Potential use of microbial electrolysis cells in domestic wastewater treatment plants for energy recovery
- Chemical and Environmental Bioprocess Engineering Group, Natural Resources Institute (IRENA), University of León, León, Spain
Globally, large amounts of electrical energy are spent every year for domestic wastewater (dWW) treatment. In the future, energy prices are expected to rise as the demand for energy resources increases and fossil fuel reserves become depleted. By using appropriate technologies, the potential chemical energy contained in the organic compounds present in dWWs might help to improve the energy and economic balance of dWW treatment plants. Bioelectrochemical systems (BESs) in general and microbial electrolysis cells (MECs) in particular represent an emerging technology capable of harvesting part of this energy. This study offers an overview of the potential of using MEC technology in domestic wastewater treatment plants (dWWTPs) to reduce the energy bill. It begins with a brief account of the basics of BESs, followed by an examination of how MECs can be integrated in dWWTPs, identifying scaling-up bottlenecks and estimating potential energy savings. A simplified analysis showed that the use of MEC technology may help to reduce up to ~20% the energy consumption in a conventional dWWTP. The study concludes with a discussion of the future perspectives of MEC technology for dWW treatment. The growing rates of municipal water and wastewater treatment markets in Europe offer excellent business prospects and it is expected that the first generation of MECs could be ready within 1–4 years. However, before MEC technology may achieve practical implementation in dWWTPs, it need not only to overcome important techno-economic challenges, but also to compete with other energy-producing technologies.
Introduction
Domestic wastewater (dWW) often consist of a complex mixture of organics that must be removed before discharge into the environment. Activated sludge (AS) systems, which have become a conventional wastewater treatment method in developed nations, usually make use of large blowers (to favor oxygen transfer from air into the mixed liquor) that are energy intensive and increase the treatment costs. In 2009, 12,800,974 m3 of wastewater was treated daily in Spain (INE, 2010), with an associated energy cost of 0.53 kWh/m3 of wastewater (IDAE, 2010) resulting in a power consumption of 2476 GWh year−1 (~1% of the Spanish electrical energy consumption) (IEA, 2011).
In addition, energy prices in Europe have been continuously rising during the second half of the past decade (and will likely continue to rise in the near future as carbon-based fuels become depleted) from an average €0.0756 kWh−1 in the EU-27 in 2005 to €0.1023 kWh−1 in 2009 (Eurostat, 2011). Hence, the operating costs of treating wastewater are bound to increase despite the fact that a significant amount of the energy investment can be recovered as biogas from anaerobic digesters. To make matters worse, aerobic treatments produce large amounts of sludge that needs to be disposed-off at a cost that can reach €500 per ton dry matter (Weemaes and Verstraete, 2001). However, the costs of operating treatment plants could be greatly reduced if we manage to use the energy in the wastewater. For example, at a conventional wastewater treatment plant in Toronto, Canada, the potential energy available in the raw wastewater exceeded the electricity requirements for the treatment process by a factor of 9.3 (Shizas, 2004); thus, turning such wastewaters into potential commodities from which bioenergy and biochemicals may be produced (Angenent et al., 2004). Nevertheless, due to its complex composition, wastewater exploitation requires flexible and robust technologies. From this perspective, biological treatment is the ideal candidate because biological conversions in natural ecosystems commonly occur in dilute aqueous environments (Rozendal, 2007). Among the several chemicals that may be extracted from wastewaters, hydrogen occupies a preeminent position because of its interesting characteristics as a fuel: it is a clean and carbon dioxide (CO2)-neutral energy carrier and can be converted directly into electrical energy very efficiently using fuel cell technology. The biological methods for generating H2 include light-dependent methods (direct and indirect bio-photolysis and photo-fermentation) and non-light-dependent methods, which traditionally include the dark fermentation process and the water–gas shift reaction mediated by photoheterotrophic bacteria (Gómez et al., 2011). However, all of these methods are characterized by low efficiency in terms of hydrogen yield (Angenent et al., 2004), which is commonly attributed to thermodynamic limitations and the methanogenic consumption of hydrogen (Hawkes et al., 2002; Kim et al., 2004). In fact, under standard conditions, complete oxidation of carbohydrates to CO2 and H2 is thermodynamically not possible because a significant amount of volatile fatty acids (VFAs) and some other organics (e.g., lactate, ethanol, butanediol, succinate) are always produced.
Alternatively, it was recently discovered that this thermodynamic barrier may be overcome by means of a small input of electrical energy (Liu et al., 2005b; Rozendal and Buisman, 2005; Rozendal et al., 2006b) in what has been called a microbial electrolysis cell (MEC). More recent developments in bioelectrochemical systems (BESs) suggest that MECs may represent a promising technology for combining wastewater treatment and energy recovery (Ditzig et al., 2007; Logan et al., 2008; Rozendal et al., 2008a,b; Tartakovsky et al., 2009; Pinto et al., 2011) by using the wastewater stream as a free electron supply.
In this work, we explore the potential of MECs as a complement to more conventional biological treatments in domestic wastewater treatment plants (dWWTPs), stressing the energy savings that this technology may bring. We also analyze some of the bottlenecks and barriers that need to be overcome in order to make MECs an economically, technically, and environmentally feasible technology suitable for commercial application in dWW treatment facilities.
Bioelectrochemical Systems: Energy Recovery from Organic Matter Contaminated Streams
A BES can be defined as an electrochemical system in which at least one of the anodic or cathodic reactions is microbially catalyzed (Rabaey et al., 2007). In BESs, microorganisms function as catalysts to convert chemical energy into other various types of energy by transferring electrons to the anode in an oxidation reaction or by accepting electrons from the cathode in a reduction reaction (Rozendal et al., 2008b; Logan, 2009). If a BES is producing electrical energy, then the system is referred to as microbial fuel cell, whereas if it consumes electrical energy to drive the electrochemical reactions it is termed a MEC (Rozendal et al., 2006b). The anodic reactions in both MFCs and MECs are quite similar, and almost any source of organic matter, such as carbohydrates and lipids (or even the complex mixtures of organics usually found in wastewaters), represent a suitable fuel for BES. However, the main difference lies on the cathode side. Whereas in MFCs the presence of an oxidative agent (usually oxygen) causes the electrical current to flow spontaneously, MECs require a certain amount of electrical input to drive the redox reactions because no oxygen (or oxidative agent) is allowed to enter the cathode chamber. Figure 1 shows a schematic representation of the operation of BESs. Electrons (e−), protons (H+), and CO2 are the main sub-products of the metabolism of the anode respiring bacteria (ARB) present in the biofilm. The electrons flow through an external circuit (either an electric load or a power source depending on whether the BES operates in electricity or hydrogen production mode). In both cases, protons migrate through the membrane into the cathodic chamber where they re-combine with electrons, producing either water when oxygen is allowed to enter into the cathodic chamber (MFC), or hydrogen when no oxygen is allowed to get into the cathodic chamber and electrons are forced to circulate by means of a power source (MEC). Further information about the underlying principles of the BES, the microbial communities involved and the mechanisms of electron transfer can be found elsewhere (Rabaey, 2010).
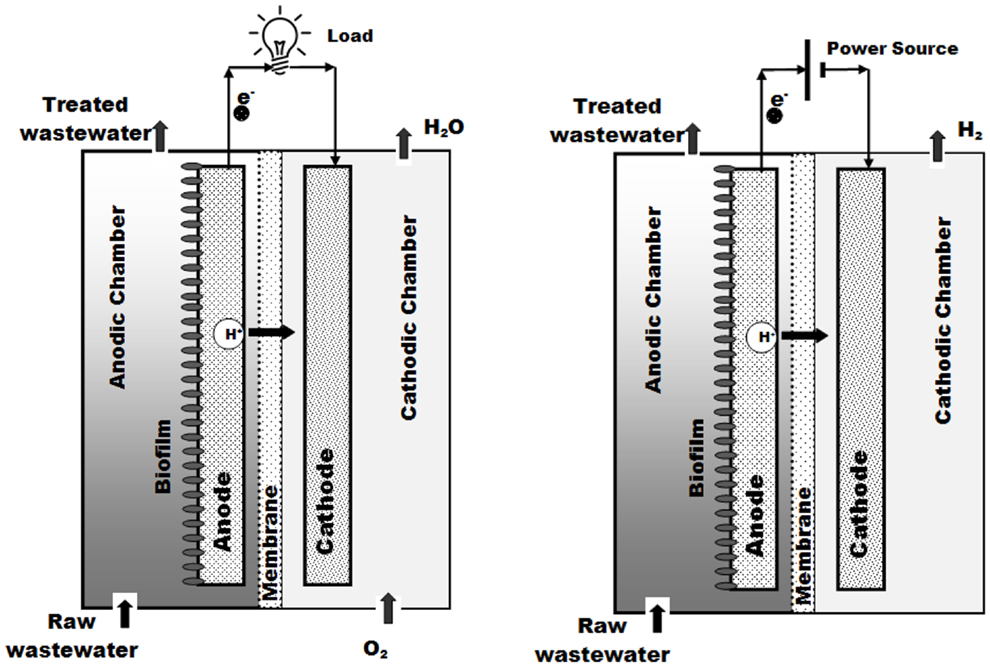
Figure 1. Schematic representation of electricity production (left) and hydrogen production (right) through MFC and MEC, respectively.
Electricity and hydrogen are the most common outcome of BES, but they are not the only ones: one of the first processes investigated (aside from hydrogen and electricity) was the production of sodium hydroxide in the cathode of an MEC (Rozendal et al., 2006a). More recently, a novel device that combines a MEC with a microbial desalination cell (MDC) had the feature of simultaneous HCl and NaOH production while reducing the organic load of a synthetic wastewater (Chen et al., 2012). Hydrogen peroxide is another valuable product that can be generated by stabilizing the oxygen reduction in the cathode of an MEC (Rozendal et al., 2009). It has also been demonstrated that CO2 can be reduced to methane in microbial biocathodes (Cheng et al., 2009). Methane production represents an attractive alternative to hydrogen in MECs due to the difficulty of obtaining pure hydrogen in the cathode of an MEC (because methane is the main contaminant). More recently, phosphorus was precipitated as struvite while simultaneously producing a significant amount of hydrogen in the cathode of an MEC (Cusick and Logan, 2012). Under microaerophilic conditions, Srikanth et al. (2012) succeeded in producing polyhydroxybutyrate (PHA) in a biocathode. Another interesting application of BES is the treatment of inorganic and recalcitrant pollutants, such as nitrates, nitrites, dyes (Mu et al., 2009; Wang et al., 2013), and chlorinated solvents (Liang et al., 2013).
In addition, novel applications have emerged, resulting in the development of new types of BESs based on the concept of MFCs and MECs. Cao et al. (2009) introduced the idea of simultaneous desalination, as well as wastewater treatment and hydrogen energy recovery, giving birth to MDCs. Microbial solar cells (MSC) represent another original development of BES because they make possible the harvesting of solar energy using photoautotrophic microorganisms or higher plants in combination with conventional BESs to generate electrical current (Strik et al., 2011). BESs can also be used as an alternative method to assess the amount of pollutant organic matter in water (Peixoto et al., 2011).
All of the processes and products described above highlight an important feature that BESs share with other biochemical systems, namely their ability to convert negative-value waste streams into value added products by using the residual energy content. MECs in particular have proved to be a robust and flexible technology capable of using pure compounds (acetate, glucose, and glycerol) (Rozendal et al., 2006b; Tartakovsky et al., 2008; Escapa et al., 2009) and complex mixtures of organics as fuels. The latter usually correspond to wastewaters, such as those produced in swine facilities (Wagner et al., 2009), food processing industries (Kiely et al., 2011), wineries (Cusick et al., 2011), and dWW (Gil-Carrera et al., 2013c).
From all of the possible potential uses of BESs and all of the available feedstock (usually wastes) briefly discussed above, this study will focus on the feasibility of using dWW as a fuel for hydrogen production in an MEC. Our choice of hydrogen rather that electricity as the output is mainly based on techno-economic considerations: the economic value of hydrogen is relatively high compared with the value of electricity, which makes MECs the more economical choice for dWW treatment, making electricity a less attractive output (Cusick et al., 2010; Harnisch et al., 2011). Moreover, the internal resistance and current density required for a BES to become cost–effective are much more restrictive for an MFC than for an MEC (Sleutels et al., 2012), confirming that the production of valuable products seems to be the most attractive use of BESs (Rabaey and Rozendal, 2010).
Integrating MEC Technology in Domestic Wastewater Treatment Plants for Energy Recovery
Wastewaters are polluted with many materials and substances, the most common being: (i) organic materials, as measured by the biological demand for oxygen (BOD) or the chemical demand for oxygen (COD); (ii) nitrogen, which includes organic nitrogen, nitrates, nitrites, and ammonium; (iii) phosphorus; (iv) suspended solids; (v) pathogenic organisms (as estimated by coliforms); and (vi) traces of persistent organics (such as chlorinated pesticides). In general, the removal of these contaminants comprises a set of physical, biological, and chemical treatment methods, the final arrangement depending on the type of WW to be treated. Although dWWTP can vary greatly in terms of their design, they often take the general form as shown in Figure 2A, which includes the most characteristic elements of the water-treatment: (i) the preliminary treatment, where the dWW is first screened to remove large debris and grit in order to protect pumps and the remainder of the unit operations. (ii) The primary treatment: its purpose is to remove all particles that are settable. (iii) The secondary treatment, which typically consists of two components: a bioreactor where soluble organic material gets converted mainly to bacterial biomass and carbon dioxide, and a settling tank (called secondary clarifier) where the bacterial biomass is recovered. (iv) Advanced treatment, which includes several polishing or cleanup processes, such as nutrients removal (mainly phosphorous and nitrogen) and disinfection. (v) Residuals management, where solids and sludge removed by other process are collected, stabilized, and subsequently disposed.
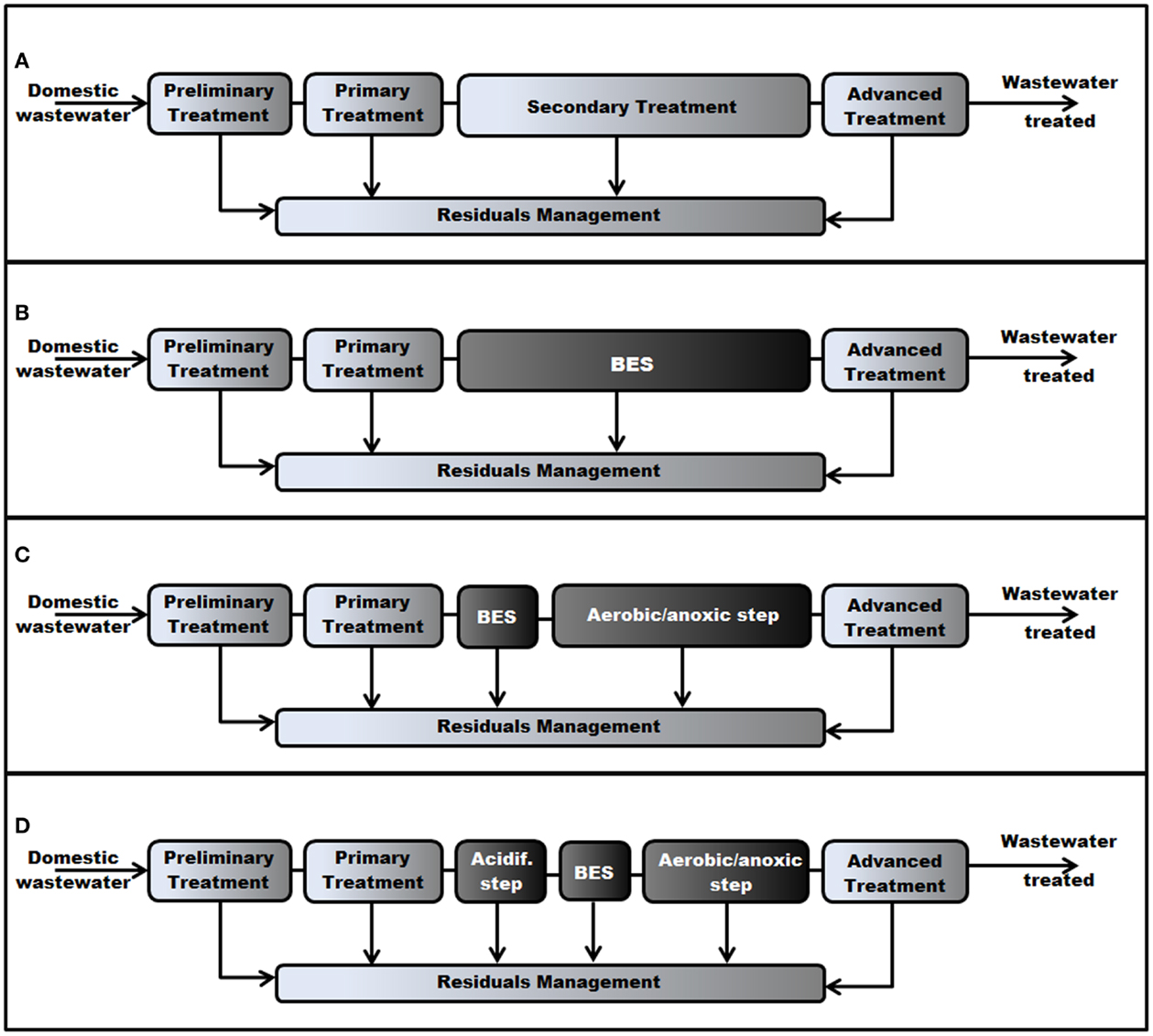
Figure 2. (A) Arrangement of a typical large wastewater treatment facility integrating a BES. (A) Traditional arrangement with no BES (drawn with modifications from Vesilind, 2003). (B) The BES replaces the bioreactor in the secondary treatment. (C) The BES is followed by a polishing (anaerobic/anoxic) step. (D) The BES is preceded by an acidification step (two-stage process) and followed by a polishing step.
The use of bioelectrochemical reactors (MFC in particular) for wastewater treatment was first proposed in 1991 by Habermann and Pommer (1991), and since then, several treatment process trains that integrate BESs in a WWTP have been envisioned (Rosenbaum et al., 2010). Because the anodic chamber of a BES usually contains undefined mixed cultures (including electrogenic microorganisms) that can oxidize a wide variety of organics with the anode as an electron acceptor (Rabaey et al., 2003; Liu et al., 2004, 2005a; He et al., 2005; Heilmann and Logan, 2006; Huang and Logan, 2008), the first and simplest process train is a “stand-alone” arrangement, where the BES could be incorporated, replacing the bioreactor in the secondary treatment (Figure 2B). Nevertheless, experiments conducted with real dWW-fed reactors have revealed that COD removal at bench-scale tests is limited (typically 40–80%) (Liu et al., 2004; Min and Logan, 2004; Rodrigo et al., 2007; Gil-Carrera et al., 2013a), and may not accomplish what local regulations allow in terms of BOD removal and BOD concentration of the effluent. In addition, the removal of nitrogen (an important contaminant in dWW) in a MFC–MEC is usually low, and it is mostly attributable to nitrogen assimilation into bacterial biomass, which accounts for only a small percent of the total removal usually needed (Freguia et al., 2007; Logan, 2008). Therefore, it seems unlikely that a BES may operate alone in a WWTP, and so a polishing step may be required. This leads us to the second configuration or niche for BESs application, which consists of a bioelectrochemical treatment followed by an aerobic/anoxic step (Figure 2C).
Usually, BESs fed with non-fermentable substrates outperform (in terms of the conversion of organic matter to electricity) those fed with readily fermentable substrates (Lee et al., 2008) because the food web in the former does not need to support hydrolysis and digestion of complex organic constituents. Therefore, wastewater acidification prior to the BES treatment (Figure 2D) would likely increase its performance, degrading high-molecular-weight organics into relatively simple VFAs, which are readily converted to electricity in a BES (Li and Yu, 2011), even at low concentrations (Lee et al., 2008; Kim et al., 2010). In addition, this “two-stage” process favors the enrichment of specific species in the individual reactors (De la Rubia et al., 2009; Fezzani and Ben Cheikh, 2010), increases the process stability (Held et al., 2002) and might help to remove compounds that may be toxic to the electrogenic bacteria (Mahmoud et al., 2014). However, a two-stage arrangement complicates the reactor configuration and operation, resulting in increased investment and operation costs. Another drawback of this arrangement is, as with the “stand-alone” arrangement, little nitrogen is removed from the effluent, and so an aerobic/anoxic polishing step might be required after the BES reactor.
Finally, BESs can also be integrated in a WWTP as a posttreatment of the effluent of AS and primary sludge (PS) anaerobic co-digestion (Rosenbaum et al., 2010). High temperatures in anaerobic digestion increase the concentrations of propionate and ammonia, leading to increased accumulation of VFAs (Bocher et al., 2008), which can inhibit methanogenesis. The use of BES as a posttreatment could easily solve this problem as VFAs can be readily converted into electricity by electrogenic microorganisms.
MEC Scale-Up for Domestic Wastewater Treatment: Experiences and Bottlenecks
Microbial electrolysis cell scale-up is, like all scale-up endeavors, a story of facing challenges and finding solutions. The use of raw dWW as a fuel for hydrogen production in an MEC was first investigated in 2007 in a 300 mL double chambered reactor operated in batch (Ditzig et al., 2007). This set-up offered good results in terms of the quality of the effluent (BOD <10 mg BOD L−1), with a removal of organic contamination above 90%. However, it required relatively long batch periods (30–108 h), and the conversion of organic matter to current (and consequently into hydrogen) was relatively low. This limited energy recovery, together with the use of expensive materials, such as Nafion (membrane) and platinum (the catalyst for the cathodic reaction), restricts the scalability of this design.
Five years later, in an effort to test the potential of MECs in conditions closer to practical application, Escapa et al. (2012a) tested a 100 mL monocameral MEC within the range of hydraulic retention times (HRTs) typically found in dWW facilities. Following the improvements made in MEC designs by other researchers (Tartakovsky et al., 2008; Zhuang et al., 2009), the MEC was built with low-cost materials (no polymeric membrane was interposed between the anode and the cathode, and nickel was used as a catalyst) and operated in continuous mode. The results were promising regarding the energy consumption: (per kilogram of COD removed) when only the electrical energy use was considered, and if the energy recovered as hydrogen was included in the balance. In both cases, the energy required to remove the organic contamination was below the energy consumption threshold typically associated with an aerobic treatment (; Metcalf & Eddy Inc, 2003) at low HRTs (3–6 h). However, the energy recovery into hydrogen was relatively low (~25%) compared with the potential of the dWW used as fuel, which was mainly attributed to the existence of electron sinks other than the anode (biomass production, nitrates, methanogens, etc.) and hydrogen loses through gas tubing and non-optimal reactor configurations.
Tubular designs, which together with planar designs represent the two main typologies of MEC reactors, have also been evaluated for dWW treatment and hydrogen production. Gil-Carrera et al. (2013a,c), examined a semi-pilot continuous-flow single-chamber MEC that consisted of two tubular units (2 L each) connected in series. These studies corroborated the results obtained at lab scale: organic contamination removal rates of up to 80% with associated energy consumption below that usually attributed to aerobic treatments. However, the absence of a polymeric membrane or other significant physical barrier between the two electrodes favored the re-oxidation of part of the cathodic hydrogen on the anode. This phenomenon, known as hydrogen recycling (Ruiz et al., 2013), results in the creation of parasitic electrical currents, thus limiting the overall performance of the MEC.
To our knowledge, the only pilot-scale MEC using raw dWW as a fuel is a 120 L MEC operated on site in North England for a period of over 3 months (Heidrich et al., 2012). The plant consisted of six independent bicameral MEC units made of low-cost materials, such as stainless steel (cathode), and a low-cost micro porous membrane (instead of expensive polymeric membranes). Despite the loss of hydrogen (the energy recovery was not enough to offset the energy input), the overall energy usage to remove the organic pollution was again below that of aerobic treatments. Still, the authors claimed that with improved future design energy, neutral or even positive dWW treatment might be possible with this design.
These experiences have proved that MECs may become a feasible technology for dWW treatment, bringing competitive advantages over conventional aerobic treatments from energy usage and environmental points of view. Moreover, these and many other studies (Lee and Rittmann, 2010; Gil-Carrera et al., 2013b; Ruiz et al., 2013; Tice and Kim, 2014) have helped to identify the main bottlenecks that need to be addressed in order to improve its scalability. Without being exhaustive, here is a list of the main bottlenecks that need to be overcome: (i) low organic matter conversion rates into electrical current, which leads to low current densities and therefore larger reactor sizes (and capital costs) to treat the same amount of organic contamination (and produce the same amount of hydrogen); (ii) high internal resistances and ohmic loses derived from the low conductivity of the electrolyte (dWW and polymeric membranes) and the electrical resistivity of the electrodes; (iii) low hydrogen recoveries originating from a poor sealing of laboratory designs. Fortunately, they could be easily avoided by improving the detailed engineering of prototypes; (iv) hydrogen recycling, which generates parasitic currents that do not improve COD removal, nor hydrogen production and only result in an extra energy consumption; and (v) techno-economical bottlenecks: MEC technology would need to compete with other energy-producing technologies, such as anaerobic digestion, with an associated capital cost several times lower than that associated with MECs.
Not all of the above mentioned restrictions and limitations have the same influence over MEC scalability, and some deserve special attention. Current density dictates the rate of organic contamination reduction and hydrogen production (and therefore the efficiency at which we exploit the energy in the dissolved organic matter), whereas internal resistance greatly influences the energy consumption. In fact, several studies have concluded that these are the two parameters that most influence the feasibility of MEC technology and therefore determine its practical applicability. In 2008, Rozendal et al. (2008a) estimated that 10 A m−2 is a typical expected current density for full-scale MECs. In turn, Sleutels et al. (2012) claimed that for the revenues to exceed costs, MECs need to achieve an internal resistance lower than 80 mΩ m−2 and a current density of ~20 A m−2. However, given the typical low COD concentration of dWW and the reduced cell thickness required to assure adequate contact between the wastewater and the anode, current densities of 10–20 A m−2 would demand organic loading rates (OLRs) between 14,000 and 28,000 g-COD m−3 day−1 (assuming a conversion rate of organic matter into current of 50%) leading to HRTs well below 1 h, which seems unlikely for dWW. In our opinion, these targets of current density are more appropriate for industrial wastewaters, which usually display higher COD concentrations. A recent case-study aimed at investigating the feasibility of using MEC technology in an already operating dWWTP concluded that current densities above 5 A m−2 would start to be feasible, at least from an economic point of view (Escapa et al., 2012b).
Estimation of Energy Savings Associated with MEC Use in dWWTPs
Typical dWWTPs employing aerobic AS technology are energy intensive (energy can account for up to 50% of the operating costs) (Ahn and Logan, 2010), requiring ~0.6 kWh m−3, half of which is dedicated to supplying air for the aeration basins (McCarty et al., 2011) (see Figure 3). Process optimization and/or the use of more efficient technologies would help not only to improve the environmental performance but also the economic balance. The potential energy of the organic matter dissolved in the wastewater (~4 kWh kg−1 CODr) (Logan, 2008) represents a valuable resource, that if captured with an appropriate technology, wastewater treatment might become a net energy producer rather than a consumer (McCarty et al., 2011). MEC technology is an ideal candidate because it is able to recover part of this potential into hydrogen, thus helping to curb the energy requirements in the plant.
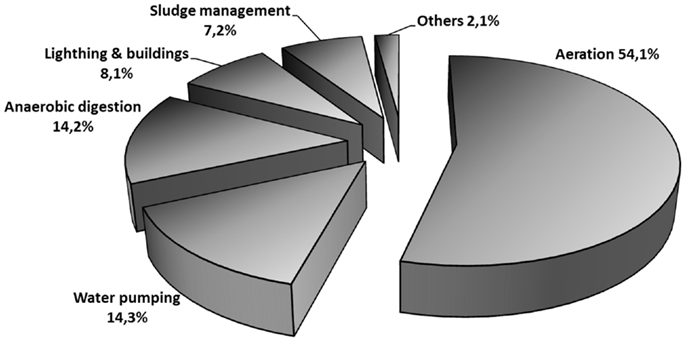
Figure 3. Typical energy requirements in a conventional domestic wastewater treatment plant. Derived from data from Water Environment Federation (2009).
As discussed in Section “Integrating MEC Technology in Domestic Wastewater Treatment Plants for Energy Recovery,” it is unlikely that MECs could operate as stand-alone reactors in potential full-scale MEC-based dWWTPs. Instead, their use as a pre-treatment step to extract the energy content of the organic matter is a more plausible option. Although dWW-fed MEC reactors have achieved 80% COD removal rates operating in continuous mode (Gil-Carrera et al., 2013a), the need to provide enough organic matter to perform nitrogen removal in the aerobic treatment would determine the actual COD removal in the MEC. For instance, in a previous case-study based on an already operating dWWTP, it was estimated that COD removal in the MEC pre-treatment should not be higher than 45% (Escapa et al., 2012b).
Because large scale MEC reactors would need to achieve at least similar performance as bench-scale reactors (Pant et al., 2012), we make use of the results reported in Escapa et al. (2012a) to advance a simplified estimation of the energy savings that MEC technology may bring to dWW treatment. In the refereed study, an OLR (which represents the amount of organic material that is daily fed to the reactor per cubic meter) of 3.1 kg-COD m−3 day−1 yielded a hydrogen production rate of 0.3 m3H2 m−3 d−1 with an associated energy consumption of 1.15 kWh kg−1 CODr. If we assume that 1.5 kWh kg−1 CODr of energy would be used in the aerobic treatment (Metcalf & Eddy Inc, 2003) and the energy required for pumping would remain the same, the energy consumption in the combined biological treatment (MEC + AS) can be computed as:
where EBT is the global energy consumption in the biological treatment , EMEC is the energy consumption in the MEC , WMEC is the COD removal efficiency in the MEC (%), EAL is the energy consumption in the aerated lagoon (1.5 kWh kg-COD-1), and WAL is the COD removal efficiency in the polishing step (%).
If local regulations allow the discharge of treated wastewaters only after 95% of COD removal (i.e., WMEC + WPS = 95), then EBT = 1.33 kWh kg-COD-1, which would represent an 11% savings in the energy consumption for organic matter removal. By including the energy captured from the organic matter and stored as hydrogen and subtracting the energy requirements for hydrogen compression (~2.1 kWh kg−1 H2), the global energy consumption in the biological treatment decreases to 1.05 kWh kg-COD-1, accounting for a 30% savings.
Further energy savings can be obtained from the reduced sludge production in MEC reactors. Although this is an issue that has not been fully studied, biomass production in the anode of a BES may lay in the range of 0.07–0.16 g of volatile suspended solids per gram of COD (g-VSS g−1-COD) (Wang and Ren, 2013), which is much lower than the biomass production in an aerobic reactor (0.35–0.45 g-VSS g−1-COD) (Metcalf & Eddy Inc, 2003). For the sake of simplicity we will assume: (i) a mean value of biomass production of 0.11 g-VSS g−1-COD in the MEC and 0.4 g-COD-biomass g−1-COD in the aerobic reactor, and (ii) a positive relationship between energy consumption and excess sludge production (Wang et al., 2012). Using a similar approach as we did for energy consumption in the biological treatment (Eq. 1) shows that energy requirements derived from reduced sludge production could help to save 37% of the energy during sludge management in the plant. Taking into account the distribution of energy use in dWWTPs shown in Figure 3, all these energy savings derived from the use of MECs for dWW treatment may bring a global savings of 19% in the energy bill of the plant.
However, for a MEC performing within the operational parameters discussed above, the revenues derived from the selling of hydrogen and the reduction in energy consumption would not be enough to offset the capital costs. Instead, it has been claimed that economic feasibility would only be achieved if future MECs could operate at current densities above 5 A m−2 and energy consumption below 0.9 kWh kg-1 COD (Escapa et al., 2012b). In this situation, the global energy savings in the potential MEC-based dWWTPs could add up to 28%.
Outlook and Future Perspectives
The estimates for the European market of municipal water and wastewater treatment (€2.37 billion in 2010) predict a growing rate of 4.1% per year (compounding rate) (Di Lorenzo et al., 2009), which highlights the excellent business prospects for MECs. The implementation of this emerging technology at the commercial scale would be driven by reliability and costs, and it is expected that the first generation of MECs could be ready within 1–4 years (European Commission, 2013). However, the reduced incomes derived from the selling of hydrogen (using dWW as a feedstock) and the relatively large capital costs (€1200–4000/m3 of reactor) (Rozendal et al., 2008a; Fornero et al., 2010; Pant et al., 2010) inevitably lead to long pay-back periods, threatening its economic feasibility. Manufacturing costs are likely to decline during the first steps of commercial development as experience accumulates and more MEC units are built. However, to benefit from this cost reduction phenomenon, MEC pioneering uses should be aimed at sectors that might bring revenues greater than those associated with dWW. Industrial WW might be the ideal candidate. Its higher COD concentrations (and consequently higher energy densities) would allow higher energy recoveries, thus improving its economic feasibility, making its use for wastewater treatment more attractive and triggering large scale production. In addition, during the first steps of implementation of this technology in dWWTP, MECs could also make use of the existing concrete structures of the aerobic basins (expensive to build), which would undoubtedly help to further reduce the capital cost.
Methane production in the anodic and/or cathodic chambers of MECs is an issue that has been challenging researchers since the proof-of-concept (Liu et al., 2005b; Rozendal et al., 2006b) and appears to have no easy solution. Methanogenic microorganisms present inside MEC reactors not only compete with electrogenic microorganism for substrate but also contaminate the cathodic hydrogen, requiring a gas-cleaning step depending on the final use of the hydrogen. However, it has been argued that fostering methane production (using biocathodes containing methanogens) rather than avoiding it, could bring additional advantages over hydrogen (Cheng et al., 2009). Therefore, methane production MECs could serve as an intermediate step that would further facilitate the implementation of MEC technology in dWWTPs.
In this study, we have seen that the use of MEC technology may bring not only substantial energy savings when used as pretreatment to the aerobic step in a dWWT but also other environmental benefits through the reduced use of energy and through the displacement of hydrogen production by conventional means (Foley et al., 2010). Significant economic benefits may also be obtained, provided future research allows MEC reactors to improve their present performance (Escapa et al., 2012b; Sleutels et al., 2012). The bottlenecks discussed in Section “MEC Scale-Up for Domestic Wastewater Treatment: Experiences and Bottlenecks” and the relatively large capital costs (Rozendal et al., 2008a; Fornero et al., 2010; Pant et al., 2010) are two vital concerns that will determine the practical implementation of MECs in dWWTPs. Consequently, and to improve the economic and energy balances in MECs (and as a result, their applicability), further enhancements would be required in areas such as: (i) reactor architecture: more efficient water circulation inside the anodic chamber would reduce the energy needs for pumping and optimize the conversion of organic matter into electricity; (ii) in the detailed engineering of the system: to improve certain aspects, such as the electrical contacts, or maximize hydrogen recovery by limiting its diffusion back into the anodic chamber and leaks into the atmosphere; (iii) in electrode and current collectors: new materials with greater specific surface, low ohmic resistances, and better catalytic properties; (iv) in the understanding of the mechanism of the transfer of electrons from the bacteria to the electrodes and interspecies interactions; and (v) other secondary issues, such as how MEC units should be electrically connected. It seems unlikely to build MEC units able to operate alone and treat several thousand of cubic meters per day. In contrast, a whole MEC reactor would need to be divided into a certain number of MEC units that would make up the whole treatment. Connecting all of the units in parallel does not seem realistic: this configuration would demand the use of a rectifier able to manage large currents at low potentials, which would be impractical at full-scale. Moreover, low-voltage rectification usually incurs substantial energy losses. Therefore, it seems necessary that a certain number of MEC units must be stacked in series.
Finally, on its road to practical application, not only would MEC technology need to overcome these economic and technological barriers, but it would also need to compete with other energy-producing technologies. Undoubtedly, the main competitor of MECs for wastewater treatment is anaerobic digestion (AD), with its reduced capital costs that may be 40–800 times lower compared with BES (Rozendal et al., 2008a). In addition, the ability of BES to operate in mild conditions, such as low organics concentrations and low temperatures, is often cited as an important competitive advantage of BESs (Pham et al., 2006; Rozendal et al., 2008a). However, several studies have shown that AD can achieve good performance levels at temperatures as low as 5°C and low HRTs (a few hours) (McCarty et al., 2011; Batstone and Virdis, 2014).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Funding for this study was provided by the Spanish Ministry of Science and Innovation (Project Number ENE 2012-33027) and Adrián Escapa thanks the postdoctoral fellowship of the Regional Government of Castilla y León.
Abbreviations
AD, anaerobic digestion; AS, aerobic sludge; BES: bioelectrochemical system; BOD, biochemical oxygen demand (mg-BOD L−1). Represents the amount of oxygen required to oxidize the biodegradable organic matter dissolved into the wastewater to CO2 and H2O. It gives an indication of the amount of biodegradable organic matter dissolved into the wastewater; COD: chemical oxygen demand (mg-COD L−1). Represents the amount of oxygen required to oxidize the organic matter dissolved into the wastewater to CO2 and H2O. It gives an indication of the amount of organic matter dissolved into the wastewater and can be used to estimate the energy content of this organic material. dWW, domestic wastewater; dWWTP, domestic wastewater treatment plant; HRT, hydraulic retention time (h); MDC, microbial desalination cell; MEC, microbial electrolysis cell; MFC, microbial fuel cell; MSC, microbial solar cell; PS, primary sludge; VFA, volatile fatty acid.
References
Ahn, Y., and Logan, B. E. (2010). Effectiveness of domestic wastewater treatment using microbial fuel cells at ambient and mesophilic temperatures. Bioresour. Technol. 101, 469–475. doi:10.1016/j.biortech.2009.07.039
Angenent, L. T., Karim, K., Al-Dahhan, M. H., Wrenn, B. A., and Domíguez-Espinosa, R. (2004). Production of bioenergy and biochemicals from industrial and agricultural wastewater. Trends Biotechnol. 22, 477–485. doi:10.1016/j.tibtech.2004.07.001
Batstone, D. J., and Virdis, B. (2014). The role of anaerobic digestion in the emerging energy economy. Curr. Opin. Biotechnol. 27, 142–149. doi:10.1016/j.copbio.2014.01.013
Bocher, B., Agler, M., Garcia, M., Beers, A., and Angenent, L. (2008). Anaerobic digestion of secondary residuals from an anaerobic bioreactor at a brewery to enhance bioenergy generation. J. Ind. Microbiol. Biotechnol. 35, 321–329. doi:10.1007/s10295-007-0295-4
Cao, X., Huang, X., Liang, P., Xiao, K., Zhou, Y., Zhang, X., et al. (2009). A new method for water desalination using microbial desalination cells. Environ. Sci. Technol. 43, 7148–7152. doi:10.1021/es901950j
Chen, S., Liu, G., Zhang, R., Qin, B., and Luo, Y. (2012). Development of the microbial electrolysis desalination and chemical-production cell for desalination as well as acid and alkali productions. Environ. Sci. Technol. 46, 2467–2472. doi:10.1021/es203332g
Cheng, S., Xing, D., Call, D. F., and Logan, B. E. (2009). Direct biological conversion of electrical current into methane by electromethanogenesis. Environ. Sci. Technol. 43, 3953–3958. doi:10.1021/es803531g
Cusick, R. D., Bryan, B., Parker, D., Merrill, M. D., Mehanna, M., Kiely, P. D., et al. (2011). Performance of a pilot-scale continuous flow microbial electrolysis cell fed winery wastewater. Appl. Microbiol. Biotechnol. 89, 2053–2063. doi:10.1007/s00253-011-3130-9
Cusick, R. D., Kiely, P. D., and Logan, B. E. (2010). A monetary comparison of energy recovered from microbial fuel cells and microbial electrolysis cells fed winery or domestic wastewaters. Int. J. Hydrogen Energy 35, 8855–8861. doi:10.1016/j.ijhydene.2010.06.077
Cusick, R. D., and Logan, B. E. (2012). Phosphate recovery as struvite within a single chamber microbial electrolysis cell. Bioresour. Technol. 107, 110–115. doi:10.1016/j.biortech.2011.12.038
De la Rubia, M. A., Raposo, F., Rincón, B., and Borja, R. (2009). Evaluation of the hydrolytic-acidogenic step of a two-stage mesophilic anaerobic digestion process of sunflower oil cake. Bioresour. Technol. 100, 4133–4138. doi:10.1016/j.biortech.2009.04.001
Di Lorenzo, M., Scott, K., Curtis, T. P., Katuri, K. P., and Head, I. M. (2009). Continuous feed microbial fuel cell using an air cathode and a disc anode stack for wastewater treatment. Energy Fuels 23, 5707–5716. doi:10.1021/ef9005934
Ditzig, J., Liu, H., and Logan, B. E. (2007). Production of hydrogen from domestic wastewater using a bioelectrochemically assisted microbial reactor (BEAMR). Int. J. Hydrogen Energy 32, 2296–2304. doi:10.1007/s00253-012-4456-7
Escapa, A., Gil-Carrera, L., García, V., and Morán, A. (2012a). Performance of a continuous flow microbial electrolysis cell (MEC) fed with domestic wastewater. Bioresour. Technol. 117, 55–62. doi:10.1016/j.biortech.2012.04.060
Escapa, A., Gómez, X., Tartakovsky, B., and Morán, A. (2012b). Estimating microbial electrolysis cell (MEC) investment costs in wastewater treatment plants: case study. Int. J. Hydrogen Energy 37, 18641–18653. doi:10.1016/j.ijhydene.2012.09.157
Escapa, A., Manuel, M. F., Morán, A., Gómez, X., Guiot, S. R., and Tartakovsky, B. (2009). Hydrogen production from glycerol in a membraneless microbial electrolysis cell. Energ. Fuel 23, 4612–4618. doi:10.1021/ef900357y
European Commission. (2013). Future Brief: Bioelectrochemical Systems. Wastewater Treatment, Bioenergy and Valuable Chemicals Delivered by Bacteria. Available at: http://ec.europa.eu/environment/integration/research/newsalert/pdf/FB5.pdf
Eurostat. (2011). Statistical Office of the European Communities. Available at: http://epp.eurostat.ec.europa.eu/portal/page/portal/statistics/themes
Fezzani, B., and Ben Cheikh, R. (2010). Two-phase anaerobic co-digestion of olive mill wastes in semi-continuous digesters at mesophilic temperature. Bioresour. Technol. 101, 1628–1634. doi:10.1016/j.biortech.2009.09.067
Foley, J. M., Rozendal, R. A., Hertle, C. K., Lant, P. A., and Rabaey, K. (2010). Life cycle assessment of high-rate anaerobic treatment, microbial fuel cells, and microbial electrolysis cells. Environ. Sci. Technol. 44, 3629–3637. doi:10.1021/es100125h
Fornero, J. J., Rosenbaum, M., and Angenent, L. T. (2010). Electric power generation from municipal, food, and animal wastewaters using microbial fuel cells. Electroanalysis 22, 832–843. doi:10.1002/elan.200980011
Freguia, S., Rabaey, K., Yuan, Z., and Keller, J. (2007). Electron and carbon balances in microbial fuel cells reveal temporary bacterial storage behavior during electricity generation. Environ. Sci. Technol. 41, 2915–2921. doi:10.1021/es062611i
Gil-Carrera, L., Escapa, A., Carracedo, B., Morán, A., and Gómez, X. (2013a). Performance of a semi-pilot tubular microbial electrolysis cell (MEC) under several hydraulic retention times and applied voltages. Bioresour. Technol. 146, 63–69. doi:10.1016/j.biortech.2013.07.020
Gil-Carrera, L., Escapa, A., Mehta, P., Santoyo, G., Guiot, S. R., Morán, A., et al. (2013b). Microbial electrolysis cell scale-up for combined wastewater treatment and hydrogen production. Bioresour. Technol. 130, 584–591. doi:10.1016/j.biortech.2012.12.062
Gil-Carrera, L., Escapa, A., Moreno, R., and Morán, A. (2013c). Reduced energy consumption during low strength domestic wastewater treatment in a semi-pilot tubular microbial electrolysis cell. J. Environ. Manage. 122, 1–7. doi:10.1016/j.jenvman.2013.03.001
Gómez, X., Fernández, C., Fierro, J., Sánchez, M. E., Escapa, A., and Morán, A. (2011). Hydrogen production: two stage processes for waste degradation. Bioresour. Technol. 102, 8621–8627. doi:10.1016/j.biortech.2011.03.055
Habermann, W., and Pommer, E. H. (1991). Biological fuel cells with sulphide storage capacity. Appl. Microbiol. Biotechnol. 35, 128–133. doi:10.1007/BF00180650
Harnisch, F., Aulenta, F., Schröder, U., and Moo-Young, M. (2011). “Microbial fuel cells and bioelectrochemical systems: industrial and environmental biotechnologies based on extracellular electron transfer,” in Comprehensive Biotechnology, 2nd Edn, ed. M. Moo-Young (Amsterdam: Elsevier), 643–659. doi:10.1016/B978-0-08-088504-9.00462-1
Hawkes, F. R., Dinsdale, R. M., Hawkes, D. L., and Hussy, I. (2002). Sustainable fermentative hydrogen production: challenges for process optimisation. Int. J. Hydrogen Energy 27, 1339–1347. doi:10.1016/S0360-3199(02)00090-3
He, Z., Minteer, S. D., and Angenent, L. T. (2005). Electricity generation from artificial wastewater using an upflow microbial fuel cell. Environ. Sci. Technol. 39, 5262–5267. doi:10.1021/es0502876
Heidrich, E. S., Dolfing, J., Scott, K., Edwards, S. R., Jones, C., and Curtis, T. P. (2012). Production of hydrogen from domestic wastewater in a pilot-scale microbial electrolysis cell. Appl. Microbiol. Biotechnol. 89, 2053–2063. doi:10.1007/s00253-012-4456-7
Heilmann, J., and Logan, B. E. (2006). Production of electricity from proteins using a microbial fuel cell. Water Environ. Res. 78, 531–537. doi:10.2175/106143005X73046
Held, C., Wellacher, M., Robra, K., and Gübitz, G. M. (2002). Two-stage anaerobic fermentation of organic waste in CSTR and UFAF-reactors. Bioresour. Technol. 81, 19–24. doi:10.1016/S0960-8524(01)00108-0
Huang, L., and Logan, B. E. (2008). Electricity production from xylose in fed-batch and continuous-flow microbial fuel cells. Appl. Microbiol. Biotechnol. 80, 655–664. doi:10.1007/s00253-008-1588-x
IDAE. (2010). Water and Energy: The Complex Interplay of Two Scarce Resources. The Energy Footprint of the Water. A First Estimate of Energy Consumption of Desalination and Urban Wastewater Treatment. Madrid, Spain: IDAE. [Spanish].
IEA. (2011). International Energy Agency. Available at: http://www.iea.org/country/m_country.asp?COUNTRY_CODE=ES
INE. (2010). National Statistics Institute. Available at: http://www.ine.es/jaxi/tabla.do
Kiely, P. D., Cusick, R. D., Call, D. F., Selembo, P. A., Regan, J. M., and Logan, B. E. (2011). Anode microbial communities produced by changing from microbial fuel cell to microbial electrolysis cell operation using two different wastewaters. Bioresour. Technol. 102, 388–394. doi:10.1016/j.biortech.2010.05.019
Kim, I. S., Hwang, M. H., Jang, N. J., Hyun, S. H., and Lee, S. T. (2004). Effect of low pH on the activity of hydrogen utilizing methanogen in bio-hydrogen process. Int. J. Hydrogen Energy 29, 1133–1140. doi:10.1016/j.ijhydene.2003.08.017
Kim, J. R., Premier, G. C., Hawkes, F. R., Rodriguez, J., Dinsdale, R. M., and Guwy, A. J. (2010). Modular tubular microbial fuel cells for energy recovery during sucrose wastewater treatment at low organic loading rate. Bioresour. Technol. 101, 1190–1198. doi:10.1016/j.biortech.2009.09.023
Lee, H., Parameswaran, P., Kato-Marcus, A., Torres, C. I., and Rittmann, B. E. (2008). Evaluation of energy-conversion efficiencies in microbial fuel cells (MFCs) utilizing fermentable and non-fermentable substrates. Water Res. 42, 1501–1510. doi:10.1016/j.watres.2007.10.036
Lee, H., and Rittmann, B. E. (2010). Significance of biological hydrogen oxidation in a continuous single-chamber microbial electrolysis cell. Environ. Sci. Technol. 44, 948–954. doi:10.1021/es9025358
Li, W., and Yu, H. (2011). From wastewater to bioenergy and biochemicals via two-stage bioconversion processes: a future paradigm. Biotechnol. Adv. 29, 972–982. doi:10.1016/j.biotechadv.2011.08.012
Liang, B., Cheng, H., Kong, D., Gao, S., Sun, F., Cui, D., et al. (2013). Accelerated reduction of chlorinated nitroaromatic antibiotic chloramphenicol by biocathode. Environ. Sci. Technol. 47, 5353–5361. doi:10.1021/es400933h
Liu, H., Ramnarayanan, R., and Logan, B. E. (2004). Production of electricity during wastewater treatment using a single chamber microbial fuel cell. Environ. Sci. Technol. 38, 2281–2285. doi:10.1021/es034923g
Liu, H., Cheng, S., and Logan, B. E. (2005a). Power generation in fed-batch microbial fuel cells as a function of ionic strength, temperature, and reactor configuration. Environ. Sci. Technol. 39, 5488–5493. doi:10.1021/es050316c
Liu, H., Grot, S., and Logan, B. E. (2005b). Electrochemically assisted microbial production of hydrogen from acetate. Environ. Sci. Technol. 39, 4317–4320. doi:10.1021/es050244p
Logan, B. E. (2009). Exoelectrogenic bacteria that power microbial fuel cells. Nat. Rev. Microbiol. 7, 375–381. doi:10.1038/nrmicro2113
Logan, B. E., Call, D. F., Cheng, S., Hamelers, H. V. M., Sleutels, T. H. J. A., Jeremiasse, A. W., et al. (2008). Microbial electrolysis cells for high yield hydrogen gas production from organic matter. Environ. Sci. Technol. 42, 8630–8640. doi:10.1021/es801553z
Mahmoud, M., Parameswaran, P., Torres, C. I., and Rittmann, B. E. (2014). Fermentation pre-treatment of landfill leachate for enhanced electron recovery in a microbial electrolysis cell. Bioresour. Technol. 151, 151–158. doi:10.1016/j.biortech.2013.10.053
McCarty, P. L., Bae, J., and Kim, J. (2011). Domestic wastewater treatment as a net energy producer – can this be achieved? Environ. Sci. Technol. 45, 7100–7106. doi:10.1021/es2014264
Min, B., and Logan, B. E. (2004). Continuous electricity generation from domestic wastewater and organic substrates in a flat plate microbial fuel cell. Environ. Sci. Technol. 38, 5809–5814. doi:10.1021/es0491026
Mu, Y., Rabaey, K., Rozendal, R. A., Yuan, Z., and Keller, J. (2009). Decolorization of azo dyes in bioelectrochemical systems. Environ. Sci. Technol. 43, 5137–5143. doi:10.1021/es900057f
Pant, D., Singh, A., Van Bogaert, G., Irving Olsen, S., Singh Nigam, P., Diels, L., et al. (2012). Bioelectrochemical systems (BES) for sustainable energy production and product recovery from organic wastes and industrial wastewaters. RSC Adv. 2, 1248–1263. doi:10.1039/c1ra00839k
Pant, D., Van Bogaert, G., De Smet, M., Diels, L., and Vanbroekhoven, K. (2010). Use of novel permeable membrane and air cathodes in acetate microbial fuel cells. Electrochim. Acta 55, 7710–7716. doi:10.1016/j.electacta.2009.11.086
Peixoto, L., Min, B., Martins, G., Brito, A. G., Kroff, P., Parpot, P., et al. (2011). In situ microbial fuel cell-based biosensor for organic carbon. Bioelectrochemistry 81, 99–103. doi:10.1016/j.bioelechem.2011.02.002
Pham, T., Rabaey, K., Aelterman, P., Clauwaert, P., De Schamphelaire, L., Boon, N., et al. (2006). Microbial fuel cells in relation to conventional anaerobic digestion technology. Eng. Life Sci. 6, 285–292. doi:10.1002/elsc.200620121
Pinto, R. P., Srinivasan, B., Escapa, A., and Tartakovsky, B. (2011). Multi-population model of a microbial electrolysis cell. Environ. Sci. Technol. 45, 5039–5046. doi:10.1021/es104268g
Rabaey, K. (2010). Bioelectrochemical Systems: From Extracellular Electron Transfer to Biotechnological Application. London: IWA Publishing, 1–16.
Rabaey, K., Lissens, G., Siciliano, S. D., and Verstraete, W. (2003). A microbial fuel cell capable of converting glucose to electricity at high rate and efficiency. Biotechnol. Lett. 25, 1531–1535. doi:10.1023/A:1025484009367
Rabaey, K., Rodriguez, J., Blackall, L. L., Keller, J., Gross, P., Batstone, D. J., et al. (2007). Microbial ecology meets electrochemistry: electricity-driven and driving communities. ISME J. 1, 9–18. doi:10.1038/ismej.2007.4
Rabaey, K., and Rozendal, R. A. (2010). Microbial electrosynthesis – revisiting the electrical route for microbial production. Nat. Rev. Microbiol. 8, 706–716. doi:10.1038/nrmicro2422
Rodrigo, M. A., Cañizares, P., Lobato, J., Paz, R., Sáez, C., and Linares, J. J. (2007). Production of electricity from the treatment of urban waste water using a microbial fuel cell. J. Power Sources 169, 198–204. doi:10.1016/j.jpowsour.2007.01.054
Rosenbaum, M., Agler, M. T., Fornero, J. J., Venkataraman, A., and Angenent, L. T. (2010). “Integrating BES in the wastewater and sludge treatment line,” in Bioelectrochemical Systems: From Extracellular Electron Transfer to Biotechnological Application, eds K. Rabaey, L. T. Angenent, U. Schroder, and J. Keller (London: IWA Publishing), 393–421.
Rozendal, R. A. (2007). Hydrogen Production Through Biocatalyzed Electrolysis. Wageningen Universiteit, Wageningen, Netherlands.
Rozendal, R. A., and Buisman, C. J. N. (2005). Process for Producing Hydrogen. Patent WO2005005981 (USA).
Rozendal, R. A., Hamelers, H. V. M., and Buisman, C. J. N. (2006a). Effects of membrane cation transport on pH and microbial fuel cell performance. Environ. Sci. Technol. 40, 5206–5211. doi:10.1021/es060387r
Rozendal, R. A., Hamelers, H. V. M., Euverink, G. J. W., Metz, S. J., and Buisman, C. J. N. (2006b). Principle and perspectives of hydrogen production through biocatalyzed electrolysis. Int. J. Hydrogen Energy 31, 1632–1640. doi:10.1016/j.ijhydene.2005.12.006
Rozendal, R. A., Hamelers, H. V. M., Rabaey, K., Keller, J., and Buisman, C. J. N. (2008a). Towards practical implementation of bioelectrochemical wastewater treatment. Trends Biotechnol. 26, 450–459. doi:10.1016/j.tibtech.2008.04.008
Rozendal, R. A., Jeremiasse, A. W., Hamelers, H. V. M., and Buisman, C. J. N. (2008b). Hydrogen production with a microbial biocathode. Environ. Sci. Technol. 42, 629–634. doi:10.1021/es071720+
Rozendal, R. A., Leone, E., Keller, J., and Rabaey, K. (2009). Efficient hydrogen peroxide generation from organic matter in a bioelectrochemical system. Electrochem. commun. 11, 1752–1755. doi:10.1016/j.elecom.2009.07.008
Ruiz, Y., Baeza, J. A., and Guisasola, A. (2013). Revealing the proliferation of hydrogen scavengers in a single-chamber microbial electrolysis cell using electron balances. Int. J. Hydrogen Energy 38, 15917–15927. doi:10.1016/j.ijhydene.2013.10.034
Shizas, I. (2004). Experimental determination of energy content of unknown organics in municipal wastewater streams. J. Energ. Eng. 130, 45. doi:10.1061/(ASCE)0733-9402(2004)130:2(45)
Sleutels, T. H. J. A., Ter-Heijne, A., Buisman, C. J. N., and Hamelers, H. V. M. (2012). Bioelectrochemical systems: an outlook for practical applications. ChemSusChem 5, 1012–1019. doi:10.1002/cssc.201100732
Srikanth, S., Venkateswar Reddy, M., and Venkata Mohan, S. (2012). Microaerophilic microenvironment at biocathode enhances electrogenesis with simultaneous synthesis of polyhydroxyalkanoates (PHA) in bioelectrochemical system (BES). Bioresour. Technol. 125, 291–299. doi:10.1016/j.biortech.2012.08.060
Strik, D. P., Timmers, R. A., Helder, M., Steinbusch, K. J. J., Hamelers, H. V. M., and Buisman, C. J. N. (2011). Microbial solar cells: applying photosynthetic and electrochemically active organisms. Trends Biotechnol. 29, 41–49. doi:10.1016/j.tibtech.2010.10.001
Tartakovsky, B., Manuel, M. F., Neburchilov, V., Wang, H., and Guiot, S. R. (2008). Biocatalyzed hydrogen production in a continuous flow microbial fuel cell with a gas phase cathode. J. Power Sources 182, 291–297. doi:10.1016/j.jpowsour.2008.03.062
Tartakovsky, B., Manuel, M. F., Wang, H., and Guiot, S. R. (2009). High rate membrane-less microbial electrolysis cell for continuous hydrogen production. Int. J. Hydrogen Energy 34, 672–677. doi:10.1016/j.ijhydene.2008.11.003
Tice, R. C., and Kim, Y. (2014). Methanogenesis control by electrolytic oxygen production in microbial electrolysis cells. Int. J. Hydrogen Energy 39, 3079–3086. doi:10.1016/j.ijhydene.2013.12.103
Wagner, R. C., Regan, J. M., Oh, S., Zuo, Y., and Logan, B. E. (2009). Hydrogen and methane production from swine wastewater using microbial electrolysis cells. Water Res. 43, 1480–1488. doi:10.1016/j.watres.2008.12.037
Wang, H., and Ren, Z. J. (2013). A comprehensive review of microbial electrochemical systems as a platform technology. Biotechnol. Adv. 31, 1796–1807. doi:10.1016/j.biotechadv.2013.10.001
Wang, X., Liu, J., Ren, N., and Duan, Z. (2012). Environmental profile of typical anaerobic/anoxic/oxic wastewater treatment systems meeting increasingly stringent treatment standards from a life cycle perspective. Bioresour. Technol. 126, 31–40. doi:10.1016/j.biortech.2012.09.009
Wang, Y., Wang, A., Liu, W., Kong, D., Tan, W., and Liu, C. (2013). Accelerated azo dye removal by biocathode formation in single-chamber biocatalyzed electrolysis systems. Bioresour. Technol. 146, 740–743. doi:10.1016/j.biortech.2013.07.082
Water Environment Federation. (2009). “Manual of practice (MOP) no. 32: energy conservation in water and wastewater facilities,” in Prepared by the Energy Conservation in Water and Wastewater Treatment Facilities Task Force of the Water Environment Federation (New York, NY: McGraw Hill), 400.
Weemaes, M., and Verstraete, W. (2001). “Other treatment techniques,” in Sludge into Biosolids, eds L. Spinosa and P. A. Vesilind (London: IWA Publishing), 364–383.
Keywords: microbial electrolysis cell, energy savings, energy recovery, domestic wastewater, biological waste treatments, hydrogen
Citation: Escapa A, San-Martín MI and Morán A (2014) Potential use of microbial electrolysis cells in domestic wastewater treatment plants for energy recovery. Front. Energy Res. 2:19. doi: 10.3389/fenrg.2014.00019
Received: 19 March 2014; Accepted: 22 May 2014;
Published online: 06 June 2014.
Edited by:
Maria Luisa Di Vona, University of Rome Tor Vergata, ItalyReviewed by:
Gu-Gon Park, Korea Institute of Energy Research, South KoreaFrancisco Jesus Fernandez Morales, University Castilla-La Mancha, Spain
Ioan Stamatin, University of Bucharest, Romania
Copyright: © 2014 Escapa, San-Martín and Morán. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Antonio Morán, Chemical and Environmental Bioprocess Engineering Group, Natural Resources Institute (IRENA), University of León, Avda. de Portugal 41, León 24009, Spain e-mail: amorp@unileon.es
 Adrián Escapa
Adrián Escapa María Isabel San-Martín
María Isabel San-Martín Antonio Morán
Antonio Morán