A theoretical study of two novel concept systems for maximum thermal-chemical conversion of biomass to hydrogen
- Department of Mechanical and Aerospace Engineering, University of Florida, Gainesville, FL, USA
Two concept systems that are based on the thermochemical process of high temperature steam gasification of lignocellulosic biomass and municipal solid waste are introduced. The primary objectives of the concept systems are (1) to develop the best scientific, engineering, and technology solutions for converting lignocellulosic biomass, as well as agricultural, forest, and municipal waste to clean energy (pure hydrogen fuel), and (2) to minimize water consumption and detrimental impacts of energy production on the environment (air pollution and global warming). The production of superheated steam is by hydrogen combustion using recycled hydrogen produced in the first concept system while in the second concept system concentrated solar energy is used for the steam production. A membrane reactor that performs the hydrogen separation and water gas shift reaction is involved in both systems for producing more pure hydrogen and CO2 sequestration. Based on obtaining the maximum hydrogen production rate the hydrogen recycled ratio is around 20% for the hydrogen combustion steam heating system. Combined with pure hydrogen production, both high temperature steam gasification systems potentially possess more than 80% in first law overall system thermodynamic efficiencies.
Introduction
In recent years, the global warming and climate change issues have created controversies and public concerns that involve the energy industry, government leaders, and society at large (Holdren, 2001; Kalicki and Goldwyn, 2005). Only renewable and sustainable carbon-neutral energy resources with high energy contents should be considered and explored for the future needs in the power, transportation and manufacturing sectors of the economy (Wilk, 2002). The production of energy from these resources should cast minimum impacts on the food chain, water supply, land use, and environment. Biomass energy is basically carbon-neutral and is one of the most important primary, renewable energy resources. The concept systems introduced in this paper would contribute to an enabling technology to convert lignocellulosic and municipal solid waste (MSW) biomass resources to useful energy, from thermodynamic efficiency and system technology development standpoints, as well as from emission impacts on the environment, water and weather. The main objectives for proposing these systems are (1) to introduce the best scientific, engineering, and technology concepts for converting lignocellulosic and MSW biomass together with agricultural and forest residues to clean energy (liquid fuels, chemical feedstock, electricity, and mechanical power), and (2) to minimize the water consumption and detrimental impacts on the environment (air pollution and global warming).
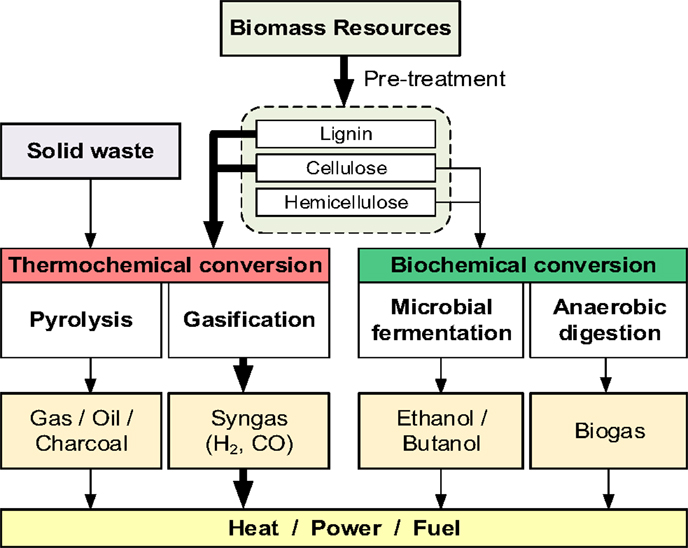
Figure 1 illustrates the biomass and solid waste conversion to energy paths. There are two parallel approaches: the first flow process focuses on the bio-chemical conversion of biomass to biofuels and the second process uses the thermal-chemical methods to produce synthesis gas (syngas) by gasification from lignocellulosic and solid waste biomass (energy crop, forest products, and agricultural residues). The concept systems developed in this study are based on the thermochemical conversion process (indicated by the bold arrows in Figure 1) of non-food based lignocellulosic biomass and MSW.
High Temperature Steam Allothermal Gasification
Existing gasification technologies use a reactor that operates in the temperature range of 400–850°C (McKendry, 2002; Pandey and Kim, 2011). They do not need external heat source as they rely on the “air-breathing combustion” of a portion of feedstock to facilitate and sustain the gasification. So they are really “partial combustor and partial gasifier.” As a result, these lower temperature air-present gasifiers produce low-quality syngas that contains undesirable char, tar, and soot. Furthermore, their harmful air pollution emissions are similar to those emitted from incinerators due to the air-blown combustion. The lower operating temperatures can not break down all the materials in the feeds that cause the gasifier to only accept selective feedstock. However, the super-high temperature gasification system, that employs clean burning hydrogen technology or requires an external clean heat source such as concentrated solar energy, is a technologically advanced and environmentally friendly process that uses a higher temperature (~1000°C) working medium in an oxygen-starved environment to completely decompose the feedstock (including char, tar, soot, and solid waste) into pure syngas and inert vitreous slag. The solid vitreous slag is easily disposed of and will not get into the air. The fundamental knowledge base of this high temperature thermal-chemical conversion process is still not a fully predictive engineering science. This major scientific deficiency, which is well recognized in the gasification industry, must be rectified by the scientific talents and experimental technologies. By defining the critical parameters affecting product yields from various biomass and solid waste, and developing optimal conditions for thermal-chemical conversion to specific products, processes may be developed for the cost-effective production of synthesis gases (mainly, CO and hydrogen) with minimum impacts on the environment.
For the concept systems, the unique innovation of the systems includes the use of supercritical high temperature steam as both the heat source and the gasification agent in an oxygen-starved (air-free) environment. As part of the hydrogen generated from the gasification is used in the production of the steam, the system is self-sufficient in heat supply and does not require any external energy. The impact on the environment in terms of air pollution and global climate control is minimized. The advanced biomass to hydrogen conversion process to be covered in the concept systems will help ensure the availability of a highly efficient and clean technology.
In summary, as mentioned above that the high temperature air-free steam gasification process has the potential to revolutionize the biomass conversion to energy technology and development such that a new dimension would be created where systems with high-efficiency, minimum water consumption and no adverse impact on the environment and climate control can be developed.
Design Criteria of the Systems
The overall goal is to develop an advanced technology to produce clean fuels for a renewable and sustainable energy future in order to become independent of foreign oil. The primary objectives of the concept systems are as follows:
(1) Develop the best scientific, engineering, and technology solutions for converting lignocellulosic and MSW biomass resources economically to carbon-neutral clean energy, through an integrated gasification and membrane reactor system.
(2) Minimize water consumption and detrimental impacts of energy production on the environment (air pollution and global warming).
Literature Review and Background
Potential of Biomass as a Solution for Future Energy Needs
Our dependence on petroleum and oil has created future energy sustainability issues as the supply and availability of fossil fuels will soon reach the limit. The search for sustainable and renewable alternatives has been the top agenda for the society of energy research and development. To plan for future energy security and independence and for the post-oil energy needs, the recent U.S. NSF-DOE Workshop report (Huber, 2007) concluded that liquid biofuels produced from lignocellulosic biomass can significantly reduce our dependence on fossil fuels, create new jobs, improve rural economics, reduce greenhouse emissions, and ensure energy security. Additionally, the report specifically pointed out that the key roadblock for lignocellulosic biomass-derived fuels is the lack of engineering technology for the efficient conversion of biomass into energy. Accordingly, new technologies are required to replace fossil fuels with biofuels from renewable and sustainable biomass resources.
Among the biomass conversion to energy methods, the thermochemical reaction infrastructure is likely to be one of the most cost-effective conversion processes. Furthermore, biomass steam gasification can be considered as part of the highly effective technology for thermochemical conversion.
Kirtay (2011) summarized that biomass is a very versatile energy resource, that can produce energy generally in the more convenient forms of gases, liquids, chemicals, or electricity. Corradetti and Desideri (2007) found that based on a thermodynamics study the hydrogen fuel can be produced from woody biomass through gasification coupled with steam-methane reforming and water gas shift (WGS) reaction in a large-scale industrial plant with an efficiency of 62% that is similar to those of the existing competitive process technologies. The U.S. Natural Resources Defense Council has projected that an aggressive plan to make lignocellulosic biofuels in the U.S. could produce 7.9 million barrels of oil per day by 2050, or more than 50% of current total oil use in the transportation sector (Greene, 2004).
The synthetic biofuels produced by the Fischer–Tropsch process from lignocellulosic materials contain no sulfur, no particulates, no aromatics, and no nitrous compounds, thus making them very clean burning and reducing the production of acid rain. Because it has exactly the same chemical properties as fossil based diesel, it can be blended with regular diesel, stored and distributed using the same infrastructure. Although chemically identical to fossil diesel, it has a higher cetane number and on a gallon for gallon basis contains 22% more energy. In general, the synthetic lignocellulosic diesel has up to 80% less combustion emissions compared to petroleum diesel that include carbon dioxide, carbon monoxide, particulate matter, sulfur oxides, and hydrocarbons (Sheehan et al., 1998). Furthermore, these green biofuels are renewable, carbon-neutral, and sustainable.
Therefore, a very promising route to liquid fuels, in particular the synthetic lignocellulosic diesel, is the woody biomass gasification to synthesis gas (syngas: CO + H2) followed by the Fischer–Tropsch process to convert the syngas to hydrocarbon products. Fischer–Tropsch technology can be briefly defined as the means used to convert synthesis gas containing hydrogen and carbon monoxide to liquid hydrocarbon products (Damartzis and Zabaniotou, 2011; Sousa et al., 2011).
Steam Gasification of Biomass
Umeki et al. (2010) and Umeki et al. (2012) have studied a high temperature gasification process to generate hydrogen-rich fuel gas from woody biomass using steam with temperatures exceeding 1200K. They discovered that both the steam temperature and the molar ratio of steam to carbon (S/C ratio) affected the reaction temperature which strongly affects the gasified gas composition. They also reported that the tar concentration in the produced gas from the high temperature steam gasification process was higher than that from the oxygen-blown gasification processes. The highest cold gas efficiency was found to be 60.4%.
Baratieri et al. (2008) presented an equilibrium model (gas–solid), based on the minimization of the Gibbs energy, to estimate the theoretical yield and the equilibrium composition of the gases produced from a biomass thermochemical conversion process. The proposed model has been applied both to partial oxidation and steam gasification processes with varying air to biomass ratio (ER) and S/C ratio values and different feedstocks; the obtained results have been compared with experimental data and with other model predictions obtaining a satisfactory agreement.
Chang et al. (2011) investigated the steam gasification of agriculture waste at temperatures between 600 and 1000°C for the production of bio-hydrogen and syngas in a fluidized bed reactor. They also developed a kinetic model to determine the order of the reaction and activation energy. Their results suggested that at the equivalent ratio of 0.2 and at 1000°C the maximum yield of bio-hydrogen (29.5%) and CO (23.6%) was achieved and the CO2 concentration at this condition is 10.9% only.
Solar Energy – Heated Steam Gasification of Biomass
Piatkowski et al. (2011) indicated that solar-driven gasification, in which concentrated solar radiation is supplied as the energy source for the high temperature process heat to the endothermic reactions, offers an attractive alternative to conventional autothermal processes. Further they discovered that it has the potential to produce high-quality synthesis gas with higher output per unit of feedstock and lower specific CO2 emissions. The conclusion is that solar-driven gasification is an efficient means of storing intermittent solar energy in a transportable and dispatchable chemical form.
Gordillo and Belghit (2011) developed a numerical model for a solar downdraft gasifier of biomass char (biochar) with steam. The model predicts the dynamic and steady state profiles of the temperature and concentration of gas and solid phases, based on the mass and heat balances. The Rosseland equation is used to calculate the radiative transfer within the bed. They found that hydrogen is the principal product followed by carbon monoxide with negligibly small amount of carbon dioxide and methane. By applying the temperature gradient theory in the steam-only gasification process for a solar gasifier design, a solar downdraft gasifier improves the energy conversion efficiency by over 20% when compared to a solar packed bed gasifier.
Summary of the Current State of Knowledge
Based on the above literature review of the research and development advances in the steam gasification of biomass, we can conclude that the present state of knowledge have been focused solely on the potential, feasibility, characterization, and modeling of the gasification process itself. Whereas, what is needed the most in the actual engineering applications is the development of innovative systems that can take the feedstock and convert it to useful products efficiently and without casting negative impacts on the environment. In the current study, two concept systems are brought up and will be used as the model systems for the current study to focus on.
Description of Concept Systems
Fundamental Core Concept: Super-High Temperature Air-Free Steam Gasification
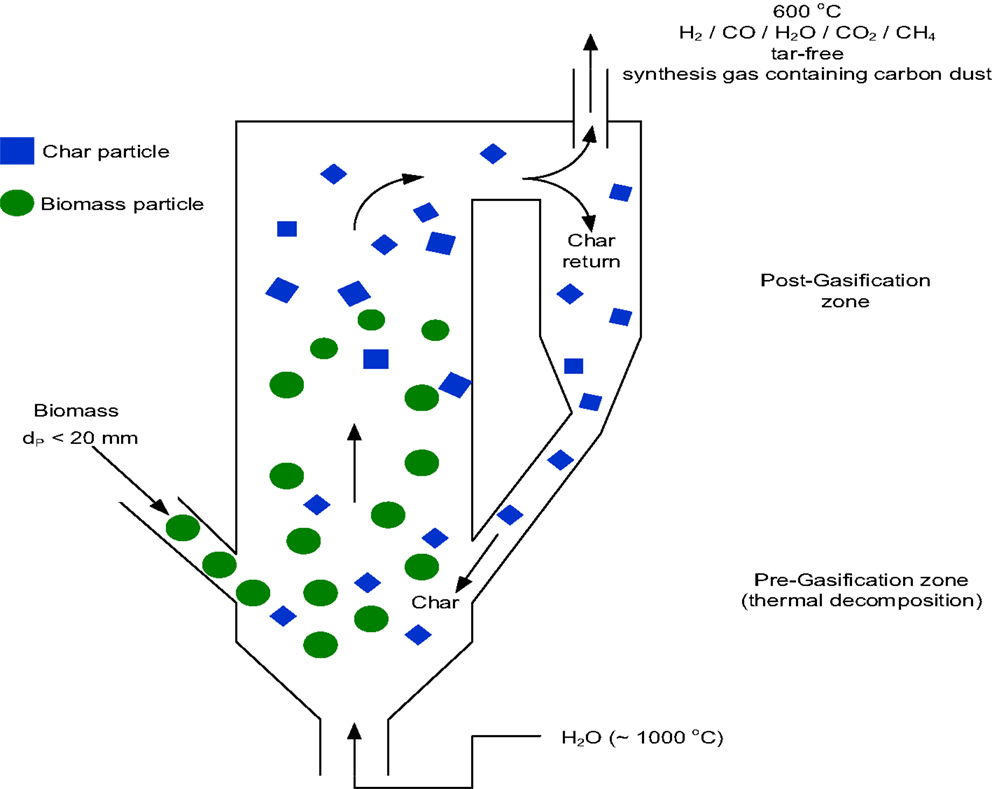
The heart of the biomass conversion to energy concept systems is the high temperature gasification unit. In an oxygen-starved environment, the gasification unit uses the supercritical high temperature (~1000°C) steam as the gasifying agent. The schematic of the gasification unit is shown in Figure 2. The gasification process that converts the lignocellulosic biomass to synthesis gas is composed of two stages as follows:
Pre-gasification:
Post-gasification:
The two-stage process given above shows the general chemical reactions taking place in an oxygen-starved super-high temperature environment. In the first stage (pre-gasification) the biomass is first thermally decomposed by the high temperature steam to flash-pyrolysis gases and residual char (carbon). The liberated gases consist primarily of higher hydrocarbons including tars, but are immediately deteriorated due to very high temperatures in the pre-gasification zone. In the second stage (post-gasification) the excess steam reacts with the residual char producing synthesis gas (syngas, a mixture of hydrogen and carbon monoxide). The recirculation loop is intended for providing more residence time for char conversion and tar cracking. It is noted that the gasification chamber integrates the pyrolysis process with the gasification process. The governing gasification reactions that occur in an oxygen-starved environment are endothermic. According to chemical equilibrium requirement, some CO2 and minor quantities of methane are also produced depending upon the specific feedstock and the gasifier operating conditions.
Concept Systems
According to the present state of knowledge based on the literature review given in Section “Literature Review and Background” above, we can concluded that all the reported research and developmental advances in the area of steam gasification of biomass have focused solely on the potential, feasibility, characterization, and modeling of the gasification process itself. Whereas, what is needed the most in the actual engineering applications is an integrated system that is the most efficient and can take the feedstock and convert it to the useful product. In the current paper, two concept systems are introduced below and will be used as the model systems for the study to focus on.
Concept system I (hydrogen combustion heat supply system)
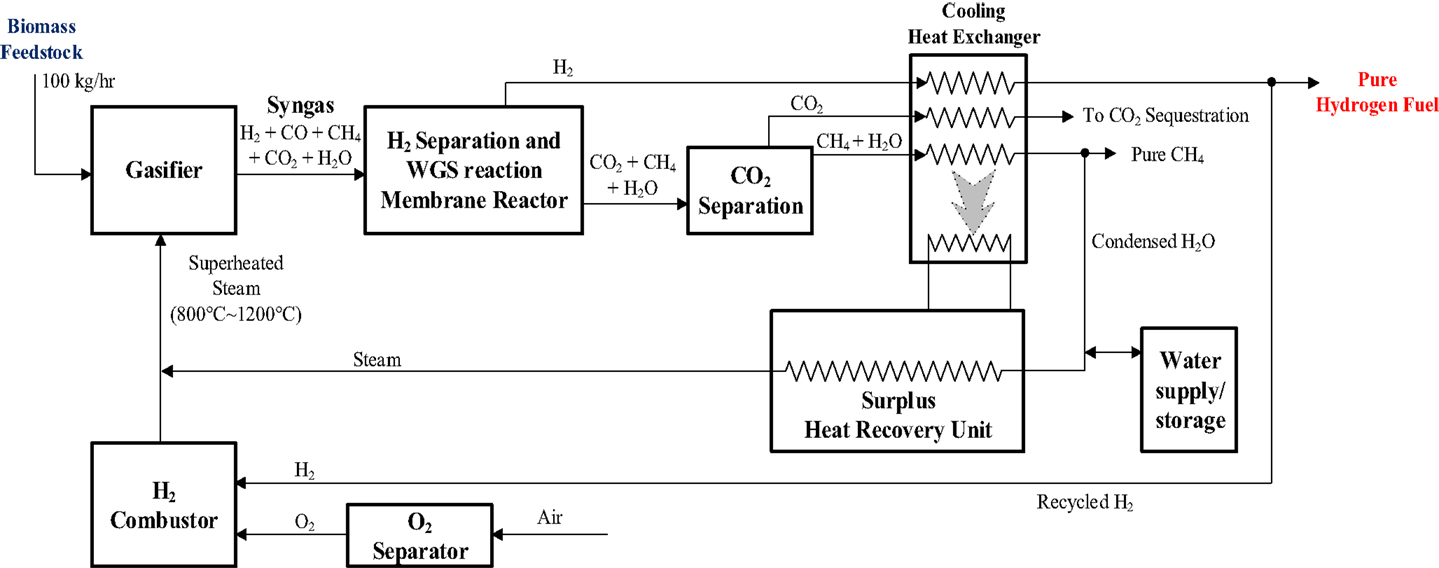
This system is basically a hydrogen combustion heated and gasification driven hydrogen fuel production infrastructure that is composed of five major components: a gasifier, a membrane reactor, a CO2 separator, a heat recovery unit, and a hydrogen combustor as explained below and shown in Figure 3.
High temperature gasification unit. Thermochemical conversion by air-free, high temperature supercritical steam at ~1000°C offers the required technology to convert biomass into a synthesis gas (mostly H2 and CO) which can be further converted to pure hydrogen or catalytically reformed into liquid hydrocarbon fuels (biodiesel or green gasoline) and chemicals. Several thermochemical reaction models have shown that syngas composition would be approximately the same when the steam temperature passes 1000°C (Gupta and Cichonski, 2007; Balu, 2013). It is noted that the steam gasification process runs at the atmospheric pressure, therefore no elevated pressure is required.
Membrane reactor. The key innovation is the integration of a novel high temperature ceramic membrane reactor whose feasibility has been established (Li et al., 2012). As indicated in Figure 3, the main functions of the reactor are to simultaneously separate hydrogen from the syngas and perform water gas shift reaction to produce more pure hydrogen and facilitate CO2 sequestration. A key role of the reactor is to separate hydrogen out of the syngas for specific applications.
CO2 separator. This component is simply an absorption bed for the sequestration of CO2.
Heat recovery unit. The core of this unit is a heat exchanger that recovers the heat associated with the high temperature syngas (~600°C) and transfers the high temperature thermal energy to heat the steam for the gasifier. The capture of the syngas thermal energy would drastically increase the system thermal efficiency.
Hydrogen combustor. This unit is used to provide the super-high temperature steam for the gasification unit. The hydrogen combustor draws the H2 fuel from the produced hydrogen by the gasification process. The produced hydrogen that is bled out to supply the H2 combustor is called the recycled H2.
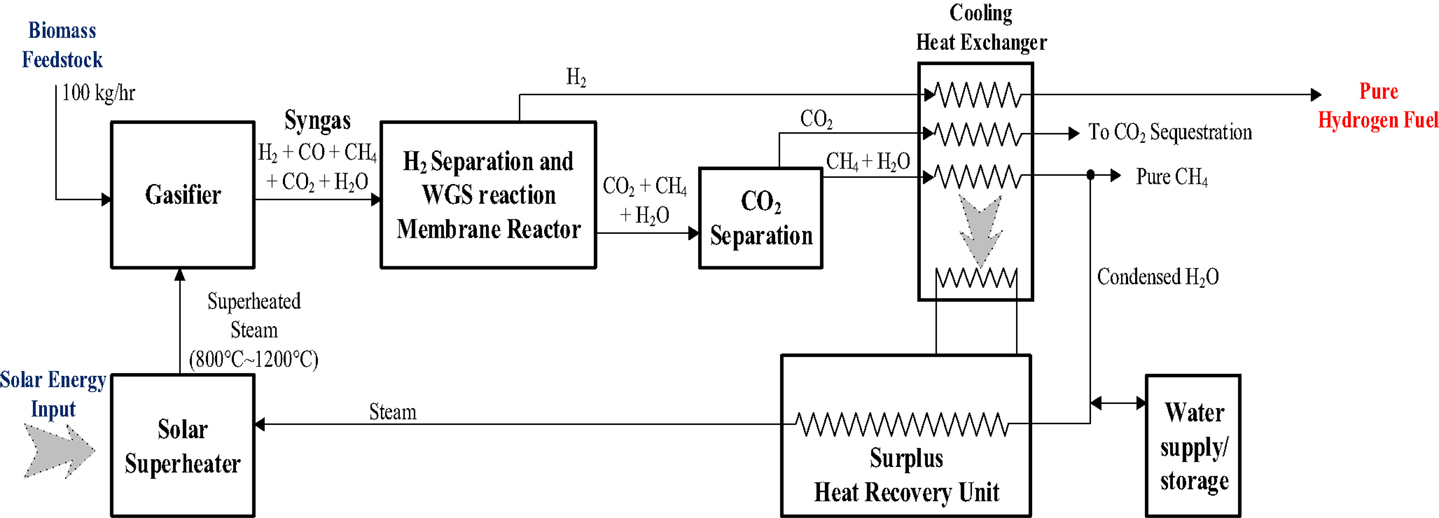
Concept system II (solar energy heat supply system)
This integrated system, shown in Figure 4, is different from the Concept System I basically in the steam production section where a concentrated solar heating unit replaces the hydrogen combustor for providing superheated steam to facilitate the gasification. The only new component is the solar heating unit that is described below:
Concentrated solar energy superheated steam generator. This unit will use concentrated solar power for the superheating of water vapor to desired gasification temperature. The water vapor will be generated from the condensed water vapor produced by the gasifier with additions from external supply.
Thermodynamic Analysis on the Performances of Both Systems
Syngas composition analysis
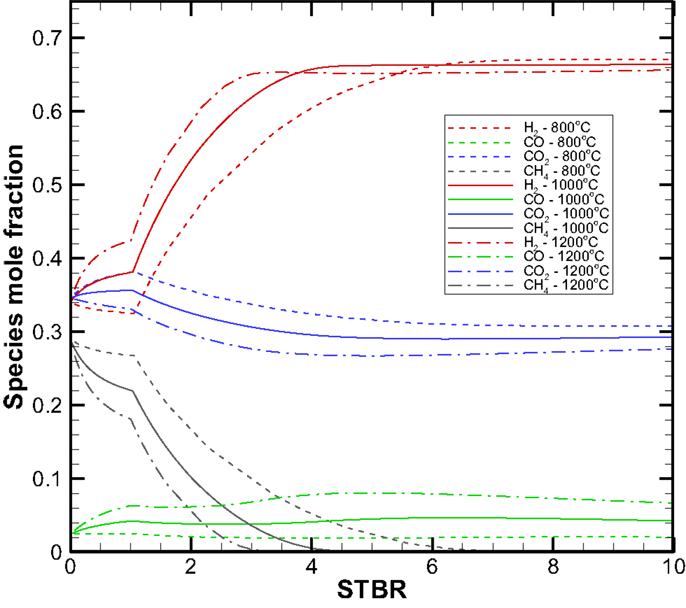
Based on a mass and energy balance analysis using the chemical reactions presented above in Section “Fundamental Core Concept: Super-High Temperature Air-Free Steam Gasification” and under thermal and chemical equilibrium conditions for the gasification unit, Balu (2013) developed a mathematicial model for predicting the syngas compositions from superheated steam gasification of biomass. Using a typical biomass with chemical composition of CH1.5O0.67, Balu (2013) provides in Figure 5 the molar fractions of gaseous species for a dry syngas (water vapor and solid carbon are excluded in the molar fraction results) for steam inlet temperatures of 800, 1000, and 1200°C. It can be seen that the molar fraction versus the steam to biomass molar ratio (STBR) results can be divided into three regions. Region 1 (0.1 < STBR < 1.0) refers to the existence of solid carbon deposit and Region 2 (1.0 < STBR < ~6.5) corresponds to no carbon deposit and CH4 production dropping down to zero. Region 3 (6.5 < STBR) starts when the molar fraction of CH4 reaches zero. After CH4 is no longer produced, there is no more carbon element left to sustain the increase in the production of CO, CO2, and H2 that results in the condition of the so called “saturated syngas composition” where the molar fractions of the gaseous species remain relatively constant and become independent of the STBR. Under the syngas composition equilibrium, the dry syngas would be composed of H2, CO, and CO2 and each component remains at a constant molar fraction.
System performance model
The system performance model is based on the following assumptions:
1. The gasifier is assumed to be perfectly insulated and in thermal and chemical equilibrium. So results given in Figure 5 are used for the performance analysis.
2. The H2 separation and WGS membrane reactor is an ideal membrane reactor that can completely separate the H2 in the syngas and those produced in the membrane by WGS. The WGS reactor, a part of the membrane, will convert all the CO in the syngas to H2 and CO2 as there is more than enough H2O vapor available.
3. The CO2 separator is also an ideal absorber that can completely remove the CO2 from CH4 and H2O.
4. The cooling heat exchanger and the surplus heat recovery unit are all ideal heat exchanger with 100% effectiveness.
5. The H2 combustor is totally insulated and operates under the adiabatic flame temperature condition.
6. The solar superheater is capable of heating the steam to the maximum of 1200°C.
Based on the above assumptions, the model that can predict the performance of the integrated system has been developed using the conservation of mass and energy principles.
System performance evaluation
The performance evaluations for both concept systems are based 100 kg/h biomass feeding rate. Also, the evaluations would only cover Regions 2 and 3, so the STBR would start from one for all the cases as the formation of solid carbon in Region 1 (STBR < 1) always means a much less efficient use of biomass resources.
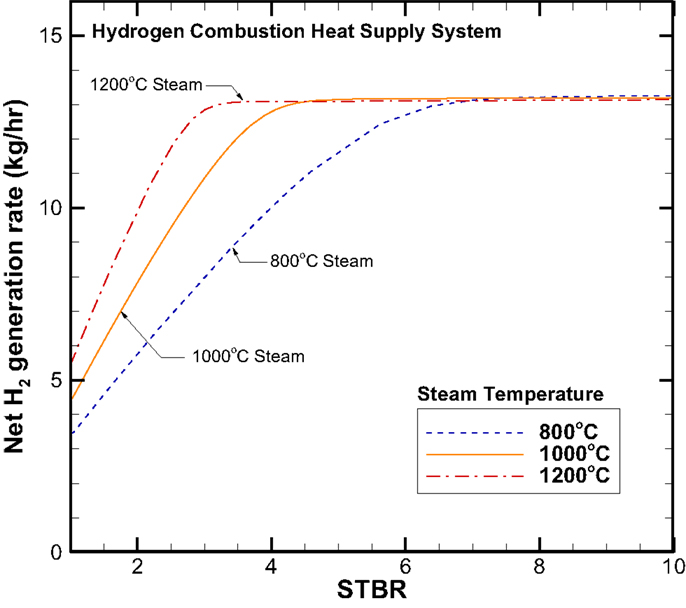
Concept system I – hydrogen combustion heat supply system. First, the net hydrogen production rate as a function of the STBR is shown in Figure 6. The net hydrogen production rate is a direct reflection of the syngas compositions profile shown in Figure 5. In Region 2, the H2 production rate increases with the STBR, while for a given STBR, a higher steam inlet temperature results in a higher H2 production rate. In Region 3, both the STBR and steam inlet temperature make no difference on the composition as the system has reached the saturation condition due to the exhaustion of carbon element. For the 100 kg/h biomass input rate, the maximum H2 production rate is around 13.2 kg/h.
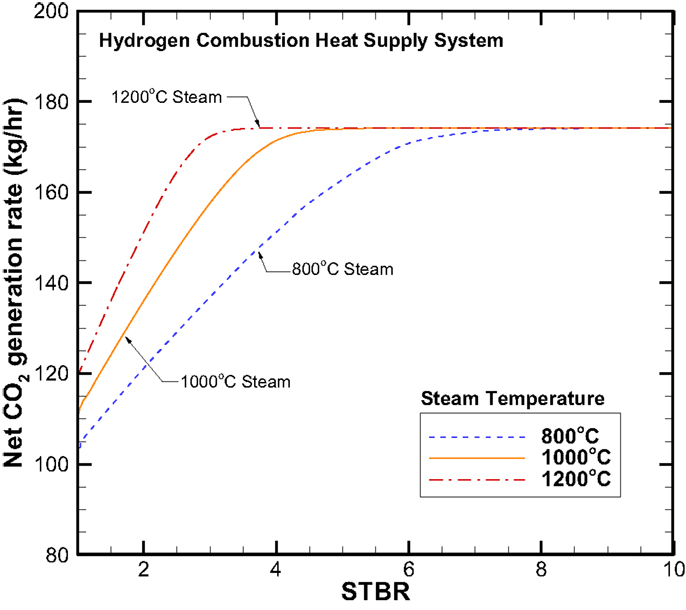
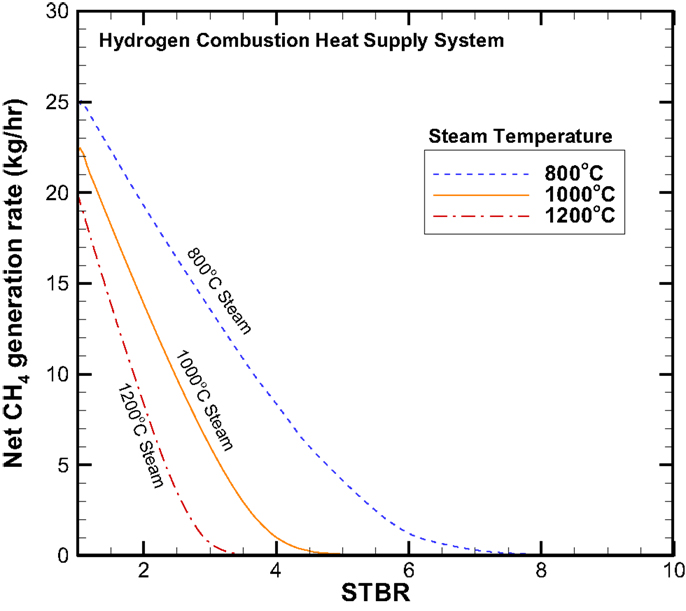
Figures 7 and 8 provide the net CO2 and CH4 production rates, respectively. While the CO2 production rate follows the same trend as that of the H2 production, however the CH4 displays a reversed trend where the higher the steam inlet temperature causes a lower CH4 production rate. The CH4 production rate also decreases with increasing STBR.
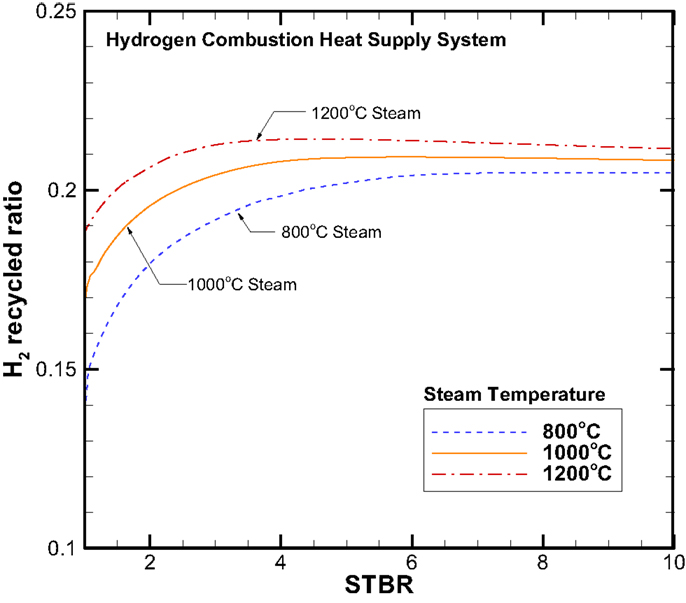
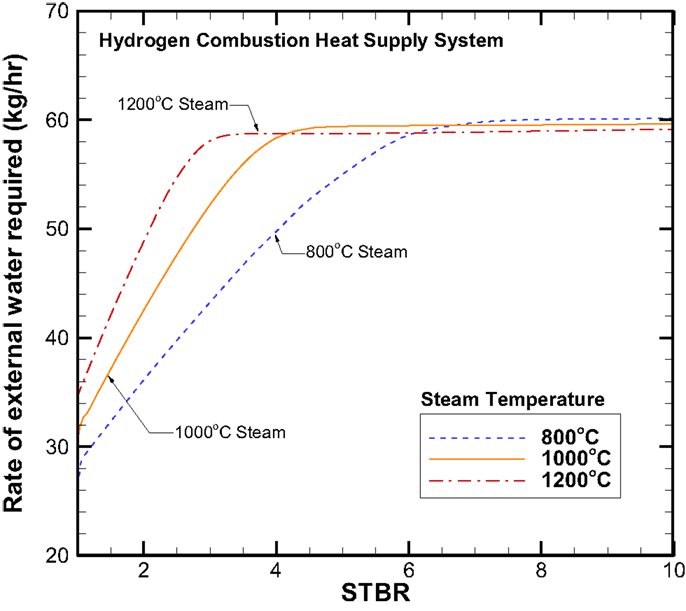
Next, we would discuss the water balance together with the H2 recycle ratio. Figure 9 gives the information for the hydrogen recycle ratio that is defined as the amount of H2 taken back to facilitate the H2 combustion for steam production to that of the total hydrogen produced. Figure 10 shows the rate of external room-temperature water supplied. It should be made clear that the results given in Figures 9 and 10 are based on the principle of minimizing the ratio of recycled H2 or maximizing the net H2 production. In Region 2 as shown in Figure 9, this recycle ratio increases with the STBR and for a given STBR it is higher for a higher steam inlet temperature. However, in Region 3, this ratio becomes independent of the STBR and it increases slightly with increasing steam inlet temperature. The maximum H2 recycle ratio is around 21%. As expected, the trend on the rate of external water required is very similar to that of H2 production rate and it maximizes around 60 kg/h.
The most useful parameter of performance evaluation is the efficiency of the system. We will take a look at three different types of efficiencies for the two systems.
The cold gas and hot gas efficiencies are applicable to both concept systems and they are defined as follows solely for the gasifier.
The overall system efficiency is defined for the entre integrated system, therefore a different definition is required for each concept system.
Concept system I – hydrogen combustion heat supply system.
It is noted that the hydrogen combustion heat supply system is self-sustainable with the help of hydrogen recirculation and thermal energy recovery.
Concept system II – solar energy heat supply system.
It is interesting to note that the solar energy heat supply system also includes the thermal energy recovery internally that reduces the input energy from the solar power.
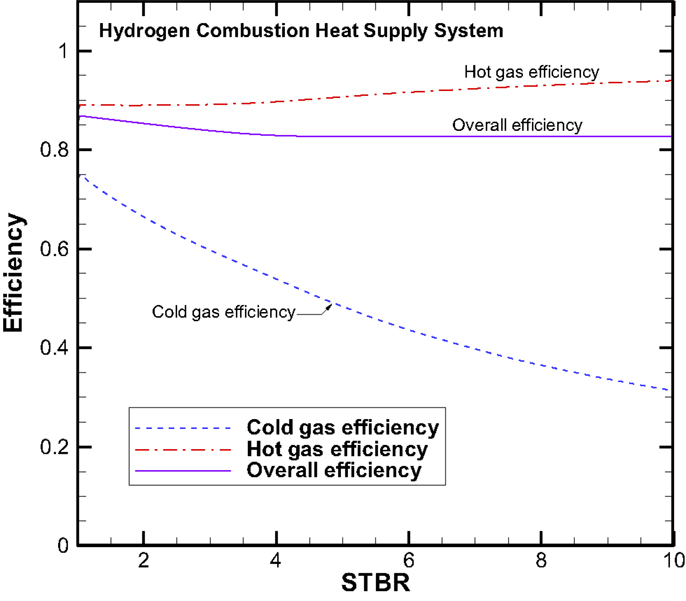
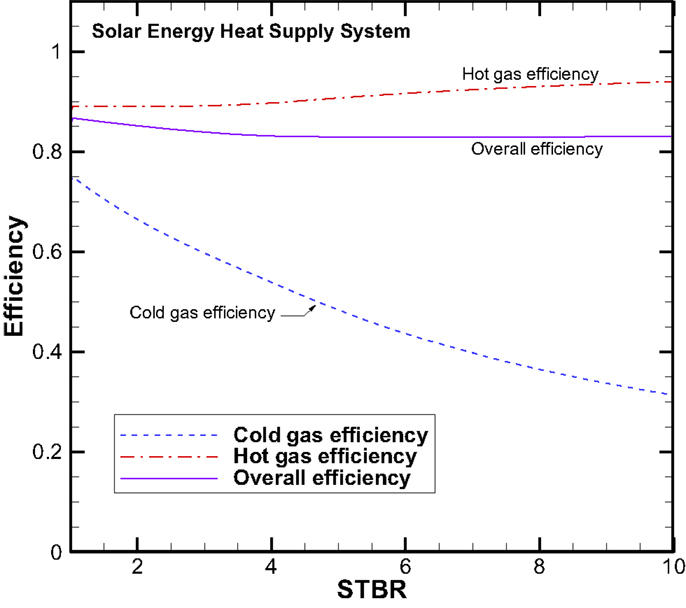
The three efficiencies for the concept system I are plotted in Figure 11. The cold gas efficiency starts at 78% for the STBR at unity and decreases monotonically with increasing STBR. At the STBR of 10, the cold gas efficiency is down to 32%. The main reason that the cold gas efficiency drops at higher STBRs is due to that more energy is needed for heating the increasing amount of steam than the chemical energy gained. However for the hot gas efficiency that includes the thermal energy of the syngas is relatively insensitive to the change in STBR. The hot gas efficiency varies between 89 and 94%. On the overall system efficiency, it is also relatively constant with values ranging between 83 and 87%. Based on the values of the three different efficiencies, it can be summarized that if our goal is to produce more chemical fuels, the STBR should be kept at lower values. The STBR is not an important parameter if we are looking at the sum of chemical energy and thermal energy together.
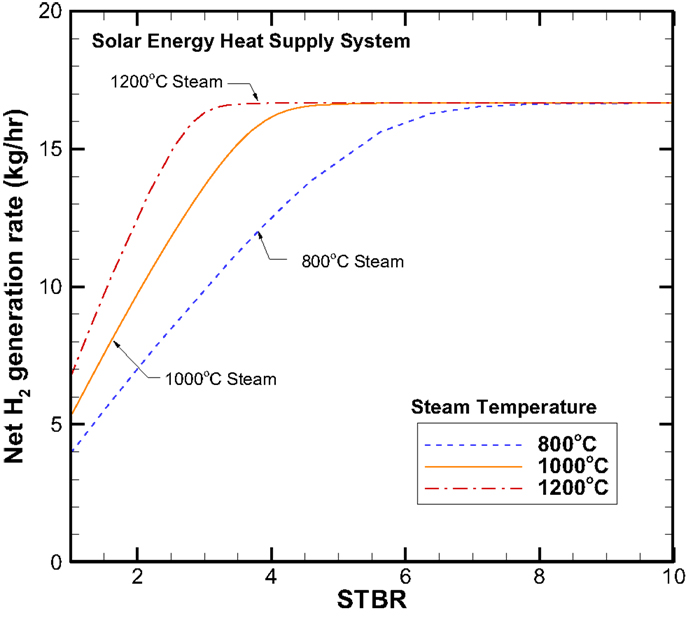
Concept system I – solar energy heat supply system. Again, let us examine the net hydrogen production rate as a function of the STBR that is shown in Figure 12. As mentioned above, the net hydrogen production rate is a direct reflection of the syngas compositions shown in Figure 5. Therefore the trends of hydrogen production rates are very similar to those of the H2 combustion heating case given in Figure 6. The only distinctive difference is that the quantitative H2 generation rate is uniformly higher for the solar energy heating case. For example, under the 100 kg/h biomass consumption rate, the maximum H2 production rate is around 16.8 kg/h as compared to the 13.2 kg/h for the H2 combustion heating case. The increase in the H2 production rate is due to the heating of the steam by solar power that eliminates the need for recycling H2 and thus results in more H2 production.
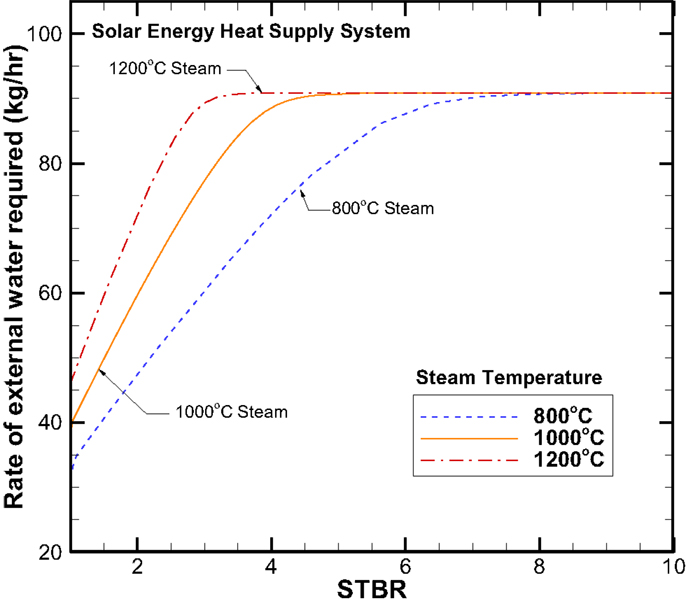
The net CO2 and CH4 production rates are identical to those in the H2 combustion heating case, therefore the plots will not be repeated here. Figure 13 provides the external water required for the system water balance. When comparing with the H2 combustion heating case (Figure 10), the trends are similar but the quantitative external water flow rate is uniformly higher. For example the maximum external water supply rate required is 91 kg/h for the solar energy heating case versus the 60 kg/h for the H2 combustion heating case.
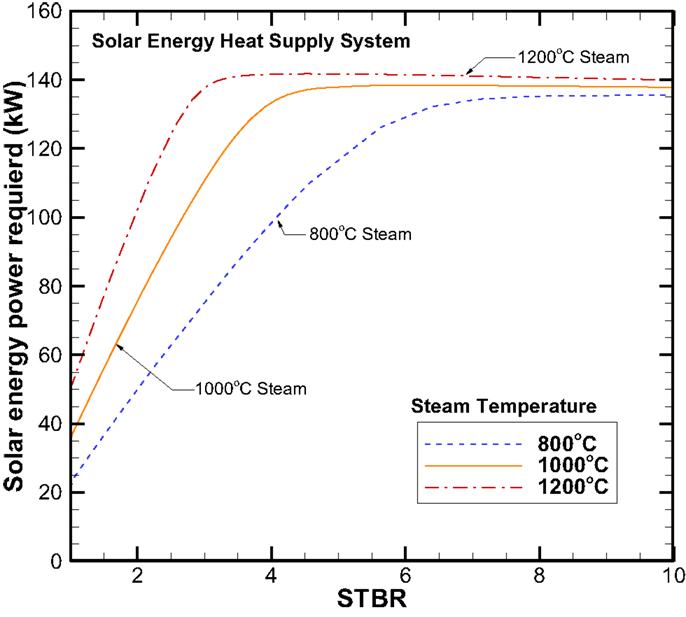
For the solar energy power required, Figure 14 shows that required solar power increases with both steam inlet temperature and STBR up to STBR equal to 8. After that, the solar power required is reaching a saturated condition with 140, 138, and 135 kW for inlet steam temperatures of 1200°C, 1000°C, and 800°C, respectively.
Again, three efficiencies for the solar energy heating case are plotted in Figure 15. As expected the cold gas and hot gas efficiencies are identical for both cases as the definitions are the same. While the overall system efficiencies should be very close to each other as the solar energy input results in more chemical energy produced that balances the energy budget.
Discussion and Conclusion
(1) The high temperature oxygen-starved (air-free) gasification uses supercritical steam at >800°C to gasify the feedstock. Almost all of the carbon is converted to pure fuel gas. As a result, all the tars and char are broken down with almost no ash. The trace amounts of inorganic materials in the biomass are converted into inert vitreous slag – very high environmental benefit.
(2) The hydrogen combustor in System I that provides the supercritical steam gasifying agent is a very clean combustion process and draws the fuel from the H2 produced by the gasification process (~20% of total H2 produced), so the system is self-sufficient and there is no need for external heat supply.
(3) The solar heating in System II that provides the heat for producing supercritical steam at 1000°C is also a very clean process with a renewable source and zero carbon release.
(4) With the membrane reactor and CO2 absorber, we can produce pure hydrogen and totally capture CO2 for sequestration.
(5) Biomass energy brings numerous environmental benefits – reducing air and water pollution, increasing soil quality and reducing erosion, and improving wildlife habitat.
(6) Due to the high temperature gasifying agent, the selectivity of the feedstock is substantially increased. Other than agricultural and forest biomass, most of MSW can be included.
(7) Combined with pure hydrogen production, both high temperature steam gasification systems potentially possess more than 80% in first law overall system thermodynamic efficiencies.
(8) This high temperature thermal-chemical transformation process consolidates system components by providing completely tar cracking and reforming catalysts, thereby increasing the thermodynamic efficiency and reducing the cost and risk of gasification-based process technology.
(9) For applications in the rural and farm infrastructure, we recognize that the biomass is inherently dispersed and abundant, making the energy costs for harvesting and transporting them prohibitive unless the fuel processing plant is nearby. As the concept systems can be designed at various scales, establishing a network of mobile and distributed energy (DE) plants would be another advantage of the biomass gasification systems.
(10) It is worth noting that the most significant benefit of using steam as the gasifying agent is the much higher production rate of hydrogen. In an air-blown autothermal partial combustion and partial gasification system, the molar ratio of H2 to CO is about unity while in a steam gasification system, this ratio is six. The higher H2 production is facilitated by the WGS reaction that converts CO and water to H2 and CO2.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The research was supported by the Florida Energy Systems Consortium and Florida Institute of Sustainable Energy. Partial support by the Andrew H. Hines, Jr., Florida Progress Energy endowment fund is also acknowledged. Mr. Uisung Lee prepared Figures 5–15.
References
Balu, E. (2013). A Study of Biomass Gasification Systems and Hydrogen Production Using High Temperature Proton Conducting Ceramic Membrane. Ph.D. Dissertation, University of Florida.
Baratieri, M., Baggio, P., Fiori, L., and Grigiante, M. (2008). Biomass as an energy source: thermodynamic constraints on the performance of the conversion process. Bioresour. Technol. 99, 7063–7073. doi:10.1016/j.biortech.2008.01.006
Chang, A. C. C., Chang, H. F., Lin, F. J., Lin, K. H., and Chen, C. H. (2011). Biomass gasification for hydrogen production. Int. J. Hydrogen Energy 36, 14252–14260. doi:10.1016/j.ijhydene.2011.05.105
Corradetti, A., and Desideri, U. (2007). Should biomass be used for power or hydrogen production? J. Eng. Gas Turb. Power 129, 629–636. doi:10.1115/1.2718226
Damartzis, T., and Zabaniotou, A. (2011). Thermochemical conversion of biomass to second generation biofuels through integrated process design – a review. Renew. Sustain. Energ. Rev. 15, 366–378. doi:10.1016/j.rser.2010.08.003
Gordillo, E. D., and Belghit, A. (2011). A downdraft high temperature steam-only solar gasifier of biomass char: a modelling study. Biomass Bioenergy 35, 2034–2043. doi:10.1016/j.biombioe.2011.01.051
Greene, N. (2004). Growing Energy: How Biofuels Can Help End America’s Oil Dependence. New York: Natural Resources Defense Council. Available from: http://www.nrdc.org/biofuels/biofuels.pdf
Gupta, A. K., and Cichonski, W. (2007). Ultrahigh temperature steam gasification of biomass and solid wastes. Environ. Eng. Sci. 24, 1179–1189. doi:10.1089/ees.2007.0120
Holdren, J. P. (2001). The energy-climate change: issues for the new US administration. Environment 43, 8–21. doi:10.1080/00139150109605143
Huber, G. W. (2007). Breaking the Chemical and Engineering Barriers to Lignocellulosic Biofuels: A Research Roadmap for Making Lignocellulosic Biofuels a Practical REALITY. Washington, DC: NSF, DOE and American Chemical Society Workshop.
Kalicki, J. H., and Goldwyn, D. L. (2005). Energy and Security: Toward a New Foreign Policy Strategy. Baltimore, MD: The Johns Hopkins University Press.
Kirtay, E. (2011). Recent advances in production of hydrogen from biomass. Energ. Convers. Manag. 52, 1778–1789. doi:10.1016/j.enconman.2010.11.010
Li, J., Yoon, H., Oh, T. K., and Wachsman, E. D. (2012). SrCe0.7 Zr0.2 Eu0.1 O3-based hydrogen transport water gas shift reactor. Int. J. Hydrogen Energy 37, 16006–16012. doi:10.1016/j.ijhydene.2012.08.040
McKendry, P. (2002). Energy production from biomass (part 3): gasification technologies. Bioresour. Technol. 83, 55–63. doi:10.1016/S0960-8524(01)00120-1
Pandey, M. P., and Kim, C. S. (2011). Lignin depolymerization and conversion: a review of thermochemical methods. Chem. Eng. Technol. 34, 29–41. doi:10.1002/ceat.201000270
Piatkowski, N., Wieckert, C., Weimer, A. W., and Steinfeld, A. (2011). Solar-driven gasification of carbonaceous feedstock – a review. Energ. Environ. Sci. 4, 73–82. doi:10.1039/c0ee00312c
Sheehan, J., Camobreco, V., Duffield, J., Graboski, M., and Shapouri, H. (1998). Life Cycle Inventory of Biodiesel and Petroleum Diesel for Use in an Urban Bus. Report, NREL/SR-580-24089 UC Category 1503. Golden: Department of Energy National Renewable Energy Laboratory.
Sousa, E. F., Noronhac, F. B., and Faro, A. (2011). The main catalytic challenges in GTL (gas-to-liquids) processes. Catal. Sci. Technol. 1, 698–713. doi:10.1039/c1cy00116g
Umeki, K., Namioka, K., and Yoshikawa, K. (2012). Analysis of an updraft biomass gasifier with high temperature steam using a numerical model. Appl. Energ. 90, 38–45. doi:10.1016/j.apenergy.2010.12.058
Keywords: thermochemical process, steam, concept system, hydrogen production, biomass, solar energy
Citation: Chung JN (2014) A theoretical study of two novel concept systems for maximum thermal-chemical conversion of biomass to hydrogen. Front. Energy Res. 1:12. doi: 10.3389/fenrg.2013.00012
Received: 15 November 2013; Accepted: 19 December 2013;
Published online: 02 January 2014.
Edited by:
Junye Wang, Athabasca University, CanadaCopyright: © 2014 Chung. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: J. N. Chung, Department of Mechanical and Aerospace Engineering, University of Florida, Gainesville, FL 32611, USA e-mail: jnchung@ufl.edu
 J. N. Chung
J. N. Chung