El Niño-southern oscillations and lathyrism epidemics
- Neurology Unit, College of Medicine, University of Ibadan, Ibadan, Nigeria
Epidemics of lathyrism, a neurological syndrome of spastic paraparesis, have occurred during severe droughts in Europe, Asia, and Africa for millenia. Causation is linked to exposure to β-N-oxalyl-L-α,β-diaminopropionic acid (β-L-ODAP), a neurotoxin in Lathyrus sativus. Lathyrism shares neurological features with konzo, a syndrome of predominantly spastic paraparesis which occurs during droughts in East and Central Africa and is linked to El Niño activity. This study was done to determine the relationship of lathyrism epidemics to phases of El Niño-southern oscillation (ENSO) and Pacific decadal oscillation (PDO), and to propose a model to explain why the geospatial distributions of lathyrism and konzo are non-overlapping. Contingency table of phases of ENSO and occurrence of lathyrism epidemics in Central Provinces, India from 1833–1902 was created and odds ratio was calculated. Wavelet spectra of time series of annual occurrence of lathyrism in Rewah district, India, and its coherence with ENSO and PDO from 1894 to 1920 were performed. Lathyrism epidemic was associated with El Niño phase of ENSO, odds ratio 378 (95% 32–4475). Global spectra showed peaks at periodicities of 2.5 and 4.6 years for lathyrism; 2.7 and 5.0 years for PDO; and 2.5, 4.6, 7.0 years for ENSO. Spectrograms showed time-varying periodicities of 2.5–3.5 and 4.5–5.5 years for lathyrism; 2.0–3.0 and 6.5–9.0 years for ENSO; and 3.5 and 5.0 years for PDO, p < 0.0001. Spectral coherence were at 2.0–3.5 and 4.5–5.0 years for ENSO and lathyrism p < 0.0001, and 5.0 years for PDO and lathyrism p < 0.05. The droughts of El Niños initiate dependence on Lathyrus sativus, which exposes the population to neurotoxic β-L-ODAP. Public health control of lathyrism epidemics should include development of models to forecast El Niños and initiate food programmes in susceptible areas.
Introduction
Lathyrism is a neurological syndrome of spastic paraparesis, which usually develops in previously healthy subjects (Gopalan, 1950; Chaudhuri et al., 1963). Prodromal symptoms, which lasts 3–15 days, include cramps in the calves, tingling sensation in the legs, but onset is sudden in about 50% of cases (Chaudhuri et al., 1963). Time to maximum deficit may be as long as 2–7 months (Gopalan, 1950; Chaudhuri et al., 1963). Males are affected predominantly, but male to female ratio vary from 3:1 (Gopalan, 1950) to 8:1 (Acton, 1922). Mortality is not associated. Although historical records show that lathyrism was documented about 500 BC by Hippocrates, its epidemics in Europe and Asia in the 17th and 18th century brought the syndrome to clinical and political attention. It was documented in 1904 that “It was in Europe that the disease appears to have first attracted attention. Reference had been made by Hippocrates and Galen to the impotentia crurum of those who fed on what was called ervum, …” (Buchanan, 1904). While lathyrism epidemics occurred during droughts in France, Germany, Spain, India, and Algeria from early nineteenth century to early twentieth century, and in Bangladesh (Haque et al., 1994), Afghanistan (Arya et al., 1988), India (Khandare et al., 2014; Mishra et al., 2014), Nepal (Hamilton, 1978), and Ethiopia (Fikre et al., 2011) from the late twentieth century to early twenty-first century, the most systematic studies were carried out in India during the Colonial rule from 1833 to 1920. It was shown that lathyrism epidemics occurred during droughts when the population depended on Lathyrus sativus (grasspea), a drought resistant crop, for supply of calories (Buchanan, 1904). β-N-oxalyl-L-α,β-diaminopropionic acid (β-L-ODAP), an amino acid which is present in Lathyrus sativus, is the putative neurotoxin.
Lathyrism shares neurological features and risk factors with konzo (Tshala-Katumbay and Spencer, 2007), a neurological syndrome of acute or subacute onset spastic paraparesis (Howlett et al., 1990). Konzo epidemics occur in parts of East, Central, and Southern Africa where the population depends exclusively on foods from poorly processed cassava roots during droughts (World Health Organisation, 1982; Ministry of Health Mozambique, 1984; Tylleskär et al., 1991; Ciglenecki et al., 2011; Mlingi et al., 2011). Cassava (Manihot Esculenta Crantz), is a cyanogenic, drought resistant crop (de Tafur et al., 1997), which causes exposure to cyanide (Hernández et al., 1995; Oluwole et al., 2002). Lathyrism epidemics in East Africa is, however, limited to Ethiopia, a country where konzo does not occur. Konzo epidemics, which is more frequent during warm climate regimes, occur following El Niños, which induce severe droughts in East, Central, and Southern Africa (Nicholson and Kim, 1997; Caviedes, 2007). This study was done to determine the relationship of lathyrism epidemics to phases of the El Niño-southern oscillation (ENSO) and Pacific Decadal Oscillation (PDO), and to propose a model to explain the non-overlapping geospatial distribution of lathyrism and konzo.
Methods
Climate Indices Data
Paleoclimate ENSO multiproxy data from 1525–1982 (Braganza et al., 2009) was obtained from ftp://ftp.ncdc.noaa.gov/pub/data/paleo/contributions_by_author/braganza2009/braganza2009enso.txt. A Pacific Decadal Oscillation record since 1470 AD reconstructed from proxy data from 1470 to 1988 (Shen et al., 2006) was downloaded from the ftp://ftp.ncdc.noaa.gov/pub/data/paleo/historical/pacific/pdo-shen2006.txt. ENSO ranks from 1871 to 2005 were downloaded from http://www.esrl.noaa.gov/psd/enso/mei.ext/rank.ext.html.
Instrument based multivariate ENSO Index (MEI) data, which were derived from sea-level pressure, zonal and meridional components of the surface wind, sea surface temperature, surface air temperature, and total cloudiness fraction of the sky of the South Pacific Ocean, from 1950 to 2014 (Wolter and Timlin, 1998), were downloaded from the website of National Oceanic and Atmospheric Administration (NOAA), USA, http://www.esrl.noaa.gov/psd/data/correlation/mei.data. Instrument based Pacific Decadal Oscillation Index was downloaded from the JISAO's Arctic Oscillation website, http://jisao.washington.edu/pdo/PDO.latest.
Lathyrism Epidemic Data
Years of occurrence of lathyrism epidemics from the seventeenth to twenty-first century were obtained from the literature. Timeline of historical epidemics were drawn. The relationship of lathyrism epidemics of the late twentieth to early twenty-first century was established by determining the ranks of El Niños during the years of the epidemics. Epidemics of lathyrism were mapped to cycle plots of ENSO and PDO from 1800 to 1949 and 1950 to 2010.
Data of systematically documented lathyrism epidemics from 1933 to 1902 in the Central Provinces, India by Major Andrew Buchanan were analyzed (Sleeman, 1844; Buchanan, 1904). Each year was categorized as epidemic or no epidemic, and as El Niño or La Niña. Since it was documented in 1844 that before the lathyrism epidemic of 1833, food crops had failed in 1829–1831 due to droughts (Sleeman, 1844), the lag before epidemic could be up to 4 years after the onset of drought. The effects of El Niño, however, could last at least 2 years (Nicholson and Kim, 1997). To be conservative, only epidemics which occurred within 2 years of onset of El Niño were associated. Contingency table of phases of ENSO and lathyrism epidemics was created, and odds ratio was calculated.
Time Series of Lathyrism Cases and ENSO Data
Time series were fitted to annual cases of lathyrism in North Rewah, India from 1894 to 1920 (Acton, 1922), and to indices of ENSO and PDO from 1894 to 1920. Time domain analysis, using autocorrelation and partial autocorrelation tests, and lag plots, were done to exclude white noise. Stationarity was assessed using the unit root test.
Time-frequency domain analysis was performed using the wavelet transform. Wavelet analyses of indices of ENSO and PDO were done to describe their frequency-time spectra from 1800 to 1949. Wavelet transform of the time series of lathyrism cases, ENSO, and PDO data from 1896 to 1920 were done to describe their spectra, while cross-wavelet transform of lathyrism cases and ENSO, and lathyrism cases and PDO were done to determine their coherence. Wavelet methods were as described for epidemiological (Torrence and Compo, 1998; Grinsted et al., 2004), human (Issartel et al., 2014), and environmental data (Cazelles et al., 2007, 2014) time series. The Morlet wavelet (Torrence and Compo, 1998; Grinsted et al., 2004) was used to transform the time series to time-frequency. The Morlet wavelet was defined as
where ω0 is dimensionless frequency, and η is dimensionless time. The continuous wavelet transform of time series (xn, n = 1, …, N)
with uniform time steps δt, was defined as the convolution of xn with the scaled and normalized wavelet (Torrence and Compo, 1998; Grinsted et al., 2004). The cross wavelet transform (Torrence and Compo, 1998; Grinsted et al., 2004)
where Zv(p) was the confidence level associated with probability p, and Pxk and Pyk were the power spectra. The wavelet coherency phase was as defined (Torrence and Compo, 1998; Grinsted et al., 2004)
The global wavelet spectra, the equivalent of the Fourier power spectrum smoothed by the Morlet wavelet function (Farge, 1992; Grinsted et al., 2004) was
Significance was set at 0.01%. Phase plots were drawn to determine phase shift between the time series.
Statistics
Packages of the R Statistical Programming and Environment (R Core Team, 2015) were used for statistical analyses and graphics. Wavelet analyses were performed using the biwavelet package, and phase plots using the WaveletComp package.
Results
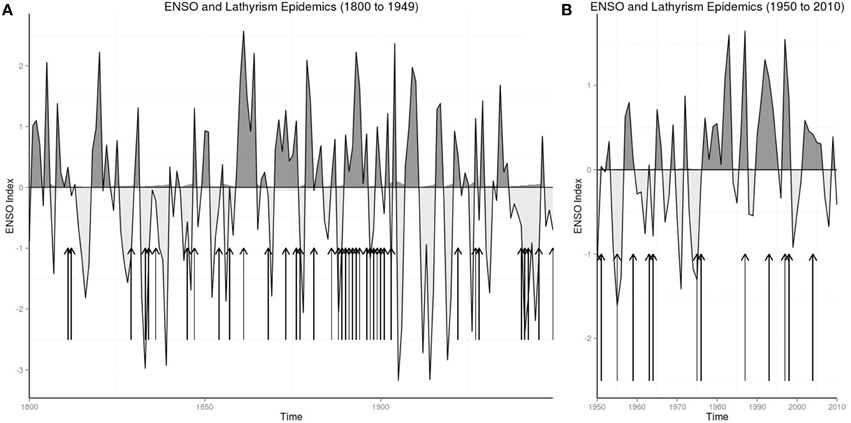
Figures 1A,B show the warm and cool phases of ENSO from 1800 to 1949 and 1950 to 2010, where arrows mapped years of lathyrism epidemics to phases of ENSO.
Historical Lathyrism Epidemics and EL Niño Ranks
The strength of El Niño from 1871 to 2005 were ranked 1–135. El Niño of 1939–1942, which ranked 132/135, was followed by the lathyrism epidemic of 1944–1945; the El Niño of 1976–1977, which ranked 115/135, was followed by the lathyrism epidemic of 1976–1977; the El Niño of 1994–1995, which ranked 124/135, was followed by the lathyrism epidemic of 1997; the El Niño of 1997–1998, which ranked 135/135, was followed by the lathyrism epidemic of 1998–2001. All the epidemics followed El Niños which ranked more than 85th percentile.
Time Series
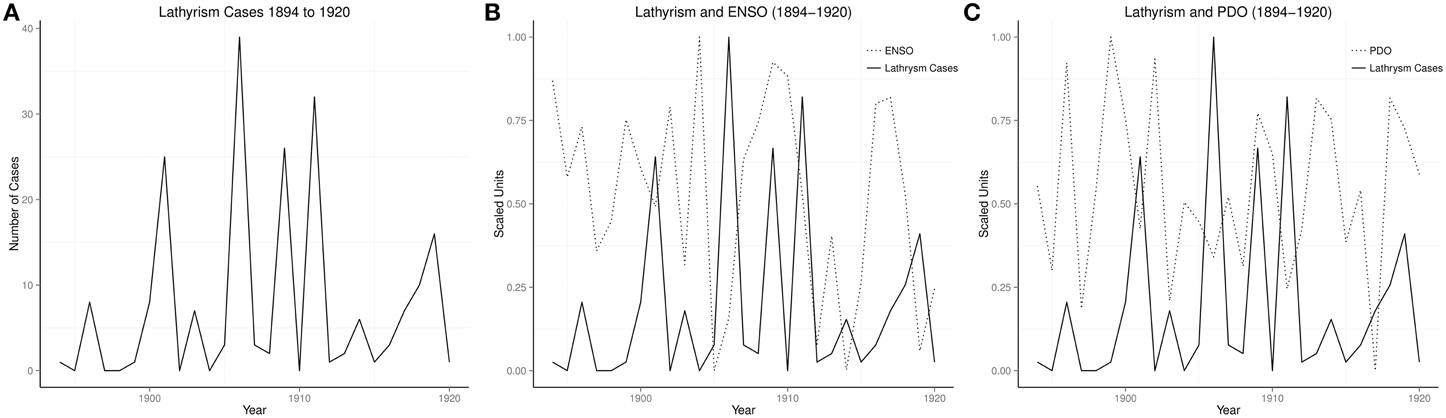
Of 70 years ENSO time series from 1933 to 1902, there were 56 cool phases and 14 warm phases, while there were 16 epidemics, 14 during warm phases, and 2 during cool phases, odds ratio 378 (95% 32–4475). The time series of cases of lathyrism from 1894 to 1920, and its relationship with ENSO and PDO are shown in (Figures 2A–C).
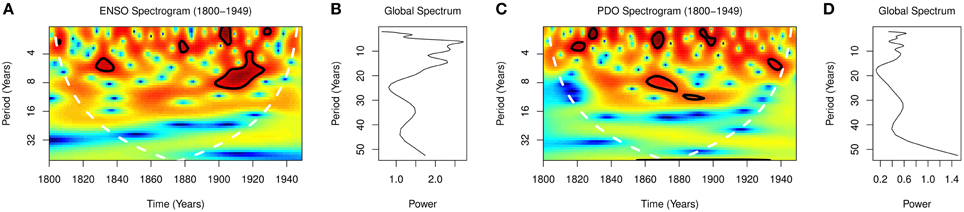
Global wavelet spectra from 1800 to 1949 showed peaks at periodicities of 2.0, 6.0, 9.0, 15.0, and 35.0 years for ENSO, and at 2.8, 8.0, 11.0, 32.0 years for PDO (Figures 3B,D). Spectograms from 1800 to 1949 showed significant time varying periodicities of 2.5–8.0 and 6.5–9.0 years for ENSO, p < 0.0001, and 3–12 years for PDO, p < 0.0001 (Figures 3A,C).
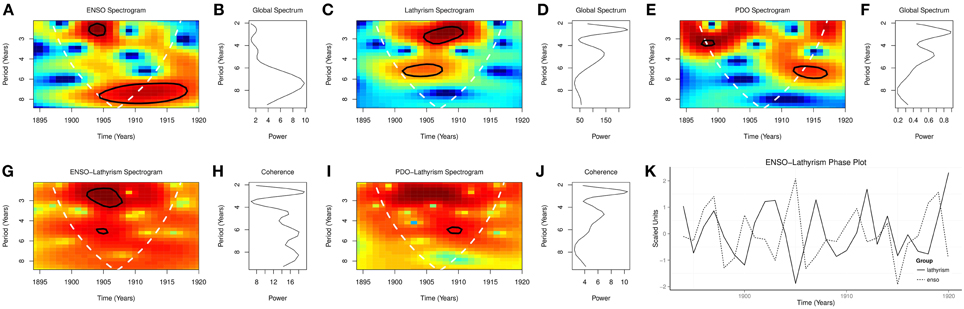
Global wavelet spectra from 1894 to 1920 showed peaks at periodicities of 2.5 and 4.6 years for lathyrism, at 2.5, 4.6, and 7.0 years for ENSO, and at 2.7, and 5.0 years for PDO (Figures 4B,D,F). Spectrograms from 1894 to 1920 showed significant time varying periodicities at 2.5–3.5 and 4.5–5.5 years for lathyrism, p < 0.0001, at 2.0–3.0 and 6.5–9.0 years for ENSO, p < 0.0001, and at 3.5 and 5.0 years for PDO, p < 0.0001 (Figures 4A,C,E). Coherence squared were at 2.5, 4.6, 6.0, 8.0 years for ENSO and lathyrism, but 2.6 and 4.6 years for PDO and lathyrism, (Figures 4H,J). Cross spectrogram showed significant coherence at 2.0–3.5 and 4.5–5.0 years for ENSO and lathyrism, p < 0.0001, and at 5.0 years for PDO and lathyrism p < 0.05 (Figures 4G,I). Phase plot showed that the rhythm of lathyrism lags that of ENSO (Figure 4K).
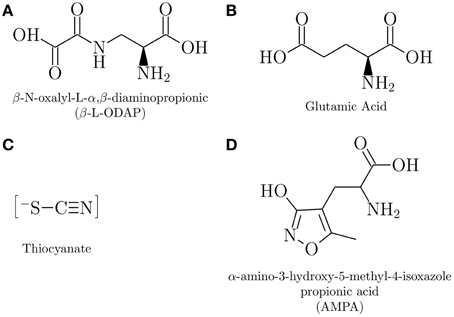
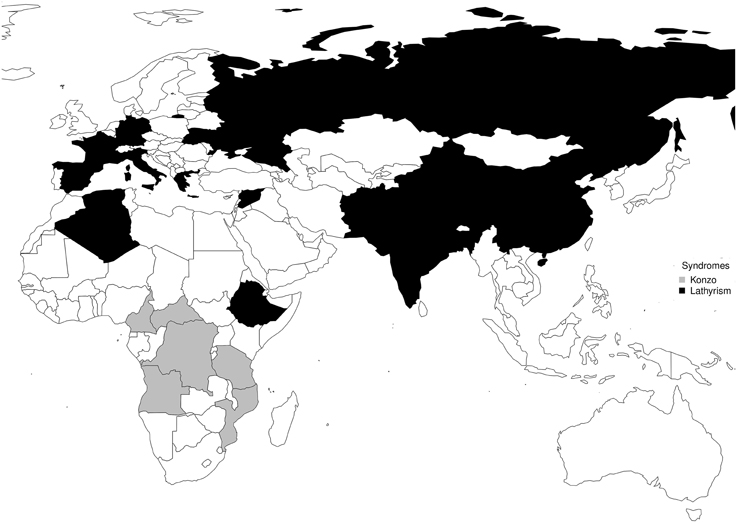
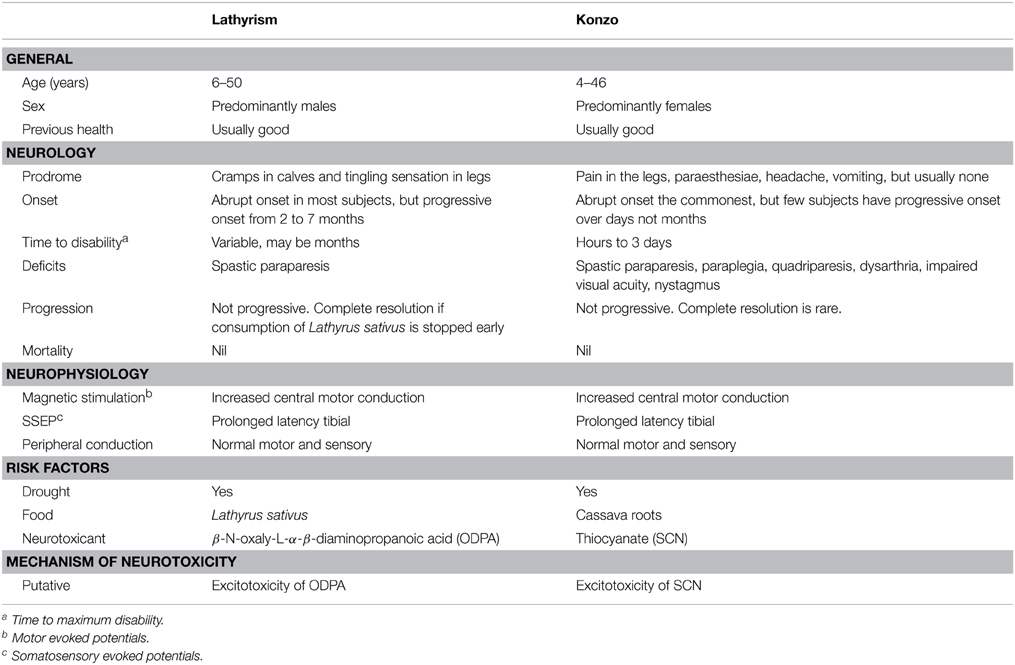
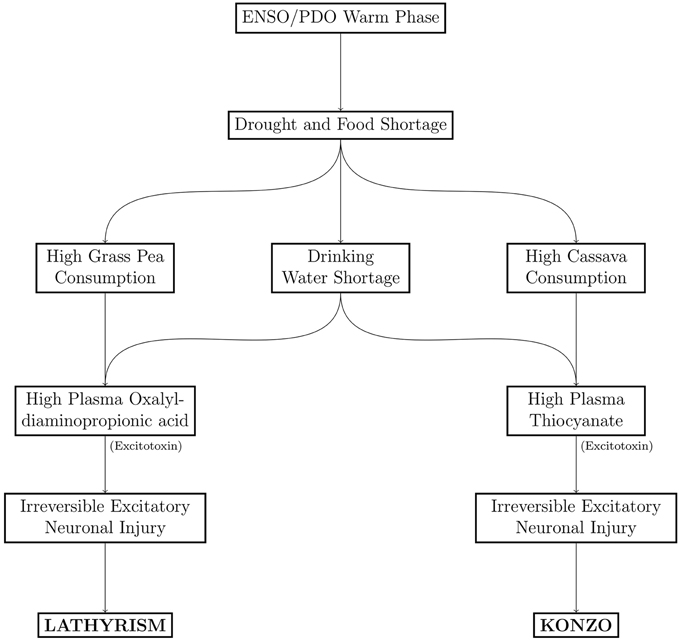
The timeline of notable epidemics of lathyrism is shown in Figure 5. The structures of compounds implicated in the causation of lathyrism and konzo are shown in Figures 6A–D. Figure 7 showed the map of countries where epidemics of lathyrism have occurred. Table 1 compares and contrasts the neurology and risk factors of lathyrism and konzo. The proposed model of causation geospatial occurrence of lathyrism and konzo is shown in Figure 8.
Discussion
The odds ratio of 378 shows the strong association of lathyrism epidemics in India and El Niño phase of the ENSO from 1833 to 1902. Graphical plot of historical lathyrism epidemics and ENSO shows that lathyrism epidemics occur following El Niños (Figure 1). The compelling account of Lt-Col Sleeman in 1844 (Sleeman, 1844) documents the observation of occurrence of lathyrism epidemics after periods of droughts, food shortages, and consumption of Lathyrus sativus. He wrote: “In 1829 the wheat and other spring crops in this and the surrounding villages were destroyed by a severe hail-storm; in 1830 they were deficient from the want of reasonable rains; and in 1831 they were destroyed by blight. … In 1831 they reaped a rich crop of Lathyrus sativus from the blighted wheat-fields, and subsisted upon its grain during that and the following years, giving the stalks and leaves only to their cattle. In 1833 the sad effects of this food began to manifest themselves. The younger part of the population of this and the surrounding villages, from the age of thirty downwards, began to be deprived of the use of their limbs below the waist by paralytic strokes, in all cases sudden, but in some cases more severe than in others” (Sleeman, 1844). This account, which shows lag of about 3 years between drought and food shortages and lathyrism epidemics, is consistent with the known duration of effects of El Niño activity (Nicholson and Kim, 1997), which has been linked to changes in the Indian monsoon (Mokhov et al., 2012). Thus, occurrence of lathyrism epidemics in India from 1833 to 1902 is attributable to El Niño activity.
The economic and health implications of severe droughts led Sir Gilbert Walker, the British Director of Meteorological Department in India, to investigate India monsoon changes in the 1920s (Walker and Bliss, 1928). Data from over 180 weather stations showed that values of sea level pressure, temperature, and precipitation alternate between west and east equatorial Pacific Ocean (Walker and Bliss, 1928). Low sea level pressure in east equatorial Pacific, but high sea pressure in west equatorial Pacific, or vice versa defines the southern oscillation, which is the basis for differences in precipitation between east and west Pacific (Walker and Bliss, 1928), and all parts of the planet through teleconnection. The Walker circulation, which was described in 1969 (Bjerknes, 1969), is the atmospheric component of ocean-atmosphere heat transfer. Under normal conditions the equitorial west pacific ocean is warmer than the equitorial east pacific ocean due to the presence of strong trade winds, which pool surface heat from east to west. Warm air rises from west Pacific Ocean, carries moisture, condenses, gives rain, blows eastwards, becomes dry and cool, and sinks in east Pacific Ocean (Bjerknes, 1969). The trade wind weakens periodically and warm waters of the western Pacific drift eastwards (Bjerknes, 1969). The appearance of warm water in east Pacific ocean is El Niño (Julian and Chervin, 1978; Caviedes, 2007), which is associated with increase precipitation in the Pacific basin, western South America, Central America, and the western half of North America, but with severe droughts in Indonesia, Australia, East and Southern Africa, and eastern parts of South America (Caviedes, 2007). Continued warming of eastern Pacific ocean is terminated by a negative feedback oscillator. La Niña occurs when the trade winds, equatorial easterlies, are very strong and move surface water westward and make the sea level temperature of the east Pacific abnormally cool (Philander, 1985). Thus, inter-annual variations of climate of all parts of the planet are attributable to changes in sea level pressure and temperature of equatorial Pacific Ocean, which is the largest ocean on the planet.
ENSO varies predominantly on interannual, but also on decadal and multidecadal timescales (Wang and Ropelewski, 1985; An and Wang, 2000). The periodicities of variations of the ENSO was 3–4 years between 1872 and 1910, 5–7 years between 1911 and 1960, and 5 years between 1970 and 1972 (Torrence and Compo, 1998; Torrence and Webster, 1999). The presence of multiple rhythms in the spectra of the ENSO is shown for the period 1800–1949, where the global spectra show peaks at 2.0, 6.0, 9.0, 15.0, and 35.0 years (Figure 3B). The time varying nature of the rhythms of the ENSO, which is shown in the spectrogram of Figure 3A, illustrates the unpredictability of occurrence of El Niños and La Niñas. Thus, the time varying nature of ENSO explains the irregularity of occurrence of severe droughts.
Climate regimes are defined by phases of the PDO, which persist much longer than that of the ENSO. Sir Gilbert Walker also described the North Pacific Oscillation (NPO), which is the oscillation of sea surface pressure and temperature between Hawaii and Alaska (Walker and Bliss, 1928). Southern oscillation was described to have negative influence on NPO simultaneously and 6 months in advance (Walker and Bliss, 1928). Extensive investigations of anomalies of sea surface temperature and pressure of the North Pacific Ocean in the late 1990s showed oscillation at interannual and decadal timescales, but predominantly at decadal timescale now known as Pacific Decadal Oscillation (Mantua et al., 1997). When the Pacific Coast of western North America and Gulf of Alaska is warm the eastern and central North Pacific between 30° and 50° N latitude is cool (Mantua et al., 1997). Climate regime is defined by the characteristic behavior of a natural phenomenon like sea level pressure, temperature or recruitment over time (Hare and Mantua, 2000). When the PDO is warm the climate regime is warm, and when it is cool the climate regime is cool. El Niños dominate warm climate regimes, while La Niñas, the cool phase of ENSO, dominate cool climate regimes (Christy et al., 2001). Thus, changes in frequency of El Niños and droughts are attributable to the teleconnections of the PDO.
Climate regime shift is defined as an abrupt change of a regime (Hare and Mantua, 2000). Such shifts from warm to cool modes occurred in 1890 and lasted until 1924, and in 1947 and lasted until 1976, while shifts from cool to warm occurred in 1925 and lasted until 1946, and in 1976 and lasted until 2000 (Peterson and Schwing, 2003; Christensen et al., 2013). PDO also oscillate at multidecadal timescales, in addition to decadal and inter-annual timescales. The presence of peaks at periodicities of 2.8, 8.0, 11.0, and 32.0 years in the global spectra from 1800 to 1949 shows that the PDO oscillate at short and long periodicities (Figure 3D). The time-varying nature of the oscillations, which are shown in the spectrogram (Figure 3C), illustrates while climate regimes are irregular. Thus, both ENSO and PDO oscillate at decadal and shorter time scales.
Time series of occurrence of lathyrism from 1894 to 1920, which show rhythmic changes, indicate that occurrence of lathyrism is not random (Figure 2A). The time series of lathyrism, which covary with ENSO and PDO, indicate the presence of relationship between the three variables (Figures 2B,C). Presence of multiple peaks in the global spectra of lathyrism cases, ENSO, and PDO from 1894 to 1920 indicate that multiple rhythms are present in these variables (Figures 4B,D,F). The spectrograms, which show that these rhythms are time-varying, agree with the irregular occurrence of El Niños and lathyrism epidemics (Figures 4A,C,E). Coherence squared show that lathyrism, ENSO, PDO have rhythms of similar periodicities (Figures 4H,J). The highly significant spectra coherence confirms the strong association of variations of the ENSO and lathyrism epidemics, while the phase plot shows that lathyrism epidemics lag the ENSO (Figure 4K). Thus, occurrence of lathyrism epidemics from 1894–1920 is attributable to El Niño activity.
It is known that El Niño strength varies from weak, moderate, strong, to very strong (Quinn and Neal, 1987, 1992). Notable lathyrism epidemics since the mid-1940s occurred following strong El Niños. The lathyrism epidemic of 1944–1945 in Bhopal State, Central India (Shourie, 1945) followed the El Niño of 1939–1942, which ranked 132/135. The lathyrism epidemic of 1976–1977 in northern Ethiopia (Gebreab et al., 1978; Haimanot et al., 1990) followed the El Niño of 1976–1977, which ranked 115/135. The lathyrism epidemic of 1997 (Getahun et al., 1999) followed the El Niño of 1994–1995, which ranked 124/135, while the 1998–2001 lathyrism epidemic in northern Ethiopia (Haimanot and Lambein, 2005) followed the El Niño of 1997–1998, which ranked 135/135. Thus, all lathyrism epidemics of the past 70 years followed strong El Niños.
The first documentation of causal link of Lathyrus sativus and paralysis is credited to the edit of the Grand Duke of Wurtemberg in 1671, which prohibited its use for bread (Buchanan, 1904) (Figure 5). Similar edicts in 1705 and 1714 (Buchanan, 1904; Sleeman, 1844) show the strength of conviction of the association. Lathyrus sativus is a legume that is grown for human and livestock feeds. Although it can be boiled and consumed, it is usually milled to flour to make meals, baked to cakes or bread, added to rice, or mixed with other flours (Campbell, 1997). The β-L-ODAP concentrations in one collection of Lathyrus sativus varied from 0.22 to 7.20 g/kg (Campbell, 1997). Toxicity, however, depends on prolonged monotonous consumption rather than on acute exposures. As documented by Major Andrew Buchannan “The disease varies in severity according to the length of time during which the grain was consumed, to the quantity that was consumed daily, to the age and sex of the persons affected” (Buchanan, 1904). Case control study shows that consumption of Lathyrus sativus is associated with lathyrism (Getahun et al., 2002, 2005). It is noteworthy that prohibition of consumption of Lathyrus sativus has been unsuccessful, and the crop is still cultivated and consumed in Asia (Khandare et al., 2014), Europe, North and East Africa, North and South America, and the Middle East (Campbell, 1997). Thus, the risk of exposure during severe droughts remain particularly in Asia and Africa, but not in areas where it is consumed sparingly and considered a source of nitric oxide (NO) from its high homoarginine content (Rao, 2011).
β-L-ODAP (Figure 6A), a structural analog of glutamate (Figure 6B) (Hugon et al., 2000), was extracted from lathyrus sativus in 1964 (Rao et al., 1964). Glutamate is a natural occurring excitatory amino acid neurotransmitter in the central nervous system (Olney and Ludolph, 2000). β-L-ODAP is a potent agonist (Bridges et al., 1989) at α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) glutamate receptor subtype (Figure 6D). Feeding experiments with Lathyrus sativus induced paraparesis in chickens (Sharan, 1973), in rats (Geiger et al., 1933), and in ascorbic acid deficient guinea pigs and primates (Jahan and Ahmad, 1993). Fortification of Lathyrus sativus flour with graded doses of β-L-ODAP induced dose dependent spastic motor changes in monkeys (Spencer et al., 1986). Intraventricular injection of β-L-ODAP suggest that it induces non-NMDA receptor mediator excitotoxicity (Ross and Spencer, 1987). In addition to exposure to β-L-ODAP and possible deficiences of vitamins as the major risk factors for lathyrism (Enneking, 2011), deficiency of methionine, a sulfur containing amino acid, has also been suggested as risk factor (Getahun et al., 2005; Kusama-Eguchi et al., 2011). Although factors that make only a subset of exposed subjects develop lathyrism are not clearly worked out, and induction of central nervous system lesions with β-L-ODAP is not consistently reproducible (Singh and Rao, 2013), β-L-ODAP remains the most likely causative agent of lathyrism.
Other theories of causation of lathyrism that were considered in the late nineteenth century and early twentieth century include the sun, rain, and wind (Buchanan, 1904). The wind theory, which was proposed by locals in India, was documented as “It is remarkable thing that even in the places in which lathyrism is most prevalent many people will be found who are firmly convinced that the disease is caused by the wind. In Hoshangabad people thought that the cold wind from the river Nerbudda was the cause. In Jubbulpore it was thought that the injurious wind blew along the valey of Bohriban. In Saugor it was said that a very cold wind blows along the eastern border” (Buchanan, 1904). Although all these theories have no direct link with occurrence, they suggest the link of occurrence to climate.
Unlike lathyrism, which has one putative neurotoxicant, several putative neurotoxicants have been investigated for konzo. Linamarin (Sreeja et al., 2003), the cyanogenic glycoside in cassava, cyanohydrin (Soler-Martin et al., 2010), the breakdown product of linamarin, cyanide (Kimani et al., 2014), which is released from cyanohydrin, and several metabolites of cyanide, which include cyanate (Tor-Agbidye et al., 1999; Kimani et al., 2014), and iminothiazolidine-4-carboxylic acid (Bitner et al., 1995) have been investigated in experimental studies, but none have induced lesions consistent konzo. Thiocyanate (SCN−), the major metabolite of cyanide, is considered the most likely neurotoxicant of konzo (Spencer, 1999; Oluwole, 2015) (Figure 6C). SCN−, a pseudohalide has 730 times more affinity than Cl− in physiological assays (van Dalen et al., 1997). Since SCN− increases the affinity of AMPA receptors for AMPA 10- to 30-folds (Arai et al., 1995) it is a likely candidate to induce the neuronal lesions of konzo. Thus, both putative neurotoxicants of lathyrism and konzo act through excitatory mechanisms.
Although spastic paraparesis is common to both lathyrism and konzo, there are clinical and non-clinical differences between the two (Table 1). The time to maximum deficit in konzo ranges from hours to about 3 days (Tylleskär et al., 1991), but maximum deficit may take weeks or months to develop in lathyrism. The neurological features of konzo are not limited to paraparesis, but include quadriparesis, nystagmus, impaired vision, and dysarthria (Ministry of Health Mozambique, 1984), which are not features of lathyrism. Konzo subjects are predominantly < 15 years of age, unlike lathyrism with older subjects (Ministry of Health Mozambique, 1984; Howlett et al., 1992). Females predominate in konzo (Ministry of Health Mozambique, 1984; Howlett et al., 1992), while males predominate in lathyrism (Buchanan, 1932). Thus, the lesions of konzo are more extensive than lathyrism, and they differ in age and sex distribution.
The geospatial distribution of lathyrism and konzo in non-overlapping areas during severe droughts can be attributed to differences in the foods which the population depends for supply of calories (Figure 7). Lathyrism occurs in areas where the population depends solely on Lathyrus sativus, while konzo occurs where the population depends solely on cassava. The proposed model to explain occurrence of either lathyrism or konzo is shown in Figure 8. Strong El Niño induces severe drought and food shortages. Populations that depend on cassava are exposed to cyanide and its metabolites, while populations that depend on Lathyrus sativus are exposed to β-L-ODAP.
Conclusions
Lathyrism has been associated with severe droughts and prolonged consumption of Lathyrus sativus for more than 150 years. Although recurrent epidemics of lathyrism and severe droughts in India led to the discovery of ENSO, the association of the two have not been investigated. ENSO has teleconnections with Indian monsoon and other monsoons around the world. Occurrence of historical lathyrism epidemics following strong El Niños, the very high odds ratio of association of lathyrism epidemics and El Niño phase of ENSO, and the spectra coherence of time series of ENSO and annual cases of lathyrism indicate that El Niño phase of ENSO is the determinant of lathyrism epidemics. Lathyrism occurs where lathyrus sativus is the dominant food during drought, while konzo occurs where cassava is the dominant food. Cassava (Oluwole, 2015) and lathyrus sativus (Singh and Rao, 2013) can, however, be save foods that will continue to contribute to sustenance of millions without neurological syndromes. Prevention of lathyrism and konzo during droughts requires food programmes for susceptible populations. Forecast models of possible epidemics are, however, crucial to realize the public health objectives.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Acton, H. W. (1922). An investigation into the causation of lathyrism in man. Indian Med. Gaz. 57, 241–247.
An, S., and Wang, B. (2000). Interdecadal change of the structure of the ENSO mode and its impact on the ENSO frequency. J. Clim. 13, 2044–2055. doi: 10.1175/1520-0442(2000)013<2044:ICOTSO>2.0.CO;2
Arai, A., Silberg, J., Kessler, M., and Lynch, G. (1995). Effect of thiocyanate on AMPA receptor mediated responses in excised patches and hippocampal slices. Neuroscience 66, 815–827. doi: 10.1016/0306-4522(94)00616-D
Arya, L. S., Qureshi, M. A., Jabor, A., and Singh, M. (1988). Lathyrism in Afghanistan. Indian J. Pediatr. 55, 440–442. doi: 10.1007/BF02810373
Bitner, R. S., Kanthasamy, A., Isom, G. E., and Yim, G. K. W. (1995). Seizures and selective CA-1 hippocampal lesions induced by an excitotoxic cyanide metabolite, 2-iminothiazolidine-4-carboxylic acid. Neurotoxicology 16, 115–122.
Bjerknes, J. (1969). Atmospheric teleconnections from the equatorial Pacific. Mon. Weather Rev. 97, 163–172. doi: 10.1175/1520-0493(1969)097<0163:ATFTEP>2.3.CO;2
Braganza, K., Gergis, J. L., Power, S. B., Risbey, J. S., and Fowler, A. M. (2009). A multiproxy index of the El Niño-southern oscillation, A.D. 1525–1982. J. Geophys. Res. Atm. 114:D05106. doi: 10.1029/2008JD010896
Bridges, R. J., Stevens, D. R., Kahle, J. S., Nunn, P. B., Kadri, M., and Cotman, C. W. (1989). Structure-function studies on N-oxalyl-diamino-dicarboxylic acids and excitatory amino acid receptors: evidence that beta-L-ODAP is a selective non-NMDA agonist. J. Neurosci. 9, 2073–2079.
Buchanan, A. (1904). Report on Lathyrism in the Central Provinces in 1896–1902. Nagpur: Albert Press.
Buchanan, J. C. R. (1932). “Chachaleh” a common disease in British Somaliland, and its relation to tropical deficiency diseases. Trans. R. Soc. Trop. Med. Hyg. XXV, 383–397. doi: 10.1016/S0035-9203(32)90158-8
Campbell, C. G. (1997). Grass Pea. Lathyrus Sativus L. Promoting the Conservation and Use of Underutilized and Neglected Crops. Rome: Institute of Plant Genetics; Crop Plant Research; Gatersleben/International Plant Genetic Resources Institute.
Caviedes, C. N. (2007). “Impacts of El Niño-Southern Oscillation on natural and human systems,” in Physical Geography of South America, eds T. T. Veblen, K. R. Young, and A. R. Orme (New York, NY: Oxford University Press), 305–321.
Cazelles, B., Chavez, M., de Magny, G. C., Guégan, J. F., and Hales, S. (2007). Time-dependent spectral analysis of epidemiological time-series with wavelets. J. R. Soc. Interface 4, 625–636. doi: 10.1098/rsif.2007.0212
Cazelles, B., Cazelles, K., and Chavez, M. (2014). Wavelet analysis in ecology and epidemiology: impact of statistical tests. J. R. Soc. Interface 11:20130585. doi: 10.1098/rsif.2013.0585
Chaudhuri, R. N., Chhetri, M. K., Saha, T. K., and Mitra, P. P. (1963). Lathyrism: a clinical and epidemiological study. J. Indian Med. Assoc. 41, 169–173.
Christensen, J. H., Kumar, K. K., Aldrian, E., An, S. I., Cavalcanti, I. F. A., de Castro, M., et al. (2013). “Climate phenomena and their relevance for future regional climate change,” in The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, eds T. F. Stocker, D. Qin, D. M. PlQin, M. Tignor, S. K. Allen, J. Boschung, et al. (Cambridge, UK: Cambridge University Press), 1217–1308.
Christy, J. R., Clarke, R. A., Gruza, G. V., Jouzel, J., Mann, M. E., Oerlemans, J., et al. (2001). “Observed climate variabibity and change,” in Climate Change 2001: The Scientific Basis. Contribution of Working Group 1 to the Third Assessment Report of the Intergovernmental Panel on Climate Change, eds J. T. Houghton, Y. Ding, D. J. Griggs, M. Noguer, P. J. van der Linden, X. Dai, et al. (Cambridge, UK: Cambridge University Press), 99–181.
Ciglenecki, I., Eyema, R., Kabanda, C., Taafo, F., Mekaoui, H., and Urbaniak, V. (2011). Konzo outbreak among refugees from Central African Republic in Eastern region, Cameroon. Food Chem. Toxicol. 49, 579–582. doi: 10.1016/j.fct.2010.05.081
de Tafur, S. M., El-Sharkawy, M. A., and Calle, F. (1997). Photosynthesis and yield performance of cassava in seasonally dry and semiarid environments. Photosynthetica 33, 249–257. doi: 10.1023/A:1022116414969
Enneking, D. (2011). The nutritive value of grasspea (Lathyrus sativus) and allied species, their toxicity to animals and the role of malnutrition in neurolathyrism. Food Chem. Toxicol. 49, 694–709. doi: 10.1016/j.fct.2010.11.029
Farge, M. (1992). Wavelet transforms and their applications to turbulence. Annu. Rev. Fluid Mech. 24, 395–457. doi: 10.1146/annurev.fl.24.010192.002143
Fikre, A., Van Moorhem, M., Ahmed, S., Lambein, F., and Gheysen, G. (2011). Studies on neurolathyrism in Ethiopia: dietary habits, perception of risks and prevention. Food Chem. Toxicol. 49, 678–684. doi: 10.1016/j.fct.2010.09.035
Gebreab, T., Wolde, G. Z., Ahmed, Z., Ayele, T., and Fanta, H. (1978). Neurolathyrism–a review and a report of an epidemic. Ethiop. Med. J. 16, 1–11.
Getahun, H., Mekonnen, A., TekleHaimanot, R., and Lambein, F. (1999). Epidemic of neurolathyrism in Ethiopia. Lancet 354, 306–307. doi: 10.1016/S0140-6736(99)02532-5
Getahun, H., Lambein, F., Vanhoorne, M., and Van der Stuyft, P. (2002). Pattern and associated factors of the neurolathyrism epidemic in Ethiopia. Trop. Med. Int. Health 7, 118–124. doi: 10.1046/j.1365-3156.2002.00836.x
Getahun, H., Lambein, F., Vanhoorne, M., and Van der Stuyft, P. (2005). Neurolathyrism risk depends on type of grass pea preparation and on mixing with cereals and antioxidants. Trop. Med. Int. Health 10, 169–178. doi: 10.1111/j.1365-3156.2004.01370.x
Gopalan, C. (1950). The lathyrism syndrome. Trans. R. Soc. Trop. Med. Hyg. 44, 333–338. doi: 10.1016/0035-9203(50)90061-7
Grinsted, A., Moore, J. C., and Jevrejeva, S. (2004). Application of the cross wavelet transform and wavelet coherence to geophysical time series. Nonlinear Proc. Geoph. 11, 561–566. doi: 10.5194/npg-11-561-2004
Haimanot, R. T., Kidane, Y., Wuhib, E., Kalissa, A., Alemu, T., Zein, Z. A., et al. (1990). Lathyrism in rural northwestern Ethiopia: a highly prevalent neurotoxic disorder. Int. J. Epidemiol. 19, 664–672. doi: 10.1093/ije/19.3.664
Haimanot, R. T. A., and Lambein, F. (2005). Is lathyrism still endemic in Northern Ethiopia? The case of Legambo Woreda (district) in the Southe Wollo Zone, Amhara National Regional State. Ethiop. J. Health Dev. 19, 230–236. doi: 10.4314/ejhd.v19i3.10003
Hamilton, D. (1978). Some experience with paraplegia in a small hospital in Nepal. Paraplegia 15, 293–301. doi: 10.1038/sc.1977.44
Haque, A., Hossain, M., Khan, J. K., Kuo, Y. H., Lambein, F., and De Reuck, J. (1994). New findings and symptomatic treatment for neurolathyrism, a motor neuron disease occurring in north west Bangladesh. Paraplegia 32, 193–195. doi: 10.1038/sc.1994.35
Hare, S. R., and Mantua, N. J. (2000). Empirical evidence for North Pacific regime shifts in 1977 and 1989. Prog. Oceanogr. 47, 103–145. doi: 10.1016/S0079-6611(00)00033-1
Hernández, T., Lundquist, P., Oliveira, L., Pérez Cristiá, R., Rodriguez, E., and Rosling, H. (1995). Fate in humans of dietary intake of cyanogenic glycosides from roots of sweet cassava consumed in Cuba. Nat. Toxins 3, 114–117. doi: 10.1002/nt.2620030210
Howlett, W. P., Brubaker, G., Mlingi, N., and Rosling, H. (1992). A geographical cluster of konzo in Tanzania. J. Trop. Geogr. Neurol. 2, 102–108.
Howlett, W. P., Brubaker, G. R., Mlingi, N., and Rosling, H. (1990). Konzo, an epidemic upper motor neuron disease studied in Tanzania. Brain 113, 223–235. doi: 10.1093/brain/113.1.223
Hugon, J., Ludolph, A. C., and Spencer, P. S. (2000). “β-N-Oxalylamino-L-alanine,” in Experimental and Clinical Neurotoxicology, 2nd Edn., eds P. S. Spencer and H. H. Schaumburg (New York, NY: Oxford), 929–938.
Issartel, J., Bardainne, T., Gaillot, P., and Marin, L. (2014). The relevance of the cross-wavelet transform in the analysis of human interaction - a tutorial. Front. Psychol. 5:1566. doi: 10.3389/fpsyg.2014.01566
Jahan, K., and Ahmad, K. (1993). Studies on neurolathyrism. Environ. Res. 60, 259–266. doi: 10.1006/enrs.1993.1035
Julian, P. R., and Chervin, R. M. (1978). A study of the southern oscillation and Walker circulation Phenomenon. Mon. Weather Rev. 106, 1433–1451. doi: 10.1175/1520-0493(1978)106<1433:ASOTSO>2.0.CO;2
Khandare, A. L., Babu, J. J., Ankulu, M., Aparna, N., and Shirfule, A, Rao, G. S. (2014). Grass pea consumption & present scenario of neurolathyrism in Maharashtra State of India. Indian J. Med. Res. 140, 96–101.
Kimani, S., Moterroso, V., Morales, P., Wagner, J., Kipruto, S., Bukachi, F., et al. (2014). Cross-species and tissue variations in cyanide detoxification rates in rodents and non-human primates on protein-restricted diet. Food Chem. Toxicol. 66, 203–209. doi: 10.1016/j.fct.2014.01.047
Kusama-Eguchi, K., Yoshino, N., Minoura, A., Watanabe, K., Kusama, T., Lambein, F., et al. (2011). Sulfur amino acids deficiency caused by grass pea diet plays an important role in the toxicity of L-β-ODAP by increasing the oxidative stress: studies on a motor neuron cell line. Food Chem. Toxicol. 49, 636–643. doi: 10.1016/j.fct.2010.07.049
Mantua, N. J., Hare, S. R., Zhang, Y., Wallace, J. M., and Francis, R. C. (1997). A Pacific Interdecadal climate oscillation with impacts on salmon production. Bull. Am. Meteorol. Soc. 78, 1069–1079. doi: 10.1175/1520-0477(1997)078<1069:APICOW>2.0.CO;2
Ministry of Health Mozambique. (1984). Mantakassa: an epidemic of spastic paraparesis associated with chronic cyanide intoxication in a cassava staple area in Mozambique. 1. Epidemiology and clinical and laboratory findings in patients. Bull. World Health Organ. 62, 477–484.
Mishra, V. N., Tripathi, C. B., Kumar, A., Nandmer, V., Ansari, A. Z., Kumar, B., et al. (2014). Lathyrism: has the scenario changed in 2013? Neurol Res. 36, 38–40. doi: 10.1179/1743132813Y.0000000258
Mlingi, N. L. V., Nkya, S., Tatala, S. R., Rashid, S., and Bradbury, J. H. (2011). Recurrence of konzo in southern Tanzania: rehabilitation and prevention using the wetting method. Food Chem. Toxicol. 49, 673–677. doi: 10.1016/j.fct.2010.09.017
Mokhov, I. I., Smirnov, D. A., Nakonechny, P. I., Kozlenko, S. S., and Kurths, J. (2012). Relationship between El-Niño/Southern Oscillation and the Indian monsoon. Atmos. Oceanic Phys. 48, 47–56. doi: 10.1134/S0001433812010082
Nicholson, S. E., and Kim, J. (1997). The relationship of the El Niño-Southern Oscillation to African rainfall. Int. J. Climatol. 17, 117–135. doi: 10.1002/(SICI)1097-0088(199702)17:2<117::AID-JOC84>3.0.CO;2-O
Olney, J. W., and Ludolph, A. C. (2000). “Glutamic acid,” in Experimental and Clinical Neurotoxicology, 2nd Edn., eds P. S. Spencer and H. H. Schaumburg (New York, NY: Oxford), 604–609.
Oluwole, O. S. A. (2015). Cyclical konzo epidemics and climate variability. Ann. Neurol. 77, 371–380. doi: 10.1002/ana.24334
Oluwole, O. S. A. (2015). Global cassava food supply and occurrence of ataxic polyneuropathy and konzo. Eur. J. Nutr. Food Saf. 5, 138–149. doi: 10.9734/EJNFS/2015/11453
Oluwole, O. S. A., Onabolu, A. O., and Sowunmi, A. (2002). Exposure to cyanide following a meal of cassava food. Toxicol. Lett. 135, 19–23. doi: 10.1016/S0378-4274(02)00232-1
Peterson, W. T., and Schwing, F. B. (2003). A new climate regime in the northeast Pacific ecosytem. Geophys. Res. Lett. 30, 1–4. doi: 10.1029/2003GL017528
Philander, S. G. H. (1985). El Niño and La Niña. J. Atmos. Sci. 42, 2652–2662. doi: 10.1175/1520-0469(1985)042<2652:ENALN>2.0.CO;2
Quinn, W. H., and Neal, V. T. (1987). El Niño occurrence over the past four and a half centuries. J. Geophys. Res. 92, 14449–14461. doi: 10.1029/JC092iC13p14449
Quinn, W. H., and Neal, V. T. (1992). “The historical record of El Nino events,” in Climate Since A.D. 1500, eds R. S. Bradley and P. D. Jones (London: Routledge), 623–648.
R Core Team. (2015). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Rao, S. L., Adiga, P. R., and Sarma, P. S. (1964). The isolation and characterisation of beta-N-oxalyl-L-alpha,beta-diaminopropionic acid: a neurotoxin from the seeds of Lathyrus sativus. Biochemistry 3, 432–436. doi: 10.1021/bi00891a022
Rao, S. L. N. (2011). A look at the brighter facets of β-N-oxalyl-l-α,β-diaminopropionic acid, homoarginine and the grass pea. Food Chem. Toxicol. 49, 620–622. doi: 10.1016/j.fct.2010.06.054
Ross, S. M., and Spencer, P. S. (1987). Specific antagonism of behavioral action of “uncommon” amino acids linked to motor-system diseases. Synapse 1, 248–253. doi: 10.1002/syn.890010305
Sharan, R. K. (1973). Experimental neurolathyrism in chicks. Paraplegia 10, 249–255. doi: 10.1038/sc.1973.47
Shen, C., Wang, W. C., Gong, W., and Hao, Z. (2006). A Pacific Decadal Oscillation record since 1470 AD reconstructed from proxy data of summer rainfall over eastern China. Geophys. Res. Lett. 33:L03702. doi: 10.1029/2005GL024804
Singh, S. S., and Rao, S. L. N. (2013). Lessons from neurolathyrism: a disease of the past & the future of Lathyrus sativus (Khesari dal). Indian J. Med. Res. 138, 32–37.
Sleeman, W. (1844). Rambles and Recollections of an Indian Official. London: Oxford University Press.
Soler-Martin, C., Riera, J., Seoane, A., Cutillas, B., Ambrosio, S., Boadas-Vaello, P., et al. (2010). The targets of acetone cyanohydrin neurotoxicity in the rat are not the ones expected in an animal model of konzo. Neurotoxicol. Teratol. 32, 289–294. doi: 10.1016/j.ntt.2009.11.001
Spencer, P. S. (1999). Food toxins, AMPA receptors, and motor neuron diseases. Drug Metab. Rev. 31, 561–587. doi: 10.1081/DMR-100101936
Spencer, P. S., Roy, D. N., Ludolph, A., Hugon, J., Dwivedi, M. P., and Schaumburg, H. H. (1986). Lathyrism: evidence for role of the neuroexcitatory aminoacid BOAA. Lancet ii, 1066–1067. doi: 10.1016/S0140-6736(86)90468-X
Sreeja, V. G., Nagahara, N., Li, Q., and Minami, M. (2003). New aspects in pathogenesis of konzo: neural cell damage directly caused by linamarin contained in cassava (Manihot esculent Crantz). Br. J. Nutr. 90, 467–472. doi: 10.1079/BJN2003902
Tor-Agbidye, J., Palmer, V. S., Spencer, P. S., Craig, A. M., Blythe, L. L., and Sabri, M. I. (1999). Sodium cyanate alters glutathione homeostasis in rodent brain: relationship to neurodegenerative diseases in protein-deficient malnourished populations in Africa. Brain Res. 820, 12–19. doi: 10.1016/S0006-8993(98)01343-2
Torrence, C., and Compo, G. P. (1998). A Practical guide to wavelet analysis. Bull. Amer. Meteor. Soc. 79, 61–78. doi: 10.1175/1520-0477(1998)079<0061:APGTWA>2.0.CO;2
Torrence, C., and Webster, P. J. (1999). Interdecadal changes in the ENSO-monsoon system. J. Clim. 12, 2679–2690. doi: 10.1175/1520-0442(1999)012<2679:ICITEM>2.0.CO;2
Tshala-Katumbay, D., and Spencer, P. S. (2007). “Toxic disorders of the upper motor neuron system,” in Handbook of Clinical Neurology, Vol. 82, eds A. A. Eisen and P. J. Shaw (Amsterdam: Elsevier Science Publishers), 361–370.
Tylleskär, T., Banea, M., Bikangi, N., Fresco, L., Persson, L. A., Rosling, H., et al. (1991). Epidemiological evidence from Zaire for a dietary etiology of Konzo, an upper motor neuron disease. Bull. World Health Organ. 69, 581–589.
van Dalen, C. J., Whitehouse, M. W., Winterbourn, C. C., and Kettle, A. J. (1997). Thiocyanate and chloride as competing substrates for myeloperoxidase. Biochem. J. 327, 487–492. doi: 10.1042/bj3270487
Wang, X. L., and Ropelewski, C. F. (1985). An asessment of ENSO-Scale secular variability. J. Clim. 8, 1584–1599. doi: 10.1175/1520-0442(1995)008<1584:AAOESS>2.0.CO;2
Keywords: lathyrism, konzo, El Niño, drought, climate change
Citation: Oluwole OSA (2015) El Niño-southern oscillations and lathyrism epidemics. Front. Environ. Sci. 3:60. doi: 10.3389/fenvs.2015.00060
Received: 08 July 2015; Accepted: 18 August 2015;
Published: 04 September 2015.
Edited by:
Yuhua Duan, US Department of Energy-National Energy Technology Laboratory, USAReviewed by:
Ju-Guang Han, University of Science and Technology of China, ChinaTianzhou Wu, Centers for Disease Control and Prevention, USA
Copyright © 2015 Oluwole. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Olusegun S. A. Oluwole, Neurology Unit, College of Medicine, University of Ibadan, Rm. 319, Clinical Sciences Building, Ibadan 200212, Nigeria, osaoluwole@hotmail.com
 Olusegun S. A. Oluwole
Olusegun S. A. Oluwole