The Role of Fibronectin in the Adherence and Inflammatory Response Induced by Enteroaggregative Escherichia coli on Epithelial Cells
- 1Centro de Estudios Moleculares, Departamento de Pediatría, Hospital Dr. Luis Calvo Mackenna, Facultad de Medicina, Universidad de Chile, Santiago, Chile
- 2Department of Pediatrics, University of Virginia School of Medicine, Charlottesville, VA, USA
- 3Programa de Microbiología, Instituto de Ciencias Biomédicas, Facultad de Medicina, Universidad de Chile, Santiago, Chile
Enteroaggregative Escherichia coli (EAEC) infections are still one of the most important etiologic pathogens of diarrhea in children worldwide. EAEC pathogenesis comprises three stages: adherence and colonization, production of toxins, and diarrhea followed by inflammation. Previous studies have demonstrated that EAEC strains have the ability to bind to fibronectin (FN); however, the role this extracellular matrix protein plays in the inflammatory response induced by EAEC remains unknown. In this study, we postulated that FN-mediated adherence of EAEC strains to epithelial cells increases the expression of pro-inflammatory genes. To verify this hypothesis, we infected HEp-2 and HT-29 cells, in both the presence and absence of FN, with EAEC reference strain 042. We quantified IL-8 secretion and the relative expression of a set of genes regulated by the NF-κB pathway. Although FN increased EAEC adherence, no changes in IL-8 protein secretion or IL8 gene expression were observed. Similar observations were found in HEp-2 cells transfected with FN-siRNA and infected with EAEC. To evaluate the involvement of AAF/II fimbriae, we infected HEp-2 and HT-29 cells, in both the presence and absence of FN, with an EAEC 042aafA mutant strain transformed with a plasmid harboring the native aafA gene with a site-directed mutation in Lys72 residue (K72A and K72R strains). No changes in IL-8 secretion were observed. Finally, SEM immunogold assay of cells incubated with FN and infected with EAEC revealed that AAF fimbriae can bind to cells either directly or mediated by FN. Our data suggests that FN participates in AAF/II fimbriae-mediated adherence of EAEC to epithelial cells, but not in the inflammatory response of cells infected by this pathogen.
Introduction
Enteroaggregative Escherichia coli (EAEC) are a major cause of acute diarrhea in developing and industrialized regions (Huang et al., 2006). Several outbreaks, such as the 2011 outbreak caused by a Shiga toxin-producing EAEC in Germany, have increased interest in investigating the molecular mechanisms involved in the interaction between this pathogen and the intestinal epithelia (Rasko et al., 2011). In general, EAEC pathogenesis involves adherence to intestinal cells, the secretion of toxins and the induction of an inflammatory response (Harrington et al., 2006). EAEC is characterized by its ability to adhere to HEp-2 cells in a “stacked-brick” aggregative adherence pattern. Clinically, EAEC induces watery diarrhea, sometimes accompanied by blood and mucus, and patients normally manifest intestinal inflammation (Steiner et al., 1998; Flores and Okhuysen, 2009).
It has been proposed that extracellular matrix (ECM) proteins act as receptors for several pathogens, including EAEC (Farfan et al., 2008). Under normal conditions, ECM proteins are restricted to the basement membrane, where they are not available for interaction with luminal bacteria (Dubreuil et al., 2002). However, several observations indicate that ECM proteins could be present on the surface of intestinal cells and may participate as bacterial receptors (Westerlund and Korhonen, 1993; Walia et al., 2004; Konkel et al., 2005). Fibronectin (FN) was the first ECM protein shown to act as a cell receptor for bacterial pathogens (Kuusela, 1978). This glycoprotein contains multiple domains that can bind to several ligands, such as fibrin, heparin, syndecan, collagens, gelatin, and integrins (Pankov and Yamada, 2002). To date, over 100 bacterial FN binding proteins have been identified, and several studies have shown that adhesion to FN would contribute to the successful colonization of bacteria on the epithelial surface, by acting as a “molecular bridge” connecting the bacteria with the host cell surface (Henderson et al., 2011; Izquierdo et al., 2014a).
EAEC adherence to intestinal cells is mediated by fimbrial adhesins, designated aggregative adherence fimbriae (AAFs). For EAEC reference strain 042, adherence to cells requires the AAF/II variant (Czeczulin et al., 1997), but the cell receptors involved in the recognition of these fimbriae have not been completely described. Our group, along with others, has previously shown the recognition of FN by AAF/II fimbriae, resulting in enhanced bacterial adherence to intestinal epithelial cells (Farfan et al., 2008; Konar et al., 2012; Izquierdo et al., 2014a).
Given the above, in this study we investigated whether the presence of FN increases the adherence of EAEC strains to epithelial cells, induces secretion of IL-8 and the expression of pro-inflammatory genes through the fimbriae. Our data suggest that FN increases AAF/II fimbriae-mediated adherence of EAEC to epithelial cells; however, in the case of cells infected with this pathogen, binding to FN is not involved in their inflammatory response.
Materials and Methods
Bacterial Strains
EAEC strains were grown overnight in Luria-Bertani (LB) broth. EAEC 042aafA mutant strains transformed with the pBAD30 plasmid harboring the native aafA gene with site-directed mutation in Lys72 (K72R and K72A) were selectively grown on LB-agar with kanamycin and 0.2% arabinose (Berry et al., 2014).
Cell Culture
HT-29 and HEp-2 cells were maintained in McCoy's 5A Medium (Gibco) and DMEM high glucose (DMEM/HG) (HyClone), respectively. Cells media were supplemented with 10% fetal bovine serum (FBS), penicillin (10 U/mL), and streptomycin (10 g/mL) at 37°C under 5% CO2.
Adherence Assays
Confluent epithelial cells were incubated for 30 min with FN (2.5, 5, 7.5, or 10 μg/well) and then infected with a multiplicity of infection (MOI) of 10 for 3 h, as previously described (Izquierdo et al., 2014b). Cells were lysed with 0.1% Triton X-100/PBS and serial dilutions of lysed cells were plated onto LB agar. The number of adherent bacteria was determined by counting colony-forming units (CFU).
IL-8 Secretion
Infection assay was performed with HEp-2 and HT-29 cells in triplicate, as described above. After infection, cells were incubated with media containing gentamicin (100 μg/ml) for 3 h to determine the amount of IL-8 by ELISA, as previously described (Harrington et al., 2005).
siRNA
Subconfluent cultures of HEp-2 cells were transfected with 20 pmol of small interfering RNA (siRNA) for FN or scrambled siRNA (Santa Cruz Biotechnology), as previously described (Izquierdo et al., 2014b). Adherence and IL-8 secretion assays were performed 48–72 h after transfection. Reduction in FN expression was determined by Western blot analysis of transfected cells lysates using primary antibodies against FN (Sigma) or actin, and the secondary HRP-conjugated antibody. The band intensity was determined with ImageJ software. Additionally, transfected HEp-2 cells grown in an 8-well Lab-Tek Chamber slide were fixed and immunostained with anti-FN primary antibodies. FN expression was examined by confocal microscopy.
Real-Time PCR
RNA obtained from HEp-2 cells infected with EAEC was reverse-transcribed into single-strands using the Transcriptor First Strand cDNA Synthesis Kit (Roche). cDNA was used as a template to quantify the expression of IL8, CCL20, TNFA, NFkB, IL1B, and FOS genes using specific primers (Vergara et al., 2015). The mRNA expression levels were normalized to those of the human housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH), as previously described (Vergara et al., 2015), and analyzed using the comparative critical threshold (CT) method.
Scanning Electron Microscopy
HEp-2 cells were infected for adherence assays, as described above. The wells were rinsed twice with PBS and fixed in 2.5% glutaraldehyde in PBS. A 1:50 dilution of immune rabbit serum against AafA was added for 1 h followed by washing and addition of the anti-rabbit IgG 10-nm gold conjugate (BBI solutions) (1:100) for 1 h. FN was detected with mouse anti-FN monoclonal antibodies (1:50) (Sigma) and goat anti-mouse Ig 20-nm gold conjugate (BBI solutions) (1:100) for 1 h each. The specimens were submitted to the Advanced Microscopy Facility (University of Virginia) for preparation; namely, post-fixed with osmium tetroxide, dehydrated in ethanol, critical point dried, mounted, coated with carbon, and imaged at 3 kV with a Zeiss Sigma HDS scope with SE2 and back scatter detectors to visualize the gold particles.
Statistical Analysis
Experimental data were expressed as mean ± SD in each group. Statistical comparisons were performed using the Student's t-test or ANOVA multiple pairwise comparisons test with Tukey's posttest. The significant P-value was established at P < 0.05.
Results
FN Increases EAEC Adherence to Epithelial Cells, but not IL-8 Secretion
HEp-2 cells were incubated with increasing concentrations of FN prior to infection with EAEC. Figure 1A shows that EAEC adherence to cultured epithelial cells increased in a dose-dependent manner. Using 7.5 μg/ml of FN, we evaluated the amount of IL-8 secreted to the media by HEp-2 and HT-29 cells 3 h post-infection with EAEC. Although there was a significant increase in EAEC adherence to both cell types (Figure 1B), no difference was observed in the amount of secreted IL-8 present in the media (Figure 1C). Thus, these data indicate that EAEC-induced IL-8 secretion in HT-29 and HEp-2 cells is FN independent. There was no observed effect of FN on the induction of IL-8 secretion in HEp-2 and HT-29 cells.
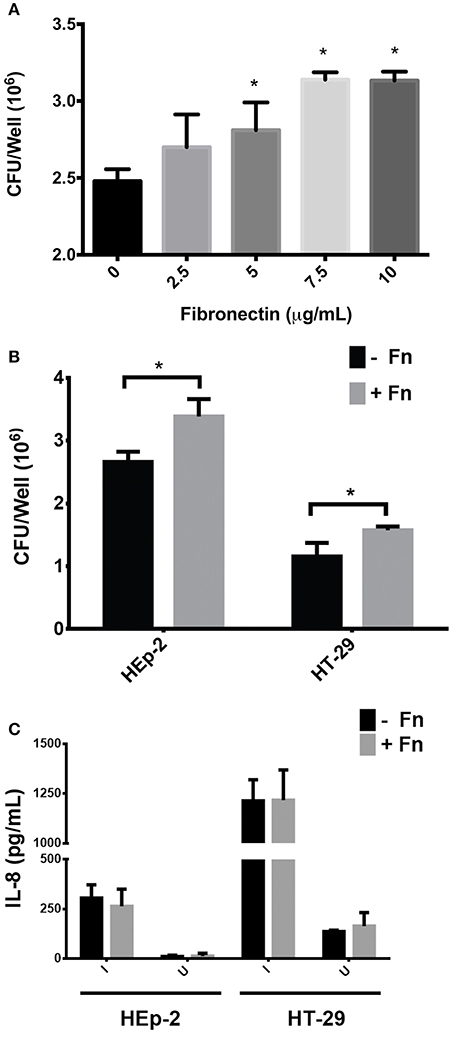
Figure 1. Participation of fibronectin in adherence and IL-8 secretion (A). HEp-2 cells were pre-incubated with increasing concentrations of fibronectin prior to infection. The number of bound bacteria was determined by plating. (B) Prior to infection, HEp-2 and HT-29 cells were pre-incubated with 7.5 μg/mL of FN (+Fn) or media only (−Fn) and then infected with EAEC 042 strains for 3 h. The number of bound bacteria was determined by plating. (C) After 3 h of infection, cell monolayers were incubated with gentamicin for 3 h and the media from cells were collected. Secreted IL-8 levels were measured by ELISA. I, infected; U, uninfected. Bars represent the mean of three experiments, with the error bars indicating one standard deviation. *Represents a statistically significant difference (P < 0.05).
FN-Mediated Adherence of EAEC to Epithelial Cells is Associated with No Changes in the Expression of the IL8 Gene
To corroborate the results described above, we evaluated the expression of several pro-inflammatory genes associated with enteric infection. HEp-2 cells were infected with EAEC strain 042 in the presence or absence of FN and the relative expression of FOS, NFkB, IL8, CCL20, IL1B, and TNFa genes was compared to uninfected cells. After 3 h of infection, there was no difference in the expression of any of the pro-inflammatory markers; however, an ~4 fold reduction in the expression of the IL8 gene was found in cells preincubated with FN as compared to uninfected cells (Figure 2A). Additionally, we performed the same analyses on cells 1, 2, and 3 h post-infection, and found no significant difference in IL8 gene expression (Figure 2B).
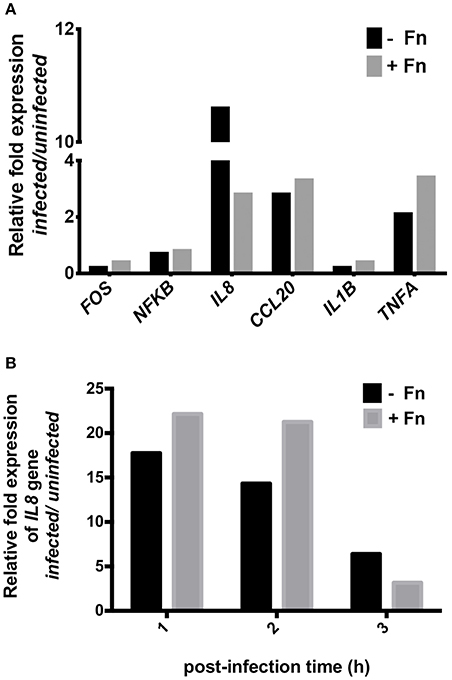
Figure 2. NF-κB pathway gene expression in cells infected with EAEC in the presence of fibronectin. Prior to infection, HEp-2 cells were pre-incubated with 7.5 μg/mL of fibronectin (+Fn) or media only (−Fn). (A) Real-time PCR analysis for FOS, NFKB, IL8, CCL20, IL1B, and TNFA genes after 3 h of infection. (B) Real-time PCR analysis for IL8 gene at 1, 2, and 3 h post-infection.
FN Gene Knockdown Reduced EAEC Adherence to Epithelial Cells, but Had No Effect on IL-8 Secretion
The involvement of cell-produced FN in EAEC adherence and IL-8 secretion was evaluated in HEp-2 cells transfected with siRNA for FN. Reduction in FN expression, compared with HEp-2 transfected with scrambled siRNA, was confirmed by Western blot (Figure 3A) and immunofluorescence assay (Figure 3B). Although we found a significant reduction in EAEC strain 042 binding to HEp-2 cells treated with FN siRNA as compared to cells treated with scrambled siRNA (Figure 3C), no difference in IL-8 secretion was observed (Figure 3D).
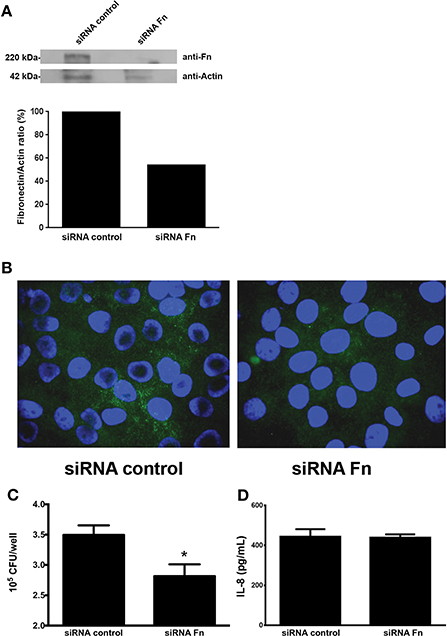
Figure 3. Knocking down the fibronectin gene reduces EAEC binding to epithelial cells. (A) Fibronectin expression determined by Western blot analysis using HEp-2 cells lysates transfected with fibronectin or scrambled siRNA (siRNA control). (B) Immunofluorescence of transfected HEp-2 cells infected with EAEC 042. Fibronectin was visualized with Alexa Fluor 488 (green), and bacterial or human DNA was stained with TO-PRO-3 (blue). (C) HEp-2 cells treated with siRNA infected with EAEC 042. The number of adherent bacteria was determined by counting CFUs. (D) Levels of secreted IL-8 at 3 h post infection. Bars represent the mean of three experiments, with the error bars indicating one standard deviation. *Denotes statistically significant difference (P < 0.05).
Involvement of AAF/II Fimbriae in FN-Mediated IL-8 Secretion
Previous reports demonstrated that the substitution of Lys72Arg (K72R) in AafA proteins did not affect FN binding, whereas the Lys72Ala (K72A) substitution yielded significant reduction in binding to this ECM protein (Berry et al., 2014). In order to evaluate whether FN/AAF fimbriae interaction plays a role in the inflammation induced by EAEC on epithelial cells, we infected HEp-2 and HT-29 cells with the EAEC 042aafA mutant strain transformed with a pBAD30 plasmid harboring the native aafA gene with a site-directed mutation in the Lys72 residue (K72A and K72R strains). As expected, this amino-acid substitution significantly increased the adherence of the AafA mutant K72R in the presence of FN to HEp-2 and HT-29 cells. On the contrary, there was no difference in the adherence of the AafA mutant K72A in either the presence or absence of FN (Figure 4A). In both cell models, there was no difference in the induction of IL-8 secretion in cells infected with the mutants tested (Figure 4B).
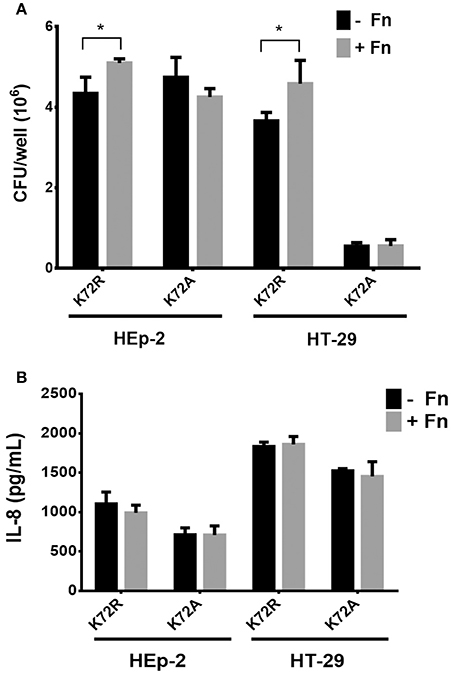
Figure 4. Involvement of AAF/II in fibronectin-mediated IL-8 secretion. (A) The number of bacteria adherent to HEp-2 or HT-29 cells pre-incubated with 7.5 μg/mL of fibronectin (+Fn) or media only (−Fn) and then infected with K72R or K72A strains. (B) Levels of secreted IL-8 at 3 h post infection. Bars represent the mean of three experiments, with the error bars indicating one standard deviation. *Denotes statistical significant difference (P < 0.05).
Interaction of FN with AAF/II Fimbriae on Epithelial Cells Infected with EAEC
Our data suggests that FN increased adherence of EAEC to cells, but this increase was not related to high levels of IL8 gene expression or IL-8 secretion. To understand these findings, we performed immunogold SEM assays on HEp-2 cell monolayers infected with EAEC in the presence of FN. This ultrastructural analysis revealed compelling evidence of the formation of an AAF/II-FN complex observed at the surface of the bacterium, as well as on the epithelial cell surface (Figure 5). These findings confirm that FN is involved in EAEC adherence to the cell surface mediated by AAF/II fimbriae.
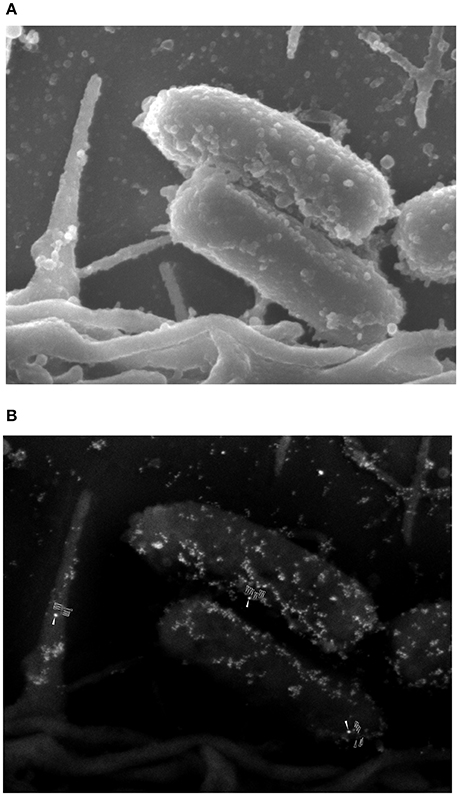
Figure 5. Ultrastructural analysis of the interaction of fibronectin with AAF/II on EAEC-infected epithelial cells. (A) An SEM image of EAEC 042 adhering to the surface of an HEp-2 cell. (B) An SEM image employing backscattered electron detection. The presence of fibronectin was evidenced with anti-fibronectin antibodies and anti-mouse IgG conjugated to 20-nm gold particles (solid white arrows). AAF/II was immunostained with rabbit anti-AafA serum plus anti-rabbit IgG conjugated to 10-nm gold particles (unfilled white arrows).
Discussion
FN is an ECM protein involved in several biological processes and may be recognized as a receptor by many bacterial pathogens (Henderson et al., 2011). However, one of the main questions regarding the ability of pathogens to adhere to FN is the biological significance of this binding in the context of disease establishment. It has been recognized for some time that FN plays a role in EAEC 042's adherence to epithelial cells through interaction with AAF/II fimbriae, the major EAEC virulence factor known to be involved in both adherence and inflammation (Farfan et al., 2008; Konar et al., 2012; Izquierdo et al., 2014a). In this study, we focused on determining whether the AAF/II-FN interaction constitutes a signal that triggers the expression of genes encoding pro-inflammatory markers resulting in an increased inflammatory response in infected host epithelial cells. We found that the addition of soluble FN to cultured epithelial cells increased adhesion of EAEC 042 in both epithelial cell models, HEp-2 and HT-29 (Figure 1B), as expected according to previously published data (Farfan et al., 2008). However, no difference in IL-8 secretion was observed in these experiments (Figure 1C). IL-8 is a cytokine responsible for the chemoattraction and recruitment of polymorphonuclear neutrophils (PMNs) at the site of infection, and is a marker widely used to evaluate inflammation response to pathogens (Szabady and McCormick, 2013). A previous report showed that AAF fimbriae were required for EAEC-induced PMN transmigration in vitro (Boll et al., 2012). To characterize the role that FN is playing in EAEC-induced PMN transmigration, inverted intestinal cells were infected with EAEC 042 in the presence or absence of FN as described (Vergara et al., 2015). No differences in the migration of PMNs were found (data not shown). These observations might be explained by the fact that similar amounts of IL-8 were secreted by cells infected with EAEC in the presence or absence of FN (Figure 1C).
Diarrheagenic E. coli strains induce secretion of IL-8 by activation of kinase mitogen activated protein (MAPK) ERK-1/2, JNK, and p38MAPK, leading to the induction of the transcription factors NF-κB and AP-1 (Khan and Konar, 2010). Therefore, we investigated expression of the NF-κB pathway genes in cells infected with EAEC in the presence or absence of FN. Our analysis showed that NF-κB pathway gene expression is FN-independent, since no significant quantitative differences in transcription were found in infected cells in the presence or absence of FN. Surprisingly though, a four-fold decrease in the expression of the IL8 gene was observed when FN was added, suggesting that perhaps FN impedes AAF/II from inducing IL-8 secretion (Figure 2A). However, no differences in the expression of the IL8 gene were found at post-infection time points (Figure 2B). These observations suggested that FN is not involved in the expression of IL8 gene induced by EAEC.
To further confirm or rebut these findings, we then sought to investigate the effect of attenuation of the FN-encoding gene on HEp-2 cells by utilizing specific siRNA. This approach reduced FN production. Quantitative adherence and IL-8 secretion experiments were then performed with FN-producing and FN-negative HEp-2 cells. While EAEC adherence was reduced, in agreement with previous data, the reduced amount of surface-associated FN on HEp-2 cells had no effect in the amount of secreted IL-8 found in the media of infected cells (Figure 3).
Based on these results, we evaluated the involvement of AAF/II in this process. An EAEC 042aafA mutant expressing recombinant AafA proteins with directed mutations Lys72Arg (K72R) and Lys72Ala (K72A) (Figure 4) were used. In the case of the K72R mutant, the strain behaved like the wild-type EAEC, as increased cell adherence was observed in the presence of FN. Yet again, IL-8 secretion remained unchanged between treatments. For the K72A strain, which is not capable of binding to FN (Berry et al., 2014), no change in cell adhesion or secretion of IL-8 was observed; demonstrating that FN participates in AAF/II-mediated adherence of EAEC to cells, but is not involved in the inflammatory IL-8 response characteristic of EAEC 042 in this model study.
Our results demonstrate the role of FN as a bridging molecule, connecting the bacterium with the epithelial cell surface. Furthermore, our data strongly suggest that FN-mediated binding is not necessary for the activation of intracellular signaling cascades involved in the inflammatory response against EAEC infection. SEM immunogold analysis clearly showed that AAF/II fimbriae bind to either FN or the cell surface (Figure 5). AAF/II fimbriae also have the ability to bind to other receptors, such as laminin, collagen IV, cytokeratin 8, epidermal growth factor receptor, Thrombospondin-1, or glucose-regulated protein (Farfan et al., 2008; Konar et al., 2012; Izquierdo et al., 2014b). Upcoming studies in our laboratory will evaluate the role of these proteins in the inflammation process, in order to decipher the pathways involved in the inflammatory response elicited by epithelial cells after EAEC infection.
Previous studies have associated the inflammatory response induced by EAEC with the AafB minor subunit of AAF/II, presumably a tip adhesin protein. IL-8 released from intestinal epithelial cells infected with the EAEC 042aafB mutant strain was reduced in comparison to the wild-type strain (Harrington et al., 2005). In contrast, the binding of EAEC to FN is mediated by the AafA major subunit of AAF/II, and the interaction between these two proteins involves electrostatic interactions attributed to the presence of basic residues in the AAF (Berry et al., 2014). Given our experimental data, we hypothesize that when EAEC reaches its target epithelial cells in the gut environment, AAF/II may bind to adherence-associated receptors, such as FN, which as others described above, are recognized by AafA, or pro-inflammatory receptors that activate intracellular signaling cascades involved in mounting an inflammatory response, which are recognized by AafB. In this context, the binding of EAEC to cellular FN through AafA increases the number of bacteria at the cell surface, leaving AafB unavailable to interact with pro-inflammatory receptors, thereby dampening the secretion of pro-inflammatory cytokines, such as IL-8. The identification of an AafB protein receptor would help clarify or support this hypothesis, as well as aid in further understanding as to the role of AAF/II in immunomodulation of the inflammatory response during EAEC infections.
In conclusion, we report that while the presence of FN favors the interaction of EAEC 042 with host cultured epithelial cells, this ECM protein is not involved in the inflammatory response induced by EAEC.
Author Contributions
DY carried out the infection assay, IL-8 measurement and interpretation of data; MI, carried out the siRNA experiments and participate in the interpretation of data, FR-P participated in the interpretation of data; JN participated in the interpretation of data; JG carried out the SEM analysis, interpretation of data and manuscript writing, RV participated in the design of the study; MF participated in the design of the study, acquisition of data, interpretation of data and manuscript writing and final approval of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by FONDECYT grants 1120809 and 1160426 to MF. We thank Abraham Medrano for technical assistance.
References
Berry, A. A., Yang, Y., Pakharukova, N., Garnett, J. A., Lee, W. C., Cota, E., et al. (2014). Structural insight into host recognition by aggregative adherence fimbriae of enteroaggregative Escherichia coli. PLoS Pathog. 10:e1004404. doi: 10.1371/journal.ppat.1004404
Boll, E. J., Struve, C., Sander, A., Demma, Z., Nataro, J. P., McCormick, B. A., et al. (2012). The fimbriae of enteroaggregative Escherichia coli induce epithelial inflammation in vitro and in a human intestinal xenograft model. J. Infect. Dis. 206, 714–722. doi: 10.1093/infdis/jis417
Czeczulin, J. R., Balepur, S., Hicks, S., Phillips, A., Hall, R., Kothary, M. H., et al. (1997). Aggregative adherence fimbria II, a second fimbrial antigen mediating aggregative adherence in enteroaggregative Escherichia coli. Infect. Immun. 65, 4135–4145.
Dubreuil, J. D., Giudice, G. D., and Rappuoli, R. (2002). Helicobacter pylori interactions with host serum and extracellular matrix proteins: potential role in the infectious process. Microbiol. Mol. Biol. Rev. 66, 617–629. doi: 10.1128/MMBR.66.4.617-629.2002
Farfan, M. J., Inman, K. G., and Nataro, J. P. (2008). The major pilin subunit of the AAF/II fimbriae from enteroaggregative Escherichia coli mediates binding to extracellular matrix proteins. Infect. Immun. 76, 4378–4384. doi: 10.1128/IAI.00439-08
Flores, J., and Okhuysen, P. C. (2009). Enteroaggregative Escherichia coli infection. Curr. Opin. Gastroenterol. 25, 8–11. doi: 10.1097/MOG.0b013e32831dac5e
Harrington, S. M., Dudley, E. G., and Nataro, J. P. (2006). Pathogenesis of enteroaggregative Escherichia coli infection. FEMS Microbiol. Lett. 254, 12–18. doi: 10.1111/j.1574-6968.2005.00005.x
Harrington, S. M., Strauman, M. C., Abe, C. M., and Nataro, J. P. (2005). Aggregative adherence fimbriae contribute to the inflammatory response of epithelial cells infected with enteroaggregative Escherichia coli. Cell. Microbiol. 7, 1565–1578. doi: 10.1111/j.1462-5822.2005.00588.x
Henderson, B., Nair, S., Pallas, J., and Williams, M. A. (2011). Fibronectin: a multidomain host adhesin targeted by bacterial fibronectin-binding proteins. FEMS Microbiol. Rev. 35, 147–200. doi: 10.1111/j.1574-6976.2010.00243.x
Huang, D. B., Nataro, J. P., DuPont, H. L., Kamat, P. P., Mhatre, A. D., Okhuysen, P. C., et al. (2006). Enteroaggregative Escherichia coli is a cause of acute diarrheal illness: a meta-analysis. Clin. Infect. Dis. 43, 556–563. doi: 10.1086/505869
Izquierdo, M., Alvestegui, A., Nataro, J. P., Ruiz-Perez, F., and Farfán, M. J. (2014a). Participation of Integrin 5 1 in the fibronectin-mediated adherence of enteroaggregative Escherichia coli to intestinal cells. Biomed. Res. Int. 2014:781246. doi: 10.1155/2014/781246
Izquierdo, M., Navarro-Garcia, F., Nava-Acosta, R., Nataro, J. P., Ruiz-Perez, F., and Farfán, M. J. (2014b). Identification of cell surface-exposed proteins involved in the fimbriae mediated adherence of enteroaggregative Escherichia coli to intestinal cells. Infect. Immun. 82, 1719–1724. doi: 10.1128/IAI.01651-13
Khan, K., and Konar, M. (2010). Enteroaggregative Escherichia coli infection induces IL-8 production via activation of mitogen-activated protein kinases and the transcription factors NF-κB and AP-1 in INT-407 cells. Mol. Cell. Biochem. 337, 17–24. doi: 10.1007/s11010-009-0282-3
Konar, M., Sachin, O., Priya, A., and Ghosh, S. (2012). Identification of key proteins of cultured human intestinal cells involved in interaction with enteroaggregative Escherichia coli. FEMS Immunol. Med. Microbiol. 66, 177–190. doi: 10.1111/j.1574-695X.2012.00998.x
Konkel, M. E., Christensen, J. E., Keech, A. M., Monteville, M. R., Klena, J. D., and Garvis, S. G. (2005). Identification of a fibronectin-binding domain within the Campylobacter jejuni CadF protein. Mol. Microbiol. 57, 1022–1035. doi: 10.1111/j.1365-2958.2005.04744.x
Kuusela, P. (1978). Fibronectin binds to Staphylococcus aureus. Nature 276, 718–720. doi: 10.1038/276718a0
Pankov, R., and Yamada, K. M. (2002). Fibronectin at a glance. J. Cell Sci. 115, 3861–3863. doi: 10.1242/jcs.00059
Rasko, D. A., Webster, D. R., Sahl, J. W., Bashir, A., Boisen, N., Scheutz, F., et al. (2011). Origins of the E. coli strain causing an outbreak of hemolytic-uremic syndrome in Germany. N. Engl. J. Med. 365, 709–717. doi: 10.1056/NEJMoa1106920
Steiner, T. S., Lima, A. A., Nataro, J. P., and Guerrant, R. L. (1998). Enteroaggregative Escherichia coli produce intestinal inflammation and growth impairment and cause interleukin-8 release from intestinal epithelial cells. J. Infect. Dis. 177, 88–96. doi: 10.1086/513809
Szabady, R. L., and McCormick, B. A. (2013). Control of neutrophil inflammation at mucosal surfaces by secreted epithelial products. Front. Immunol. 4:220. doi: 10.3389/fimmu.2013.00220
Vergara, A. F., Vidal, R. M., Torres, A. G., and Farfán, M. J. (2015). Long polar fimbriae participates in the induction of neutrophils transepithelial migration across intestinal cells infected with enterohemorrhagic E. coli O157:H7. Front. Cell Infect. Microbiol. 4:185. doi: 10.3389/fcimb.2014.00185
Walia, B., Castaneda, F. E., Wang, L., Kolachala, V. L., Bajaj, R., Roman, J., et al. (2004). Polarized fibronectin secretion induced by adenosine regulates bacterial-epithelial interaction in human intestinal epithelial cells. Biochem. J. 382, 589–596. doi: 10.1042/BJ20040021
Keywords: enteroaggregative E. coli, adherence aggregative fimbriae, fibronectin, IL-8
Citation: Yáñez D, Izquierdo M, Ruiz-Perez F, Nataro JP, Girón JA, Vidal RM and Farfan MJ (2016) The Role of Fibronectin in the Adherence and Inflammatory Response Induced by Enteroaggregative Escherichia coli on Epithelial Cells. Front. Cell. Infect. Microbiol. 6:166. doi: 10.3389/fcimb.2016.00166
Received: 26 August 2016; Accepted: 15 November 2016;
Published: 08 December 2016.
Edited by:
Philip R. Hardwidge, Kansas State University, USAReviewed by:
John K. Crane, University at Buffalo, USAMonica Konar, University of California, San Francisco, USA
Copyright © 2016 Yáñez, Izquierdo, Ruiz-Perez, Nataro, Girón, Vidal and Farfan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mauricio J. Farfan, mfarfan@med.uchile.cl
 Dominique Yáñez1
Dominique Yáñez1  Roberto M. Vidal
Roberto M. Vidal Mauricio J. Farfan
Mauricio J. Farfan