- 1 Division of Neurobiology, Department of Neurology and Neuroscience, Weill Cornell Medical College, New York, NY, USA
- 2 Weill Cornell/Rockefeller/Sloan-Kettering Tri-Institutional MD-PhD Program, New York, NY, USA
- 3 Harold and Margaret Milliken Hatch Laboratory of Neuroendocrinology, The Rockefeller University, New York, NY, USA
- 4 College of Pharmacy, Nova Southeastern University, Ponce, Puerto Rico
Opioids play a critical role in hippocampally dependent behavior and plasticity. In the hippocampal formation, mu opioid receptors (MOR) are prominent in parvalbumin (PARV) containing interneurons. Previously we found that gonadal hormones modulate the trafficking of MORs in PARV interneurons. Although sex differences in response to stress are well documented, the point at which opioids, sex, and stress interact to influence hippocampal function remains elusive. Thus, we used quantitative immunocytochemistry in combination with light and electron microscopy for the phosphorylated MOR (pMOR) at the SER375 carboxy-terminal residue in male and female rats to assess these interactions. In both sexes, pMOR-immunoreactivity (ir) was prominent in axons and terminals and in a few neuronal somata and dendrites, some of which contained PARV in the mossy fiber pathway region of the dentate gyrus (DG) hilus and CA3 stratum lucidum. In unstressed rats, the levels of pMOR-ir in the DG or CA3 were not affected by sex or estrous cycle stage. However, immediately following 30 min of acute immobilization stress (AIS), males had higher levels of pMOR-ir whereas females at proestrus and estrus (high estrogen stages) had lower levels of pMOR-ir within the DG. In contrast, the number and types of neuronal profiles with pMOR-ir were not altered by AIS in either males or proestrus females. These data demonstrate that although gonadal steroids do not affect pMOR levels at resting conditions, they are differentially activated both pre and postsynaptic MORs following stress. These interactions may contribute to the reported sex differences in hippocampally dependent behaviors in stressed animals.
Introduction
Estrogens influence fundamental processes within the hippocampus. When estrogen levels are high (either at the proestrus stage of the estrous cycle or in ovariectomized animals replaced with estradiol), spine density, synaptic proteins, and glutamatergic receptors are elevated in the hippocampus (Gazzaley et al., 1996; Woolley, 1998; McEwen and Milner, 2007; Spencer et al., 2008; Waters et al., 2009). Estrogen fluctuations affect long term potentiation (LTP) and long term depression, two forms of synaptic plasticity considered to be cellular models of memory trace formation (reviewed by Foy, 2011). Moreover, estrogens likely contribute to sex differences in associative learning behaviors important in addiction, particularly in relation to relapse (Roberts et al., 1989; Lynch et al., 2002; Roth et al., 2002; Hu et al., 2004).
One potential way that estrogens influence hippocampal learning relevant to addictive processes is through the modulation of the stress response. Drug addiction, particularly relapse, is often provoked by stress (reviewed by Bruchas et al., 2010; Shalev et al., 2010). Sex differences in response to both chronic and acute stress have been reported (Bowman et al., 2003; Conrad et al., 2003; Luine et al., 2007). In males, chronic stress is detrimental to learning processes, decreases in LTP, and results in atrophy of CA3 pyramidal cell dendrites as well as increased packing density of small synaptic vesicles near active zones of mossy fiber terminals (Magariños et al., 1997, and reviewed by McEwen and Milner, 2007). However, in females, chronic stress either does not effect or slightly increases spatial learning performance, particularly at the proestrus phase of the estrus cycle, and does not result in the dramatic morphological changes seen in the male hippocampus (Shors and Thompson, 1992; Galea et al., 1997; Luine et al., 2007; McEwen and Milner, 2007). The relative preservation of hippocampal morphology and learning processes in females following stress could contribute to accelerated course of addiction seen in females (Robbins et al., 1999; Elman et al., 2001).
Several lines of evidence suggest that the endogenous hippocampal opioid system is involved in these sexually dimorphic responses to stress. The mossy fiber-CA3 pathway, which is most vulnerable to stress in males, is rich in opioid peptides and receptors as well as gonadal steroid receptors (Drake et al., 2007; Hajszan et al., 2007). Moreover, LTP in the mossy fiber – CA3 pathway is opioid dependent, requiring activation of the mu opioid receptors (MORs; Derrick et al., 1992; Derrick and Martinez, 1994). In other brain regions, chronic stress alters enkephalin levels as well as MOR binding (Kalivas and Abhold, 1987; Stein et al., 1992; Drolet et al., 2001; Lucas et al., 2004; Dantas et al., 2005). Ovarian hormones, particularly estrogens, also can alter the levels of opioid peptides in the mossy fiber pathway (Torres-Reveron et al., 2008, 2009b) and MOR binding throughout the hippocampus (Piva et al., 1995; Šlamberová et al., 2003). Furthermore, ovarian hormones can alter the trafficking as well as the availability at the cell membrane of MORs and delta opioid receptors in hippocampal interneurons and principal cells, respectively (Sinchak and Micevych, 2001; Torres-Reveron et al., 2009b; Williams et al., 2011). However, whether ovarian hormones can affect the responses of the hippocampal opioid system, particularly the MORs, to stress has not been explored.
Therefore, the present study aimed to determine if MORs are activated in the hippocampus following stress exposure and, if so, is the activation of MORs affected by sex and/or gonadal steroid levels. For this, an antibody to MOR phosphorylated at ser375 (pMOR) was localized by light and electron microscopy in male and cycling female rats following acute immobilization stress (AIS).
Materials and Methods
Animals
All procedures were approved by the Weill Cornell Medical College and Rockefeller University Institutional Animal Care and Use Committees and were in accordance with the National Institutes of Health guidelines. Adult male and female Sprague Dawley rats (approximately 60 days old; N = 76) were obtained from Charles River Laboratories (Wilmington, MA, USA). Rats were group housed (three to four per cage) and given access to food and water ad libitum with 12:12 light/dark cycles. Two cohorts of animals were used in these studies. The first cohort, that was used to assess the levels of pMOR-ir in males compared to females at each estrous stage, was the same cohort of animals used in our previous studies (Torres-Reveron et al., 2008, 2009b; D:Williamsetal:2011]). The second cohort was used to assess the effects of AIS in males and in females from different estrous stages. These two cohorts of animals were housed in different animal facilities (cohort 1: WCMC; cohort 2: RU) and their brains were collected approximately 3 years apart.
Estrous Cycle Determination
Female rats were allowed to acclimate for 1 week after arrival and then estrous stage was determined daily between 9:00 and 10:00 am using vaginal smear cytology (Turner and Bagnara, 1971). Only female rats that showed two consecutive, regular 4–5 day estrous cycles were used in the study. Diestrus-II rats rather than metestrus (diestrus I) were chosen to insure that rats were completely out of the estrus phase. The stage of the estrous cycle was confirmed by measuring uterine weight and plasma serum estradiol levels by radioimmunoassay as previously described (Torres-Reveron et al., 2009b). To control for handling effects, male rats were removed from their cages daily to perform mock estrous cycling.
Acute Immobilization Stress
Acute immobilization stress was conducted between 9:00 am and 1:00 pm and was performed as previously described (Lucas et al., 2007; Shansky et al., 2010). Rats were transported from their home room to a procedure room. Rats were placed in plastic cone shaped polyethylene bags with a Kotex mini-pad underneath them to collect urine and a small hole at the apex of the cone. Each animal was sealed with tape in the bag with the nose opposed to the hole allowing for unrestricted breathing. The immobilized rats were placed on a counter top undisturbed for 30 min. Immediately following the AIS, rats were anesthetized in the procedure room and their brains fixed by perfusion as described below. Control rats remained in their cages in the home room and were anesthetized prior to transfer to the procedure room for perfusion.
Immunocytochemistry
Antibodies
A rabbit polyclonal antibody generated against a synthetic phosphopeptide corresponding to residues surrounding Ser377 of human (homologous to Ser375 of mouse) of the pMOR (#3451 Cell Signaling, Danvers, MA, USA) was used in this study. This antibody has been used to measure pMOR-ir using immunofluorescence (Chu et al., 2008), western blots, and immunoprecipitation in both HEK293 cells and neuronal cells (Schulz et al., 2004). In the presence of opioid receptor agonists (DAMGO) or antagonist (naloxone), immunoreactivity for pMOR is increased and decreased, respectively, in HEK293 cells (Schulz et al., 2004). Moreover, mutating the SER 375 residue of the MOR in HEK293 cells abolished morphine induced phosphorylation as assessed by immunoprecipitation (Chu et al., 2008), further demonstrating specificity of this antibody. To additionally test the specificity of the pMOR antibody, a preadsorption control was also performed. For this, 2 ml of the working dilution of the antiserum (1:800) was divided into two scintillation vials. Fifty micrograms of the peptide to which the antibody was generated (Cell Signaling #5976B) in 50 μl saline was added to one of the vials and 50 μl saline was added to the other vial and the vials were placed on a shaker overnight at 4°C. The following day, the solutions were spun at 10,000 RPM for 10 min on a microfuge and the supernatant collected and used for immunocytochemistry as described below.
The mouse monoclonal antibody directed against the calcium binding protein parvalbumin (PARV; Sigma-Aldrich) was utilized in our previous studies (Torres-Reveron et al., 2009b). This antibody has been previously characterized by immunoblots, radioimmunoassay, and has ability to recognize PARV in brain tissue (Celio et al., 1988).
Section preparation
Rats were deeply anesthetized with sodium pentobarbital (150 mg/kg) and perfused sequentially through the ascending aorta with: (1) 10–15 ml of 0.9% saline containing 2% heparin; (2) 50 ml 3.75% acrolein and 2% paraformaldehyde in 0.1 M phosphate buffer (PB; pH 7.4); and (3) 200 ml 2% paraformaldehyde in PB. Brains then were cut into 5 mm thick coronal blocks and post-fixed for 30 min in the latter fixative before being switched into PB. Coronal sections (40 μm thick) through the hippocampus were cut on a vibratome into PB, placed in cryoprotectant solution (30% sucrose and 30% ethylene glycol in PB) and stored at −20°C until processing for immunocytochemistry. Prior to immunocytochemistry, sections were rinsed in PB, coded with hole-punches and pooled into single containers to insure identical exposure to immunoreagents (Pierce et al., 1999). Sections then were incubated in 1% sodium borohydride in PB for 30 min, and rinsed in PB.
Light microscopic immunocytochemistry and analysis
To examine changes in pMOR-ir levels between different experimental groups, sections were processed as previously described (Torres-Reveron et al., 2008). Briefly, sections were transferred to 0.1 M Tris-buffered saline (TS; pH 7.6) and then blocked in 0.5% BSA in TS for 30 min. Sections were placed in rabbit polyclonal pMOR antibody (1:800) for 24 h at room temperature, and then 24 h at 4°C. Sections then were processed in a 1:400 dilution of biotinylated goat anti-rabbit immunoglobulin (IgG; Vector Laboratories, Burlingame, CA, USA) for 30 min followed by a 1:100 dilution of avidin–biotin complex (ABC; Vectastain elite kit, Vector Laboratories) for 30 min. All incubations were separated by washes of TS. Sections were reacted in 3,3′-diaminobenzidine (DAB; Sigma-Aldrich, St. Louis, MO, USA) and 3% H202 in TS for 4–6 min and rinsed in TS followed by PB. Sections were mounted on gelatin-coated slides, dehydrated and coverslipped from xylene with DPX mounting media (Sigma-Aldrich).
Sections from the dorsal hippocampus (−3.6 to −4.0 mm from Bregma; Swanson, 2000) were chosen for analysis. All histological evaluation was done by an experimenter who was blind to treatment group. For quantitative densitometry, photomicrographs through regions of interest (ROI) were captured using a Dage MTI CCD-72 camera on a Nikon Eclipse 80i microscope and converted to gray-scale values MicroComputer Imaging Device (MCID) software. The mean gray value (of 256 gray levels) for each selected ROI was determined as previously described (Torres-Reveron et al., 2008). To control for variations in illumination and background staining, the average pixel density from three regions lacking labeling was subtracted. Optical density values were measured using MCID and net optical density values obtained after subtracting background values were converted to a percentage scale of 256 preset gray values ranging from 0 to 100%. To determine the levels of pMOR-ir in individual cells, optical density measurements from the cytoplasm of two to three cells were section were averaged. For statistical analyses, percentages were transformed by calculating the inverse sine of the proportion (EisenHart and Hastay, 1947). All data was analyzed using a one-way ANOVA followed by preplanned Student-Newman–Keuls post hoc analysis (p < 0.05).
Immunofluorescence
For immunofluorescence, the same procedures above were followed with the following changes: (1) rats were perfusion fixed with 4% paraformaldehyde in PB; (2) the sodium borohydride step was eliminated; (3) sections were incubated in pMOR antiserum (diluted 1:500) for 24 h at RT and 48 h at 4°C; (4) sections were incubated in biotinylated goat anti-rabbit IgG and ABC for 60 min each, followed by an incubation in streptavidin conjugated Alexa Fluor 488 goat anti-rabbit IgG (Invitrogen) diluted 1:200 for 90 min. Sections then were incubated in a mouse monoclonal antibody directed against PARV (1:6000) for 6 h at room temperature and 48 h at 4°C followed by a goat anti-mouse Alexa Fluor 568 IgG for 90 min. Sections were rinsed in PB, air-dried, and coverslipped with VectorShield (Vector laboratories). Images were collected with a Leica TCS SP5 confocal microscope using an argon laser (488) and a HeNe laser (543). Sequential scans were acquired to avoid any overlap between the wavelengths for the two dyes. Z stack images (approx. 20 μm at 1 μm each) were taken and combined into a single image using the Leica Application Suite Software.
Electron Microscopic Immunocytochemistry and Analysis
Single label immunocytochemistry
Sections were processed for immunoperoxidase as described above for light microscopy. Sections were post-fixed in 2% osmium tetroxide for 1 h, rinsed in PB, dehydrated in ascending concentrations of ethanol and propylene oxide and then embedded in EMbed 812 (Electron Microscopy Sciences, Fort Washington, PA, USA). Ultrathin sections (70–72 nm thick) through the ROI were cut on a Leica UCT ultratome, collected on copper grids and counterstained with uranyl acetate and Reynolds lead citrate. Sections were examined with a Philips CM10 transmission electron microscope equipped with an Advanced Microscopy Techniques digital camera (Danvers, PA, USA). For figures, images were prepared in Adobe Photoshop 9.0 to sharpness and imported into PowerPoint 2008 on a MacBook Pro where final adjustments to brightness and contrast were made. These changes did not alter the original content of the raw image.
Dual label immunocytochemistry
A subset of sections was processed for dual label immunoelectron microscopy. For this, sections were incubated in a cocktail of rabbit anti-pMOR (diluted 1:800) and mouse anti-PARV (diluted 1:3000). PARV was visualized using the ABC procedure as described for light microscopy except that sections were incubated in biotinylated horse anti-mouse IgG. Immunoreactivity for pMOR was visualized using the silver enhanced immunogold technique (Chan et al., 1990). Briefly, sections were rinsed in TS and incubated in a 1:50 dilution of goat anti-rabbit IgG conjugated to 1 nm gold particles (1:50, EMS) in 0.08% BSA and 0.001% gelatin in 0.01 M phosphate-buffered saline (PBS; pH 7.4) for 2 h at RT. Sections then were rinsed in PBS, post-fixed in 2% glutaraldehyde for 10 min and rinsed in PBS followed by 0.2 M sodium citrate buffer (pH 7.4). The gold particles were enhanced by incubation of a silver solution (RPN491; GE Healthcare) for 5–7 min. The sections were then prepared for electron microscopy as described above.
Analysis
For quantitative single label electron microscopic studies, ultrathin sections from three to four rats in each experimental condition were analyzed. All of the sections from each experimental group were co-processed and used identical sampling methods, to minimize variables that could affect between-group comparisons. To minimize differences due to antibody penetration, analysis was conducted at the tissue–plastic interface (Pierce et al., 1999). In each ROI, all profiles from eight random, but non-overlapping, micrographs (65 μm2/micrograph) were counted. All analysis was done by a experimenter who was blind to experimental group. Immunolabeled profiles were classified using criteria defined by Peters et al. (1991. Dendritic profiles contained regular microtubule arrays and were usually postsynaptic to axon terminal profiles. Unmyelinated axons were profiles smaller than 0.2 μm in diameter, contained a few small synaptic vesicles and lacked a synaptic junction in the plane of section. Axon terminal profiles had numerous small synaptic vesicles and had a cross-sectional diameter greater than 0.2 μm. Astrocytic profiles were distinguished by their tendency to conform to the boundaries of surrounding profiles, by the absence of microtubules and/or by the presence of glial filaments. All statistical analyses were performed using a two-way ANOVA (p < 0.05), followed by preplanned, pairwise comparisons using Student-Newman–Keuls post hoc analysis (p < 0.05).
Results
pMOR-ir is Concentrated in the Mossy Fiber Pathway Region
At the light microscopy level, pMOR-ir was limited to select subregions (Figure 1). Diffuse pMOR-ir was found in the stratum lucidum of the CA3, particularly in CA3b and c, and the central hilus of the dentate gyrus (DG; Figure 1A). Moreover, cells containing pMOR-ir were found primarily in the dorsal portion of the dentate hilus (Figure 1B) and sparsely scattered near the CA3 pyramidal cell layer. The pattern of pMOR labeling appeared identical in male and female rats. Moreover, neither the density of pMOR-ir in six subregions from the mossy fiber pathway nor the density of pMOR-ir in individual cell somata was significantly different from males and females, regardless of estrous stage [p > 0.05; cohort 1 (see Materials and Methods)]. All pMOR-ir was abolished when the antibody was incubated with the peptide against which the antiserum was raised (Figure 1C).
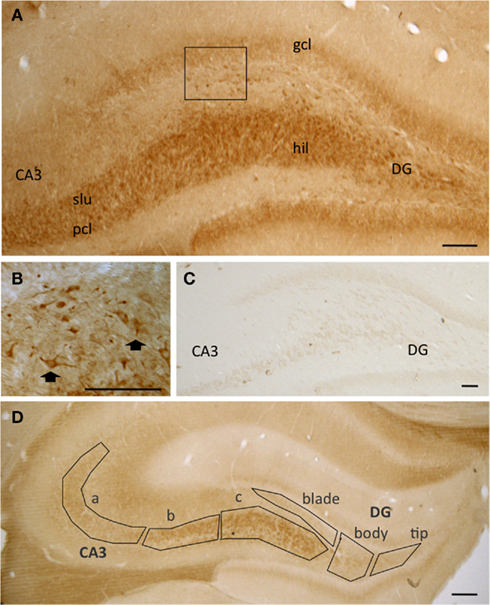
Figure 1. (A) At the light level, pMOR-immunoreactivity (pMOR-ir) is most dense in stratum lucidum (slu) of CA3 and in the hilus (hil) of the dentate gyrus (DG). (B) Higher magnification of the inset in A shows that several cells contain pMOR-ir in the dorsal hilus (arrows). (C) No labeling was seen in these regions following preadsorption of the pMOR antibody with antigenic peptide. (D) The mossy fiber pathway zone was divided into six regions for analysis: CA3a, CA3b, and CA3c and the denate gyrus tip, blade, and body. gcl, granule cell layer; pcl pyramidal cell layer. Scale bars = 100 μm.
Confocal microscopy revealed that some cells with pMOR-ir co-expressed PARV, particularly in the dorsal blade of the hilus of the DG (Figures 2A–C). Rarely, cells near the CA3 pyramidal cell layer contained pMOR- and PARV-ir (Figures 2D–F); however, in these instances the pMOR labeling was less robust. These observations indicate that pMOR may be expressed within a subset of interneurons, consistent with previous reports demonstrating that MOR-ir is frequently co-expressed within PARV-containing hippocampal interneurons (Drake and Milner, 2006).
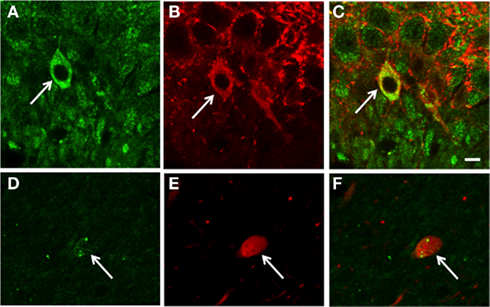
Figure 2. Confocal microscopy shows that pMOR-ir is contained in some PARV-labeled interneurons. In dorsal blade of the dentate gyrus, pMOR-ir (A) and PARV-ir (B) are co-expressed a neuron be [merged image (C)]. In the CA3, sparse pMOR-ir (D) and PARV-ir (E) are co-expressed in a neuron [merged image (F)]. gcl, granule cell layer. Scale bar = 10 μm.
pMOR-ir is Primarily in Axons and in Some Dendrites
Since CA3b contained dense pMOR-ir, this region initially was chosen for electron microscopic examination in normal non-stressed rats (Figure 1D). Ultrastructurally, pMOR-ir was found exclusively in neuronal profiles. As there was no effect of sex or estrous cycle phase on the types or proportion of profiles (p > 0.05), data were collapsed from all animals (N = 16). The majority (74.6 ± 1.4% out of 1862 total profiles) of pMOR-ir was found in small unmyelinated axons (Figures 3A,C). A minority of axons with pMOR-ir was myelinated (2.3 ± 1.3%). Within stratum lucidum, pMOR-labeled axons were often found in bundles, indicative of mossy fiber axons (Figure 3A). pMOR-ir in axons was usually patchy, often affiliated with small vesicles (Figure 3C). In fortuitous planes of section, pMOR-labeled axons gave rise to terminals that formed asymmetric synapses with dendritic spines (Figure 3C).
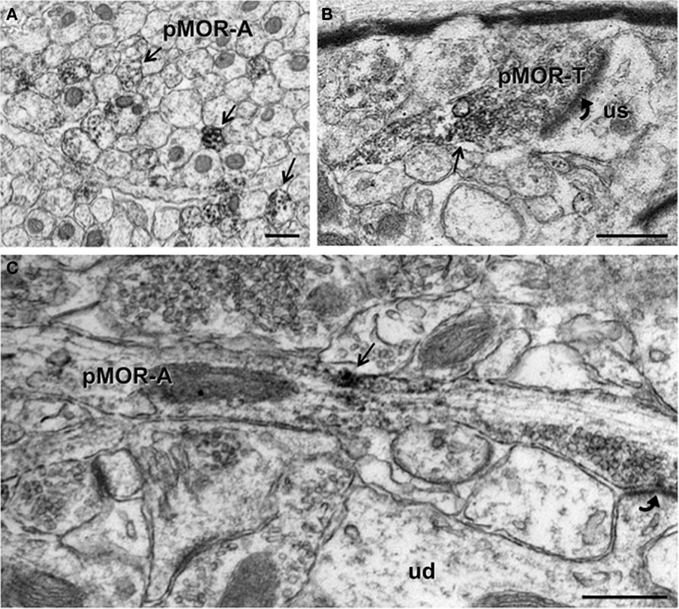
Figure 3. By electron microscopy, pMOR-ir is found in axons and axon terminals. (A) pMOR-ir is found in numerous small, unmyelinated axons (pMOR-A; arrows) within the stratum lucidum of the CA3. (B) A cluster of pMOR-ir is found in a terminal (pMOR-T) containing numerous small synaptic vesicles and forming an asymmetric synapse (curved arrow) with an unlabeled dendritic spine (us). (C) pMOR-ir is affiliated with small synaptic vesicles in an enpassant axon that forms an asymmetric synapse (curved arrow) with a dendritic spine emanating from an unlabeled dendritic shaft (ud). Scale bar = 500 nm.
A few (3.3 ± 0.5%) axon terminals contained pMOR-ir. pMOR-labeled terminals were usually between 0.3 and 0.6 μm in diameter, contained numerous small synaptic vesicles (Figure 3B). Like axons, pMOR-ir in terminals was usually patchy and affiliated with vesicles. Few pMOR-labeled terminals formed synapses; most of these were asymmetric on unlabeled dendritic spines.
About one-sixth (16.0 ± 1.7%) of the pMOR-labeled profiles were dendritic shafts. pMOR-ir was usually distributed throughout the dendrites, with no particular affiliation with select organelles (Figure 4A). Dendritic shafts with pMOR-ir ranged in size from 0.4 to 1 μm in diameter, lacked spines and were contacted by several axon terminals. In some cases, terminals contacting pMOR-labeled dendritic shafts had the morphology of mossy fiber terminals (Figure 4A). Rarely, pMOR-ir was found in dendritic spines (3.8 ± 0.4%). Consistent with the confocal data, dual label electron microscopy revealed that pMOR-ir is co-expressed within a few dendritic shafts containing PARV-ir (Figure 4B). Dual labeled dendrites lacked dendritic spines and received numerous contacts (usually asymmetric) from unlabeled terminals.
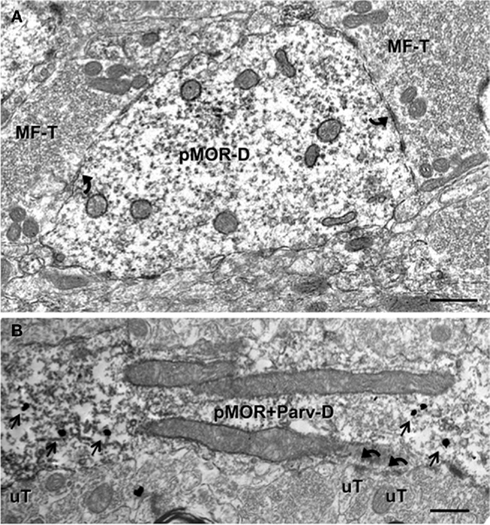
Figure 4. pMOR-ir is found in dendrites, some of which colocalize PARV. (A) A large dendritic shaft containing diffuse pMOR-ir (pMOR-D) is contacted (arrows) by mossy fiber terminals (MF-T). (B) pMOR-ir identified by silver intensified gold particles (arrows) is in a PARV-labeled dendritic shaft identified by diffuse peroxidase reaction product. Unlabeled terminals (uT) form asymmetric synapses (arrows) on the dual labeled dendrite (pMOR–Parv-D). Scale bars for all images = 500 nm.
Alterations in pMOR-ir Following AIS Vary Depending on Region and Hormonal Status
The levels of pMOR-ir in six subregions of the mossy fiber pathway (Figure 1D) were quantified using densitometry in males and females from each estrous stage following AIS. Although no effect of hormonal cycle or sex was observed in control rats [p > 0.05; cohort 2 (see Materials and Methods)], AIS produced robust sex differences in the density of pMOR-ir in the CA3 and DG that is anatomically and gonadal steroid status specific. However, like unstressed rats, no sex or cycle differences in the density of pMOR-ir were found in individual cell somata sampled from the dentate hilus in AIS compared to control rats (p > 0.05).
CA3
Overall, pMOR-ir was not altered by estrous stage or sex in non-stressed rats within stratum lucidum of CA3. However, when rats were exposed to AIS, significant sex differences in the levels of pMOR-ir in CA3 emerged. In general, pMOR-ir in males increased following AIS compared to unstressed males and to both unstressed and stressed proestrus females.
The CA3 was analyzed first as a single region and then subdivided into three separate areas (CA3a, CA3b, and CA3c) as seen in Figure 5. When analyzed as a single brain area (Figure 5A), two-way ANOVA revealed a significant main effect of sex/cycle (F3,46 = 3.198) and a significant interaction of sex/cycle by stress (F3,46 = 3.373, p < 0.05). Post hoc analysis revealed that AIS induced pMOR-ir in males (p < 0.05) but not in females. Furthermore, AIS males had greater pMOR-ir than did females at proestrus (p < 0.01) and estrus (p < 0.05). Within the CA3a (Figure 5B), two-way ANOVA showed no effect of sex/cycle (F3,46 = 2.464) or stress (F1,46 = 2.129), and no significant interaction (F3,46 = 2.129, p > 0.05 for all) on the density of pMOR-ir. However, in the CA3b (Figure 5C), a two-way ANOVA revealed a strong trend of sex/cycle (F3,46 = 2.792, p = 0.053) and a significant sex/cycle by stress interaction (F3,46 = 2.939, p < 0.05). Post hoc analysis showed that AIS increased pMOR-ir in males (p < 0.05) but not in females at any point in the cycle. Sex differences in the levels pMOR-ir were only found between males and proestrus females (p < 0.01) after AIS. Furthermore, a small, but significant effect of estrus cycle emerged such that diestrus females had more pMOR-ir than did proestrus females (p = 0.050). Consistent with these observations, within the CA3c (Figure 6D) a two-way ANOVA revealed an effect of sex/cycle (F3,46 = 2.919, p < 0.05), as well as a significant interaction between sex/cycle and stress (F3,46 = 3.312, p < 0.05). Post hoc analysis showed that AIS increased pMOR-ir in males (p < 0.05), but not in females at any stage. Within stressed animals, males expressed significantly more pMOR-ir than did females at proestrus (p < 0.005) and estrus (p < 0.05).
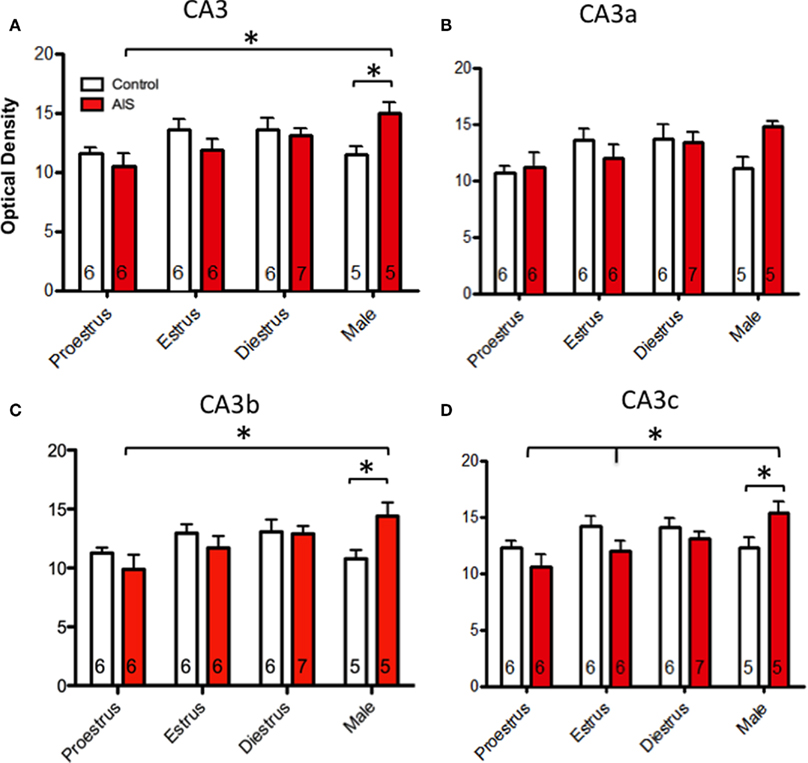
Figure 5. Effects of sex and estrous stage on the levels of pMOR-ir within CA3 following AIS. Neither sex nor estrous cycle stage significantly affected the density of pMOR-ir in non-stressed rats when the CA3 was examined as a whole (A) or divided into subregions (B–D). After exposure to AIS, however, significant differences emerged. When the CA3 was analyzed as a whole, AIS males had significantly more pMOR-ir than control males and AIS proestrus females (A). When analyzed as separate regions, AIS significantly increased pMOR-ir in males within the CA3b (C) and CA3c (D) [with a trend (p = 0.077) in the CA3a (B)] compared to AIS proestrus females. In CA3c (D), AIS males also had significantly more pMOR-ir than diestrus females. *p < 0.05, **p < 0.01.
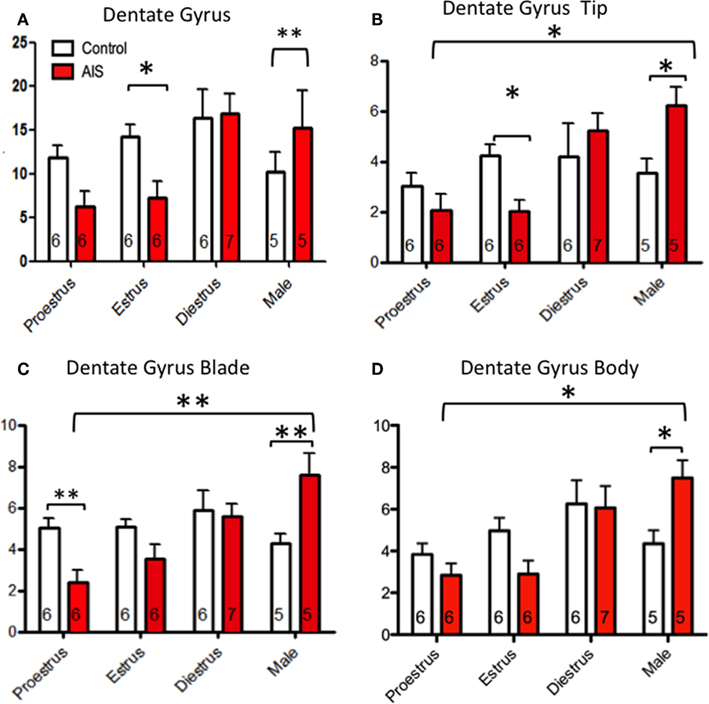
Figure 6. Effects of sex and estrous stage on the levels of pMOR-ir within dentate gyrus following AIS. Neither sex nor estrous cycle stage significantly affected the density of pMOR-ir in non-s]ssed animals when the dentate gyrus was examined as a whole (A) or divided into subregions (B–D). However, sex and cycle differences in pMOR-ir emerged after AIS. When analyzed as a whole, AIS males had significantly more pMOR-ir compared to controls whereas AIS estrus females had less pMOR-ir compared to their controls (A). When analyzed as separate subregions however, additional differences were emerged. AIS significantly increased pMOR-ir in males compared to proestrus females in the tip (B), blade (C), and body (D) of the DG. AIS significantly decreased pMOR-ir in proestrus females within the DG blade (B). *p < 0.05, **p < 0.01.
Dentate gyrus
Similar to the CA3, pMOR-ir was highly influenced by cycle stage and sex in the DG. Interestingly, these differences were more apparent in the DG compared to the CA3. Overall, AIS induced pMOR-ir in males and decreased pMOR-ir in females.
When analyzed as a single brain area (Figure 6A), a two-way ANOVA revealed a main effect of sex/cycle (F3,46 = 6.337, p > 0.005) and an interaction of sex/cycle and stress (F3,46 = 4.967, p > 0.01). Post hoc analysis shows that stressed females, regardless of cycle stage, have less pMOR-ir than do non-stressed controls. However, pMOR-ir was increased in males after AIS when compared to controls (p < 0.01). Within the DG tip (Figure 6B), two-way ANOVA revealed a significant main effect of sex (F3,46 = 5.115, p < 0.005), and an interaction between sex/cycle and stress (F3,46 = 4.225, p > 0.05). Post hoc analysis showed a reduction of pMOR-ir within estrus females following AIS (p < 0.05). Conversely, AIS increased pMOR-ir in males when compared to controls (p < 0.05). Furthermore, significant sex differences were observed in rats exposed to AIS such that males expressed more pMOR-ir than did estrus (p < 0.005) and proestrus (p < 0.0005) females. In addition, an effect of cycle was observed such that females during diestrus had more pMOR-ir than did females at estrus (p < 0.01) and proestrus (p > 0.005). Consistent with these observations, a two-way ANOVA showed a main effect of sex/cycle (F3,46 = 5.087, p < 0.01) and an interaction between sex/cycle and stress (F3,46 = 6.474, p < 0.005) within the DG blade (Figure 6C). Post hoc analysis revealed that AIS reduced pMOR-ir in proestrus females and increased pMOR-ir in males when compared to controls. Overall, males had significantly more pMOR-ir than did females at proestrus, estrus, and diestrus after AIS (p < 0.05 for all). Furthermore, diestrus females had more pMOR-ir than proestrus and estrus females following AIS (p > 0.05 for all). Within the DG body (Figure 6D), a two-way ANOVA revealed a main effect of sex/cycle (F3,46 = 6.315, p < 0.005) and an interaction between sex/cycle and stress (F3,46 = 3.566, p < 0.05). Post hoc analysis showed an induction of pMOR-ir in males after stress (p < 0.015). After AIS exposure males had more pMOR-ir than proestrus and estrus females (p < 0.005); diestrus females also had more pMOR-ir than did proestrus and estrus females (p > 0.05 for both).
Since the greatest differences in pMOR-ir following AIS were found between males and proestrus females in the DG, further analysis was performed at the ultrastructural level. For this, the types and proportions pMOR-labeled profiles were counted in the central hilus of control and AIS proestrus female and male rats (N = 4 per group). A two-way ANOVA revealed no significant difference between groups (p > 0.05) when either total number of profiles, or types profiles (e.g., axons, dendrites, terminals) were compared.
Discussion
This is the first report to demonstrate that pMOR-ir is restricted to the mossy fiber pathway region where it is found primarily in axons and dendrites, some of which arise from PARV-containing interneurons. The levels of pMOR-ir are not altered by sex or estrous cycle stage in unstressed rats. However, following AIS, pMOR-ir significantly decreased in proestrus and estrous females and increased in males. These interactions may contribute to the reported sex differences in hippocampally dependent behaviors in stressed animals.
Methodological Considerations
We used a well-characterized antiserum to pMOR(Ser375), the expression of which is regulated by opioid agonists and antagonists in HEK cells, neuronal cultures from hippocampus, and spinal cord (Narita et al., 2004; Schulz et al., 2004; Chu et al., 2008). Moreover, in AT20 and HEK cell cultures the distribution of the pMOR(Ser 375) was similar to that of MOR-P1(Thr180) and MOR-P2(Thr370 and Ser375), two non-commercial antibodies to the activated form of MOR (Petraschka et al., 2007). This is the first report to use the pMOR(Ser375) antiserum in acrolein/paraformaldehyde fixed neuronal tissue. The demonstration that preadsorption with the peptide that the antiserum was raised against completely abolished any immunoreactivity within the entire hippocampus further supports the specificity of this antiserum. However, the possibility exists that the antiserum recognizes similar peptide sequences within the brain.
Phosphorylation of G-protein coupled receptors such as MORs is one of the first steps following ligand activation controlling receptor signaling and is critical to their desensitization and internalization (Koch and Höllt, 2008). Several lines of evidence demonstrate that the actions giving rise to desensitization and internalization can be separated. In particular, mutation of MOR at Thr180 eliminates ligand-induced desensitization but does not affect internalization (Celver et al., 2004). Conversely, ligand-stimulated activation of the MOR at Thr370 and Ser375 is important for internalization (El Kouhen et al., 2001) and morphine but not DAMGO-induced desensitization (Schulz et al., 2004). Thus, it is possible that the pMOR(Ser 375) antibody used in the present study discriminates between internalization and desensitization processes.
The Distribution of pMOR is Consistent with that of MOR in the Hippocampus
The current study demonstrates that pMOR-ir is found primarily in the region of the DG and CA3 known to contain the mossy fibers (Drake et al., 2007). Our electron microscopic studies revealed that most pMOR-ir is in small unmyelinated axons and in dendrites, some of which contain PARV. These observations are consistent with previous reports describing the localization of MORs within the hippocampus. The abundance of pMOR-ir within small axons in the mossy fiber pathway suggests that the antibody primarily recognizes activated forms of the MOR-1D splice variant (Abbadie et al., 2000). The localization of pMOR-ir in axons suggests that MOR activation could impact synaptic transmission either by influencing the transduction of electrical signals to the terminal and/or protein transport (Cunnane and Stjärne, 1984; Cheung, 1990). Interestingly, dynorphin, which is also localized in mossy fibers (Drake et al., 2007), has a high affinity for the MOR-1D (Petraschka et al., 2007) and is released by high frequency stimulation following seizures or chronic stress (Wagner et al., 1991; Mazarati et al., 1999; Redila and Chavkin, 2008). Thus, release of dynorphins following high frequency stimulation could provide an auto-regulation of granule cell mossy fibers through a presynaptic mechanism. Moreover, localization of pMOR-ir in a few small terminals forming asymmetric synapses with dendritic spines suggests that MOR activation could particularly affect excitatory transmission (Peters et al., 1991).
The presence of pMOR-ir in some PARV-containing interneurons is consistent with previous reports localizing MOR-ir and mRNA in the hippocampus (Drake and Milner, 1999, 2006); Stumm et al., 2004; Torres-Reveron et al., 2009b). PARV-containing interneurons comprise one group of basket cells that form inhibitory-type synapses with pyramidal cell somata and proximal dendrites (for reviews see Freund and Buzsáki, 1996; and Drake et al., 2007) as well as cholescystokinin-containing basket interneurons (Karson et al., 2009). Activation of MORs profoundly disinhibits pyramidal cells (Jin and Chavkin, 1999). Moreover, LTP in the mossy fiber – CA3 pathway is opioid dependent, requiring activation of the MORs (Derrick et al., 1992; Derrick and Martinez, 1994). Thus, in addition to presynaptic mechanisms, activation of MORs could affect pyramidal cells and other interneurons through postsynaptic mechanisms.
The present localization of pMOR-ir in cells, however, was more limited than that described previously for MORs in hippocampus (Stumm et al., 2004; Drake et al., 2007). A previous study (Petraschka et al., 2007) localizing pMOR using MOR-P1 and MOR-P2 in other brain regions also noticed a discrepancy between MOR distribution by non-phosphorylated antibodies. Petraschka et al. (2007) suggested that the pMOR antibodies might not detect newly synthesized or recently internalized MORs which are distant to a regulatory kinase. Regardless, the localization of pMOR-ir in GABAergic interneurons is consistent with previous reports (Drake et al., 2007).
Following AIS, the Level of PMOR-ir are Influenced by Sex and Estrous Cycle Phase
In unstressed animals, neither estrus cycle stage nor sex significantly affected levels of pMOR-ir in any of the subregions of the mossy fiber pathway and within individual cell somata. These results were in contrast to those observed with the opioid peptides and receptors. In particular, when estrogen levels are high (either in proestrus or in ovariectomized rats replaced with estradiol) the levels of enkephalin and dynorphin in the mossy fiber pathway as well as the availability of MORs on the plasma membrane of PARV-labeled dendrites are elevated (Torres-Reveron et al., 2008, 2009a,b). Moreover, we recently have found that LTP in the mossy fiber – CA3 pathway, which is known to be opioid dependent (Derrick et al., 1992; Derrick and Martinez, 1994), is greater in proestrus and estrus females compared to males and diestrus females (Varga-Wesson et al., 2011). Several factors may have contributed to lack of detection of sex or cycle differences of pMOR-ir in our study. As mentioned above, the antisera may distinguish between desensitization and internalization processes and/or may recognize only a subset of MOR-containing neurons. Moreover, since the majority of pMOR-ir was in axons, the current light microscopic approaches lacked the resolution to detect changes in the levels of pMOR-ir dendrites. Additionally, phosphorylation MORs would not be expected to change until the opioid peptides are released from dense core vesicles following some form of high frequency stimulation (Thureson-Klein and Klein, 1990). Thus, our finding that sex and cycle differences in pMOR-ir emerged following stress, which is known to release opioid peptides (Redila and Chavkin, 2008), was not unexpected.
In general, the levels of pMOR-ir in the mossy fiber zone increased in males and decreased in proestrus and estrus females after AIS. Moreover, our ultrastuctural findings showing no changes in the number or proportion (e.g., axons and dendrites) of pMOR-containing profiles following AIS suggests that the observed level changes do not reflect recruitment of new profiles. The differences in the magnitude of the changes in the levels of pMOR-ir in subregions varied. In part, the observed differences likely reflect the profile and/or receptor makeup. In particular, the dentate tip and body contain more enkephalin-containing axons and small terminals compared to any of the CA3 subregions (Torres-Reveron et al., 2008). Moreover, the distribution of estrogen and progestin receptors varies between hippocampal subregions (Milner et al., 2005; Waters et al., 2008). Given our prior ultrastructural observations that opioid levels and well as MORs available for ligand binding in PARV interneurons increase in proestrus rats (Torres-Reveron et al., 2008, 2009a,b), the observed decreases in pMOR-ir in the proestrus rats after AIS were unexpected. However, our findings are consistent with reports that after AIS males have enhanced learning processes whereas females have decreased learning processes (Luine et al., 2007).
Functional Considerations
Activation of MORs has been shown to play an important role in LTP and cognitive processes within the hippocampus (Meilandt et al., 2004; Yoo et al., 2004). The current report demonstrating a significant sex and estrous cycle differences in the levels of activated MORs after AIS suggests that gonadal steroid status may influence LTP or plasticity following stress. Indeed, stress induced changes in LTP in males have been found to be reduced by acute application of estradiol (Foy et al., 2008; Foy, 2011). The present results also suggest that MOR trafficking changes in response to ovarian hormones may not be the only mechanism that regulates MOR-activated responses. Although the impact of these changes in MOR activation in the female brain is currently unknown, studies are in progress exploring this question.
A growing body of literature demonstrates that the effects of stress are sexually dimorphic (Shors and Thompson, 1992; Beck and Luine, 2002; Bowman et al., 2003; Conrad et al., 2003). The results from this study support this idea demonstrating that pMOR-ir is regulated in a sex specific fashion, as well as over the estrus cycle following AIS. These observations are consistent with other studies demonstrating that males and females perform in opposing manners on hippocampally dependent behavioral tasks following both acute and chronic stress (Beck and Luine, 2002; Luine, 2002; Luine et al., 2007). Moreover, our findings support other studies that the opioid system is involved in stress responses. In particular, acute and chronic stress induce the release of opioid peptides and alter MOR binding in many brain regions (Kalivas and Abhold, 1987; Nabeshima et al., 1992; Stein et al., 1992; Drolet et al., 2001; Lucas et al., 2004; Dantas et al., 2005). Moreover, the release of opioid peptides following stress can affect drug-induced reinstatement in males (McLaughlin et al., 2003; Redila and Chavkin, 2008; Bruchas et al., 2010; Shalev et al., 2010). Although the direct mechanisms for sexually dimorphic stress responses are not fully understood, the current report suggests that the role of the hippocampal opioid system in learning behaviors following stress merit further study.
In conclusion, these data demonstrate that the endogenous hippocampal opioid system responds to stress in an opposing fashion in males and females. These differences could potentially play a role in many of the reported sex differences in hippocampal function, such as susceptibility to seizures, differences in cognitive performance, and drug addiction (Brady and Randall, 1999; Doucette et al., 2007). Together, these data suggest that gonadal hormones can play a critical role in the excitability and plasticity of the hippocampus by modulating the endogenous opioid system.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Ms. Jessica Lin for technical assistance and Ms. Louisa Thompson for help with figure preparation. GRANT SUPPORT: NIH grants DA08259, HL096571, and HL098351 (Teresa A. Milner), DA08259-S1 (Keith L. Gonzales), T32 DA007274 (Jeanette D. Chapleau), DK07313 & AG059850 (Elizabeth M. Waters), NS007080 (Bruce S. McEwen), MSTP grant GM07739 (Tanya J. Williams).
References
Abbadie, C., Pan, Y. X., Drake, C. T., and Pasternak, G. W. (2000). Comparative immunohistochemical distributions of carboxy terminus epitopes from the mu-opioid receptor splice variants MOR-1D, MOR-1 and MOR-1C in the mouse and rat CNS. Neuroscience 100, 141–153.
Beck, K. D., and Luine, V. N. (2002). Sex differences in behavioral and neurochemical profiles after chronic stress: role of housing conditions. Physiol. Behav. 75, 661–673.
Bowman, R. E., Beck, K. D., and Luine, V. N. (2003). Chronic stress effects on memory: sex differences in performance and monoaminergic activity. Horm. Behav. 43, 48–59.
Brady, K. T., and Randall, C. L. (1999). Gender differences in substance use disorders. Psychiatr. Clin. North Am. 22, 241–252.
Bruchas, M., Land, B., and Chavkin, C. (2010). The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain Res. 1314, 44–55.
Celio, M. R., Baier, W., Scharer, L., De Viragh, P., and Gerday, C. (1988). Monoclonal antibodies directed against the calcium binding protein parvalbumin. Cell Calcium 9, 81–86.
Celver, J., Xu, M., Jin, W., Lowe, J., and Chavkin, C. (2004). Distinct domains of the μ-opioid receptor control uncoupling and internalization. Mol. Pharmacol. 65, 528–537.
Chan, J., Aoki, C., and Pickel, V. M. (1990). Optimization of differential immunogold-silver and peroxidase labeling with maintenance of ultrastructure in brain sections before plastic embedding. J. Neurosci. Methods 33, 113–127.
Cheung, D. (1990). Synaptic transmission in the guinea-pig vas deferens: the role of nerve action potentials. Neuroscience 37, 127–134.
Chu, J., Zheng, H., Loh, H. H., and Law, P. Y. (2008). Morphine-induced mu-opioid receptor rapid desensitization is independent of receptor phosphorylation and beta-arrestins. Cell. Signal. 20, 1616–1624.
Conrad, C. D., Grote, K. A., Hobbs, R. J., and Ferayorni, A. (2003). Sex differences in spatial and non-spatial Y-maze performance after chronic stress. Neurobiol. Learn. Mem. 79, 32–40.
Cunnane, T., and Stjärne, L. (1984). Frequency dependent intermittency and ionic basis of impulse conduction in postganglionic sympathetic fibres of guinea-pig vas deferens. Neuroscience 11, 211–229.
Dantas, G., Torres, I. L. D. S., Crema, L. M., Lara, D. R., and Dalmaz, C. (2005). Repeated restraint stress reduces opioid receptor binding in different rat CNS structures. Neurochem. Res. 30, 1–7.
Derrick, B., and Martinez, J. (1994). Opioid receptor activation is one factor underlying the frequency dependence of mossy fiber LTP induction. J. Neurosci. 14, 4359–4367.
Derrick, B. E., Rodriguez, S. B., Lieberman, D. N., and Martinez, J. L. (1992). Mu opioid receptors are associated with the induction of hippocampal mossy fiber long-term potentiation. J. Pharmacol. Exp. Ther. 263, 725–733.
Doucette, T. A., Ryan, C. L., and Tasker, R. A. (2007). Gender-based changes in cognition and emotionality in a new rat model of epilepsy. Amino Acids 32, 317–322.
Drake, C. T., Chavkin, C., and Milner, T. A. (2007). Opioid systems in the dentate gyrus. Prog. Brain Res. 163, 245–263.
Drake, C. T., and Milner, T. A. (1999). Mu opioid receptors are in somatodendritic and axonal compartments of GABAergic neurons in rat hippocampal formation. Brain Res. 849, 203–215.
Drake, C. T., and Milner, T. A. (2006). Mu opioid receptors are extensively co-localized with parvalbumin, but not somatostatin, in the dentate gyrus. Neurosci. Lett. 403, 176–180.
Drolet, G., Dumont, É. C., Gosselin, I., Kinkead, R., Laforest, S., and Trottier, J. F. (2001). Role of endogenous opioid system in the regulation of the stress response. Prog. Neuropsychopharmacol. Biol. Psychiatry 25, 729–741.
EisenHart, C., and Hastay, M. W. (1947). Techniques of Statistical Analysis. New York, NY: McGraw-Hill.
El Kouhen, R., Burd, A. L., Erickson-Herbrandson, L. J., Chang, C.-Y., Law, P.-Y., and Loh, H. H. (2001). Phosphorylation of ser363, thr370, and ser375 residues within the carboxyl tail differentially regulates μ-opioid receptor internalization. J. Biol. Chem. 276, 12774–12780.
Elman, I., Karlsgodt, K. H., and Gastfriend, D. R. (2001). Gender differences in cocaine craving among non-treatment-seeking individuals with cocaine dependence. Am. J. Drug Alcohol Abuse 27, 193–202.
Foy, M. R. (2011). Ovarian hormones, aging and stress on hippocampal synaptic plasticity. Neurobiol. Learn. Mem. 95, 134–144.
Foy, M. R., Baudry, M., Foy, J. G., and Thompson, R. F. (2008). 17β-estradiol modifies stress-induced and age-related changes in hippocampal synaptic plasticity. Behav. Neurosci. 122, 301–309.
Galea, L. A. M., McEwen, B. S., Tanapat, P., Deak, T., Spencer, R. L., and Dhabhar, F. S. (1997). Sex differences in dendritic atrophy of CA3 pyramidal neurons in response to chronic restraint stress. Neuroscience 81, 689–697.
Gazzaley, A. H., Weiland, N. G., McEwen, B. S., and Morrison, J. H. (1996). Differential regulation of NMDAR1 mRNA and protein by estradiol in the rat hippocampus. J. Neurosci. 16, 6830.
Hajszan, T., Milner, T. A., and Leranth, C. (2007). Sex steroids and the dentate gyrus. Prog. Brain Res. 163, 399–415.
Hu, M., Crombag, H. S., Robinson, T. E., and Becker, J. B. (2004). Biological basis of sex differences in the propensity to self-administer cocaine. Neuropsychopharmacology 29, 81–85.
Jin, W., and Chavkin, C. (1999). Mu opioids enhance mossy fiber synaptic transmission indirectly by reducing GABAB receptor activation. Brain Res. 821, 286–293.
Kalivas, P. W., and Abhold, R. (1987). Enkephalin release into the ventral tegmental area in response to stress: modulation of mesocorticolimbic dopamine. Brain Res. 414, 339–348.
Karson, M. A., Tang, A. H., Milner, T. A., and Alger, B. E. (2009). Synaptic cross talk between perisomatic-targeting interneuron classes expressing cholecystokinin and parvalbumin in hippocampus. J. Neurosci. 29, 4140–4154.
Koch, T., and Höllt, V. (2008). Role of receptor internalization in opioid tolerance and dependence. Pharmacol. Ther. 117, 199–206.
Lucas, L., Celen, Z., Tamashiro, K., Blanchard, R., Blanchard, D., Markham, C., Sakai, R., and McEwen, B. (2004). Repeated exposure to social stress has long-term effects on indirect markers of dopaminergic activity in brain regions associated with motivated behavior. Neuroscience 124, 449–457.
Lucas, L. R., Wang, C. J., Mccall, T. J., and McEwen, B. S. (2007). Effects of immobilization stress on neurochemical markers in the motivational system of the male rat. Brain Res. 1155, 108–115.
Luine, V., Beck, K., Bowman, R., Frankfurt, M., and Maclusky, N. (2007). Chronic stress and neural function: accounting for sex and age. J. Neuroendocrinol. 19, 743–751.
Lynch, W. J., Roth, M. E., and Carroll, M. E. (2002). Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology (Berl.) 164, 121–137.
Magariños, A. M., Verdugo, J. M. G., and McEwen, B. S. (1997). Chronic stress alters synaptic terminal structure in hippocampus. Proc. Natl. Acad. Sci. U.S.A. 94, 14002–14008.
Mazarati, A., Liu, H., and Wasterlain, C. (1999). Opioid peptide pharmacology and immunocytochemistry in an animal model of self-sustaining status epilepticus. Neuroscience 89, 167–173.
McEwen, B. S., and Milner, T. A. (2007). Hippocampal formation: shedding light on the influence of sex and stress on the brain. Brain Res. Rev. 55, 343–355.
McLaughlin, J. P., Marton-Popovici, M., and Chavkin, C. (2003). Opioid receptor antagonism and prodynorphin gene disruption block stress-induced behavioral responses. J. Neurosci. 23, 5674–5683.
Meilandt, W. J., Barea-Rodriguez, E., Harvey, S. a. K., and Martinez, J. L. (2004). Role of hippocampal CA3 μ-opioid receptors in spatial learning and memory. J. Neurosci. 24, 2953–2962.
Milner, T. A., Ayoola, K., Drake, C. T., Herrick, S. P., Tabori, N. E., McEwen, B. S., Warrier, S., and Alves, S. E. (2005). Ultrastructural localization of estrogen receptor immunoreactivity in the rat hippocampal formation. J. Comp. Neurol. 491, 81–95.
Nabeshima, T., Katoh, A., Wada, M., and Kameyama, T. (1992). Stress-induced changes in brain met-enkephalin, leu-enkephalin and dynorphin concentrations. Life Sci. 51, 211–217.
Narita, M., Kuzumaki, N., Suzuki, M., O, K., Yamazaki, M., Yajima, Y., and Suzuki, T. (2004). Increased phosphorylated-mu-opioid receptor immunoreactivity in the mouse spinal cord following sciatic nerve ligation. Neurosci. Lett. 354, 148–152.
Peters, A., Palay, S. L., and Webster, H. D. (1991). The Fine Structure of the Nervous System, 3rd Edn. New York: Oxford University Press.
Petraschka, M., Li, S., Gilbert, T. L., Westenbroek, R. E., Bruchas, M. R., Schreiber, S., Lowe, J., Low, M. J., Pintar, J. E., and Chavkin, C. (2007). The absence of endogenous beta-endorphin selectively blocks phosphorylation and desensitization of mu opioid receptors following partial sciatic nerve ligation. Neuroscience 146, 1795–1807.
Pierce, J. P., Kurucz, O. S., and Milner, T. A. (1999). Morphometry of a peptidergic transmitter system: dynorphin B-like immunoreactivity in the rat hippocampal mossy fiber pathway before and after seizures. Hippocampus 9, 255–276.
Piva, F., Limonta, P., Dondi, D., Pimpinelli, F., Martini, L., and Maggi, R. (1995). Effects of steroids on the brain opioid system. J. Steroid Biochem. Mol. Biol. 53, 343–348.
Redila, V. A., and Chavkin, C. (2008). Stress-induced reinstatement of cocaine seeking is mediated by the kappa opioid system. Psychopharmacology (Berl.) 200, 59–70.
Robbins, S. J., Ehrman, R. N., Childress, A. R., and O’Brien, C. P. (1999). Comparing levels of cocaine cue reactivity in male and female outpatients. Drug Alcohol Depend. 53, 223–230.
Roberts, D., Bennett, S., and Vickers, G. (1989). The estrous cycle affects cocaine self-administration on a progressive ratio schedule in rats. Psychopharmacology (Berl.) 98, 408–411.
Roth, M. E., Casimir, A. G., and Carroll, M. E. (2002). Influence of estrogen in the acquisition of intravenously self-administered heroin in female rats. Pharmacol. Biochem. Behav. 72, 313–318.
Schulz, S., Mayer, D., Pfeiffer, M., Stumm, R., Koch, T., and Höllt, V. (2004). Morphine induces terminal -opioid receptor desensitization by sustained phosphorylation of serine-375. EMBO J. 23, 3282–3289.
Shalev, U., Erb, S., and Shaham, Y. (2010). Role of CRF and other neuropeptides in stress-induced reinstatement of drug seeking. Brain Res. 1314, 15–28.
Shansky, R. M., Hamo, C., Hof, P. R., Lou, W., McEwen, B. S., and Morrison, J. H. (2010). Estrogen promotes stress sensitivity in a prefrontal cortex–amygdala pathway. Cereb. Cortex 20, 2560–2567.
Shors, T. J., and Thompson, R. F. (1992). Acute stress impairs (or induces) synaptic long term potentiation (LTP) but does not affect paired pulse facilitation in the stratum radiatum of rat hippocampus. Synapse 11, 262–265.
Sinchak, K., and Micevych, P. E. (2001). Progesterone blockade of estrogen activation of opioid receptors regulates reproductive behavior. J. Neurosci. 21, 5723–5729.
Šlamberová, R., Rimanóczy, Á., Bar, N., Schindler, C. J., and Vathy, I. (2003). Density of opioid receptors in the hippocampus of adult male and female rats is altered by prenatal morphine exposure and gonadal hormone treatment. Hippocampus 13, 461–471.
Spencer, J. L., Waters, E. M., Romeo, R. D., Wood, G. E., Milner, T. A., and McEwen, B. S. (2008). Uncovering the mechanisms of estrogen effects on hippocampal function. Front. Neuroendocrinol. 29, 219–237.
Stein, E., Hiller, J., and Simon, E. (1992). Effects of stress on opioid receptor binding in the rat central nervous system. Neuroscience 51, 683–690.
Stumm, R. K., Zhou, C., Schulz, S., and Höllt, V. (2004). Neuronal types expressing and opioid receptor mRNA in the rat hippocampal formation. J. Comp. Neurol. 469, 107–118.
Swanson, L. W. (2000). Brain Maps: Structure of the Rat Brain, 2nd Edn. San Diego, CA: Academic Press.
Thureson-Klein, Å. K., and Klein, R. L. (1990). Exocytosis from neuronal large dense-cored vesicles. Int. Rev. Cytol. 121, 67–126.
Torres-Reveron, A., Khalid, S., Williams, T. J., Waters, E. M., Drake, C. T., McEwen, B. S., and Milner, T. A. (2008). Ovarian steroids modulate leu-enkephalin levels and target leu-enkephalinergic profiles in the female hippocampal mossy fiber pathway. Brain Res. 1232, 70–84.
Torres-Reveron, A., Khalid, S., Williams, T. J., Waters, E. M., Jacome, L., Luine, V. N., Drake, C. T., McEwen, B. S., and Milner, T. A. (2009a). Hippocampal dynorphin immunoreactivity increases in response to gonadal steroids and is positioned for direct modulation by ovarian steroid receptors. Neuroscience 159, 204–216.
Torres-Reveron, A., Williams, T. J., Chapleau, J. D., Waters, E. M., McEwen, B. S., Drake, C. T., and Milner, T. A. (2009b). Ovarian steroids alter mu opioid receptor trafficking in hippocampal parvalbumin GABAergic interneurons. Exp. Neurol. 219, 319–327.
Varga-Wesson, A., Milner, T. A., and Scharfman, H. E. (2011). “Sex differences in adult rat hippocampal mossy fiber plasticity,” in 2011 Neuroscience Meeting Planner (Washington, DC: Society for Neurosceince Meeting 2011). [Online].
Wagner, J. J., Evans, C. J., and Chavkin, C. (1991). Focal stimulation of the mossy fibers releases endogenous dynorphins that bind K1 opioid receptors in guinea pig hippocampus. J. Neurochem. 57, 333–343.
Waters, E. M., Mitterling, K., Spencer, J. L., Mazid, S., McEwen, B. S., and Milner, T. A. (2009). Estrogen receptor alpha and beta specific agonists regulate expression of synaptic proteins in rat hippocampus. Brain Res. 1290, 1–11.
Waters, E. M., Torres Reveron, A., McEwen, B. S., and Milner, T. A. (2008). Ultrastructural localization of extranuclear progestin receptors in the rat hippocampal formation. J. Comp. Neurol. 511, 34–46.
Williams, T. J., Torres-Reveron, A., Chapleau, J. D., and Milner, T. A. (2011). Hormonal regulation of delta opioid receptor immunoreactivity in interneurons and pyramidal cells in the rat hippocampus. Neurobiol. Learn. Mem. 95, 206–220.
Woolley, C. S. (1998). Estrogen-mediated structural and functional synaptic plasticity in the female rat hippocampus. Horm. Behav. 34, 140–148.
Keywords: opioids, sex differences, estrogens, mossy fiber pathway
Citation: Gonzales KL, Chapleau JD, Pierce JP, Kelter DT, Williams TJ, Torres-Reveron A, McEwen BS, Waters EM and Milner TA (2011) The influences of reproductive status and acute stress on the levels of phosphorylated mu opioid receptor immunoreactivity in rat hippocampus. Front. Endocrin. 2:18. doi: 10.3389/fendo.2011.00018
Received: 30 June 2011;
Accepted: 26 July 2011;
Published online: 19 August 2011.
Edited by:
Hubert Vaudry, University of Rouen, FranceReviewed by:
James A. Carr, Texas Tech University, USAGábor B. Makara, Hungarian Academy of Sciences, Hungary
Copyright: © 2011 Gonzales, Chapleau, Pierce, Kelter, Williams, Torres-Reveron, McEwen, Waters and Milner. This is an open-access article subject to a non-exclusive license between the authors and Frontiers Media SA, which permits use, distribution and reproduction in other forums, provided the original authors and source are credited and other Frontiers conditions are complied with.
*Correspondence: Keith L. Gonzales and Teresa A. Milner, Division of Neurobiology, Department of Neurology and Neuroscience, Weill Cornell Medical College, 407 East 61st Street, RM 307, New York, NY 10065, USA. e-mail: keg2008@med.cornell.edu; tmilner@med.cornell.edu
