- 1Division of Endocrinology, Georgetown University, Washington, DC, USA
- 2Department of Biostatistics, Medstar Health Research Institute, Hyattsville, MD, USA
- 3Department of Nuclear Medicine, Georgetown University, Washington, DC, USA
Objective: Symptoms of salivary gland dysfunction frequently develop after radioactive iodine (RAI) therapy, but have generally not been correlated with assessment of salivary gland functioning. The aim of this study was to determine whether there was a correlation between salivary symptoms and salivary functioning as assessed by salivary scan parameters.
Methods: This was a non-randomized observational study. Fifteen patients receiving RAI therapy for differentiated thyroid cancer completed a questionnaire assessing their salivary and nasal symptoms prior to their therapy and 3 and 12 months after their therapy. Salivary gland scanning using technetium-99m pertechnetate was performed at the same time points. In addition, protective measures used at the time of radioiodine administration, such as use of fluids and sour candy, were also documented. Measures of salivary gland accumulation and secretion were correlated with scores of salivary and nasal symptomatology and any effects of protective measures were assessed.
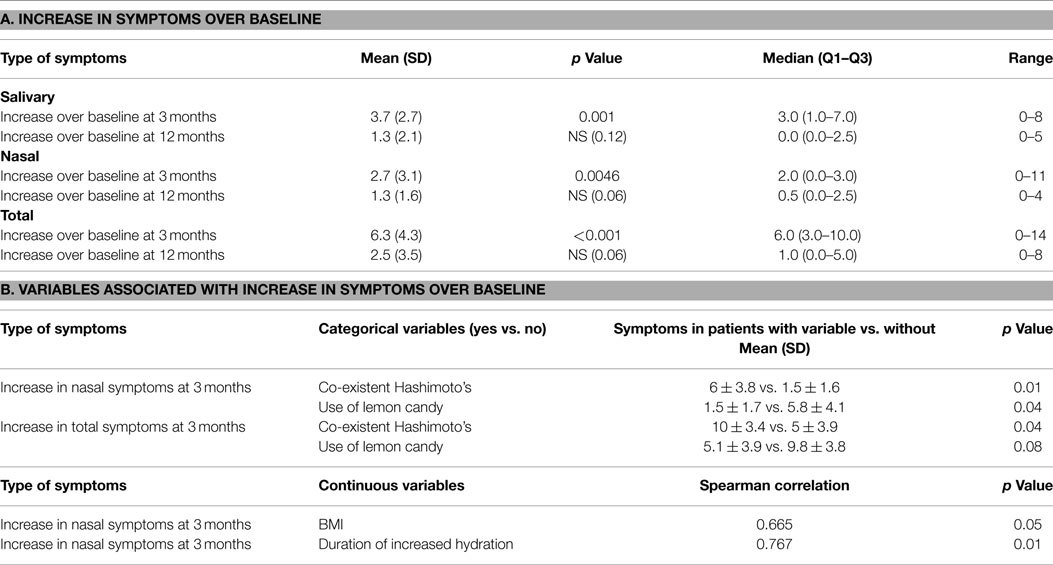
Results: The mean number of salivary, nasal, and total symptoms at 3 months increased significantly over the number of symptoms at baseline by 3.7, 2.7, and 6.3 symptoms, respectively (p values 0.001, 0.0046, and <0.001, respectively). The mean increases in the number of salivary, nasal, and total symptoms at 12 months were non-significant at 1.3, 1.3, and 2.5 symptoms, respectively. The mean right parotid gland accumulation and secretion of radioisotope declined significantly at 3 months, compared with baseline. The changes in left parotid and right and left submandibular function were non-significant. There was no association between the increase in salivary, nasal, or total symptoms and the change in scintigraphy measures. However, the increases in nasal and total symptoms were significantly greater in those with co-existent Hashimoto’s disease, compared with those without this condition (p values 0.01 and 0.04, respectively). Nasal symptoms decreased (p value 0.04) and total symptoms trended to decrease (p value 0.08) in those who used sour candies, compared with those who did not. Increasing body mass index was significantly associated with increasing nasal symptoms (p value 0.05). Greater decline in salivary parameters at 3 months compared with baseline was generally associated with heavier body weight, decreased thyroid cancer stage, absence of Hashimoto’s thyroiditis, and pre-menopausal status.
Conclusion: Salivary and nasal symptoms increased and salivary scintigraphy parameters decreased after radioiodine therapy. However, the increased symptoms did not correlate with decrements in salivary gland accumulation or secretion. Moreover, the variables associated with symptoms and changes in salivary scan parameters differed. Therefore, a better understanding of the relationship between salivary gland symptoms and functioning is needed. Factors affecting susceptibility to salivary and nasal damage after radioiodine therapy need to be better elucidated, so that modifiable factors can be identified.
Introduction
Administered radioactive iodine (RAI) is actively transported via the sodium iodide symporter and concentrated primarily by benign and malignant thyroid cells (1). Despite the proven efficacy of RAI in the treatment of thyroid cancer, there are several serious side effects associated with its use (2). These side effects, which are due to the impact of RAI on non-thyroid tissue, are one of the major causes of morbidity in thyroid cancer survivors (2, 3). Salivary glands are one of the most affected tissues.
The sodium iodide symporter has also been shown to be present in salivary tissue (1). Consequently, salivary glands have the ability to specifically concentrate iodine (1, 4), although its physiological role is not as yet elucidated. Salivary gland dysfunction after RAI treatment occurs quite frequently, even with a first therapy or low-dose therapy (5, 6). Salivary gland damage occurs in a dose-dependent manner but reported incidence rates vary widely from 5 to 86% (2, 7–10). More damage also seems to occur with treatment protocols involving withdrawal from thyroid hormone than with recombinant human thyrotropin (rhTSH) protocols (5). The parotid glands appear to suffer damage more frequently than the submandibular glands (4, 6, 10). Salivary gland dysfunction can present as sialadenitis, xerostomia, taste alterations, hypogeusia, sialolithiasis, dental caries, stomatitis, salivary gland or oral infections, facial nerve damage, and even salivary gland neoplasia (6, 9). Although side effects such as xerostomia may be transient, they may also persist for longer periods, be delayed in onset, or even be permanent (11, 12). Such events can profoundly decrease patient quality of life (2, 3).
Radioactivity has been demonstrated in tears (13) and contact lenses (14) after RAI administration, and the lacrimal system is another organ system affected by RAI. Lacrimal dysfunction can be manifest as epiphora, tearing, ocular dryness or xerophthalmia, and recurrent or chronic conjunctivitis. Xerophthalmia and abnormal lacrimal function tests were noted in 18 (9) and 92% (15) of patients, respectively after RAI therapy. Various rates of lacrimal dysfunction have been reported in other studies. These include conjunctivitis in 23% (16), significant tearing in 5% (17), “watery eyes” in 10% (18), and various symptoms of lacrimal dysfunction in 9.7% (19).
Nasal tissues are also affected by RAI. Nasopharyngeal tissues do not express the sodium iodide symporter (20), in contrast to the finding of its expression in extra-thyroidal tissues such the salivary glands (20) and lacrimal system (21). However, iodine may be transported non-specifically from the lacrimal system into the nasal ducts, and then from the interstitial surface of nasal ducts into the ductal lumen and out onto the nasal mucosal surface. A recent retrospective study (19) showed that the nasal side effects of RAI declared themselves within 1–2 weeks of the therapeutic dose. The mean time of onset of nasal symptoms was 11 days. The rate of nasal dysfunction was 10.5%: similar to the occurrence rate of 9.7% for lacrimal dysfunction. The nasal complaints included nasal pain, nasal tenderness, bloody nasal discharge, and dry nose. RAI dose and body mass index were significantly positively and negatively correlated with sustaining nasal side effects. Being prepared for treatment using a withdrawal protocol was associated with increased risk of nasal side effects.
This study was designed to prospectively study symptoms of salivary dysfunction after RAI therapy, along with simultaneously documenting objective measures of salivary gland functioning. Nasal symptoms were concurrently assessed.
Materials and Methods
This was an observational, prospective, non-randomized study. The aim of this study was to determine whether there was a correlation between salivary symptoms and salivary functioning as assessed by salivary scan parameters. The study was approved by the Georgetown University Institutional Review Board.
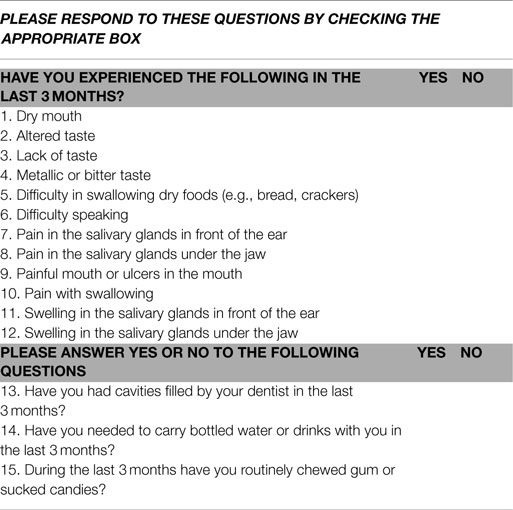
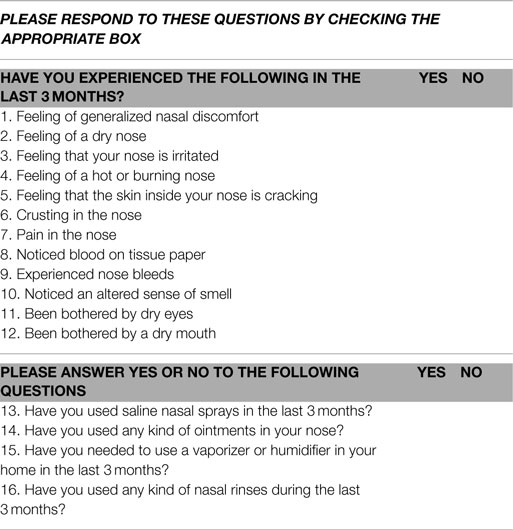
Patients who were scheduled to undergo RAI treatment with I-131 for differentiated thyroid cancer were recruited for the study. Details of their thyroid cancer features, including thyroid cancer stage as defined using the National Thyroid Cancer Treatment Cooperative Study Group staging system (22, 23) were documented. Participation involved completion of two questionnaires about salivary and nasal symptoms respectively and undergoing a salivary gland scan using technetium-99m (99mTc) pertechnetate performed prior to receiving RAI and 3 months and 12 months after RAI treatment. Participation thus involved completion of two questionnaires and a salivary scan on three separate occasions. The salivary gland questionnaire included 15 questions and is shown in Table 1. The nasal symptoms questionnaire included 16 questions and is shown in Table 2. Question number 12 on the nasal questionnaire asked about a dry mouth, as did question 1 on the salivary gland questionnaire. Question 12 from the nasal questionnaire was not included in the questionnaire score, but was used to help determine whether patients answered the questions consistently. Each positive response from the two questionnaires was assigned one point, thus the score for each questionnaire could potentially range from 0 to 15.
Salivary gland function was assessed using salivary gland scintigraphy. Scintigraphy was performed following intravenous injection of 8 mCi (296 MBq) of 99mTc-pertechnetate. Following isotope administration, the patient was placed supine with the head slightly extended. A support was placed behind the patient’s neck and to the sides of the head to minimize head motion. Camera settings included a 128 × 128 matrix with a 2.67 zoom, energy window at the 140 KeV 99mTc photopeak with 15% symmetric window. A high-resolution parallel hole collimator was used. Sequential parotid and sublingual gland images were acquired every 20 s for 20 min. Two milliliters of a 100% lemon juice solution were then administered with a syringe and images were acquired at the same frame rate for a further 20 min. Regions of interest were drawn for the parotid and submandibular glands. Regions of interests for the mouth and the respective backgrounds (temporal area for parotid gland and submental area for submandibular gland) were also obtained. Background-corrected time activity curves were then derived. Parotid and sublingual gland functioning were assessed in the same study. The general pattern of radiotracer changes during salivary scanning was as follows. Immediately after radiotracer administration, tracer accumulation in the major salivary glands was observed, with more accumulation in the parotid than in the submandibular glands. After lemon juice administration, rapid secretion of activity from all salivary glands was observed, with nadir levels reached within 3–4 min from the start of stimulation. This washout of activity was greater in the parotid than in the submandibular glands.
Two parameters were calculated: accumulation (uptake) of 99mTc-petechnatate and secretion (excretion) of 99mTc-petechnatate. Accumulation was defined as the ratio of gland to background counts at the point of maximum counts. Secretion was defined as the ratio of gland to background counts at the nadir achieved after lemon juice stimulation subtracted from the ratio of gland to background counts at the point of maximum counts. The methodology used was similar to that described by Aung and colleagues (24). Accumulation and secretion of both the right and left salivary glands were obtained prior to RAI treatment as a baseline for each individual patient. The baseline values were compared with those obtained at the two time points after RAI treatment. These time points after RAI therapy were 3 months and 12 months. The 3-month background-corrected uptake ratio and the 12-month background-corrected uptake ratio were each subtracted from the background-corrected uptake ratio at baseline in order to generate the change that occurred following RAI therapy.
During the study period, the isotope used for diagnostic RAI scanning was I-123, with I-131 used for therapy. None of the conditions surrounding the patient’s RAI therapy were manipulated because of study participation. Patients received the RAI activity and used the method of TSH elevation prescribed by their treating physician. In addition, recommendations in place regarding the use of salivary stimulants and hydration were those given by the prescribing physician and/or nuclear medicine physician. During the period of the study, patients were routinely admitted to an isolation room in the hospital for 22 h after receiving their RAI therapy. Patients were permitted to drink fluids, use sialogogues, and eat 2 h after RAI administration. As part of the protocol, use of lemon candy, use of fluids, and urination frequency were carefully documented. With respect to lemon candy, the type of lemon candy, amount, frequency, and duration of its use was documented. Similarly for fluid intake, the type of fluid consumed, and the amount, frequency, and duration of its use, was documented. Patients were also asked to quantify the length of time for which they consumed more than their usual volume of fluids. For urine output, the frequency of voiding during the initial 22 h after RAI treatment was recorded.
The baseline characteristics of study population were calculated. Due to the very small sample size (n = 15), the assumption of normal distribution could not be assessed. Non-parametric tests without assumption of distribution were used for the analysis. The Spearman correlation coefficient was used to assess the correlation among symptom score, scan results, and continuous patient variables. The Wilcoxon rank sum test or Kruskal–Wallis test was used to compare the distribution of symptom score and scan results between the categories of patient variables.
Results
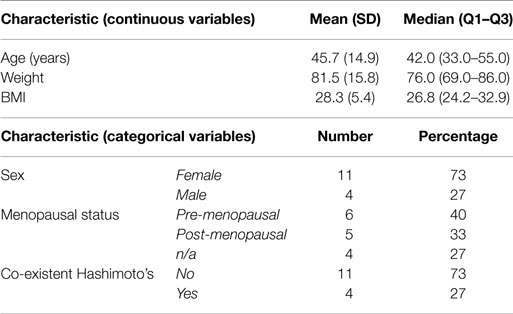
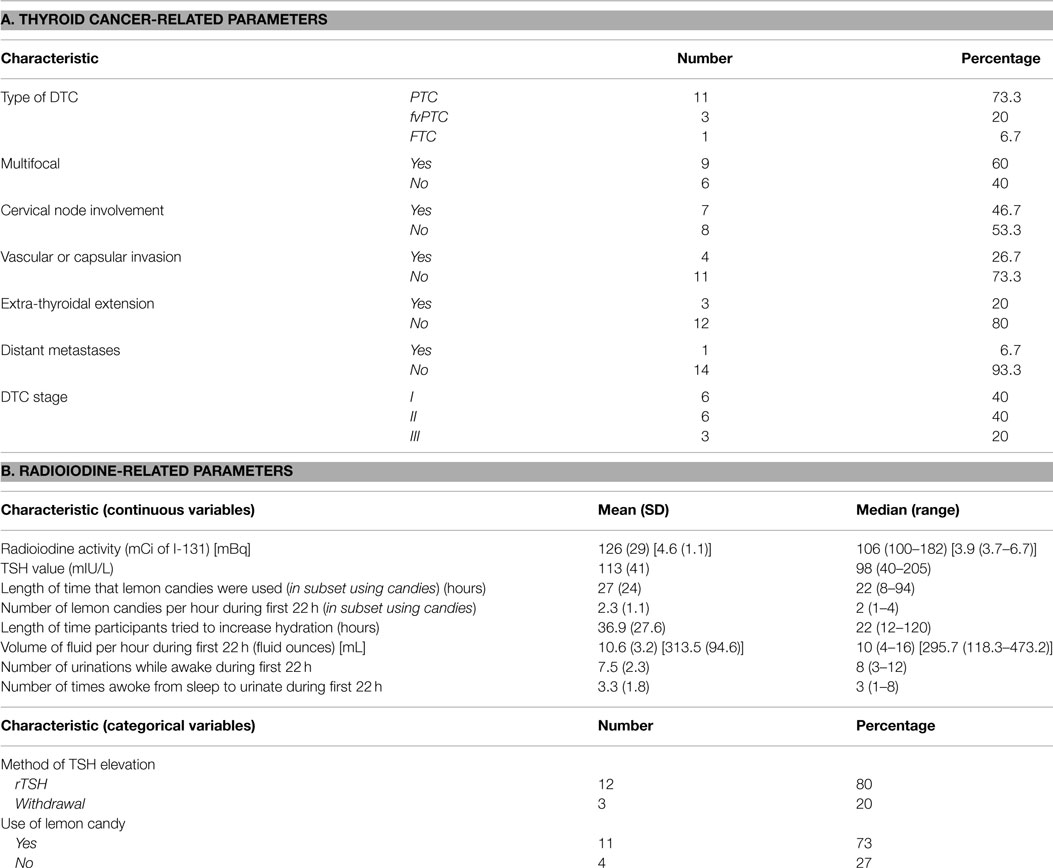
Patients were treated with RAI and underwent salivary scanning during the period February 2009 to June 2010. Fifteen participants were consented for the study. All 15 patients completed the baseline and 3-month symptom questionnaire and salivary scan. Funding was only sufficient for eight patients to complete the 12-month scan. The same eight patients also completed the symptom questionnaires at 12 months. The question used to assess the reliability of response to the questions was consistently answered in all cases. The characteristics of the participants are displayed in Table 3. The mean age of the patients was 45.7 years and 73% of them were female. The relevant details about the patient thyroid cancer characteristics are shown in Table 4A. The majority of patients had papillary thyroid cancer of typical histology. Approximately, 50% had multifocal disease and/or cervical lymph node involvement. One patient had distant metastases.
Twelve out of 15 patients were prepared for RAI administration using a recombinant rhTSH protocol. Details regarding RAI administration and attendant use of sialogogues and fluids are shown in Table 4B. Mean and median TSH values for all 15 patients were 113 and 98 mIU/L, respectively. Mean and median RAI activities administered were 126 mCi (4.6 GBq) and 106 mCi (3.9 GBq), respectively. Patients attempted to drink more than their usual volume of fluids for a mean of 36.9 h. The mean volume of fluid consumed during the initial 22 h of participant hospitalization was 10.6 fluid ounces (313.6 mL) per hour. Fluids consumed included water, orange juice, apple juice, lemon juice, and Gatorade®. Eleven out of 15 patients used sour candies following RAI administration. Those that used lemon candies used a mean of 2.3 candies per hour during the initial 22 h. The mean length of lemon candy use was 27 h. The lemon candies used included the following: Lemonheads, Brachs, Jolly Ranchers, Sour Beans, Citron, sour gummies (various brands), Sour Patch, and Warheads. During their inpatient stay, participants urinated a mean of 7.5 times during waking hours and 3.3 times during the night.
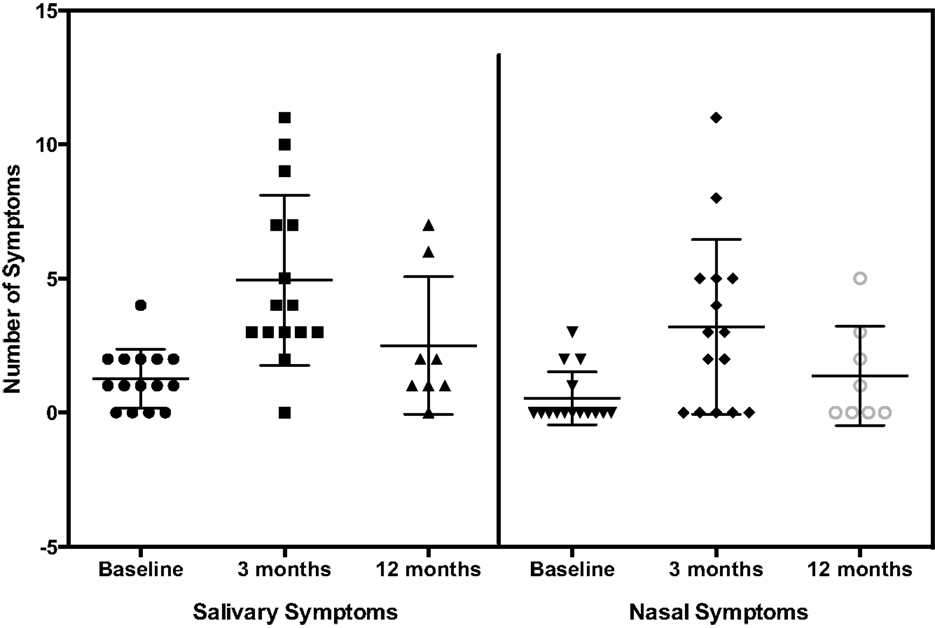
The number of salivary and nasal symptoms reported by patients at baseline, 3 months, and 12 months are shown in Figure 1. The mean increases in the number of symptoms at 3 and 12 months over baseline are shown in Table 5A. The mean number of salivary, nasal, and total symptoms at 3 months increased significantly over the number of symptoms at baseline by 3.7, 2.7, and 6.3 symptoms, respectively (p values 0.001, 0.0046, <0.001). The mean increases in the number of salivary, nasal, and total symptoms at 12 months were non-significant at 1.3, 1.3, and 2.5 symptoms, respectively.
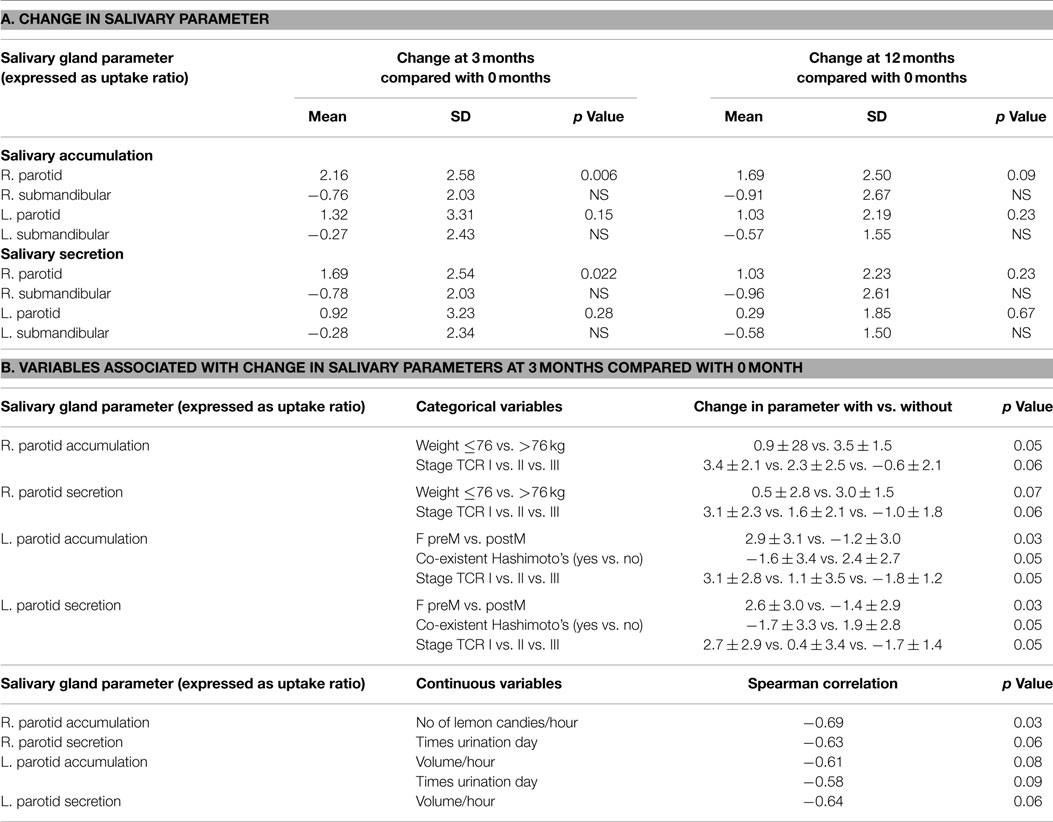
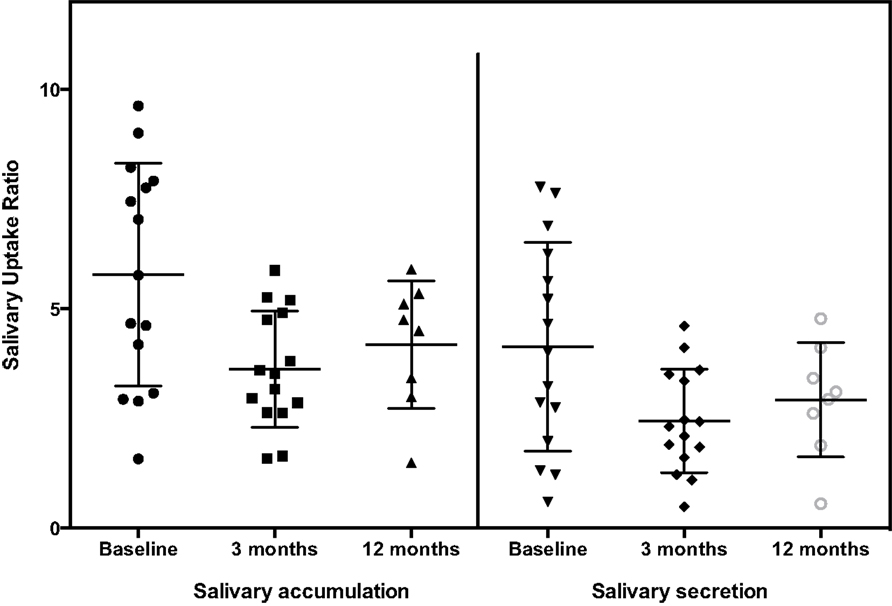
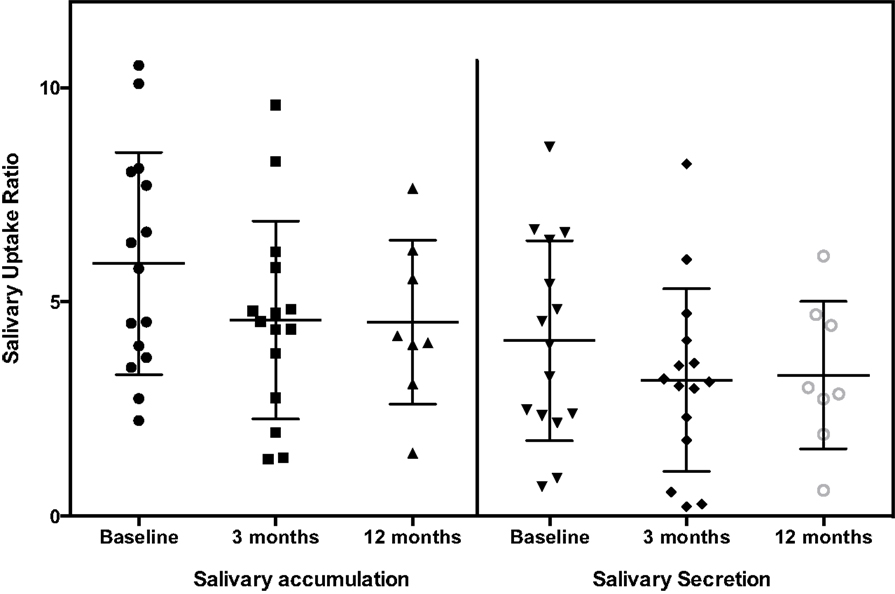
The general pattern of radiotracer changes during salivary scanning was described in the Section “Materials and Methods.” A representative example of salivary gland accumulation and secretion is shown in Figure 2. The mean parotid gland accumulation and secretion of radioisotope generally declined at 3 months, compared with baseline. The declines in right parotid accumulation and secretion at 3 months were significant (see Table 6A; Figure 3). The changes in left parotid accumulation and secretion at 3 months, however, were not significant (see Table 6A; Figure 4). None of the changes in submandibular functioning or parotid gland functioning at 12 months, were significant (see Table 6A.) All the changes in salivary function were characterized by great variability, as can be seen in Table 6A and Figures 3 and 4.
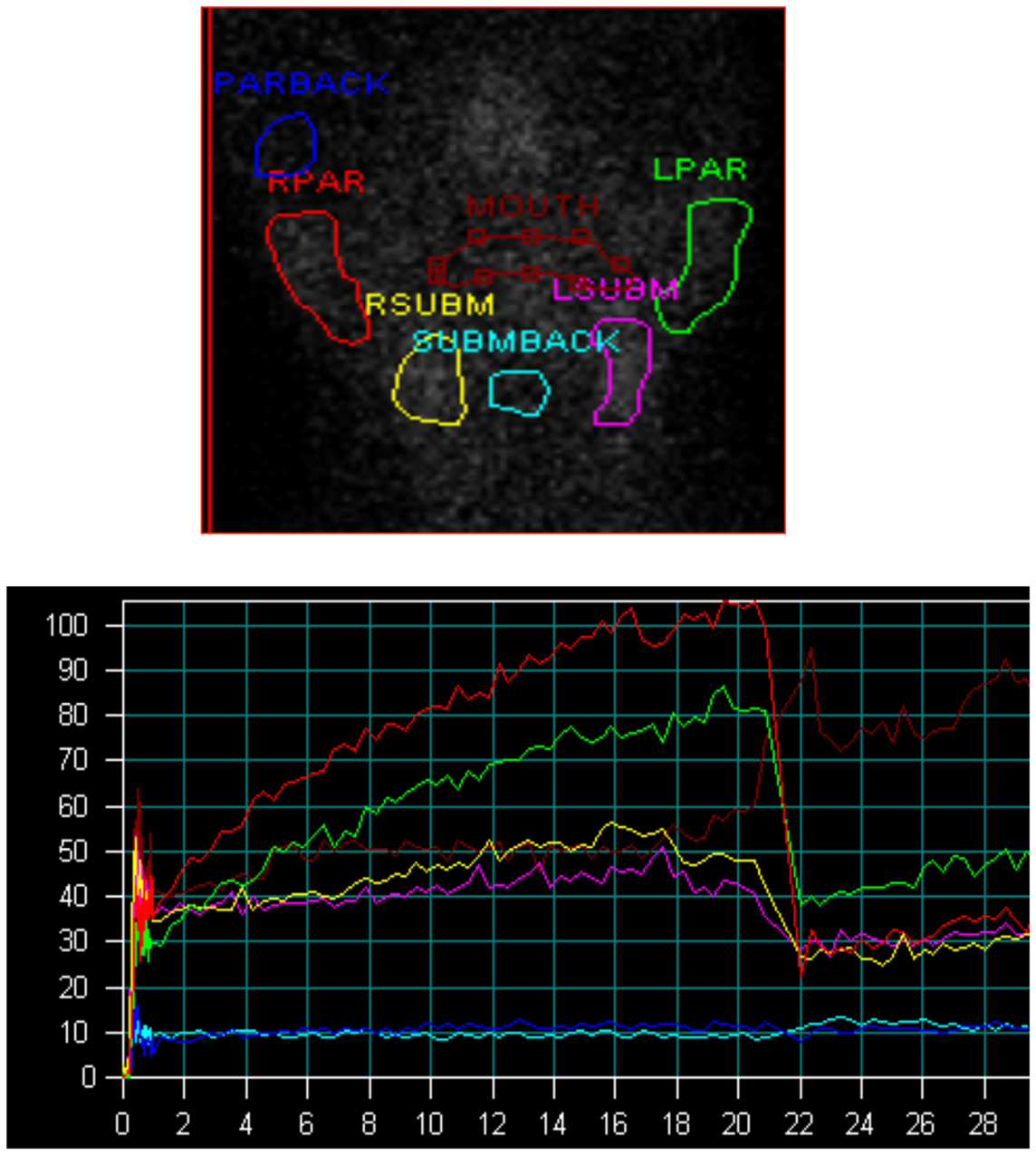
Figure 2. Salivary scintigraphy. Upper figure shows regions of interest, lower figure shows time activity curves (y axis = counts per second, x axis = minutes, lemon juice was administered at the 20 min time point). Red = right parotid, green = left parotid, yellow = right submandibular gland, pink = left submandibular gland, blue = parotid background, turquoise = submandibular background, brown = mouth.
There was no association between the increase in salivary, nasal, or total symptoms and the change in scintigraphy measures. However, the increases in nasal and total symptoms were significantly greater in those with co-existent Hashimoto’s disease, compared with those without this condition (p values 0.01 and 0.04, respectively) (see Table 5B). The increase in nasal symptoms was of lesser magnitude (p value 0.04), and the increase in total symptoms trended to be less (p value 0.08), in those who used sour candies, compared with those who did not. Body mass index was significantly associated with increasing nasal symptoms at 3 months (p values 0.05). Increased nasal symptoms at 3 months were positively associated with duration of increased hydration (p value 0.01) (see Table 5B). With respect to the categorical variables that were associated with greater decrement in salivary parameters, these included increased body weight (p value 0.05), lower thyroid cancer stage (p value 0.05), pre-menopausal status (p value 0.03), and absence of Hashimoto’s disease (p value 0.05) (see Table 6B). The only continuous variable with a significant association was fewer lemon candies per hour being associated a greater decrement in parotid gland accumulation at 3 months (p value 0.03). In this small study, neither symptoms nor scintigraphy measures appeared to be affected by the method of TSH elevation or the RAI dose administered.
Discussion
This study showed an increase in symptoms of salivary gland dysfunction 3 months following RAI treatment followed by a decrease in symptoms by 12 months. At the same time, there was a decline in scintigraphy parameters that reflect parotid salivary gland functioning. Both accumulation and secretion of radiotracer were affected, with the right parotid being more affected than the left parotid. However, surprisingly, there was lack of correlation between salivary symptoms and scintigraphy measures of salivary function.
This is in contrast to a recent study in which all eight patients who had oral symptoms consistent with salivary dysfunction 12 months after RAI therapy were reported to have their salivary dysfunction confirmed by salivary scintigraphy (25). However, the particular deficit noted on scintigraphy (parotid vs. submandibular gland, accumulation vs. secretion) was not reported. However, another study also did not show correlation between symptoms and objective measures of salivary function (26). In this study of patients who received or did not receive RAI therapy, salivary scintigraphy parameters or salivary flow measured by sialometry were not associated with xerostomia or dysphagia (26). However, dysphagia and reduced parotid secretion were each associated with RAI therapy. An additional study examining correlation of symptoms with objective measures of salivary function in patients with thyroid cancer found that xerostomia was correlated with the number of salivary glands showing worsening scintigraphy parameters and that xerostomia correlated better with scintigraphic measures of submandibular dysfunction than parotid dysfunction (27).
The failure of symptoms of salivary function to correlate with salivary parameters in this study is most likely due to under-powering of the study. However, given that similar lack of correlation has been reported in other studies too (26, 27), perhaps additional factors contributing to symptomatology need to be investigated. The lack of correlation between symptoms and function may also be due to failure to collect data about other more pertinent symptoms that perhaps better mirror functioning. If true, this highlights the need for better, validated symptom questionnaires.
Variables associated with either salivary symptoms or scintigraphy changes have been elucidated. Higher activities of RAI have been associated with a greater frequency of xerostomia and greater changes in scintigraphy parameters (27). Another study identified patient age as being associated with decreased parotid accumulation and decreased salivary flow, whereas having received RAI therapy was associated with decreased parotid secretion and decreased salivary flow (27). In this study, the variables that were associated with increased salivary symptoms were different, and even of opposite impact, than the variables associated with a decline in salivary scan parameters. By way of illustration, co-existent Hashimoto’s thyroiditis was associated with an increase in symptoms at 3 months but a lesser decline in parotid parameters at 3 months. It is possible that the lesser decline in salivary function in patients with Hashimoto’s occurred because some dysfunction already existed prior to RAI due to underlying autoimmunity. In this present study, use of lemon candy, which has been shown in another study by Kulkarni and coworkers to decrease the radiation dose absorbed by the salivary glands (28), was associated with less nasal symptoms and less total symptoms. A greater number of lemon candies used per hour was also associated with less decrement in right parotid accumulation at 3 months in the present study. BMI and weight were positively associated with number of symptoms and greater decrement in salivary parameters; advancing thyroid cancer stage was associated with lesser decline in salivary parameters. If confirmed, these findings could be associated with altered handling of iodine associated with body composition, and the proportion of RAI taken up by non-thyroid tissue vs. residual thyroid cancer.
In confirmation of other studies, we show that there is a lesser effect of RAI therapy on submandibular functioning (4, 6, 10). The greater impact of ionizing radiation on the parotid glands is thought to due to their greater activity and greater content of serous cells, compared with the mixed serous and mucinous cell content of the submandibular glands (6). We are not aware of prior studies that suggest a greater effect of RAI therapy on the right parotid, as opposed to the left. Most studies report combined bilateral salivary gland parameters, but one prior study that reported separate function for right and left glands did not report any differential effect (27).
This study also gives new information about the duration of nasal symptoms after radioiodine therapy. A prior retrospective study has shown a mean time of onset of nasal symptoms of 11 days and no documentation of continuing symptoms from chart review of 3-month and 12-month endocrinology follow-up visits (19). This study, however, shows that, as demonstrated by questionnaire completion during a prospective study, nasal symptoms were still clearly present at 3 months.
The limitations of this study include the very small sample size, a non-validated questionnaire, lack of a control group not treated with RAI, and lack of randomization. To illustrate the need for a control group who did not receive RAI, some salivary scintigraphic parameters were more robust at 3 months than at baseline; a control group would have given insight as to whether this reflected a natural variation in salivary function and/or in the measurement techniques used. However, a positive aspect of the study is that the data were collected prospectively.
There is a clear need for better understanding of the cause of symptoms of salivary dysfunction after RAI administration. If these symptoms are confirmed in additional larger studies not to correlate well with objective measures of salivary functioning, then an investigation of potential alternative causes of these symptoms is needed. In addition, risk factors for both symptoms and altered scintigraphy parameters need to be better documented, especially given the opposing effects of some of these risk factors documented here.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This project has been funded in part with Federal funds (UL1TR000101 previously UL1RR031975) from the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health, through the Clinical and Translational Science Awards Program (CTSA), a trademark of DHHS, part of the Roadmap Initiative, “Re-Engineering the Clinical Research Enterprise.” Funding for salivary scanning was provided by intramural funds from Georgetown University. Biostatistics services were provided by the CTSA. The authors would like to thank Rebecca Taylor-Tillery for her skilled performance of the salivary scans.
References
1. Dohan O, De la Vieja A, Paroder V, Riedel C, Artani M, Reed M, et al. The sodium/iodide symporter (NIS): characterization, regulation, and medical significance. Endocr Rev (2003) 24(1):48–77. doi: 10.1210/er.2001-0029
2. Mendoza A, Shaffer B, Karakla D, Mason ME, Elkins D, Goffman TE. Quality of life with well-differentiated thyroid cancer: treatment toxicities and their reduction. Thyroid (2004) 14(2):133–40. doi:10.1089/105072504322880373
3. Almeida JP, Vartanian JG, Kowalski LP. Clinical predictors of quality of life in patients with initial differentiated thyroid cancers. Arch Otolaryngol Head Neck Surg (2009) 135(4):342–6. doi:10.1001/archoto.2009.16
4. Newkirk KA, Ringel MD, Wartofsky L, Burman KD. The role of radioactive iodine in salivary gland dysfunction. Ear Nose Throat J (2000) 79(6):460–8.
5. Rosario PW, Borges MA, Purisch S. Preparation with recombinant human thyroid-stimulating hormone for thyroid remnant ablation with 131I is associated with lowered radiotoxicity. J Nucl Med (2008) 49(11):1776–82. doi:10.2967/jnumed.108.050591
6. Mandel SJ, Mandel L. Radioactive iodine and the salivary glands. Thyroid (2003) 13(3):265–71. doi:10.1089/105072503321582060
7. Lin WY, Shen YY, Wang SJ. Short-term hazards of low-dose radioiodine ablation therapy in postsurgical thyroid cancer patients. Clin Nucl Med (1996) 21(10):780–2. doi:10.1097/00003072-199610000-00006
8. Caglar M, Tuncel M, Alpar R. Scintigraphic evaluation of salivary gland dysfunction in patients with thyroid cancer after radioiodine treatment. Clin Nucl Med (2002) 27(11):767–71. doi:10.1097/00003072-200211000-00003
9. Solans R, Bosch JA, Galofre P, Porta F, Rosello J, Selva-O’Callagan A, et al. Salivary and lacrimal gland dysfunction (sicca syndrome) after radioiodine therapy. J Nucl Med (2001) 42(5):738–43.
10. Malpani BL, Samuel AM, Ray S. Quantification of salivary gland function in thyroid cancer patients treated with radioiodine. Int J Radiat Oncol Biol Phys (1996) 35(3):535–40. doi:10.1016/S0360-3016(96)80016-2
11. Laupa MS, Toth BB, Keene HJ, Sellin RV. Effect of radioactive iodine therapy on salivary flow rates and oral Streptococcus mutans prevalence in patients with thyroid cancer. Oral Surg Oral Med Oral Pathol (1993) 75(3):312–7. doi:10.1016/0030-4220(93)90143-R
12. Mandel SJ, Mandel L. Persistent sialadenitis after radioactive iodine therapy: report of two cases. J Oral Maxillofac Surg (1999) 57(6):738–41. doi:10.1016/S0278-2391(99)90444-5
13. Bakheet SM, Hammami MM, Hemidan A, Powe JE, Bajaafar F. Radioiodine secretion in tears. J Nucl Med (1998) 39(8):1452–4.
14. Zettinig G, Karanikas G, Hanselmayer G, Havlik E, Dudczak R. Radioactive contamination of contact lenses during radioiodine therapy. Nucl Med Commun (2000) 21(10):955–7. doi:10.1097/00006231-200010000-00010
15. Zettinig G, Hanselmayer G, Fueger BJ, Hofmann A, Pirich C, Nepp J, et al. Long-term impairment of the lacrimal glands after radioiodine therapy: a cross-sectional study. Eur J Nucl Med Mol Imaging (2002) 29(11):1428–32. doi:10.1007/s00259-002-0969-0
16. Alexander C, Bader JB, Schaefer A, Finke C, Kirsch CM. Intermediate and long-term side effects of high-dose radioiodine therapy for thyroid carcinoma. J Nucl Med (1998) 39(9):1551–4.
17. Burns JA, Morgenstern KE, Cahill KV, Foster JA, Jhiang SM, Kloos RT. Nasolacrimal obstruction secondary to I(131) therapy. Ophthal Plast Reconstr Surg (2004) 20(2):126–9. doi:10.1097/01.IOP.0000117340.41849.81
18. Schlumberger M, Catargi B, Borget I, Deandreis D, Zerdoud S, Bridji B, et al. Strategies of radioiodine ablation in patients with low-risk thyroid cancer. N Engl J Med (2012) 366(18):1663–73. doi:10.1056/NEJMoa1108586
19. Jonklaas J. Nasal symptoms after radioiodine therapy: a rarely described side effect with similar frequency to lacrimal dysfunction. Thyroid (2014) 24(12):1806–14. doi:10.1089/thy.2014.0162
20. Spitzweg C, Joba W, Eisenmenger W, Heufelder AE. Analysis of human sodium iodide symporter gene expression in extrathyroidal tissues and cloning of its complementary deoxyribonucleic acids from salivary gland, mammary gland, and gastric mucosa. J Clin Endocrinol Metab (1998) 83(5):1746–51. doi:10.1210/jcem.83.5.4839
21. Riesco-Eizaguirre G, Santisteban P. A perspective view of sodium iodide symporter research and its clinical implications. Eur J Endocrinol (2006) 155(4):495–512. doi:10.1530/eje.1.02257
22. Jonklaas J, Sarlis NJ, Litofsky D, Ain KB, Bigos ST, Brierley JD, et al. Outcomes of patients with differentiated thyroid carcinoma following initial therapy. Thyroid (2006) 16(12):1229–42. doi:10.1089/thy.2006.16.1229
23. Sherman SI, Brierley JD, Sperling M, Ain KB, Bigos ST, Cooper DS, et al. Prospective multicenter study of thyroid carcinoma treatment: initial analysis of staging and outcome. National Thyroid Cancer Treatment Cooperative Study Registry Group. Cancer (1998) 83(5):1012–21. doi:10.1002/(SICI)1097-0142(19980901)83:5<1012::AID-CNCR28>3.0.CO;2-9
24. Aung W, Murata Y, Ishida R, Takahashi Y, Okada N, Shibuya H. Study of quantitative oral radioactivity in salivary gland scintigraphy and determination of the clinical stage of Sjogren’s syndrome. J Nucl Med (2001) 42(1): 38–43.
25. Rosario PW, Calsolari MR. Salivary and lacrimal gland dysfunction after remnant ablation with radioactive iodine in patients with differentiated thyroid carcinoma prepared with recombinant human thyrotropin. Thyroid (2013) 23(5):617–9. doi:10.1089/thy.2012.0050
26. Almeida JP, Sanabria AE, Lima EN, Kowalski LP. Late side effects of radioactive iodine on salivary gland function in patients with thyroid cancer. Head Neck (2011) 33(5):686–90. doi:10.1002/hed.21520
27. Jeong SY, Kim HW, Lee SW, Ahn BC, Lee J. Salivary gland function 5 years after radioactive iodine ablation in patients with differentiated thyroid cancer: direct comparison of pre- and postablation scintigraphies and their relation to xerostomia symptoms. Thyroid (2013) 23(5):609–16. doi:10.1089/thy.2012.0106
Keywords: salivary symptoms, salivary dysfunction, salivary scintigraphy, nasal symptoms, radioiodine therapy
Citation: Jonklaas J, Wang H and Esposito G (2015) Salivary function after radioiodine therapy: poor correlation between symptoms and salivary scintigraphy. Front. Endocrinol. 6:100. doi: 10.3389/fendo.2015.00100
Received: 07 April 2015; Accepted: 01 June 2015;
Published: 17 June 2015
Edited by:
Yuji Nagayama, Nagasaki University, JapanReviewed by:
Jeffrey Knauf, Memorial Sloan-Kettering Cancer Center, USATakashi Kudo, Nagasaki University, Japan
Copyright: © 2015 Jonklaas, Wang and Esposito. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jacqueline Jonklaas, Division of Endocrinology, Georgetown University, Suite 230, Building D, 4000 Reservoir Road, NW, Washington, DC 20007, USA, jonklaaj@georgetown.edu
 Jacqueline Jonklaas
Jacqueline Jonklaas Hong Wang2
Hong Wang2 Giuseppe Esposito
Giuseppe Esposito