NADPH-generating dehydrogenases: their role in the mechanism of protection against nitro-oxidative stress induced by adverse environmental conditions
- 1Group of Antioxidants, Free Radicals and Nitric Oxide in Biotechnology, Food and Agriculture, Department of Biochemistry, Cell and Molecular Biology of Plants, Estación Experimental del Zaidín, Consejo Superior de Investigaciones Científicas, Granada, Spain
- 2Group of Biochemistry and Cell Signaling in Nitric Oxide, Department of Biochemistry and Molecular Biology, University of Jaén, Jaén, Spain
NADPH is an essential reductive coenzyme in biosynthetic processes such as cell growth, proliferation, and detoxification in eukaryotic cells. It is required by antioxidative systems such as the ascorbate-glutathione cycle and is also necessary for the generation of superoxide radicals by plant NADPH oxidases and for the generation of nitric oxide (NO) by L-arginine-dependent nitric oxide synthase. This coenzyme is principally re-generated by a group of NADP-dehydrogenases enzymes including glucose-6-phosphate dehydrogenase (G6PDH) and 6-phosphogluconate dehydrogenase (6PGDH), both belonging to the pentose phosphate pathway, the NADP-malic enzyme (NADP-ME), and NADP-isocitrate dehydrogenase (NADP-ICDH). In this study, current perspectives on these enzymes in higher plants under different stress situations are reviewed and it is also pointed out that this group of NADPH-generating dehydrogenases is a key element in supporting the mechanism of response to nitro-oxidative stress situations.
Introduction
The supply of reducing equivalents in the form of NADPH (the reduced form of the nicotinamide adenine dinucleotide phosphate) is essential in all living organisms (Pandolfi et al., 1995; Barroso et al., 1998; Ying, 2008). Thus, NADPH is required for cell growth and proliferation which are necessary in several metabolic pathways including fatty acid biosynthesis, biosynthesis of sugars in the Calvin cycle, biosynthesis of carotenoids, conversion of ribonucleotide (RNA) to deoxy-ribonucleotide (DNA) and regulation of chloroplast protein import via the metabolic redox status of the chloroplast, specifically in the Tic62, (a component of the translocon at the inner envelope of chloroplasts, Tic complex) (Stengel et al., 2008; Kovács-Bogdán et al., 2010). NADPH is also required by NADPH-cytochrome P450 reductases (Ro et al., 2002), the generation of superoxide radicals by the NADPH oxidase (NOX) (Sagi and Fluhr, 2006) and is a necessary cofactor for the generation of nitric oxide (NO) by L-arginine-dependent nitric oxide synthase (NOS) activity (Corpas et al., 2009). NADPH is also essential by different antioxidative systems including the activity of glutathione reductase (GR), a key enzyme in the ascorbate-glutathione cycle to protect against oxidative damage (Noctor et al., 2006; Gill et al., 2013), and by NADPH-dependent thioredoxin reductases (NTRs) in the regulation of metabolic pathways through thiol group reduction (Spinola et al., 2008; Cha et al., 2014). Curiously, in this last case it has been reported that the chloroplastic G6PDH activity can undergo a redox regulation by thioredoxin (Née et al., 2014) which suggests a complex interaction between the source of NADPH and the NTR system. In consequence, the ultimate antioxidant capacity of the cell must be determined by the availability of reducing equivalents. Figure 1 summarizes the main pathways in plant cells where NADPH is required.
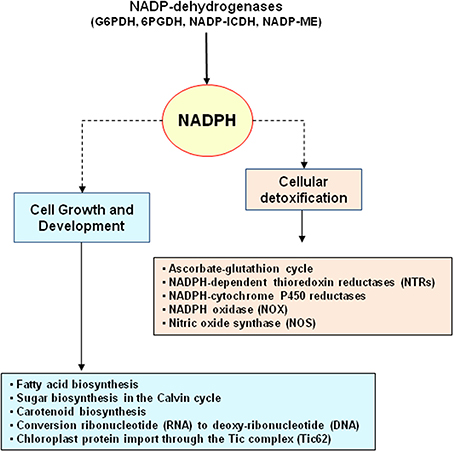
Figure 1. NADP-dehydrogenases as enzymatic sources of NAPDH in plant cells and its implications in cellular detoxification, cell growth and development.
There are several enzymatic components involved in the maintenance of the pool of NADP and NADPH. NAD kinases (NADKs) catalyze the direct phosphorylation of NAD to NADP and therefore contribute to the generation of the cellular NADP pool (Pollak et al., 2007; Agledal et al., 2010). On the other hand, ferrodoxin-NADP reductase (FNR) in photosynthetic cells during the light phase is recognized as a principal source of NADPH. However, in non-photosynthetic cells during the dark phase of photosynthesis, the main enzymes capable of generating power reduction in the form of NADPH are the following: glucose-6-phosphate dehydrogenase (G6PDH, EC 1.1.1.49) and 6-phosphogluconate dehydrogenase (6PGDH, EC 1.1.1.44) (both belonging to the pentose phosphate pathway), NADP-isocitrate dehydrogenase (NADP-ICDH, EC 1.1.1.42) and NADP-malic enzyme (NADP-ME, EC 1.1.1.40), also known as NADP-malate dehydrogenase. This mini-review will focus on these groups of NADPH recycling dehydrogenases, principally in relation to their role as second lines of defense against nitro-oxidative stress.
Subcellular NADP-Dehydrogenase Compartmentalization as a NADPH Supply Regulation Mechanism
The NADPH pool is required in many processes while the contribution of each NADP-dehydrogenase in specific situations is difficult to determine. However, cell compartmentalization is required as an additional control mechanism in order to keep the NADPH supply close to the system when required, particularly given that NADPH is part of a network containing other energy-rich molecules such as NADH and ATP (Scheibe and Dietz, 2012). In addition, given that NADPH is not easily transported across membranes but, rather, operates through indirect shuttle systems, all these NADP-dehydrogenases usually have different isozymes which are localized in the different subcellular compartments. Although the localization of some of these NADP-dehydrogenases in the different organelles has been described in different plant species (Gálvez and Gadal, 1995; Corpas et al., 1998, 1999; Debnam and Emes, 1999; Hodges et al., 2003; Kruger and von Schaewen, 2003; Leterrier et al., 2007), the availability of genomes in higher plants such as Arabidopsis thaliana and Oryza sativa has facilitated a more systematic analysis of different NADP-dehydrogenases (Chi et al., 2004; Wakao and Benning, 2005; Wheeler et al., 2005).
Function of NADP-Dehydrogenases Under Environmental Stress Conditions
Under diverse biotic and abiotic stress conditions, plants have developed a whole battery of response mechanisms in order to overcome any potential cellular damage. In many cases, these processes could be accompanied by an uncontrolled increase in reactive oxygen and nitrogen species (ROS and RNS) which might generate nitro-oxidative stress (Corpas et al., 2007; Corpas and Barroso, 2013). As all these processes usually involve a redox response, an additional NADPH supply may be required for all the pathways using it.
To support this hypothesis, there is a body of evidence to show that, under specific stress conditions, one or more NADP-dehydrogenases are regulated at the level of activity and protein/gene expression (Valderrama et al., 2006; Liu et al., 2007, 2013; Marino et al., 2007; Mhamdi et al., 2010; Airaki et al., 2012). Moreover, the importance of some of these NADP-dehydrogenases has been confirmed by reverse genetic studies (Scharte et al., 2009; Dal Santo et al., 2012; Voll et al., 2012; Siddappaji et al., 2013).
In olive plants (Olea europaea) under salinity-induced nitro-oxidative stress, a general increase in the activity of the main antioxidative systems (catalase, superoxide dismutase and enzymes of the ascorbate-glutathione cycle) was accompanied by a significant increase in the activity and protein expression of G6PDH, NADP-ME, and NADP-ICDH (Valderrama et al., 2006, 2007). Similar behavior has been reported in leaves from pepper plants (Capsicum annum) exposed to cadmium stress which generates oxidative stress and a concomitant increase in the activity of all NADP-dehydrogenases (G6PDH, 6PGDH, NADP-ME, and NADP-ICDH) (León et al., 2002). In pepper plant leaves exposed to low temperatures (8°C) for different periods of time (1–3 d) after 24 h treatments, we observed alterations in the metabolism of ROS and RNS (an increase in lipid oxidation and protein nitration) and a general rise in the activity of the main NADPH-generating enzymes (G6PDH, 6PGDH, NADP-ME, and NADP-ICDH) which appeared to contribute to cold acclimation (Airaki et al., 2012). Arabidopsis seedlings grown under salinity conditions (100 mM NaCl) also displayed nitro-oxidative stress. Among the NADPH-generating dehydrogenases (G6PDH, 6PGDH, NADP-ME, and NADP-ICDH) analyzed under these conditions, NADP-ICDH showed maximum activity levels, mainly attributable to the root NADP-ICDH (Leterrier et al., 2012c). Another study of NADP-ICDH activity in Arabidopsis has demonstrated that this enzyme's kinetic parameters vary depending on the organ involved, being the specific activity much higher in roots than in leaves. In vitro analysis of NADP-ICDH activity in the presence of different ROS and RNS showed that H2O2 does not affect this activity in either organ; however, reduced glutathione (GSH) inhibited activity in leaves but not in roots. On the other hand, S-nitrosoglutathione, a cellular S-nitrosothiol used as a NO donor, and peroxynitrite (ONOO−) depressed NADP-ICDH activity in leaves and roots (Leterrier et al., 2012b). Modulation of NADP-ICDH activity by RNS was also observed in pea roots (Pisum sativum) during natural senescence which is associated with nitro-oxidative stress since there are increases in the ONOO− levels and in the number of nitrated proteins. Thus, cytosolic NADP-ICDH activity was shown to be inhibited by nitration at Tyr392 during senescence in a process mediated by peroxynitrite (Begara-Morales et al., 2013).
Depending on the plant species involved, the organs analyzed and the intensity of stress, the response of the NAPD-dehydrogenases could also be vary. Thus, in tomato roots (Solanum lycopersicum) under salinity conditions (120 mM NaCl) accompanied by oxidative stress, an overall decrease in NADPH content and the enzymatic activities of the main NADPH-generating dehydrogenases has been reported, especially NADP-ICDH activity which recorded a drastic reduction of 94% (Manai et al., 2014). This could be explained by the sensitivity of this enzyme to post-translational modification mediated by ONOO− as observed in pea roots during senescence (Begara-Morales et al., 2013). However, in Arabidopsis thaliana seedlings exposed to arsenic (1 mM KH2AsO4) which also generates nitro-oxidative stress based in the concomitant increase of tyrosine-nitration and lipid peroxidation, the activity of NADP-dehydrogenases (G6PDH, 6PGDH, and NADP-ICDH) did not vary significantly, suggesting that the supply of NAPDH was sufficient to withstand this stress (Leterrier et al., 2012a). Alternatively, the involvement of Arabidopsis cytosolic NADP-ICDH in leaves has been demonstrated to contribute to the maintenance of redox homeostasis under biotic stress caused by Pseudomonas syringe (Mhamdi et al., 2010). In the leaves of tobacco plants (Nicotiana tabacum), NADP-ME activity increased significantly in response to drought (Doubnerová-Hısková et al., 2014). On the other hand, in Lotus japonicus exposed to water stress, differential and spatially distributed nitro-oxidative stress was reported in roots and leaves. Analysis of NADP-dehydrogenase activities in roots revealed that, whereas G6PDH and NADP-ICDH activity decreased 6.5- and 1.5-fold, respectively, 6PGDH and NADP-ME increased 1.5- and 1.3-fold, respectively. However, no leaf NADP-dehydrogenase appeared to be affected, except for G6PDH which decreased by around 50% under water stress conditions (Signorelli et al., 2013). Table 1 summarizes some examples of the response of NADP-dehydrogenases to nitro-oxidative stresses generated by different abiotic stresses.
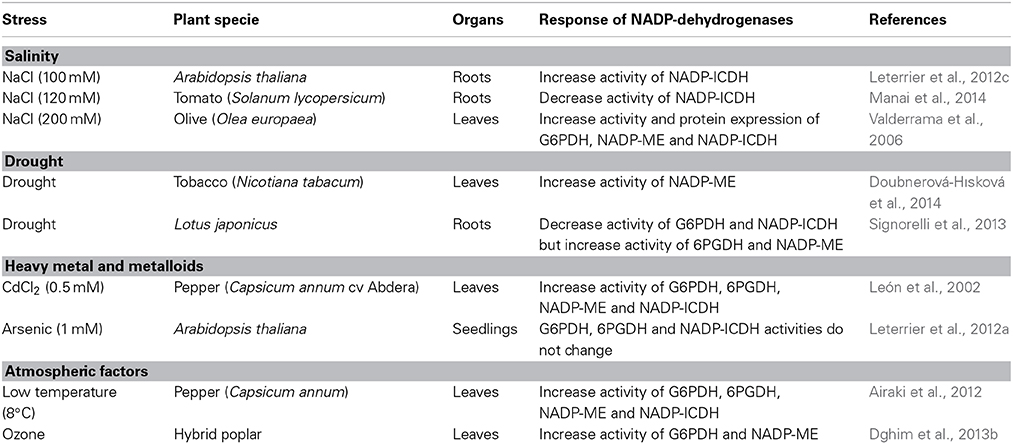
Table 1. Examples of the response of NADP-dehydrogenases to nitro-oxidative stresses generated by different abiotic stresses.
As mentioned above, certain post-translation modifications could negatively affect activity under stress conditions although up-regulation has also been reported. For example, in Arabidopsis thaliana under salinity (150 mM NaCl) stress conditions, the cytosolic G6PDH isozyme (G6PD6) is targeted by phosphorylation at Thr-467 whose activity increased. The important role played by this dehydrogenase was corroborated using Arabidopsis thaliana knockout mutants of cytosolic G6PDH (G6PD6) where the cellular redox state was altered and plants were more sensitive to salt stress (Dal Santo et al., 2012). The importance of cytosolic G6PDH in the leaves of tobacco plants (Nicotiana tabacum) at an early stage of defense against the Phytophthora nicotianae pathogen which is accompanied by oxidative burst has also been reported. This was demonstrated using a genetic approach involving over-expression of this G6PDH isozyme which improved NADPH provision for pathogen-activated NOXs at the plasma membrane during early oxidative burst (Scharte et al., 2009). In addition, these tobacco plants showed heightened resistance to drought stress. In the same way, transgenic tobacco plants over-expressing the cytosolic G6PDH from Populus suaveolens have enhanced cold (4°C) tolerance. Beside of the increased G6PDH activity, these transgenic plants showed lower level of lipid oxidation and higher activity of antioxidant enzymes such as superoxide dismutase and peroxidase. Moreover, these plants have activated the expression of stress-related genes. Therefore, these data clearly show the regulatory function of G6PDH during low temperature stress (Lin et al., 2013).
There are other examples of certain specific NADP-dehydrogenases being regulated at the level of activity and gene expression under diverse stress conditions. For instance, G6PDH mRNA expression in wheat seedlings under salt stress conditions of 150 mM NaCl reached a maximum level at 12 h of the treatment (Nemoto and Sasakuma, 2000). A similar response was observed in the expression of the 6PGDH gene which was up-regulated in rice shoots under salt stress (150 mM NaCl) (Huang et al., 2003). By using the Arabidopsis cytosolic NADP-ICDH knockout mutant, it has been reported that the loss of this isozyme function does not markedly affect the response of Arabidopsis to ozone. However, other cytosolic NADPH-producing enzymes (G6PDH and NADP-ME) showed a significant increase which contributed to maintaining the status of NADPH redox (Dghim et al., 2013a). A similar increase in G6PDH and NADP-ME has also been reported in hybrid poplar leaves in response to ozone (Dghim et al., 2013b).
Conclusions
Together with NADH, NADPH participates in the equilibrium of cellular redox homeostasis and also maintains certain antioxidant systems such as the ascorbate-glutathione cycle and NTRs. Thus, NADP-dehydrogenase systems should be regarded as a second line of defense in order to maintain the effective functioning of the main antioxidative systems. Biochemical and genetic approaches provide a strong data basis to confirm the essential involvement of NADP-dehydrogenases in the mechanism of response to nitro-oxidative stress situations. Organ distribution and subcellular compartmentalization are regarded as additional regulatory mechanisms of these systems to ensure that the NADPH supply is at the required location. Future research will be essential to identify the specific involvement of each NADP-dehydrogenase in the different organs and cellular compartments supporting a particular pathway as all these enzymes are also involved in nitrogen and carbohydrate metabolisms.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Work in our laboratories is supported by ERDF-cofinanced grants from the Ministry of Science and Innovation (BIO2012-33904 and Recupera 2020-20134R056). We apologize for all the authors we could not cite in this mini review article due to space limitation.
Abbreviations
FNR, ferrodoxin-NADP reductase (FNR ferrodoxin-NADP reductase); G6PDH, glucose-6-phosphate dehydrogenase; GR, glutathione reductase; NADKs, NAD kinases; NADP-ICDH, NADP-isocitrate dehydrogenase; NADP-ME, NADP-malic enzyme; NO, nitric oxide; NOS, nitric oxide synthase; NOX, NADPH oxidase; NTRs, NADPH-dependent thioredoxin reductases; ONOO−, peroxynitrite; 6PGDH, 6-phosphogluconate dehydrogenase; ROS, reactive oxygen species; RNS, reactive nitrogen species; Tic, The Inner envelope of Chloroplasts.
References
Agledal, L., Niere, M., and Ziegler, M. (2010). The phosphate makes a difference: cellular functions of NADP. Redox Rep. 15, 2–10. doi: 10.1179/174329210X12650506623122
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Airaki, M., Leterrier, M., Mateos, R. M., Valderrama, R., Chaki, M., Barroso, J. B., et al. (2012). Metabolism of reactive oxygen species and reactive nitrogen species in pepper (Capsicum annuum L.) plants under low temperature stress. Plant Cell Environ. 35, 2812–2895. doi: 10.1111/j.1365-3040.2011.02310.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Barroso, J. B., Peragón, J., Contreras-Jurado, C., García-Salguero, L., Corpas, F. J., Esteban, F. J., et al. (1998). Impact of starvation-refeeding on kinetics and protein expression of trout liver NADPH-production systems. Am. J. Physiol. 274(6 Pt 2), R1578–R1587.
Begara-Morales, J. C., Chaki, M., Sánchez-Calvo, B., Mata-Pérez, C., Leterrier, M., Palma, J. M., et al. (2013). Protein tyrosine nitration in pea roots during development and senescence. J. Exp. Bot. 64, 1121–1134. doi: 10.1093/jxb/ert006
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Cha, J.-Y., Kim, J. Y., Jung, I. J., Kim, M. R., Melencion, A., Alam, S. S., et al. (2014). NADPH-dependent thioredoxin reductase A (NTRA) confers elevated tolerance to oxidative stress and drought. Plant Physiol. Biochem. 80, 184–191. doi: 10.1016/j.plaphy.2014.04.008
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Chi, W., Yang, J., Wu, N., and Zhang, F. (2004). Four rice genes encoding NADP malic enzyme exhibit distinct expression profiles. Biosci. Biotechnol. Biochem. 68, 1865–1874. doi: 10.1271/bbb.68.1865
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Corpas, F. J., and Barroso, J. B. (2013). Nitro-oxidative stress vs oxidative or nitrosative stress in higher plants. New Phytol. 199, 633–635. doi: 10.1111/nph.12380
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Corpas, F. J., Barroso, J. B., Sandalio, L. M., Distefano, S., Palma, J. M., Lupiáñez, J. A., et al. (1998). A dehydrogenase-mediated recycling system of NADPH in plant peroxisomes. Biochem. J. 330, 777–784.
Corpas, F. J., Barroso, J. B., Sandalio, L. M., Palma, J. M., Lupiáñez, J. A., and del Río, L. A. (1999). Peroxisomal NADP-dependent isocitrate dehydrogenase. Characterization and activity regulation during natural senescence. Plant Physiol. 121, 921–928.
Corpas, F. J., del Río, L. A., and Barroso, J. B. (2007). Need of biomarkers of nitrosative stress in plants. Trends Plant Sci. 12, 436–438. doi: 10.1016/j.tplants.2007.08.013
Corpas, F. J., Palma, J. M., del Río, L. A., and Barroso, J. B. (2009). Evidence supporting the existence of L-arginine-dependent nitric oxide synthase activity in plants. New Phytol. 184, 9–14. doi: 10.1111/j.1469-8137.2009.02989.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Dal Santo, S., Stampfl, H., Krasensky, J., Kempa, S., Gibon, Y., Petutschnig, E., et al. (2012). Stress-induced GSK3 regulates the redox stress response by phosphorylating glucose-6-phosphate dehydrogenase in Arabidopsis. Plant Cell 24, 3380–3392. doi: 10.1105/tpc.112.101279
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Debnam, P. M., and Emes, M. J. (1999). Subcellular distribution of enzymes of the oxidative pentose phosphate pathway in root and leaf tissues. J. Exp. Bot. 50, 1653–1661. doi: 10.1093/jxb/50.340.1653
Dghim, A. A., Dumont, J., Hasenfratz-Sauder, M. P., Dizengremel, P., Le Thiec, D., and Jolivet, Y. (2013b). Capacity for NADPH regeneration in the leaves of two poplar genotypes differing in ozone sensitivity. Physiol. Plant. 148, 36–50. doi: 10.1111/j.1399-3054.2012.01686.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Dghim, A. A., Mhamdi, A., Vaultier, M. N., Hasenfratz-Sauder, M. P., Le Thiec, D., Dizengremel, P., et al. (2013a). Analysis of cytosolic isocitrate dehydrogenase and glutathione reductase 1 in photoperiod-influenced responses to ozone using Arabidopsis knockout mutants. Plant Cell Environ. 36, 1981–1991. doi: 10.1111/pce.12104
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Doubnerová-Hısková, V. Miedziñska, L., Dobrá, J., Vankova, R., Ryšlavá, H.(2014). Phosphoenolpyruvate carboxylase, NADP-malic enzyme, and pyruvate, phosphate dikinase are involved in the acclimation of Nicotiana tabacum L. to drought stress. J. Plant Physiol. 171, 19–25. doi: 10.1016/j.jplph.2013.10.017
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Gálvez, S., and Gadal, P. (1995). On the function of the NADP-dependent isocitrate dehydrogenase isoenzymes in living organisms. Plant Sci. 105, 1–14. doi: 10.1016/0168-9452(94)04041-E
Gill, S. S., Anjum, N. A., Hasanuzzaman, M., Gill, R., Trivedi, D. K., Ahmad, I., et al. (2013). Glutathione and glutathione reductase: a boon in disguise for plant abiotic stress defense operations. Plant Physiol. Biochem. 70, 204–212. doi: 10.1016/j.plaphy.2013.05.032
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Hodges, M., Flesch, V., Galvez, S., and Bismuth, E. (2003). Higher plant NADP-dependent isocitrate dehydrogenases, ammonium assimilation and NADPH production. Plant Physiol. Biochem. 41, 577–585. doi: 10.1016/S0981-9428(03)00062-7
Huang, J., Zhang, H., Wang, J., and Yang, J. (2003). Molecular cloning and characterization of rice 6-phosphogluconate dehydrogenase gene that is up-regulated by salt stress. Mol. Biol. Rep. 30, 223–227. doi: 10.1023/A:1026392422995
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kovács-Bogdán, E., Soll, J., and Bölter, B. (2010). Protein import into chloroplasts: the Tic complex and its regulation. Biochim. Biophys. Acta 1803, 740–747. doi: 10.1016/j.bbamcr.2010.01.015
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kruger, N. J., and von Schaewen, A. (2003). The oxidative pentose phosphate pathway: structure and organization. Curr. Opin. Plant Biol. 6, 236–246. doi: 10.1016/S1369-5266(03)00039-6
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
León, A. M., Palma, J. M., Corpas, F. J., Gómez, M., Romero-Puertas, M. C., Chatterjee, D., et al. (2002). Antioxidative enzymes in cultivars of pepper plants with different sensitivity to cadmium. Plant Physiol. Biochem. 40, 813–820. doi: 10.1016/S0981-9428(02)01444-4
Leterrier, M., Airaki, M., Palma, J. M., Chaki, M., Barroso, J. B., and Corpas, F. J. (2012a). Arsenic triggers the nitric oxide (NO) and S-nitrosoglutathione (GSNO) metabolism in Arabidopsis. Environ. Pollut. 166, 136–143. doi: 10.1016/j.envpol.2012.03.012
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Leterrier, M., Barroso, J. B., Palma, J. M., and Corpas, F. J. (2012b). Cytosolic NADP-isocitrate dehydrogenase in Arabidopsis leaves and roots. Biol. Plantarum 56, 705–710. doi: 10.1007/s10535-012-0244-6
Leterrier, M., Barroso, J. B., Valderrama, R., Palma, J. M., and Corpas, F. J. (2012c). NADP-dependent isocitrate dehydrogenase (NADP-ICDH) from Arabidopsis roots contributes in the mechanism of defence against the nitro-oxidative stress induced by salinity. ScientificWorldJournal 2012:694740. doi: 10.1100/2012/694740
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Leterrier, M., del Río, L. A., and Corpas, F. J. (2007). Cytosolic NADP-isocitrate dehydrogenase of pea plants: genomic clone characterization and functional analysis under abiotic stress conditions. Free Radic. Res. 41, 191–199. doi: 10.1080/10715760601034055
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Lin, Y., Lin, S., Guo, H., Zhang, Z., and Chen, X. (2013). Functional analysis of PsG6PDH, a cytosolic glucose-6-phosphate dehydrogenase gene from Populus suaveolens, and its contribution to cold tolerance improvement in tobacco plants. Biotechnol. Lett. 35, 1509–1518. doi: 10.1007/s10529-013-1226-2
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Liu, J., Wang, X., Hu, Y., Hu, W., and Bi, Y. (2013). Glucose-6-phosphate dehydrogenase plays a pivotal role in tolerance to drought stress in soybean roots. Plant Cell Rep. 32, 415–429. doi: 10.1007/s00299-012-1374-1
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Liu, S., Cheng, Y., Zhang, X., Guan, Q., Nishiuchi, S., Hase, K., et al. (2007). Expression of an NADP-malic enzyme gene in rice (Oryza sativa. L) is induced by environmental stresses; over-expression of the gene in Arabidopsis confers salt and osmotic stress tolerance. Plant Mol. Biol. 64, 49–58. doi: 10.1007/s11103-007-9133-3
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Manai, J., Gouia, H., and Corpas, F. J. (2014). Redox and nitric oxide homeostasis are affected in tomato (Solanum lycopersicum) roots under salinity-induced oxidative stress. J. Plant Physiol. 171, 1028–1035. doi: 10.1016/j.jplph.2014.03.012
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Marino, D., González, E. M., Frendo, P., Puppo, A., and Arrese-Igor, C. (2007). NADPH recycling systems in oxidative stressed pea nodules: a key role for the NADP-dependent isocitrate dehydrogenase. Planta 225, 413–421. doi: 10.1007/s00425-006-0354-5
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Mhamdi, A., Mauve, C., Gouia, H., Saindrenan, P., Hodges, M., and Noctor, G. (2010). Cytosolic NADP-dependent isocitrate dehydrogenase contributes to redox homeostasis and the regulation of pathogen responses in Arabidopsis leaves. Plant Cell Environ. 33, 1112–1123. doi: 10.1111/j.1365-3040.2010.02133.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Née, G., Aumont-Nicaise, M., Zaffagnini, M., Nessler, S., Valerio-Lepiniec, M., and Issakidis-Bourguet, E. (2014). Redox regulation of chloroplastic G6PDH activity by thioredoxin occurs through structural changes modifying substrate accessibility and cofactor binding. Biochem. J. 457, 117–125. doi: 10.1042/BJ20130337
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Nemoto, Y., and Sasakuma, T. (2000). Specific expression of glucose-6-phosphate dehydrogenase (G6PDH) gene by salt stress in wheat (Triticum aestivum L.). Plant Sci. 158, 53–60. doi: 10.1016/S0168-9452(00)00305-8
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Noctor, G., Queval, G., and Gakière, B. (2006). NAD(P) synthesis and pyridine nucleotide cycling in plants and their potential importance in stress conditions. J. Exp. Bot. 57, 1603–1620. doi: 10.1093/jxb/erj202
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Pandolfi, P. P., Sonati, F., Rivi, R., Mason, P., Grosveld, F., and Luzzatto, L. (1995). Targeted disruption of the housekeeping gene encoding glucose 6-phosphate dehydrogenase (G6PD): G6PD is dispensable for pentose synthesis but essential for defense against oxidative stress. EMBO J. 14, 5209–5215.
Pollak, N., Dölle, C., and Ziegler, M. (2007). The power to reduce: pyridine nucleotides–small molecules with a multitude of functions. Biochem. J. 402, 205–218. doi: 10.1042/BJ20061638
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ro, D. K., Ehlting, J., and Douglas, C. J. (2002). Cloning, functional expression, and subcellular localization of multiple NADPH-cytochrome P450 reductases from hybrid poplar. Plant Physiol. 130, 1837–1851. doi: 10.1104/pp.008011
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Sagi, M., and Fluhr, R. (2006). Production of reactive oxygen species by plant NADPH oxidases. Plant Physiol. 141, 336–340, doi: 10.1104/pp.106.078089
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Scharte, J., Schön, H., Tjaden, Z., Weis, E., and von Schaewen, A. (2009). Isoenzyme replacement of glucose-6-phosphate dehydrogenase in the cytosol improves stress tolerance in plants. Proc. Natl. Acad. Sci. U.S.A. 106, 8061–8066. doi: 10.1073/pnas.0812902106
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Scheibe, R., and Dietz, K. J. (2012). Reduction-oxidation network for flexible adjustment of cellular metabolism in photoautotrophic cells. Plant Cell Environ. 35, 202–216. doi: 10.1111/j.1365-3040.2011.02319.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Siddappaji, M. H., Scholes, D. R., Bohn, M., and Paige, K. N. (2013). Overcompensation in response to herbivory in Arabidopsis thaliana: the role of glucose-6-phosphate dehydrogenase and the oxidative pentose-phosphate pathway. Genetics 195, 589–598. doi: 10.1534/genetics.113.154351
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Signorelli, S., Corpas, F. J., Borsani, O., Barroso, J. B., and Monza, J. (2013). Water stress induces a differential and spatially distributed nitro-oxidative stress response in roots and leaves of Lotus japonicus. Plant Sci. 201–202, 137–146. doi: 10.1016/j.plantsci.2012.12.004
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Spinola, M. C., Perez-Ruiz, J. M., Pulido, P., Kirchsteiger, K., Guinea, M., Gonzalez, M., et al. (2008). NTRC new ways of using NADPH in the chloroplast. Physiol. Plant. 133, 516–524. doi: 10.1111/j.1399-3054.2008.01088.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Stengel, A., Benz, P., Balsera, M., Soll, J., and Bölter, B. (2008). TIC 62 redox-regulated translocon composition and dynamics. J. Biol. Chem. 283, 6656–6667. doi: 10.1074/jbc.M706719200
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Valderrama, R., Corpas, F. J., Carreras, A., Fernández-Ocaña, A., Chaki, M., Luque, F., et al. (2007). Nitrosative stress in plants. FEBS Lett. 581, 453–461. doi: 10.1016/j.febslet.2007.01.006
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Valderrama, R., Corpas, F. J., Carreras, A., Gómez-Rodríguez, M. V., Chaki, M., Pedrajas, J. R., et al. (2006). The dehydrogenase-mediated recycling of NADPH is a key antioxidant system against salt-induced oxidative stress in olive plants. Plant Cell Environ. 29, 1449–1459. doi: 10.1111/j.1365-3040.2006.01530.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Voll, L. M., Zell, M. B., Engelsdorf, T., Saur, A., Wheeler, M. G., Drincovich, M. F., et al. (2012). Loss of cytosolic NADP-malic enzyme 2 in Arabidopsis thaliana is associated with enhanced susceptibility to Colletotrichum higginsianum. New Phytol. 195, 189–202. doi: 10.1111/j.1469-8137.2012.04129.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Wakao, S., and Benning, C. (2005). Genome-wide analysis of glucose-6-phosphate dehydrogenases in Arabidopsis. Plant J. 41, 243–256. doi: 10.1111/j.1365-313X.2004.02293.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Wheeler, M. C., Tronconi, M. A., Drincovich, M. F., Andreo, C. S., Flügge, U. I., and Maurino, V. G. (2005). A comprehensive analysis of the NADP-malic enzyme gene family of Arabidopsis. Plant Physiol. 139, 39–51. doi: 10.1104/pp.105.065953
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ying, W. (2008). NAD+/NADH and NADP+/NADPH in cellular functions and cell death: regulation and biological consequences. Antioxid. Redox Signal. 10, 179–206. doi: 10.1089/ars.2007.1672
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Keywords: G6PDH, 6PGDH, ICDH, NAPDH, NADP-ME, nitric oxide, nitrosative stress, oxidative stress
Citation: Corpas FJ and Barroso JB (2014) NADPH-generating dehydrogenases: their role in the mechanism of protection against nitro-oxidative stress induced by adverse environmental conditions. Front. Environ. Sci. 2:55. doi: 10.3389/fenvs.2014.00055
Received: 02 October 2014; Accepted: 14 November 2014;
Published online: 02 December 2014.
Edited by:
Naser A. Anjum, University of Aveiro, PortugalReviewed by:
Naser A. Anjum, University of Aveiro, PortugalMargarete Baier, Freie Universität Berlin, Germany
Yogesh Abrol, Bhagalpur University, India
Copyright © 2014 Corpas and Barroso. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Francisco J. Corpas, Profesor Albareda 1, 18008-Granada, Spain e-mail: javier.corpas@eez.csic.es
 Francisco J. Corpas
Francisco J. Corpas Juan B. Barroso
Juan B. Barroso