Biophysical and Biochemical Markers of Metal/Metalloid-Impacts in Salt Marsh Halophytes and Their Implications
- 1Centre for Environmental and Marine Studies and Department of Chemistry, University of Aveiro, Aveiro, Portugal
- 2Faculty of Sciences, Marine and Environmental Sciences Centre, University of Lisbon, Lisbon, Portugal
- 3UR: MaNE, Faculté des Sciences de Bizerte, Université de Carthage, Bizerte, Tunisie
As a major sink, estuarine/salt marsh ecosystem can receive discharges laden with myriads of contaminants including metals/metalloids from man-made activities. Two among the major consequences of metal/metalloid-exposure in estuarine/salt marsh ecosystem flora such as halophytic plants are: (a) the excessive accumulation of light energy that in turn leads to severe impairments in the photosystem II (PS II), and (b) metal/metalloids-accrued elevation in the cellular reactive oxygen species (ROS) that causes imbalance in the cellular redox homeostasis. On one hand, plants adopt several strategies to dissipate excessive energy hence eventually to avoid damage in the PS II and maintain optimum photosynthesis. On the other hand, components of the cellular redox system quickly respond to metal/metalloid-exposure, where plants try to maintain a fine-tuning among these components, and tightly control the level of ROS and its potential consequences. Herein, major insights into, and the significance and implications of important biophysical and biochemical markers in metal/metalloid-exposed halophytes are overviewed and also highlighted main aspects so far least explored in the present context. Discussion advocates to regularly monitor and integrate studies on the highlighted herein biophysical and biochemical markers taking into account the missing aspects such as essential and non-essential metal/metalloid-speciation, -availability, and -methylation, role of the obvious microbial activities, and a comparative account of the outcomes of the studies on mixture of metal/metalloid performed in laboratory and field conditions. Thus, consideration of these missing aspects in future studies on the subject can help us to: (a) unveil the status of the metal/metalloid-contamination and -impact; (b) understand adaptive responses of salt marsh halophyte to metals/metalloids, and also (c) to devise sustainable strategies for the environmental or ecosystem management and safety.
Background
Impacts of about 40% of the world population living in 60 km radius of a coastal area can be obvious on the nearby ecosystem (WHO, 2005). This is particularly in the case of highly urbanized estuaries, where the anthropogenic pressure has higher impacts. The anthropogenic load of trace elements in coastal ecosystems is one of the most important sources of allochthonous matter into the oceans, throughout river discharge in the estuarine ocean-river mixing zone. As a consequence of these mixing processes, estuaries are often considered as filters of the river signal inputs into the ocean (Zhou et al., 1998). Salt marshes are natural deposits of metals/metalloids in the estuarine system (Williams et al., 1994; Doyle and Otte, 1997). When located near polluted areas, these ecosystems receive large amounts of chemical pollutants from industrial and urban wastes that either drifts downstream within the river flow or waste dumping from the near industrial and urban areas (Reboreda and Caçador, 2007; Anjum et al., 2014a). The misconceived idea that estuaries had the ability to dilute and disperse pollutants led to urban and industrial discharges into estuarine waters without pre-treatment of wastes (Pedro et al., 2015). Once entered into salt marshes metals/metalloids can spread along with the tides and periodic floods and also interact with soil-sediment and the biotic community as well (Suntornvongsagul et al., 2007). Most salt marsh plants accumulate large amounts of metals/metalloids in their aerial and belowground organs (Caçador et al., 1996). Although they are subjected to all these anthropogenic impacts, salt marshes have great ecological value for the ecosystem, namely in nutrient regeneration, primary production, habitat for wildlife species and as shoreline stabilizers (Anjum et al., 2014a). Their important role has been recently admitted also by the inclusion of these ecosystems in the Water Framework Directive (WFD) highlighting the establishment of monitoring programs to transitional and coastal waters in order to assess their ecological and chemical status (Best et al., 2007). Despite, the fact that some salt marsh halophyte species can withstand some degree of metal/metalloid contamination, excessive concentration of metals/metalloids in the soil, driven from long-term accumulation or toxic discharges, can not only cause damages toward the plants, but also be potentially harmful to human health, through food chain (Santos et al., 2014). Besides these facts, halophytes inhabit naturally harsh environments subjected to a variety of stresses like flooding, and excessive salinities, and metal/metalloid levels (Duarte et al., 2013a, 2014; Anjum et al., 2014a). Previous facts together are indicative of a strong stress-adaptive potential of halophytes. Notably, severe impairments in the photosystem II (PS II) can be one of the first impacts (at biophysical level) caused by metal/metalloid-accrued elevations in the accumulation of light energy (Duarte et al., 2014, 2015, in press; Santos et al., 2014). On the other hand, at biochemical level, metal/metalloid-accrued elevation in reactive oxygen species (ROS) in halophytic plant cells can cause imbalance in cellular redox homeostasis (Pietrini et al., 2003; Schroder et al., 2009; Gill and Tuteja, 2010; Li et al., 2011; Anjum et al., 2012a,b, 2014a,b,c, 2015a; Santos et al., 2014, 2015). Despite above facts, discussion is meager in literature on the significance and important implications of major biophysical and biochemical markers in halophytes exposed to rapidly increasing metal/metalloid pollution.
Herein, major insights into and the significance and implications of significant biophysical and biochemical markers in metal/metalloid-exposed halophytes are overviewed, and main points so far unexplored in the current context are briefly highlighted.
Biophysical Markers—Role and Implication in Metal/Metalloid-Exposed Halophytes
As any other kind of stress, any adverse conditions to which halophytes are exposed have its more evident expression on the plant primary productivity (Duarte et al., 2013a, 2014; Santos et al., 2014). The impacts of metal stress on halophytes are no exception and can thus be assessed at the primary productivity basis, the light harvesting mechanisms. Pulse Modulated Amplitude (PAM) Fluorescence arises as a highly efficient non-invasive tool to address any failure or disturbance on the electronic energy transduction pathway. This technique evaluates the photonic energy capture processes and transformation to electronic energy throughout the assessment of the chlorophyll (Chl) fluorescence signals. Thus, any disturbance at the primary productivity level can be efficiently assessed by this technique.
One of the most common techniques is the Saturating Pulse technique, where a high-energy pulse hits the photosystem II (PS II) and its fluorescence eco is captured, reflecting the PS II quantum efficiency on its more general aspect. For example, in Juncus acutus exposed to increased doses of Zn (Figure 1), shows an evident pattern of PS II Quantum efficiency decrease characteristic of a Zn toxicity situation (Santos et al., 2014). This was also reported in non-halophytic species by Monnet et al. (2001) as consequence of the antennae pigments destruction due to excessive heavy metal accumulation, disturbing maximum PS II photochemistry. This decrease in PS II quantum yields is mostly due to a decrease in basal fluorescence, F0, and can be interpreted as a clear reflection of changes in thylakoid structure on the PS II electron donor sites (Skorzynska-Polit and Baszynski, 1997). This reduction suggests a change in thylakoids membrane structure, inevitably affecting electron transport, and/or leading to the destruction of Chl pigment (Vaillant et al., 2005).
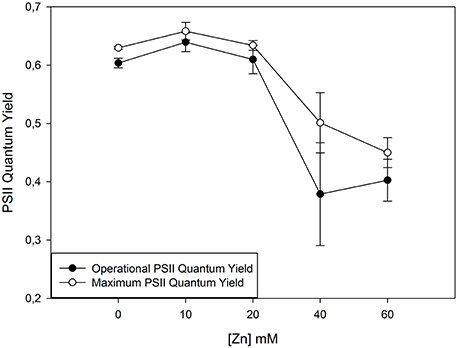
Figure 1. Photosystem II (PS II) quantum efficiencies in normal light conditions (operational) and in optimum conditions (maximum) when all the PS II reaction centres are available for capturing light energy in Juncus accutus. Data is presented as average ± standard deviation. The concept of this figure is based on Santos et al. (2014).
The electronic transport chain (ETC) appears this way as another efficient approach while addressing metal/metalloid stress in halophytes. When subjected to stress plants can suffer damages at the ETC. A stressed plant is not able to deal with the same amount of light energy as a healthy plant and thus the assessment of the ETC efficiency at under a light gradient reveals to be an efficient tool while evaluating the possible toxic effects derived from metal/metalloid exposure. Moreover, this approach is also provided of an ecological meaning. Exposing the plants to different light levels provides valuable insights on the plant ability to survival under a normal light environment to which is normally exposed. Observing a halophyte grass (Spartina patens) exposed to different doses of Cu this becomes evident (Figure 2). Not only the control individuals present higher electron transport rates (ETR) at all the exposed light levels, but also these healthy individuals can withstand higher light levels before reaching a saturation point. When exposed to excessive Cu concentrations these individuals chloroplasts decrease their ETR, producing lower amounts of chemical energy, being photo-inhibited at low photosynthetic active radiation (PAR) levels. At photo-inhibitory PAR values all PS II centers are saturated and thus the plant enters into a state of energy burst that has to be released, for example throughout respiration or heat dissipation. This has inevitable consequences at the photosynthetic efficiency, reducing the balance between produced and dissipated energy. Although, heavy metals can cause the arresting of Chl synthesis, plastoquinone, and carotenoids, it also restricts the PS II related electron transport, due to structural and functional changes in thylakoid membranes, reduced activity of ferrodoxin NADP+ oxireductase and arrested plastoquinone synthesis (Baszynki et al., 1980; Barceló et al., 1988; Krupa and Baszynski, 1995). This electron obstruction has inevitable effects on the photosynthetic efficiency itself and on the maximum capacity to transport electrons (ETRmax) throughout the ETC.
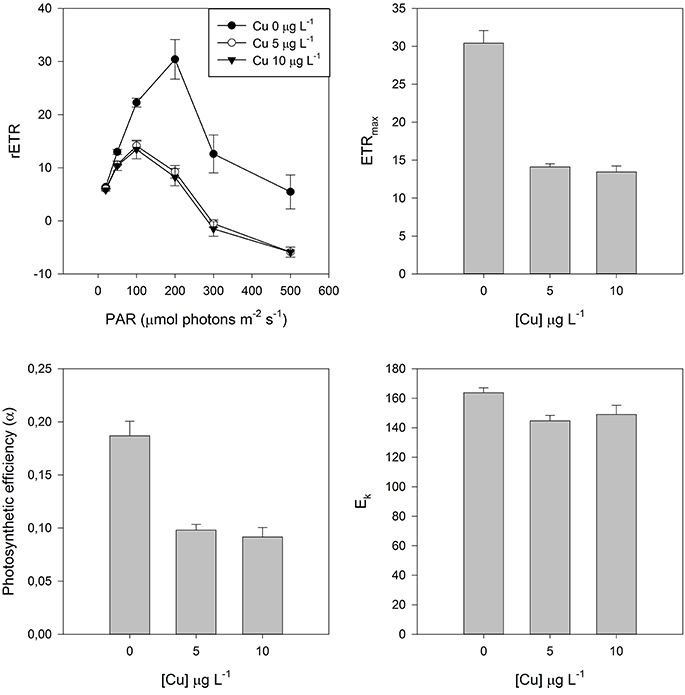
Figure 2. Rapid Light Curves (RLC) and derived parameters in dark adapted leaves of Spartina patens exposed to increasing copper (Cu) doses. Data is presented as average ± standard deviation.
Again this points out to the final outcome of metal/metalloid stress, leaving several doubts on the causes behind these effects. For example, this photosynthetic efficiency decrease as result of lower ETRs can be caused either by disruptions at the energy reception or at its transduction. Although, important from the physiological and ecological points of view, these techniques leaves some questions unanswered, like whether there are or not impacts at the PS II structure or in the energetic balance between the PS II and the PS I. For this a deeper approach proved to be valuable.
When photosynthetic samples, kept in darkness (e.g., for 10 min) are illuminated, Chl a fluorescence intensity shows characteristic changes called fluorescence induction, fluorescence transient, or simply the Kautsky effect, named after Hans Kautsky (1891–1966; Stribet and Govindjee 2011). This transient has inflection points: the fast phase is labeled as OJIP, where O corresponds to the basal fluorescence F0, the first measured minimal level, J and I are intermediate levels, and P is the peak corresponding to the dark-adapted maximum fluorescence or FM. Fluorescence changes during the fast part of the can be primarily correlated with the events taking place in the course of successive reduction of the electron acceptors of the entire photosynthetic electron transport chain. Thus, using this methodology the entire process since photon capture, energy transduction, and dissipation can be evaluated as well as its intervenient (quinones, photosystems donor, and acceptor sides, PS II antennae). This proved to be very useful to evaluate the effects of stress conditions on plant productivity giving important insights on its effects at the energetic transduction level (Duarte et al., 2014, 2015, in press; Santos et al., 2014). This is even evident on a first look to the Kautsky curves resulting from the transient analysis (Figure 3). In this case, Tamarix gallica individuals were exposed to 500 mM of As and of Al. Compared to the control there is a generalized decrease in the fluorescence signal in all the phases of the Kautsky curves in individuals exposed to Al and more severely to As. This already points out to several problems within the energy transduction pathway. The decomposition of these curves can reveal where heavy metals are impairing this energetic pathway.
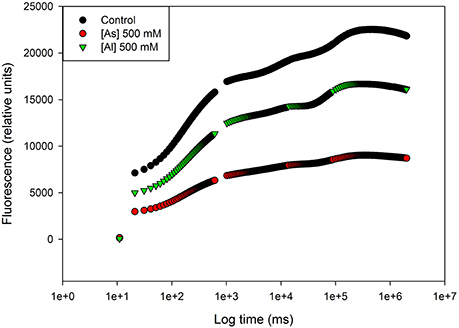
Figure 3. Kautsky curves in dark adapted leaves of Tamarix gallica exposed to arsenic (As) and aluminum (Al; average). The concept of this figure is based on Sghaier et al. (2015).
On the basis of the energetic transduction pathway are four simple phenomological processes expressed on a leaf cross-section basis (CS). When a photon hits the PS II reaction centers (RC) it is absorbed (ABS) and trapped into the PS II (TR). This photon will generate an electronic transport (ET) throughout the ETC hitting the end acceptors or can be dissipated (DI) in the form of heat or fluorescence. If we take a good look to these four simple fluxes, we can already detect where the effects of the heavy metals are being felt. One of two things can happen. For example, in J. accutus exposed to increasing concentrations of Zn there is a decrease in the absorbed light energy, and consequently in all the ongoing processes, without severe changes in the number of RCs available to capture light (Santos et al., 2014; Figure 4). Another possible impact is the observed for e.g., while exposing S. patens to Cu. In this case there is an evident increase in the energy absorption as well as in its entrapment within the RCs, without a concomitant increase in the electron transport leading, to an accumulation of excessive energy that has to be dissipated in order to prevent the PS II burnout. This is now known to be a counteractive measure to an inefficient energy processing, in order to increase the energetic balance toward the electron transport, in stressed and less efficient systems (Duarte et al., 2014). The increase in the ABS/RC is normally associated to low energy processing levels (Strasser et al., 2004; Panda et al., 2008). Both outcomes have its origin in the same process. Like in several other cases, a cell under stress cannot cope with the same amounts of energy as a healthy cell. This leads inevitably to an accumulation of large amounts of energy that, as stated before, can destroy the D1 protein, impairing all photochemical machinery (Demmig-Adams and Adams, 1992; Qiu et al., 2003; Duarte et al., 2013a).
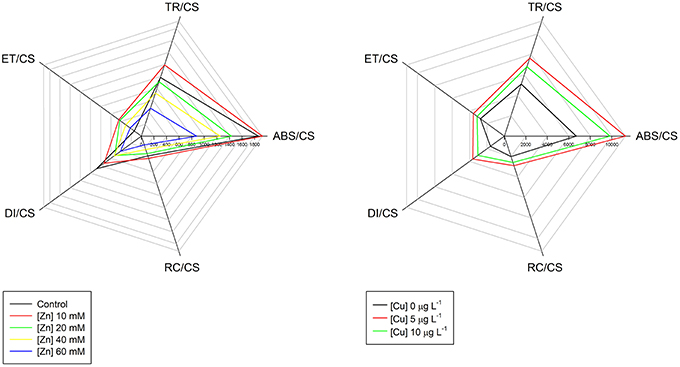
Figure 4. Phenomological energy processes in Juncus accutus exposed to increased doses of zinc (Zn) and in Spartina patens exposed to increased doses of copper (Cu). The concept of this figure is based on Santos et al. (2014).
Nevertheless, this is still a very narrow view of the energy transduction pathway, without uncovering the molecular reasons behind metal-induced photoinhibition. Let's take as an example a halophyte exposed to increasing doses of Pb. Figure 5 shows the typical and more often outcomes derived from heavy metal stress at the PS II and ETC structural level. Not only electron transport is processed at lower rates in Pb-stressed plants, but also the size of quinone pools (directly proportional to the area above the Kautsky curve) is severely reduced at the PS II acceptor side (Strasser et al., 1995; Joliot and Joliot, 2002). Alongside this reduction in the oxidized quinone pool available to be reduced, there can be also observed a significant increase in the damage at the oxygen evolving complexes (OECs) level at the PS II. The appearance of an intermediate K-step in stressed individuals is exclusively related to damage to the oxygen-evolving complex of PS II (Srivastava et al., 1997). Strasser et al. (2000) found that altering the OEC enables alternative internal electron donors to donate electrons to PS II creating a short-lived increase in the fraction of Pheo− and QA−, which causes an increase to the K fluorescence step in the 300 ms range, measurable as an increase in its amplitude (Wk). Krüger et al. (2014) suggests that this K-band indicates accessibility of internal non-water electron donors to PS II (e.g., ascorbate red) competing with the OEC. The OJIP-test also provides means to calculate the overall grouping probability (PG) or connectivity between PS II antennae. This parameter accounts for all energetic communication pathways between neighbor PS II antennae (Strasser and Stirbet, 2001; Panda et al., 2006), being inversely proportional to the health state of this connectivity. There is a gradual loss of connectivity between PS II antennae units, being more evident at the highest Zn concentrations, directly related to lacks of energetic connectivity between the two PS II subunits and therefore impairing energetic transport (Strasser and Stirbet, 2001). All these structural impairments have as inevitable consequences a reduction in the electronic flow and thus in the energy production.
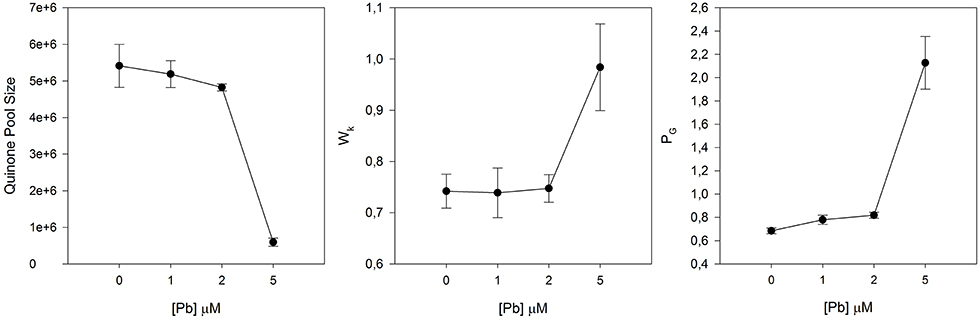
Figure 5. Quinone pool size, oxygen evolving complexes (OECs) damage (Wk), and Grouping probability (PG) in Juncus accutus exposed to increased doses of lead (Pb). Data is presented as average ± standard deviation.
By affecting the structure of the PS II and its connection ends with the PS I, metal stress will eventually affect both the balance between the light and dark reactions but also the energetic balance between the PSI and the PS II (Figure 6). In this case Cd appears to shift the equilibrium between PS toward the PS I, by reducing the PS II efficiency instead of an increase in the PS I efficiency. Samson et al. (1988) suggests that the inhibitory effect of Cd on PS II activity seems to be located on its oxidizing side, probably because Cd inactivates some PS II reactions centers (Cid et al., 1995). With this, both the light and dark reactions occurring in the PS II are dramatically reduced, impairing both the contribution of the light reaction to the primary photochemistry and the quinone restoring reaction occurring in the dark.
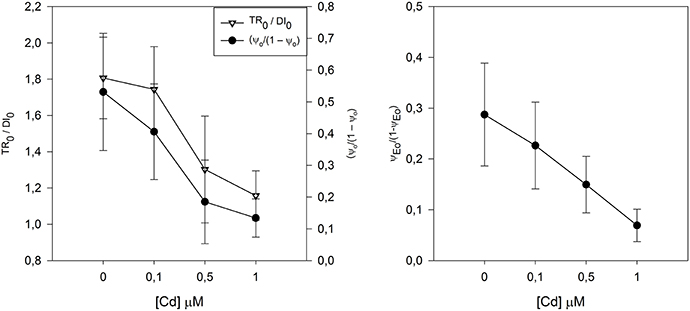
Figure 6. Contribution of the light reactions (TR0/DI0) and dark reactions (ψo/(1 – ψo)) and correspondent redox reactions between PS II and PSI equilibrium constant (ψEo/(1 − ψEo)), in Juncus accutus exposed to increased doses of cadmium (Cd). Data is presented as average ± standard deviation. The concept of this figure is based on Santos et al. (2014).
Another common features derived from excessive metal/metalloid within the chloroplast are structural modifications of the Chl pigment. Prasad and Strzalka (1999) suggested that the substitution of Mg by Zn, Cu, and Cd might also damage the Chl synthesis system, leading to the accumulation of Chl degradation products like, pheophythins. Recently, Küpper et al. (2007) developed a method that allows to accurately determining heavy metal-substituted Chl concentrations. Overlooking the data obtained throughout this technique (Figure 7), it can be observed an evident substitution of the Mg atom in plants exposed to Zn, Cu, and Cd, with a consequent decrease in the functional Mg-Chl concentration, by heavy metal-induced Mg replacement. Hence, the formation of minor proportions of heavy metal-substituted Chl relative to the total Chl may already inhibit photosynthesis completely, affecting the overall cell bioenergetics and redox homeostasis (Küpper et al., 2002). This substitution effect is rather well known and was already described in algae (Küpper et al., 1996) and in higher plants (Ngo and Zhao, 2007; Santos et al., 2014, 2015).
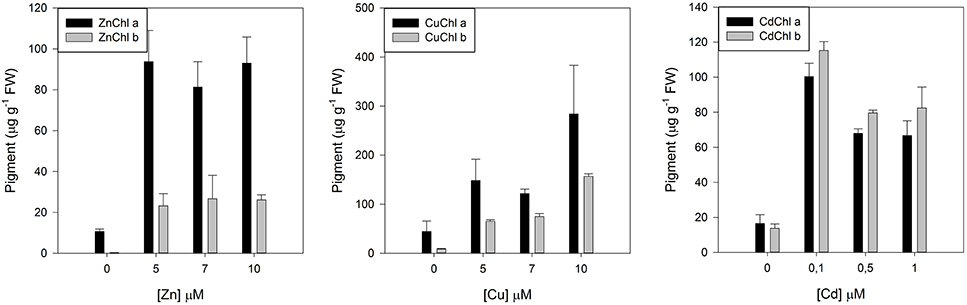
Figure 7. Heavy metal substituted chlorophylls in Juncus accutus plants exposed to increasing concentrations of zinc (Zn), copper (Cu), and cadmium (Cd). Data is presented as average ± standard deviation. The concept of this figure is based on Santos et al. (2014, 2015).
On the other hand, the interaction of Chl with the ROS generated due to heavy metal stress, namely 1O2, results in the formation of Chl allomers (Hynninen, 1991; Wang et al., 2000). In vitro, it is inhibited by the presence of β-carotene (Hynninen, 1991), which is believed to primarily scavenge 1O2 (Bumann and Oesterhelt, 1995). This conformational change of the Chl molecule has already been identified as one of the causes of heavy metal stress (Küpper et al., 1996). Taking as an example J. accutus plants exposed to an increasing Cu gradient there is an evident allomerization effect of the Chl molecule (Figure 8). In this case the antennal Chl is unable to pass on excitation energy to similarly energized neighbors.
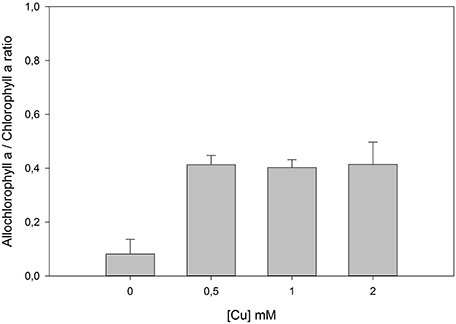
Figure 8. Ratio between forms of allomerized and normal chlorophyll a in Juncus accutus plants exposed to increasing concentrations of copper (Cu). Data is presented as average ± standard deviation.
This lack of signal transduction either by changes and impairments in the photosystems and in the ETC or by structural modifications of the Chl, have as primary and more evident consequence an accumulation of excessive reducing power inside the chloroplast. The non-dissipation of this excessive energy can, in extreme cases, lead to the destruction of D1 protein (Rintamäki et al., 1995), and thus stop irreversibly all PS II activity. This energy dissipation can be accomplished by the activation of what is known as the xanthophyll cycle. When this cycle is activated there is an increased de-epoxidation of the luminal violaxanthin pool toward zeanxanthin, decreasing this way the overload of the photosynthetic light harvesting apparatus, by dissipating the elevated reducing power accumulated within the stroma (Demmig-Adams, 1990). Although, the high amounts of energy dissipated in plants exposed to Zn (Figure 4), there was no activation of the xanthophyll cycle as it can be observed by the reduced de-epoxidation state index (DES, Figure 9). On the other hand, these same plants, presented an evident increase in the concentration of auroxanthin (Figure 9), a violaxanthin analog (Wentworth et al., 2000). Auroxanthin is a C5,8 epoxy carotenoid that has previously been shown to enhance aggregation-associated quenching in isolated LHC IIb (Ruban et al., 1998). The effectiveness of auroxanthin has been suggested to come from the fact that its S1 energy level is higher than that of violaxanthin (Wentworth et al., 2000). In fact, this C5,8 epoxide is in the basis of the its higher efficiency, while compared to zeaxanthin, and thus an efficient energy quencher under stress conditions (Ruban et al., 1998; Horton et al., 1999). Inevitably these physiological changes at the biophysical level of the photosystems have impacts arresting the primary productivity of the individuals, in ecological terms this has a far greater impact. Metal/metalloid contamination induces damages at the photosystem level that can go from a simple slow-down of the photosynthetic rates to a complete arrest of the energy transduction process, both ways this implies a lower primary productivity and thus a lower carbon harvesting. In a time of global changes, this acquires a reinforced importance, and halophytes inhabit one of the most productive ecosystems of the planet (Caçador et al., 2015). At the interspecific level, this also implies that plants with different tolerance degrees will respond differently to metal-metalloid stress; and thus, will have their ecological fitness reduced, changing the landscape of the ecosystem by simple competition and reduction of the weaker/less tolerant species. Apart from these ecological consequences, the abovementioned biophysical implications can be for certain an added value tool as non-invasive sentinels of the ecosystem health and/or as ecotoxicological tools for preventive tests using model plants.
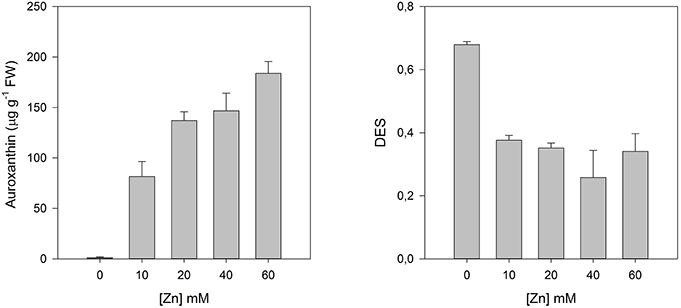
Figure 9. Auroxanthin contents and de-epoxidation state (DES) index in Juncus accutus plants exposed to increasing concentrations of copper (Cu). Data is presented as average ± standard deviation. The concept of this figure is based on Santos et al. (2014).
Biochemical Markers—Role and Implication in Metal/Metalloid-Exposed Halophytes
Several biochemical markers of plant tolerance to a range of abiotic stress factors have been reported to have information those can explain abiotic stress-impact on plant growth, development, productivity, and stress-protective mechanisms (Gill and Tuteja, 2010; Yildiz et al., 2010). Markers of both (oxidative)stress [reactive oxygen species (ROS) and their consequences such as oxidation of proteins, membrane lipids, nucleic acids] and defense (enzymatic and non-enzymatic antioxidants) are among major biochemical markers enlightening plant health under stressful conditions (Gill and Tuteja, 2010; Anjum et al., 2012a,b; Duarte et al., 2013b). Notably, a simultaneous presence of both oxidized and reduced forms of electron carriers is essential for the efficient flux through plant electron transport cascades that in turn contributes to the generation of superoxide and other ROS (Foyer and Noctor, 2005). Though specific ROS concentrations play vital roles in cell signaling, negative effects of elevated ROS concentrations cause an oxidative environment and eventually severe impairments in cellular redox homeostasis and halting of cellular metabolism (reviewed by Das et al., 2015).
Plants are endowed with machinery for keeping ROS levels under control to avoid ROS-accrued oxidative stress and its subsequent consequences. Various components of antioxidant defense system together constitute and govern ROS-scavenging machinery in plants (Anjum et al., 2010; Gill and Tuteja, 2010). Both enzymatic (such as superoxide dismutase, SOD, E.C. 1.15.1.1; catalase, CAT, E.C. 1.11.1.6; glutathione reductase, GR, E.C. 1.6.4.2; peroxidase, POD, E.C. 1.11.1.7; ascorbate peroxidase, APX, E.C. 1.11.1.11; guaiacol peroxidases, GOPX, E.C. 1.11.1.7) and non-enzymatic (such as ascorbic acid, AsA; glutathione, GSH; tocopherols; phenolics, proline etc.) components comprise plant antioxidant defense system. APX and/or GOPX and CAT, localized in the cytoplasm and other cellular compartments decompose ROS (such as H2O2) to water and oxygen. As a complementary enzyme of the ascorbate (AsA)-glutathione (GSH) cycle, GR maintains a high GSH/oxidized glutathione (GSSG) ratio for protection against oxidative damages caused by a host of stress factors (Anjum et al., 2010, 2012a,b; Gill and Tuteja, 2010). Among major non-enzymatic antioxidants, AsA (a hydrophilic redox buffer) and GSH (a tripeptide redox compound, γ-Glu-Cys-Gly) are major low molecular weight antioxidants and beside influencing plant growth and development AsA and GSH are significantly contributes in the maintenance of cellular redox state through their involvement in multiple stress-defense pathways (Anjum et al., 2010; Gill and Tuteja, 2010; Anjum et al., 2012a,b). To maintain cellular redox homeostasis and avoid ROS-mediated anomalies, the highlighted above enzymatic antioxidants must work coordinately, and also AsA and GSH must be present in their reduced state. However, stress factors including excessive metals/metalloids exogenous concentrations provoke increases in the ROS production that eventually impacts fine tuning among various enzymatic and non-enzymatic components. Nevertheless, elevated and/or non-metabolized ROS cause the oxidation of AsA and GSH in to dehydroascorbate (DHA) and glutathione disulfide (GSSG), respectively, and modulate their redox couples (AsA/DHA and GSH/GSSG; Anjum et al., 2010, 2012a,b; Gill and Tuteja, 2010). Despite, the previous facts, reports are meager on the modulation and significance of cellular redox homeostasis managers AsA and GSH, and major enzymatic antioxidants in salt marsh halophytes under metals/metalloids stress conditions (Tables 1, 2).
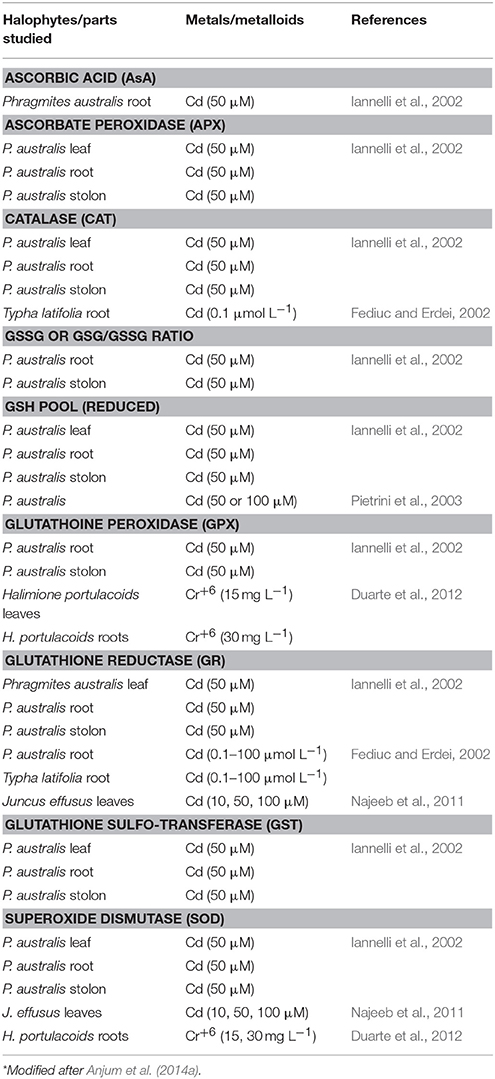
Table 1. Representative studies reporting increases in major components of antioxidant defense system in metal/metalloid-exposed salt marsh halophytes*.
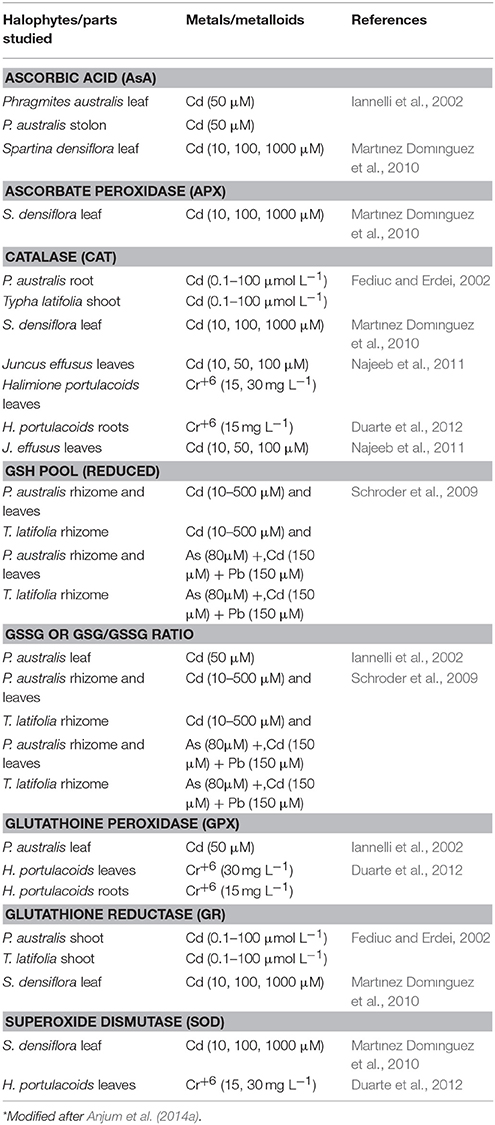
Table 2. Representative studies reporting decreases in major components of antioxidant defense system in metal/metalloid-exposed salt marsh halophytes*.
Considered as natural deposits of metals/metalloids in the estuarine system (Anjum et al., 2014a), the health of salt marsh halophytes in salt marshes can obviously be impacted by varied metals/metalloids available therein. It is a fact that plant capacity to successfully metabolize metal/metalloid-burden-accrued ROS in above (such as leaves) and below (roots/rhizomes) ground plant parts largely decides their sustenance under such scenario (Patra and Sharma, 2000; Anjum et al., 2014b,c, 2015a). To tolerate (and execute metal/metalloid-remediation) under metal/metalloid-exposure, salt marsh halophyte organs (roots and leaves) were reported to exhibit a differential modulation of cellular redox homeostasis managers. Exhibition of higher concentrations of AsA and GSH in Phragmites australis roots was suggested as a marker of tolerance of this macrophyte to Cd (50 μM)- exposure (Iannelli et al., 2002). In Cd-exposed P. australis, reduced GSH pool was advocated to protect the activity of many key photosynthetic enzymes against the thiophilic binding of Cd (Pietrini et al., 2003). However, the impact of a mixture of metals/metalloids was indicated by decreased concentrations of GSH and GSSG and the GSH:GSSG ratio in leaves and rhizomes of P. australis exposed to As (80 μM) + Cd (150 μM) + Pb (150 μM; Schroder et al., 2009).
Salt marsh macrophyte Juncus maritimus performs major role of Hg phytostabilizer under environmental Hg exposure by restricting the entry of Hg accumulated at root level (Anjum et al., 2011, 2014a, 2015a; Marques et al., 2011; Figueira et al., 2012). However, a portion of the root-accumulated Hg can be transferred to aboveground parts. Thus, the efficiency of antioxidant defense system at both root and shoot levels can coordinately control plant-tolerance to stress-accrued ROS elevation and anomalies. GSH, its redox couple and associated enzymes responded deferentially to Hg-contamination and accumulation in roots and shoots. To this end, under environmental Hg-exposure, a maintenance of a fine synchronization between H2O2-metabolizing enzymes (non-GSH based: APX and CAT; GSH-based: GPX and GST) and GSH-regenerating enzyme (GR) was argued to protect J. maritimus roots exhibiting significantly elevated contents of both reduced and oxidized GSH maximally at the site with the highest Hg-contamination (Anjum et al., 2015a). In J. maritimus shoots exhibiting differential Hg-burdens, non-GSH (APX; CAT) based H2O2-decomposing enzyme system dominated over GSH (GPX, GST) based H2O2-scavenging enzyme system (Anjum et al., 2014b). Additionally, the failure of coordination between GSH-based H2O2-metabolizing system and enhanced GSH-regenerating enzyme can be possible in shoots exhibiting differential Hg-burdens. Because plant capacity to successfully metabolize ROS (including H2O2) in leaves generated due to Hg-burden largely control photosynthetic functions (that provide energy required for plant life; Patra and Sharma, 2000; Anjum et al., 2012a,b, 2014c) status of non-GSH (APX; CAT) and GSH (GPX, GST) based H2O2-decomposing enzyme system can be implicated in macrophyte sustenance in the metal/metalloid-contaminated marine system. A clear organ (root- and leaf)-based Hg-stress-adaptive strategies of another macrophyte H. portulacoides was revealed through physiological/biochemical characterization of its below (roots)- and above (leaves) ground organs under environmental Hg-exposure (Anjum et al., 2014c). In roots, GSH regenerating (GR), GSH-utilizing (GPX, GST), and also H2O2-detoxifying (i.e., APX, CAT, GPX, GST) enzymes stood alone, and were not well coordinated. However, therein, new polypeptides emerged as an important biomarker of stress tolerance. Further, a close relation of the occurrence of Hg-induced new polypeptides (225, 100, 90, 60, 45, and 35 kDa at site with high Hg contamination, L1; 95, 65, and 25 kDa at site with moderate Hg contamination, L2) was revealed with increased GSSG but decreased GSH levels and GSH/GSSG ratio (Anjum et al., 2014c). In contrast, H. portulacoides was reported to took the advantage of the physiological/biochemical strategy adapted by its leaves where, a finely tuned coordination among the responses of enzymatic-defense endpoints was argued result into decreased GSH-oxidation but enhanced pools of reduced GSH and GSH/GSSG ratio, which subsequently lowered the extent of Hg-accrued damages (Anjum et al., 2014c).
Antioxidative feedback was monitored in a number of salt marsh plants including H. portulacoides, Sarcocornia fruticosa, and Spartina maritima in a contaminated and non-contaminated marsh (Duarte et al., 2013b). These species were biochemically separated and also differed in the activity of SOD as well APX and GOPX. However, exhibition of a degree of contamination-dependent differential biochemical characteristics (especially enzymatic defense) was argued a basis of “bioindicator” role of S. maritima for estuarine sediment quality assessment studies (Duarte et al., 2013b). Specific peroxidases (such as APX and GOPX) were reported as a marker of contamination in salt marsh, where these peroxidases were involved in Cd-driven ROS detoxification in H. portulacoides, S. fruticosa, and S. maritima (Duarte et al., 2013b). Elevated activity of SOD, that yields H2O2 production was argued to produce stimulatory effect on APX in Zn-hyperacumulating J. acutus (Santos et al., 2014), and on GPX in the same plant under Cd stress (Santos et al., 2015). Exhibition of several oscillations in SOD activity but no change in CAT, APX, and GOPX were argued to indicate the response-modulating role of salinity stress in Zn-exposed H. portulacoides (da Silva, 2010). In contrast, under Cd-exposure, CAT was reported to exhibit no (Santos et al., 2014), a linear response (Liu et al., 2012) or elevated activity (Li et al., 2011). Enhanced activity of SOD and CAT was suggested as biomarkers of Cd-tolerance in Spartina densiflora (Martınez Domınguez et al., 2010) and Spartina alterniflora (Li et al., 2011). Notably, diminished activities of GPX and GST at high Cd level (2.0 mg L−1 Cd2+) were reported to indicate an increased seedling-senescence and plant-death in Suaeda salsa at the same Cd level (Liu et al., 2012). Increasing Cd-mediated decrease in the growth of Juncus effusus was indicated by a diminished activity of CAT (and also APX, and peroxidase) (Najeeb et al., 2011).
Conclusions and Way Forward
Characterization of plant responses to metal/metalloid exposure can unveil the tolerance/adaptive potential plants to metal/metalloid-contaminated conditions (Anjum et al., 2014c). Biophysical and biochemical markers can stand second to none among plant response-characterization under metal/metalloid exposure. Despite, previous facts discussion is meager on the major biophysical and biochemical markers, those can be used to dissect halophytic plant health and eventually be implicated in devising metal/metalloid-cleanup strategies. Literature is full on isolated studies on the responses of biophysical and biochemical markers in metal/metalloid-exposed halophytes. Nevertheless, besides separately highlighting the pools of and tuning between AsA and GSH these important biochemical markers must be cross-talked with other enzymatic defense components, and also with discussed above biophysical biomarkers. Future studies considering major biophysical and biochemical markers together and critically cross-talking these markers can enlighten so far unexplored facts in the current context.
A list of missing aspects important in the present context needs to be highlighted here. The suitability of empirical “soil to plant transfer models” has been tested to predict levels and availability of potentially toxic elements in crops (Rodrigues et al., 2012). Additionally, the incorporation of the estimation of the “chemical availability” has been considered must in order to assess potential risks for major elements such as Cd, Cu, Pb, and Zn in urban soils (Rodrigues et al., 2013). However, such studies are meager on plants representing salt marsh/estuarine system. The phytotoxicity metals/metalloids largely depend on the status of their tissue-burdens (Anjum et al., 2012b, 2014a,b,c, 2015a). Despite this fact, most of the discussed herein studies on salt marsh halophytes did not consider the role of elemental speciation in soils that in fact controls the availability, rate of uptake; and hence, their effects/toxicity in plants (Ge et al., 2000; Weis and Weis, 2004). Less emphasis has been given to the role of methylation of metalloids that can determine their toxicity in plants (Bentley and Chasteen, 2002; Weis and Weis, 2004). Additionally, marsh ecosystem is the home of the microbial activities (Weis and Weis, 2004; Anjum et al., 2014a), and the role of later in the methylation of metalloids has been reported (Bentley and Chasteen, 2002). Nevertheless, plants can also exhibit quite different responses to elements essential and non-essential for their growth and development (Anjum et al., 2015b). However, reports are meager on the previous aspects in connection with their role in the control of the biophysical and biochemical markers in salt marsh halophytes. Notably, major results obtained are from laboratory studies (considering one and/or two/three elements) that can be of little value in and cannot be implicated for understanding metal/metalloid-toxicity and -tolerance in halophytes under field conditions.
Thus, a regular monitoring of and cross-talks on the highlighted herein biophysical and biochemical markers taking into account the mentioned above missing aspects in salt marsh/estuarine system with potential metal/metalloid contamination is advocated. If considered, the previous approach can yield triple benefits of: (a) unveiling the status of the metal-metalloid contamination, (b) understanding adaptive responses of salt marsh halophyte to metals/metalloids, and finally (c) devising sustainable strategies for the environmental or ecosystem management and safety.
Author Contributions
NAA and BD conceived the idea of this paper. NAA, BD, and NS prepared the manuscript. IC, ACD, and EP supervised the work and corrected the manuscript. All authors read and approved the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
NAA (SFRH/BPD/84671/2012), ACD and EP are grateful to European Funds through COMPETE and by National Funds through the Portuguese Science Foundation (FCT) (PEst-C/MAR/LA0017/2013, PTDC/MAR-BIO/3533/2012) and the Aveiro University Research Institute/Centre for Environmental and Marine Studies (CESAM) for partial financial supports. BD (SFRH/BD/75951/2011) and IC would also like to thank to the FCT for funding the research in the Marine and Environmental Sciences Centre (MARE) throughout the project UID/MAR/04292/2013.
References
Anjum, N. A., Ahamd, I., Mohmood, I., Pacheco, M., Duarte, A. C., Pereira, E., et al. (2012b). Modulation of glutathione and its related enzymes in plants' responses to toxic metals and metalloids-a review. Environ. Exp. Bot. 75, 307–324. doi: 10.1016/j.envexpbot.2011.07.002
Anjum, N. A., Ahmad, I., Valega, M., Mohmood, I., Gill, S. S., Tuteja, N., et al. (2014a). Salt marsh halophyte services to metal-metalloid remediation: assessment of the processes and underlying mechanisms. Crit. Rev. Environ. Sci. Technol. 44, 2038–2106. doi: 10.1080/10643389.2013.828271
Anjum, N. A., Ahmad, I., Válega, M., Pacheco, M., Figueira, E., Duarte, A. C., et al. (2011). Impact of seasonal fluctuations on the sediment-mercury, its accumulation and partitioning in Halimione portulacoides and Juncus maritimus collected from Ria de Aveiro Coastal Lagoon (Portugal). Water Air Soil Pollut. 222, 1–15. doi: 10.1007/s11270-011-0799-4
Anjum, N. A., Duarte, A. C., Pereira, E., and Ahmad, I. (2014b). Oxidative stress status, antioxidant metabolism and polypeptide patterns in Juncus maritimus shoots exhibiting differential mercury burdens in Ria de Aveiro coastal lagoon (Portugal). Environ. Sci. Pollut. Res. 21, 6652–6661. doi: 10.1007/s11356-014-2578-4
Anjum, N. A., Duarte, A. C., Pereira, E., and Ahmad, I. (2015a). Juncus maritimus root biochemical assessment for its mercury stabilization potential in Ria de Aveiro coastal lagoon (Portugal). Environ. Sci. Pollut. Res. 22, 2231–2238. doi: 10.1007/s11356-014-3455-x
Anjum, N. A., Israr, M., Duarte, A. C., Pereira, M. E., and Ahmad, I. (2014c). Halimione portulacoides (L.) physiological/biochemical characterization for its adaptive responses to environmental mercury exposure. Environ. Res. 131, 39–49. doi: 10.1016/j.envres.2014.02.008
Anjum, N. A., Singh, H. P., Khan, M. I. R., Masood, A., Per, T. S., Negi, A., et al. (2015b). Too much is bad - an appraisal of phytotoxicity of elevated plant-beneficial heavy metal ions. Environ. Sci. Pollut. Res. 22, 3361–3382. doi: 10.1007/s11356-014-3849-9
Anjum, N. A., Umar, S., and Ahmad, A. (2012a). Oxidative Stress in Plants: Causes, Consequences and Tolerance, 1st Edn. New Delhi: IK International Publishing House.
Anjum, N. A., Umar, S., and Chan, M. T. (2010). Ascorbate-Glutathione Pathway and Stress Tolerance in Plants, 1st Edn. Dordrecht: Springer.
Barceló, J., Vásquez, M. D., and Poschenrieder, C. H. (1988). Structural and ultrastructural disorders in cadmium-treated bush bean plants (Phaseolus vulgaris L.). New Phytol. 108, 37–49. doi: 10.1111/j.1469-8137.1988.tb00202.x
Baszynki, T., Wajda, L., Król, M., Wolinska, D., Krupa, Z., and Tukendorf, A. (1980). Photosynthetic activities of cadmium-treated tomato plants. Physiol. Plant. 48, 365–370. doi: 10.1111/j.1399-3054.1980.tb03269.x
Bentley, R., and Chasteen, T. G. (2002). Microbial methylation of metalloids: arsenic, antimony, and bismuth. Microbiol. Mol. Biol. Rev. 66, 250–271. doi: 10.1128/MMBR.66.2.250-271.2002
Best, M., Massey, A., and Prior, A. (2007). Developing a saltmarsh classification tool for the European water framework directive. Mar. Pollut. Bull. 55, 205–214. doi: 10.1016/j.marpolbul.2006.08.036
Bumann, D., and Oesterhelt, D. (1995). Destruction of a single chlorophyll is correlated with the photoinhibition of photosystem II with a transiently inactive donor site. Proc. Natl. Acad. Sci. U.S.A. 92, 12195–12199. doi: 10.1073/pnas.92.26.12195
Caçador, I., Duarte, B., Marques, J. C., and Sleimi, N. (2015). “Carbon mitigation: a salt marsh ecosystem service in times of change,” in Halophytes for Food Security in Dry Lands, eds M. A. Khan, M. Ozturk, B. Gul, and M. Z. Ahmed (Amsterdam: Elsevier Inc.), 83–110.
Caçador, I., Vale, C., and Catarino, F. (1996). The influence of plants on concentration and fractionation of Zn, Pb, and Cu in salt marsh sediments (Tagus Estuary, Portugal). J. Aquat. Ecosys. Health 5, 193–198. doi: 10.1007/BF00124106
Cid, A., Herrero, C., Torres, E., and Abalde, J. (1995). Copper toxicity on the marine microalga Phaeodactylum tricornutum: effects on photosynthesis and related parameters. Aquat. Toxicol. 31, 165–174. doi: 10.1016/0166-445X(94)00071-W
da Silva, V. F. N. (2010). Response of Salt Marsh Plants to Heavy Metals in the Tagus Estuary. thesis, Departamento de Biologia Vegetal, Faculdade de Ciências, Universidade de Lisboa, Portugal.
Das, P., Nutan, K. K., Singla-Pareek, S. L., and Pareek, A. (2015). Oxidative environment and redox homeostasis in plants: dissecting out significant contribution of major cellular organelles. Front. Environ. Sci. 2:70. doi: 10.3389/fenvs.2014.00070
Demmig-Adams, B. (1990). Carotenoids and photoprotection in plants: a role for the xanthophyll zeaxanthin. Biochim. Biophys. Acta 1020, 1–24. doi: 10.1016/0005-2728(90)90088-L
Demmig-Adams, B., and Adams, W. W. II (1992). Photoprotection and other responses of plants to light stress. Annu. Rev. Plant Physiol. Plant Mol. Biol. 43, 599–626. doi: 10.1146/annurev.pp.43.060192.003123
Doyle, M., and Otte, M. (1997). Organism-induced accumulation of Fe, Zn and AS in wetland soils. Environ. Pollut. 96, 1–11. doi: 10.1016/S0269-7491(97)00014-6
Duarte, B., Marques, J. C., and Caçador, I. (in press). Ecophysiological responses of native invasive Spartina species to extreme temperature events in Mediterranean marshes. Biol. Invas. doi: 10.1007/s10530-015-0958-4
Duarte, B., Santos, D., and Caçador, I. (2013b). Halophyte anti-oxidant feedback seasonality in two salt marshes with different degrees of metal contamination: search for an efficient biomarker. Funct. Plant Biol. 40, 922–930. doi: 10.1071/fp12315
Duarte, B., Santos, D., Marques, J. C., and Caçador, I. (2013a). Ecophysiological adaptations of two halophytes to salt stress: photosynthesis, PS II photochemistry and anti-oxidant feedback - implications for resilience in climate change. Plant Physiol. Biochem. 67, 178–188. doi: 10.1016/j.plaphy.2013.03.004
Duarte, B., Santos, D., Marques, J. C., and Caçador, I. (2014). Biophysical probing of Spartina maritima Photo-system II changes during increased submersion periods: possible adaptation to sea level rise. Plant Physiol. Biochem. 77, 122–132. doi: 10.1016/j.plaphy.2014.01.023
Duarte, B., Santos, D., Marques, J. C., and Caçador, I. (2015). Ecophysiological constraints of two invasive plant species under a saline gradient: halophytes versus glycophytes. Estuar. Coast. Shelf Sci. 167, 154–165. doi: 10.1016/j.ecss.2015.04.007
Duarte, B., Silva, V., and Caçador, I. (2012). Hexavalent chromium reduction, uptake and oxidative biomarkers in Halimione portulacoides. Ecotoxicol. Environ. Saf. 83, 1–7. doi: 10.1016/j.ecoenv.2012.04.026
Fediuc, E., and Erdei, L. (2002). Physiological and biochemical aspects of cadmium toxicity and protective mechanisms induced in Phragmites australis and Typha latifolia. J. Plant Physiol. 159, 265–271. doi: 10.1078/0176-1617-00639
Figueira, E., Freitas, R., Pereira, E., and Duarte, A. (2012). Mercury uptake and allocation in Juncus maritimus: implications for phytoremediation and restoration of a mercury contaminated salt marsh. J. Environ. Monit. 14, 2181–2188. doi: 10.1039/c2em30076a
Foyer, C. H., and Noctor, G. (2005). Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell. 17, 1866–1875. doi: 10.1105/tpc.105.033589
Ge, Y., Murray, P., and Hendershot, W. H. (2000). Trace metal speciation and bioavailability in urban soils. Environ. Pollut. 107, 137–144. doi: 10.1016/S0269-7491(99)00119-0
Gill, S. S., and Tuteja, N. (2010). Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 48, 909–930. doi: 10.1016/j.plaphy.2010.08.016
Horton, P., Ruban, A. V., and Young, A. J. (1999). “Regulation of the structure and function of the light-harvesting complexes of photosystem II by the xanthophyll cycle,” in The Photochemistry of Carotenoids: Applications in Biology, eds H. A. Frank, A. J. Young, and J. C. Cogdell (Dordrecht: Kluwer Academic Publishers), 271–291.
Hynninen, P. H. (1991). “Chemistry of chlorophyll: modifications,” in Chlorophylls, ed H. Scheer (Boca Raton, FL: CRC Press), 145–210.
Iannelli, M. A., Pietrini, F., Fiore, L., Petrilli, L., and Massacci, A. (2002). Antioxidant response to cadmium in Phragmites australis plants. Plant Physiol. Biochem. 40, 977–982. doi: 10.1016/S0981-9428(02)01455-9
Joliot, P., and Joliot, A. (2002). Cyclic electron transport in plant leaf. Proc. Natl. Acad. Sci. U.S.A. 99, 10209–10214. doi: 10.1073/pnas.102306999
Krüger, G. H. J., De Villiers, M. F., Strauss, A. J., de Beer, M., van Heerden, P. D. R., Maldonado, R., et al. (2014). Inhibition of photosystem II activities in soybean (Glycine max) genotypes differing in chilling sensitivity. South Afr. J. Bot. 95, 85–96. doi: 10.1016/j.sajb.2014.07.010
Krupa, Z., and Baszynski, T. (1995). Some aspects of heavy metals toxicity towards photosynthetic apparatus: direct and indirect effects on light and dark reactions. Acta Physiol. Plant 17, 177–190.
Küpper, H., Kupper, F., and Spiller, M. (1996). Environmental relevance of heavy metal-substituted chlorophylls using the example of water plants. J. Exp. Bot. 47, 259–266. doi: 10.1093/jxb/47.2.259
Küpper, H., Seibert, S., and Aravind, P. (2007). A fast, sensitive and inexpensive alternative to analytical pigment HPLC: quantification of chlorophylls and carotenoids in crude extracts by fitting with Gauss-Peak-Spectra. Anal. Chem. 79, 7611–7627. doi: 10.1021/ac070236m
Küpper, H., Setlík, I., Spiller, M., Küpper, F. C., and Prásil, O. (2002). Heavy metal-induced inhibition of photosynthesis: targets of in vivo heavy metal chlorophyll formation. J. Phycol. 48, 429–441. doi: 10.1046/j.1529-8817.2002.t01-1-01148.x
Li, J. M., Shi, G. Y., and Wei, Y. (2011). Effects of cadmium on growth and physiological characteristics of Spartina alterniflora. J. Anhui Agric. Sci. 39, 2174–2176.
Liu, S., Yang, C., Xie, W., Xia, W., and Fan, P. (2012). The effects of cadmium on germination and seedling growth of Suaeda salsa. Procedia. Environ. Sci. 16, 293–298. doi: 10.1016/j.proenv.2012.10.041
Marques, B., Lillebø, A. I., Pereira, E., and Duarte, A. C. (2011). Mercury cycling and sequestration in salt marshes sediments: an ecosystem service provided by Juncus maritimus and Scirpus maritimus. Environ. Pollut. 159, 1869–1876. doi: 10.1016/j.envpol.2011.03.036
Martınez Domınguez, D., C'ordoba Garcıa, F., Canalejo Raya, A., and Torronteras Santiago, R. (2010). Cadmium-induced oxidative stress and the response of the antioxidative defense system in Spartina densiflora. Physiol. Plant 139, 289–302. doi: 10.1111/j.1399-3054.2010.01368.x
Monnet, F., Vaillant, N., Vernay, P., Coudret, A., Sallanon, H., and Hitmi, A. (2001). Relationship between PS II activity, CO2 fixation, and Zn, Mn and Mg contents of Lolium perenne under zinc stress. J. Plant Physiol. 158, 1137–1144. doi: 10.1078/S0176-1617(04)70140-6
Najeeb, U., Jilani, G., Ali, S., Sarwar, M., Xu, L., and Zhou, W. (2011). Insights into cadmium induced physiological and ultra-structural disorders in Juncus effuses L. and its remediation through exogenous citric acid. J. Hazard Mater. 186, 565–574. doi: 10.1016/j.jhazmat.2010.11.037
Ngo, T., and Zhao, Y. (2007). Formation of zinc-chlorophyll-derivative complexes in thermally processed green pears (Pyrus communis L.). J. Food Sci. 77, 397–404. doi: 10.1111/j.1750-3841.2007.00465.x
Panda, D., Rao, D. N., Sharma, S. G., Strasser, R. J., and Sarkar, R. K. (2006). Submergence effects on rice genotypes during seedling stage: probing of submergence driven changes of photosystem 2 by chlorophyll a fluorescence induction O-J-I-P transients. Photosynthetica 44, 69–75. doi: 10.1007/s11099-005-0200-1
Panda, D., Sharma, S. G., and Sarkar, R. K. (2008). Chlorophyll fluorescence parameters, CO2 photosynthetic rate and regeneration capacity as a result of complete submergence and subsequent reemergence in rice (Oryza sativa L.). Aquat. Bot. 88, 127–133. doi: 10.1016/j.aquabot.2007.08.012
Patra, M., and Sharma, A. (2000). Mercury toxicity in plants. Bot. Rev. 66, 379–422. doi: 10.1007/BF02868923
Pedro, S., Duarte, B., Almeida, P. R., and Caçador, I. (2015). Metal speciation in salt marsh sediments: Influence of halophyte vegetation in salt marshes with different morphology. Estuar. Coast. Shelf Sci. 167, 248–255. doi: 10.1016/j.ecss.2015.05.034
Pietrini, F., Iannelli, M. A., Pasqualini, S., and Massacci, A. (2003). Interaction of cadmium with glutathione and photosynthesis in developing leaves and chloroplasts of Phragmites australis (Cav.) Trin. ex Steudel. Plant Physiol. 133, 829–837. doi: 10.1104/pp.103.026518
Prasad, M. N. V., and Strzalka, K. (1999). Impact of heavy metals on photosynthesis. in Heavy Metal Stress in Plants, eds M. N. V. Prasad and J. Hagemeyer (Berlin: Springer), 117–138.
Qiu, N., Lu, Q., and Lu, C. (2003). Photosynthesis, photosystem II efficiency and the xanthophyll cycle in the salt-adapted halophyte Atriplex centralasiatica. New Phytol. 159, 479–486. doi: 10.1046/j.1469-8137.2003.00825.x
Reboreda, R., and Caçador, I. (2007). Coer, zinc and lead speciation in salt marsh sediments colonized by Halimione portulacoides and Spartina maritima. Chemosphere 69, 1655–1661. doi: 10.1016/j.chemosphere.2007.05.034
Rintamäki, E., Salo, R., Lehtonen, E., and Aro, E. M. (1995). Regulation of D1 protein-degradation during photoinhibition of photosystem-II in vivo-phosphorylation of the D1 protein in various plant groups. Planta 195, 379–386. doi: 10.1007/BF00202595
Rodrigues, S. M., Cruz, N., Coelho, C., Henriques, B., Carvalho, L., Duarte, A. C., et al. (2013). Risk assessment for Cd, Cu, Pb and Zn in urban soils: chemical availability as the central concept. Environ. Pollut. 183, 234–242. doi: 10.1016/j.envpol.2012.10.006
Rodrigues, S. M., Pereira, E., Duarte, A. C., and Römkens, P. F. A. M. (2012). Derivation of soil to plant transfer functions for metals and metalloids: impact of contaminants availability. Plant Soil 361, 329–341. doi: 10.1007/s11104-012-1249-9
Ruban, A. V., Phillip, D., Young, A. J., and Horton, P. (1998). Excited state energy level does not determine the differential effect of violaxanthin and zeaxanthin on chlorophyll fluorescence quenching in the isolated light-harvesting complex of Photosystem II. Photochem. Photobiol. 68, 829–834. doi: 10.1111/j.1751-1097.1998.tb05291.x
Samson, G., Morisette, J. C., and Popovic, R. (1988). Copper quenching of the variable fluorescence in Dunaliella tertiolecta. New evidence for a copper inhibition effect on PS II photochemistry. Photochem. Photobiol. 48, 329–332. doi: 10.1111/j.1751-1097.1988.tb02829.x
Santos, D., Duarte, B., and Caçador, I. (2014). Unveiling Zn hyperacumulation in Juncus acutus: implications on the electronic energy fluxes and on oxidative stress with emphasis on non-functional Zn-Chlorophylls. J. Photochem. Photobiol. B Biol. 140, 228–239. doi: 10.1016/j.jphotobiol.2014.07.019
Santos, D., Duarte, B., and Caçador, I. (2015). Biochemical and photochemical feedbacks of acute Cd toxicity in Juncus acutus seedlings: the role of non-functional Cd-chlorophylls. Estuar. Coast. Shelf Sci. 167, 228–239. doi: 10.1016/j.ecss.2015.10.005
Schroder, P., Lyubenova, L., and Huber, C. (2009). Do heavy metals and metalloids influence the detoxification of organic xenobiotics in plants? Environ. Sci. Pollut. Res. 16, 795–804. doi: 10.1007/s11356-009-0168-7
Sghaier, D., Duarte, B., Bankaji, I., Caçador, I., and Sleimi, N. (2015). Growth, chlorophyll fluorescence and mineral nutrition in the halophyte Tamarix gallica cultivated in combined stress conditions: arsenic and NaCl. J. Photochem. Photobiol. B 149, 204–214. doi: 10.1016/j.jphotobiol.2015.06.003
Skorzynska-Polit, E., and Baszynski, T. (1997). Differences in sensitivity of the photosynthetic apparatus in Cd-stressed runner bean plants in relation to their age. Plant Sci. 128, 11–21. doi: 10.1016/S0168-9452(97)00126-X
Srivastava, A., Guisse, B., Greppin, H., and Strasser, R. J. (1997). Regulation of antenna structure and electron transport in PS II of Pisum sativum under elevated temperature probed by the fast polyphasic chlorophyll a fluorescence transient: OKJIP. Biochim. Biophys. Acta 1320, 95–106. doi: 10.1016/S0005-2728(97)00017-0
Strasser, R. J., Srivastava, A., and Govindjee, G. (1995). Polyphasic chlorophyll a fluorescence transient in plants and cyanobacteria. Photochem. Photobiol. 61, 32–42. doi: 10.1111/j.1751-1097.1995.tb09240.x
Strasser, R. J., Srivastava, A., and Tsimilli-Michael, M. (2000). “The fluorescence transient as a tool to characterize and screen photosynthetic samples,” in Probing Photosynthesis: Mechanisms, Regulation and Adaptation, eds M. Yunus, U. Pathre, and P. Mohanty (London: Taylor & Francis), 445–483.
Strasser, R. J., and Stirbet, A. D. (2001). Estimation of the energetic connectivity of PS II centres in plants using the fluorescence rise O–J–I–P. Fitting of experimental data to three different PS II models. Mathemat. Comput. Simulat. 56, 451–461. doi: 10.1016/S0378-4754(01)00314-7
Strasser, R. J., Tsimilli-Michael, M., and Srisvastava, A. (2004). “Analysis of the chlorophyll-a fluorescence transiente,” in Advances in Photosynthesis and Respiration, eds G. C. Papageorgiou and Govindjee (Berlin: Springer), 321–362.
Stribet, A., and Govindjee (2011). On the relation between the Kautsky effect (chlorophyll a fluorescence induction) and Photosystem II: basics and applications of the OJIP fluorescence transient. J. Photochem. Photobiol. B Biol. 104, 236–257. doi: 10.1016/j.jphotobiol.2010.12.010
Suntornvongsagul, K., Burke, D., Hamerlynck, E., and Hahn, D. (2007). Fate and effects of heavy metals in salt marsh sediments. Environ. Pollut. 149, 79–91. doi: 10.1016/j.envpol.2006.12.010
Vaillant, N., Monnet, F., Hitmi, A., Sallanon, H., and Coudret, A. (2005). Comparative study of responses in four datura species to zinc stress. Chemosphere 59, 1005–1013. doi: 10.1016/j.chemosphere.2004.11.030
Wang, J. W., Asami, T., Murofushi, N., and Yoshida, S. (2000). Isolation and initial characterization of 132-hydroxychlorophyll a induced by cyclohexanedione derivatives in tobacco cell suspension cultures. Photochem. Photobiol. 71, 84–89. doi: 10.1562/0031-8655(2000)0710084IAICOH2.0.CO2
Weis, J. S., and Weis, P. (2004). Metal uptake, transport and release by wetland plants: implications for phytoremediation and restoration. Environ. Intl. 30, 685–700. doi: 10.1016/j.envint.2003.11.002
Wentworth, M., Ruban, A. V., and Horton, P. (2000). Chlorophyll fluorescence quenching in isolated light-harvesting complexes induced by zeaxanthin. FEBS Lett. 471, 71–74. doi: 10.1016/S0014-5793(00)01369-7
World Health Organization (WHO) (2005). Millenium Ecosystem Assessment. (Washington, DC: United Nations).
Williams, T. P., Bubb, J. M., and Lester, J. N. (1994). Metal accumulation within salt marsh environments: a review. Mar. Pollut. Bull. 28, 277–290. doi: 10.1016/0025-326X(94)90152-X
Yildiz, M., Terzi, H., Cenkci, S., Arikan Terzi, E. S., and Uruşak, B. (2010). Physiological and biochemical markers of salinity tolerance in plants. Anadolu Univ. J. Sci. Tech. C. Life Sci. Biotechnol. 1, 1–33.
Keywords: Metal/metalloid toxicity, Halophytes, Biophysical biomarkers, Biochemical biomarkers, Photosynthesis, Redox active compounds
Citation: Anjum NA, Duarte B, Caçador I, Sleimi N, Duarte AC and Pereira E (2016) Biophysical and Biochemical Markers of Metal/Metalloid-Impacts in Salt Marsh Halophytes and Their Implications. Front. Environ. Sci. 4:24. doi: 10.3389/fenvs.2016.00024
Received: 23 December 2015; Accepted: 17 March 2016;
Published: 05 April 2016.
Edited by:
Rathinam Arthur James, Bharathidasan University, IndiaReviewed by:
Jia-Jang Hung, National Sun Yat-sen University, TaiwanWei-Yu Chen, Kaohsiung Medical University, Taiwan
Copyright © 2016 Anjum, Duarte, Caçador, Sleimi, Duarte and Pereira. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Naser A. Anjum, anjum@ua.pt
†These authors have contributed equally to this work.
 Naser A. Anjum
Naser A. Anjum Bernardo Duarte
Bernardo Duarte Isabel Caçador
Isabel Caçador Noomene Sleimi
Noomene Sleimi Armando C. Duarte
Armando C. Duarte Eduarda Pereira1
Eduarda Pereira1