Relationships between biomass composition and liquid products formed via pyrolysis
- 1Department of Microbiology and Plant Biology, University of Oklahoma, Norman, OK, USA
- 2School of Chemical, Biological, and Materials Engineering, University of Oklahoma, Norman, OK, USA
Thermal conversion of biomass is a rapid, low-cost way to produce a dense liquid product, known as bio-oil, that can be refined to transportation fuels. However, utilization of bio-oil is challenging due to its chemical complexity, acidity, and instability – all results of the intricate nature of biomass. A clear understanding of how biomass properties impact yield and composition of thermal products will provide guidance to optimize both biomass and conditions for thermal conversion. To aid elucidation of these associations, we first describe biomass polymers, including phenolics, polysaccharides, acetyl groups, and inorganic ions, and the chemical interactions among them. We then discuss evidence for three roles (i.e., models) for biomass components in the formation of liquid pyrolysis products: (1) as direct sources, (2) as catalysts, and (3) as indirect factors whereby chemical interactions among components and/or cell wall structural features impact thermal conversion products. We highlight associations that might be utilized to optimize biomass content prior to pyrolysis, though a more detailed characterization is required to understand indirect effects. In combination with high-throughput biomass characterization techniques, this knowledge will enable identification of biomass particularly suited for biofuel production and can also guide genetic engineering of bioenergy crops to improve biomass features.
Biomass can be a renewable and sustainable source of transportation fuels not associated with fossil CO2 release. Numerous studies highlight the advantages of displacing petroleum fuels with industrial production of liquid fuels from thermochemical conversion of biomass (Bridgwater et al., 1999; Perlack et al., 2005; Mohan et al., 2006; NSF, 2008). Thermochemical conversion entails heating of biomass in an anoxic environment; condensation of organic liquid products, known as bio-oil; and subsequent treatment of the products with catalysts to create liquid fuels, i.e., refined bio-oil, similar to petroleum-derived gasoline or diesel. This is in contrast to biochemical conversion, which utilizes enzymes to release sugars followed by microbial production of ethanol or other fuel molecules (Somerville, 2007; Youngs and Somerville, 2012). Relative to biochemical approaches, thermal conversion has the potential to make use of all carbon (C)-containing biomass components, would allow society to retain existing infrastructure associated with liquid hydrocarbon fuels, and, due to the rapidity of the process, may reduce production costs by permitting scalability and distribution of production (Huber et al., 2006; Mettler et al., 2012). For both thermochemical and biochemical biofuels, lowering processing costs and improving fuel yields per hectare are major engineering challenges that hinder economic viability. Thermochemical fuel production also faces challenges related to maintaining a high C-yield while obtaining a fungible fuel. We posit that this latter challenge might be addressed by understanding the relationships between biomass composition and bio-oil components and using this information to alter biomass through genetic, chemical, or thermal means.
Thermal Conversion Challenges
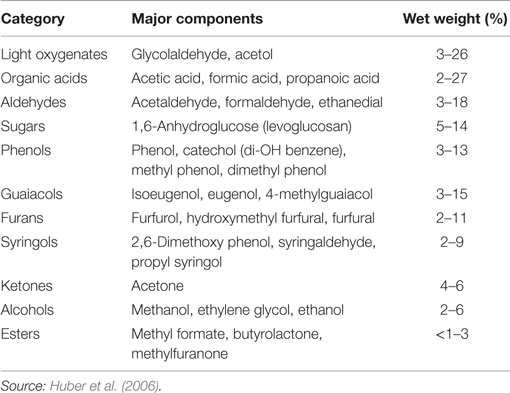
Two types of pyrolysis have been developed: fast pyrolysis and slow pyrolysis. Slow pyrolysis is usually performed over several hours and has a high solid yield, and as such has little relevance for liquid fuels production. Fast pyrolysis, however, is typically performed quickly, in seconds, at temperatures between 400 and 600°C and decomposes most of the solid biomass into a volatile mixture of various organic molecules, water, and CO/CO2. Pyrolysis oil or bio-oil constitutes the condensable portion of this vapor. Non-condensable components (primarily CO2 and CO) and a mineral-rich solid (char) are other product classes that will not be addressed here, except in that they detract from the overall C-yield of raw and refined bio-oil. Bio-oil comprises water (15–30%) plus compounds from several chemical families including the following (Table 1): organic acids, light (C1–C3) oxygenates, furan and furan derivatives, phenolic species with various methyl and methoxy substituents, pyrones, and sugar derivatives like levoglucosan (Faix et al., 1991a,b). Bio-oil’s chemically complex nature prohibits its direct use in combustion applications or petroleum refining. The reasons for this include low heating value; ignition difficulty; high chemical reactivity, which results in oligomerization and polymerization over time and upon heating, prohibiting distillative separation (Oasmaa and Czernik, 1999; Demirbas, 2011; Patwardhan et al., 2011a); immiscibility with petroleum; and high corrosivity (Oasmaa and Czernik, 1999). Many of these features are associated with the high oxygen content of biomass and the resulting bio-oil, relative to fossil fuels.
In order to obtain desirable fuel properties and allow integration with the existing transportation fuels infrastructure (gasoline and diesel engines), the bio-oil must be chemically converted to reduce the undesirable characteristics mentioned above. Catalytic upgrading is typically used to refine bio-oil, improving its stability and making it an acceptable liquid fuel. The simplest method is hydrotreating or hydrodeoxygenation, which removes oxygen via catalytic hydrogenation (Furimsky, 2000), decreasing both the chemical reactivity and corrosivity. However, this process converts any C1–C5 oxygenates, representing as much as half of the carbon in bio-oil, to C1–C5 hydrocarbons that are too volatile for liquid fuels (Resasco, 2011). Another straightforward approach is to “crack” the pyrolysis vapors using acidic zeolite catalysts into light olefins and aromatic hydrocarbons (primarily benzene, toluene, and o/m/p-xylene) (Bridgwater, 1994; Carlson et al., 2008, 2009). This approach is appealing because of the lack of an external H2 requirement and the simplicity of the product streams. Furthermore, since zeolite cracking is widely used in traditional petroleum refining/valorization (Wan et al., 2015), other advantages are the product compatibility with existing refinery infrastructure and the maturity of the process (Wan et al., 2015). However, zeolite cracking is crippled by poor usable carbon yield due to the high amounts of coke, CO, and CO2 formed during the catalytic process (Carlson et al., 2008) and the concomitant rapid catalyst deactivation. Additionally, further catalytic oligomerization and reforming for olefins and aromatics, respectively, are needed to make these products suitable for addition to refinery fuel product streams, increasing the process costs and further reducing overall carbon yield.
More advanced strategies propose to use reactions such as ketonization, condensation, alkylation, and others to retain a higher fraction of the biomass carbon into liquid fuel-range molecules (Zhu et al., 2011; Zapata et al., 2012; Pham et al., 2013; Gonzalez-Borja and Resasco, 2015). However, catalytic upgrading of any one family of compounds (e.g., light oxygenates) typically requires a catalyst and reaction conditions different than those required for another family of compounds (e.g., substituted phenolics). Moreover, catalysts used for upgrading one family of compounds may be ill-suited for other families, either facilitating undesirable reactions (breaking C–C bonds unnecessarily or increasing H:C ratios above the 2:1 optimum) or undergoing rapid deactivation due to reactions with other non-targeted bio-oil oxygenates. These upgrading challenges suggest the desirability of thermal conversion producing more selective product streams, i.e., each stream comprising fewer families of chemical compounds. Developing such thermal conversion processes would be aided by clearer knowledge of the relationship between biomass composition and thermal conversion products.
Biomass Composition and Chemical Structures
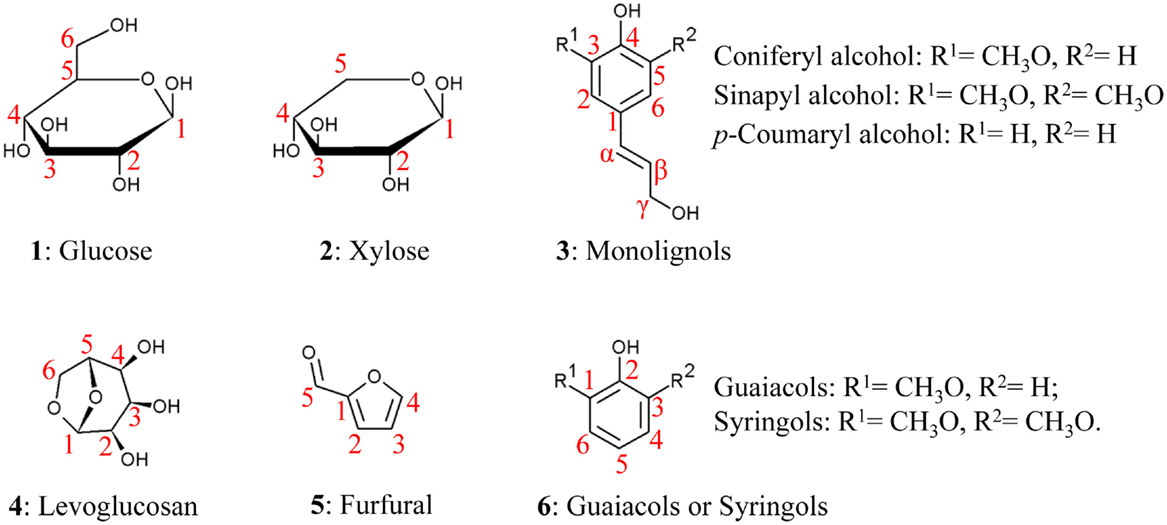
Recent reviews have addressed the general relationships between biomass composition and thermal products, such as increasing the content of phenolics relative to carbohydrates to reduce the oxygen content of bio-oil (Tanger et al., 2013). Here, we provide a more detailed description of the chemical structure and interactions among major cell wall components to aid in understanding more subtle relationships between biomass and bio-oil content. Biomass consists of cell walls that establish the structure of the plant and, to a lesser extent, non-structural components (Table 2). Cell walls determine the shape of leaves and stems and the cells that compose them and consist of cellulose, hemicellulose, lignin, as well as structural proteins and wall-associated mineral components (O’Neill and York, 2009; Vogel et al., 2011; Tanger et al., 2013). Non-structural components include sugars, proteins, and additional minerals (O’Neill and York, 2009; Vogel et al., 2011; Tanger et al., 2013). For example, in switchgrass, an important potential bioenergy crop, dry biomass consists of ~70% cell walls, 9% intrinsic water, 8% minerals, 6% proteins, and 5% non-structural sugars (Vogel et al., 2011). The relative fractions of different components, chemical linkages within and between polymers, and cellular patterning vary among plant species, organs, developmental stages, and growth conditions (Adler et al., 2006; El-Nashaar et al., 2009; Singh et al., 2012; Zhao et al., 2012). Here, we review the components of secondary cell walls, which are formed as plant growth ceases, as they constitute the majority of plant biomass (Pauly and Keegstra, 2008), and then discuss evidence for interactions among components. Table 2 lists the different major and minor components of biomass and the broad ranges of their representation within biomass for biofuel conversion. Figure 1(1–3) shows the chemical structures and atom numbering of the most abundant cell wall monomeric species.
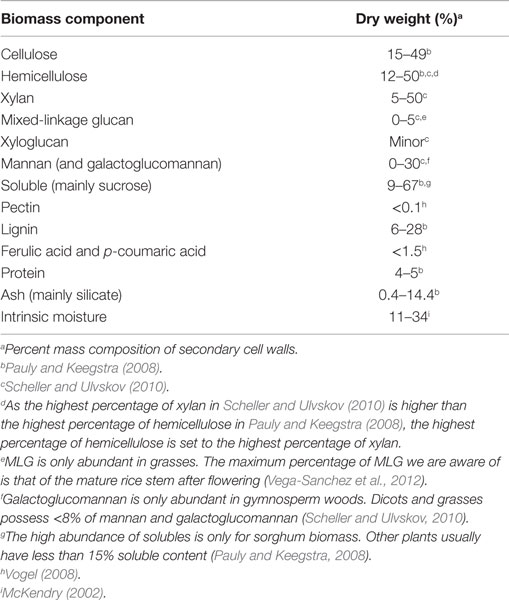
Table 2. The variation of biomass components among vascular plants including grasses, softwoods, and hardwoods.
Cellulose and hemicellulose represent 15–49% and 12–50% of biomass by dry weight, respectively (Pauly and Keegstra, 2008; Vogel, 2008; Zhao et al., 2012). Cellulose is an unbranched homopolymer of >500 β-(1,4)-linked glucose units. In plant cell walls, cellulose is primarily in the form of crystalline microfibrils consisting of approximately 36 hydrogen-bonded cellulose chains, but also has amorphous regions (Somerville, 2006; Newman et al., 2013).
Hemicelluloses are typically branched polysaccharides substituted with various sugars and acyl groups. As discussed further in the Section “Evidence Relating Biomass Content and Bio-oil Composition,” the different sugar composition and linkages of hemicelluloses influence thermal products (Shafizadeh et al., 1972; Mante et al., 2014). The structure and composition of hemicellulosic polysaccharides differ depending on plant species classification, i.e., taxonomy. Major taxonomic divisions with relevance to bioenergy production are grasses, such as switchgrass and wheat; woody dicots, i.e., hardwoods, such as poplar; and woody gymnosperms, i.e., softwoods, such as pine. The most abundant grass hemicelluloses are mixed-linkage glucan (MLG) and glucuronoarabinoxylan (GAX) (Scheller and Ulvskov, 2010; Vega-Sanchez et al., 2013); the hemicelluloses of hardwood are primarily composed of glucuronoxylans (GX) but also contain a small amount of galactomannans (GM) (Pauly and Keegstra, 2008); and softwood hemicelluloses are largely galactoglucomannan (GGM) and GAXs (Scheller and Ulvskov, 2010). MLG is an unbranched glucose polymer similar to cellulose but containing both β-(1-3)- and β-(1-4)-linkages (Vega-Sanchez et al., 2012). MLG is nearly unique to the order Poales, which includes the grasses, but has also been found in horsetail (Equisetum). Its abundance in mature tissues and secondary cell walls has recently been recognized (Vega-Sanchez et al., 2013). Xylans consist of a β-(1-4)-linked xylose backbone with various substitutions. GXs are xylans substituted mostly by glucuronic acid and 4-O-methyl glucuronic acid through α-(1-2)-linkages. GAXs are not only substituted by glucuronic acid but also substituted by arabinofuranoses at the O-3, which can be further substituted by the phenylpropanoid acids, to form feruloyl- and p-coumaryl esters linked at the O-5 (Scheller and Ulvskov, 2010). Acetyl groups are often attached to the O-3 of backbone xyloses but also attach to the O-2. Unlike xylans, which mainly consist of pentoses, mannans consist of hexoses like mannose, glucose, and galactose. GM and GGM have a β-(1-4)-linked backbone with mannose or a combination of glucose and mannose, respectively. Both GM and GGM can be acetylated and substituted by α-(1-6)-linked galactoses (Scheller and Ulvskov, 2010; Rodriguez-Gacio Mdel et al., 2012; Pauly et al., 2013). Relatively depleted in secondary walls, but rich in growing primary walls of dicot species, xyloglucan and pectins are two other polysaccharides in cell walls. Xyloglucan consists of β-(1-4)-linked glucose residues, modified by xylose and other sugar residues; and pectin is another branched or unbranched polymer that is rich in galacturonic acid, rhamnose, galactose, and several other monosaccharide residues (Somerville et al., 2004; Scheller and Ulvskov, 2010).
Lignin is a cross-linked, heteropolyphenol mainly assembled from three monolignols – sinapyl (S), coniferyl (G), and p-coumaryl (H) alcohols. As waste products are often selected as biofuel feedstocks, it is also relevant to note that lignin derived from other monolignols such as caffeyl alcohol and 5-hydroxyconiferyl have been found in the seedcoat of both monocots and dicots (Chen et al., 2012, 2013). Lignin structural heterogeneity and various types of incorporated groups can lead to a variety of different depolymerization reactions during pyrolysis (Kawamoto et al., 2007). Often traceable to the corresponding bio-oil components, the three major lignin units differ in the degree of methoxylation of their carbon ring. S-units are methoxylated at both O-3 and O-5 ring positions; G-unit have one methoxy group at the O-3 position; and H-units lack ring methoxy groups (3, Figure 1) (Boerjan et al., 2003). Lignin units undergo oxidative coupling in the cell wall to form many types of dimers, including β–O–4, β–5, β–β, 5–5, 5–O–4, and β–1, leaving other atoms free to further polymerize, which significantly increases the structural heterogeneity of lignin. Lignin units can also be esterified with p-coumaryl, p-hydroxybenzoyl, and acetyl groups, primarily at the γ position of terminal units (Petrik et al., 2014; Lu et al., 2015). Lignin compositions and the acylation groups vary among plant clades (Boerjan et al., 2003). Woody dicot lignins have G- and S-units and trace amount of H-units. Poplar wood, for example, has a G:S:H ratio of 55:45:1 (Vanholme et al., 2013). The lignin of many hardwoods is acylated by p-hydroxybenzoates (Lu et al., 2015) and acetyl groups in low amounts (Sarkanen et al., 1967). Biomass from other species, such as palms and kenaf, possess a high degree of lignin acetylation (Lu and Ralph, 2002). Grass lignins also contain G- and S-units with slightly higher amount of H-units than woody dicots. Wheat straw, for example, has a G:S:H ratio of 64:30:6 (Bule et al., 2013). Grass lignin possesses high levels of p-coumarate esters (Hatfield et al., 2008) and can also be etherified by tricin and ferulic acid (Ralph et al., 1995; Lan et al., 2015), as discussed further below. Woody gymnosperm lignins are different from angiosperm lignins, being primarily composed of G-units and a lower amount of H-units (Boerjan et al., 2003).
Biomass also contains inorganic elements including Ca, K, Si, Mg, Al, S, Fe, P, Cl, and Na and some trace elements (<0.1%) such as Mn and Ti, according to ash analysis, formed by oxidation of biomass at 575°C (Masia et al., 2007; Vassilev et al., 2010). As with other biomass components, the abundance of mineral elements varies among species. In general, compared with grass biomass, woody biomasses contain less ash, Cl, K, N, S, and Si, but more Ca (Vassilev et al., 2010).
Plant biomass components do not accumulate independently of each other, though their relationships are still an active area of research (Dick-Perez et al., 2011; Tan et al., 2013; Mikkelsen et al., 2015). Biomass component amounts can correlate because they are physically bound to each other through covalent and non-covalent bonds or because they accumulate in the same plant organ or stage of plant development, though a physical interaction may not exist. Because the abundance of some biomass components is correlated, the thermal products from one biomass component may also correlate with other components. For example, the abundance of cellulose correlates with the abundance of lignin in five different biomass sources (Pearson’s correlation coefficient = 0.83) and lignin-derived thermal products correlate with cellulosic glucose (Mante et al., 2014). Many mineral elements are also correlated with each other, for example, N, S, and Cl; Si, Al, Fe, Na, and Ti; Ca, Mg, and Mn; K, P, S, and Cl (Vassilev et al., 2010). Numerous interactions between lignins and hemicelluloses and among hemicelluloses have been observed. Among the best-studied examples, GAXs of grasses and other recently evolved monocot species covalently link to lignin through ether bonds with ferulate esters on arabinose moieties of arabinoxylan (Bunzel et al., 2004). In poplar and spruce wood, NMR results indicate that lignin and carbohydrates are directly bonded through several types of ether linkages (Yuan et al., 2011; Du et al., 2014). The data provide evidence for ether bonds between lignin and C1, C5, and C6 atoms of pentoses and hexoses (Yuan et al., 2011). Generally, xylan is the most closely associated polysaccharide to lignin, and NMR studies have also clearly identified lignin–glucuronic acid ester bonds (Yuan et al., 2011). Also, MLGs closely coat low-substituted xylan regions, likely via non-covalent interactions (Carpita et al., 2001; Kozlova et al., 2014). Furthermore, some components can also affect the distribution of other components. For example, rice plants that overexpress an enzyme that cleaves MLG exhibit reduced MLG and have an altered distribution profile of Si though maintain the same total amount of Si (Kido et al., 2015). In sum, mounting evidence supports covalent and non-covalent interactions among cell wall polymers and components; however, these connections have been difficult to study with questions persisting related to how different cell wall preparations and manipulation may alter observations.
Models for Relationships Between Biomass Components and Bio-oil Product Composition
Reaction pathways of individual biomass components to formation of thermal products have been described (Collard and Blin, 2014). However, the pyrolysis literature suggests that biomass components tend to have more complex effects on bio-oil yield and product composition than simply their quantity. Here, we introduce three possible “models” of how biomass components may influence the yield or composition of thermal products, and in Section “Evidence Relating Biomass Content and Bio-oil Composition,” we discuss evidence supporting each of them. Figure 2 provides schematic representations of the following models:
Model 1: Biomass components are the direct sources of thermal products. Components are converted to products through depolymerization and secondary reactions such as cracking, i.e., splitting, and recombination (Figure 2A).
Model 2: Components or their derived products act as catalysts that accelerate thermal reactions of other components, altering product yields and ratios (Figure 2B).
Model 3: Chemical interactions or structural relationships among cell wall components alter bio-oil composition and/or yield (Figure 2C). This “indirect” model applies when variation in a biomass component alters the yield of a chemically unrelated product in a manner not easily explained by a catalytic effect. Chemical interactions that alter products may either be covalent or non-covalent chemical bonds between cell wall components. Structural relationships refer to correlations between components, often minor ones, and physical features of the biomass. For example, the abundance of a cell wall component may be indicative of the structure of the plant material, such as biomass bulk density differences caused by different leaf to stem ratios, but do not reflect chemical bonding between components. As of the preparation of this review, very little evidence addresses how biological correlations effect bio-oil products, so the discussion focuses on potential chemical interactions.
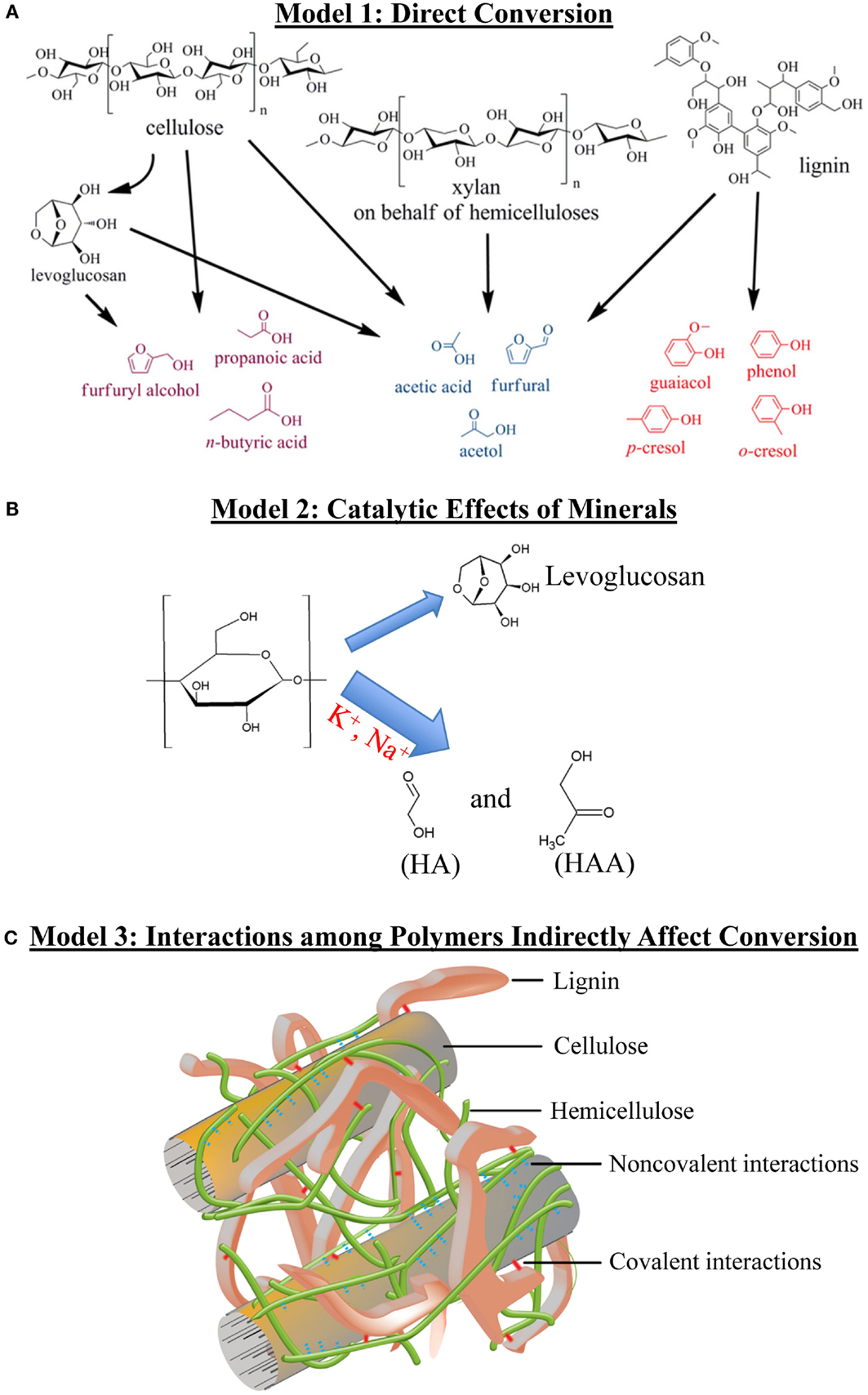
Figure 2. Three models of how biomass components and their interactions affect the formation of thermal products. HA, hydroxyacetone; HAA, hydroxyacetaldehyde. (A) Direct conversion. (B) Catalytic effect of minerals. (C) Interactions among polymers indirectly affect conversion. (A) and (C) were adapted and modified with permission (Vanholme et al., 2010; Zhang et al., 2013).
Evidence Relating Biomass Content and Bio-oil Composition
Evidence in the literature for the three models described above is presented in Table 3 and discussed below. In the reviewed experiments, relationships between biomass components and pyrolysis products have been identified by varying the starting biomass, either through experimentation on purified components, via naturally occurring variation among different biomass sources, or via pretreatment of the biomass. Most studies included in this discussion report the chemical products derived from pyrolysis of biomass or biomass components. Studies that only reported weight losses or elemental balances were not considered. The two dominant techniques present in this corpus of literature are either pyrolysis-gas chromatography/mass spectroscopy, where pyrolysis vapors from microgram- to milligram-scale samples are directly transported to a GC for analysis, or pyrolysis in a gram- to kilogram-scale reactor system followed by condensation of the vapors and subsequent chromatographic analysis of the liquid.
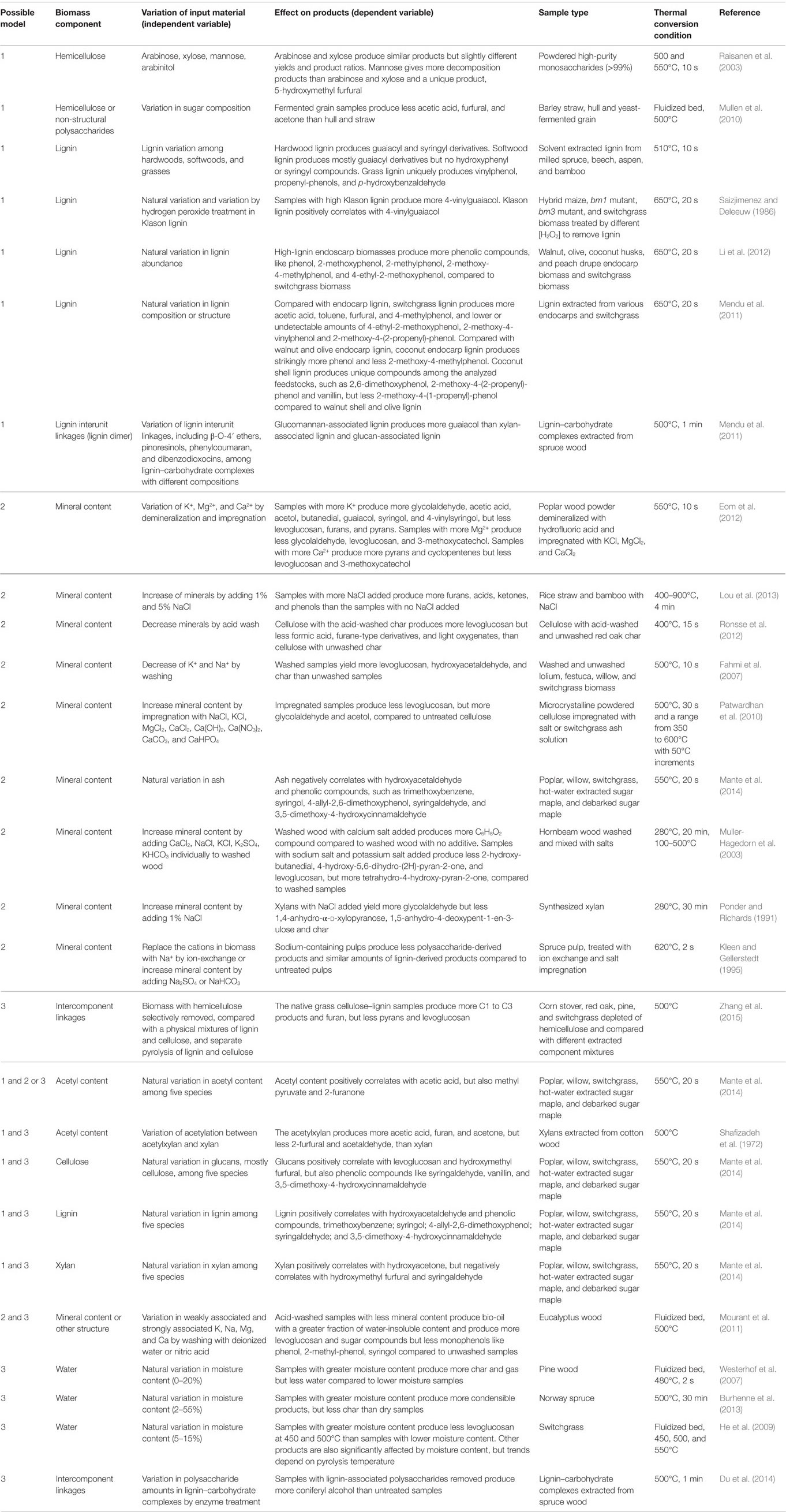
Table 3. Possible models of how biomass components affect the composition and yield of liquid thermal products.
Model 1: Direct Products of Cellulose, Hemicellulose, and Lignin
Thermal breakdown of purified cellulose, hemicellulose, and lignin has been relatively well studied. Levoglucosan, a six-carbon 1,6-anhyrosugar (see Figure 1), was identified as the main product of cellulose pyrolysis nearly a century ago (Pictet and Sarasin, 1918). Levoglucosan is formed alongside other smaller decomposition products, with maximum levoglucosan production occurring at 500°C (Shafizadeh et al., 1979). Minor products of cellulose pyrolysis are dominated by other anhydrosugars that retain all six carbons of glucose, such as 1,6-anhydroglucofuranose and 5-hydroxymethyl furfural, but also smaller molecules, like furfural (5, Figure 1), formic acid, and glycolaldehyde, among others (Patwardhan et al., 2011b).
As with cellulose, hemicellulose pyrolysis products depend mostly on the number of carbons in the monosaccharide residues of the starting polymer (Shafizadeh et al., 1972). Pentoses and hexoses produce similar light C1–C3 oxygenates but differ in the types and selectivities (i.e., relative ratios) of heavier C4–C6 products. Consistent with expectations, pyrolysis of monosaccharides reveals that hexoses can form more unique compounds than pentoses, including pyranic species; additionally, pentoses yield more lighter fragmentation products than hexoses and only trace amounts of C6 and higher products (Raisanen et al., 2003).
Lignin thermal degradation products generally retain the characteristic ring decoration of the monolignols from which they originate (3, Figure 1). For example, syringol derivatives are bio-oil products derived from S-lignin units and guaiacols are products derived from G-lignin units (6, Figure 1). The derivative groups possess 1–3 carbons and/or oxygenate moieties at the fourth position (6, Figure 1). Consistent with expectations, softwood lignins yield almost exclusively guaiacyl derivatives, while hardwood lignins yield both guaiacyl and syringyl derivatives. Grasses yield not only guaiacyl, syringyl, and p-hydroxyphenyl derivatives but also vinylphenol, propenyl-phenols, and p-hydroxybenzaldehyde that are not produced during pyrolysis of softwood and hardwood (Saizjimenez and Deleeuw, 1986; Mante et al., 2014) and are likely derived from ferulate and coumarate esters (Penning et al., 2014b). Phenol derivatives are the large majority of the products formed from lignin pyrolysis; aromatic hydrocarbons and some furan derivatives are also detectable, but at very low amounts that might represent lignin sample contaminants (Saizjimenez and Deleeuw, 1986). Lignins from spruce wood with different dimer compositions also show different product distributions, including variations in the yield of major products like guaiacol (Du et al., 2014). This suggests that bonds between lignin units and the lignin structure determined by those bonds may impact pyrolysis as well.
Model 2: Secondary Reactions Catalyzed by Inorganic Components
The biopolymers that make up the majority of the biomass by weight are established as the primary source of bio-oil products formed during thermal degradation. However, secondary reactions occur during the pyrolysis process involving other components present within the biomass (Ponder and Richards, 1991; Kleen and Gellerstedt, 1995; Muller-Hagedorn et al., 2003; Fahmi et al., 2007; Patwardhan et al., 2010; Ronsse et al., 2012; Lou et al., 2013; Mante et al., 2014). As products form, they can interact with catalytic minerals in the residual solid. For example, levoglucosan has been shown to react on minerals present in the residual char from pyrolysis of biomass. The products formed include levoglucosenone, furan derivatives, and lighter oxygenates such as acetic acid, acetone, and acetol. Demineralization prohibits the formation of these products (Fahmi et al., 2007; Ronsse et al., 2012).
Different inorganics are responsible for different kinds of secondary reactions. In general, the presence of metal cations enhances the homolytic cleavage of pyranose ring bonds over the heterolytic cleavage of glycosidic linkages, leading to the increased formation of light oxygenate decomposition products at the expense of levoglucosan formation. While Na+, K+, Mg2+, and Ca2+ all catalyze levoglucosan decomposition, the effects of group 1 (alkali metals) and group 2 (alkaline) elements differ. Increased Na+ and K+ alkali metal loading increased formic acid, glycolaldehyde, and acetol more than similar amounts of the alkaline metals, Mg2+ and Ca2+, though more furfural is produced with increasing concentrations of Mg2+ and Ca2+. Additionally, the alkali metals reduce levoglucosan production at very low thresholds. This suggests that Na+ and K+ ultimately promote cracking reactions while Mg2+ and Ca2+ promote dehydration reactions (Muller-Hagedorn et al., 2003; Patwardhan et al., 2010; Eom et al., 2012).
Model 3: Interactions and Linkages Between Primary Components
While the first two models address the direct conversion of biopolymer organic components to related bio-oil products and their further reaction catalyzed by biomass inorganics, the third addresses compositional and structural relationships among cell wall components and their impact on products. Interactions between polysaccharides and lignin have been shown to alter pyrolysis products (Du et al., 2014; Zhang et al., 2015). The cellulose–lignin interaction can lead to a decrease in levoglucosan yield and an increase in light (C1–C3) compounds, especially glycolaldehyde and furans. Based on the nature of the small products, Zhang et al. (2015) hypothesized that the cellulose–lignin interaction occupies the C6 position, disfavoring glycosidic bond cleavage that is required for the formation of levoglucosan and favoring light compound and furan formation through ring scission, rearrangement, and dehydration reactions. The strength of this effect on pyrolysis products is most pronounced in grasses, followed by softwood and then hardwood, possibly due to the increased prevalence of covalent bonds between cellulose and lignin in grass cell walls (Jin et al., 2006; Zhou et al., 2010). Hemicellulose–lignin interactions, especially the xylan–lignin interaction revealed in NMR experiments (Yuan et al., 2011), may also affect pyrolysis. Indeed, enzymatic removal of hemicelluloses from lignin–carbohydrate complexes increased coniferyl alcohol yields (Du et al., 2014).
An example of a compositional feature that may impact product distribution is the degree of acetylation of the biopolymers. As mentioned, acetyl groups decorate hemicellulose side chains and are also present in the lignin. The increased abundance of these groups in biomass correlates with increasing yields of acetic acid, methyl pyruvate, acetone, and furan; additionally, this acetylation correlates with decreasing yields of furfural and acetaldehyde (Shafizadeh et al., 1972; Mante et al., 2014). While the acetic acid and perhaps the methyl pyruvate can be explained by the direct production of these compounds upon pyrolytic decomposition (Model 1), the nature of the relationship between acetate and the furanic and other 4-carbon species has not been clearly defined. The production of the 4-carbon species may be due to an indirect effect (Model 3) or may be the result of catalytic reaction of acetate with itself (Model 2).
Several investigations (Westerhof et al., 2007; He et al., 2009; Burhenne et al., 2013) suggest that feedstock moisture content can also play a role in the yield and product distribution of the organic fraction of the bio-oil. As previously discussed, the presence of water in bio-oil prohibits its direct use and creates challenges to catalytic valorization. For these reasons, biomass is typically subjected to drying prior to pyrolysis, which both reduces the required energy of the pyrolysis step and limits the water in the liquid condensate to water produced by decomposition reactions. However, the degree to which the feedstock moisture content should be eliminated is still under investigation. Burhenne et al. (2013) found that higher feedstock moisture content led to slightly lower char and gas yields upon pyrolysis with minimal changes to the elemental composition of the char. However, this is in disagreement with Westerhof et al. (2007) who observed slightly higher char yields with increasing moisture content. The water weight fraction distribution of the feedstocks in the two studies were quite different, 2.4–55.4% in Burhenne et al. versus 0–20% in Westerhof et al. Beyond impacts to the yields, He et al. (2009) studied the change in selectivity to the organic fraction produced upon pyrolysis of switchgrass with 5, 10, and 15% feedstock moisture contents. The authors found that at 500°C, the lowest moisture content feedstock produced the highest amounts of levoglucosan and acetic acid. The authors note that while significant differences in pyrolysis products were observed, they could not identify clear trends in their data. Among these studies, the observable but sometimes contradictory or unclear trends suggest that the feedstock moisture content may have multiple impacts on the pyrolysis process, possibly related to the physical location of the water in biomass.
In addition to compositional factors, morphological factors also influence the bio-oil product distributions. Biomass undergoing thermal decomposition retains its morphology even in harsh thermal treatment regimes (Pohlmann et al., 2014). Biomass is a poor conductor of heat (conductivity <0.1 W/m K) (Bridgwater et al., 1999), and large temperature gradients occur in heated biomass particles (Bryden et al., 2002). Most reactor systems for thermal degradation require size reduction of biomass particles; as an example, fluidized beds require particle sizes no larger than 2 mm (Bridgwater et al., 1999) to ensure rapid reaction. These particle sizes are larger than the tissue structures present in biomass. While the overall tissue and cellular morphology remain intact, micropore formation and shrinkage during the reaction process can occur in a non-uniform manner throughout the biomass (Davidsson and Pettersson, 2002; Pohlmann et al., 2014). Piskorz and colleagues observed decreasing liquid yields with increasing particle size, attributed to increasing incidence of secondary reactions with in wood particles (Scott and Piskorz, 1984). The principles of internal and external diffusion and the impacts of tortuosity, surface area, and diffusion path lengths are all fundamental to catalytic reaction engineering, and in the case of thermal biomass conversion, these important parameters are all dictated by the reacting feedstock (Fogler, 2006). Some evidence supports the notion that different plant developmental stages, which are related to the ratio of leaves to stems and biomass density, result in different pyrolysis products. For example, switchgrass harvested at later times during the growing season produced increased yields of condensable products, relative to that from younger, leafier material (Boateng et al., 2006), though compositional and developmental differences of the starting material were not carefully assessed.
Conclusion
Years of research have led to understanding of the direct pyrolysis conversion pathways of the major monomeric and polymeric constituents of biomass (Model 1, Table 2). The observation that these constituents often represent minor components in raw bio-oil (Table 1) highlights the importance of catalytic degradation (Model 2) and possibly indirect effects (Model 3) on pyrolysis products. The latter model is only recently receiving attention as knowledge of cell wall structures and analytical repertoires blossom (Mante et al., 2014; Zhang et al., 2015). Detailed examination of the relationships between components and products is still sparse, with the biological literature providing detailed characterization of cell wall components, while the engineering literature analyzes the chemical components, or often just total yields, of different pyrolysis fractions. We would argue that further investigations on the relationships between biomass components and thermal products will allow improvement of thermal product “quality.” Short of attaining (or improving on) petroleum fuel-like properties, even the criteria for a high-quality thermal product remain unclear. As discussed, this is, in part, because methods for upgrading are so dependent on bio-oil composition. Thus, methods that economically separate and/or simplify the different product streams, while still maintaining C–C bonds and overall C-content, are more likely to be amenable to catalytic upgrading.
Greater and more systematic analysis of biomass composition and pyrolysis products within species that show significant compositional variation will aid in better understanding biomass–bio-oil relationships. Much of the existing literature relies on comparisons of thermal degradation products across diverse taxonomic groups that vary greatly in cell wall composition beyond the biomass components measured (Table 3). An analysis of more subtle compositional differences, in which compositional factors are varied across different samples, may aid in refining biomass–bio-oil relationships. For example, genetic mutants that vary in only one component relative to near isogenic, unmutated “wild-type” plants can directly address relationships between starting components and products (Li et al., 2012). In addition to genetically determined compositional differences, biomass composition also depends on growth conditions and developmental stage, which relates to harvest time. Taken together, the scale of the problem points to the value of developing high-throughput methods to help identify species and genotypes that are most suitable for production of specific thermal products and to guide the optimization of genetic stocks and growth condition for bioenergy crops. Methods available to identify such “high-quality” biomass include near-infrared reflectance spectroscopy (Vogel et al., 2011), Fourier transform near-infrared spectroscopy (Liu et al., 2010), and pyrolysis molecular beam mass spectrometry, at least for lignin components (Sykes et al., 2009; Penning et al., 2014b). In general, these methods can be trained, either rationally or in a model-independent manner, to detect spectroscopic or molecular signatures in biological materials with linear or non-linear relationships to thermal products.
Besides selecting or breeding for natural variation in biomass composition (Wegrzyn et al., 2010; Penning et al., 2014a), it is also possible to genetically modify biomass composition (Bartley and Ronald, 2009). Most simply, genetic engineering of bioenergy plants can be achieved by modifying the plant’s genome to (1) express genes from other organisms, (2) increase expression of native genes, or (3) reduce expression of native genes. More complex schemes are also possible, in which expression patterns of genes are altered through synthetic biology approaches that recombine various genetic elements (Yang et al., 2013). The most common method for plant genetic engineering co-opts the molecular machinery of a bacterial pathogen that introduces genes into plant chromosomes to facilitate its pathogenesis.
Genetic engineering to improve bio-oil production would aim to increase biomass components that enhance the yield of favored products and/or to decrease components that produce disfavored products or interfere with upgrading strategies. Advances in understanding cell wall biosynthesis, including genes responsible for synthesizing the major polymer classes (Bonawitz and Chapple, 2010; Scheller and Ulvskov, 2010; Pauly et al., 2013) and covalent interactions among them (Chiniquy et al., 2012; Bartley et al., 2013; Schultink et al., 2015); regulation of expression of the cell wall biosynthesis genes (Zhao and Dixon, 2011); and metal ion transport proteins that determine the abundance and distribution of plant mineral content (Ma et al., 2006; Yamaji and Ma, 2009; Zhong and Ye, 2015), lay the foundation for genetically engineering bioenergy crop cell wall content and structure. For example, lignin is an important target for genetic engineering for pyrolysis since the major lignin-derived products have a lower O:C ratio, a higher energy value, and are more stable than sugar-derived products (Tanger et al., 2013; Mante et al., 2014). Some important genes that participate in or regulate lignin synthesis have already been modified in energy crops without major interference with plant biomass yield (Baxter et al., 2014, 2015; reviewed in Bartley et al., 2014). However, current genetic engineering strategies are focused on developing low lignin biomass for saccharification and biochemical conversion to fuels. Therefore, more work is required to develop biomass with high-lignin content for thermal conversion. Producing corrosive acetic acid in bio-oil (Mante et al., 2014), acetyl groups on cell wall polymers are another potential target for genetic engineering of “pyrolysis crops.” Three enzyme classes, including the reduced wall acetylation (RWA) proteins, Trichome birefringence-like (TBL) and Altered Xyloglucan (AXY) proteins acetylate cell wall polysaccharides (Lee et al., 2011; Xiong et al., 2013; Schultink et al., 2015). A mutant of the dicot reference plant, Arabidopsis thaliana, which lacks expression of all four RWA genes, shows a 40% reduction in secondary wall-associated acetyl groups (Lee et al., 2011). Reducing expression of this family of genes in bioenergy crops may help to solve the problems caused by acetic acid in bio-oil produced from such plants.
Pretreatments such as washing/leaching and torrefaction are another class of strategies to improve biomass quality by changing biomass composition (Zheng et al., 2013; Banks et al., 2014). For example, by washing biomass with detergent (Triton) or acid to remove minerals, the yield of bio-oil is increased and reaction water content is reduced (Banks et al., 2014). Coupling biochemical conversion of biomass, which depletes the polysaccharide fraction, with pyrolysis of the resulting residue, or bagasse, is another avenue to explore further (Islam et al., 2010; Cunha et al., 2011). Torrefaction is a low-temperature (200–400°C) thermal pretreatment that decomposes hemicellulose and may segregate disfavored products such as water and acid into intermediate streams before the next stage of pyrolysis (Zheng et al., 2013). More efficient torrefaction may be achieved by changing the composition or chemical structure of hemicellulose through genetic methods to further separate the decomposition temperatures of hemicellulose from lignin and cellulose. By identifying and studying the roles of key biomass components during thermal conversion, it will be possible to maximize the economic and environmental benefits of plant biomass-derived biofuels in the future.
Author Contributions
FL and CW drafted and revised the manuscript. LB, LL, and RM revised the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This material is based upon work supported by the National Institute of Food and Agriculture, U.S. Department of Agriculture, under award number 2010-38502-21836, through the South Central Sun Grant Program.
References
Adler, P. R., Sanderson, M. A., Boateng, A. A., Weimer, P. I., and Jung, H. J. G. (2006). Biomass yield and biofuel quality of switchgrass harvested in fall or spring. Agron. J. 98, 1518–1525. doi: 10.2134/agronj2005.0351
Banks, S. W., Nowakowski, D. J., and Bridgwater, A. V. (2014). Fast pyrolysis processing of surfactant washed Miscanthus. Fuel Process. Technol. 128, 94–103. doi:10.1016/j.fuproc.2014.07.005
Bartley, L. E., Peck, M. L., Kim, S. R., Ebert, B., Manisseri, C., Chiniquy, D. M., et al. (2013). Overexpression of a BAHD acyltransferase, OsAt10, alters rice cell wall hydroxycinnamic acid content and saccharification. Plant Physiol. 161, 1615–1633. doi:10.1104/pp.112.208694
Bartley, L. E., and Ronald, P. C. (2009). Plant and microbial research seeks biofuel production from lignocellulose. Calif. Agric. 63, 178–184. doi:10.3733/ca.v063n04p178
Bartley, L. E., Tao, X., Zhang, C., Nguyen, H., and Zhou, J. (2014). “Switchgrass biomass content, synthesis, and biochemical conversion to biofuels,” in Compendium of Bioenergy Plants, eds Luo H., and Wu Y. (Boca Raton, FL: Science), 109–169.
Baxter, H. L., Mazarei, M., Labbe, N., Kline, L. M., Cheng, Q., Windham, M. T., et al. (2014). Two-year field analysis of reduced recalcitrance transgenic switchgrass. Plant Biotechnol. J. 12, 914–924. doi:10.1111/pbi.12195
Baxter, H. L., Poovaiah, C. R., Yee, K. L., Mazarei, M., Rodriguez, M., Thompson, O. A., et al. (2015). Field evaluation of transgenic switchgrass plants overexpressing PvMYB4 for reduced biomass recalcitrance. Bioenerg. Res. 8, 910–921. doi:10.1007/s12155-014-9570-1
Boateng, A. A., Hicks, K. B., and Vogel, K. P. (2006). Pyrolysis of switchgrass (Panicum virgatum) harvested at several stages of maturity. J. Anal. Appl. Pyrolysis 75, 55–64. doi:10.1016/j.jaap.2005.03.005
Boerjan, W., Ralph, J., and Baucher, M. (2003). Lignin biosynthesis. Annu. Rev. Plant Biol. 54, 519–546. doi:10.1146/annurev.arplant.54.031902.134938
Bonawitz, N. D., and Chapple, C. (2010). The genetics of lignin biosynthesis: connecting genotype to phenotype. Ann. Rev. Genet. 44, 337–363. doi:10.1146/annurev-genet-102209-163508
Bridgwater, A. V. (1994). Catalysis in thermal biomass conversion. Appl. Catal. A Gen. 116, 5–47. doi:10.1016/0926-860x(94)80278-5
Bridgwater, A. V., Meier, D., and Radlein, D. (1999). An overview of fast pyrolysis of biomass. Org. Geochem. 30, 1479–1493. doi:10.1016/S0146-6380(99)00120-5
Bryden, K. M., Ragland, K. W., and Rutland, C. J. (2002). Modeling thermally thick pyrolysis of wood. Biomass Bioenergy 22, 41–53. doi:10.1016/S0961-9534(01)00060-5
Bule, M. V., Gao, A. H., Hiscox, B., and Chen, S. (2013). Structural modification of lignin and characterization of pretreated wheat straw by ozonation. J. Agric. Food Chem. 61, 3916–3925. doi:10.1021/jf4001988
Bunzel, M., Ralph, J., Lu, F., Hatfield, R. D., and Steinhart, H. (2004). Lignins and ferulate-coniferyl alcohol cross-coupling products in cereal grains. J. Agric. Food Chem. 52, 6496–6502. doi:10.1021/jf040204p
Burhenne, L., Damiani, M., and Aicher, T. (2013). Effect of feedstock water content and pyrolysis temperature on the structure and reactivity of spruce wood char produced in fixed bed pyrolysis. Fuel 107, 836–847. doi:10.1016/j.fuel.2013.01.033
Carlson, T. R., Tompsett, G. A., Conner, W. C., and Huber, G. W. (2009). Aromatic production from catalytic fast pyrolysis of biomass-derived feedstocks. Top. Catal. 52, 241–252. doi:10.1007/s11244-008-9160-6
Carlson, T. R., Vispute, T. R., and Huber, G. W. (2008). Green gasoline by catalytic fast pyrolysis of solid biomass derived compounds. ChemSusChem 1, 397–400. doi:10.1002/cssc.200800018
Carpita, N. C., Defernez, M., Findlay, K., Wells, B., Shoue, D. A., Catchpole, G., et al. (2001). Cell wall architecture of the elongating maize coleoptile. Plant Physiol. 127, 551–565. doi:10.1104/pp.010146
Chen, F., Tobimatsu, Y., Havkin-Frenkel, D., Dixon, R. A., and Ralph, J. (2012). A polymer of caffeyl alcohol in plant seeds. Proc. Natl. Acad. Sci. U.S.A. 109, 1772–1777. doi:10.1073/pnas.1120992109
Chen, F., Tobimatsu, Y., Jackson, L., Nakashima, J., Ralph, J., and Dixon, R. A. (2013). Novel seed coat lignins in the Cactaceae: structure, distribution and implications for the evolution of lignin diversity. Plant J. 73, 201–211. doi:10.1111/tpj.12012
Chiniquy, D., Sharma, V., Schultink, A., Baidoo, E. E., Rautengarten, C., Cheng, K., et al. (2012). XAX1 from glycosyltransferase family 61 mediates xylosyltransfer to rice xylan. Proc. Natl. Acad. Sci. U.S.A. 109, 17117–17122. doi:10.1073/pnas.1202079109
Collard, F. X., and Blin, J. (2014). A review on pyrolysis of biomass constituents: mechanisms and composition of the products obtained from the conversion of cellulose, hemicelluloses and lignin. Renew. Sustain. Energ. Rev. 38, 594–608. doi:10.1016/j.rser.2014.06.013
Cunha, J. A., Pereira, M. M., Valente, L. M. M., De La Piscina, P. R., Homs, N., and Santos, M. R. L. (2011). Waste biomass to liquids: low temperature conversion of sugarcane bagasse to bio-oil. The effect of combined hydrolysis treatments. Biomass Bioenergy 35, 2106–2116. doi:10.1016/j.biombioe.2011.02.019
Davidsson, K. O., and Pettersson, J. B. C. (2002). Birch wood particle shrinkage during rapid pyrolysis. Fuel 81, 263–270. doi:10.1016/S0016-2361(01)00169-7
Demirbas, A. (2011). Competitive liquid biofuels from biomass. Appl. Energy 88, 17–28. doi:10.1016/j.apenergy.2010.07.016
Dick-Perez, M., Zhang, Y., Hayes, J., Salazar, A., Zabotina, O. A., and Hong, M. (2011). Structure and interactions of plant cell-wall polysaccharides by two- and three-dimensional magic-angle-spinning solid-state NMR. Biochemistry 50, 989–1000. doi:10.1021/bi101795q
Du, X., Perez-Boada, M., Fernandez, C., Rencoret, J., Del Rio, J. C., Jimenez-Barbero, J., et al. (2014). Analysis of lignin-carbohydrate and lignin-lignin linkages after hydrolase treatment of xylan-lignin, glucomannan-lignin and glucan-lignin complexes from spruce wood. Planta 239, 1079–1090. doi:10.1007/s00425-014-2037-y
El-Nashaar, H. M., Banowetz, G. M., Griffith, S. M., Casler, M. D., and Vogel, K. P. (2009). Genotypic variability in mineral composition of switchgrass. Bioresour. Technol. 100, 1809–1814. doi:10.1016/j.biortech.2008.09.058
Eom, I. Y., Kim, J. Y., Kim, T. S., Lee, S. M., Choi, D., Choi, I. G., et al. (2012). Effect of essential inorganic metals on primary thermal degradation of lignocellulosic biomass. Bioresour. Technol. 104, 687–694. doi:10.1016/j.biortech.2011.10.035
Fahmi, R., Bridgwater, A. V., Darvell, L. I., Jones, J. M., Yates, N., Thain, S., et al. (2007). The effect of alkali metals on combustion and pyrolysis of Lolium and Festuca grasses, switchgrass and willow. Fuel 86, 1560–1569. doi:10.1016/j.fuel.2006.11.030
Faix, O., Fortmann, I., Bremer, J., and Meier, D. (1991a). Thermal-degradation products of wood – a collection of electron-impact (EI) mass-spectra of polysaccharide derived products. Holz Roh Werkst. 49, 299–304. doi:10.1007/Bf02663795
Faix, O., Fortmann, I., Bremer, J., and Meier, D. (1991b). Thermal-degradation products of wood – gas-chromatographic separation and mass-spectrometric characterization of polysaccharide derived products. Holz Roh Werkst. 49, 213–219. doi:10.1007/Bf02613278
Fogler, H. S. (2006). Elements of Chemical Reaction Engineering. Upper Saddle River, NJ: Prentice Hall PTR.
Furimsky, E. (2000). Catalytic hydrodeoxygenation. Appl. Catal. A Gen. 199, 147–190. doi:10.1016/S0926-860x(99)00555-4
Gonzalez-Borja, M. A., and Resasco, D. E. (2015). Reaction pathways in the liquid phase alkylation of biomass-derived phenolic compounds. AIChE J. 61, 598–609. doi:10.1002/aic.14658
Hatfield, R. D., Marita, J. M., and Frost, K. (2008). Characterization of p-coumarate accumulation, p-coumaroyl transferase, and cell wall changes during the development of corn stems. J. Sci. Food Agric. 88, 2529–2537. doi:10.1002/jsfa.3376
He, R., Ye, X. P., English, B. C., and Satrio, J. A. (2009). Influence of pyrolysis condition on switchgrass bio-oil yield and physicochemical properties. Bioresour. Technol. 100, 5305–5311. doi:10.1016/j.biortech.2009.02.069
Huber, G. W., Iborra, S., and Corma, A. (2006). Synthesis of transportation fuels from biomass: chemistry, catalysts, and engineering. Chem. Rev. 106, 4044–4098. doi:10.1021/cr068360d
Islam, M. R., Parveen, M., and Haniu, H. (2010). Properties of sugarcane waste-derived bio-oils obtained by fixed-bed fire-tube heating pyrolysis. Bioresour. Technol. 101, 4162–4168. doi:10.1016/j.biortech.2009.12.137
Jin, Z., Katsumata, K. S., Lam, T. B., and Iiyama, K. (2006). Covalent linkages between cellulose and lignin in cell walls of coniferous and nonconiferous woods. Biopolymers 83, 103–110. doi:10.1002/bip.20533
Kawamoto, H., Horigoshi, S., and Saka, S. (2007). Pyrolysis reactions of various lignin model dimers. J. Wood Sci. 53, 168–174. doi:10.1007/s10086-006-0834-z
Kido, N., Yokoyama, R., Yamamoto, T., Furukawa, J., Iwai, H., Satoh, S., et al. (2015). The matrix polysaccharide (1;3,1;4)-beta-d-glucan is involved in silicon-dependent strengthening of rice cell wall. Plant Cell Physiol. 56, 268–276. doi:10.1093/pcp/pcu162
Kleen, M., and Gellerstedt, G. (1995). Influence of inorganic species on the formation of polysaccharide and lignin degradation products in the analytical pyrolysis of pulps. J. Anal. Appl. Pyrolysis 35, 15–41. doi:10.1016/0165-2370(95)00893-J
Kozlova, L. V., Ageeva, M. V., Ibragimova, N. N., and Gorshkova, T. A. (2014). Arrangement of mixed-linkage glucan and glucuronoarabinoxylan in the cell walls of growing maize roots. Ann. Bot. 114, 1135–1145. doi:10.1093/aob/mcu125
Lan, W., Lu, F., Regner, M., Zhu, Y., Rencoret, J., Ralph, S. A., et al. (2015). Tricin, a flavonoid monomer in monocot lignification. Plant Physiol. 167, 1284–1295. doi:10.1104/pp.114.253757
Lee, C., Teng, Q., Zhong, R., and Ye, Z. H. (2011). The four Arabidopsis reduced wall acetylation genes are expressed in secondary wall-containing cells and required for the acetylation of xylan. Plant Cell Physiol. 52, 1289–1301. doi:10.1093/pcp/pcr075
Li, M., Foster, C., Kelkar, S., Pu, Y., Holmes, D., Ragauskas, A., et al. (2012). Structural characterization of alkaline hydrogen peroxide pretreated grasses exhibiting diverse lignin phenotypes. Biotechnol. Biofuels 5, 38. doi:10.1186/1754-6834-5-38
Liu, L., Ye, X. P., Womac, A. R., and Sokhansanj, S. (2010). Variability of biomass chemical composition and rapid analysis using FT-NIR techniques. Carbohydr. Polym. 81, 820–829. doi:10.1016/j.carbpol.2010.03.058
Lou, R., Wu, S. B., Lv, G. J., and Zhang, A. L. (2013). Factors related to minerals and ingredients influencing the distribution of pyrolysates derived from herbaceous biomass. Bioresources 8, 1345–1360. doi:10.15376/biores.8.1.1345-1360
Lu, F., Karlen, S. D., Regner, M., Kim, H., Ralph, S. A., Sun, R.-C., et al. (2015). Naturally p-hydroxybenzoylated lignins in palms. Bioenergy Res. 8, 934–952. doi:10.1007/s12155-015-9583-4
Lu, F. C., and Ralph, J. (2002). Preliminary evidence for sinapyl acetate as a lignin monomer in kenaf. Chem. Commun. 90–91. doi:10.1039/b109876d
Ma, J. F., Tamai, K., Yamaji, N., Mitani, N., Konishi, S., Katsuhara, M., et al. (2006). A silicon transporter in rice. Nature 440, 688–691. doi:10.1038/nature04590
Mante, O. D., Babu, S. P., and Amidon, T. E. (2014). A comprehensive study on relating cell-wall components of lignocellulosic biomass to oxygenated species formed during pyrolysis. J. Anal. Appl. Pyrolysis 108, 56–67. doi:10.1016/j.jaap.2014.05.016
Masia, A. A. T., Buhre, B. J. P., Gupta, R. P., and Wall, T. F. (2007). Characterising ash of biomass and waste. Fuel Process. Technol. 88, 1071–1081. doi:10.1016/j.fuproc.2007.06.011
McKendry, P. (2002). Energy production from biomass (part 1): overview of biomass. Bioresour. Technol. 83, 37–46. doi:10.1016/S0960-8524(01)00118-3
Mendu, V., Harman-Ware, A. E., Crocker, M., Jae, J., Stork, J., Morton, S. III, et al. (2011). Identification and thermochemical analysis of high-lignin feedstocks for biofuel and biochemical production. Biotechnol. Biofuels 4, 43. doi:10.1186/1754-6834-4-43
Mettler, M. S., Vlachos, D. G., and Dauenhauer, P. J. (2012). Top ten fundamental challenges of biomass pyrolysis for biofuels. Energy Environ. Sci. 5, 7797–7809. doi:10.1039/C2EE21679E
Mikkelsen, D., Flanagan, B. M., Wilson, S. M., Bacic, A., and Gidley, M. J. (2015). Interactions of arabinoxylan and (1,3)(1,4)-beta-glucan with cellulose networks. Biomacromolecules 16, 1232–1239. doi:10.1021/acs.biomac.5b00009
Mohan, D., Pittman, C. U., and Steele, P. H. (2006). Pyrolysis of wood/biomass for bio-oil: a critical review. Energy Fuel 20, 848–889. doi:10.1021/ef0502397
Mourant, D., Wang, Z. H., He, M., Wang, X. S., Garcia-Perez, M., Ling, K. C., et al. (2011). Mallee wood fast pyrolysis: effects of alkali and alkaline earth metallic species on the yield and composition of bio-oil. Fuel 90, 2915–2922. doi:10.1016/j.fuel.2011.04.033
Mullen, C. A., Boateng, A. A., Hicks, K. B., Goldberg, N. M., and Moreau, R. A. (2010). Analysis and comparison of bio-oil produced by fast pyrolysis from three barley biomass/byproduct streams. Energy Fuel 24, 699–706. doi:10.1021/ef900912s
Muller-Hagedorn, M., Bockhorn, H., Krebs, L., and Muller, U. (2003). A comparative kinetic study on the pyrolysis of three different wood species. J. Anal. Appl. Pyrolysis 6, 231–249. doi:10.1016/S0165-2370(03)00065-2
Newman, R. H., Hill, S. J., and Harris, P. J. (2013). Wide-angle x-ray scattering and solid-state nuclear magnetic resonance data combined to test models for cellulose microfibrils in mung bean cell walls. Plant Physiol. 163, 1558–1567. doi:10.1104/pp.113.228262
NSF. (2008). “Selective thermal processing of cellulosic biomass and lignin” in Breaking the Chemical and Engineering Barriers to Lignocellulosic Biofuels: Next Generation Hydrocarbon Biorefineries. ed. Huber G. W. (Washington, DC: University of Massachusetts, Amherst), 30–47.
Oasmaa, A., and Czernik, S. (1999). Fuel oil quality of biomass pyrolysis oils state of the art for the end users. Energy Fuel 13, 914–921. doi:10.1021/ef980272b
O’Neill, M. A., and York, W. S. (2009). “The plant cell wall,” in Annual Plant Reviews. ed. Rose J. K. C. (Blackwell: Wiley), 1–44.
Patwardhan, P. R., Brown, R. C., and Shanks, B. H. (2011a). Understanding the fast pyrolysis of lignin. ChemSusChem 4, 1629–1636. doi:10.1002/cssc.201100133
Patwardhan, P. R., Dalluge, D. L., Shanks, B. H., and Brown, R. C. (2011b). Distinguishing primary and secondary reactions of cellulose pyrolysis. Bioresour. Technol. 102, 5265–5269. doi:10.1016/j.biortech.2011.02.018
Patwardhan, P. R., Satrio, J. A., Brown, R. C., and Shanks, B. H. (2010). Influence of inorganic salts on the primary pyrolysis products of cellulose. Bioresour. Technol. 101, 4646–4655. doi:10.1016/j.biortech.2010.01.112
Pauly, M., Gille, S., Liu, L., Mansoori, N., De Souza, A., Schultink, A., et al. (2013). Hemicellulose biosynthesis. Planta 238, 627–642. doi:10.1007/s00425-013-1921-1
Pauly, M., and Keegstra, K. (2008). Cell-wall carbohydrates and their modification as a resource for biofuels. Plant J. 54, 559–568. doi:10.1111/j.1365-313X.2008.03463.x
Penning, B. W., Sykes, R. W., Babcock, N. C., Dugard, C. K., Held, M. A., Klimek, J. F., et al. (2014a). Genetic determinants for enzymatic digestion of lignocellulosic biomass are independent of those for lignin abundance in a maize recombinant inbred population. Plant Physiol. 165, 1475–1487. doi:10.1104/pp.114.242446
Penning, B. W., Sykes, R. W., Babcock, N. C., Dugard, C. K., Klimek, J. F., Gamblin, D., et al. (2014b). Validation of PyMBMS as a high-throughput screen for lignin abundance in lignocellulosic biomass of grasses. Bioenerg. Res. 7, 899–908. doi:10.1007/s12155-014-9410-3
Perlack, R. D., Wright, L. L., Turhollow, A., Graham, R. L., Stokes, B. J., and Erbach, D. C. (2005). Biomass as Feedstock for a Bioenergy and Bioproducts Industry: The Technical Feasibility of a Billion-Ton Annual Supply. Oak Ridge, TN: Oak Ridge National Laboratory.
Petrik, D. L., Karlen, S. D., Cass, C. L., Padmakshan, D., Lu, F., Liu, S., et al. (2014). p-Coumaroyl-CoA:monolignol transferase (PMT) acts specifically in the lignin biosynthetic pathway in Brachypodium distachyon. Plant J. 77, 713–726. doi:10.1111/tpj.12420
Pham, T. N., Sooknoi, T., Crossley, S. P., and Resasco, D. E. (2013). Ketonization of carboxylic acids: mechanisms, catalysts, and implications for biomass conversion. ACS Catal. 3, 2456–2473. doi:10.1021/cs400501h
Pictet, A., and Sarasin, J. (1918). Sur la distillation de la cellulose et de l’amidon sous pression réduite. Helv. Chim. Acta 1, 87–96. doi:10.1002/hlca.19180010109
Pohlmann, J. G., Osorio, E., Vilela, A. C. F., Diez, M. A., and Borrego, A. G. (2014). Integrating physicochemical information to follow the transformations of biomass upon torrefaction and low-temperature carbonization. Fuel 131, 17–27. doi:10.1016/j.fuel.2014.04.067
Ponder, G. R., and Richards, G. N. (1991). Mechanisms of pyrolysis of polysaccharides. 4. Thermal synthesis and pyrolysis of a xylan. Carbohydr. Res. 218, 143–155. doi:10.1016/0008-6215(91)84093-T
Raisanen, U., Pitkanen, I., Halttunen, H., and Hurtta, M. (2003). Formation of the main degradation compounds from arabinose, xylose, mannose and arabinitol during pyrolysis. J. Therm. Anal. Calorim. 72, 481–488. doi:10.1023/A:1024557011975
Ralph, J., Grabber, J. H., and Hatfield, R. D. (1995). Lignin-ferulate cross-links in grasses – active incorporation of ferulate polysaccharide esters into ryegrass lignins. Carbohydr. Res. 275, 167–178. doi:10.1016/0008-6215(95)00237-N
Resasco, D. E. (2011). What should we demand from the catalysts responsible for upgrading biomass pyrolysis oil? J. Phys. Chem. Lett. 2, 2294–2295. doi:10.1021/jz201135x
Rodriguez-Gacio Mdel, C., Iglesias-Fernandez, R., Carbonero, P., and Matilla, A. J. (2012). Softening-up mannan-rich cell walls. J. Exp. Bot. 63, 3976–3988. doi:10.1093/jxb/ers096
Ronsse, F., Bai, X. L., Prins, W., and Brown, R. C. (2012). Secondary reactions of levoglucosan and char in the fast pyrolysis of cellulose. Environ. Prog. Sustain. 31, 256–260. doi:10.1002/ep.11633
Saizjimenez, C., and Deleeuw, J. W. (1986). Lignin pyrolysis products – their structures and their significance as biomarkers. Org. Geochem. 10, 869–876. doi:10.1016/S0146-6380(86)80024-9
Sarkanen, K. V., Chang, H., and Allan, G. G. (1967). Species variation in lignins. 3. Hardwood lignins. Tappi 50, 587.
Scheller, H. V., and Ulvskov, P. (2010). Hemicelluloses. Annu. Rev. Plant Biol. 61, 263–289. doi:10.1146/annurev-arplant-042809-112315
Schultink, A., Naylor, D., Dama, M., and Pauly, M. (2015). The role of the plant-specific ALTERED XYLOGLUCAN9 protein in Arabidopsis cell wall polysaccharide O-acetylation. Plant Physiol. 167, 1271–U1243. doi:10.1104/pp.114.256479
Scott, D. S., and Piskorz, J. (1984). The continuous flash pyrolysis of biomass. Can. J. Chem. Eng. 62, 404–412. doi:10.1016/j.biortech.2012.11.114
Shafizadeh, F., Mcginnis, G. D., and Philpot, C. W. (1972). Thermal degradation of xylan and related model compounds. Carbohydr. Res. 25, 23–33. doi:10.1016/S0008-6215(00)82742-1
Shafizadeh, F., Furneaux, R. H., Cochran, T. G., Scholl, J. P., and Sakai, Y. (1979). Production of levoglucosan and glucose from pyrolysis of cellulosic materials. J. Appl. Polym. Sci. 23, 3525–3539. doi:10.1002/app.1979.070231209
Singh, M. P., Erickson, J. E., Sollenberger, L. E., Woodard, K. R., Vendramini, J. M. B., and Fedenko, J. R. (2012). Mineral composition and biomass partitioning of sweet sorghum grown for bioenergy in the southeastern USA. Biomass Bioenergy 47, 1–8. doi:10.1016/j.biombioe.2012.10.022
Somerville, C. (2006). Cellulose synthesis in higher plants. Annu. Rev. Cell Dev. Biol. 22, 53–78. doi:10.1146/annurev.cellbio.22.022206.160206
Somerville, C., Bauer, S., Brininstool, G., Facette, M., Hamann, T., Milne, J., et al. (2004). Toward a systems approach to understanding plant cell walls. Science 306, 2206–2211. doi:10.1126/science.1102765
Sykes, R., Yung, M., Novaes, E., Kirst, M., Peter, G., and Davis, M. (2009). “High-throughput screening of plant cell-wall composition using pyrolysis molecular beam mass spectroscopy,” in Biofuels, ed. Mielenz J. R. (New York City: Humana Press), 169–183.
Tan, L., Eberhard, S., Pattathil, S., Warder, C., Glushka, J., Yuan, C., et al. (2013). An Arabidopsis cell wall proteoglycan consists of pectin and arabinoxylan covalently linked to an arabinogalactan protein. Plant Cell 25, 270–287. doi:10.1105/tpc.112.107334
Tanger, P., Field, J. L., Jahn, C. E., Defoort, M. W., and Leach, J. E. (2013). Biomass for thermochemical conversion: targets and challenges. Front. Plant Sci. 4:218. doi:10.3389/fpls.2013.00218
Vanholme, B., Cesarino, I., Goeminne, G., Kim, H., Marroni, F., Van Acker, R., et al. (2013). Breeding with rare defective alleles (BRDA): a natural Populus nigra HCT mutant with modified lignin as a case study. New Phytol. 198, 765–776. doi:10.1111/nph.12179
Vanholme, R., Van Acker, R., and Boerjan, W. (2010). Potential of Arabidopsis systems biology to advance the biofuel field. Trends Biotechnol. 28, 543–547. doi:10.1016/j.tibtech.2010.07.008
Vassilev, S. V., Baxter, D., Andersen, L. K., and Vassileva, C. G. (2010). An overview of the chemical composition of biomass. Fuel 89, 913–933. doi:10.1016/j.fuel.2009.10.022
Vega-Sanchez, M. E., Verhertbruggen, Y., Christensen, U., Chen, X., Sharma, V., Varanasi, P., et al. (2012). Loss of cellulose synthase-like F6 function affects mixed-linkage glucan deposition, cell wall mechanical properties, and defense responses in vegetative tissues of rice. Plant Physiol. 159, 56–69. doi:10.1104/pp.112.195495
Vega-Sanchez, M. E., Verhertbruggen, Y., Scheller, H. V., and Ronald, P. C. (2013). Abundance of mixed linkage glucan in mature tissues and secondary cell walls of grasses. Plant Signal. Behav. 8, e23143. doi:10.4161/psb.23143
Vogel, J. (2008). Unique aspects of the grass cell wall. Curr. Opin. Plant Biol. 11, 301–307. doi:10.1016/j.pbi.2008.03.002
Vogel, K. P., Dien, B. S., Jung, H. G., Casler, M. D., Masterson, S. D., and Mitchell, R. B. (2011). Quantifying actual and theoretical ethanol yields for switchgrass strains using NIRS analyses. Bioenergy Res. 4, 96–110. doi:10.1007/s12155-010-9104-4
Wan, S. L., Waters, C., Stevens, A., Gumidyala, A., Jentoft, R., Lobban, L., et al. (2015). Decoupling HZSM-5 catalyst activity from deactivation during upgrading of pyrolysis oil vapors. ChemSusChem 8, 552–559. doi:10.1002/cssc.201402861
Wegrzyn, J. L., Eckert, A. J., Choi, M., Lee, J. M., Stanton, B. J., Sykes, R., et al. (2010). Association genetics of traits controlling lignin and cellulose biosynthesis in black cottonwood (Populus trichocarpa, Salicaceae) secondary xylem. New Phytol. 188, 515–532. doi:10.1111/j.1469-8137.2010.03415.x
Westerhof, R. J. M., Kuipers, N. J. M., Kersten, S. R. A., and Van Swaaij, W. P. M. (2007). Controlling the water content of biomass fast pyrolysis oil. Ind. Eng. Chem. Res. 46, 9238–9247. doi:10.1021/ie070684k
Xiong, G. Y., Cheng, K., and Pauly, M. (2013). Xylan O-acetylation impacts xylem development and enzymatic recalcitrance as indicated by the Arabidopsis mutant tbl29. Mol. Plant. 6, 1373–1375. doi:10.1093/mp/sst014
Yamaji, N., and Ma, J. F. (2009). A transporter at the node responsible for intervascular transfer of silicon in rice. Plant Cell 21, 2878–2883. doi:10.1105/tpc.109.069831
Yang, F., Mitra, P., Zhang, L., Prak, L., Verhertbruggen, Y., Kim, J. S., et al. (2013). Engineering secondary cell wall deposition in plants. Plant Biotechnol. J. 11, 325–335. doi:10.1111/Pbi.12016
Youngs, H., and Somerville, C. (2012). Growing better biofuel crops. Scientist 26, 46–52. doi:10.2134/jeq2013.05.0171
Yuan, T. Q., Sun, S. N., Xu, F., and Sun, R. C. (2011). Characterization of lignin structures and lignin-carbohydrate complex (LCC) linkages by quantitative 13C and 2D HSQC NMR spectroscopy. J. Agric. Food Chem. 59, 10604–10614. doi:10.1021/jf2031549
Zapata, P. A., Faria, J., Ruiz, M. P., and Resasco, D. E. (2012). Condensation/hydrogenation of biomass-derived oxygenates in water/oil emulsions stabilized by nanohybrid catalysts. Top. Catal. 55, 38–52. doi:10.1007/s11244-012-9768-4
Zhang, J., Choi, Y. S., Yoo, C. G., Kim, T. H., Brown, R. C., and Shanks, B. H. (2015). Cellulose-hemicellulose and cellulose-lignin interactions during fast pyrolysis. ACS Sustain. Chem. Eng. 3, 293–301. doi:10.1021/sc500664h
Zhang, X. S., Yang, G. X., Jiang, H., Liu, W. J., and Ding, H. S. (2013). Mass production of chemicals from biomass-derived oil by directly atmospheric distillation coupled with co-pyrolysis. Sci. Rep. 3, 1120. doi:10.1038/srep01120
Zhao, Q., and Dixon, R. A. (2011). Transcriptional networks for lignin biosynthesis: more complex than we thought? Trends Plant Sci. 16, 227–233. doi:10.1016/j.tplants.2010.12.005
Zhao, Y. L., Steinberger, Y., Shi, M., Han, L. P., and Xie, G. H. (2012). Changes in stem composition and harvested produce of sweet sorghum during the period from maturity to a sequence of delayed harvest dates. Biomass Bioenergy 39, 261–273. doi:10.1016/j.biombioe.2012.01.020
Zheng, A., Zhao, Z., Chang, S., Huang, Z., Wang, X., He, F., et al. (2013). Effect of torrefaction on structure and fast pyrolysis behavior of corncobs. Bioresour. Technol. 128, 370–377. doi:10.1016/j.biortech.2012.10.067
Zhong, R., and Ye, Z. H. (2015). Secondary cell walls: biosynthesis, patterned deposition and transcriptional regulation. Plant Cell Physiol. 56, 195–214. doi:10.1093/pcp/pcu140
Zhou, Y., Stuart-Williams, H., Farquhar, G. D., and Hocart, C. H. (2010). The use of natural abundance stable isotopic ratios to indicate the presence of oxygen-containing chemical linkages between cellulose and lignin in plant cell walls. Phytochemistry 71, 982–993. doi:10.1016/j.phytochem.2010.03.001
Keywords: thermochemical conversion, plant biomass, bio-oil, lignin, polysaccharides, cell wall, fast pyrolysis, minerals
Citation: Lin F, Waters CL, Mallinson RG, Lobban LL and Bartley LE (2015) Relationships between biomass composition and liquid products formed via pyrolysis. Front. Energy Res. 3:45. doi: 10.3389/fenrg.2015.00045
Received: 22 July 2015; Accepted: 29 September 2015;
Published: 21 October 2015
Edited by:
Jason Lupoi, Joint BioEnergy Institute, USA; University of Queensland, AustraliaReviewed by:
Suyin Gan, The University of Nottingham Malaysia Campus, MalaysiaXu Fang, Shandong University, China
Copyright: © 2015 Lin, Waters, Mallinson, Lobban and Bartley. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Laura E. Bartley, lbartley@ou.edu
†Fan Lin and Christopher L. Waters have contributed equally to this work.
 Fan Lin
Fan Lin Christopher L. Waters
Christopher L. Waters Richard G. Mallinson
Richard G. Mallinson Lance L. Lobban
Lance L. Lobban Laura E. Bartley
Laura E. Bartley