease evolution, reflected by considerable variation in time to morbidity (Mallick et al., 2012), thus greatly complicating detailed investigation of stage-specific pathology.
In the current study, we altered two features of the existing CR(ΦStx2dact) model. First, because C. rodentium anaerobiosis is associated with enhanced acid-resistance and a transcriptomic profile more similar that of host-adapted, hyper-infectious bacteria (Smith and Bhagwat, 2013), we changed our culturing procedures by growing inocula without shaking. Studies with other enteric pathogens have revealed that the method of inoculum preparation can have dramatic effects on virulence in animal models (Clark et al., 1998; Brady et al., 2011). Second, we infected mice by feeding to avoid potential physical trauma. In addition, gavage is associated with host stress, reflected in an increase in heart rate, mean arterial pressure, and fecal corticosteroids (Hoggatt et al., 2010; Walker et al., 2012), factors that might contribute to mouse-to-mouse variability. Mice infected using this modified protocol were efficiently colonized even at doses as low as 3 × 104 bacteria, as reflected by titers of CR(ΦStx2dact) in feces and colonic homogenates. (In fact, further infection studies suggest that food-borne inoculation of doses fewer than 104 CR(ΦStx2dact), 2.35 × 103 and 6 × 103, also promotes colonization and disease; LJF, unpublished data). Although we did not perform a parallel dose-response study with gavage-delivered bacteria, this 3 × 104 dose is three or four orders of magnitude lower than what has been typically used in gavage studies (Vallance et al., 2003; Tennant et al., 2008; Bergstrom et al., 2010; Diez et al., 2011; Kamada et al., 2012). In contrast to this study, a previous study with gavage-inoculated mice suggested that fecal shedding of CR(ΦStx2dact) was slightly higher than that of CR(ΦStx2dact::kanR) (Mallick et al., 2012, 2014). However, detection of a statistically significant difference in colonization required much larger numbers of mice (n ≥ 20) than utilized in this study.
Importantly, compared with the previously described gavage model, infection using our model produced relatively synchronous disease; when four independent experiments were analyzed together, 70% of mice reached a threshold of >4.5% body weight loss on day 5 post-infection, and all mice required euthanasia on day 6 or 7 post-infection. Given that oral gavage is by far the most common method of inoculation for models of intestinal pathogens, we speculate that the preparation and inoculation methods described here might be widely applied to improve the reliability and synchronicity of other infection models.
It is important to note that several aspects of human HUS are not faithfully replicated in mice. Although murine Stx-injection or EHEC infection models have been associated with increased renal (as well as systemic) cytokines, renal damage is typically limited to tubule pathology and lacks the characteristic glomerular damage associated with HUS (Mallick et al., 2012; Melton-Celsa et al., 2012). This difference has been postulated to be due to the paucity of Gb3, the Stx receptor, on murine podocytes and renal endothelial cells, whose counterparts in humans are important targets of Stx intoxication (Keepers et al., 2006). Genetic manipulation of mice to ectopically produce Gb3 on critical target cells, in combination with the improved CR(ΦStx2dact) infection protocol described here, might facilitate the systematic investigation of the evolution of Stx-mediated disease upon EHEC infection.
Author Contributions
LF: Designed and performed the experiments and wrote the paper. EB: Suggested critical parameters in design of experiments and edited paper. JL: Provided advice in design, performance of experiments and co-wrote paper.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Emily Mallick for critical advice, Stacie Clark and Haley BrinJones for technical assistance and Dakshina Jandhyala, Cheleste Thorpe, Marcia Osbourne, and Cammie Lesser for critical reading and discussion of the manuscript. LF was supported by NIH T32AI07422 and T32GM007310 and EB is a Howard Hughes Medical Institute Fellow of Life Sciences Research Foundation. This work was also supported by NIH R21AI107587 and R01-AI46454 to JL.
References
Battesti, A., Majdalani, N., and Gottesman, S. (2011). The RpoS-mediated general stress response in Escherichia coli. Annu. Rev. Microbiol. 65, 189–213. doi: 10.1146/annurev-micro-090110-102946
Bergstrom, K. S., Kissoon-Singh, V., Gibson, D. L., Ma, C., Montero, M., Sham, H. P., et al. (2010). Muc2 protects against lethal infectious colitis by disassociating pathogenic and commensal bacteria from the colonic mucosa. PLoS Pathog. 6:e1000902. doi: 10.1371/journal.ppat.1000902
Bou Ghanem, E. N., Jones, G. S., Myers-Morales, T., Patil, P. D., Hidayatullah, A. N., and D'Orazio, S. E. (2012). InlA promotes dissemination of Listeria monocytogenes to the mesenteric lymph nodes during food borne infection of mice. PLoS Pathog. 8:e1003015. doi: 10.1371/journal.ppat.1003015
Brady, M. J., Campellone, K. G., Ghildiyal, M., and Leong, J. M. (2007). Enterohaemorrhagic and enteropathogenic Escherichia coli Tir proteins trigger a common Nck-independent actin assembly pathway. Cell. Microbiol. 9, 2242–2253. doi: 10.1111/j.1462-5822.2007.00954.x
Brady, M. J., Radhakrishnan, P., Liu, H., Magoun, L., Murphy, K. C., Mukherjee, J., et al. (2011). Enhanced actin pedestal formation by enterohemorrhagic Escherichia coli O157:H7 adapted to the mammalian host. Front. Microbiol. 2:226. doi: 10.3389/fmicb.2011.00226
Bunger, J. C., Melton-Celsa, A. R., and O'Brien, A. D. (2013). Shiga toxin type 2dact displays increased binding to globotriaosylceramide in vitro and increased lethality in mice after activation by elastase. Toxins 5, 2074–2092. doi: 10.3390/toxins5112074
Clark, M. A., Hirst, B. H., and Jepson, M. A. (1998). Inoculum composition and Salmonella pathogenicity island 1 regulate M-cell invasion and epithelial destruction by Salmonella typhimurium. Infect. Immun. 66, 724–731.
Davis, T. K., Van De Kar, N. C., and Tarr, P. I. (2014). Shiga toxin/verocytotoxin-producing Escherichia coli infections: practical clinical perspectives. Microbiol. Spectr. 2:EHEC-0025-2014. doi: 10.1128/microbiolspec.EHEC-0025-2014
Diez, E., Zhu, L., Teatero, S. A., Paquet, M., Roy, M. F., Loredo-Osti, J. C., et al. (2011). Identification and characterization of Cri1, a locus controlling mortality during Citrobacter rodentium infection in mice. Genes Immun. 12, 280–290. doi: 10.1038/gene.2010.76
Eaton, K. A., Friedman, D. I., Francis, G. J., Tyler, J. S., Young, V. B., Haeger, J., et al. (2008). Pathogenesis of renal disease due to enterohemorrhagic Escherichia coli in germ-free mice. Infect. Immun. 76, 3054–3063. doi: 10.1128/IAI.01626-07
Freedman, S. B., Xie, J., Neufeld, M. S., Hamilton, W. L., Hartling, L., Tarr, P. I., et al. (2016). Shiga toxin-producing Escherichia coli infection, antibiotics, and risk of developing hemolytic uremic syndrome: a meta-analysis. Clin. Infect. Dis. 62, 1251–1258. doi: 10.1093/cid/ciw099
Hoggatt, A. F., Hoggatt, J., Honerlaw, M., and Pelus, L. M. (2010). A spoonful of sugar helps the medicine go down: a novel technique to improve oral gavage in mice. J. Am. Assoc. Lab. Anim. Sci. 49, 329–334.
Kamada, N., Kim, Y. G., Sham, H. P., Vallance, B. A., Puente, J. L., Martens, E. C., et al. (2012). Regulated virulence controls the ability of a pathogen to compete with the gut microbiota. Science 336, 1325–1329. doi: 10.1126/science.1222195
Kaper, J. B., and O'Brien, A. D. (2014). Overview and historical perspectives. Microbiol. Spectr. 2:EHEC-0028-2014. doi: 10.1128/microbiolspec.EHEC-0028-2014
Keepers, T. R., Psotka, M. A., Gross, L. K., and Obrig, T. G. (2006). A murine model of HUS: shiga toxin with lipopolysaccharide mimics the renal damage and physiologic response of human disease. J. Am. Soc. Nephrol. 17, 3404–3414. doi: 10.1681/ASN.2006050419
Lai, Y., Rosenshine, I., Leong, J. M., and Frankel, G. (2013). Intimate host attachment: enteropathogenic and enterohaemorrhagic Escherichia coli. Cell. Microbiol. 15, 1796–1808. doi: 10.1111/cmi.12179
Mallick, E. M., Garber, J. J., Vanguri, V. K., Balasubramanian, S., Blood, T., Clark, S., et al. (2014). The ability of an attaching and effacing pathogen to trigger localized actin assembly contributes to virulence by promoting mucosal attachment. Cell. Microbiol. 16, 1405–1424. doi: 10.1111/cmi.12302
Mallick, E. M., McBee, M. E., Vanguri, V. K., Melton-Celsa, A. R., Schlieper, K., Karalius, B. J., et al. (2012). A novel murine infection model for Shiga toxin-producing Escherichia coli. J. Clin. Invest. 122, 4012–4024. doi: 10.1172/JCI62746
Melton-Celsa, A., Mohawk, K., Teel, L., and O'Brien, A. (2012). Pathogenesis of Shiga-toxin producing Escherichia coli. Curr. Top. Microbiol. Immunol. 357, 67–103. doi: 10.1007/82_2011_176
Melton-Celsa, A. R., and O'Brien, A. D. (2014). New therapeutic developments against shiga toxin-producing Escherichia coli. Microbiol. Spectr. 2:EHEC-0013-2013. doi: 10.1128/microbiolspec.EHEC-0013-2013
Mohawk, K. L., and O'Brien, A. D. (2011). Mouse models of Escherichia coli O157:H7 infection and shiga toxin injection. J. Biomed. Biotechnol. 2011:258185. doi: 10.1155/2011/258185
Molofsky, A. B., and Swanson, M. S. (2004). Differentiate to thrive: lessons from the Legionella pneumophila life cycle. Mol. Microbiol. 53, 29–40. doi: 10.1111/j.1365-2958.2004.04129.x
Obata, F., Tohyama, K., Bonev, A. D., Kolling, G. L., Keepers, T. R., Gross, L. K., et al. (2008). Shiga toxin 2 affects the central nervous system through receptor globotriaosylceramide localized to neurons. J. Infect. Dis. 198, 1398–1406. doi: 10.1086/591911
Obrig, T. G. (2010). Escherichia coli shiga toxin mechanisms of action in renal disease. Toxins 2, 2769–2794. doi: 10.3390/toxins2122769
Obrig, T. G., Louise, C. B., Lingwood, C. A., Boyd, B., Barley-Maloney, L., and Daniel, T. O. (1993). Endothelial heterogeneity in Shiga toxin receptors and responses. J. Biol. Chem. 268, 15484–15488.
Proulx, F., Seidman, E. G., and Karpman, D. (2001). Pathogenesis of Shiga toxin-associated hemolytic uremic syndrome. Pediatr. Res. 50, 163–171. doi: 10.1203/00006450-200108000-00002
Robinson, C. M., Sinclair, J. F., Smith, M. J., and O'Brien, A. D. (2006). Shiga toxin of enterohemorrhagic Escherichia coli type O157:H7 promotes intestinal colonization. Proc. Natl. Acad. Sci. U.S.A. 103, 9667–9672. doi: 10.1073/pnas.0602359103
Smith, A., and Bhagwat, A. A. (2013). Hypervirulent-host-associated Citrobacter rodentium cells have poor acid tolerance. Curr. Microbiol. 66, 522–526. doi: 10.1007/s00284-012-0298-x
Sonenshein, A. L. (2005). CodY, a global regulator of stationary phase and virulence in Gram-positive bacteria. Curr. Opin. Microbiol. 8, 203–207. doi: 10.1016/j.mib.2005.01.001
Teel, L. D., Melton-Celsa, A. R., Schmitt, C. K., and O'Brien, A. D. (2002). One of two copies of the gene for the activatable shiga toxin type 2d in Escherichia coli O91:H21 strain B2F1 is associated with an inducible bacteriophage. Infect. Immun. 70, 4282–4291. doi: 10.1128/IAI.70.8.4282-4291.2002
Tennant, S. M., Hartland, E. L., Phumoonna, T., Lyras, D., Rood, J. I., Robins-Browne, R. M., et al. (2008). Influence of gastric acid on susceptibility to infection with ingested bacterial pathogens. Infect. Immun. 76, 639–645. doi: 10.1128/IAI.01138-07
Thorpe, C. M., Smith, W. E., Hurley, B. P., and Acheson, D. W. (2001). Shiga toxins induce, superinduce, and stabilize a variety of C-X-C chemokine mRNAs in intestinal epithelial cells, resulting in increased chemokine expression. Infect. Immun. 69, 6140–6147. doi: 10.1128/IAI.69.10.6140-6147.2001
Vallance, B. A., Deng, W., Jacobson, K., and Finlay, B. B. (2003). Host susceptibility to the attaching and effacing bacterial pathogen Citrobacter rodentium. Infect. Immun. 71, 3443–3453. doi: 10.1128/IAI.71.6.3443-3453.2003
Vingadassalom, D., Kazlauskas, A., Skehan, B., Cheng, H. C., Magoun, L., Robbins, D., et al. (2009). Insulin receptor tyrosine kinase substrate links the E. coli O157:H7 actin assembly effectors Tir and EspF(U) during pedestal formation. Proc. Natl. Acad. Sci. U.S.A. 106, 6754–6759. doi: 10.1073/pnas.0809131106
Wadolkowski, E. A., Burris, J. A., and O'Brien, A. D. (1990a). Mouse model for colonization and disease caused by enterohemorrhagic Escherichia coli O157:H7. Infect. Immun. 58, 2438–2445.
Wadolkowski, E. A., Sung, L. M., Burris, J. A., Samuel, J. E., and O'Brien, A. D. (1990b). Acute renal tubular necrosis and death of mice orally infected with Escherichia coli strains that produce Shiga-like toxin type II. Infect. Immun. 58, 3959–3965.
Walker, M. K., Boberg, J. R., Walsh, M. T., Wolf, V., Trujillo, A., Duke, M. S., et al. (2012). A less stressful alternative to oral gavage for pharmacological and toxicological studies in mice. Toxicol. Appl. Pharmacol. 260, 65–69. doi: 10.1016/j.taap.2012.01.025
Keywords: Citrobacter rodentium, shiga toxin, hemolytic uremic syndrome, enterohemorrhagic E. coli, food-borne inoculation
Citation: Flowers LJ, Bou Ghanem EN and Leong JM (2016) Synchronous Disease Kinetics in a Murine Model for Enterohemorrhagic E. coli Infection Using Food-Borne Inoculation. Front. Cell. Infect. Microbiol. 6:138. doi: 10.3389/fcimb.2016.00138
Received: 09 June 2016; Accepted: 07 October 2016;
Published: 03 November 2016.
Edited by:
Nikhil A. Thomas, Dalhousie University, CanadaReviewed by:
Leigh A. Knodler, Washington State University, USASiouxsie Wiles, University of Auckland, New Zealand
Copyright © 2016 Flowers, Bou Ghanem and Leong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: John M. Leong, john.leong@tufts.edu
 Laurice J. Flowers
Laurice J. Flowers Elsa N. Bou Ghanem
Elsa N. Bou Ghanem John M. Leong
John M. Leong