- The MRC Lifecourse Epidemiology Unit, Southampton General Hospital, University of Southampton, Southampton, UK
Osteoporosis causes considerable morbidity and mortality in later life, and the risk of the disease is strongly determined by peak bone mass, which is achieved in early adulthood. Poor intrauterine and early childhood growth are associated with reduced peak bone mass, and increased risk of osteoporotic fracture in older age. In this review we describe the regulatory aspects of intrauterine bone development, and then summarize the evidence relating early growth to later fracture risk. Physiological systems include vitamin D, parathyroid hormone, leptin, GH/IGF-1; finally the potential role of epigenetic processes in the underlying mechanisms will be explored. Thus factors such as maternal lifestyle, diet, body build, physical activity, and vitamin D status in pregnancy all appear to influence offspring bone mineral accrual. These data demonstrate a likely interaction between environmental factors and gene expression, a phenomenon ubiquitous in the natural world (developmental plasticity), as the potential key process. Intervention studies are now required to test the hypotheses generated by these epidemiological and physiological findings, to inform potential novel public health interventions aimed at improving childhood bone health and reducing the burden of osteoporotic fracture in future generations.
The Burden of Osteoporotic Fracture
Osteoporosis is the commonest bone disorder in Western populations. It is characterized by low bone mass and microarchitectural deterioration of bone tissue, which lead to increased bone fragility and the potentially devastating consequences of fragility fracture (Melton and Cooper, 2001). It has been estimated that at age 50 the remaining lifetime risk of fracture at the wrist, spine, or hip is 39% among women and 13% among men (Sambrook and Cooper, 2006). The number of fractures sustained is likely to rise substantially over coming years, as fracture rates seem to be rising in many parts of the world, and elderly people are the fastest growing age group worldwide. Even if age-adjusted incidence rates for hip fracture increase by only 1% per year, the estimated number of hip fractures worldwide will rise from 1.7 million in 1990 to 8.2 million in 2050 (Sambrook and Cooper, 2006).
Hip fractures are the most devastating consequence of osteoporosis: they require hospital admission and are associated with an excess mortality of 10–20% in the first year after the fracture (Center et al., 1999). Beyond this they are associated with a loss of independence, and a subsequent need for residential care. Vertebral fractures secondary to osteoporosis can also cause substantial disability from increased thoracic kyphosis, and long-term back pain. Unlike hip fractures only about a third of vertebral fractures result from falls; most result from routine activities such as bending or lifting (Cooper et al., 1992), therefore interventions aimed at reducing falls are unlikely to substantially reduce this problem. The third type of fracture typically associated with osteoporosis is distal radial fracture, although commonly viewed as the mildest outcome of osteoporosis, up to 30% of affected individuals may suffer a long-term complication (O’Neill et al., 2001). Osteoporotic fracture also has a huge economic impact, the annual cost to the United States has been estimated as $20 billion, and to the European Union as $30 billion (Felsenberg et al., 2002).
It appears that an individual’s bone mass (a composite measure of bone size and volumetric density) tracks through childhood and adolescence to reach a peak in early adulthood (Ferrari et al., 1998). Therefore, an individual mostly stays in the same position in the distribution relative to peers throughout growth, although with some potential for temporary deviation from this track as a result of changes in nutrition, physical activity, or during puberty. Bone mass then declines into older age, with an accelerated rate of decline at the female menopause. Osteoporotic fracture risk increases continuously as bone mineral density (BMD) declines, with a 1.5- to 3-fold increase in the risk for each SD fall in BMD (Cummings et al., 1993). The ability to predict fracture risk from BMD is at least as good, if not better, than the ability to detect heart disease from blood cholesterol levels, and to predict stroke from blood pressure values (Marshall et al., 1996).
Preventative strategies against osteoporosis may be aimed at either optimizing the peak bone mass obtained, or reducing the rate of bone loss. The former approach may be more appropriate for a population based program and modeling studies have shown that the magnitude of peak bone mass achieved is a major predictors of later osteoporosis risk (Hernandez et al., 2003). Peak bone mass is partly inherited, but the currently identified genetic markers only explain a small amount of the variation in individual peak bone mass and fracture risk (Ralston, 1998; Paternoster et al., 2010). Environmental influences during early life have been shown to impact upon bone mineral accrual. It is likely that this is due to an interaction between environmental factors and the child’s genome, which establishes a functional level in a variety of metabolic processes involved in skeletal growth. This leads to interest in potentially modifiable factors in pregnancy, which could have important implications for the bone health of future generations.
Skeletal Development
For adequate bone development to occur the human fetus requires approximately 30 g of calcium during development, which is actively transported across the placenta (Widdowson et al., 1988). This process starts as early as 20 weeks gestation (Forestier et al., 1987). To supply the demand for calcium the mother increases both calcium absorption from the gut, and bone resorption. Thus there may be a decrease in maternal bone mass of around 10% during pregnancy (Gambacciani et al., 1995), with potentially further bone loss during lactation but recovery of bone mass in the long term (Laskey and Prentice, 1999).
A miniature version of the skeleton is laid down in the embryonic period, and primary ossification centers form in the vertebrae and long bones between the 8th and 12th weeks, but it is not until the third trimester that the bulk of mineralization occurs (Moore and Persaud, 1998).
The main determinant of skeletal mineralization in utero appears to be the fetal plasma calcium concentration (Kovacs, 2003), this is influenced by the placental transfer (Mughal et al., 1989), and fetal management, of calcium (Heaney and Skillman, 1971). Placental calcium transfer is thought to be regulated by PTHrP, and injection of PTHrP into hypothyroidectomized sheep has been shown to increase placental calcium flux (Care et al., 1986). Fetal levels of PTHrP are increased in response to a low fetal plasma calcium levels (Kovacs et al., 1996, 2001b).
Fetal management of calcium is mainly influenced by fetal parathyroid hormone (PTH). Lack of parathyroids in fetal mice leads to low fetal calcium levels and decreased skeletal mineralization (Kovacs et al., 2001a). Fetal PTH seems to act by increasing calcium resorption from the kidney, and possibly bone, to increase calcium concentration, but does not affect placental calcium transfer. Maternal PTH does not cross the placenta, but affects the fetus by altering the concentration of maternal circulating calcium, and so the calcium load presented to the fetus, which in turn affects levels of fetal PTH. As such maternal hypoparathyroidism can lead to fetal parathyroid hyperplasia, and generalized neonatal skeletal demineralization (Kovacs, 2003). In addition to its effects on placental calcium transport PTH and PTHrP directly influence fetal bone growth. For example PTHrP has been shown to act on prehypertrophic chondrocytes in the fetal growth plate to inhibit differentiation to hypertrophic chondrocytes (Weir et al., 1996; Calvi and Schipani, 2000). Both over and under expression of PTHrP or its receptor are associated with short-limbed dwarfism in animals and humans (Kato et al., 1990; Iwamoto et al., 1994; Karaplis et al., 1994; Lanske et al., 1996; Vortkamp et al., 1996).
It is unclear how the actions of fetal PTH and PTHrP interact with the characterized molecular apparatus in the placenta: Placental calcium transfer occurs in the syncytiotrophoblast and proceeds through a sequence of events consisting of facilitated apical entry through a calcium transport channel, cytosolic diffusion of calcium bound to calbindin and finally, basolateral extrusion of calcium ions through a plasma membrane calcium dependent ATPase (Belkacemi et al., 2005). This last group of transport channels includes four individual plasma membrane Ca2+ ATPase isoforms (PMCA 1–4). These have been demonstrated in human placenta, as well as in fetal skeletal muscle and brain. PMCA 1 and 4 are present in most tissues while PMCA 2 and 3 are found in more specialized cell types (Stauffer et al., 1993; Zylinska et al., 2002). In the rat it has been found that a two to threefold increase in PMCA gene expression is associated with a 72-fold increase in calcium transport across the placenta during late gestation (Glazier et al., 1992). The regulation of this process is as yet unknown, but at least one of the isoforms of PMCA has been shown to be regulated by 1,25(OH)-vitamin D (Kip and Strehler, 2004), and in some animals, but not others, 1,25(OH)2-vitamin D appears necessary for maintenance of the maternofetal calcium gradient (Lester, 1986). Work in human subjects has shown that the level of mRNA expression of an active placental calcium transporter (PMCA3), thought to be situated on the basal membrane of the placenta, is positively correlated with whole body bone mineral content (BMC) in the offspring at birth (Martin et al., 2005). These observations may suggest a possible mechanism for the influence of maternal vitamin D status on placental calcium transport and intrauterine bone mineral accrual.
Influence of the Early Environment on Bone Development
Several lines of evidence suggest that environmental factors acting early in development, for example in utero or early postnatal life, may lead to long-term modifications to skeletal development. Thus data have accrued from adult cohorts in whom birth records existed, mother–offspring cohorts, human physiological studies, animal work, and finally investigations into the potential epigenetic mechanisms which may underlie these observations.
Early Growth and Adult BMC and Risk of HIP Fracture
The link between the environment in early life and osteoporosis risk was first identified in an epidemiological study of 21-year-old women born in Bath in 1968 and 1969 (Cooper et al., 1995). A statistically significant relationship was found between the girl’s weight at 1 year and their adult BMC at the lumbar spine and femoral neck, independent of adult weight and body mass index. Older adults (60–75 years) were studied in the Hertfordshire Cohort Study, demonstrating a positive relationship between weight at 1 year and adult BMC (Dennison et al., 2005). The relationships remained after adjusting for lifestyle characteristics in adulthood including physical activity, dietary calcium intake, cigaret smoking, and alcohol consumption. These findings corroborate observations from United States, Australia, Sweden, and the Netherlands (Cooper et al., 2008).
Hip structure analysis using DXA scan images of the participant hips from the Hertfordshire Cohort Study demonstrated a positive relationship between weight at 1 year and the inter-trochanteric width of the femur, but not femoral neck length at age 60–75 years (Javaid et al., 2006b). The association remained after adjustment for adult body weight and was independent of proximal femoral BMC. This suggests that poor growth in utero and during the first year of life is associated with disproportion of the proximal femur in later life leading to a narrower neck but preserved axis length. This would correspond to a reduction in the mechanical strength of the region, over and above that attributable to BMC alone. Data directly linking early growth with adult risk of hip fracture have come from the Helsinki Cohort Study (Cooper et al., 2001). Birth and childhood growth data from 7000 men and women born in Helsinki University Central Hospital during 1924–1933 were linked to hospital discharge records for hip fracture (n = 112). After adjustment for age and gender three independent determinants of hip fracture risk were identified: tall maternal stature (p < 0.001), shortness at birth (p = 0.03), and low rate of childhood growth (height p = 0.006, weight p = 0.01). Additionally, people who had hip fractures were more likely to be shorter at birth but of average height by age 7 years. This suggested that hip fracture risk may be particularly elevated among children in whom the growth of the skeletal envelope had been forced ahead of its capacity to mineralize.
Maternal Lifestyle, Body Build, and Physical Activity: Evidence from Mother–Offspring Cohorts
There are now data from several mother–offspring cohorts demonstrating relationships between maternal factors in pregnancy and offspring bone mass. One of the first studies have examined the relationships between maternal body build, nutrition, lifestyle, and physical activity during pregnancy and offspring bone size and density at birth assessed by DXA. Maternal triceps skinfold thickness at 28 weeks positively correlated with neonatal whole body BMC, and mothers who smoked in pregnancy had, on average, babies with an 11% lower whole body BMC than mothers who did not smoke (Godfrey et al., 2001). Interestingly smoking at the time of the last menstrual period was not associated with neonatal BMC or areal bone mineral density (BMD = bone mineral content divided by bone area), suggesting a fundamental importance of the immediate intrauterine environment. High levels of maternal vigorous physical activity in late gestation also predicted that reduced BMC at birth.
The Southampton Women’s Survey provided the opportunity to further investigate these interactions in a larger sample of 841 infants (Harvey et al., 2010). This confirmed that independent predictors of greater neonatal whole body bone area and BMC included greater maternal birthweight, height, parity, fat stores (triceps skinfold thickness), and lower physical activity in late pregnancy. Maternal smoking was again statistically significantly (and independently) associated with lower neonatal bone mass. Additionally great parity and maternal fat stores, and lower physical activity predicted greater bone width in the offspring. These relationships were observed in both male and female neonates.
Vitamin D and Calcium Nutrition
The mother–offspring cohorts also provide a platform to investigate the mechanism of interactions between intrauterine environment and later bone health. Calcium and vitamin D, being key nutrients in bone development, are prime candidates for investigation. In a study of 9-year-old children born to mothers involved in a nutrition in pregnancy study in Southampton, whole body BMC, and BMD in childhood were positively associated with ionized calcium level in the umbilical cord of the child at birth (Javaid et al., 2006a). This association appeared to mediate the effects of maternal fat stores, smoking, and socio-economic status. In this study around 31% of the mothers had insufficient and 18% had deficient circulating concentrations of 25(OH)-vitamin D during late pregnancy (11–20 and <11 μg/l respectively). Lower concentrations of serum 25(OH)-vitamin D in mothers during late pregnancy were associated with reduced whole body BMC and BMD in children at age 9 years (Javaid et al., 2006a). Estimated exposure to ultraviolet B radiation during late pregnancy (p < 0.0001) and the maternal use of vitamin D supplements (p = 0.01) both predicted maternal 25(OH)-vitamin D concentration, and childhood BMC (p = 0.03). Adjunctive evidence supporting a role for maternal vitamin D status was obtained in the Southampton Women’s Survey, where maternal vitamin D concentrations again correlated with neonatal bone mass (Harvey et al., 2008). Findings from the Avon Longitudinal Study of Parents and Children (Sayers and Tobias, 2009) further reinforced the importance of maternal 25(OH)-vitamin D levels, demonstrating a positive association between ambient ultraviolet B radiation in pregnancy and offspring BMC at 9 years old.
In developing southern hemisphere populations where sunshine is in abundance, often calcium nutrition is a greater issue than that of 25(OH)-vitamin D status. Thus in a cohort of mothers and children from Pune, India (Ganpule et al., 2006), offspring of women who had a higher frequency of intake of calcium-rich foods during pregnancy had higher total and lumbar spine BMC and BMD, independent of parental size, and bone composition. Circulating maternal 25(OH)-vitamin D concentrations in this cohort were relatively high, and were not associated with childhood skeletal measures. Thus, in populations in nutritional transition, where maternal sunlight exposure is sufficient to maintain adequate vitamin D status, the availability of calcium becomes a more critical determinant of fetal and childhood bone mineral accrual.
Moving beyond maternal calcium homeostasis, it is apparent that there is a broader relationship between maternal dietary pattern and childhood bone mass. Using principal component analysis of food frequency questionnaires, a particular pattern of dietary intake was observed in the mothers of the Southampton 9 year follow-up study. Because this pattern matched closely current guidelines on health eating, it was termed the “prudent diet” pattern (Robinson et al., 2004; Crozier et al., 2006). Women with high prudent diet scores had diets characterized by high intakes of fruit, vegetables, wholemeal bread, rice, and pasta, with low intakes of confectionary, added sugar, white bread, and crisps. Greater compliance with this “healthy” dietary pattern during pregnancy was associated with increased offspring whole body and lumbar spine BMD. At age 9 years (p < 0.001), independent of maternal body build, smoking, and physical activity (Cole et al., 2009).
Physiological Studies
Analysis of samples acquired from mother–offspring cohorts has allowed the relationships between physiological markers such as leptin and IGF-1 and offspring bone mineral accrual to be investigated.
Leptin
Leptin is a hormone which is best known for its role in fat metabolism in adults, but laboratory based studies have shown that it has a positive effect on mesenchymal cell differentiation into osteoblasts (Thomas et al., 1999; Kiyokawa et al., 2001). Leptin receptors have been found on osteoblasts, chondrocytes, and bone marrow stromal cells. Leptin is produced in the feto-placental circulation by fetal fat, heart, liver, muscle, and interestingly the placenta itself (Masuzaki et al., 1997; Lepercq et al., 2001). Using umbilical cord samples from 93 neonates Javaid et al. (2005) were able to show that serum leptin at birth was related strongly to neonatal whole body BMC, bone area, and estimated volumetric BMD. In a more recent study, growth velocity of fetal abdominal circumference, which is a composite measure of adiposity and liver volume, positively predicted estimated volumetric bone density at 4 years (Harvey et al., 2009). Thus fetal leptin may be an important regulator of bone development. The human placenta is likely to be instrumental in this regulation, both through its role as a barrier between maternal and fetal leptin, and through fetal leptin production. The detailed regulation of fetal leptin has yet to be elucidated, as has the relationship between leptin, adiposity, and fetal bone mass.
Growth Hormone and IGF-1
Developing chondrocytes have been found to express insulin like growth factor-1 (IGF-1) mRNA, and it has been shown to stimulate their proliferation (Olney and Mougey, 1999). The placenta acts as a barrier between the maternal and fetal IGF systems, with fetal IGF predominantly produced in the fetal liver. Using the same subjects as the leptin study Javaid et al. (2004) found that umbilical cord serum IGF-1 concentration positively correlated with neonatal whole body BMC, lean mass, and fat mass. Cord serum IGF-1 levels partly explained the effect of maternal smoking upon offspring skeletal development, but did not account for the other previously demonstrated parental characteristics, such as height, weight, and fat mass. This suggested that both IGF-1 dependent and independent factors influence bone parameters of the neonate.
In adults profiles of circulating growth hormone (GH) and cortisol were compared to bone density and birth records of patients in the Hertfordshire cohort study (Fall et al., 1998; Phillips et al., 1998; Dennison et al., 1999). It was found that weight at 1 year positively correlated with median GH concentration and negatively correlated with cortisol concentration at age 61–72 years, suggesting a “memory” of a difficult intrauterine, or early life environment. Furthermore the profiles of these two hormones were found to be determinants of prospectively determined bone loss rate, suggesting a physiological outcome of this “memory.” This suggests that, unlike leptin, the GH–IGF axis is instrumental in both the formation and maintenance of skeletal health, additionally the ability of this system to maintain skeletal health may be influenced by the events of intrauterine life.
Developmental Plasticity and Gene–Environment Interactions
There is a strong biological basis for a model of disease pathogenesis in which a single genotype can give rise to several different phenotypes, allowing the organism to adapt future generations to prevailing environmental conditions: this phenomenon is termed developmental plasticity (Bateson et al., 2004). There are striking illustrations of such phenomena in the natural world: for example the thickness of the meadow vole’s coat is determined exclusively by the amount of light the mother is exposed to prenatally (Lee, 1993). In this way the vole offspring is able to adapt for the season into which it will be born. Clearly such an adaptation could not arise through fixed genetic change. Evidence for a prolonged effect of an adverse intrauterine environment on genetically identical individuals come from studies of monozygotic twins. Thus in a UK study of 445 monozygotic (MZ) and 966 dizygotic (DZ) healthy female twins, at a mean age of 47 years, birth weight was found to positively predict BMC and BMD (Antoniades et al., 2003). The MZ twins, despite being genetically identical, had greater intra-pair variability in birth weight than DZ twins. This highlights the crucial role of the placenta in development, as two-thirds of the MZ twins will have shared a placenta, whereas the DZ twins will have had a placenta each. A majority of the MZ twins therefore had to compete for placental resources, with a subsequent compromise in their growth potential. Furthermore, in the MZ twins compared to the DZ twins there was a stronger intra-pair association between birth weight and bone density. This suggests that the early adverse environment of the smaller MZ twin, when competing for placental resources, strongly influenced their future adult skeletal status.
In humans the influence of the intrauterine environment was initially demonstrated by the observation that the risk of hypertension, coronary heart disease, and diabetes is increased in those individuals born with low birthweight (Godfrey et al., 1997; Barker, 1998; Gluckman and Hanson, 2004). The data presented above demonstrate that similar associations are observed for osteoporosis. The key mechanistic point is that it is likely to be not just genes or environment that is important, but an interaction between the two. Thus in the Hertfordshire Cohort Study there was no significant association between either vitamin D receptor (VDR) genotype and adult BMD in the cohort as a whole (Dennison et al., 2001). However the relationship between lumbar spine BMD and VDR varied according to birth weight. After adjusting for age, gender, and adult weight individuals in the lowest third for birthweight had a higher spine BMD if they were of the BB genotype (p = 0.01). People of the same genotype who were in the highest third for birth weight had a lower spine BMD compared to those of bb genotype in the same birth weight category (p = 0.04). Similar interactions between birthweight and genome were observed for the GH gene (Dennison et al., 2004). What is needed therefore, is a scientific mechanism that can explain the interaction between the environment and genome, and this is provided by considering the epigenetic modification of gene expression.
Epigenetics
Epigenetics refers to heritable information that is not contained in the underlying DNA sequence (Jaenisch and Bird, 2003). Each cell in the body is said to acquire a unique “epigenetic signature” reflecting the genotype, developmental history, and environmental influences upon the cell (Morgan et al., 2005). These changes then influence cell function, and so the phenotype of the organism. The two most studied forms of epigenetic marking are DNA methylation and histone modification. DNA methylation involves the addition of a methyl group to cytosine residues at the carbon-5 position of CpG dinucleotides. DNA methylation is generally associated with gene repression, either by decreased binding of transcription factors or by attracting methyl-CpG-binding proteins that act as transcriptional repressors (Gluckman et al., 2007; Gicquel et al., 2008). Histone modification refers to post-translational modification of histone tails. Histones are involved in the packaging of DNA into chromatin, and if the way that DNA is wrapped around the histones changes, gene expression can also change.
With the formation of a new organism most, but not all, of the epigenetic markings of the previous generation are wiped out. This occurs during two major points in development: gametogenesis and preimplantation development (Morgan et al., 2005). During gametogenesis the primordial germ cells undergo demethylation in the embryo, including at imprinted genes. The genomes of the gametes then acquire imprints and undergo de novo methylation in differing ways according to the sex of the embryo. In males this process finishes during embryological development, whilst in females this continues until adult life in maturing oocytes. When these gametes are fertilized, but before implantation, the new embryo then undergoes a second round of demethylation, however this time imprinted genes are not demethylated. As the new embryo implants new methylation patterns become established via de novo methylation by the activities of DNA methyltransferases.
From this baseline level of methylation epigenetic mechanisms are then thought to influence a range of processes, including the commitment of cells to develop a particular lineage (Morgan et al., 2005), and adaptations the organism makes in response to its environment (Bird, 2007). Thus it has been found that humans exposed to the Dutch hunger winter of 1944–1945 at the time of conception had less DNA methylation of the imprinted IGF-2 gene (a key factor in growth and development) 60 years later, compared to their same sex siblings (Heijmans et al., 2008). More recent analysis of the children born during the Dutch hunger winter has now identified genes which show epigenetic changes associated with famine exposure later in pregnancy (Tobi et al., 2009). The early life environment has been shown to have an influence upon epigenetic marking of the glucocorticoid receptor in rats (Weaver et al., 2004). Rat pups nursed by mothers with a particular style of nursing behavior, with more licking and grooming, have been found to be less fearful and better able to cope with stress. Rat pups nursed by the attentive mothers were shown to have an altered pattern of methylation of their glucocorticoid receptors; these differences emerged in the first week of life, were reversed by cross fostering, and persisted into adulthood (Weaver et al., 2004).
In rats protein restriction during pregnancy is associated with reduced expression of a particular DNA methyl transferase (Dnmt1; Lillycrop et al., 2007) in the liver of the adult offspring. Dnmt1 is involved in maintaining patterns of DNA methylation through mitosis (Bird, 2002). Dnmt1 methylation has been demonstrated in human umbilical cord, and in both the human umbilical cord and the rat liver Dnmt1 expression was associated with the degree of methylation of the glucocorticoid receptor promoter. Indeed, in human umbilical cord Dnmt1 expression predicted 49% of the variation in methylation of the glucocorticoid receptor promoter (Lillycrop et al., 2007). As would be expected methylation of the glucocorticoid receptor promoter was associated with reduced expression of the receptor protein (Lillycrop et al., 2007). It is therefore suggested that maternal dietary restriction in pregnancy may result in reduced fetal Dnmt1 expression, leading to reduced methylation of the glucocorticoid receptor promoter, causing increased expression of the glucocorticoid receptor. An effect of this could be an increased sensitivity of osteoblasts to cortisol, which may act to decrease bone mineral density. This would be consistent with the findings of a positive association between birth weight and basal levels of cortisol (Phillips et al., 1998), and the negative association between integrated cortisol concentration and bone density in adults from the Hertfordshire cohort study (Dennison et al., 1999).
The second area of current research into the epigenomic changes associated with osteoporosis has directly focused on the placenta. It is known that placental calcium transport is dependent upon a series of transport proteins in the syncytiotrophoblast, and as discussed earlier umbilical cord calcium levels correlated with BMC when the child was age 9 years (Javaid et al., 2006a). A family of plasma membrane ATPase isoforms (PMCA1–4) are thought to be a rate limiting step in the placental calcium transportation. Using placental tissue samples from 70 placentae it was found that expression of PMCA3 mRNA predicted neonatal BMC (Martin et al., 2007). Furthermore this relationship was independent of maternal height, pre-pregnancy fat stores, parity, physical activity, smoking, and calcium intake. 1,25(OH)-vitamin D has been shown to regulate other PMCA isoforms (Kip and Strehler, 2004) and is therefore a potential candidate. Figure 1 summarizes the path of calcium across the placenta from mother to fetus.
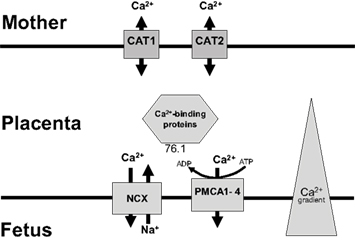
Figure 1. Placental calcium transport: Calcium enters from the maternal circulation via facilitated transporters (CAT 1 and 2), crosses the cytosol bound to proteins such as calbindin and is then actively extruded into the fetal circulation by Na/Ca exchanger and PMCA proteins (plasma membrane calcium ATPases). Used with permission (Williams et al., 2009).
Conclusion
Osteoporosis is a major cause of morbidity and mortality through its association with age-related fractures. It is becoming increasingly clear that there is a relationship between growth and development in early life and bone health in older age. It is likely that this is mediated, at least in part, by the main interface between the mother and her fetus, the placenta. Epigenetic processes may be the mechanism by which the “memory” of early life environment in stored in the offspring. Future understanding of the nature, timing and transduction of epigenetic changes could point toward potential biomarkers for later life diseases, and suggest focused interventions. It is envisaged that the placenta will play a central role in this research, which ultimately aims to develop novel strategies to improve skeletal health throughout the life course, and reduce osteoporotic fracture in future generations.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Antoniades, L., MacGregor, A. J., Andrew, T., and Spector, T. D. (2003). Association of birth weight with osteoporosis and osteoarthritis in adult twins. Rheumatology 42, 791–796.
Barker, D. J. P. (1998). Mothers, Babies and Health in Later Life. Edinburgh: Churchhill Livingstone.
Bateson, P., Barker, D., Clutton-Brock, T., Deb, D., D’Udine, B., Foley, R. A., Gluckman, P., Godfrey, K., Kirkwood, T., Lahr, M. M., McNamara, J., Metcalfe, N. B., Monaghan, P., Spencer, H. G., and Sultan, S. E. (2004). Developmental plasticity and human health. Nature 430, 419–421.
Belkacemi, L., Bedard, I., Simoneau, L., and Lafond, J. (2005). Calcium channels, transporters and exchangers in placenta: a review. Cell Calcium 37, 1–8.
Calvi, L. M., and Schipani, E. (2000). The PTH/PTHrP receptor in Jansen’s metaphyseal chondrodysplasia. J. Endocrinol. Invest. 23, 545–554.
Care, A. D., Caple, I. W., Abbas, S. K., and Pickard, D. W. (1986). The effect of fetal thyroparathyroidectomy on the transport of calcium across the ovine placenta to the fetus. Placenta 7, 417–424.
Center, J. R., Nguyen, T. V., Schneider, D., Sambrook, P. N., and Eisman, J. A. (1999). Mortality after all major types of osteoporotic fracture in men and women: an observational study. Lancet 353, 878–882.
Cole, Z. A., Gale, C. R., Kassim Javaid, M., Robinson, S. M., Law, C., Boucher, B. J., Crozier, S. R., Godfrey, K. M., Dennison, E. M., and Cooper, C. (2009). Maternal dietary patterns during pregnancy and childhood bone mass: a longitudinal study. J. Bone Miner. Res. 24, 663–668.
Cooper, C., Atkinson, E. J., O’Fallon, W. M., and Melton, L. J. III. (1992). Incidence of clinically diagnosed vertebral fractures: a population-based study in Rochester, Minnesota, 1985–1989. J. Bone Miner. Res. 7, 221–227.
Cooper, C., Cawley, M., Bhalla, A., Egger, P., Ring, F., Morton, L., and Barker, D. (1995). Childhood growth, physical activity, and peak bone mass in women. J. Bone Miner. Res. 10, 940–947.
Cooper, C., Eriksson, J. G., Forsén, T., Osmond, C., Tuomilehto, J., and Barker, D. J. P. (2001). Maternal height, childhood growth and risk of hip fracture in later life: a longitudinal study. Osteoporos. Int. 12, 623–629.
Cooper, C., Harvey, N., Javaid, K., Hanson, M., and Dennison, E. (2008). Growth and bone development. Nestle Nutr. Workshop Ser. Pediatr. Program 61, 53–66.
Crozier, S. R., Robinson, S. M., Borland, S. E., and Inskip, H. M. (2006). Dietary patterns in the Southampton women’s survey. Eur. J. Clin. Nutr. 60, 1391–1399.
Cummings, S. R., Black, D. M., Nevitt, M. C., Browner, W., Cauley, J., Ensrud, K., Genant, H. K., Palermo, L., Scott, J., and Vogt, T. M. (1993). Bone density at various sites for prediction of hip fractures. Lancet 341, 72–75.
Dennison, E., Hindmarsh, P., Fall, C., Kellingray, S., Barker, D., Phillips, D., and Cooper, C. (1999). Profiles of endogenous circulating cortisol and bone mineral density in healthy elderly men. J. Clin. Endocrinol. Metab. 84, 3058–3063.
Dennison, E. M., Arden, N. K., Keen, R. W., Syddall, H., Day, I. N. M., Spector, T. D., and Cooper, C. (2001). Birthweight, vitamin D receptor genotype and the programming of osteoporosis. Paediatr. Perinat. Epidemiol. 15, 211–219.
Dennison, E. M., Syddall, H. E., Rodriguez, S., Voropanov, A., Day, I. N. M., and Cooper, C. (2004). Polymorphism in the growth hormone gene, weight in infancy, and adult bone mass. J. Clin. Endocrinol. Metab. 89, 4898–4903.
Dennison, E. M., Syddall, H. E., Sayer, A. A., Gilbody, H. J., and Cooper, C. (2005). Birth weight and weight at 1 year are independent determinants of bone mass in the seventh decade: the hertfordshire cohort study. Pediatr. Res. 57, 582–586.
Fall, C., Hindmarsh, P., Dennison, E., Kellingray, S., Barker, D., and Cooper, C. (1998). Programming of growth hormone secretion and bone mineral density in elderly men: a hypothesis. J. Clin. Endocrinol. Metab. 83, 135–139.
Felsenberg, D., Silman, A. J., Lunt, M., Armbrecht, G., Ismail, A. A., Finn, J. D., Cockerill, W. C., Banzer, D., Benevolenskaya, L. I., Bhalla, A., Bruges Armas, J., Cannata, J. B., Cooper, C., Dequeker, J., Eastell, R., Felsch, B., Gowin, W., Havelka, S., Hoszowski, K., Jajic, I., Janott, J., Johnell, O., Kanis, J. A., Kragl, G., Lopes Vaz, A., Lorenc, R., Lyritis, G., Masaryk, P., Matthis, C., Miazgowski, T., Parisi, G., Pols, H. A. P., Poor, G., Raspe, H. H., Reid, D. M., Reisinger, W., Scheidt-Nave, C., Stepan, J. J., Todd, C. J., Weber, K., Woolf, A. D., Yershova, O. B., Reeve, J., and O’Neill, T. W. (2002). Incidence of vertebral fracture in Europe: results from the European prospective osteoporosis study (EPOS). J. Bone Miner. Res. 17, 716–724.
Ferrari, S., Rizzoli, R., Slosman, D., and Bonjour, J. P. (1998). Familial resemblance for bone mineral mass is expressed before puberty. J. Clin. Endocrinol. Metab. 83, 358–361.
Forestier, F., Daffos, F., and Rainaut, M. (1987). Blood chemistry of normal human fetuses at midtrimester of pregnancy. Pediatr. Res. 21, 579–583.
Gambacciani, M., Spinetti, A., Gallo, R., Cappagli, B., Teti, G. C., and Facchini, V. (1995). Ultrasonographic bone characteristics during normal pregnancy: longitudinal and cross-sectional evaluation. Am. J. Obstet. Gynecol. 173, 890–893.
Ganpule, A., Yajnik, C. S., Fall, C. H. D., Rao, S., Fisher, D. J., Kanade, A., Cooper, C., Naik, S., Joshi, N., Lubree, H., Deshpande, V., and Joglekar, C. (2006). Bone mass in Indian children – Relationships to maternal nutritional status and diet during pregnancy: the Pune maternal nutrition study. J. Clin. Endocrinol. Metab. 91, 2994–3001.
Gicquel, C., El Osta, A., and Le Bouc, Y. (2008). Epigenetic regulation and fetal programming. Best Pract. Res. Clin. Endocrinol. Metab. 22, 1–16.
Glazier, J. D., Atkinson, D. E., Thornburg, K. L., Sharpe, P. T., Edwards, D., Boyd, R. D., and Sibley, C. P. (1992). Gestational changes in Ca2+ transport across rat placenta and mRNA for calbindin9K and Ca(2+)-ATPase. Am. J. Physiol. 263(4 Pt 2), R930–R935.
Gluckman, P. D., and Hanson, M. A. (2004). The developmental origins of the metabolic syndrome. Trends Endocrinol. Metab. 15, 183–187.
Gluckman, P. D., Hanson, M. A., and Beedle, A. S. (2007). Non-genomic transgenerational inheritance of disease risk. Bioessays 29, 145–154.
Godfrey, K., Walker-Bone, K., Robinson, S., Taylor, P., Shore, S., Wheeler, T., and Cooper, C. (2001). Neonatal bone mass: influence of parental birthweight, maternal smoking, body composition, and activity during pregnancy. J. Bone Miner. Res. 16, 1694–1703.
Godfrey, K. M., Barker, D. J. P., Robinson, S., and Osmond, C. (1997). Maternal birthweight and diet in pregnancy in relation to the infant’s thinness at birth. Br. J. Obstet. Gynaecol. 104, 663–667.
Harvey, N., Javaid, M. K., Arden, N. K., Poole, J. R., Crozier, S. R., Robinson, S. M., Inskip, H. M., Goldberg, J., Dennison, E. M., Cooper, C., and “the SWS study team”. (2010). Maternal predictors of neonatal bone size and geometry: the Southampton Women’s Survey. J. Dev. Orig. Health Dis. 1, 35–41.
Harvey, N., Mahon, P., Robinson, S., Nisbet, C., Javaid, M., Crozier, S., Inskip, H., Godfrey, K., Arden, N., Dennison, E., and Cooper, C. (2009). Different indices of fetal growth predict bone size and volumetric density at 4 years old. J. Bone Miner. Res. Available at: PM:19839768
Harvey, N. C., Javaid, M. K., Poole, J. R., Taylor, P., Robinson, S. M., Inskip, H. M., Godfrey, K. M., Cooper, C., Dennison, E. M., Barker, D. J. P., Law, C. M., Cox, V., Coakley, P., and Hammond, J. (2008). Paternal skeletal size predicts intrauterine bone mineral accrual. J. Clin. Endocrinol. Metab. 93, 1676–1681.
Heaney, R. P., and Skillman, T. G. (1971). Calcium metabolism in normal human pregnancy. J. Clin. Endocrinol. Metab. 33, 661–670.
Heijmans, B. T., Tobi, E. W., Stein, A. D., Putter, H., Blauw, G. J., Susser, E. S., Slagboom, P. E., and Lumey, L. H. (2008). Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc. Natl. Acad. Sci. U.S.A. 105, 17046–17049.
Hernandez, C. J., Beaupré, G. S., and Carter, D. R. (2003). A theoretical analysis of the relative influences of peak BMD, age-related bone loss and menopause on the development of osteoporosis. Osteoporos. Int. 14, 843–847.
Iwamoto, M., Jikko, A., Murakami, H., Shimazu, A., Nakashima, K., Iwamoto, M., Takigawa, M., Baba, H., Suzuki, F., and Kato, Y. (1994). Changes in parathyroid hormone receptors during chondrocyte cytodifferentiation. J. Biol. Chem. 269, 17245–17251.
Jaenisch, R., and Bird, A. (2003). Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat. Genet. 33(Suppl.), 245–254.
Javaid, M. K., Crozier, S. R., Harvey, N. C., Gale, C. R., Dennison, E. M., Boucher, B. J., Arden, N. K., Godfrey, K. M., and Cooper, C. (2006a). Maternal vitamin D status during pregnancy and childhood bone mass at age 9 years: a longitudinal study. Lancet 367, 36–43.
Javaid, M. K., Lekamwasam, S., Clark, J., Dennison, E. M., Syddall, H. E., Loveridge, N., Reeve, J., Beck, T. J., and Cooper, C. (2006b). Infant growth influences proximal femoral geometry in adulthood. J. Bone Miner. Res. 21, 508–512.
Javaid, M. K., Godfrey, K. M., Taylor, P., Robinson, S. M., Crozier, S. R., Dennison, E. M., Robinson, J. S., Breier, B. R., Arden, N. K., and Cooper, C. (2005). Umbilical cord leptin predicts neonatal bone mass. Calcif. Tissue Int. 76, 341–347.
Javaid, M. K., Godfrey, K. M., Taylor, P., Shore, S. R., Breier, B., Arden, N. K., and Cooper, C. (2004). Umbilical venous IGF-1 concentration, neonatal bone mass, and body composition. J. Bone Miner. Res. 19, 56–63.
Karaplis, A. C., Luz, A., Glowacki, J., Bronson, R. T., Tybulewicz, V. L., Kronenberg, H. M., and Mulligan, R. C. (1994). Lethal skeletal dysplasia from targeted disruption of the parathyroid hormone-related peptide gene. Genes Dev. 8, 277–289.
Kato, Y., Shimazu, A., Nakashima, K., Suzuki, F., Jikko, A., and Iwamoto, M. (1990). Effects of parathyroid hormone and calcitonin on alkaline phosphatase activity and matrix calcification in rabbit growth-plate chondrocyte cultures. Endocrinology 127, 114–118.
Kip, S. N., and Strehler, E. E. (2004). Vitamin D3 upregulates plasma membrane Ca2+-ATPase expression and potentiates apico-basal Ca2+ flux in MDCK cells. Am. J. Physiol. Renal Physiol. 286, F363–F369.
Kiyokawa, N., Mori, T., Taguchi, T., Saito, M., Mimori, K., Suzuki, T., Sekino, T., Sato, N., Nakajima, H., Katagiri, Y. U., Takeda, T., and Fujimoto, J. (2001). Reciprocal control of osteoblast/chondroblast and osteoblast/adipocyte differentiation of multipotential clonal human marrow stromal F/STRO-1+ cells. J. Cell. Biochem. 81, 23–38.
Kovacs, C. S. (2003). “Skeletal physiology: fetus and neonate,” in Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism, 5th Edn, ed. M. J. Favus (Washington: ASBMR), 65–71.
Kovacs, C. S., Chafe, L. L., Fudge, N. J., Friel, J. K., and Manley, N. R. (2001a). PTH regulates fetal blood calcium and skeletal mineralization independently of PTHrP. Endocrinology 142, 4983–4993.
Kovacs, C. S., Manley, N. R., Moseley, J. M., Martin, T. J., and Kronenberg, H. M. (2001b). Fetal parathyroids are not required to maintain placental calcium transport. J. Clin. Invest. 107, 1007–1015.
Kovacs, C. S., Lanske, B., Hunzelman, J. L., Guo, J., Karaplis, A. C., and Kronenberg, H. M. (1996). Parathyroid hormone-related peptide (PTHrP) regulates fetal-placental calcium transport through a receptor distinct from the PTH/PTHrP receptor. Proc. Natl. Acad. Sci. U.S.A. 93, 15233–15238.
Lanske, B., Karaplis, A. C., Lee, K., Luz, A., Vortkamp, A., Pirro, A., Karperien, M., Defize, L. H., Ho, C., Mulligan, R. C., Abou-Samra, A. B., Juppner, H., Segre, G. V., and Kronenberg, H. M. (1996). PTH/PTHrP receptor in early development and Indian hedgehog-regulated bone growth. Science 273, 663–666.
Laskey, M. A., and Prentice, A. (1999). Bone mineral changes during and after lactation. Obstet. Gynecol. 94, 608–615.
Lee, T. M. (1993). Development of meadow voles is influenced postnatally by maternal photoperiodic history. Am. J. Physiol. 265, R749–R755.
Lepercq, J., Challier, J. C., Guerre-Millo, M., Cauzac, M., Vidal, H., and Hauguel-De Mouzon, S. (2001). Prenatal leptin production: evidence that fetal adipose tissue produces leptin. J. Clin. Endocrinol. Metab. 86, 2409–2413.
Lillycrop, K. A., Slater-Jefferies, J. L., Hanson, M. A., Godfrey, K. M., Jackson, A. A., and Burdge, G. C. (2007). Induction of altered epigenetic regulation of the hepatic glucocorticoid receptor in the offspring of rats fed a protein-restricted diet during pregnancy suggests that reduced DNA methyltransferase-1 expression is involved in impaired DNA methylation and changes in histone modifications. Br. J. Nutr. 97, 1064–1073.
Marshall, D., Johnell, O., and Wedel, H. (1996). Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ 312, 1254–1259.
Martin, R., Harvey, N. C., Crozier, S. R., Javaid, M. K., Taylor, P., Dennison, E. M., Inskip, H. M., Godfrey, K. M., Cooper, C., and Lewis, R. M. (2005). Placental calcium transporter gene (PMCA3) expression predicts intrauterine bone mineral accrual. J. Bone Miner. Res. 20(Suppl. 1), S3.
Martin, R., Harvey, N. C., Crozier, S. R., Poole, J. R., Javaid, M. K., Dennison, E. M., Inskip, H. M., Hanson, M., Godfrey, K. M., Cooper, C., and Lewis, R. (2007). Placental calcium transporter (PMCA3) gene expression predicts intrauterine bone mineral accrual. Bone 40, 1203–1208.
Masuzaki, H., Ogawa, Y., Sagawa, N., Hosoda, K., Matsumoto, T., Mise, H., Nishimura, H., Yoshimasa, Y., Tanaka, I., Mori, T., and Nakao, K. (1997). Nonadipose tissue production of leptin: leptin as a novel placenta- derived hormone in humans. Nat. Med. 3, 1029–1033.
Melton, L. J., and Cooper, C. (2001). “Magnitude and impact of osteoporosis and fractures,” in Osteoporosis, 2nd Edn, eds R. Marcus, D. Feldman, and J. Kelsey (San Diego: Academic Press), 557–567.
Moore, K. L., and Persaud, T. V. N. (1998). The Developing Human, 6th Edn. Philadelphia: W. B. Saunders.
Morgan, H. D., Santos, F., Green, K., Dean, W., and Reik, W. (2005). Epigenetic reprogramming in mammals. Hum. Mol. Genet. 14(Suppl. 1), R47–R58.
Mughal, M. Z., Ross, R., and Tsang, R. C. (1989). Clearance of calcium across in situ perfused placentas of intrauterine growth-retarded rat fetuses. Pediatr. Res. 25, 420–422.
Olney, R. C., and Mougey, E. B. (1999). Expression of the components of the insulin-like growth factor axis across the growth-plate. Mol. Cell. Endocrinol. 156, 63–71.
O’Neill, T. W., Cooper, C., Finn, J. D., Lunt, M., Purdie, D., Reid, D. M., Rowe, R., Woolf, A. D., and Wallace, W. A. (2001). Incidence of distal forearm fracture in British men and women. Osteoporos. Int. 12, 555–558.
Paternoster, L., Lorentzon, M., Vandenput, L., Karlsson, M. K., Ljunggren, O., Kindmark, A., Mellstrom, D., Kemp, J. P., Jarett, C. E., Holly, J. M., Sayers, A., St, P. B., Timpson, N. J., Deloukas, P., Davey, S. G., Ring, S. M., Evans, D. M., Tobias, J. H., and Ohlsson, C. (2010). Genome-wide association meta-analysis of cortical bone mineral density unravels allelic heterogeneity at the RANKL locus and potential pleiotropic effects on bone. PLoS Genet. 6, e1001217. doi: 10.1371/journal.pgen.1001217
Phillips, D. I. W., Barker, D. J. P., Fall, C. H. D., Seckl, J. R., Whorwood, C. B., Wood, P. J., and Walker, B. R. (1998). Elevated plasma cortisol concentrations: a link between low birth weight and the insulin resistance syndrome? J. Clin. Endocrinol. Metab. 83, 757–760.
Ralston, S. H. (1998). Do genetic markers aid in risk assessment? Osteoporos. Int. 8(Suppl. 1), S37–S42.
Robinson, S. M., Crozier, S. R., Borland, S. E., Hammond, J., Barker, D. J. P., and Inskip, H. M. (2004). Impact of educational attainment on the quality of young women’s diets. Eur. J. Clin. Nutr. 58, 1174–1180.
Sayers, A., and Tobias, J. H. (2009). Estimated maternal ultraviolet B exposure levels in pregnancy influence skeletal development of the child. J. Clin. Endocrinol. Metab. 94, 765–771.
Stauffer, T. P., Hilfiker, H., Carafoli, E., and Strehler, E. E. (1993). Quantitative analysis of alternative splicing options of human plasma membrane calcium pump genes. J. Biol. Chem. 268, 25993–26003.
Thomas, T., Gori, F., Khosla, S., Jensen, M. D., Burguera, B., and Riggs, B. L. (1999). Leptin acts on human marrow stromal cells to enhance differentiation to osteoblasts and to inhibit differentiation to adipocytes. Endocrinology 140, 1630–1638.
Tobi, E. W., Lumey, L. H., Talens, R. P., Kremer, D., Putter, H., Stein, A. D., Slagboom, P. E., and Heijmans, B. T. (2009). DNA methylation differences after exposure to prenatal famine are common and timing- and sex-specific. Hum. Mol. Genet. 18, 4046–4053.
Vortkamp, A., Lee, K., Lanske, B., Segre, G. V., Kronenberg, H. M., and Tabin, C. J. (1996). Regulation of rate of cartilage differentiation by Indian hedgehog and PTH-related protein. Science 273, 613–622.
Weaver, I. C. G., Cervoni, N., Champagne, F. A., D’Alessio, A. C., Sharma, S., Seckl, J. R., Dymov, S., Szyf, M., and Meaney, M. J. (2004). Epigenetic programming by maternal behavior. Nat. Neurosci. 7, 847–854.
Weir, E. C., Philbrick, W. M., Amling, M., Neff, L. A., Baron, R., and Broadus, A. E. (1996). Targeted overexpression of parathyroid hormone-related peptide in chondrocytes causes chondrodysplasia and delayed endochondral bone formation. Proc. Natl. Acad. Sci. U.S.A. 93, 10240–10245.
Widdowson, E. M., Southgate, D. A. T., and Hey, E. (1988). “Fetal growth and body composition,” in Perinatal Nutrition, ed. B. S. Linblad (New York: Academic Press), 3–14.
Williams, E. L., Harvey, N. C., Dennison, E. M., Edwards, C. J., and Cooper, C. (2009). Maternal nutrition and bone health in the offspring. Int. J. Clin. Rheumatol. 4, 133–145.
Keywords: bone, placenta, osteoporosis, fracture, BMC, vitamin D, fetus, neonate
Citation: Goodfellow LR, Cooper C and Harvey NC (2011) Regulation of placental calcium transport and offspring bone health. Front. Endocrin. 2:3. doi: 10.3389/fendo.2011.00003
Received: 13 December 2010;
Paper pending published: 17 January 2011;
Accepted: 31 January 2011;
Published online: 14 February 2011.
Edited by:
Mark Stuart Cooper, University of Birmingham, UKReviewed by:
Kate Anna Ward, Medical Research Council Human Nutrition Research, UKMuhammad Kassim Javaid, University of Oxford, UK
Copyright: © 2011 Goodfellow, Cooper and Harvey. This is an open-access article subject to an exclusive license agreement between the authors and Frontiers Media SA, which permits unrestricted use, distribution, and reproduction in any medium, provided the original authors and source are credited.
*Correspondence: Nicholas C. Harvey, MRC Lifecourse Epidemiology Unit, Southampton General Hospital, University of Southampton, Southampton SO16 6YD, UK. e-mail: nch@mrc.soton.ac.uk
