Microbial regulation of nitrogen dynamics along the hillslope of a natural forest
- 1Graduate School of Agricultural and Life Sciences, The University of Tokyo, Tokyo, Japan
- 2Field Science Education and Research Center, Kyoto University, Kyoto, Japan
Topography affects the soil physicochemistry, soil N dynamics, and plant distribution and growth in forests. In Japan, many forests are found in mountainous areas and these traits are often highly variable along steep slopes. In this study, we investigated how the microbial population dynamics reflected the bioavailable N dynamics with the physicochemical gradient along the slope in soils of a natural forest in Japan. We measured the gross rates of NH+4 production, nitrification, and NH+4/ NO−3 immobilization using the N isotope dilution method to analyze the N dynamics in the soils. We also determined the abundance of the bacterial 16S rRNA gene and bacterial and archaeal ammonia monooxygenase gene (amoA) using qPCR to assess the populations of total bacteria and nitrifiers. We found that gross rates of NH+4 production and nitrification were higher in the lower part of the slope, they were positively correlated with the abundance of the bacterial 16S rRNA gene and archaeal amoA, respectively; and the availability of N, particularly NO−3, for plants was higher in the lower part of the slope because of the higher microbial nitrification activity and low microbial NO−3 immobilization activity. In addition, path analysis indicated that gross rates of NH+4 production and nitrification were regulated mainly by the substrate (dissolved organic N and NH+4) concentrations and population sizes of total bacteria and nitrifiers, respectively, and their population sizes were strongly affected by the soil physicochemistry such as pH and water content. Our results suggested that the soil physicochemical gradient along the slope caused the spatial gradient of gross rates of NH+4 production and nitrification by altering the communities of ammonifiers and nitrifiers in the forest slope, which also affected plant distribution and growth via the supply of bioavailable N to plants.
Introduction
In forests, the topography affects the soil environment such as the soil depth, soil formation processes, moisture content, and nutrient status, as well as the local climate (Hook and Burke, 2000; Tromp-van Meerveld and McDonnell, 2006; Penna et al., 2009). It may enhance the plant diversity in a forest, because spatial heterogeneity of environmental conditions allows plants with various physiologies to coexist. In Japan, many forests are found in mountainous areas; thus, the soil environments and plant diversity are highly variable along a steep slope (Enoki, 2003; Tateno and Takeda, 2003; Koyama et al., 2013). In general, a hydrological gradient is observed along a slope, from dry soil at the top part to wet soil at the bottom part. The soil chemistry such as pH and nutrient availability as well as the light intensity also exhibit gradients from the upper to lower parts. These gradients could affect the transient vegetation dynamics directly or indirectly (i.e., growth and distribution). The spatial differentiation of plants (e.g., plants adapted only to upper or lower slope conditions, or ubiquitously distributed plants) has often been observed and has been explained according to the availabilities of water, nutrients, or light for plants (Hook and Burke, 2000; Tromp-van Meerveld and McDonnell, 2006; Engelbrecht et al., 2007). Nitrogen is considered to be a limiting nutrient for plant growth in most temperate forests (Vitousek and Howarth, 1991). The N availability for plants can be strongly affected by the hydrological factors on a slope (Hill and Kemp, 1999; Band et al., 2001). Therefore, many studies have focused on the patterns of soil N dynamics and NO−3 production as well as their controlling factors in forest slopes (Hirobe et al., 1998, 2003; Tokuchi et al., 2000; Nishina et al., 2009a,b; Koyama et al., 2013).
Microorganisms are responsible for the production of NH+4 and NO−3, which are the main forms of bioavailable N to plants, via the degradation of organic N and nitrification, respectively (Isobe et al., 2011a; Isobe and Ohte, 2014). Given that microbial activity levels reflect the dynamics of the bioavailable N in soil, we can expect that microorganisms will also have direct or indirect associations with the growth and distribution of plants via the supply of bioavailable N to plants. This association can also be found in the competition for N between microorganisms and plants. This competition can be harder for plants in N-limiting environments, because microorganisms generally have a higher affinity for bioavailable N than plants (Kuzyakov and Xu, 2013). Microbial communities and their activities can be influenced by environmental conditions. Therefore, an environmental gradient may cause changes in the bioavailable N supply for plants via alterations in the microbial activity levels. In particular, many recent studies have shown that the rates of dissimilatory N transformation, such as nitrification and denitrification, could be explained by the sizes of nitrifier or denitrifier populations (Hawkes et al., 2005; Philippot et al., 2009; Isobe et al., 2012). Therefore, although few previous studies on forest slopes have focused on microbial population dynamics, we can expect that soil environmental gradients affect the transient vegetation dynamics on forest slopes by altering the population size of microorganisms responsible for the supply of bioavailable N to plants. With this, we can obtain a more mechanistic understanding of how soil environmental gradients affect the dynamics of bioavailable N and the plant diversity on forest slopes by considering microbial population dynamics and their associated activities.
The research site in the present study, Ashiu Forest Research Station, is a broadleaved deciduous forest located in Kyoto, Japan. Previous studies have found the gradient of soil physicochemistry and transient vegetation dynamics on a hill slope in this forest (Tateno and Takeda, 2003, 2010; Tateno et al., 2004, 2005). These studies showed that the soil properties, i.e., the water content, pH, and N content, increased from the upper part to the lower part of the slope. The dominant plant species also differed between the upper and lower parts of the slope. Fagus crenata, a dominant and ubiquitously distributed plant on the slope, exhibited different growth rates and N utilization patterns. In the lower part of the slope, F. crenata grew faster, and its biomass distribution was more likely to be weighted to the aboveground sections; moreover, it utilized more NO−3 than the upper slope. Previous studies did not analyze the N dynamics on the basis of gross rates and microbial populations responsible for the N dynamics on the forest slope. However, we hypothesized that the population dynamics of ammonifiers and nitrifiers in the soil environment along the slope affects the production and consumption of NH+4 and NO−3 which can influence plant distribution or growth rate and the N utilization pattern of F. crenata.
In the present study, we investigated how the microbial population dynamics reflected the bioavailable N dynamics with the physicochemical gradient along a slope in the Ashiu forest. We specifically analyzed how the soil environmental gradient affected the NH+4 and NO−3 production rates by altering populations of ammonifiers and nitrifiers. We also addressed how these relationships affected N uptake and growth of plants. We hypothesized that we could consider almost all bacteria to be ammonifiers because NH+4 production can occur via the assimilation of small organic N compounds such as amino acids, amino sugars, and nucleotides which all bacteria can be involved in (Schimel and Bennett, 2004; Myrold and Bottomley, 2008; Bottomley et al., 2012; Isobe and Ohte, 2014). We also analyzed ammonia-oxidizing bacteria and archaea as nitrifiers, which are responsible for the rate-limiting step of nitrification, ammonia oxidation (Isobe et al., 2011a).
Materials and Methods
Study Site
The study was conducted in a cool-temperate broadleaved deciduous forest in the Kyoto University Ashiu Forest Research Station, Kyoto Prefecture, Japan (35°18′N, 135°43′E). The forest is located in a mountainous area at elevations of 680–720 masl. In this area, forests are dominated by broadleaved deciduous tree species, including F. crenata Blume and Quercus crispula Blume, and they have remained intact since 1898 or earlier. The mean annual temperature and precipitation over a 56-year period at a weather station (640 masl) located approximately 1 km from the study site were approximately 10°C and 2495 mm, respectively. More detailed information about the site was reported by Tateno and Takeda (2003).
Soil Sampling
Soil sampling was conducted in June 2013. A 30–200-m transect (0.6 ha) from the valley bottom to the ridge top on a northwest-facing slope was established in a previous study. We sampled approximately 500 g of soil from the surface 0–10 cm in the mineral layer at 11 points every 20 m from the top to the bottom along the center line of the transect. The soil was sieved through a 2-mm mesh.
Soil Chemistry Analysis
The soil water content was measured by drying 20 g of soil at 105°C for 24 h in a ventilated oven. The soil pH was measured using a pH meter (Horiba, Kyoto, Japan) after extracting 5 g of soil in 25 mL of water. The NH+4 and NO−3 concentrations in soil samples were determined using the indophenol and denitrifier methods (Isobe et al., 2011b), respectively, after extracting 7 g of soil with 35 mL of 2-M KCl solution. The concentration of dissolved organic nitrogen (DON) in the soil extracted with KCl solution was determined using a TOC/TN analyzer (TOC-V; Shimadzu, Kyoto, Japan).
Measurement of the Gross and Net Rates of NH+4 Production and Nitrification
The gross rates of NH+4 production and nitrification in soils were determined using the isotope dilution method (Hart et al., 1994). Two subsamples (7 g each, equivalent to about 3.5 g of dry soil) from each soil sample were used for the analysis of NH+4 production or nitrification during 24-h incubation. The detailed procedures have been reported previously (Isobe et al., 2011b,c; Urakawa et al., 2014). The concentrations and N isotope ratios of NH+4 or NO−3 in the 2M KCl extracts were determined according to the method of Isobe et al. (2011b). The gross soil NH+4 production and nitrification rates were calculated according to the equations of Kirkham and Bartholomew (1954) using the concentrations and N isotope ratios for NH+4 and NO−3, respectively. The gross NH+4 and NO−3 consumption rates were calculated in the same manner. The gross NH+4 immobilization rate was calculated by substituting the gross nitrification rate with the gross NH+4 consumption rate. The gross NO−3 immobilization rate was considered to be the same as the NO−3 consumption rate because denitrification can be minimized during aerobic incubation. Immobilization includes microbial assimilation and any other consumption process, but we assumed that the immobilization process during the 24-h incubation was the microbial assimilation process because abiotic consumption processes are more likely to occur in a short period after the 15N addition (i.e., <2 h). The percentage of nitrification was calculated by gross NH+4 production rate divided by gross nitrification rate which presented the proportion of the produced NH+4 that is being converted to NO−3 via nitrification. The net rates of NH+4 production and nitrification were calculated as the concentration changes in NH+4 and NO−3, respectively, during the 24-h incubation.
Quantification of the Bacterial 16S rRNA Gene and Bacterial and Archaeal AmoA Genes
The bacterial 16S rRNA gene and bacterial and archaeal AmoA genes (amoA) were quantified to estimate the sizes of the populations of ammonifiers and nitrifiers. The bacterial 16S rRNA gene was determined by qPCR using the primers, 357f-520r, and the StepOne real-time PCR system (Applied Biosystems, Tokyo, Japan). The bacterial and archaeal amoA genes were also determined by qPCR using the primers amoA1f and amoA2r and primers CrenamoA23f and CrenamoA616r (Nicol et al., 2008), respectively. Each reaction mixture (20 μ L) contained the KOD SYBR green PCR master mixture (Toyobo, Tokyo, Japan), 0.2 μ M of each primer, 0.5 μ g mL−1 of bovine serum albumin, and 10 ng of DNA template. To generate standard curves (101–107 copies per reaction mixture), we used the bacterial 16S rRNA gene fragment of Pseudomonas stuzeri, the bacterial amoA fragment of Nitrosospira multiformis ATCC 25196, and the archaeal amoA fragment of the soil clone obtained in a previous study (Isobe et al., 2012). The reactions were performed in the following conditions: initial annealing at 98°C for 2 min, followed by 45 cycles at 98°C for 10 s, 58°C for 30 s for the bacterial 16S rRNA gene or 55°C for 30 s for bacterial/archaeal amoA, and 72°C for 30 s. The amplification efficiency of all the genes during standard curve generation was >90% and the standard curves had high correlation coefficients (R2 > 0.95). The amplification of DNA fragments of the correct size was confirmed by dissociation curve analysis and agarose gel electrophoresis.
Data Analysis
To highlight the differences in the soil properties, N dynamics, and gene abundances between the upper and lower parts of the slope, we divided the data obtained into two categories [data derived from soils sampled at points 0–120 m from the top as the upper part (N = 5), and from 120 to 200 m as the lower part (N = 6)], which we compared using an unequal variance t-test. Plant distribution was clearly different between upper and lower parts of the slope (Tateno and Takeda, 2003). The relationship between the two variables in all samples was assessed by correlation analysis to identify possible factors that affected the gross rate of N transformation and gene abundances (N = 11). An α < 0.05 was considered statistically significant for both tests. Path analysis was also used to determine the factors that affected the gross rates and abundances. In the path analysis, an experimentally supported theory was used to formulate a simple model of the causal and noncausal relationships between the variables (Figure 1A). Specifically, the gross N transformation rate can be affected by the N-substrate supply, the population size of microorganisms that utilize the substrate for its transformation, and the water content. The population size is also affected by the N-substrate supply, soil pH, and soil water content. The substrate and microorganisms should be the minimum requirements for N transformation. Water mediates all physiological reactions and microbial growth, and pH also has a great impact on growth. In the analysis of the gross NH+4 production rate, we used the DON concentration as the substrate supply and the abundance of the bacterial 16S rRNA gene as the population size. In the analysis of the gross nitrification rate, we used the NH+4 concentration and the gross NH+4 production rate as the substrate supply, and the abundance of the bacterial and archaeal amoA as the population size. Reduced models were created by eliminating the paths with the highest probability values in a stepwise manner until all paths between two variables were significant (p < 0.05). Because of the small number of samples and variables, we performed these processes manually and only one reduced model maintained all paths between two variables with significance (Figures 1B,C). To account for differences in the magnitudes of data, the measurement scales were changed by standardizing all data to a similar numeric magnitude before calculating the variance–covariance matrices. All statistical analyses were performed using the R software (version 3.1.1, R Development Core Team, 2007) and path analysis was performed using the structural equation modeling function in the sem package in R.
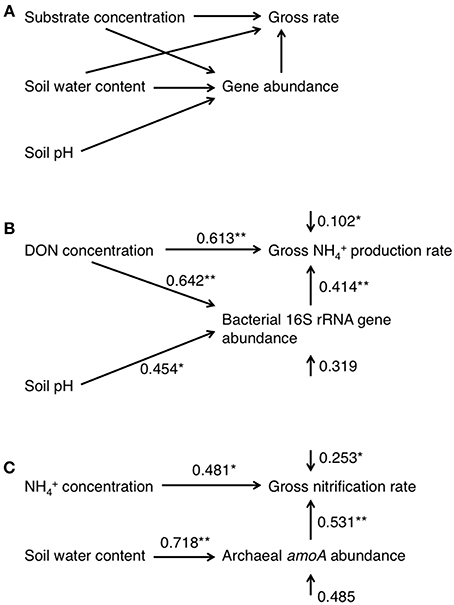
Figure 1. Path diagrams representing the full model (A) and the final models used to describe the patterns observed in the gross NH+4 production rate (B) and gross nitrification rate (C). The numbers associated with the arrows between two variables are the partial regression coefficients derived from multiple regressions. The numbers associated with the arrows of single variables represent the unexplained variation (1-R2), which represent the effects of unmeasured variables and measurement error. All pathways are significant in the final model (*p < 0.05; **p < 0.01).
Results
Table 1 shows the soil properties (water content, pH, and DON/NH+4/NO−3 concentrations), N transformation rates (gross rates of NH+4 production/consumption/immobilization, nitrification, and NO−3 immobilization, and net rates of NH+4 production and nitrification), and gene abundances (abundances of the bacterial 16S rRNA gene and archaeal amoA) in the upper and lower parts of the slope. Bacterial amoA was detected only in the sampling points at the lowest positions (7.1 × 106 copy/g-dry soil). With respect to the soil properties, the water content, soil pH, and NH+4 concentration did not differ significantly between the upper and lower parts, whereas the DON and NO−3 concentrations were higher in the lower part. The net NH+4 production rate was higher in the upper part, whereas the net nitrification rate was higher in the lower part. The gross rates of N transformation were higher in the lower part, except for NH+4 and NO−3 immobilization. The percentage of nitrification was also higher in the lower part. The abundances of the bacterial 16S rRNA gene and archaeal amoA were higher in the lower part.
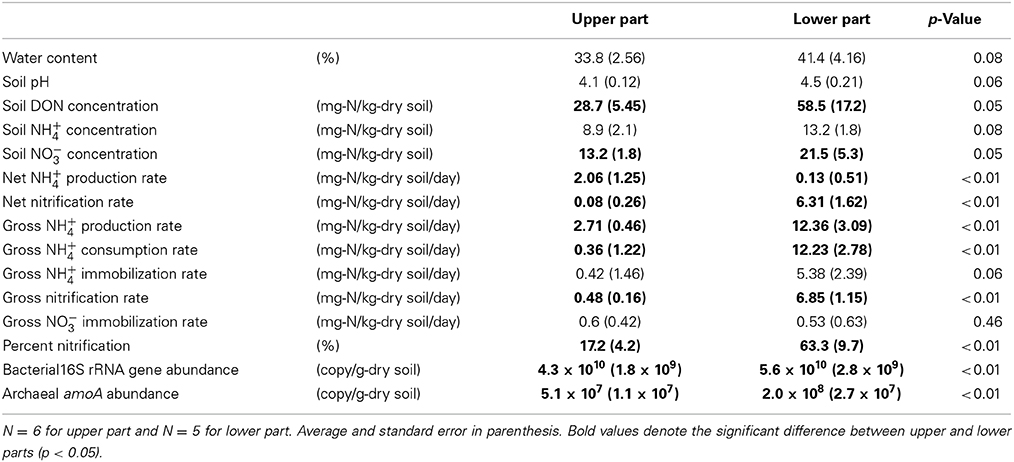
Table 1. Soil properties, N transformation rates, and gene abundances in upper and lower parts on the forest slope.
To estimate the factors that affected the gross rates of NH+4 production and nitrification, we performed correlation analyses of the gross rates and soil physicochemical properties or gene abundances and the results are shown in Table 2. In particular, the gross rate of NH+4 production correlated with the abundance of the bacterial 16S rRNA gene (Figure 2). The gross rate of nitrification correlated with the abundance of archaeal amoA (Figure 2). The correlation analysis (Table 2) showed that the soil water content, DON concentration, and the abundance of the bacterial 16S rRNA gene were possible factors that affected the gross NH+4 production rate, whereas the gross NH+4 production rate, NH+4 concentration, and the abundance of archaeal amoA were possible factors that affected the gross nitrification rate. These variables do not always have direct effects on the gross rates of NH+4 production or nitrification; thus, we performed path analysis based on the assumptions, described in Figure 1A, to consider the indirect influences on the gross rates. The results of the analysis (Figures 1B,C) suggest that the gross NH+4 production rate was affected by both the DON concentration and the abundance of the bacterial 16S rRNA gene, whereas the abundance of the bacterial 16S rRNA gene was affected by both the DON concentration and soil pH. The gross nitrification rate was affected by both the NH+4 concentration and the abundance of archaeal amoA, whereas the abundance of archaeal amoA was affected by the soil water content. We did not use the gross NH+4 production rate as the resource parameter (Figure 1A) instead of or in addition to the NH+4 concentration in the analysis of the gross nitrification rate, because there was high multicollinearity between the gross NH+4 production rate and the abundance of archaeal amoA, which interfered with the statistical evaluation of the influence of the gross NH+4 production rate.
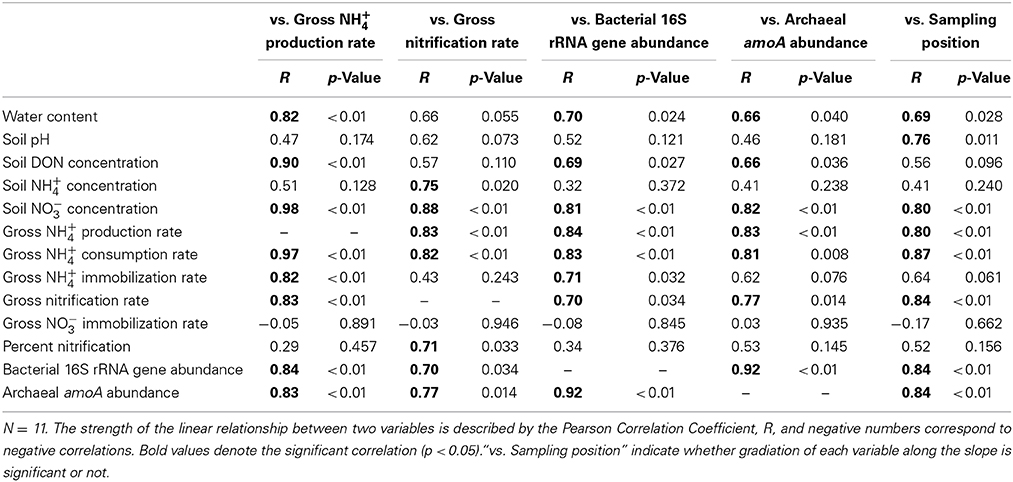
Table 2. Correlation with gross rates of NH+4 production and nitrification, abundance of bacterial 16S rRNA gene and archaeal amoA, or sampling position.
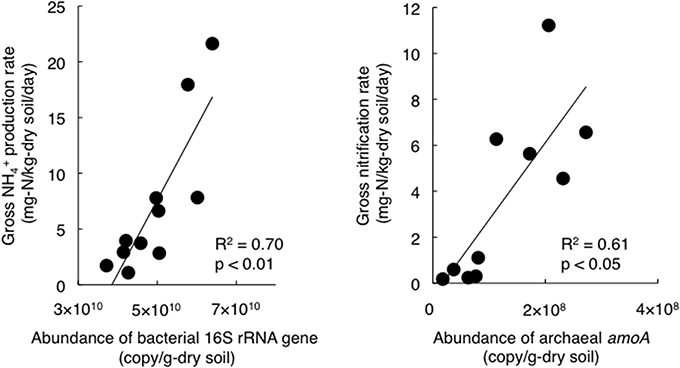
Figure 2. Relationships between the gross NH+4 production rate and the bacterial 16S rRNA gene abundance (Left panel, y = 7 × 10−10 x - 25.5, R2 = 0.70, p < 0.05), and between the gross nitrification rate and the archaeal amoA abundance (Right panel, y = 3 × 10−8 x - 0.5, R2 = 0.61, p < 0.05).
Discussion
Soil Chemistry and N Dynamics on the Slope
The soil water content and pH did not differ significantly between the upper and lower parts of the slope because of the large standard error, but they increased gradually down the slope from the top to the bottom (see “vs. sampling position” in Table 2), which was consistent with the results of a previous study conducted in 1997, as reported previously (Tateno and Takeda, 2003). The net rates of NH+4 production and nitrification suggest that NH+4 tended to accumulate in the upper part of the slope whereas NO−3 tended to accumulate in the lower part (Table 1). This indicates that the available N for plants in the surface 0–10 cm mineral soil was likely to be NH+4 in the upper part and NO−3 in the lower part. NH+4 accumulation was not observed in the lower part, but the gross NH+4 production rates in the lower part were much larger (Table 1), thereby suggesting that the concurrent production and consumption of larger amounts of NH+4 occurred in the lower part. Nitrification and NH+4 immobilization were the dominant pathways of NH+4 consumption, with similar rates (Table 1). The higher gross nitrification rates in the lower part showed that larger amounts of NO−3 were produced, and the higher percentage of nitrification in the lower part suggests that more of the NH+4 produced via DON degradation was oxidized to NO−3 via nitrification. In addition, the gross rates of NH+4 immobilization and nitrification were much higher than the gross NO−3 immobilization rate in the lower part. This suggested that soil microorganisms were likely to utilize NH+4 rather than NO−3 for N assimilation, and a large amount of the NO−3 produced via nitrification was not utilized. These results suggest that the N dynamics related to NH+4 and NO−3 were more prominent in the lower part because of the greater microbial NH+4 production and consumption activities and the N availability, particularly the NO−3 availability, for plants was greater in the lower part because of the higher microbial nitrification activity but lower NO−3 immobilization activity.
Microbial Population Responsible for N Transformation
Similar to the gross rates of NH+4 production and nitrification, the populations of total bacteria and NH+4-oxidizing archaea were higher in the lower part (Table 1). We hypothesized that almost all bacteria were involved in NH+4 production, and the positive correlation between the gross NH+4 production rates and abundances of the bacterial 16S rRNA gene supported our hypothesis. In general, N mineralization via DON degradation is measured in the form of NH+4 production as a single process, but it is actually the sum of multiple distinct physiological processes (Isobe and Ohte, 2014). Therefore, it is difficult to identify the microorganisms responsible for NH+4 production solely based on genetic information such as amoA for ammonia oxidizers or nirK/S for denitrifiers (Hawkes et al., 2005; Philippot et al., 2009; Isobe et al., 2012). Alternatively, NH+4 can potentially be produced via the direct enzymatic cleavage of a free amino group, amine-N, or amide-N (R-NH2) inside cells that take up R-NH2, and deaminase and deamidase enzymes can be produced by most bacteria (Schimel and Bennett, 2004; Myrold and Bottomley, 2008; Bottomley et al., 2012; Isobe and Ohte, 2014); thus, we hypothesized that almost all bacteria are involved in NH+4 production. There are few studies on microbial ecology associating with NH+4 production; however, our study presents an alternative method for analyzing the mechanisms that regulate NH+4 production rate in soils. The positive correlation between the gross nitrification rates and the abundance of archaeal amoA (Figure 1) suggests that NH3-oxidizing archaea were mainly responsible for NH3 oxidation. Because NH3-oxidizing bacteria were detected only at the lowest point, their contribution to NH3 oxidation on the slope could be limited or site-specific. Previous studies have shown that gross or net nitrification rates correlate with the abundance of bacterial amoA in grassland or agricultural fields (Hawkes et al., 2005; Di et al., 2009; Jia and Conrad, 2009), but with the abundance of archaeal amoA in forests (Isobe et al., 2012). This supports the results of the present study, thereby suggesting that NH3-oxidizing archaea are likely to predominate in forests.
Regulation of N Dynamics on the Slope
The results of the path analysis of the regulation of the gross rates of NH+4 production and nitrification appeared to be reasonable because a substrate supply and microorganisms that utilize the substrate for transformation are the primary requirements for N transformation. The soil pH was suggested to be a major factor that affected the total bacterial population size. Previous study has also shown that the bacterial population size can be strongly affected by the soil pH (Rousk et al., 2010). The soil water content was suggested to be a strong factor that affected the population size of NH+4-oxidizing archaea. Recently, Bustamante et al. (2012) also showed that the population size of NH+4-oxidizing archaea responded positively to water availability. The gross NH+4 production rate was indicated to be a factor that affected the gross nitrification rate, although we did not use it in the analysis. A positive correlation between the gross rates of NH+4 production and nitrification is observed in many forests (Booth et al., 2005; Kuroiwa et al., 2011). Petersen et al. (2012) demonstrated the mutual correlations among the gross NH+4 production rate, abundance of bacterial amoA, and the net nitrification rate in various ecosystems in Alaska. Therefore, multicollinearity between the gross NH+4 production rate and the abundance of amoA during the analysis of gross nitrification may be observed in many forests. However, the gross NH+4 production rate is not always a dominant factor that affects the gross nitrification rate. Hawkes et al. (2005) showed that the gross NH+4 production rate was not considered to be a factor that affected either the gross nitrification rate or the abundance of bacterial amoA in a grassland soil, despite a strong correlation between them. We also have found that the gross nitrification rates positively correlated with the abundance of archaeal amoA in subtropical forest soils with different gross NH+4 production rates (Isobe, 2011). In the present study, the lack of statistical evaluation of the effect of the gross NH+4 production rate on the gross nitrification rate and the abundance of archaeal amoA was attributable to the low number of samples examined. Microbial NH+4 immobilization was one of main N transformation processes on the slope. As demonstrated by the correlation between the gross NH+4 immobilization rate and the abundance of the bacterial 16S rRNA gene (Table 2), gross NH+4 immobilization rate could be affected strongly by the size of the bacterial population because NH+4, but not NO−3, was the main form of N assimilated by microorganisms in the lower part of the slope. The results of the present study suggest that the gradients of soil environmental properties such as the pH and water content along the forest slope affected N transformation rates by altering the sizes of the microbial populations responsible for the N transformations. Figure 3 shows a conceptual diagram of the possible regulation of N transformation on the slope.
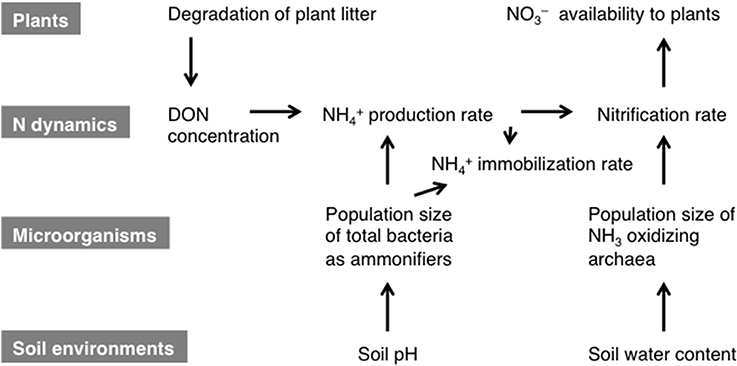
Figure 3. Conceptual diagram showing the regulation of N transformation on the examined slope. The effects of the gradients in the soil environmental properties on the bioavailable N dynamics are mediated via the microbial population dynamics. The relationships among the three categories are influenced by plant litter fall, which affects the plant N availability. The gross NH+4 production rate is affected by the concentration of DON and the total bacterial population size as ammonifiers. The total bacterial population size is affected by the soil water content. The gross nitrification rate is affected by the NH+4 concentration and the NH3-oxidizing archaea population size as nitrifiers. The NH3-oxidizing archaea population size is affected by the soil pH. The NO−3 produced is available to plants because soil microorganisms prefer NH+4 for N assimilation.
Interactions between Microorganisms and Plants Via the Regulation of N Dynamics
The N availability for plants was higher in the lower part of the slope. In particular, the larger population size of NH3-oxidizing archaea was related to the higher NO−3 availability for plants. In addition, the microbial communities preferred NH+4 for N assimilation and supplied free NO−3 via nitrification. These results indicate that partitioning the bioavailable N can occur between microbial communities and plants. This could facilitated the higher growth and more active utilization of NO−3 by F. crenata on the lower slope, as shown in a previous study (Tateno et al., 2005). The higher water availability in the lower part, which is one of the general characteristics of forest slopes, also led to increases in the population size of NH3-oxidizing archaea and acceleration of the diffusion of the NO−3 produced, which could relieved the plants from water- and N-limiting conditions. However, the lower part could presented severe conditions for plants that selectively uptake NH+4 for N assimilation. The increased concentration of DON of which supply originates from the degradation of the plant litter could facilitate increases in the population size of total bacteria as the ammonifiers and the NH+4 production rate.
In summary, the results of this study suggest that the soil physicochemical gradient along the slope caused the spatial gradient of gross rates of NH+4 production and nitrification by altering the communities of ammonifiers and nitrifiers in the forest slope, which also affected plant growth via the supply of bioavailable N to plants. Many studies have investigated microorganism–plant interactions in forests by focusing on direct interactions such as ectomycorrhizal or endomycorrhizal symbiosis (Kohzu et al., 1999; Toljander et al., 2006; Hobbie and Hobbie, 2008). However, our study suggests that microorganism–plant interactions occur indirectly via microbial regulation of supply of the bioavailable N. Let us be cautioned, however, that this study was performed in a single forest slope. Obtaining a definitive picture of the microbial regulation of bioavailable N dynamics in forest soils will require studies in forests with different vegetation types (e.g., planted forests) or different pattern of N dynamics, which should be the objective of future work.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by Grants-in-Aid for Scientific Research from the Japanese Society for the Promotion of Science (Nos. 25252026, 25550009, 26292085, and 26712015) and the GRENE/Ecoinformatics project from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
References
Band, L. E., Tague, C. L., Groffman, P., and Belt, K. (2001). Forest ecosystem processes at the watershed scale: hydrological and ecological controls of nitrogen export. Hydrol. Process. 15, 2013–2028. doi: 10.1002/hyp.253
Booth, M. S., Stark, J. M., and Rastetter, E. (2005). Controls on nitrogen cycling in terrestrial ecosystems: a synthetic analysis of literature data. Ecol. Monogr. 75, 139–157. doi: 10.1890/04-0988
Bottomley, P. J., Taylor, A. E., and Myrold, D. D. (2012). A consideration of the relative contributions of different microbial subpopulations to the soil N cycle. Front. Microbiol. 3:373. doi: 10.3389/fmicb.2012.00373
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Bustamante, M., Verdejo, V., Zúñiga, C., Espinosa, F., Orlando, J., and Carú, M. (2012). Comparison of water availability effect on ammonia-oxidizing bacteria and archaea in microcosms of a Chilean semiarid soil. Front. Microbiol. 3:282. doi: 10.3389/fmicb.2012.00282
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Di, H. J., Cameron, K. C., Shen, J. P., Winefield, C. S., O'Callaghan, M., Bowatte, S., et al. (2009). Nitrification driven by bacteria and not archaea in nitrogen-rich grassland soils. Nat. Geosci. 2, 621–624. doi: 10.1038/ngeo613
Engelbrecht, B. M. J., Comita, L. S., Condit, R., Kursar, T. A., Tyree, M. T., Turner, B. L., et al. (2007). Drought sensitivity shapes species distribution patterns in tropical forests. Nature 447, 80–82. doi: 10.1038/nature05747
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Enoki, T. (2003). Microtopography and distribution of canopy trees in a subtropical evergreen broad-leaved forest in the northern part of Okinawa Island, Japan. Ecol. Res. 18, 103–113. doi: 10.1046/j.1440-1703.2003.00549.x
Hart, S. C., Stark, J. M., Davidson, E. A., and Firestone, M. K. (1994). “Nitrogen mineralization, immobilization, and nitrification,” in Methods of Soil Analysis. Part 2, Biochemical and Microbiological Properties, eds R. Weaver, S. Angle, P. Bottomley, D. Bezdicek, S. Smith, A. Tabatabai, and A. Wollum (Madison: Soil Science Society of America), 985–1018.
Hawkes, C. V., Wren, I. F., Herman, D. J., and Firestone, M. K. (2005). Plant invasion alters nitrogen cycling by modifying the soil nitrifying community. Ecol. Lett. 8, 976–985. doi: 10.1111/j.1461-0248.2005.00802.x
Hill, A., and Kemp, W. (1999). Nitrogen chemistry of subsurface storm runoff on forested Canadian Shield hillslopes. Water Resour. Res. 35, 811–821. doi: 10.1029/1998WR900083
Hirobe, M., Koba, K., and Tokuchi, N. (2003). Dynamics of the internal soil nitrogen cycles under moder and mull forest floor types on a slope in a Cryptomeria japonica D. Don plantation. Ecol. Res. 18, 53–64. doi: 10.1046/j.1440-1703.2003.00532.x
Hirobe, M., Tokuchi, N., and Iwatsubo, G. (1998). Spatial variability of soil nitrogen transformation patterns along a forest slope in a Cryptomeria japonica D. Don plantation. Eur. J. Soil Biol. 34, 123–131. doi: 10.1016/S1164-5563(00)88649-5
Hobbie, E. A., and Hobbie, J. E. (2008). Natural abundance of 15N in nitrogen-limited forests and tundra can estimate nitrogen cycling through mycorrhizal fungi: a review. Ecosystems 11, 815–830. doi: 10.1007/s10021-008-9159-7
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Hook, P., and Burke, I. (2000). Biogeochemistry in a shortgrass landscape: control by topography, soil texture, and microclimate. Ecology 81, 2686–2703. doi: 10.1890/0012-9658(2000)081[2686:BIASLC]2.0.CO;2
Isobe, K. (2011). Nitrogen Flow and Nitrifying Microbial Communities in Subtropical Forest Soils Receiving High N Deposition in CHINA. Doctoral dissertation of The University of Tokyo, Tokyo.
Isobe, K., Koba, K., Otsuka, S., and Senoo, K. (2011a). Nitrification and nitrifying microbial communities in forest soils. J. For. Res. 16, 351–362. doi: 10.1007/s10310-011-0266-5
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Isobe, K., Koba, K., Suwa, Y., Ikutani, J., Fang, Y., Yoh, M., et al. (2012). High abundance of ammonia-oxidizing archaea in acidified subtropical forest soils in southern China after long-term N deposition. FEMS Microbiol. Ecol. 80, 193–203. doi: 10.1111/j.1574-6941.2011.01294.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Isobe, K., Koba, K., Ueda, S., Senoo, K., Harayama, S., and Suwa, Y. (2011c). A simple and rapid GC/MS method for the simultaneous determination of gaseous metabolites. J. Microbiol. Methods 84, 46–51. doi: 10.1016/j.mimet.2010.10.009
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Isobe, K., and Ohte, N. (2014). Ecological perspectives on microbes involved in N-cycling. Microbes Environ. 29, 4–16. doi: 10.1264/jsme2.ME13159
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Isobe, K., Suwa, Y., Ikutani, J., Kuroiwa, M., Makita, T., Takebayashi, Y., et al. (2011b). Analytical techniques for quantifying 15N/14N of nitrate, nitrite, total dissolved nitrogen and ammonium in environmental samples using a gas chromatograph equipped with a quadrupole mass spectrometer. Microbes Environ. 26, 46–53. doi: 10.1264/jsme2.ME10159
Jia, Z., and Conrad, R. (2009). Bacteria rather than Archaea dominate microbial ammonia oxidation in an agricultural soil. Environ. Microbiol. 11, 1658–1671. doi: 10.1111/j.1462-2920.2009.01891.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kirkham, D., and Bartholomew, W. V. (1954). Equations for following nutrient transformations in soil, utilizing tracer data1. Soil Sci. Soc. Am. J. 18, 33. doi: 10.2136/sssaj1954.03615995001800010009x
Kohzu, A., Yoshioka, T., Ando, T., Takahashi, M., Koba, K., and Wada, E. (1999). Natural 13 C and 15 N abundance of field-collected fungi and their ecological implications. New Phytol. 144, 323–330. doi: 10.1046/j.1469-8137.1999.00508.x
Koyama, L., Hirobe, M., Koba, K., and Tokuchi, N. (2013). Nitrate-use traits of understory plants as potential regulators of vegetation distribution on a slope in a Japanese cedar plantation. Plant Soil 362, 119–134. doi: 10.1007/s11104-012-1257-9
Kuroiwa, M., Koba, K., Isobe, K., Tateno, R., Nakanishi, A., Inagaki, Y., et al. (2011). Gross nitrification rates in four Japanese forest soils: heterotrophic versus autotrophic and the regulation factors for the nitrification. J. For. Res. 16, 363–373. doi: 10.1007/s10310-011-0287-0
Kuzyakov, Y., and Xu, X. (2013). Competition between roots and microorganisms for nitrogen?: mechanisms and ecological relevance. New Phytol. 198, 656–669. doi: 10.1111/nph.12235
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Myrold, D. D., and Bottomley, P. J. (2008). “Nitrogen mineralization and immobilization,” in Nitrogen in Agricultural Systems, eds J. S. Schepers and W. R. Raun (Madison, WI: ASA-CSSA-SSSA), 153–168.
Nicol, G. W., Leininger, S., Schleper, C., and Prosser, J. I. (2008). The influence of soil pH on the diversity, abundance and transcriptional activity of ammonia oxidizing archaea and bacteria. Environ. Microbiol. 10, 2966–2978. doi: 10.1111/j.1462-2920.2008.01701.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Nishina, K., Takenaka, C., and Ishizuka, S. (2009a). Spatial variations in nitrous oxide and nitric oxide emission potential on a slope of Japanese cedar (Cryptomeria japonica) forest. Soil Sci. Plant Nutr. 55, 179–189. doi: 10.1111/j.1747-0765.2007.00315.x
Nishina, K., Takenaka, C., and Ishizuka, S. (2009b). Spatiotemporal variation in N2O flux within a slope in a Japanese cedar (Cryptomeria japonica) forest. Biogeochemistry 96, 163–175. doi: 10.1007/s10533-009-9356-2
Penna, D., Borga, M., Norbiato, D., and Dalla Fontana, G. (2009). Hillslope scale soil moisture variability in a steep alpine terrain. J. Hydrol. 364, 311–327. doi: 10.1016/j.jhydrol.2008.11.009
Petersen, D. G., Blazewicz, S. J., Firestone, M., Herman, D. J., Turetsky, M., and Waldrop, M. (2012). Abundance of microbial genes associated with nitrogen cycling as indices of biogeochemical process rates across a vegetation gradient in Alaska. Environ. Microbiol. 14, 993–1008. doi: 10.1111/j.1462-2920.2011.02679.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Philippot, L., Cuhel, J., Saby, N. P. A., Chèneby, D., Chronáková, A., Bru, D., et al. (2009). Mapping field-scale spatial patterns of size and activity of the denitrifier community. Environ. Microbiol. 11, 1518–1526. doi: 10.1111/j.1462-2920.2009.01879.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Rousk, J., Bååth, E., Brookes, P. C., Lauber, C. L., Lozupone, C., Caporaso, J. G., et al. (2010). Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J. 4, 1340–1351. doi: 10.1038/ismej.2010.58
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Schimel, J. P., and Bennett, J. (2004). Nitrogen mineralization: challenges of a changing paradigm. Ecology 85, 591–602. doi: 10.1890/03-8002
Tateno, R., Hishi, T., and Takeda, H. (2004). Above- and belowground biomass and net primary production in a cool-temperate deciduous forest in relation to topographical changes in soil nitrogen. For. Ecol. Manag. 193, 297–306. doi: 10.1016/j.foreco.2003.11.011
Tateno, R., Osada, N., Terai, M., Tokuchi, N., and Takeda, H. (2005). Inorganic nitrogen source utilization byFagus crenata on different soil types. Trees 19, 477–481. doi: 10.1007/s00468-005-0409-4
Tateno, R., and Takeda, H. (2003). Forest structure and tree species distribution in relation to topography-mediated heterogeneity of soil nitrogen and light at the forest floor. Ecol. Res. 18, 559–571. doi: 10.1046/j.1440-1703.2003.00578.x
Tateno, R., and Takeda, H. (2010). Nitrogen uptake and nitrogen use efficiency above and below ground along a topographic gradient of soil nitrogen availability. Oecologia 163, 793–804. doi: 10.1007/s00442-009-1561-0
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Tokuchi, N., Hirobe, M., and Koba, K. (2000). Topographical differences in soil N transformation using15N dilution method along a slope in a conifer plantation forest in Japan. J. For. Res. 5, 13–19. doi: 10.1007/BF02762758
Toljander, J. F., Eberhardt, U., Toljander, Y. K., Paul, L. R., and Taylor, A. F. S. (2006). Species composition of an ectomycorrhizal fungal community along a local nutrient gradient in a boreal forest. New Phytol. 170, 873–883. doi: 10.1111/j.1469-8137.2006.01718.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Tromp-van Meerveld, H. J., and McDonnell, J. J. (2006). On the interrelations between topography, soil depth, soil moisture, transpiration rates and species distribution at the hillslope scale. Adv. Water Resour. 29, 293–310. doi: 10.1016/j.advwatres.2005.02.016
Urakawa, R., Ohte, N., Shibata, H., Tateno, R., Hishi, T., Fukushima, K., et al. (2014). Biogeochemical nitrogen properties of forest soils in the Japanese archipelago. Ecol Res. doi: 10.1007/s11284-014-1212-8. (in press)
Keywords: ammonia-oxidizing archaea, ammonification, hillslope, nitrification, nitrogen dynamics
Citation: Isobe K, Ohte N, Oda T, Murabayashi S, Wei W, Senoo K, Tokuchi N and Tateno R (2015) Microbial regulation of nitrogen dynamics along the hillslope of a natural forest. Front. Environ. Sci. 2:63. doi: 10.3389/fenvs.2014.00063
Received: 30 September 2014; Accepted: 07 December 2014;
Published online: 07 January 2015.
Edited by:
Wilfred Otten, Abertay University, UKReviewed by:
Peter S. Hooda, Kingston University London, UKMiguel Ángel Sánchez-Monedero, Spanish National Research Council, Spain
Copyright © 2015 Isobe, Ohte, Oda, Murabayashi, Wei, Senoo, Tokuchi and Tateno. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kazuo Isobe, Department of Applied Biological Chemistry, Graduate School of Agricultural and Life Sciences, The University of Tokyo, 1-1-1 Yayoi, Bunkyo-ku, Tokyo 113-8657, Japan e-mail: akisobe@mail.ecc.u-tokyo.ac.jp
 Kazuo Isobe
Kazuo Isobe Nobuhito Ohte
Nobuhito Ohte Tomoki Oda1
Tomoki Oda1  Wei Wei
Wei Wei Keishi Senoo
Keishi Senoo Ryunosuke Tateno
Ryunosuke Tateno