Role of nutraceuticals in hypolipidemic therapy
- Biomedical Department of Internal Medicine and Specialistics (DIBIMIS), University of Palermo, Palermo, Italy
Nutraceuticals are food components or active ingredients present in foods and used in therapy. This article analyzes the characteristics of the molecules with a lipid-lowering effect. The different nutraceuticals may have different mechanisms of action: inhibition of cholesterol synthesis primarily through action on the enzyme HMG-CoA reductase (policosanol, polyphenols, garlic and, above all, red yeast rice), increase in LDL receptor activity (berberine), reduction of intestinal cholesterol absorption (garlic, plant sterols, probiotics), and also the ability to interfere with bile metabolism (probiotics, guggul). Based on the different mechanisms of action, some nutraceuticals are then able to enhance the action of statins. Nutraceuticals are often used without relevant evidence: mechanisms of action are not clearly confirmed; most of clinical data are derived from small, uncontrolled studies, and finally, except for fermented red rice, there are no clinical trials which may document the relationship between these interventions and the reduction of clinical events. Therefore, among all nutraceuticals, it is necessary to extrapolate those having a really documentable efficacy. However, these kinds of treatments are usually well-tolerated by patients. Overall, subjects with a middle or low cardiovascular risk are the best indication of nutraceuticals, but they may also be useful for patients experiencing side effects during classical therapies. Finally, in consideration of the additive effect of some nutraceuticals, a combination therapy with classical drugs may improve the achievement of clinical targets. Thus, nutraceuticals may be a helpful alternative in hypolipidemic treatment and, if properly used, might represent a valid strategy of cardiovascular prevention.
Nutraceutics is an area of pharmacology regarding food components or active ingredients that may be used as therapeutic agents. This includes a large number of compounds, such as an active ingredient, food supplements (i.e., supplements the normal diet), and functional foods (i.e., foods enriched with components with specific therapeutic or protective functions), as well as preparations based on medicinal herbs. Most compounds are vegetable originated, but there are also substances with animal origin (e.g., fish oil). Recent studies have shown promising results for these drugs in various pathological complications such as diabetes, atherosclerosis, cardiovascular diseases, cancer, and neurological disorders. These conditions involve many changes, including alterations redox state, and most of nutraceuticals have antioxidant activity with the ability to counteract this situation. Hence, nutraceuticals are considered as sources of health promotion (1), and they, nowadays, have received a considerable interest. A market research recently proposed that the worldwide nutraceuticals market is expanding and would reach US $250 billion by 2018 (1). Since nutraceuticals are generally considered like “foods,” their use do not strictly follow the same rules of classical drugs and have not patent protection. Thus, a large amount of preparations have been suggested to have a therapeutic effect and are rapidly available for patients. The process of market release for a drug is a very lengthy process, starting from the “in vitro” demonstration of the possible effects, followed by evaluation in animal models and then in humans (in healthy volunteers first and then in patients with a specific disease) analyzing effectiveness and tolerability of therapies (2). Following the approval and the market availability, there is also a strict monitoring of side effects. Large controlled clinical trials will finally validate clinical outcomes. In contrast, nutraceuticals are used in therapy without relevant evidence. They might be involved in a wide variety of biological processes, including activation of signal transduction pathways, antioxidant defenses, gene expression, cell proliferation, differentiation, and preservation of mitochondrial integrity, but the mechanistic actions are not always fully clear and sometimes they are not, or not particularly robust, for the theoretical basis of their effectiveness. Clinical efficacy often derives only from data produced by small-scale, uncontrolled studies. Thus, among all nutraceuticals, it is necessary to extrapolate those having a really documentable efficacy. Also, the “natural” origin does not protect from side effects in itself, since many classical drugs derive from plants and, in nature, there are also a number of toxic substances (such as derived from mushrooms); moreover, the insufficient clinical monitoring makes adverse events less predictable.
There are a variety of nutraceuticals with a potential lipid-lowering effect (Table 1), and therefore useful in the cardiovascular prevention (3). Nevertheless, in relation to the scarcity of experimental studies, these molecules do not always have solid scientific evidence with regard to both mechanisms of action and clinical efficacy (4). Data produced by small studies were often disavowed by larger and controlled studies or meta-analytic data. Some nutraceuticals are also able to enhance the action of the classic drugs (including statins), due to different mechanisms of action. Finally, except for the red yeast rice, there are no clinical trials which may document the relationship between any of these treatments and the reduction of clinical events, and this represents a great limit in their reasoned prescription (Table 2).
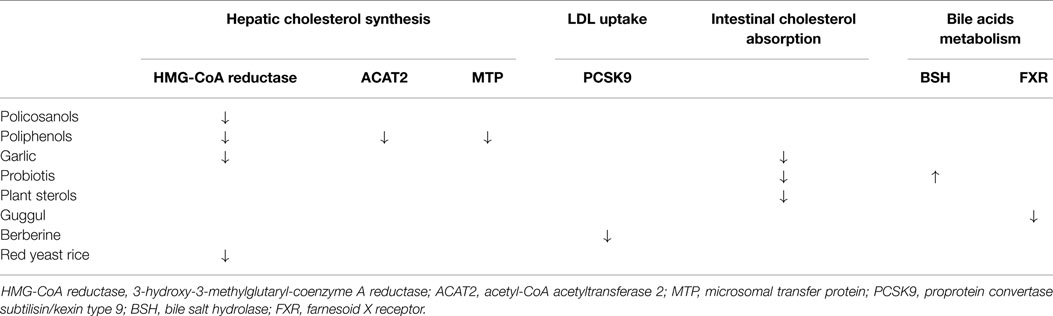
Table 1. Mechanisms of action of different hypothetical nutraceuticals with recommended lipid-lowering effect.
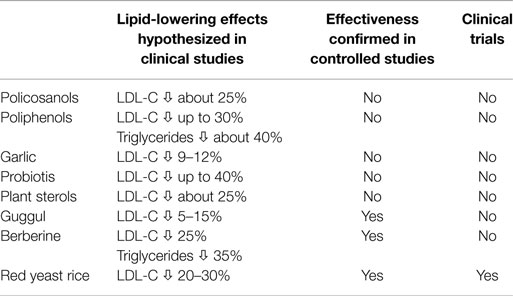
Table 2. Summary of clinical data of different nutraceuticals with recommended lipid-lowering effect.
Here, we review the major nutraceuticals with lipid-lowering effect: this should also include omega-3 polyunsaturated fatty acids (fish oil), but the scientific history of this drug followed pathways different than that of other nutraceuticals, and therefore will not be considered in this review.
Policosanols
These are a mixture of natural long chain aliphatic alcohols obtained from a wide variety of plants. It has been suggested that policosanols might inhibit the activity of HMG-CoA reductase, but this is not definitively confirmed (5). In the early 90s, a number of clinical studies suggested a lipid-lowering effect of policosanols in different types of patients (healthy volunteers, hypercholesterolemics, diabetics, or postmenopausal women), with reduction in LDL-cholesterol similar to that of statins (about 25%), and a 10% increase of HDL-C (6). Some reports suggested benefits even on clinical outcomes, including coronary ischemia or claudication; these treatments appeared also very well tolerated by patients (7, 8). Nevertheless, studies were of limited samples but especially on a limited number of clinical centers, often in Cuba, with reduction in LDL-cholesterol similar to that of statins (about 25%), and a 10% increase of HDL-C (9, 10).
Polyphenols
This is a very large family of substances available in the plant world. The main feature is the presence of multiple phenolic groups having a potent anti-oxidant effect; for this reason, polyphenols present in some foods typical of the Mediterranean diet (olive oil, red wine, fruits, vegetables) are considered to account for the protective effect of this nutritional model; drug preparations, maybe for a different bioavailability, do not seem to have the same clinical effect (11). It has also been postulated that polyphenols are able to inhibit HMG-CoA reductase, as well as ACAT2 and MTP, justifying a hypocholesterolemic effect (12). Nevertheless, even if in an open study, Mollace and colleagues have suggested the possibility of a great reduction of LDL-C (>30%) and also triglycerides levels (>40%) with polyphenols extracted from bergamot (13), a controlled study failed to demonstrate any effect in subjects treated with two different polyphenols (hesperidin and naringin) compared to a group on placebo (14). Thus, the hypolipidemic effect of polyphenols still remains an open question.
Garlic
Allicin, a substance contained in the bulb of garlic, seems to be able to reduce both synthesis (perhaps through the inactivation of HMG-CoA reductase) and intestinal absorption of cholesterol (15), and therefore to have lipid-lowering properties, with reductions of total cholesterol reported between 9 and 12% (16). These data have recently been substantially refuted by Gardner and colleagues: these authors, in a randomized placebo-controlled trial, have not documented any cholesterol-lowering effect with different formulations of garlic (17). Overall, garlic, even without a significant lipid-lowering effect, could have other protective effects on the cardiovascular system for its ability to reduce blood pressure and platelet aggregation (18), but this needs to be better investigated in large controlled trials.
Probiotics
They have received a lot of attention due to the potential benefits that they seem to have in different fields. Regarding lipid metabolism, probiotics could lower cholesterol absorption through direct cellular effects or mediated by bile metabolism (19). Several studies on different patients have documented significant reductions in total cholesterol, up to 40% (20–22). However, exact mechanisms of action were not identified, and those proposed (such as the inhibition of intestinal absorption of cholesterol) are usually dependent on bacterial strains and methods of execution of experiments, often very different from the “in vivo” conditions. More recently, it has been postulated a role of expression of the gene of bile salt hydrolase activity in the lactobacilli strains to explain the cholesterol-lowering action, even if this hypothesis itself does not seem completely convincing (23). A number of confounding factors, which also include local conditions or functional anatomy, make complex the question and, at the present time, additional data are certainly necessary to give a definitive answer.
Guggul
This is a resin extracted from the bark of Commiphora mukul, a small thorny tree, also known as the tree of myrrh, used medically in India for hundreds of years (24). The active components, guggulsterone E and Z, have been demonstrated to have an antagonistic action of FXR, a nuclear receptor involved in the bile metabolism. Based on this data, published in Science in 2002 (25), and given the close relationship between cholesterol and bile metabolisms, it has been also proposed a role in modulating plasma lipid levels. Different studies, mostly uncontrolled trials, were conducted in India and showed a massive efficacy of guggul in LDL-cholesterol levels reductions with no effect on triglyceride or HDL-cholesterol concentrations (26). More recently, a randomized placebo-controlled study in healthy subjects with hyperlipidemia demonstrated no effect on plasma lipids of guggul, which also caused allergic reactions in some individuals as well (27), clarifying that guggul not only does not seem to have a real lipid-lowering effect but it could also be dangerous in predisposed individuals.
Plant Sterols
Plant sterols decrease intestinal absorption of cholesterol through the reduction of the content of cholesterol within the micelles and a consequent lower proportion of absorbable cholesterol (28). In addition, some studies suggest that phytosterols are able to compete with cholesterol in the carrier of the intracellular incorporation (NPC1L1), and also increase the activity of transmembrane proteins responsible for the excretion of cholesterol (ABCA1) and plant sterols (ABCG5 and ABCG8) in the intestine and liver, with the net effect of increasing the release of both sterols into the intestinal lumen by enterocytes and in the bile ducts in the liver (29, 30). The lower intestinal absorption of cholesterol induced by plant sterols decreases cholesterol pool of liver, which responds by increasing the expression of LDL receptors, finally resulting in higher uptake of plasma LDL and therefore in a net hypocholesterolemic effect.
For many years, there was a great interest in phytosterols, which led to the development of a rich scientific literature. Phytosterols have been added and investigated in different food carriers. Initially, they were tested in margarines but other carriers in which it is possible to add plant sterols were subsequently identified: oils, salad dressings, meat products, low-calorie beverages, cereals, and finally drinks based on fermented milk (31–33). Clinical studies with beverages based on fermented milk enriched with phytosterols have shown that the effectiveness of phytosterols in reducing cholesterol is almost equivalent to that obtained with their introduction into margarine (34).
The cholesterol-lowering properties of phytosterols have been documented in a series of clinical trials in different categories of subjects: normolipidemic and hypercholesterolemic adults with and without cardiovascular disease, patients with type 2 diabetes, and children with familial hypercholesterolemia (35–39). In a multicenter Italian study, 1.6 g phytosterols/day, taken with a fermented milk, produced a reduction of LDL cholesterol from 166.2 ± 2.0 to 147.4 ± 2.8 mg/dL, and at the same time had an anti-oxidant effect, demonstrated by a significant reduction in the levels of isoprostane (40). Overall, clinical data suggest that the daily consumption of 1–3 g of plant sterols reduces LDL-cholesterol by 5–15%, with no significant effects on HDL cholesterol or triglycerides (41). Many clinical trials in patients with hypercholesterolemia treated with statins or fibrates are also available. The associations of plant sterols-fortified foods with these drugs determine an additive effect on the reduction in plasma levels of total cholesterol and LDL-cholesterol (41, 42). Some doubt, however, remains on safety. Beyond a possible interference with the absorption of fat-soluble vitamins, some observational studies have suggested an independent association between plasma levels of plant sterols and the risk of coronary heart disease. However, in vivo studies in animals fed with high doses of plant sterols seem to show a different effect, atherosclerosis, in terms of both, development of new plaques and size or lipid accumulation of lesions seem to improve (43). Recently, it has been suggested that the state of cholesterol “absorber” (as opposed to the state of “synthesizer”) may be the real atherogenic condition: thus, elevated plasma levels of phytosterols should indicate a hyperabsorptive pattern which might increase the risk (44). Longitudinal data in humans are not conclusive, and therefore this issue appears far from solved and the possibility of an increased of coronary risk, even if not likely, remains a problem to keep into account during this kind of treatment.
Berberine
This substance, with a bitter taste and intense yellow color, is present in the bark, roots and stems, including underground (rhizome) of plants of the genus Berberis, such as barberry (Berberis vulgaris L.). For the antimicrobial and antisecretive properties, berberin is traditionally used in the treatment of infections (45). In recent years, most attentions have been on the metabolic properties of berberine. In 2004, Kong et al., have shown that berberine reduced plasma cholesterol by 29%, triglycerides by 35%, and LDL cholesterol by 25%, whereas did not modify HDL-cholesterol levels (46). Berberine increases the number of LDL receptors on the hepatic cell surface, similarly to statins. However, during statin therapy, the exposure of LDL-receptors on cell membranes follows the decrease of the endogenous cholesterol synthesis and the subsequent reduction of intracellular cholesterol pool, whereas the action of berberine seems linked to the ability to inhibit a protein (PCSK9) responsible for the partial degradation of LDL receptors in the liver. For these reasons, berberine may have synergistic effects with statins (47, 48). It is relevant to remember that inhibition of PCSK9 by monoclonal antibodies is a new lipid-lowering strategy and several clinical trials are currently in progress (49, 50). Synergy between berberine and statins has been demonstrated by both cell culture and experimental animals studies. In humans, Bertolini et al. documented additive effects of treatment with berberine/statins in patients with heterozygous familial hypercholesterolemia, even higher to those of ezetimibe/statins; a recent meta-analysis confirmed the cholesterol-lowering effect of berberine, indicating also the necessity of further data from randomized trials (51). Other studies have even highlighted hypoglycemic effects of berberine in patients with diabetes mellitus type 2 by increasing the expression of insulin receptors and the improving of insulin resistance (52). The action on PCSK9, the ability to operate at multiple levels and the absence of significant side effects, suggests that berberine represents one of the most interesting available nutraceutic drugs.
Fermented Red Rice
The fermentation of red rice by a fungus (Monascus purpureus) produces a substance called monacolin K, which inhibits the synthesis of cholesterol (53). The monacolin K is also known as lovastatin, a statin available in the market worldwide. The red yeast rice also produces other monacolins that may enhance the inhibition of HMG-CoA reductase. In addition, recent data show that, compared to the classical lovastatin, monakolin K extracted from red yeast rice have even a higher bioavailability with a higher efficacy at the same dosage (54).
Red yeast rice represents an important drug of traditional Chinese medicine, and a considerable amount of experimental data have shown the efficacy and tolerability of red yeast rice. Heber et al. in a controlled study in 83 healthy hyperlipidemic subjects treated with 2.4 g/day of red yeast rice or placebo, observed large reductions in LDL-cholesterol (39 ± 19 mg/dL), significantly different from placebo (55). Studies in other populations, as summarized by Liu et al. in a meta-analysis of 93 randomized trials, led to similar results, demonstrating a significant effect on total and LDL-cholesterol levels but not on triglycerides or HDL-cholesterol concentrations (56). In a study that evaluated the efficacy of simvastatin (40 mg/day) vs. alternative treatments (including red yeast rice) for 12 weeks in dyslipidemic subjects with a history of statin myalgias, Becker et al. showed comparable reduction of LDL-C in the two groups. These authors assessed also tolerability, measured either as changes in biochemical parameters or pain severity, and, behind the cholesterol lowering efficacy, observed a good tolerability of red yeast rice (57). Red yeast rice was even similar to pravastatin in the reduction of LDL-C (27 vs. 30%), but it was associated with a lower rate of withdrawn from the treatment (58). Overall, red yeast rice seems to have a lower incidence of muscle disorders than statins. Myotoxicity, in the form of myopathy, myalgia, myositis, or rhabdomyolysis, is the most severe adverse effect of statins and the main cause of discontinuation. Statin therapy is associated with muscle problems in approximately 10–25% of patients treated in clinical practice (59). The exact pathophysiology of statin-induced myopathy is not fully known, and multiple mechanisms may contribute to that. One of these should be represented by a possible depletion of mevalonate metabolites (such as ubiquinone), and red yeast rice might be less efficient in blocking this metabolic cascade. Psychological components may also have a role, and the possibility to replace statins with natural drugs may have the consequent effect of removing this anxiety (60). Thus, red rice might be a real therapeutic alternative in patients intolerant to statins. Recently, in a group of hypercholesterolemic patients with a mild/moderate risk previously intolerant to statins or refusing classical pharmaceutical treatment, we examined in a double-blind, placebo-controlled, randomized study, the efficacy, safety, and tolerability of a product containing red yeast rice, but also the pulse wave velocity as expression of arterial stiffness and endothelial function. Patients received daily either a nutraceutical-combined pill, containing red yeast rice 200 mg (corresponding to monakoline 3 mg) or placebo for 6 weeks, and we observed, among subjects treated with red yeast rise, a significative reduction of total cholesterol and LDL-cholesterol (respectively by 10.4 and 12.2%), as well as of pulse wave velocity (by 6.5%); triglyceride and HDL-cholesterol levels did not change. Safety parameters did not change during the study and no patient reported muscle pain (61). Finally, in a 5-year long secondary prevention trial in 5000 Chinese subjects, Lu and colleagues demonstrated that an extract of red yeast rice, the Xuenzhikang (XZK), beside a 20% decrease of LDL-cholesterol, led to a significant reduction of coronary events in comparison with placebo (5.7 vs. 10.4%), with a reduction of relative and absolute risk of 45 and 4.7%, respectively, and together with high tolerability of treatment (62). To date, this unique trial of cardiovascular prevention with a nutracetical drug is available.
Recent European Guidelines of cardiovascular prevention include treatment with nutraceuticals for their clinical effects and tolerability (63). However, it should be emphasized that some categories of patients may particularly benefit from treatment with nutraceuticals. The best indication is subjects with a middle or lower cardiovascular risk in whom a modest reduction in LDL-C may be sufficient to reach LDL-C levels internationally recommended to reduce the risk (the so-called “target values”). In these patients, usually only on dietary treatment, therapy with nutraceuticals allows a better, rapid, and more stable achievement of aims. Also, subjects experiencing side effects with statins may be treated by nutraceuticals. In these subjects, a small but stable LDL-cholesterol reduction may be useful when statin therapy at standard doses is not feasible. Another potential use of those nutraceuticals having a mechanism of action different form statins, e.g., berberin or plant sterols, is the combination therapy with statins in order to either reduce statin dose or increase the hypolipidemic efficacy. The clinical use of these preparations may be also extended to other categories of patients. In those who are afraid of side effects of classical drugs or simply do not want to be considered a patient, the psychological effect of a “natural” treatment may be helpful to permit to treat these subjects and therefore to reduce their risk level. The cost issue raised by the treatment with nutraceuticals is still unresolved, since these therapies are usually more expensive than classical drugs and, in order for them to be efficient in reducing cardiovascular risk, should be lifelong.
In conclusion, nutraceuticals represent a valid alternative hypolipidemic treatment, and thus have a role in cardiovascular prevention strategies. Nutraceuticals have multiple physiological benefits and their use may be a valid alternative or complementary therapy to conventional treatment in many fields. A proper and reasoned use may help to prevent chronic diseases, increase life expectancy, support the structure or function of the body, delay the aging process, and help maintain overall good health (64). Due to the growing industrial and commercial interest in the future, it is desirable to have better regulations and improve the scientific agreement to warrant safe usage and clinical effectiveness (65).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Das L, Bhaumik E, Raychaudhuri U, Chakraborty R. Role of nutraceuticals in human health. J Food Sci Technol (2012) 49:173–83. doi: 10.1007/s13197-011-0269-4
2. Coppens P, da Silva MF, Pettman S. European regulations on nutraceuticals, dietary supplements and functional foods: a framework based on safety. Toxicology (2006) 221:59–74. doi:10.1016/j.tox.2005.12.022
3. Nijjar PS, Burke FM, Bloesch A, Rader DJ. Role of dietary supplements in lowering low-density lipoprotein cholesterol: a review. J Clin Lipidol (2010) 4:248–58. doi:10.1016/j.jacl.2010.07.001
4. Chen ZY, Jiao R, Ma KY. Cholesterol-lowering nutraceuticals and functional foods. J Agric Food Chem (2008) 56:8761–73. doi:10.1021/jf801566r
5. Varady KA, Wang Y, Jones PJ. Role of policosanols in the prevention and treatment of cardiovascular disease. Nutr Rev (2003) 61:376–83. doi:10.1301/nr.2003.nov.376-383
6. Gouni-Berthold I, Berthold HK. Policosanol: clinical pharmacology and therapeutic significance of a new lipid-lowering agent. Am Heart J (2002) 143:356–65. doi:10.1067/mhj.2002.119997
7. Stüsser R, Batista J, Padrón R, Sosa F, Pereztol O. Long-term therapy with policosanol improves treadmill exercise-ECG testing performance of coronary heart disease patients. Int J Clin Pharmacol Ther (1998) 36: 469–73.
8. Castaño G, Más R, Roca J, Fernández L, Illnait J, Fernández JC, et al. A double-blind, placebo-controlled study of the effects of policosanol in patients with intermittent claudication. Angiology (1999) 50:123–30. doi:10.1177/000331979905000205
9. Berthold HK, Unverdorben S, Degenhardt R, Bulitta M, Gouni-Berthold I. Effect of policosanol on lipid levels among patients with hypercholesterolemia or combined hyperlipidemia: a randomized controlled trial. JAMA (2006) 295:2262–9. doi:10.1001/jama.295.19.2262
10. Marinangeli CP, Jones PJ, Kassis AN, Eskin MN. Policosanols as nutraceuticals: fact or fiction. Crit Rev Food Sci Nutr (2010) 50:259–67. doi:10.1080/10408391003626249
11. Zern TL, Fernandez ML. Cardioprotective effects of dietary polyphenols. J Nutr (2005) 135:2291–4.
12. Dávalos A, Fernández-Hernando C, Cerrato F, Martínez-Botas J, Gómez-Coronado D, Gómez-Cordovés C, et al. Red grape juice polyphenols alter cholesterol homeostasis and increase LDL-receptor activity in human cells in vitro. J Nutr (2006) 136:1766–73.
13. Mollace V, Sacco I, Janda E, Malara C, Ventrice D, Colica C, et al. Hypolipemic and hypoglycaemic activity of bergamot polyphenols: from animal models to human studies. Fitoterapia (2011) 82:309–16. doi:10.1016/j.fitote.2010.10.014
14. Demonty I, Lin Y, Zebregs YE, Vermeer MA, van der Knaap HC, Jäkel M, et al. The citrus flavonoids hesperidin and naringin do not affect serum cholesterol in moderately hypercholesterolemic men and women. J Nutr (2010) 140:1615–20. doi:10.3945/jn.110.124735
16. Silagy C, Neil A. Garlic as a lipid lowering agent: a meta-analysis. J R Coll Physicians Lond (1994) 28:39–45.
17. Gardner CD, Lawson LD, Block E, Chatterjee LM, Kiazand A, Balise RR, et al. Effect of raw garlic vs commercial garlic supplements on plasma lipid concentrations in adults with moderate hypercholesterolemia: a randomized clinical trial. Arch Intern Med (2007) 167:346–53. doi:10.1001/archinte.167.4.346
18. Ernst E. Cardiovascular effects of garlic (Allium sativum): a review. Pharmatherapeutica (1987) 5:83–9.
19. Kumar M, Nagpal R, Kumar R, Hemalatha R, Verma V, Kumar A, et al. Cholesterol-lowering probiotics as potential biotherapeutics for metabolic diseases. Exp Diabetes Res (2012) 2012:902–17. doi:10.1155/2012/902917
20. Taranto MP, Medici M, Perdigon G, Ruiz Holgado AP, Valdez GF. Evidence for hypocholesterolemic effect of Lactobacillus reuteri in hypercholesterolemicmice. J Dairy Sci (1998) 81:2336–40. doi:10.3168/jds.S0022-0302(98)70123-7
21. Kumar R, Grover S, Batish VK. Hypocholesterolaemic effect of dietary inclusion of two putative probiotic bile salt hydrolase-producing Lactobacillus plantarum strains in Sprague-Dawley rats. Br J Nutr (2010) 12:1–12. doi:10.1017/S0007114510003740
22. Abd El-Gawad A, El-Sayed EM, Hafez SA, El-Zeini HM, Saleh FA. The hypocholesterolaemic effect of milk yoghurt and soy-yoghurt containing bifidobacteria in rats fed on a cholesterol-enriched diet. Int Dairy J (2005) 15:37–44. doi:10.1016/j.idairyj.2004.06.001
23. Liong MT, Shah NP. Bile salt deconjugation ability, bile salt hydrolase activity and cholesterol co-precipitation ability of lactobacilli strains. Int Dairy J (2005) 15:391–8. doi:10.1016/j.idairyj.2004.08.007
24. Nityanand S, Kapoor NK. Cholesterol lowering activity of the various fractions of the guggul. Indian J Exp Biol (1973) 11:395–6.
25. Urizar NL, Liverman AB, Dodds DT, Silva FV, Ordentlich P, Yan Y, et al. A natural product that lowers cholesterol as an antagonist ligand for FXR. Science (2002) 296:1703–6. doi:10.1126/science.1072891
26. Ulbricht C, Basch E, Szapary P, Hammerness P, Axentsev S, Boon H, et al. Natural standard research collaboration. guggul for hyperlipidemia: a review by the natural standard research collaboration. Complement Ther Med (2005) 13:279–90. doi:10.1016/j.ctim.2005.08.003
27. Szapary PO, Wolfe ML, Bloedon LT, Cucchiara AJ, DerMarderosian AH, Cirigliano MD, et al. Guggulipid for the treatment of hypercholesterolemia: a randomized controlled trial. JAMA (2003) 290:765–72. doi:10.1001/jama.290.6.765
28. Patch CS, Tapsell LC, Williams PG, Gordon M. Plant sterols as dietary adjuvants in the reduction of cardiovascular risk: theory and evidence. Vasc Health Risk Manag (2006) 2:157–62. doi:10.2147/vhrm.2006.2.2.157
29. Jones PJ, Raeini-Sarjaz M, Ntanios FY, Vanstone CA, Feng JY, Parsons WE. Modulation of plasma lipid levels and cholesterol kinetics by phytosterol versus phytostanol esters. J Lipid Res (2000) 41:697–705.
30. von Bergmann K, Sudhop T, Lütjohann D. Cholesterol and plant sterol absorption: recent insights. Am J Cardiol (2005) 96:10D–4D. doi:10.1016/j.amjcard.2005.03.014
31. Hallikainen MA, Sarkkinen ES, Gylling H, Erkkila AT, Uusitupa MI. Comparison of the effects of plant sterol ester and plant stanol esterenriched margarines in lowering serum cholesterol concentrations in hypercholesterolaemic subjects on a low-fat diet. Eur J Clin Nutr (2000) 54:715–25. doi:10.1038/sj.ejcn.1601083
32. Davidson MH, Maki KC, Umporowicz DM, Ingram KA, Dicklin MR, Schaefer E, et al. Safety and tolerability of esterified phytosterols administered in reduced-fat spread and salad dressing to healthy adult men and women. J Am Coll Nutr (2001) 20:307–19. doi:10.1080/07315724.2001.10719051
33. Devaraj S, Jialal I, Vega-Lopez S. Plant sterol-fortified orange juice effectively lowers cholesterol levels in mildly hypercholesterolemic healthy individuals. Arterioscler Thromb Vasc Biol (2004) 24:25–8. doi:10.1161/01.ATV.0000120784.08823.99
34. Volpe R, Niittynen L, Korpela R, Sirtori C, Bucci A, Fraone N, et al. Effects of yoghurt enriched with plant sterols on serum lipids in patients with moderate hypercholesterolaemia. Br J Nutr (2001) 86:233–9. doi:10.1079/BJN2001395
35. Miettinen TA, Puska P, Gylling H, Vanhanen H, Vartiainen E. Reduction of serum cholesterol with sitostanol-ester margarine in a mildly hypercholesterolemic population. N Engl J Med (1995) 333:1308–12. doi:10.1056/NEJM199511163332002
36. Hallikainen MA, Sarkkinen ES, Uusitupa MI. Plant stanol esters affect serum cholesterol concentrations of hypercholesterolemic men and women in a dose-dependent manner. J Nutr (2000) 130:767–76.
37. Vanstone CA, Raeini-Sarjaz M, Parsons WE, Jones PJ. Unesterified plant sterols and stanols lower LDL-cholesterol concentrations equivalently in hypercholesterolemic persons. Am J Clin Nutr (2002) 76:1272–8.
38. Demonty I, Ras RT, van der Knaap HC, Duchateau GS, Meijer L, Zock PL, et al. Continuous doseresponse relationship of the LDL-cholesterol-lowering effect of phytosterol intake. J Nutr (2009) 139:271–84. doi:10.3945/jn.108.095125
39. Laitinen K, Gylling H. Dose-dependent LDL-cholesterol lowering effect by plant stanol ester consumption: clinical evidence. Lipids Health Dis (2012) 11:140. doi:10.1186/1476-511X-11-140
40. Mannarino E, Pirro M, Cortese C, Lupattelli G, Siepi D, Mezzetti A, et al. Effects of a phytosterol-enriched dairy product on lipids, sterols and 8-isoprostane in hypercholesterolemic patients: a multicenter Italian study. Nutr Metab Cardiovasc Dis (2009) 19:84–90. doi:10.1016/j.numecd.2008.03.012
41. Goldberg AC, Ostlund RE Jr., Bateman JH, Schimmoeller L, McPherson TB, Spilburg CA. Effect of plant stanol tablets on lowdensity lipoprotein cholesterol lowering in patients on statin drugs. Am J Cardiol (2006) 97:376–9. doi:10.1016/j.amjcard.2005.08.056
42. Miettinen TA, Strandberg TE, Gylling H. Noncholesterol sterols and cholesterol lowering by long-term simvastatin treatment in coronary patients: relation to basal serum cholestanol. Arterioscler Thromb Vasc Biol (2000) 20:1340–6. doi:10.1161/01.ATV.20.5.1340
43. Rocha M, Banuls C, Bellod L, Jover A, Victor VM, Hernandez-Mijares A. A review on the role of phytosterols: new insights into cardiovascular risk. Curr Pharm Des (2011) 17:4061–75. doi:10.2174/138161211798764852
44. Silbernagel G, Chapman MJ, Genser B, Kleber ME, Fauler G, Scharnagl H, et al. High intestinal cholesterol absorption is associated with cardiovascular disease and risk alleles in ABCG8 and ABO: evidence from the LURIC and YFS cohorts and from a meta-analysis. J Am Coll Cardiol (2013) 62:291–9. doi:10.1016/j.jacc.2013.01.100
45. Lau CW, Yao XQ, Chen ZY, Ko WH, Huang Y. Cardiovascular actions of berberine. Cardiovasc Drug Rev (2001) 19:234–44. doi:10.1111/j.1527-3466.2001.tb00068.x
46. Kong W, Wei J, Abidi P, Lin M, Inaba S, Li C, et al. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat Med (2004) 10:1344–51. doi:10.1038/nm1135
47. Cameron J, Ranheim T, Kulseth MA, Leren TP, Berge KE. Berberine decreases PCSK9 expression in HepG2 cells. Atherosclerosis (2008) 201:266–73. doi:10.1016/j.atherosclerosis.2008.02.004
48. Kong WJ, Wei J, Zuo ZY, Wang YM, Song DQ, You XF, et al. Combination of simvastatin with berberine improves the lipid-lowering efficacy. Metabolism (2008) 57:1029–37. doi:10.1016/j.metabol.2008.01.037
49. Urban D, Poss J, Bohm M, Laufs U. Targeting the proprotein convertase subtilisin/kexin type 9 for the treatment of dyslipidemia and atherosclerosis. J Am Coll Cardiol (2013) 62:1401–8. doi:10.1016/j.jacc.2013.07.056
50. Dadu RT, Ballantyne CM. Lipid lowering with PCSK9 inhibitors. Nat Rev Cardiol (2014) 11:563–75. doi:10.1038/nrcardio.2014.84
51. Pisciotta L, Bellocchio A, Bertolini S. Nutraceutical pill containing berberine versus ezetimibe on plasma lipid pattern in hypercholesterolemic subjects and its additive effect in patients with familial hypercholesterolemia on stable cholesterol-lowering treatment. Lipids Health Dis (2012) 11:123. doi:10.1186/1476-511X-11-123
52. Derosa G, Maffioli P, Cicero AF. Berberine on metabolic and cardiovascular risk factors: an analysis from preclinical evidences to clinical trials. Expert Opin Biol Ther (2012) 12:1113–24. doi:10.1517/14712598.2012.704014
53. Endo A. Monacolin K, a new hypocholesterolemic agent produced by a Monascus species. J Antibiot (1979) 32:852–4. doi:10.7164/antibiotics.32.852
54. Chena C-H, Yangb J-C, Uangc Y-U, Lina C-J. Improved dissolution rate and oral bioavailability of lovastatin in red yeast rice products. Int J Pharm (2013) 444:18–24. doi:10.1016/j.ijpharm.2013.01.028
55. Heber D, Yip I, Ashley JM, Elashoff DA, Elashoff RM, Go VL. Cholesterol-lowering effects of a proprietary Chinese red-yeast-rice dietary supplement. Am J Clin Nutr (1999) 69:231–6.
56. Liu J, Zhang J, Shi Y, Grimsgaard S, Alraek T, Fonnebo V. Chinese red yeast rice (Monascus purpureus) for primary hyperlipidemia: a meta- analysis of randomized controlled trials. Chin Med (2006) 1:4. doi:10.1186/1749-8546-1-4
57. Becker DJ, Gordon RY, Halbert SC, French B, Morris PB, Rader DJ. Red yeast rice for dyslipidemia in statin-intolerant patients: a randomized trial. Ann Intern Med (2009) 150:830–9. 10.7326/0003-4819-150-12-200906160-00006
58. Halbert SC, French B, Gordon RY, Farrar JT, Schmitz K, Morris PB, et al. Tolerability of red yeast rice (2,400 mg twice daily) versus pravastatin (20 mg twice daily) in patients with previous statin intolerance. Am J Cardiol (2010) 105:198–204. doi:10.1016/j.amjcard.2009.08.672
59. Armitage J. The safety of statins in clinical practice. Lancet (2007) 370:1781–90. doi:10.1016/S0140-6736(07)60716-8
60. Vaklavas C, Chatzizisis YS, Ziakas A, Zamboulis C, Giannoglou GD. Molecular basis of statin-associated myopathy. Atherosclerosis (2009) 202:18–28. doi:10.1016/j.atherosclerosis.2008.05.021
61. Barbagallo CM, Longo F, Noto D, Cefalù AB, Ganci A, Cusumano G, et al. Reduction of cholesterol with nutraceuticals: result of a double-blind study. Int J Cardiovasc Dis (2015) (in press).
62. Lu Z, Kou W, Du B, Wu Y, Zhao S, Brusco OA, et al. Effect of Xuezhikang, an extract from red yeast Chinese rice, on coronary events in a Chinese population with previous myocardial infarction. Am J Cardiol (2008) 101:1689–93. doi:10.1016/j.amjcard.2008.02.056
63. European Association for Cardiovascular Prevention & Rehabilitation, Reiner Z, Catapano AL, De Backer G, Graham I, Taskinen MR, et al. ESC/EAS guidelines for the management of dyslipidaemias: the task force for the management of dyslipidaemias of the European society of cardiology (ESC) and the European atherosclerosis society (EAS). Eur Heart J (2011) 32:1769–818.
64. Nasri H, Baradaran A, Shirzad H, Rafieian-Kopaei M. New concepts in nutraceuticals as alternative for pharmaceuticals. Int J Prev Med (2014) 5:1487–99. doi:10.1093/eurheartj/ehr158
Keywords: nutraceuticals, lipids, LDL-cholesterol, hypolipidemic therapy, cardiovascular prevention
Citation: Barbagallo CM, Cefalù AB, Noto D and Averna M (2015) Role of nutraceuticals in hypolipidemic therapy. Front. Cardiovasc. Med. 2:22. doi: 10.3389/fcvm.2015.00022
Received: 16 February 2015; Accepted: 22 April 2015;
Published: 11 May 2015
Edited by:
Manfredi Tesauro, University of Rome Tor Vergata, ItalyReviewed by:
Jeonga Kim, University of Alabama at Birmingham, USAToshio Hayashi, Nagoya University Graduate School of Medicine, Japan
Copyright: © 2015 Barbagallo, Cefalù, Noto and Averna. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carlo M. Barbagallo, Biomedical Department of Internal Medicine and Specialistics (DIBIMIS), University of Palermo, Via del Vespro, 141, Palermo 90127, Italia, carlo.barbagallo@unipa.it
 Carlo M. Barbagallo
Carlo M. Barbagallo Angelo Baldassare Cefalù
Angelo Baldassare Cefalù Davide Noto
Davide Noto Maurizio R. Averna
Maurizio R. Averna