- 1Queen’s Medical Centre, School of Life Sciences, University of Nottingham Medical School, Nottingham, UK
- 2Division of Nutritional Sciences, School of Biosciences, University of Nottingham, Loughborough, UK
The vgf gene (non-acronymic) is highly conserved and was identified on the basis of its rapid induction in vitro by nerve growth factor, although can also be induced by brain-derived neurotrophic factor, and glial-derived growth factor. The VGF gene gives rise to a 68 kDa precursor polypeptide, which is induced robustly, relatively selectively and is synthesized exclusively in neuronal and neuroendocrine cells. Post-translational processing by neuroendocrine specific prohormone convertases in these cells results in the production of a number of smaller peptides. The VGF gene and peptides are widely expressed throughout the brain, particularly in the hypothalamus and hippocampus, in peripheral tissues including the pituitary gland, the adrenal glands, and the pancreas, and in the gastrointestinal tract in both the myenteric plexus and in endocrine cells. VGF peptides have been associated with a number of neuroendocrine roles, and in this review, we aim to describe these roles to highlight the importance of VGF as therapeutic target for a number of disorders, particularly those associated with energy metabolism, pain, reproduction, and cognition.
Introduction
VGF (non-acronymic) is a neurotrophin-induced gene, which was first identified as VGF8a, NGF33.1, and a2 on the basis of its rapid induction in PC12 cells treated with nerve growth factor (NGF) (1–3). Subsequent studies demonstrated that VGF is similarly upregulated by numerous neurotrophins, including brain-derived neurotrophic factor (BDNF) and neurotrophin-3 (NT-3), in neuronal targets such as cortical or hippocampal neurons (4). However, VGF mRNA levels are only marginally increased by other growth factors including epidermal growth factor (EGF), fibroblast growth factor (FGF), interleukin-6 (IL-6), and insulin, despite the capacity of these proteins to robustly induce transcription of other immediate early genes in the PC12 cell line (3, 5, 6).
The VGF polypeptide, which is robustly and exclusively synthesized in neuronal and neuroendocrine cells (1, 3, 7, 8), is processed by the prohormone convertases (PC), PC1/3 and PC2 (9). VGF derived peptides with specific neuronal bioactivities include TLQP-62, TLQP-21, HHPD-41, AQEE-30, AQEE-11, LQEQ-19, and neuroendocrine regulatory peptides-1 and -2 (NERP-1 and -2; 9–11). Studies have shown that TLQP-62 and AQEE-30 increase the firing rate of hippocampal neurons, induce neurogenesis, and have anti-depressive properties (4, 12, 13), whereas HHPD-41, AQEE-30, AQEE-11, and LQEQ-19 stimulate sympathetic outflow and facilitate penile erection in rats (14–16); and TLQP-21 and NERP-2 regulate energy balance (17–20). Furthermore, TLQP-21 regulates contractile activity in the gastrointestinal tract, has analgesic properties, reduces neuronal apoptosis in vitro and decreases rodent blood pressure (21–23) and NERP-1 and -2 regulate water homeostasis and suppress vasopressin release (11, 20, 24). Here, we review the regulation of VGF and the neuroendocrine role of its derived peptides.
The Transcriptional Regulation of VGF
In vitro
The gene itself is highly conserved among mammalian species in respect to the coding region and the promoter sequence (25). The VGF promoter region contains a CCAAT box, various specificity protein 1 (SP-1), and activating protein 2 (AP-2) sites and a silencer element similar to the one involved in tissue-specific expression of neuronal genes (3, 25). Furthermore, it contains a cyclic AMP response element (CRE), which is embedded within a 14bp palindromic sequence, mutations of which abolish NGF and cAMP responses (6). VGF expression in response to neurotrophins that requires the combined actions of several regulator complexes; in addition to the CRE, the CCAAT box was shown to be important for NGF induction (26), possibly in association with the activity of a large complex containing a CRE binding protein (CREB), mammalian achaete-scute homolog-1 (MASH-1), and p300 (27).
In vivo
A genomic fragment extending from 800-bp 5’ to the transcriptional start site and including the first 700-bp of 5’-untranslated sequence results in reporter gene expression in a tissue-restricted pattern similar to that of the endogenous VGF gene (28). Interestingly, this region of the promoter contains a putative silencer element that is located 400-bp 5’ to the transcriptional start site, which prevents expression in non-neuronal cell lines (25). VGF mRNA in the hypothalamus alters in response to feeding/fasting (14, 15, 20), salt loading (29), adrenalectomy (30), and seasonal rhythms (31). Furthermore, VGF mRNA varies in the pituitary during the estrous cycle (32) and in the suprachiasmatic nucleus (SCN) according to circadian rhythmicity (33); while gastric damage increases VGF mRNA in the nucleus tractus solitarius (NTS) and dorsomedial nucleus of the vagus (34). VGF mRNA is also modulated in other diverse conditions, which have been well described elsewhere (35).
The Structure and Processing of the VGF Polypeptide
VGF is a 68 kDa polypeptide comprising 615 (human) or 617 (mouse/rat) amino acids with a typical secretory leader sequence of 22 amino acids at the N-terminal of VGF, which promotes translocation to the endoplasmic reticulum (ER) (36). Subsequent sequencing of the polypeptide in the mouse, horse, and bovine has confirmed extensive sequence conservation with approximately >85% identity (35). The most prominent VGF-derived peptides have apparent molecular masses of 20 (NAPP-129) and 10 kDa (TLQP-62), respectively (9) (Figure 1). However, the mouse and human sequences contain a minimum of 10 conserved regions of basic amino acid residues, which represent potential PC cleavage sites (37) (Figure 2). Indeed cleavage at the Arg-Pro-Arg555 sequence in the rat has been shown to give rise to the TLQP peptides (9). It is possible, however, that the number and function of VGF derived peptides are greater than currently known (38). The extensive review by Ferri et al. (35) describes this in more detail.
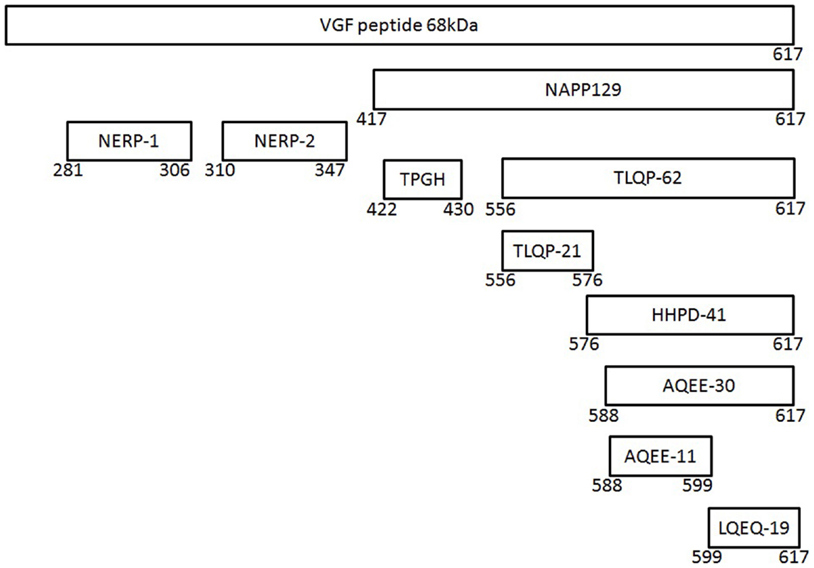
Figure 1. The vgf gene and its derived peptides. The VGF polypeptide is the precursor of several biologically active peptides, which are released and play a role in intercellular communication. The gene contains a number of specific sequences, which are highly conserved between the species and these represent potential cleavage sites for the convertases of the kexin/subtilisin-like series proteinases family, namely prohormone convertases-1/3 and -2.

Figure 2. Comparison of the human and mouse VGF polypeptide sequences. * indicates the position which have a single, fully conserved residue. “:” indicates conservation between groups of strongly similar properties scoring >0.5 in the Gonnect PAM 250 matrix. “ . ” indicates conservation between groups of weakly similar properties scoring <0.5 in the Gonnect PAM 250 matrix. Clusters of basic amino acids, which represent potential cleavage sites, are boxed. Sequence identity was >85%.
Distribution of VGF and Its Derived Peptides
VGF mRNA
VGF mRNA is widely expressed throughout the nervous system. During embryogenesis VGF mRNA is expressed in distinct neurotrophin-responsive targets in the central and peripheral nervous system (CNS and PNS, respectively) in the rat (39, 40). At birth, VGF mRNA is expressed in neurons throughout the brain and in peripheral endocrine and neuroendocrine tissues. While in the adult brain VGF mRNA has the highest expression in the hypothalamus and the granular layer of the cerebellum, it is also expressed in a number of other brain areas including the main and accessory olfactory bulbs, hippocampus, cortex, basal ganglia, thalamus, amygdala, midbrain, and the brainstem. Within the hypothalamus, the highest concentrations of VGF mRNA have been found in the ventromedial hypothalamus, in particular the arcuate nucleus (ARC), as well as in the SCN (7, 39, 41). VGF mRNA expression in the mouse is similar to the rat (14).
VGF Peptides
VGF and its derived peptides are found in dense core vesicles and are released in response to depolarizing signals from neuronal and neuroendocrine cells through the regulated secretory pathway (10, 42, 43). Antibodies raised to synthetic peptides corresponding to the C- or N-termini of potential or actual cleavage products have been utilized to study VGF derived peptide distribution. In animal tissues, VGF immunoreactivity was restricted to central and peripheral neurons (41, 44), as well as to endocrine cells of the pituitary, adrenal medulla, gut, and pancreas (44). The highest concentrations of VGF immunoreactivity have correspondingly been found in the medial hypothalamus, particularly in the ARC, in the SCN, and in the parvocellular and magnocellular cells of the paraventricular nucleus (PVN) and the supraoptic nucleus (SON) (41). Weak immunoreactivity was also detected in the hippocampus, amygdala, thalamus, and cerebral cortex (41). VGF immunoreactivity was also displayed in the female rat in the pars distalis, mainly with C-terminal antibodies (32). This, however, disappeared in accordance with the estrous peak of luteinizing hormone (LH) secretion, along with an induction of VGF mRNA in the pituitary. Additionally, VGF-derived peptides are prominent in the adult spinal cord, in α- and γ-motor neurons of the ventral horn and in the dorsal horn neurons, as well as cells of the inner nuclear and ganglion cell layers of the retina (45). In the PNS, both sympathetic ganglia and dorsal root ganglia of primary sensory neurons are important sites of localization of VGF-derived peptides (40). This is comparable to the expression of neuropeptide Y (NPY), ghrelin, and cholecystokinin (CCK), all of which regulate feeding and in some cases, gastrointestinal motility (46, 47). VGF-derived peptides are also present in mouse brown adipose tissue (BAT), where they are reduced in response to a high fat diet (HFD) (48).
VGF Receptors
Of all the VGF derived peptides, TLQP-21 has had the most interest (17, 19, 23, 49–52). Previously, TLQP-21 was shown to bind to adipocyte membranes in a saturable manner, (53) and atomic force microscopy of living cells revealed the existence of a single class of binding sites for TLQP-21 (54). Taken together these results suggested a cell surface receptor for TLQP-21. Two possible receptors have recently been identified for TLQP-21. Chen et al. (55) identified gC1qR, showing that TLQP-21 activated rat macrophages through gC1qR, which then caused mechanical hypersensitivity in rats. gC1qR protein was expressed by both brain and spinal cord derived microglia (55) and is indispensable for adipogenesis and insulin signaling (56). Furthermore, obese mice fed a HFD demonstrated increased density of TLQP-21 binding in adipose tissues (54). However, neither TLQP-62 nor LQEQ-19 elicited a response in their experimental model, both of which had been previously implicated in pain processing (22, 57). This supports the hypothesis of different receptors for the VGF derived peptides. Hannedouche et al. (58) reported the complement receptor, C3A receptor-1 (C3AR1), as a receptor for TLQP-21, which mediated activity for TLQP-21 in two different rodent cell lines. C3AR1 was originally thought to be restricted to the innate immune response, its role limited to the complement cascade. However, it has subsequently been shown to have a role in cancer (59), neurogenesis (60), and hormone release from the pituitary gland (61). However, C3AR1-/- mice are transiently resistant to diet-induced obesity (DIO) and are protected against HFD-induced insulin resistance (62). The discovery of these receptors will help identify the mechanisms by which TLQP-21 and possible other derived peptide may modulate its actions.
Physiological Roles of VGF Gene and Derived Peptides
Energy Balance
The high expression of VGF in the hypothalamus and the change in expression of the vgf gene in the ARC following acute altered energy balance first suggested the importance of VGF in the regulation of energy balance (14, 20). Indeed fasting has been shown to increase VGF mRNA expression, while administration of leptin prevents the fasting induced increase in VGF mRNA (15). These changes in VGF can be observed in models of chronic energy imbalance; VGF mRNA levels resemble that of fasted wild-type mice in the ARC of the leptin deficient ob/ob mouse and in the leptin resistance db/db mouse (15). It is well known that the ARC has two neuronal populations that respond to the fed and fasted state as well as to leptin signaling, the pro-opiomelanocortin (POMC) and neuropeptide Y (NPY) neurons (63). VGF immunoreactivity has been shown to be co-localized with both these neuronal populations in the ARC, however, expression is modulated with energy state. In the ad libitum fed state and re-fed animals, VGF mRNA is co-localized with POMC (15, 64). On the other hand, fasting increases co-localization of VGF in the NPY neurons (64).
Energy balance and lack of functional VGF
The function of VGF and its extension derived peptides was first assessed through the development of mice lacking a functional copy of the vgf gene (VGF-/-) via homologous recombination (14). At birth, the homozygous VGF-/- mice are indistinguishable from either their heterozygous or wild-type littermates. No defects in development were detected in either the CNS or the PNS. However, in the weeks following birth, the VGF-/- mice were visibly smaller than their wild-type littermates and adults were found to weigh 50-70% less due to a 50% reduction in adiposity compared to wild-type littermates (14). Consistent with the reduction in adiposity, leptin levels, serum glucose and insulin levels, and liver glycogen were reduced (48). The mice consumed considerably more calories per gram body weight, but this increase in food intake was not sufficient to maintain the same body weight as wild-type mice. The VGF-/- mice utilized twice as much oxygen at rest and displayed increased locomotor activity compared to wild-type littermates (14). Overall, the major change in VGF-/- mice is an increase in energy consumption; indeed vgf gene deletion did not block obesity via monosodium glutamate administration (15) suggesting that the thermogenic pathways resulting in the VGF-/- phenotype are blocked. These initial observations led Hahm et al. (14) to suggest that VGF may play a non-redundant role in the regulation of energy homeostasis and antagonism of the gene may constitute a basis for the treatment of obesity. Furthermore, vgf gene deletion blocked the development of obesity as a result of a HFD, gold thioglucose treatment, as well as in the agouti mouse, and suggesting that VGF functions in outflow pathways regulating energy expenditure downstream of the hypothalamic melanocortin receptors (15).
Energy balance and VGF-derived peptides
Thus from the phenotype of the VGF-/- mice one might predict that VGF promotes an anabolic drive. Surprisingly, this view has not been supported by subsequent studies in mice and Siberian hamsters. Chronic intracerebroventricular (ICV) infusion of TLQP-21 in mice fed a normal lab chow resulted in a small increase in resting energy expenditure and rectal temperature (17). The changes in metabolic parameters were mirrored by increased epinephrine content in BAT, upregulation of BAT β2-adrenergic receptor (AR), uncoupling protein 1 (UCP-1) mRNA, higher expression of peroxisome proliferator-activated receptor-δ (PPAR-δ), and β3-AR in white adipose tissue (WAT). However, hypothalamic expressions of agouti-related protein (AgRP), NPY, α-melanocyte-stimulating hormone (α-MSH), POMC, and corticotrophin-releasing hormone (CRH) were unchanged (17). In mice, switched to a HFD treatment with TLQP-21 halted the expected increase in body weight and WAT, attenuated rises in leptin, and normalized ghrelin levels (17). In rats, ICV infusion of TLQP-21 significantly decreased gastric emptying, an effect that was blocked by ICV infusion of indomethacin, which blocks prostaglandin release (65).
A similar catabolic effect was noted in Siberian hamsters, a seasonal model of energy balance. Not only is VGF mRNA significantly increased in the winter weight-loss state in the dorsal medial posterior arcuate nucleus (dmpArc) (31) but ICV infusion of TLQP-21 at the onset of the dark phase was found to significantly and dose dependently decrease food intake and body weight (19). However, there was no effect on energy expenditure as Siberian hamsters pair-fed to the treated group lost a similar amount of body weight (19). Weight loss was, therefore, attributable to reduced caloric intake rather than energy expenditure.
One of the possible explanations for this contradiction between the functional in vivo studies and the VGF-/- mice, where all the VGF peptides have been ablated, is that some of these peptides may have opposing roles in energy balance. Interestingly, Bartolomucci et al. (66) have suggested that HHPD-41 increased food intake following ICV infusion, and more recently ICV infusion of NERP-2 in rats has been shown to increase food intake, body temperature, oxygen consumption, and locomotor activity (20). Furthermore, intravenous administration of NERP-2 significantly augmented glucose stimulated insulin secretion in anesthetized rats or following intraperitoneal injection to conscious mice (67). Thus VGF may have a biphasic role in the regulation of energy balance and further characterization of the other VGF-derived peptides is required.
Energy balance and circadian rhythm
It is well known that food intake and energy metabolism in mammals are regulated by their circadian clock, and food intake is one such signal that can entrain the circadian clock (68). As previously described, VGF is expressed in the SCN, the circadian pacemaker in animals, while the E-box contained in the vgf gene promoter region is similar to the many clock genes such as the per gene (33). Therefore, it is not unexpected that the vgf gene exhibits circadian rhythm in the SCN even under constant dark conditions, while VGF mRNA levels are increased in response to light simulation in the SCN when light would be expected to cause a phase shift in locomotor rhythms (33). Indeed VGF-/- mice can maintain circadian rhythm of wheel running in constant darkness, however, the period length was found to be slightly but significantly shorter than wild-type littermates (14). Thus, this raises the question could the metabolic phenotype of the VGF-/- mice be attributed, in part, to the disruption of the circadian system.
VGF and Water Balance
Water deprivation and salt loading in rats increases VGF mRNA levels in both the SON and PVN, along with vasopressin mRNA (29). ICV injection of NERP-1 and NERP-2 suppresses hypertonic saline or angiotensin II induced increases in plasma vasopressin in rats (69). Additionally, ICV infusion of NERP-1 and -2 attenuated the increase in vasopressin as a result of water deprivation in rats, an effect which was reversed following immunoneutralisation by ICV infusion of anti-NERP-1 and -2 antibodies (69). Taken together, these data suggest that NERP-1 and -2 may be involved in the central control of body fluid balance.
VGF and Reproduction
The role of VGF signaling in reproduction was inferred from the observation that VGF gene deletion resulted in infertility in both male and female mice (14). In male VGF-/- mice, the onset of puberty and sexual maturation was delayed, and the weights of the testes, albeit having mobile spermatozoa in the lumen, were significantly lower than those of wild-type littermates (14). While in the female VGF-/- mice histological examination revealed no mature follicles or corpus lutea, and the ovaries, ovidut, and uteri weighed 30% less than those of the wild-type littermates (14). However, transplanting ovaries from VGF-/- mice into ovariectomized wild-type females restored fertility, suggesting that the reproductive deficits of VGF-/- mice were not the result of pathology but arose from deficits in the hypothalamic-pituitary-gonadal axis (14). However, Ferri et al. (32) showed that VGF gene expression varied during estrous; there was an increase in VGF mRNA and VGF peptide/s degranulation, suggesting perturbation of anterior pituitary function.
It is common knowledge that alterations in energy metabolism and fat stores can affect reproductive function. VGF-/- mice have reduced leptin and altered energy status, therefore, it could be suggested that the deficit may be due to gonadotropin releasing hormone (GnRH) synthesis or secretion. However, while GnRH levels are not affected, LH and follicle-stimulating hormone (FSH) mRNA levels were reduced in VGF-/- mice (14) suggesting decreased GnRH secretion. Indeed it has been shown that central administration of TLQP-21 in female rats during the pubertal transition advanced the timing of vaginal opening and increased the number of animals with signs of ovulation (70). These effects of TLQP-21 may be via stimulation of the GnRH release, as TLQP-21 has been shown to induce LH secretion in vitro (71). Furthermore, Pinilla et al. (71) have shown that chronic administration of TLQP-21 was able to prevent the hypogonadotropic state induced by food deprivation.
There is further evidence of VGF peptides and a possible role in the regulation of reproduction. While HHPD-41, AQEE-30, and LQEQ-19 have been shown to induce penile erection in rats following infusion into the PVN in a dose dependent manner, NERP-1 has a pro-erectile effect when injected into the lateral ventricles or the ARC of rats (72). The effect on penile erection is thought to be via nitric oxide mediated activation of oxytocinergic pathways (16).
VGF and Pain
VGF is a gene commonly upregulated in sensory neurons in clinically relevant models of neuropathic pain, namely, varicella zoster infection, HIV-associated neuropathy, and peripheral nerve trauma (55). Furthermore, VGF has been shown to be upregulated in the dorsal root ganglia and spinal cord in a number of neuropathic and inflammatory pain models (22, 57, 73–76). In these areas, VGF is co-localized with substance P, calcitonin gene related peptide, TrkA, and P2 × 3 (22, 51). A functional role for VGF-derived peptides has been identified in pain pathways. Indeed intrathecal infusion of TLQP-62 results in cold behavioral hypersensitivity in rats; while injection of TLQP-21 into the hind paw of mice resulted in hypersensitivity in both control animals and the formalin model of inflammatory pain (51) as well as inducing thermal hyperalgesia in the warm-water immersion tail-withdrawal test (77). Additionally, both LQEQ-19 and AQEE-30 have been shown to induce p38 MAP kinase phosphorylation in spinal microglia (22), suggesting that VGF-derived peptides have pro-nociceptive and hyperalgesic functions.
VGF and Memory and Learning
As previously stated VGF mRNA is expressed in the hippocampus, and it has been shown that VGF transcription is accompanied by translation within 3 hours of BDNF exposure in hippocampal slices in vitro (4). Additionally, VGF mRNA has been shown to be upregulated by activities, such as memory and learning (8), while VGF-/- mice have demonstrated impaired hippocampal-dependent spatial learning and contextual fear conditioning tasks (78). Indeed more recently, TLQP-62 has been shown to induce transient potentiation in hippocampal slices (78), enhance synaptic activity (4), and increase neurogenesis in early phase neural progenitor cells in the adult hippocampus (12, 79), as well as shown to have effect on cognitive mechanism (80), thus suggesting that VGF may be important in memory processes.
To further support this notion, proteomic studies have demonstrated a reduction in VGF-derived peptides in the cerebrospinal fluid of patients affected by Alzheimer’s disease (AD) (81–83). Similarly, there was a reduction in VGF-derived peptides in the parietal cortex of AD patients (84) and a reduction in TPGH and NERP-1 in the parietal cortex of Parkinson’s disease patients (84).
VGF and Depression
VGF protein expression is reduced in both the learned helplessness and forced swim test depression paradigms (85), while VGF is increased by antidepressant drugs and voluntary exercise (12). Exercise regulates VGF mRNA and protein expression in the rodent hippocampus and induces an antidepressant response; an opposing phenotype is observed in the heterozygous VGF-/+ mouse (86). Recently, inhibition of phosphodiesterase-4 or -5 was shown to result in increases in cAMP, activating CREB, BDNF, and VGF, which produces antidepressant-like effects on behavior in mice (87). Similarly, microinjection of TLQP-62 into the hippocampal CA1 regions demonstrated antidepressant-like behavioral effects in mice (88), possibly via a BDNF-dependent mechanism (78).
Conclusion
The evidence presented in this review indicates that the gene and gene product have a key neuroendocrine role and that VGF or its derived peptides may act as biomarkers or therapeutic targets in a number of disorders such as obesity, dementia, depression, and pain. The mechanisms by which VGF and its derived peptides are involved remains to be identified, however, the discovery of the new receptors will help advancements in this area both in vitro and in vivo.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Professor Francis Ebling and Dr. Perry Barrett for their continued support.
References
1. Levi A, Eldridge JD, Paterson BM. Molecular cloning of a gene sequence regulated by nerve growth factor. Science (1985) 229:393–5. doi: 10.1126/science.3839317
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
2. Cho KO, Skarnes WC, Minsk B, Palmieri S, Jackson-Grusby L, Wagner JA. Nerve growth factor regulates gene expression by several distinct mechanisms. Mol Cell Biol (1989) 9:135–43.
3. Salton SR, Fischberg DJ, Dong KW. Structure of the gene encoding VGF, a nervous system-specific mRNA that is rapidly and selectively induced by nerve growth factor in PC12 cells. Mol Cell Biol (1991) 11:2335–49.
4. Alder J, Thakker-Varia S, Bangasser DA, Kuroiwa M, Plummer MR, Shors TJ. Brain-derived neurotrophic factor-induced gene expression reveals novel actions of VGF in hippocampal synaptic plasticity. J Neurosci (2003) 23:10800–8.
5. Possenti R, Di Rocco G, Nasi S, Levi A. Regulatory elements in the promoter region of vgf, a nerve growth factor-inducible gene. Proc Natl Acad Sci U S A (1992) 89:3815–9. doi:10.1073/pnas.89.9.3815
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
6. Hawley RJ, Scheibe RJ, Wagner JA. NGF induces the expression of the VGF gene through a cAMP response element. J Neurosci (1992) 12:2573–81.
7. van den Pol AN, Bina K, Decavel C, Ghosh P. VGF expression in the brain. J Comp Neurol (1994) 347:455–69. doi:10.1002/cne.903470311
8. Snyder SE, Cheng HW, Murray KD, Isackson PJ, McNeill TH, Salton SR. The messenger RNA encoding VGF, a neuronal peptide precursor, is rapidly regulated in the rat central nervous system by neuronal activity, seizure and lesion. Neuroscience (1998) 82:7–19. doi:10.1016/S0306-4522(97)00280-7
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
9. Trani E, Giorgi A, Canu N, Amadoro G, Rinaldi AM, Halban PA. Isolation and characterization of VGF peptides in rat brain. Role of PC1/3 and PC2 in the maturation of VGF precursor. J Neurochem (2002) 81:565–74. doi:10.1046/j.1471-4159.2002.00842.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
10. Possenti R, Eldridge JD, Paterson BM, Grasso A, Levi A. A protein induced by NGF in PC12 cells is stored in secretory vesicles and released through the regulated pathway. EMBO J (1989) 8:2217–23.
11. Yamaguchi H, Sasaki K, Satomi Y, Shimbara T, Kageyama H, Mondal MS. Peptidomic identification and biological validation of neuroendocrine regulatory peptide-1 and -2. J Biol Chem (2007) 282(26354–60):M701665200. doi:10.1074/jbc.M701665200
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
12. Thakker-Varia S, Alder J. Neuropeptides in depression: role of VGF. Behav Brain Res (2009) 197:262–78. doi:10.1016/j.bbr.2008.10.006
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
13. Cattaneo A, Sesta A, Calabrese F, Nielsen G, Riva MA, Gennarelli M. The expression of VGF is reduced in leukocytes of depressed patients and it is restored by effective antidepressant treatment. Neuropsychopharmacology (2010) 35:1423–8. doi:10.1038/npp.2010.11
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
14. Hahm S, Mizuno TM, Wu TJ, Wisor JP, Priest CA, Kozak CA. Targeted deletion of the Vgf gene indicates that the encoded secretory peptide precursor plays a novel role in the regulation of energy balance. Neuron (1999) 23:537–48. doi:10.1016/S0896-6273(00)80806-5
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
15. Hahm S, Fekete C, Mizuno TM, Windsor J, Yan H, Boozer CN. VGF is required for obesity induced by diet, gold thioglucose treatment, and agouti and is differentially regulated in pro-opiomelanocortin- and neuropeptide Y-containing arcuate neurons in response to fasting. J Neurosci (2002) 22:6929–38.
16. Succu S, Cocco C, Mascia MS, Melis T, Melis MR, Possenti R. Pro-VGF-derived peptides induce penile erection in male rats: possible involvement of oxytocin. Eur J Neurosci (2004) 20:3035–40. doi:10.1111/j.1460-9568.2004.03781.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
17. Bartolomucci A, La Corte G, Possenti R, Locatelli V, Rigamonti AE, Torsello A. TLQP-21, a VGF-derived peptide, increases energy expenditure and prevents the early phase of diet-induced obesity. Proc Natl Acad Sci U S A (2006) 103:14584–9. doi:10.1073/pnas.0606102103
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
18. Jethwa PH, Warner A, Brameld JM, Keyte JW, Nilaweera NK, Morgan PJ. The role of a VGF derived peptide in the regulation of food intake in a seasonal rodent. Front Neuroendocrinol (2006) 27:5–6. doi:10.1016/j.yfrne.2006.03.012
19. Jethwa PH, Warner A, Nilaweera KN, Brameld JM, Keyte JW, Carter WG. VGF-derived peptide, TLQP-21, regulates food intake and body weight in Siberian hamsters. Endocrinology (2007) 148:4044–55. doi:10.1210/en.2007-0038
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
20. Toshinai K, Yamaguchi H, Kageyama H, Matsuo T, Koshinaka K, Sasaki K. Neuroendocrine regulatory peptide-2 regulates feeding behavior via the orexin system in the hypothalamus. Am J Physiol Endocrinol Metab (2010) 299:E394–401. doi:10.1152/ajpendo.00768.2009
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
21. Severini C, Ciotti MT, Biondini L, Quaresima S, Rinaldi AM, Levi A. TLQP-21, a neuroendocrine VGF-derived peptide, prevents cerebellar granule cells death induced by serum and potassium deprivation. J Neurochem (2008) 104:534–44. doi:10.1111/j.1471-4159.2007.05068.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
22. Riedl MS, Braun PD, Kitto KF, Roiko SA, Anderson LB, Honda CN. Proteomic analysis uncovers novel actions of the neurosecretory protein VGF in nociceptive processing. J Neurosci (2009) 29:13377–88. doi:10.1523/JNEUROSCI.1127-09.2009
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
23. Fargali S, Garcia AL, Sadahiro M, Jiang C, Janssen WG, Lin WJ. The granin VGF promotes genesis of secretory vesicles, and regulates circulating catecholamine levels and blood pressure. FASEB J (2014) 28:2120–33. doi:10.1096/fj.13-239509
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
24. D’Amato F, Cocco C, Noli B, Cabras T, Messana I, Ferri GL. VGF peptides upon osmotic stimuli: changes in neuroendocrine regulatory peptides 1 and 2 in the hypothalamic-pituitary-axis and plasma. J Chem Neuroanat (2012) 44:57–65. doi:10.1016/j.jchemneu.2012.05.001
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
25. Canu N, Possenti R, Rinaldi AM, Trani E, Levi A. Molecular cloning and characterization of the human VGF promoter region. J Neurochem (1997) 68:1390–9. doi:10.1046/j.1471-4159.1997.68041390.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
26. D’Arcangelo G, Habas R, Wang S, Halegoua S, Salton SR. Activation of codependent transcription factors is required for transcriptional induction of the vgf gene by nerve growth factor and Ras. Mol Cell Biol (1996) 16:4621–31.
27. Mandolesi G, Gargano S, Pennuto M, Illi B, Molfetta R, Soucek L. NGF-dependent and tissue-specific transcription of vgf is regulated by a CREB-p300 and bHLH factor interaction. FEBS Lett (2002) 510:50–6. doi:10.1016/S0014-5793(01)03227-6
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
28. Piccioli P, Di Luzio A, Amann R, Schuligoi R, Surani MA, Donnerer J. Neuroantibodies: ectopic expression of a recombinant anti-substance P antibody in the central nervous system of transgenic mice. Neuron (1995) 15:373–84. doi:10.1016/0896-6273(95)90041-1
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
29. Mahata SK, Mahata M, Fischer-Colbrie R, Winkler H. In situ hybridization: mRNA levels of secretogranin II, VGF and peptidylglycine alpha-amidating monooxygenase in brain of salt-loaded rats. Histochemistry (1993) 99:287–93. doi:10.1007/BF00269101
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
30. Mahata SK, Mahata M, Hortnag H, Fischer-Colbrie R, Steiner HJ, Dietze O. Concomitant changes of messenger ribonucleic acid levels of secretogranin II, VGF, vasopressin and oxytocin in the paraventricular nucleus of rats after adrenalectomy and during lactation. J Neuroendocrinol (1993) 5:323–30. doi:10.1111/j.1365-2826.1993.tb00489.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
31. Barrett P, Ross AW, Balik A, Littlewood PA, Mercer JG, Moar KM. Photoperiodic regulation of histamine H3 receptor and VGF messenger ribonucleic acid in the arcuate nucleus of the Siberian hamster. Endocrinology (2005) 146:1930–9. doi:10.1210/en.2004-1452
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
32. Ferri GL, Gaudio RM, Cossu M, Rinaldi AM, Polak JM, Berger P. The “VGF” protein in rat adenohypophysis: sex differences and changes during the estrous cycle and after gonadectomy. Endocrinology (1995) 136:2244–51. doi:10.1210/endo.136.5.7720674
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
33. Wisor JP, Takahashi JS. Regulation of the vgf gene in the golden hamster suprachiasmatic nucleus by light and by the circadian clock. J Comp Neurol (1997) 378:229–38. doi:10.1002/(SICI)1096-9861(19970210)378:2<229::AID-CNE6>3.3.CO;2-6
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
34. Kanemasa K, Okamura H, Kodama T, Ibata Y. Induction of VGF mRNA in neurons of the rat nucleus tractus solitarius and the dorsal motor nucleus of vagus in duodenal ulceration by cysteamine. Brain Res Mol Brain Res (1995) 32:55–62. doi:10.1016/0169-328X(95)00059-2
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
35. Ferri GL, Noli B, Brancia C, D’Amato F, Cocco C. VGF: an inducible gene product, precursor of a diverse array of neuro-endocrine peptides and tissue-specific disease biomarkers. J Chem Neuroanat (2011) 42:249–61. doi:10.1016/j.jchemneu.2011.05.007
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
36. Jethwa PH, Ebling FJP. Role of VGF-derived peptides in the control of food intake, body weight and reproduction. Neuroendocrinology (2008) 88:80–7. doi:10.1159/000127319
37. Salton SR, Ferri GL, Hahm S, Snyder SE, Wilson AJ, Possenti R, et al. VGF: a novel role for this neuronal and neuroendocrine polypeptide in the regulation of energy balance. Front Neuroendocrinology (2000) 21:199–219. doi:10.1006/frne.2000.0199
38. Levi A, Ferri GL, Watson E, Possenti R, Salton SR. Processing, distribution, and function of VGF, a neuronal and endocrine peptide precursor. Cell Mol Neurobiol (2004) 24:517–33. doi:10.1023/B:CEMN.0000023627.79947.22
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
39. Snyder SE, Salton SR. Expression of VGF mRNA in the adult rat central nervous system. J Comp Neurol (1998) 394:91–105. doi:10.1002/(SICI)1096-9861(19980427)394:1<91::AID-CNE7>3.3.CO;2-B
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
40. Snyder SE, Pintar JE, Salton SR. Developmental expression of VGF mRNA in the prenatal and postnatal rat. J Comp Neurol (1998) 394:64–90. doi:10.1002/(SICI)1096-9861(19980427)394:1<64::AID-CNE6>3.0.CO;2-F
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
41. van den Pol AN, Decavel C, Levi A, Paterson B. Hypothalamic expression of a novel gene product, VGF: immunocytochemical analysis. J Neurosci (1989) 9:4122–37.
42. Laslop A, Mahata SK, Wolkersdorfer M, Mahata M, Srivastava M, Seidah NG. Large dense-core vesicles in rat adrenal after reserpine: levels of mRNAs of soluble and membrane-bound constituents in chromaffin and ganglion cells indicate a biosynthesis of vesicles with higher secretory quanta. J Neurochem (1994) 62:2448–56. doi:10.1046/j.1471-4159.1994.62062448.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
43. Benson DL, Salton SR. Expression and polarization of VGF in developing hippocampal neurons. Brain Res Dev Brain Res (1996) 96:219–28. doi:10.1016/0165-3806(96)00108-3
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
44. Ferri GL, Levi A, Possenti R. A novel neuroendocrine gene product: selective VGF8a gene expression and immuno-localisation of the VGF protein in endocrine and neuronal populations. Brain Res Mol Brain Res (1992) 13:139–43. doi:10.1016/0169-328X(92)90053-E
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
45. Zhao Z, Lange DJ, Ho L, Bonini S, Shao B, Salton SR. Vgf is a novel biomarker associated with muscle weakness in amyotrophic lateral sclerosis (ALS), with a potential role in disease pathogenesis. Int J Med Sci (2008) 5:92–9. doi:10.7150/ijms.5.92
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
46. Wren AM, Small CJ, Ward HL, Murphy KG, Dakin CL, Taheri S. The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology (2000) 141:4325–8. doi:10.1210/endo.141.11.7873
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
47. Wren AM, Small CJ, Abbott CR, Jethwa PH, Kennedy AR, Murphy KG. Hypothalamic actions of neuromedin U. Endocrinology (2002) 143:4227–34. doi:10.1210/en.2002-220308
48. Watson E, Fargali S, Okamoto H, Sadahiro M, Gordon RE, Chakraborty T. Analysis of knockout mice suggests a role for VGF in the control of fat storage and energy expenditure. BMC Physiol (2009) 9:19. doi:10.1186/1472-6793-9-19
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
49. Bartolomucci A, Rigamonti AE, Bulgarelli I, Torsello A, Locatelli V, Pavone F. Chronic intracerebroventricular TLQP-21 delivery does not modulate the GH/IGF-1-axis and muscle strength in mice. Growth Horm IGF Res (2007) 17:342–5. doi:10.1016/j.ghir.2007.02.002
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
50. Bartolomucci A, Moles A, Levi A, Possenti R. Pathophysiological role of TLQP-21: gastrointestinal and metabolic functions. Eat Weight Disord (2008) 13:e49–54.
51. Rizzi R, Bartolomucci A, Moles A, D’Amato F, Sacerdote P, Levi A. The VGF-derived peptide TLQP-21: a new modulatory peptide for inflammatory pain. Neurosci Lett (2008) 441:129–33. doi:10.1016/j.neulet.2008.06.018
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
52. Brancia C, Cocco C, D’Amato F, Noli B, Sanna F, Possenti R. Selective expression of TLQP-21 and other VGF peptides in gastric neuroendocrine cells and modulation by feeding. J Endocrinol (2010) 207:329–41. doi:10.1677/JOE-10-0189
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
53. Possenti R, Muccioli G, Petrocchi P, Cero C, Cabassi A, Vulchanova L. Characterization of a novel peripheral pro-lipolytic mechanism in mice: role of VGF-derived peptide TLQP-21. Biochem J (2012) 441:511–22. doi:10.1042/BJ20111165
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
54. Cassina V, Torsello A, Tempestini A, Salerno D, Brogioli D, Tamiazzo L. Biophysical characterization of a binding site for TLQP-21, a naturally occurring peptide which induces resistance to obesity. Biochim Biophys Acta (2013) 1828:455–60. doi:10.1016/j.bbamem.2012.10.023
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
55. Chen YC, Pristera A, Ayub M, Swanwick RS, Karu K, Hamada Y. Identification of a receptor for neuropeptide VGF and its role in neuropathic pain. J Biol Chem (2013) 288:34638–46. doi:10.1074/jbc.M113.510917
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
56. Kim KB, Kim BW, Choo HJ, Kwon YC, Ahn BY, Choi JS. Proteome analysis of adipocyte lipid rafts reveals that gC1qR plays essential roles in adipogenesis and insulin signal transduction. Proteomics (2009) 9:2373–82. doi:10.1002/pmic.200800811
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
57. Moss A, Ingram R, Koch S, Theodorou A, Low L, Baccei M. Origins, actions and dynamic expression patterns of the neuropeptide VGF in rat peripheral and central sensory neurones following peripheral nerve injury. Mol Pain (2008) 4:62. doi:10.1186/1744-8069-4-62
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
58. Hannedouche S, Beck V, Leighton-Davies J, Beibel M, Roma G, Oakeley EJ. Identification of the C3a receptor (C3AR1) as the target of the VGF-derived peptide TLQP-21 in rodent cells. J Biol Chem (2013) 288:27434–43. doi:10.1074/jbc.M113.497214
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
59. Opstal-van Winden AW, Vermeulen RC, Peeters PH, Beijnen JH, van Gils CH. Early diagnostic protein biomarkers for breast cancer: how far have we come? Breast Cancer Res Treat (2012) 134:1–12. doi:10.1007/s10549-011-1907-2
60. Klos A, Tenner AJ, Johswich KO, Ager RR, Reis ES, Kohl J. The role of the anaphylatoxins in health and disease. Mol Immunol (2009) 46:2753–66. doi:10.1016/j.molimm.2009.04.027
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
61. Francis K, Lewis BM, Akatsu H, Monk PN, Cain SA, Scanlon MF. Complement C3a receptors in the pituitary gland: a novel pathway by which an innate immune molecule releases hormones involved in the control of inflammation. FASEB J (2003) 17:2266–8. doi:10.1096/fj.02-1103fje
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
62. Mamane Y, Chung Chan C, Lavallee G, Morin N, Xu LJ, Huang J. The C3a anaphylatoxin receptor is a key mediator of insulin resistance and functions by modulating adipose tissue macrophage infiltration and activation. Diabetes (2009) 58:2006–17. doi:10.2337/db09-0323
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
63. Schwartz MW, Woods SC, Porte D Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature (2000) 404:661–71. doi:10.1038/35007534
64. Saderi N, Buijs FN, Salgado-Delgado R, Merkenstein M, Basualdo MC, Ferri GL. A role for VGF in the hypothalamic arcuate and paraventricular nuclei in the control of energy homeostasis. Neuroscience (2014) 265:184–95. doi:10.1016/j.neuroscience.2014.01.060
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
65. Severini C, La Corte G, Improta G, Broccardo M, Agostini S, Petrella C. In vitro and in vivo pharmacological role of TLQP-21, a VGF-derived peptide, in the regulation of rat gastric motor functions. Br J Pharmacol (2009) 157:984–93. doi:10.1111/j.1476-5381.2009.00192.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
66. Bartolomucci A, Possenti R, Levi A, Pavone F, Moles A. The role of the vgf gene and VGF-derived peptides in nutrition and metabolism. Genes Nutr (2007) 2:169–80. doi:10.1007/s12263-007-0047-0
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
67. Moin AS, Yamaguchi H, Rhee M, Kim JW, Toshinai K, Waise TM. Neuroendocrine regulatory peptide-2 stimulates glucose-induced insulin secretion in vivo and in vitro. Biochem Biophys Res Commun (2012) 428:512–7. doi:10.1016/j.bbrc.2012.10.073
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
68. Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E. Obesity and metabolic syndrome in circadian clock mutant mice. Science (2005) 308:1043–5. doi:10.1126/science.1108750
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
69. Toshinai K, Nakazato M. Neuroendocrine regulatory peptide-1 and -2: novel bioactive peptides processed from VGF. Cell Mol Life Sci (2009) 66:1939–45. doi:10.1007/s00018-009-8796-0
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
70. Aguilar E, Pineda R, Gaytan F, Sanchez-Garrido MA, Romero M, Romero-Ruiz A. Characterization of the reproductive effects of the Vgf-derived peptide TLQP-21 in female rats: in vivo and in vitro studies. Neuroendocrinology (2013) 98:38–50. doi:10.1159/000350323
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
71. Pinilla L, Pineda R, Gaytan F, Romero M, Garcia-Galiano D, Sanchez-Garrido MA. Characterization of the reproductive effects of the anorexigenic VGF-derived peptide TLQP-21: in vivo and in vitro studies in male rats. Am J Physiol Endocrinol Metab (2011) 300:E837–47. doi:10.1152/ajpendo.00598.2010
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
72. Melis MR, Sanna F, Succu S, Ferri GL, Argiolas A. Neuroendocrine regulatory peptide-1 and neuroendocrine regulatory peptide-2 influence differentially feeding and penile erection in male rats: sites of action in the brain. Regul Pept (2012) 177:46–52. doi:10.1016/j.regpep.2012.04.007
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
73. Costigan M, Befort K, Karchewski L, Griffin RS, D’Urso D, Allchorne A. Replicate high-density rat genome oligonucleotide microarrays reveal hundreds of regulated genes in the dorsal root ganglion after peripheral nerve injury. BMC Neurosci (2002) 3:16. doi:10.1186/1471-2202-3-16
74. Wang H, Sun H, Della Penna K, Benz RJ, Xu J, Gerhold DL. Chronic neuropathic pain is accompanied by global changes in gene expression and shares pathobiology with neurodegenerative diseases. Neuroscience (2002) 114:529–46. doi:10.1016/S0306-4522(02)00341-X
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
75. Valder CR, Liu JJ, Song YH, Luo ZD. Coupling gene chip analyses and rat genetic variances in identifying potential target genes that may contribute to neuropathic allodynia development. J Neurochem (2003) 87:560–73. doi:10.1046/j.1471-4159.2003.02016.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
76. Maratou K, Wallace VC, Hasnie FS, Okuse K, Hosseini R, Jina N. Comparison of dorsal root ganglion gene expression in rat models of traumatic and HIV-associated neuropathic pain. Eur J Pain (2009) 13:387–98. doi:10.1016/j.ejpain.2008.05.011
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
77. Fairbanks CA, Peterson CD, Speltz RH, Riedl MS, Kitto KF, Dykstra JA. The VGF-derived peptide TLQP-21 contributes to inflammatory and nerve injury-induced hypersensitivity. Pain (2014) 155:1229–37. doi:10.1016/j.pain.2014.03.012
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
78. Bozdagi O, Rich E, Tronel S, Sadahiro M, Patterson K, Shapiro ML. The neurotrophin-inducible gene Vgf regulates hippocampal function and behavior through a brain-derived neurotrophic factor-dependent mechanism. J Neurosci (2008) 28:9857–69. doi:10.1523/JNEUROSCI.3145-08.2008
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
79. Thakker-Varia S, Behnke J, Doobin D, Dalal V, Thakkar K, Khadim F. VGF (TLQP-62)-induced neurogenesis targets early phase neural progenitor cells in the adult hippocampus and requires glutamate and BDNF signaling. Stem Cell Res (2014) 12:762–77. doi:10.1016/j.scr.2014.03.005
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
80. Brouillette J, Young D, During MJ, Quirion R. Hippocampal gene expression profiling reveals the possible involvement of Homer1 and GABA(B) receptors in scopolamine-induced amnesia. J Neurochem (2007) 102:1978–89. doi:10.1111/j.1471-4159.2007.04666.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
81. Carrette O, Demalte I, Scherl A, Yalkinoglu O, Corthals G, Burkhard P. A panel of cerebrospinal fluid potential biomarkers for the diagnosis of Alzheimer’s disease. Proteomics (2003) 3:1486–94. doi:10.1002/pmic.200300470
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
82. Ruetschi U, Zetterberg H, Podust VN, Gottfries J, Li S, Hviid Simonsen A. Identification of CSF biomarkers for frontotemporal dementia using SELDI-TOF. Exp Neurol (2005) 196:273–81. doi:10.1016/j.expneurol.2005.08.002
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
83. Selle H, Lamerz J, Buerger K, Dessauer A, Hager K, Hampel H. Identification of novel biomarker candidates by differential peptidomics analysis of cerebrospinal fluid in Alzheimer’s disease. Comb Chem High Throughput Screen (2005) 8:801–6. doi:10.2174/138620705774962391
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
84. Cocco C, D’Amato F, Noli B, Ledda A, Brancia C, Bongioanni P. Distribution of VGF peptides in the human cortex and their selective changes in Parkinson’s and Alzheimer’s diseases. J Anat (2010) 217:683–93. doi:10.1111/j.1469-7580.2010.01309.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
85. Thakker-Varia S, Krol JJ, Nettleton J, Bilimoria PM, Bangasser DA, Shors TJ. The neuropeptide VGF produces antidepressant-like behavioral effects and enhances proliferation in the hippocampus. J Neurosci (2007) 27:12156–67. doi:10.1523/JNEUROSCI.1898-07.2007
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
86. Hunsberger JG, Newton SS, Bennett AH, Duman CH, Russell DS, Salton SR. Antidepressant actions of the exercise-regulated gene VGF. Nat Med (2007) 13:1476–82. doi:10.1038/nm1669
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
87. Wang C, Zhang J, Lu Y, Lin P, Pan T, Zhao X. Antidepressant-like effects of the phosphodiesterase-4 inhibitor etazolate and phosphodiesterase-5 inhibitor sildenafil via cyclic AMP or cyclic GMP signaling in mice. Metab Brain Dis (2014) 29:673–82. doi:10.1007/s11011-014-9533-4
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
88. Lin P, Wang C, Xu B, Gao S, Guo J, Zhao X. The VGF-derived peptide TLQP62 produces antidepressant-like effects in mice via the BDNF/TrkB/CREB signaling pathway. Pharmacol Biochem Behav (2014) 120:140–8. doi:10.1016/j.pbb.2014.03.003
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Keywords: VGF, energy homeostasis, pain, cognition, reproduction
Citation: Lewis JE, Brameld JM and Jethwa PH (2015) Neuroendocrine role for VGF. Front. Endocrinol. 6:3. doi: 10.3389/fendo.2015.00003
Received: 28 October 2014; Accepted: 12 January 2015;
Published online: 02 February 2015.
Edited by:
Hubert Vaudry, University of Rouen, FranceReviewed by:
Alena Sumova, Academy of Sciences of the Czech Republic, Czech RepublicRuud Buijs, Universidad Autónoma de México, Mexico
Copyright: © 2015 Lewis, Brameld and Jethwa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Preeti H. Jethwa, Division of Nutritional Sciences, School of Biosciences, University of Nottingham, Sutton Bonington Campus, Loughborough, LE12 5RD, UK e-mail: preeti.jethwa@nottingham.ac.uk
 Jo E. Lewis
Jo E. Lewis John M. Brameld2
John M. Brameld2 Preeti H. Jethwa
Preeti H. Jethwa