A new DNA extraction protocol for screwworm fly Cochliomyia species (Diptera: Calliphoridae)
- 1International Centre for Zoonoses, Central University of Ecuador, Quito, Ecuador
- 2Faculty of Veterinary Medicine, Central University of Ecuador, Quito, Ecuador
Modifications to the DNA isolation protocol from Cochliomyia spp., are suggested based on the Chelex® 100 reactive. To apply the sterile insect technique (SIT) program it is necessary to study the molecular variations of endemic populations with efficient, fast and low costs techniques. The test samples were collected in the Pichincha province of Ecuador. The isolation protocol had 3 steps: (a) pretreatment (optional), (b) mechanic and chemical lysis, (c) two incubations; then the supernatant were separated by centrifugation. Furthermore, variations in concentrations of magnesium chloride present in the master mix were evaluated. Results showed a high efficiency in isolation with approximately 1.20 h of manipulation (without pretreatment). Additionally, the quality of the amplicon that was visualized on 2% agarose (w/v) showed that the magnesium chloride concentration was influential in the PCR reaction mix.
Introduction
Cochliomyia hominivorax (Coquerel) (Diptera: Calliphoridae), is a New World screwworm (NWS) which in its larval stage is an obligate parasite that causes parasitic and zoonotic disease known as traumatic myiasis in warm-blooded animals including humans (Forero et al., 2008; Bárcenas, 2010). The illness is included in the list of multiple species diseases, infections and infestations and their presence is notifiable (OIE, 2013). The NWS is also considered part of Transboundary Diseases in the Americas (GF-TADs, 2007).
NWS is endemic to tropical and subtropical regions of America, where it is widely distributed, mainly in South America and the Caribbean (Wyss and Galvin, 1996; Wall and Shearer, 1997). The annual losses caused by screwworm infestations in the United States, Mexico and Central America were significant; while in some Caribbean islands and South America it remains as a latent problem (Forero et al., 2007; Rodríguez et al., 2011). The programs based on the sterile insect technique eradicated the NWS caused by C. hominivorax from the southern United States, Mexico, Central America and some Caribbean islands (Robinson et al., 2009). For the period 2012–2021 the FAO has provided strategies for collecting information about the incidence of populations of screwworm and the impact of NWS on public health, in order to establish the eradication program in South America (FAO, 2011).
In Ecuador, two studies surveyed on animals from tropical areas reported presence of screwworm larvae in 4132 and 830 animals, respectively (Miño et al., 2005; Arteaga et al., 2012). Besides, studies carried out at the International Centre for Zoonosis of the Central University of Ecuador (CIZ-UCE) on adult Cochliomyia spp. have shown difficulties in the morphological differentiation of C. macellaria and C. hominivorax (unpublished data).
Griffiths et al. (2009), Torres and Azeredo-Espin (2009), Robinson et al. (2009) and Lyra et al. (2009) have reported the use of different molecular techniques and several modifications of the DNA isolation protocols for Cochliomyia spp. The principal changes were based on the type of sample (legs, abdomen and complete specimen) to be analyzed and focused to phenol-chloroform or commercial kit methods, but the quantification and purity was not reported. The aim of this study was to prove a fast and user-friendly protocol of DNA isolation, that will allow to study the genetic variability and molecular taxonomy of the species in Ecuador. In order to contribute to an efficient biological control method with more genetic information based on a large number of samples in a short period of time (Klassen and Curtis, 2005; McDonagh et al., 2009).
Materials and Methods
Sample
The specimens were collected as larvae from animals with myiasis and bovine corpses, which were in-vitro cultured in the laboratory until adult stage. All specimens were from the northwest of the Pichincha province. All specimens were labeled and deposited in the bank of specimens of CIZ-UCE; each specimen was preserved dry individually in a tube of 1.5 ml at—20°C for ≈3 months until laboratory process. Details of 9 adults specimens selected for this analysis are shown in Table 2. Additionally, all flies were previously identified morphologically as Cochliomyia spp., using dichotomous keys published by FAO (1993).
Isolation and Quantification of DNA
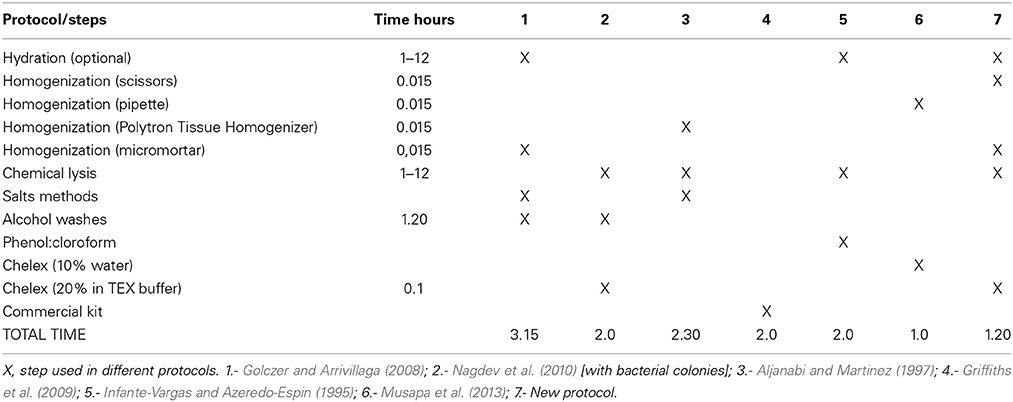
Modified and standardized according to Musapa et al. (2013), Golczer and Arrivillaga (2008), Nagdev et al. (2010), Arrivillaga et al. (2002) (Table 1). Pretreatment—Hydration of the sample (optional): The section of the specimen (Table 2) was suspended and incubated in 250 μ l of Buffer H [100 mM Tris-HCl (Invitrogen), 50 mM EDTA (Invitrogen), 100 mM NaCl (Schalau), 0.5% SDS (Sigma), 200 mM sucrose (Santa Cruz Biotechnology)] at room temperature overnight if the samples are drying or 30 min if the samples are fresh.
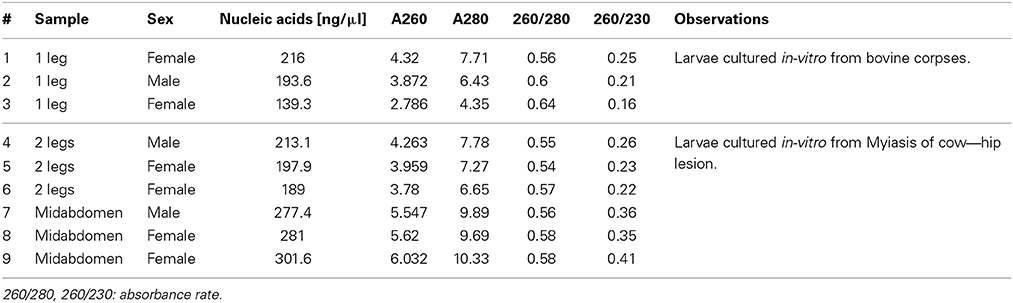
Table 2. Results of DNA quantifications from samples of Cochliomyia spp., collected in Pichincha—Ecuador.
Sample Homogenization and Chemical Lysis
Mechanical lysis was divided into three steps: (a) grinding of the sample with scissors for 15 s; (b) homogenization with battery micromortar (Kontes, Vineland, NJ) for 15 s; and (c) centrifugation for 5 min at 13,000 rpm to separate the Buffer H from the crushed sample. Then, the supernatant was discarded and 200 μ l of TEX buffer [10 mM Tris pH 8.0 (Invitrogen), 0.5 mM EDTA (Invitrogen), 1% Triton X-100 (Promega)] with Chelex ® 100 (Biorad) at 20% was added. 35 μ l of proteinase K (20 mg/ml in PK buffer −50 mM Tris-HCl pH 7.4, 10 mM CaCl2) was added to the tube and the solution was incubated first for 1 h at 60°C with shaking of 1300 rpm and followed by 10 min at 100°C with shaking of 1000 rpm. Finally, the solution was centrifuged for 5 min at 13,000 rpm the supernatant was transferred to a new tube. A measurement of 2 μ l of each sample was performed with spectrophotometry as per the Nanodrop 2000c program, by following the supplier's instructions. The results obtained were presented in Table 2 and the samples were stored at -20 °C till further us.
Polymerase Chain Reaction
A genetic mitochondrial DNA (COI) fragment was amplified based on modifications to Arrivillaga et al. (2002) using the primers CIJ1632 (5′-TGATCAAATTTATAAT-3′—eurofins mwg operon) and CIN2191 (5′-GGTAAAATTAAAATATAAACTTC-3′—eurofins mwg operon). The concentrations were adjusted to obtain the appropriate master mix reagents quantities (see Table 3). To assess the optimal concentrations, the quality of the band (sharpness and intensity), the presence of non-specific bands and primers excess were considered. The amplification conditions were as follows: initial denaturation step of 95°C for 3 min was followed by 35 cycles of 95°C for 0.3 min, 45°C for 1 min and 72°C for 1 min, and final elongation step of 72°C for 7 min. Finally, the amplicons were separated by electrophoresis on a 2% agarose gel (w/v) (Figure 1).
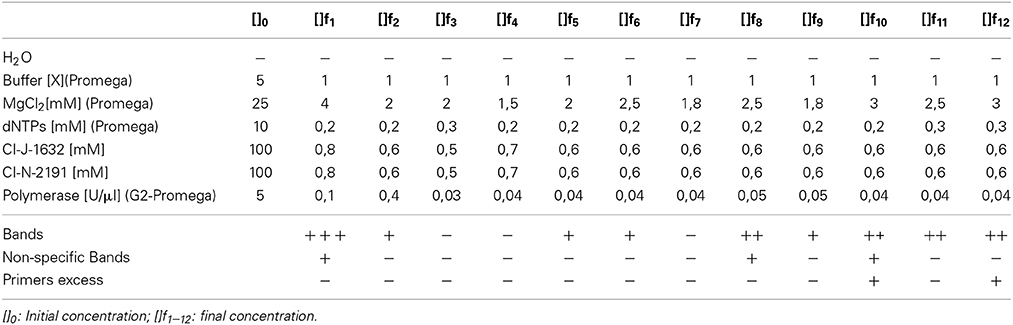
Table 3. Final concentrations of the different master mixes tested and qualitative results obtained for samples Cochliomyia spp., collected in Pichincha—Ecuador.
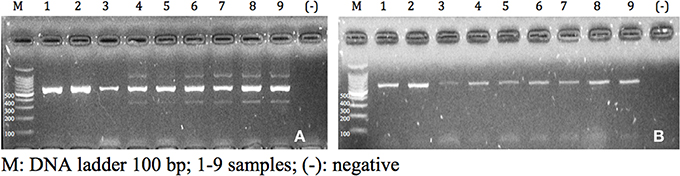
Figure 1. Amplicons visualized in agarose gel of 2% (w/v) of samples of Cochliomyia spp., collected in Pichincha-Ecuador. (A) f1 concentration; (B) f2 concentration.
Results and Discussion
The application of several steps and buffers permitted to obtain suitable DNA extracts with better PCR amplification in a short period of time. Resulting in, less cost in reagents compared with other methods, due to the correct sequence of the steps of the different protocols. Previous reports about DNA isolation from samples of Cochliomyia spp., using phenol-chloroform and salts reported an average of 2–3 h (Infante-Vargas and Azeredo-Espin, 1995; Aljanabi and Martinez, 1997). Although, the method 6 had 1 h because the authors used a complete specimen without chemical lysis, the results present more inhibition components and less efficient. Due to the characteristics of the preserved specimens, a hydration step is required to perform a pre-treatment and prepare the samples for cell lysis. The use of TEX buffer with 20% of Chelex® 100 provided the conditions to chemical attraction between the components of the sample with the chelating ion. Chelex contained paired ions to pH 7–12, which act as chelating groups in binding polyvalent ions. The resin is highly selective for positive ions and it is much higher bond strength with the components (Walsh et al., 1991). Although variations were found in the absorbance that did not relate with the standards, the PCR's performance was not affected. DNA quantification is shown in Table 2. The analysis of the data provided a reference for the state of the samples and final results showed in the PCR and with an efficient sequencing. The absorbance at 260 nm (0.1–1 optimum values according to the Lambert-Beer law and the wavelength of maximum absorption of the components) refers to a good quality of DNA. The results obtained present high values compared with the standards due to presence of double and single stranded DNA and RNA because RNase or alcohols purification were not used. In 260/280 (1.8–2.0 optimum values) (Chen et al., 2010), the results obtained (0.54–0.64) demonstrated the presence of DNA but with the presence of proteins that does not interfere with the PCR reaction. Chelex® 100 reagent could not have a total efficiency to capture the excess of proteins due to the saturation of the chelating resin. Finally, 260/230 (2.00–2.2, optimum values), the results obtained were between 0.16 and 0.41 that indicated the presence of reaction inhibitors (e.g., carbohydrates, peptides, phenols, degraded molecules of RNA) (Linton et al., 2001), these contaminants did not interfere with the PCR amplification.
In the PCR results, the variation of final concentrations of the chemical components allowed the observation of amplicons with good quality according to the parameters evaluated (quality of bands, non-specifc bands, and primer excess). Variation in absorbance values showed interference or relation of the different components of the sample. Further studies are necessary to detect possible inhibitors that intergrade the sample.
The qualitative assessments demonstrated variations among assays, as shown in Table 3. These variations were in the reaction and concentration of MgCl2/polymerase. The highest concentration of magnesium chloride, i.e., 3.0 mM, had the best results, discarding problems for primers and total amount of polymerase. The appropriate concentration of MgCl2 improves the catalytic activity of the polymerase (ref). Due to the characteristic of the sample could be precipitate the reagent induce negative PCR. The results showed that f12 test was the best (Table 3). Additionally, the reproducibility of the test was assessed by different laboratory technicians and with more samples.
Conclusions
Contrary to reported protocols, this assay permitted to obtain suitable molecular results in a short period of time (≈1.2 h, except hydration) using few reagents, friendly and with simple steps. Properties of Chelex® 100 reagent facilitated the purification of DNA from the complex molecules present in the initial sample. The results of the method shows good quality of the bands in agarose, despite not removing all necessary components shown in absorbance allowing adequate amplifications. It was feasible to demonstrate that chemical variations of the PCR and the relative concentration of magnesium chloride/polymerase are proportional to the quality of the band, the presence of non-specific fragments and primers excess.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
To Juan Carlos Navarro, Alfonso Molina and Sandra Páez for their valuable advices. Special thanks for the financial support in this study to The Central University of Ecuador. Project—“Epidemiología molecular de parásitos y microorganismos de interés zoonósico y de Salud Pública: Gusano Barrenador del Ganado, Garrapatas.”
References
Aljanabi, S., and Martinez, I. (1997). Universal and rapid salt-extraction of high quality genomic DNA for PCR-based techniques. Nucleic Acids Res. 25, 4692–4693. doi: 10.1093/nar/25.22.4692
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Arrivillaga, J., Norris, D., Feliciangeli, M., and Lanzaro, G. (2002). Phylogeography of the Neotropical sand fly Lutzomyia longipalpis inferred from mitochondrial DNA sequences. Infect. Genet. Evol. 2, 83–95. doi: 10.1016/S1567-1348(02)00087-4
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Arteaga, F. G., Rodríguez Diego, J. G., and Olivares, J. L. (2012). Comportamiento de Cochliomyia hominivorax (Coquerel) y relación con otros agentes causantes de myasis, en un cantón de la región de manabí, Ecuador. Rev. Salud Anim. 34, 19–24.
Bárcenas, A. (2010). Miasis por Gusano Barrenador del Ganado Cochliomyia hominivorax. Universidad Michoacana de San Nicolás de Hidalgo. Pre-grade thesis, Universidad Michoacana de San Nicolás de Hidalgo, México.
Chen, H., Rangasamy, M., Tan, S., Wang, H., and Siegfried, B. (2010). Evaluation of five methods for total DNA extraction from western corn rootworm beetles. PLoS ONE. 5:e11963. doi: 10.1371/journal.pone.0011963
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Food and Agriculture Organization of The United Nations (FAO). (eds.). (1993). Manual para el Control de la Mosca del Gusano Barrenador del Ganado, Vol. 1, 2 (Roma, 1993). Available online at: http://www.rlc.fao.org/es/prioridades/transfron/miasis/pdf/doc2.pdf (Accessed July 18, 2013).
Food and Agriculture Organization of The United Nations (FAO). (eds.). (2011). La Cooperación Internacional en el Control, Erradicación y Prevención del Gusano Barrenador del Ganado (Roma, 2014). Available online at: http://www.fao.org/alc/file/media/pubs/2004/cooperagbg.pdf (Accessed February 2, 2014).
Forero, E., Cortés, J., and Villamil, L. (2007). Ecología y epidemiología del Gusano Barrenador del Ganado, Cochliomyia hominivorax (Coquerel, 1858). Rev. Med. Vet. 14, 37–49.
Forero, E., Cortés, J., and Villamil, L. (2008). Problemática del Gusano Barrenador del Ganado, Cochliomyia hominivorax (Coquerel, 1858). Rev. Med. Vet. Córdoba 13, 1400–1414.
Global Framework for the Progressive Control of Transboundary Animal Diseases (GF-TADs). (eds). (2007). Enfermedades Transfronterizas en las Américas (Panama, 2007). Available online at: http://www.rlc.fao.org/es/prioridades/transfron/gftads/webactividades.htm (Accessed March 15, 2014).
Golczer, G., and Arrivillaga, J. (2008). Modificación de un protocolo estándar de extracción de ADN para flebotominos pequeños (Phlebotominae: Lutzomyia). Rev. Colomb. Entomol. 34, 199–202.
Griffiths, A., Evans, L., and Stevens, J. (2009). Characterization and utilization of microsatellite loci in the New World screwworm fly, Cochliomyia hominivorax. Med. Vet. Entomol. 23(Suppl. 1), 8–13. doi: 10.1111/j.1365-2915.2008.00770.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Infante-Vargas, M., and Azeredo-Espin, A. (1995). Genetic variability in mitochondrial DNA of Cochliomyia hominivorax (Diptera: Calliphoridae) from Brazil. Biochem. Genet. 33, 237–256. doi: 10.1007/BF00553622
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Klassen, W., and Curtis, F. (2005). “History of the sterile insect technique,” in Rome: Sterile Insect Technique, eds V. Dyck, J. Hendrichs, and A. Robinson (Dordrecht: Springer), 3–36.
Linton, Y., Harbach, R., Seng, C., Anthony, T., and Matusop, A. (2001). Morphological and molecular identity of Anopheles (Cellia) sundaicus (Diptera: Culicidae), the nominotypical member of a malaria vector species complex in Southeast Asia. Syst. Entomol. 26, 357–366. doi: 10.1046/j.1365-3113.2001.00153.x
Lyra, M., Klaczko, L., and Azeredo-Espin, A. (2009). Complex patterns of genetic variability in populations of the New World screwworm fly revealed by mitochondrial DNA markers. Med. Vet. Entomol. 23(Suppl. 1), 32–42. doi: 10.1111/j.1365-2915.2008.00776.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
McDonagh, L., García, R., and Stevens, J. (2009). Phylogenetic analysis of New World screwworm fly, Cochliomyia hominivorax, suggests genetic isolation of some Caribbean island populations following colonization from South America. Med. Vet. Entomol. 23 (Suppl. 1), 14–22. doi: 10.1111/j.1365-2915.2008.00777.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Miño, G., Falconí, F., and Vinueza, R. (2005). Estudio Sobre la Presencia de Miasis (Gusaneras) en las Ganaderías del Ecuador. Quito: SESA, MAG, COMEXA.
Musapa, M., Kumwenda, T., Mkulama, M., Chishimba, S., Norris, D., Thuma, P., et al. (2013). A Simple Chelex Protocol for DNA Extraction from Anopheles spp. J. Vis. Exp. e3281. doi: 10.3791/3281
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Nagdev, K., Kashyap, R., Deshpande, P., Purohit, H., Taori, G., and Daginawala, H. (2010). Determination of polymerase chain reaction efficiency for diagnosis of tuberculous meningitis in Chelex-100® extracted DNA samples. Int. J. Tuberc. Lung Dis. 14, 1032–1038.
Robinson, A., Vreysen, M., Hendrichs, J., and Feldmann, U. (2009). Enabling technologies to improve area-wide integrated pest management programmes for the control of screwworms. Med. Vet. Entomol. 23(Suppl. 1), 1–7. doi: 10.1111/j.1365-2915.2008.00769.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Rodríguez, H., Rojas, F., Álvarez, L., and Parra, A. (2011). “Epidemiología y control de Cochliomyia hominivorax (Coquerel) gusano barrenador del ganado del nuevo mundo,” in Rome: Epidemiología de Enfermedades Parasitarias en Animales Domésticos, eds H. Quiroz, J. Figueroa, F. Ibarra, and M. López (México DF), 403–416.
Torres, T., and Azeredo-Espin, A. (2009). Population genetics of New World screwworm from the Caribbean: insights from microsatellite data. Med. Vet. Entomol. 23(Suppl. 1), 23–31. doi: 10.1111/j.1365-2915.2008.00786.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Walsh, P. S., Meltzger, D. A., and Higuchi, R. (1991). DNA extraction for PCR. BioTechniques 10, 506.
World Organisation for Animal Health (OIE). (eds.). (2013). Organización Mundial de Sanidad Animal (France, 2013). Available online at: http://www.oie.int/es/sanidad-animal-en-el-mundo/enfermedades-de-la-lista-de-la-oie-2013/ (Accessed 20 August 2013).
Wyss, J. H., and Galvin, T. J. (1996). Central America regional screwworm eradication program (benefit/cost study). Ann. N.Y. Acad. Sci. 791, 241–247. doi: 10.1111/j.1749-6632.1996.tb53531.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Keywords: Cochliomyia spp., DNA extraction, magnesium chloride
Citation: Echeverría-Fonseca G, Mera-Ruiz PA, Carrillo-Toro J and Rodriguez-Hidalgo R (2015) A new DNA extraction protocol for screwworm fly Cochliomyia species (Diptera: Calliphoridae). Front. Environ. Sci. 2:68. doi: 10.3389/fenvs.2014.00068
Received: 11 July 2014; Accepted: 23 December 2014;
Published online: 29 January 2015.
Edited by:
Thandavarayan Ramamurthy, National Institute of Cholera and Enteric Diseases, IndiaReviewed by:
Ashima Kushwaha Bhardwaj, Indian Institute of Advanced Research, IndiaM. Jahangir Alam, University of Houston College of Pharmacy, USA
Thandavarayan Ramamurthy, National Institute of Cholera and Enteric Diseases, India
Copyright © 2015 Echeverría-Fonseca, Mera-Ruiz, Carrillo-Toro and Rodriguez-Hidalgo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Richar Rodriguez-Hidalgo, Central University of Ecuador, Faculty of Veterinary Medicine, International Centre for Zoonoses, Av. America s/n., 170517 Quito, Ecuador e-mail: rrodriguez@uce.edu.ec
 Gustavo Echeverría-Fonseca
Gustavo Echeverría-Fonseca Patricia A. Mera-Ruiz
Patricia A. Mera-Ruiz Jenny Carrillo-Toro2
Jenny Carrillo-Toro2  Richar Rodriguez-Hidalgo
Richar Rodriguez-Hidalgo