- Department of Endocrinology and Metabolism, Hirosaki University Graduate School of Medicine, Hirosaki, Aomori, Japan
While stress activates the hypothalamic–pituitary–adrenal (HPA) axis, it suppresses the hypothalamic–pituitary–gonadal (HPG) axis. Corticotropin-releasing factor (CRF) is a major regulatory peptide in the HPA axis during stress. Urocortin 1 (Ucn1), a member of the CRF family of peptides, has a variety of physiological functions and both CRF and Ucn1 contribute to the stress response via G protein-coupled seven transmembrane receptors. Ucn2 and Ucn3, which belong to a separate paralogous lineage from CRF, are highly selective for the CRF type 2 receptor (CRF2 receptor). The HPA and HPG axes interact with each other, and gonadal function and reproduction are suppressed in response to various stressors. In this review, we focus on the regulation of gonadotropins by CRF and Ucn2 in pituitary gonadotrophs and of gonadotropin-releasing hormone (GnRH) via CRF receptors in the hypothalamus. In corticotrophs, stress-induced increases in CRF stimulate Ucn2 production, which leads to the inhibition of gonadotropin secretion via the CRF2 receptor in the pituitary. GnRH in the hypothalamus is regulated by a variety of stress conditions. CRF is also involved in the suppression of the HPG axis, especially the GnRH pulse generator, via CRF receptors in the hypothalamus. Thus, complicated regulation of GnRH in the hypothalamus and gonadotropins in the pituitary via CRF receptors contributes to stress responses and adaptation of gonadal functions.
Introduction
A variety of stressors have been shown to suppress gonadal function (Chand and Lovejoy, 2011). Proteins that play key roles in vertebrate reproduction include the neuropeptides gonadotropin-releasing hormone (GnRH) and kisspeptin and their receptors (Kim et al., 2012): kisspeptin stimulates GnRH release from hypothalamic GnRH neurons via Gpr54, a G protein-coupled receptor (Messager et al., 2005), while the gonadal steroid estrogen mediates its inhibitory effect on GnRH secretion by acting on kisspeptin-expressing neurons of the arcuate nucleus (Oakley et al., 2009; Ohkura et al., 2009). The expression of kisspeptin and kisspeptin receptor mRNA is downregulated by stressors including restraint, hypoglycemia, and lipopolysaccharide, which suggests that kisspeptin/kisspeptin receptor signaling plays a critical role in the transduction of stress-induced suppression of reproduction (Kinsey-Jones et al., 2009). In fact, kisspeptin–GPR54 signaling in the arcuate nucleus of the mediobasal hypothalamus is a critical neural component of the hypothalamic GnRH pulse generator (Li et al., 2009).
Gonadotropin-inhibitory hormone (GnIH), an RFamide-related peptide, can also modulate the reproduction of vertebrates (Ubuka et al., 2008). GnIH neurons interact directly with GnRH neurons, and the action of GnIH is mediated by a novel G protein-coupled receptor, Gpr147 (Ubuka et al., 2008). In mice, higher concentrations of GnIH-like substances are expressed in the hypothalamus and GnIH reduces GnRH release from the mouse hypothalamus (Bentley et al., 2010). The glucocorticoid and corticotropin-releasing factor (CRF) receptors are expressed in a large population of GnIH/RFamide-related peptide-expressing cells (Kirby et al., 2009). Glucocorticoids increase the inhibitory actions of GnIH on GnRH secretion (Kirby et al., 2009), while the regulation of GnIH via the CRF receptor remains to be determined.
Corticotropin-releasing factor activates and regulates the hypothalamic–pituitary–adrenal (HPA) axis during stress (Vale et al., 1981, 1997). Stress-induced CRF synthesis and secretion from the hypothalamic paraventricular nucleus (PVN) stimulates adrenocorticotropic hormone (ACTH) release from pituitary corticotrophs (Gillies et al., 1982; Mouri et al., 1993), which, in turn, stimulates the release of glucocorticoids from the adrenal glands (Whitnall, 1993). These glucocorticoids then moderate the stress response by inhibiting hypothalamic PVN production of CRF and pituitary production of ACTH (Whitnall, 1993). Urocortin 1 (Ucn1), a 40-amino acid peptide originally cloned from the Edinger–Westphal nucleus, is a member of the CRF family of peptides (Vaughan et al., 1995). Both CRF and Ucn1 contribute to stress responses and cardiovascular and gonadal functions via G protein-coupled seven transmembrane receptors (Vale et al., 1997; Kageyama et al., 1999a; Suda et al., 2004). CRF exhibits high affinity for CRF type 1 receptor (CRF1 receptor; IC50 = 1.6 nM) but not for CRF type 2b receptor (CRF2b receptor; IC50 = 42 nM), while Ucn1 exhibits similar affinity for CRF1 receptor (IC50 = 0.16 nM) and CRF2b receptor (IC50 = 0.86 nM; Jahn et al., 2004). CRF1 receptor is predominately expressed in the brain and pituitary gland (Chang et al., 1993; Chen et al., 1993; Vita et al., 1993; Potter et al., 1994). In the pituitary, the CRF1 receptor is mainly expressed by corticotrophs and is responsible for mediating the effects of hypothalamic CRF on ACTH secretion in response to stress (Wynn et al., 1985; Antoni, 1986).
Ucn2 and Ucn3 prohormones were identified in the human genome database and in mouse genomic DNA, respectively (Hsu and Hsueh, 2001; Lewis et al., 2001; Reyes et al., 2001), from which the identity and existence of endogenous peptides were predicted (Fekete and Zorrilla, 2007). Ucn2 and Ucn3 are more similar to each other than to CRF with regard to receptor binding (Kishimoto et al., 1995; Lovenberg et al., 1995a; Perrin et al., 1995; Stenzel et al., 1995). Ucn2 exhibits high affinity for CRF2b receptor (IC50 = 0.25 nM) but low affinity for CRF1 receptor (IC50>350 nM; Jahn et al., 2004). Similarly, Ucn3 binds with moderate affinity to CRF2b receptor (IC50 = 14 nM), but its specific binding to CRF1 receptor is not detectable (IC50>2000 nM; Jahn et al., 2004). It is hypothesized that an ancient gene duplication event is behind why Ucn1 belongs to the CRF lineage and why Ucn2 and Ucn3 represent a separate paralogous lineage (Fekete and Zorrilla, 2007).
The CRF1 receptor is primarily involved in stress responses and depression, while the CRF2 receptor is believed to mediate “stress-coping” responses in the brain, such as anxiolysis (Suda et al., 2004), because mice deficient in the CRF2 receptor or treated with a CRF2 receptor antagonist display increased anxiety-like behaviors and hypersensitive stress responses (Bale et al., 2000). Furthermore, both Ucn2 and Ucn3 act as anorexigenic neuropeptides via the CRF2 receptor (Fekete et al., 2011; Chao et al., 2012) and Ucn3 regulates glucose-stimulated insulin secretion and energy homeostasis (Li et al., 2007). Ucn3 signaling through the CRF2 receptor is also a critical molecular mediator in the ventromedial nucleus of the hypothalamus in regulating feeding and peripheral energy metabolism (Chao et al., 2012).
Corticotropin-releasing factor is involved in the suppression of the hypothalamic–pituitary–gonadal (HPG) axis (Rivier et al., 1986), especially the GnRH pulse generator in the hypothalamus (Knobil, 1992). Stress profoundly inhibits the reproductive function by suppressing the pulsatile release of GnRH and consequently luteinizing hormone (LH), at least in part via the CRF system as well as through the GABAergic system (Lin et al., 2012). Although CRF and Ucn clearly have potent effects on the HPG system, their possible roles and how they are regulated have yet to be fully determined. In this review, we focus on the regulation and the roles of Ucn2 in pituitary gonadotrophs and discuss the regulation of GnRH via CRF receptors in the hypothalamus.
Regulation of Gonadotropins by CRF and Ucn2 in the Pituitary
Changes in CRF1 receptor expression and desensitization of the receptor in pituitary corticotrophs play a major role in modulating adaptive responses to stressors (Kageyama et al., 2006). CRF, vasopressin, lipopolysaccharides, cytokines, and glucocorticoids can negatively modulate the levels of pituitary CRF1 receptor mRNA (Pozzoli et al., 1996; Sakai et al., 1996; Aubry et al., 1997). However, CRF2 receptor mRNA is also found in the anterior pituitary and combined immunohistochemistry and in situ hybridization have demonstrated that CRF2 receptor mRNA colocalizes mainly with gonadotrophs, not corticotrophs (Figure 1).
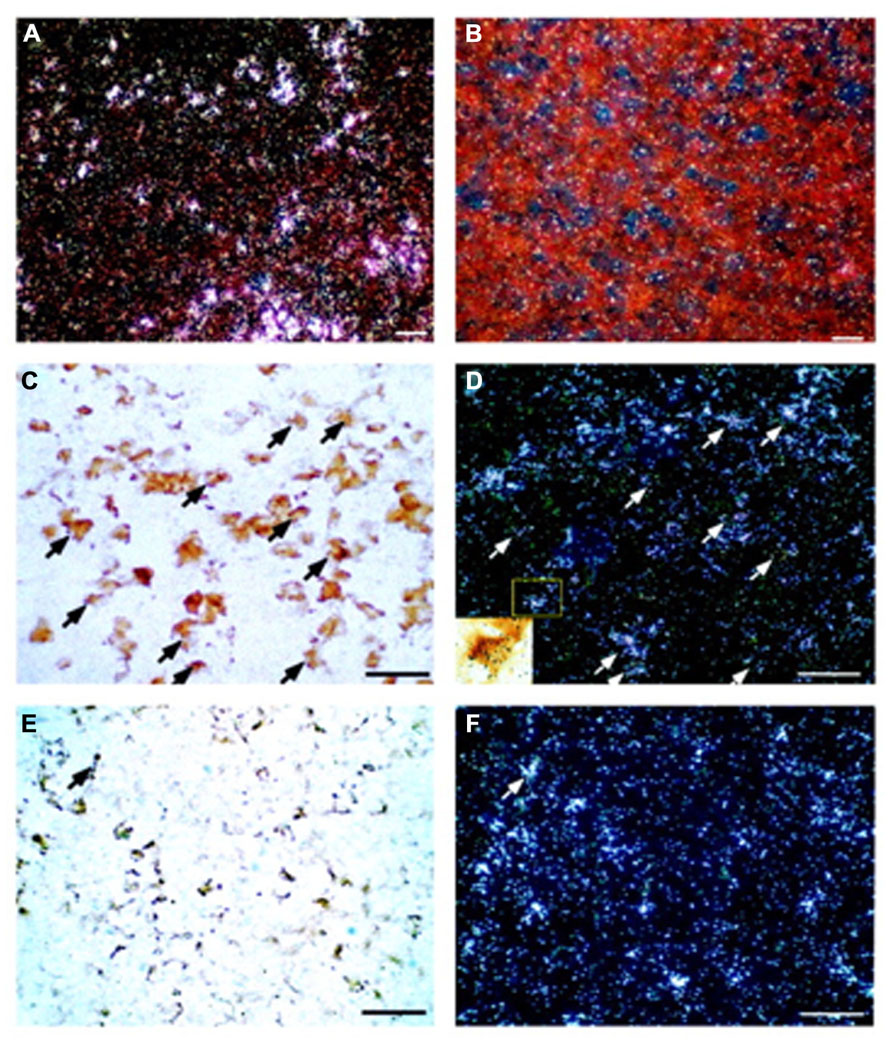
FIGURE 1. Localization of CRF2 receptor mRNA in the rat anterior pituitary gland. Representative dark-field photomicrographs showing anterior pituitary sections probed with either antisense (A) or sense (B) in situ hybridization probes for CRF2 receptor mRNA. Positive signals (indicated by silver grain clusters) were found only in the tissues probed with antisense probes; no signals were found in the sense control. (C) Bright-field photomicrograph showing LH-immunoreactive cells in the rat anterior pituitary. Gonadotrophs (visualized by the brown DAB precipitate) represent LH-immunoreactive cells. (D) Dark-field image of the same area as panel (C) showing CRF2 receptor mRNA-positive cells (silver grain clusters). Some of the cells that are double-labeled by immunostaining for LH and in situ hybridization for CRF2 receptor mRNA are indicated by arrows. Inset: High-power magnification of the boxed area of panel (D) to illustrate a LH-positive cell that shows a positive signal for CRF2 receptor mRNA (scattered black silver grains). (E) Representative bright-field photomicrograph of the anterior pituitary showing ACTH-immunoreactive cells (brown DAB precipitate). (F) Dark-field image of the same area as panel (E) showing CRF2 receptor mRNA signals (silver grain clusters). Only a few ACTH-immunoreactive and CRF2 receptor mRNA double-labeled cells (arrow) were observed. Scale bar, 50 μm. Reproduction from Kageyama et al. (2003) with permission of the publisher. Copyright 2003, The Endocrine Society.
RNase protection assays of anterior pituitary mRNA show that the dominant receptor type is the CRF type 2a receptor (CRF2a) receptor and not the CRF2b receptor (Lovenberg et al., 1995a; Kageyama et al., 2003). Rat CRF2a receptor, linked to various roles in the brain, is expressed primarily in several discrete brain regions, including the hypothalamus, lateral septum, and raphe nuclei (Lovenberg et al., 1995b), whereas the CRF2b receptor is found predominately in peripheral tissues such as the heart, gastrointestinal tract, arterioles, and muscles (Kageyama et al., 1999b). These data suggest that the CRF2a receptor in pituitary gonadotrophs is involved in the modulation of gonadotropin secretion and/or gonadal function.
Activation of the stress system could potentially influence reproduction at any level of the HPG axis (Tilbrook et al., 2002). The stress-induced decreases in LH/follicle-stimulating hormone (FSH) secretion influence gonadal functions such as sex steroidogenesis and sperm production (Demura et al., 1989; Tilbrook et al., 2002). Ucn2 is expressed mainly in corticotrophs of rat pituitary (Yamauchi et al., 2005), and its secretion and expression levels are increased by CRF and suppressed by glucocorticoids (Nemoto et al., 2007).
The CRF2 receptor-selective ligand Ucn2 suppresses both expression and secretion of gonadotropins in rats, while a CRF2 receptor antagonist increases the secretion of gonadotropins (Nemoto et al., 2009). In addition, an anti-CRF antibody blocks stress-induced increases in plasma ACTH and corticosterone, and an anti-Ucn2 antibody blocks stress-induced suppression of LH secretion without affecting stress-induced ACTH and corticosterone release (Nemoto et al., 2010). Stress-induced increases in microRNA-325-3p also suppress gonadotropin secretion (Nemoto et al., 2012). Although the presence and/or secretion of mature Ucn2 has not been determined in the pituitary or other tissues, it is possible that stress-induced increases in CRF stimulate Ucn2 in corticotrophs, which inhibits gonadotropin secretion via CRF2 receptors in the pituitary.
Regulation of GnRH by CRF and Ucn Via CRF Receptors in the Hypothalamus
Although peripheral administration of CRF fails to affect LH secretion (D’Agata et al., 1984; Rivier and Vale, 1984), central injection of CRF inhibits secretion of gonadotropins (Rivier et al., 1986). These effects of CRF probably reflect a central mechanism that involves modulation of the activity of GnRH neurons in the hypothalamus (Petraglia et al., 1987; Li et al., 2010). Indeed, in monkeys, a CRF antagonist attenuates suppression of the GnRH pulse generator in response to hypoglycemic stress (Chen et al., 1996). Furthermore, a recent in vivo rat study indicated that CRF innervation of the dorsolateral bed nucleus of the stria terminalis plays a central role in stress-induced suppression of the GnRH pulse generator (Li et al., 2011).
Corticotropin-releasing factor also suppresses GnRH gene expression levels in murine GnRH GT1-7 cells (Kinsey-Jones et al., 2006). In fact, GT1-7 GnRH-producing cells have been used extensively in studies of the basic control mechanisms involved in GnRH neuronal function. Belsham and colleagues have managed to develop cell lines that are representative of the enormous range of cell types of the hypothalamus (Dalvi et al., 2011). N39, developed from primary mouse fetal hypothalamic culture, is one of these homologous neuronal cell lines. To further understand the possible function of Ucn and the regulation of GnRH by CRF receptors in the hypothalamus, hypothalamic N39 cells have been studied because they express both CRF1 and CRF2 receptor mRNA and protein (Kageyama et al., 2012). It has been shown in these cells that a CRF1 receptor antagonist, antalarmin, inhibits CRF-induced decreases in GnRH mRNA levels, which suggests that CRF decreases GnRH mRNA levels via the CRF1 receptor (Figure 2).
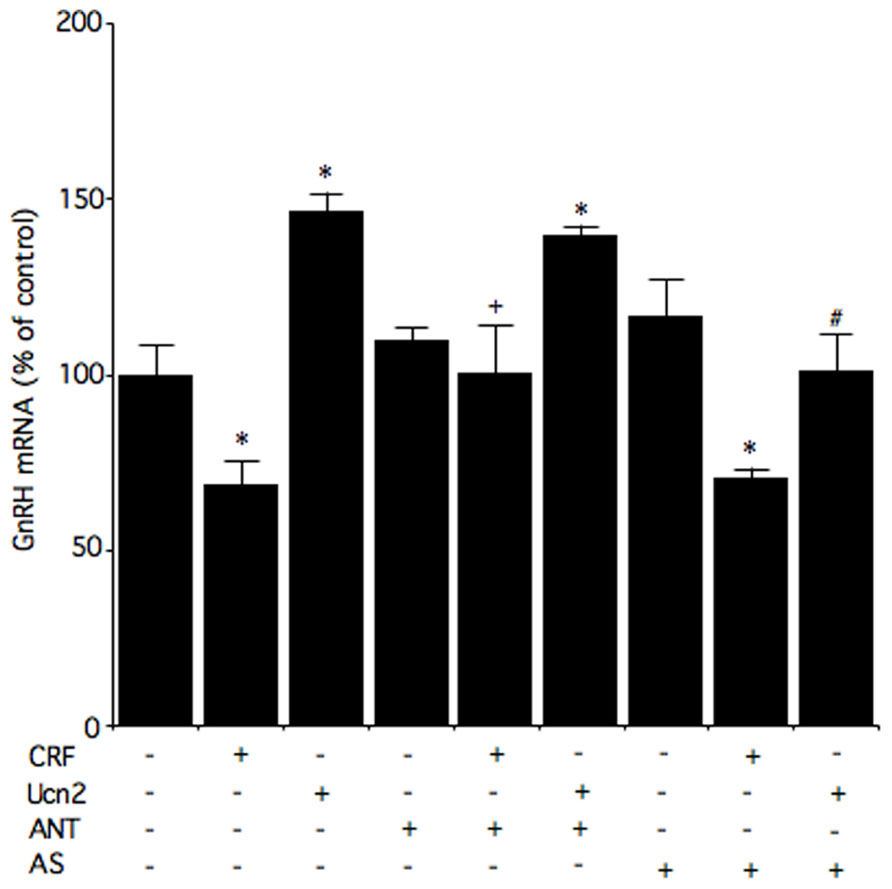
FIGURE 2. Effects of a CRF receptor antagonist on the effects of CRF and Ucn2 on GnRH mRNA levels in N39 cells. Cells were pre-incubated with medium containing 1 μM antalarmin (ANT), antisauvagine-30 (AS), or vehicle for 30 min, and then incubated for 6 h with medium containing 100 nM CRF, 100 nM Ucn2, or vehicle. Reproduction from Kageyama et al. (2012) with permission of the publisher. Copyright 2012, Elsevier.
The CRF2 receptor may also be involved in the regulation of GnRH gene expression. It has been reported that CRF regulates GnRH mRNA levels via, at least in part, the CRF2 receptor in GT1-7 cells (Kinsey-Jones et al., 2006). In N39 cells, Ucn2 increases GnRH mRNA levels, and these Ucn2-induced increases in GnRH mRNA levels are blocked by the CRF2 receptor antagonist antisauvagine-30 (Figure 2). These results suggest that Ucn2 stimulates GnRH mRNA levels via the CRF2 receptor in hypothalamic cells. In an in vivo study, hypoglycemia- and lipopolysaccharide-induced suppression of LH involves activation of CRF2 receptor while restraint stress-induced inhibition of LH pulses involves both CRF1 and CRF2 receptors (Li et al., 2006). On the other hand, a more recent in vivo study showed that a CRF1 receptor antagonist blocks the acute stress-induced increases in gonadotropin secretion on the morning of proestrus while a CRF2 receptor antagonist weakly blocks the increase in FSH secretion (Traslaviña and Franci, 2012). Although GnRH production and secretion may be differentially modulated via CRF receptors under different stressors, further study will be required to elucidate the involvement of CRF receptors.
Glucocorticoids were recently shown to increase CRF2a receptor expression while simultaneously inhibiting CRF1 receptor expression in pancreatic β cell-derived insulinoma MIN6 cells expressing glucocorticoid receptors (Huising et al., 2011). The differential effects of the glucocorticoids on the expression of these receptors in the endocrine pancreas represent a mechanism of shifting sensitivity from CRF1 to CRF2 receptor ligands (Huising et al., 2011). In the hypothalamus, glucocorticoids, released in response to stress, inhibit GnRH and gonadotropins through activation of GnIH (Kirby et al., 2009). It has yet to be determined whether glucocorticoid-induced changes in CRF and Ucn are involved in the regulation of GnRH and gonadotropins.
Relation between Sexual Differences and the CRF System in the Hypothalamus
Sexual dimorphism is associated with stress sensitivity and interaction of the HPA and HPG axes (Chand and Lovejoy, 2011). Estrogens are implicated in the differing stress responses between the sexes and modulate activation of the HPA axis; females, but not males, generally have slight hypercortisolism (Magiakou et al., 1997). Estrogen replacement increases the basal levels of ACTH in ovariectomized rats (Ochedalski et al., 2007) and in postmenopausal women (Fonseca et al., 2001). Moreover, women in the midluteal phase, when both progesterone and estrogen levels are relatively high, show enhanced ACTH levels in response to a stressor (Altemus et al., 2001).
Estrogens acting centrally, including in the pituitary corticotrophs and the hypothalamus, are able to modulate the stress responses (Nakano et al., 1991), and direct estrogenic regulation of CRF gene expression has also been demonstrated in various tissues (Vamvakopoulos and Chrousos, 1993; Dibbs et al., 1997). As high levels of estrogen replacement increase the basal levels of CRF mRNA in the PVN of ovariectomized rats (Ochedalski et al., 2007), estrogen would regulate the HPA axis in vivo by stimulating CRF gene expression in the hypothalamus. CRF mRNA levels in the PVN are not affected by estrogen treatment in either gonadectomized estrogen receptor (ER) type β (ERβ) knockout mice or wild-type male mice (Nomura et al., 2002). Therefore, it is likely that estrogen modulates CRF gene expression in a sex-dependent manner.
Hypothalamic 4B cells show characteristics of the parvocellular neurons of the PVN because these cells express CRF, vasopressin, CRF1 receptor, and glucocorticoid receptors. Estrogen directly stimulates CRF gene expression in hypothalamic 4B cells (Ogura et al., 2008), suggesting that estrogen is involved in the positive regulation of CRF gene expression in the parvocellular region of the PVN in vitro. Neurons expressing both CRF and ERβ are found in the medial parvocellular division (Miller et al., 2004) and project to the median eminence, and CRF in parvocellular PVN neurons exerts effects on corticotroph ACTH secretion (Gillies et al., 1982; Mouri et al., 1993). Therefore, estrogen and ERβ would contribute to the enhancement of stress responses through stimulation of CRF neurons of the hypothalamus, and may constitute the basis of sexual dimorphism in the regulation of the CRF gene (Straub, 2007). In addition, estrogen also enhances CRF- and stress-induced suppression of pulsatile LH secretion (Cates et al., 2004), and upregulation of the CRF2 receptor may contribute to the sensitizing influence of estradiol on the CRF- and stress-induced suppression of the GnRH pulse generator (Kinsey-Jones et al., 2006).
Meanwhile, Ucn1 in the non-preganglionic Edinger–Westphal nucleus plays an important role in stress adaptation. Estrogens exert a differential transcriptional regulation of the Ucn1 gene through either ER type α (ERα) or ERβ receptors (Haeger et al., 2006). Ucn1 mRNA levels in the non-preganglionic Edinger–Westphal nucleus of male rats are much higher than those of females (Derks et al., 2010), and estrogens may contribute to stress adaptation through modulation of Ucn1 production.
Conclusion
In summary, Ucn2, mainly produced in corticotrophs in response to CRF, acts on gonadotrophs expressing the CRF2 receptor and inhibits the production of gonadotropins in the pituitary (Figure 3). CRF is involved in the suppression of the HPG axis, especially the GnRH pulse generator in the hypothalamus, and also decreases GnRH mRNA levels via the CRF1 receptor (Figure 3). The CRF2 receptor may be involved in the regulation of GnRH production and secretion. GnRH production and secretion may be differentially modulated via CRF receptors in response to different stressors. Thus, complicated regulation of GnRH and gonadotropins via the CRF receptors contributes to stress responses and adaptation in gonadal functions.
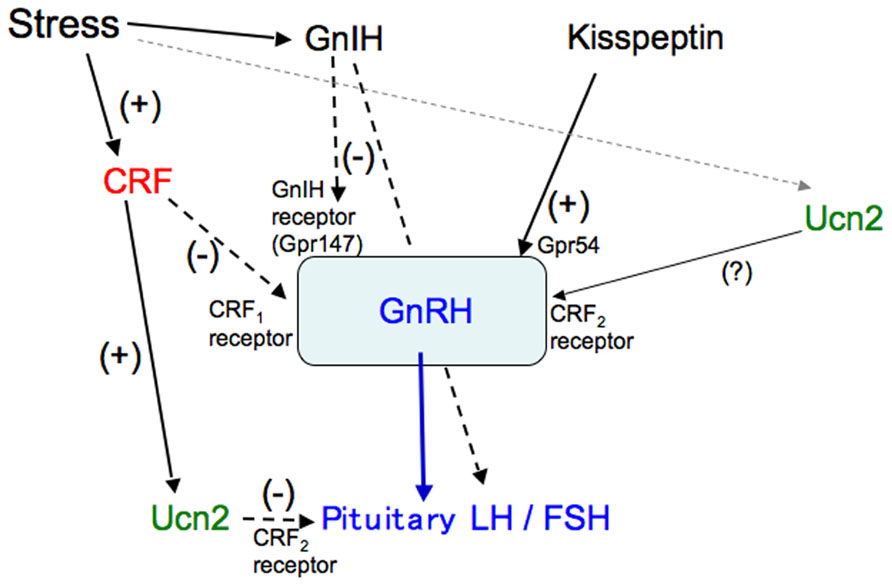
FIGURE 3. A schematic model of the regulation of gonadotropins by CRF and Ucn. Stress-induced increases in CRF stimulate Ucn2 in corticotrophs, which inhibits gonadotropin secretion via the CRF2 receptor in the pituitary. CRF inhibits the stress-induced suppression of the GnRH pulse generator and decreases GnRH mRNA levels via the CRF1 receptor in the hypothalamus. The CRF2 receptor may also be involved in the regulation of GnRH.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgment
This work was supported in part by Health and Labour Sciences Research Grants (Research on Measures for Intractable Diseases) from the Ministry of Health, Labour, and Welfare of Japan.
References
Altemus, M., Roca, C., Galliven, E., Romanos, C., and Deuster, P. (2001). Increased vasopressin and adrenocorticotropin responses to stress in the midluteal phase of the menstrual cycle. J. Clin. Endocrinol. Metab. 86, 2525–2530.
Antoni, F. A. (1986). Hypothalamic control of adrenocorticotropin secretion: advances since the discovery of 41-residue corticotropin-releasing factor. Endocr. Rev. 7, 351–378.
Aubry, J.-M., Turnbull, A. V., Pozzzoli, G., Rivier, C., and Vale, W. (1997). Endotoxin decreases corticotropin-releasing factor receptor 1 messenger ribonucleic acid levels in rat pituitary. Endocrinology 138, 1621–1626.
Bale, T. L., Contarino, A., Smith, G. W., Chan, R., Gold, L. H., Sawchenko, P. E., et al. (2000). Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress. Nat. Genet. 24, 410–414.
Bentley, G. E., Tsutsui, K., and Kriegsfeld, L. J. (2010). Recent studies of gonadotropin-inhibitory hormone (GnIH) in the mammalian hypothalamus, pituitary and gonads. Brain Res. 1364, 62–71.
Cates, P. S., Li, X. F., and O’Byrne, K. T. (2004). The influence of 17beta-oestradiol on corticotrophin-releasing hormone induced suppression of luteinising hormone pulses and the role of CRH in hypoglycaemic stress-induced suppression of pulsatile LH secretion in the female rat. Stress 7, 113–118.
Chand, D., and Lovejoy, D. A. (2011). Stress and reproduction: controversies and challenges. Gen. Comp. Endocrinol. 171, 253–257.
Chang, C. P., Pearse, R. I., O’Connell, S., and Rosenfeld, M. G. (1993). Identification of a seven transmembrane helix receptor for corticotropin-releasing factor and sauvagine in mammalian brain. Neuron 11, 1187–1195.
Chao, H., Digruccio, M., Chen, P., and Li, C. (2012). Type 2 corticotropin-releasing factor receptor in the ventromedial nucleus of hypothalamus is critical in regulating feeding and lipid metabolism in white adipose tissue. Endocrinology 153, 166–176.
Chen, M. D., Ordög, T., O’Byrne, K. T., Goldsmith, J. R., Connaughton, M. A., and Knobil, E. (1996). The insulin hypoglycemia-induced inhibition of gonadotropin-releasing hormone pulse generator activity in the rhesus monkey: roles of vasopressin and corticotropin-releasing factor. Endocrinology 137, 2012–2021.
Chen, R., Lewis, K. A., Perrin, M. H., and Vale, W. W. (1993). Expression cloning of a human corticotropin-releasing-factor receptor. Proc. Natl. Acad. Sci. U.S.A. 90, 8967–8971.
D’Agata, R., Cavagnini, F., Invitti, C., Mongioi, A., Fossati, R., Scapagnini, U., et al. (1984). Effect of CRF on the release of anterior pituitary hormones in normal subjects and patients with Cushing’s disease. Pharmacol. Res. Commun. 16, 303–311.
Dalvi, P. S., Nazarians-Armavil, A., Tung, S., and Belsham, D. D. (2011). Immortalized neurons for the study of hypothalamic function. Am. J. Physiol. Regul. Integr. Comp. Physiol. 300, R1030–R1052.
Demura, R., Suzuki, T., Nakamura, S., Komatsu, H., Odagiri, E., and Demura, H. (1989). Effect of immobilization stress on testosterone and inhibin in male rats. J. Androl. 10, 210–213.
Derks, N. M., Gaszner, B., Roubos, E. W., and Kozicz, L. T. (2010). Sex differences in urocortin 1 dynamics in the non-preganglionic Edinger–Westphal nucleus of the rat. Neurosci. Res. 66, 117–123.
Dibbs, K. I., Anteby, E., Mallon, M. A., Sadovsky, Y., and Adler, S. (1997). Transcriptional regulation of human placental corticotropin-releasing factor by prostaglandins and estradiol. Biol. Reprod. 57, 1285–1292.
Fekete, E. M., Zhao, Y., Szücs, A., Sabino, V., Cottone, P., Rivier, J., et al. (2011). Systemic urocortin 2, but not urocortin 1 or stressin 1-A, suppresses feeding via CRF2 receptors without malaise and stress. Br. J. Pharmacol. 164, 1959–1975.
Fekete, E. M., and Zorrilla, E. P. (2007). Physiology, pharmacology, and therapeutic relevance of urocortins in mammals: ancient CRF paralogs. Front. Neuroendocrinol. 28, 1–27.
Fonseca, E., Basurto, L., Velazquez, S., and Zarate, A. (2001). Hormone replacement therapy increases ACTH/dehydroepiandrosterone sulfate in menopause. Maturitas 39, 57–62.
Gillies, G. E., Linton, E. A., and Lowry, P. J. (1982). Corticotropin releasing activity of the new CRF is potentiated several times by vasopressin. Nature 299, 355–357.
Haeger, P., Andrés, M. E., Forray, M. I., Daza, C., Araneda, S., and Gysling, K. (2006). Estrogen receptors alpha and beta differentially regulate the transcriptional activity of the urocortin gene. J. Neurosci. 26, 4908–4916.
Hsu, S. Y., and Hsueh, A. J. (2001). Human stresscopin and stresscopin-related peptide are selective ligands for the type 2 corticotropin-releasing hormone receptor. Nat. Med. 7, 605–611.
Huising, M. O., Pilbrow, A. P., Matsumoto, M., van der Meulen, T., Park, H., Vaughan, J. M., et al. (2011). Glucocorticoids differentially regulate the expression of CRFR1 and CRFR2α in MIN6 insulinoma cells and rodent islets. Endocrinology 152, 138–150.
Jahn, O., Tezval, H., van Werven, L., Eckart, K., and Spiess, J. (2004). Three-amino acid motifs of urocortin II and III determine their CRF receptor subtype selectivity. Neuropharmacology 47, 233–242.
Kageyama, K., Bradbury, M. J., Zhao, L., Blount, A. L., and Vale, W. W. (1999a). Urocortin messenger ribonucleic acid: tissue distribution in the rat and regulation in thymus by lipopolysaccharide and glucocorticoids. Endocrinology 140, 5651–5658.
Kageyama, K., Gaudriault, G. E., Bradbury, M. J., and Vale, W. W. (1999b). Regulation of corticotropin-releasing factor receptor type 2 β messenger ribonucleic acid in the rat cardiovascular system by urocortin, glucocorticoids, and cytokines. Endocrinology 141, 2285–2293.
Kageyama, K., Hanada, K., Moriyama, T., Nigawara, T., Sakihara, S., and Suda, T. (2006). G protein-coupled receptor kinase 2 involvement in desensitization of corticotropin-releasing factor (CRF) receptor type 1 by CRF in murine corticotrophs. Endocrinology 147, 441–450.
Kageyama, K., Hasegawa, G., Akimoto, K., Yamagata, S., Tamasawa, N., and Suda, T. (2012). Differential regulation of gonadotropin-releasing hormone by corticotropin-releasing factor family peptides in hypothalamic N39 cells. Peptides 33, 149–155.
Kageyama, K., Li, C., and Vale, W. W. (2003). Corticotropin-releasing factor receptor type 2 messenger ribonucleic acid in rat pituitary: localization and regulation by immune challenge, restraint stress, and glucocorticoids. Endocrinology 144, 1524–1532.
Kim, D. K., Cho, E. B., Moon, M. J., Park, S., Hwang, J. I., Do Rego, J. L., et al. (2012). Molecular coevolution of neuropeptides gonadotropin-releasing hormone and kisspeptin with their cognate G protein-coupled receptors. Front. Neurosci. 6:3. doi: 10.3389/fnins.2012.00003
Kinsey-Jones, J. S., Li, X. F., Bowe, J. E., Lightman, S. L., and O’Byrne, K. T. (2006). Corticotrophin-releasing factor type 2 receptor-mediated suppression of gonadotrophin-releasing hormone mRNA expression in GT1-7 cells. Stress 9, 215–222.
Kinsey-Jones, J. S., Li, X. F., Knox, A. M., Wilkinson, E. S., Zhu, X. L., Chaudhary, A. A., et al. (2009). Down-regulation of hypothalamic kisspeptin and its receptor, Kiss1r, mRNA expression is associated with stress-induced suppression of luteinising hormone secretion in the female rat. J. Neuroendocrinol. 21, 20–29.
Kirby, E. D., Geraghty, A. C., Ubuka, T., Bentley, G. E., and Kaufer, D. (2009). Stress increases putative gonadotropin inhibitory hormone and decreases luteinizing hormone in male rats. Proc. Natl. Acad. Sci. U.S.A. 106, 11324–11329.
Kishimoto, T., Pearse, R. V. II, Lin, C. R., and Rosenfield, M. G. (1995). A sauvagine/corticotropin-releasing factor receptor expressed in heart and skeletal muscle. Proc. Natl. Acad. Sci. U.S.A. 92, 1108–1112.
Knobil, E. (1992). Remembrance: the discovery of the hypothalamic gonadotropin-releasing hormone pulse generator and of its physiological significance. Endocrinology 131, 1005–1006.
Lewis, K., Li, C., Perrin, M. H., Blount, A., Kunitake, K., Donaldson, C., et al. (2001). Identification of urocortin III, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Proc. Natl. Acad. Sci. U.S.A. 98, 7570–7575.
Li, C., Chen, P., Vaughan, J., Lee, K. F., and Vale, W. (2007). Urocortin 3 regulates glucose-stimulated insulin secretion and energy homeostasis. Proc. Natl. Acad. Sci. U.S.A. 104, 4206–4211.
Li, X. F., Bowe, J. E., Kinsey-Jones, J. S., Brain, S. D., Lightman, S. L., and O’Byrne, K. T. (2006). Differential role of corticotrophin-releasing factor receptor types 1 and 2 in stress-induced suppression of pulsatile luteinising hormone secretion in the female rat. J. Neuroendocrinol. 18, 602–610.
Li, X. F., Kinsey-Jones, J. S., Cheng, Y., Knox, A. M., Lin, Y., Petrou, N. A., et al. (2009). Kisspeptin signalling in the hypothalamic arcuate nucleus regulates GnRH pulse generator frequency in the rat. PLoS ONE 4:e8334. doi: 10.1371/journal.pone.0008334
Li, X. F., Knox, A. M., and O’Byrne, K. T. (2010). Corticotrophin-releasing factor and stress-induced inhibition of the gonadotrophin-releasing hormone pulse generator in the female. Brain Res. 1364, 153–163.
Li, X. F., Lin, Y. S., Kinsey-Jones, J. S., Milligan, S. R., Lightman, S. L., and O’Byrne, K. T. (2011). The role of the bed nucleus of the stria terminalis in stress-induced inhibition of pulsatile luteinising hormone secretion in the female rat. J. Neuroendocrinol. 23, 3–11.
Lin, Y. S., Li, X. F., Shao, B., Hu, M. H., Goundry, A. L., Jeyaram, A., et al. (2012). The role of GABAergic signalling in stress-induced suppression of gonadotrophin-releasing hormone pulse generator frequency in female rats. J. Neuroendocrinol. 24, 477–488.
Lovenberg, T. W., Chalmers, D. T., Liu, C., and De Souza, E. B. (1995a). CRF 2 α and CRF2 β receptor mRNAs are differentially distributed between the rat central nervous system and peripheral tissues. Endocrinology 136, 4139–4142.
Lovenberg, T. W., Liaw, C. W., Grigoriadis, D. E., Clevenger, W., Chalmers, D. T., De Souza, E. B., et al. (1995b). Cloning and characterization of a functionally distinct corticotropin-releasing factor receptor subtype from rat brain. Proc. Natl. Acad. Sci. U.S.A. 92, 836–840.
Magiakou, M. A., Mastorakos, G., Webster, E., and Chrousos, G. P. (1997). The hypothalamic–pituitary–adrenal axis and the female reproductive system. Ann. N. Y. Acad. Sci. 816, 42–56.
Messager, S., Chatzidaki, E. E., Ma, D., Hendrick, A. G., Zahn, D., Dixon, J., et al. (2005). Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc. Natl. Acad. Sci. U.S.A. 102, 1761–1766.
Miller, W. J., Suzuki, S., Miller, L. K., Handa, R., and Uht, R. M. (2004). Estrogen receptor (ER) beta isoforms rather than ERalpha regulate corticotropin-releasing hormone promoter activity through an alternate pathway. J. Neurosci. 24, 10628–10635.
Mouri, T., Itoi, K., Takahashi, K., Suda, T., Murakami, O., Yoshinaga, K., et al. (1993). Colocalization of corticotropin-releasing factor and vasopressin in the paraventricular nucleus of the human hypothalamus. Neuroendocrinology 57, 34–39.
Nakano, Y., Suda, T., Sumitomo, T., Tozawa, F., and Demura, H. (1991). Effects of sex steroids on beta-endorphin release from rat hypothalamus in vitro. Brain Res. 553, 1–3.
Nemoto, T., Iwasaki-Sekino, A., Yamauchi, N., and Shibasaki, T. (2007). Regulation of the expression and secretion of urocortin 2 in rat pituitary. J. Endocrinol. 192, 443–452.
Nemoto, T., Iwasaki-Sekino, A., Yamauchi, N., and Shibasaki, T. (2010). Role of urocortin 2 secreted by the pituitary in the stress-induced suppression of luteinizing hormone secretion in rats. Am. J. Physiol. Endocrinol. Metab. 299, E567–E575.
Nemoto, T., Mano, A., and Shibasaki, T. (2012). Increased expression of miR-325-3p by urocortin 2 and its involvement in stress-induced suppression of LH secretion in rat pituitary. Am. J. Physiol. Endocrinol. Metab. 302, E781–E787.
Nemoto, T., Yamauchi, N., and Shibasaki, T. (2009). Novel action of pituitary urocortin 2 in the regulation of expression and secretion of gonadotropins. J. Endocrinol. 201, 105–114.
Nomura, M., McKenna, E., Korach, K. S., Pfaff, D. W., and Ogawa, S. (2002). Estrogen receptor-beta regulates transcript levels for oxytocin and arginine vasopressin in the hypothalamic paraventricular nucleus of male mice. Brain Res. Mol. Brain Res. 109, 84–94.
Oakley, A. E., Clifton, D. K., and Steiner, R. A. (2009). Kisspeptin signaling in the brain. Endocr. Rev. 30, 713–743.
Ochedalski, T., Subburaju, S., Wynn, P. C., and Aguilera, G. (2007). Interaction between oestrogen and oxytocin on hypothalamic–pituitary–adrenal axis activity. J. Neuroendocrinol. 19, 189–197.
Ogura, E., Kageyama, K., Hanada, K., Kasckow, J., and Suda, T. (2008). Effects of estradiol on regulation of corticotropin-releasing factor gene and interleukin-6 production via estrogen receptor type beta in hypothalamic 4B cells. Peptides 29, 456–464.
Ohkura, S., Uenoyama, Y., Yamada, S., Homma, T., Takase, K., Inoue, N., et al. (2009). Physiological role of metastin/kisspeptin in regulating gonadotropin-releasing hormone (GnRH) secretion in female rats. Peptides 30, 49–56.
Perrin, M., Donaldson, C., Chen, R., Blount, A., Berggren, T., Bilezikjian, L., et al. (1995). Identification of a second CRF receptor gene and characterization of a cDNA expressed in heart. Proc. Natl. Acad. Sci. U.S.A. 92, 2969–2973.
Petraglia, F., Sutton, S., Vale, W., and Plotsky, P. (1987). Corticotropin-releasing factor decreases plasma luteinizing hormone levels in female rats by inhibiting gonadotropin-releasing hormone release into hypophysial-portal circulation. Endocrinology 120, 1083–1088.
Potter, E., Sutton, S., Donaldson, C., Chen, R., Perrin, M., Lewis, K., et al. (1994). Distribution of corticotropin-releasing factor receptor mRNA expression in the rat brain and pituitary. Proc. Natl. Acad. Sci. U.S.A. 91, 8777–8781.
Pozzoli, G., Bilezikjian, L. M., Perrin, M. H., Blount, A. L., and Vale, W. W. (1996). Corticotropin-releasing factor (CRF) and glucocorticoids modulate the expression of type 1 CRF receptor messenger ribonucleic acid in rat anterior pituitary cell cultures. Endocrinology 137, 65–71.
Reyes, T. M., Lewis, K., Perrin, M. H., Kunitake, K. S., Vaughan, J., Arias, C. A., et al. (2001). Urocortin II: a member of the corticotropin-releasing factor (CRF) neuropeptide family that is selectively bound by type 2 CRF receptors. Proc. Natl. Acad. Sci. U.S.A. 98, 2843–2848.
Rivier, C., Rivier, J., and Vale, W. (1986). Stress-induced inhibition of reproductive functions: role of endogenous corticotropin-releasing factor. Science 231, 607–609.
Rivier, C., and Vale, W. (1984). Influence of corticotropin-releasing factor on reproductive functions in the rat. Endocrinology 114, 914–921.
Sakai, K., Horiba, N., Sakai, Y., Tozawa, F., Demura, H., and Suda, T. (1996). Regulation of corticotropin-releasing factor receptor messenger ribonucleic acid in rat anterior pituitary. Endocrinology 137, 1758–1763.
Stenzel, P., Kesterson, R., Yeung, W., Cone, R. D., Rittenberg, M. B., and Stenzel-Poore, M. P. (1995). Identification of a novel murine receptor for corticotropin-releasing hormone expressed in the heart. Mol. Endocrinol. 9, 637–645.
Suda, T., Kageyama, K., Sakihara, S., and Nigawara, T. (2004). Physiological roles of urocortins, human homologues of fish urotensin 1, and their receptors. Peptides 25, 1689–1701.
Tilbrook, A. J., Turner, A. I., and Clarke, I. J. (2002). Stress and reproduction: central mechanisms and sex differences in non-rodent species. Stress 5, 83–100.
Traslaviña, G. A., and Franci, C. R. (2012). Divergent roles of the CRH receptors in the control of gonadotropin secretion induced by acute restraint stress at proestrus. Endocrinology 153, 4838–4848.
Ubuka, T., Kim, S., Huang, Y. C., Reid, J., Jiang, J., Osugi, T., et al. (2008). Gonadotropin-inhibitory hormone neurons interact directly with gonadotropin-releasing hormone-I and -II neurons in European starling brain. Endocrinology 149, 268–278.
Vale, W., Spiess, J., Rivier, C., and Rivier, J. (1981). Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science 213, 1394–1397.
Vale, W., Vaughan, J., and Perrin, M. H. (1997). Corticotropin-releasing factor (CRF) family ligands and their receptors. Endocrinologist 7, 3S–9S.
Vamvakopoulos, N. C., and Chrousos, G. P. (1993). Evidence of direct estrogenic regulation of human corticotropin-releasing hormone gene expression. Potential implications for the sexual dimorphism of the stress response and immune/inflammatory reaction. J. Clin. Invest. 92, 1896–1902.
Vaughan, J., Donaldson, C., Bittencourt, J., Perrin, M. H., Lewis, K., Sutton, S., et al. (1995). Urocortin, a mammalian neuropeptide related to fish urotensin I and to corticotropin-releasing factor. Nature 378, 287–292.
Vita, N., Laurent, P., Lefort, S., Chalon, P., Lelias, J. M., Kaghad, M., et al. (1993). Primary structure and functional expression of mouse pituitary and human brain corticotrophin releasing factor receptors. FEBS Lett. 335, 1–5.
Whitnall, M. H. (1993). Regulation of the hypothalamic corticotropin-releasing hormone neurosecretory system. Prog. Neurobiol. 40, 573–629.
Wynn, P. C., Harwood, J. P., Catt, K. J., and Aguilera, G. (1985). Regulation of corticotropin-releasing factor (CRF) receptors in the rat pituitary gland: effects of adrenalectomy on CRF receptors and corticotroph responses. Endocrinology 116, 1653–1659.
Keywords: corticotropin-releasing factor, urocortin, stress, gonadotropin
Citation: Kageyama K (2013) Regulation of gonadotropins by corticotropin-releasing factor and urocortin. Front. Endocrin. 4:12. doi: 10.3389/fendo.2013.00012
Received: 09 May 2012; Accepted: 30 January 2013;
Published online: 20 February 2013.
Edited by:
Hubert Vaudry, University of Rouen, FranceReviewed by:
David Lovejoy, University of Toronto, CanadaJae Young Seong, Korea University, South Korea
Copyright: © 2013 Kageyama. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in other forums, provided the original authors and source are credited and subject to any copyright notices concerning any third-party graphics etc.
*Correspondence: Kazunori Kageyama, Department of Endocrinology and Metabolism, Hirosaki University Graduate School of Medicine, 5 Zaifu-cho, Hirosaki, Aomori 036-8562, Japan. e-mail: kkageyama@hkg.odn.ne.jp
