Use of Archived Neonatal Bloodspots for Examining Associations between Prenatal Exposure to Potentially Traumatic or Stressful Life Events, Maternal Herpesvirus Infection and Lifetime History of Generalized Anxiety Disorder in Offspring
- 1Joseph J. Zilber School of Public Health, University of Wisconsin-Milwaukee, Milwaukee, WI, USA
- 2Department of Psychology, Carl R. Woese Institute for Genomic Biology, University of Illinois at Urbana-Champaign, Urbana, IL, USA
- 3Stanley Laboratory of Developmental Neurovirology, Johns Hopkins School of Medicine, Baltimore, MD, USA
- 4Department of Epidemiology, Gillings School of Global Public Health, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA
Background: Lifetime prevalence of anxiety disorders is over 30% among U.S. adolescents, warranting further investigation into early life risk factors for such conditions. We conducted a pilot study to examine the role that maternal herpesvirus infection may play in the pathway between maternal trauma and stress during pregnancy and offspring generalized anxiety disorder (GAD).
Methods: Participants included 69 women in the Detroit Neighborhood Health Study with data on past exposure to 19 potentially traumatic (PTEs) and 9 stressful life events (SLEs). Lifetime history of GAD in the youngest biologic child between 6 and 17 years old born in Michigan (i.e., index child) of each woman was ascertained via the Diagnostic Interview Schedule for Children, 4th edition, parent version. We obtained written informed consent from participants for retrieval of archived neonatal bloodspot samples corresponding to their index child from the Michigan Neonatal Biobank (MNB) and testing of these samples for markers of maternal herpes simplex virus (HSV)-1 and cytomegalovirus (CMV) seropositivity. Logistic regression was used to examine the association between maternal PTEs or SLEs during pregnancy and offspring GAD.
Results: A total of 18.1 and 31.9% of women experienced ≥1 PTE or SLE during pregnancy, respectively, and 10.8% of offspring met the criteria for lifetime history of GAD. We obtained maternal consent for retrieval of and tested bloodspot samples corresponding to the index child of 22 women (38.0%), of which 4.5 and 40.9% were seropositive for HSV-1 and CMV, respectively. We observed positive, although not statistically significant associations between ≥1 PTE or SLE during pregnancy and offspring lifetime history of GAD. While a greater proportion of offspring with lifetime history of GAD were born to women seropositive for CMV and HSV-1, compared to those without lifetime history, these differences were not statistically significant and we did not further examine the mediating role of maternal herpesvirus seropositivity in this pathway.
Conclusion: Findings from this study support the feasibility of utilizing neonatal bloodspots archived in the MNB to examine the role of herpesviruses as mediators between maternal trauma or stress during pregnancy and offspring anxiety disorders in larger Michigan cohorts.
Introduction
Nearly 32% of adolescents in the U.S. are estimated to have experienced an anxiety disorder in their lifetime, with a median age of onset of 6 years (Merikangas et al., 2010). While much research has focused on the role that early life trauma plays in the development of anxiety disorders among children (Stein et al., 1996; Pynoos et al., 1999; Heim and Nemeroff, 2001; Hovens et al., 2010, 2012, 2015; Lochner et al., 2010; Klauke et al., 2011; Kuo et al., 2011; Zikic et al., 2015), a growing body of studies suggest that the adverse effects of traumatic events as well as other stressors on the development of anxiety disorders in children may begin before birth (Yehuda et al., 2001; Najman et al., 2010; Rice et al., 2010; Davis and Sandman, 2012; Betts et al., 2014; Park et al., 2014). Researchers have hypothesized that excess exposure to maternal stress hormones during gestation plays a key role in fetal programming of offspring anxiety disorders via causing permanent alterations to hypothalamic-pituitary-adrenal (HPA) axis function and/or via contributing to epigenetic modifications that adversely shape offspring stress reactivity (Weaver et al., 2004; Skinner et al., 2008; Glover et al., 2010; Davis et al., 2011; Radtke et al., 2011; Perroud et al., 2014). Alternatively, trauma or stress during pregnancy may influence maternal health behaviors for which prenatal exposure has also been associated with anxiety disorders in offspring such as tobacco and alcohol use (Indredavik et al., 2007; Ashford et al., 2008; Ekblad et al., 2010; Hellemans et al., 2010; Moylan et al., 2015). The adverse effects of stress on immune function are, however, also well-documented (Segerstrom and Miller, 2004) and a converging body of evidence suggests that maternal infection during pregnancy may serve as an alternative pathway by which prenatal exposure to trauma and life stressors adversely shapes the development of offspring mental health disorders, including anxiety.
First, maternal immunoglobulin G (IgG) antibodies triggered in response to infection can be transferred across the placenta beginning in the second trimester (Simister, 2003) and cross-reaction of maternal antibodies with fetal tissues during critical periods of development is thought to adversely affect neurodevelopment and contribute to morphologic changes to the brain that influence the development of neuropsychiatric disorders (Marques et al., 2013). Indeed, maternal exposure to bacterial and viral mimics during pregnancy have been shown in murine models to impact neurodevelopment in the amygdala and prefrontal cortex (Machado et al., 2015; Zhou, 2015)–regions of the brain for which increased activity has been observed in human populations with anxiety disorders (Miguel-Hidalgo, 2013). Second, pro-inflammatory cytokines triggered by maternal infection can also cross the placental barrier and may adversely affect neurodevelopment via inducing inflammation in fetal brain tissues (Dammann and Leviton, 1997). While we are unaware of any human studies that examine the association between maternal inflammation during pregnancy and offspring anxiety disorders, numerous animal-based studies have demonstrated that maternal immune activation during pregnancy is associated with anxiety-like behaviors in offspring (Enayati et al., 2012; Babri et al., 2014; Depino, 2015). Given that individuals undergoing increased psychosocial stress are more susceptible to and have worse immunologic control of infections once acquired (Segerstrom and Miller, 2004), maternal infection may represent a key biologic mechanism by which the adverse effects of maternal trauma and stress during pregnancy contribute to the development of anxiety disorders across generations.
While acute infections elicit a short-term immune response, latent infections such as herpesviruses, are never cleared from the body (Glaser and Kiecolt-Glaser, 1994; Pedersen et al., 2010; Uddin et al., 2010) and have been shown to reactivate over time-particularly in response to stress (Glaser and Kiecolt-Glaser, 1994). Reactivation has, in turn, been hypothesized to trigger increased production of antibodies specific for such pathogens (Dowd et al., 2008, 2012; Dowd and Aiello, 2009; Christian et al., 2012; Rector et al., 2014), and contribute to elevated levels of circulating pro-inflammatory cytokines such as C-reactive protein and interleukin-6 (Nazmi et al., 2010; Bennett et al., 2012). To our knowledge no studies have examined the association between maternal infection with herpesviruses and offspring anxiety disorders, however, prenatal exposure to such pathogens have been implicated in the etiology of other neuropsychiatric disorders such as schizophrenia (Buka et al., 2008). Taken together, herpesviruses may represent a particularly salient maternal infection in the etiology of offspring anxiety disorders and therefore, an important mediator of the association between maternal trauma and/or stress during pregnancy and such outcomes.
Since July 1984, filter paper cards containing leftover bloodspots collected from newborns as part of the Michigan Newborn Screening Program have been transferred from the Michigan Department of Health and Human Services (MDHHS) public health laboratory to the Michigan Neonatal Biobank (MNB) for long-term storage and are available for use in research. These archived neonatal bloodspots serve as a valuable resource for assessing markers of maternal infection during pregnancy as antibodies detected in neonatal blood at birth reflect those of maternal origin that have crossed the placenta during gestation in the otherwise immunologically naïve neonate (Schelonka and Infante, 1998). The aims of the present pilot study were to (1) re-contact 369 women in the Detroit Neighborhood Health Study (DNHS) with existing data on lifetime history of potentially traumatic (PTEs) or SLEs and re-interview those with minor children born in Michigan to ascertain data on lifetime history of generalized anxiety disorder (GAD) in their youngest child between the ages of 6–17 (i.e., index child), (2) obtain maternal consent for retrieval and testing of neonatal bloodspot samples archived in the MNB corresponding to each woman's index child for markers of maternal infection with cytomegalovirus (CMV) and herpes simplex virus (HSV)-1, and (3) examine whether maternal herpesvirus seropositivity partially mediates the association between maternal PTEs or SLEs during pregnancy and offspring GAD.
Materials and Methods
Study Population and Design
The DNHS is a longitudinal, population-based study designed to investigate correlates of mental health disorders among individuals living in the city of Detroit, MI. A probability sample of 1547 individuals (aged ≥ 18 years) living within the Detroit city limits was recruited from 2008 to 2009 and individuals participated in up to 4 follow-up visits. During Wave 5 of DNHS (2013), we identified 369 female DNHS participants who previously reported living with children under 18 years of age to be re-contacted to determine eligibility for participation in the present pilot study. Eligibility criteria included having a biologic child between the ages of 6–17 for whom lifetime history of GAD could therefore be ascertained via parental interview and who was born in Michigan and therefore had neonatal bloodspots archived in the MNB that were available for use in research. Of the 369 women identified as potentially eligible for the study, 140 (37.9%) were successfully re-contacted via telephone, 76 (54.3%) of whom met the above eligibility criteria. Of those eligible for participation, 69 (90.8%) women agreed to participate in the telephone interview portion of the pilot study during which women were asked to recall whether any PTEs or SLEs previously reported during their lifetime at baseline (2008–2009), occurred during their pregnancy with their youngest child between the ages of 6–17 born in Michigan (i.e., index child). Women were also interviewed regarding their index child's lifetime history of GAD and invited to provide written informed consent for retrieval and testing of the index child's neonatal bloodspots that were archived in the MNB. A total of 54 (78.3%) women interviewed agreed to be mailed a consent form for retrieval and testing of archived neonatal bloodspot samples corresponding to their index child for markers of maternal herpesvirus infection. Of these women, 24 (44.4%) provided written informed consent for the bloodspot portion of the study and bloodspot samples corresponding to the index child of 22 (91.7%) women were tested for markers of maternal herpesvirus infection.
Ethics Approval
The DNHS was approved by the institutional review boards (IRB) at the University of Michigan and University of North Carolina and the present pilot study conducted during Wave 5 of DNHS was also approved by the MDHHS IRB as well as the Biotrust for Health, Inc. Scientific Advisory Board and Community Values Advisory Board. All participants provided oral informed consent for participation in the DNHS Wave 5 telephone interview. Women eligible for the pilot study portion of the study were given the opportunity to provide written informed consent for retrieval and testing of their index child's neonatal bloodspots archived in the MNB under a waiver of child assent. Participants were mailed consent materials with a pre-paid return envelope and provided a nominal monetary incentive for the time required to review and return consent materials to study staff via mail or during a home visit. Individuals could also, at any time, have opted-out of use of archived neonatal bloodspot samples corresponding to their offspring in all research studies by submitting a request to MDHHS. Last, in the event that the index child of a pilot study participant turned 18 years of age before testing of their samples was completed, we obtained informed written consent directly from that individual for testing of their samples (N = 2).
Measures
Maternal PTEs and SLEs during pregnancy were the main exposures of interest in this study. During Wave 1 of DNHS (2008–2009) data on lifetime history of 19 PTEs based upon a set of traumatic events that had been used in prior research in the Detroit metropolitan area (Breslau et al., 1998), and that met Criterion A of the Diagnostic and Statistical Manual of Mental Disorders-IV (American Psychiatric Association, 1994) PTSD diagnosis was ascertained via telephone interview. PTEs included events that can be characterized as (1) assaultive violence, (2) other injuries or shocking experiences, (3) learning of traumatic events experienced by close friends or relatives, or (4) the sudden, unexpected death of a close friend or relative and individuals were also asked to report the occurrence of any other traumatic event (Breslau et al., 1998). Individuals were also asked about lifetime history of nine non-traumatic stressors, including events such as divorce or “break up” with a significant other, serious drug or alcohol problem of a parent or close relative, emotional mistreatment, serious financial problems, legal problems, and problems accessing health care which were modified from previous studies (Boardman, 2004). During Wave 5 of DNHS (2013), participants in the present pilot study were asked as part of a telephone interview to recall whether any PTEs or SLEs previously reported, occurred specifically during their pregnancy with their index child. Women were categorized as having experienced 0 vs. ≥ 1 PTE or SLE during pregnancy with the index child, respectively.
The outcome of interest in this study was offspring lifetime history of GAD. During Wave 5 of DNHS (2013), pilot study participants were administered, via telephone, the Diagnostic Interview Schedule for Children, 4th edition (DISC-IV), parent version: a comprehensive, structured interview that covers 36 mental health disorders for children and adolescents aged 6–18 years, based upon DSM-IV criteria (American Psychiatric Association, 1994). For the purpose of this study, we limited the interview to contain questions from the diagnostic section pertaining to GAD and included the “whole life” module, which determines whether diagnoses not present in the past year occurred prior to the past year but after age 5. Offspring who met the criteria for GAD in the past year or based upon the “whole life” module were categorized as having a lifetime history of GAD and those not meeting the criteria were categorized as having no lifetime history of GAD.
Maternal HSV-1 and CMV seropositivity were the mediators of interest in this study. Two, 3.22 mm punches from archived bloodspot samples corresponding to the index child of each pilot study participant, that had been stored in temperature and humidity controlled space in the MNB, were shipped to Stanley Laboratory of Developmental Neurovirology at Johns Hopkins University School of Medicine. Samples were tested for presence and level of IgG antibodies targeted against HSV-1 and CMV using a solid phase enzyme immunoassay as described previously (Mortensen et al., 2007). Briefly, the bloodspots were eluted in 200 ul of phosphate buffered saline PH7.4 for 1 h at 35 degrees and overnight at room temperature. Eluates were collected and assayed for IgG class antibodies to HSV-1 and CMV using commercially available assays (Focus Diagnostic Assays, Cypress, CA, and Immuno-Biological Laboratories Inc, Minneapolis, MN, respectively). Antibody binding was manifested by the conversion of visual enzyme substrate as quantified by means of a microplate colorimeter. HSV-1 and CMV IgG antibody levels were computed by subtracting the absorbance generated by a blank sample from that generated by the test sample. Individuals with levels ≥ 0.02 absorbance units were categorized as seropositive.
Several covariates of interest were also collected in this study. During the Wave 5 phone interview, participants self-reported several factors we hypothesize as potential confounders including age (years) at time of birth of their child, race/ethnicity (Non-Hispanic white, black, or other), household income level during 12 months prior to the birth of their child (< $15000, $15000-$35000, >$35000), and current education level (< High School, High School and > High School). Women also self-reported several other behavioral characteristics of interest including smoking status and alcohol use during pregnancy and were categorized as ever verses never smoked and ever vs. never drank alcohol. Offspring sociodemographic characteristics reported by women during the phone interview included gestational age at birth (reported as weeks or months and converted to corresponding weeks), current age (years), and gender (female vs. male).
Statistical Analyses
Univariate distributions of sociodemographic and behavioral characteristics of women and offspring as well as the bivariate associations between covariates of interest and offspring lifetime history of GAD were estimated. For continuous, but non-normally distributed variables (i.e., maternal age, CMV, and HSV-1 IgG antibody level, income level and offspring age at interview) Wilcoxon rank sum tests were performed and median and interquartile range (IQR) was estimated for each group. Differences in proportions for categorical variables were estimated using Fisher's exact tests to accommodate small cell sizes. Logistic regression was used to estimate the association between maternal exposure to ≥1 PTE or SLE during pregnancy and lifetime history of GAD in offspring, respectively. Models were first unadjusted and then adjusted for hypothesized confounders of interest including maternal age at time of offspring birth, race/ethnicity and education level. While hypothesized as a potential confounder, we did not control for household income level during the 12 months prior to their index child's birth as 27.5% of individuals were missing data on this variable. Statistical analyses were carried out using SAS, version 9.2 (SAS Institute, Cary, NC).
Results
Descriptive characteristics of women and offspring as well as the bivariate relationships between covariates of interested and offspring lifetime history of GAD are shown in Table 1. Of the 69 women who participated in our pilot study, the median age at time of their index child's birth was 31 years (IQR: 25–35.5), 82.6% self-identified as African-American or Black, 22.1% had ≤ high school education, 4.4% drank alcohol during pregnancy, 8.7% smoked during pregnancy, and 18.2 and 31.9% experienced ≥1 PTE or SLE during pregnancy, respectively. PTEs experienced by women during pregnancy included “other injuries or shocking experiences” such as a natural disaster or witnessing violence directed at someone else (e.g., someone being killed; N = 3), “learning of traumatic events to close friends or relatives” (e.g., learning that a close friend or relative was raped or sexually assaulted, seriously physically attacked, or seriously injured in another type of accident; N = 4), and/or experiencing the “sudden, unexpected death of a close friend or relative”(N = 5). Among women who experienced SLEs during pregnancy (N = 22), the greatest proportion experienced socioeconomic stressors (59.1%; e.g., job loss, seeking employment for at least 3 months, or serious financial problems) and/or inter-personal stressors (54.5%; e.g., parent or a family member who had a problem with drugs or alcohol, divorce or “break up,” or emotional mistreatment). Of the women for whom neonatal bloodspots corresponding to their index child were retrieved and tested (N = 22), 40.9 and 4.5% were seropositive for CMV and HSV-1, respectively. The majority of offspring included in our study had a gestational age of 37 (range 26–42) weeks or greater (78.3%), the median (IQR) age of offspring at time of maternal interview was 12.0 (8–15) years, 50.7% were female, and 1.5% and 10.8% met the criteria for past year and lifetime history of GAD, respectively. Lower maternal education level (p = 0.1001) and younger gestational age (p = 0.0664) were the only factors to be marginally statistically significantly associated with lifetime history of GAD in offspring.
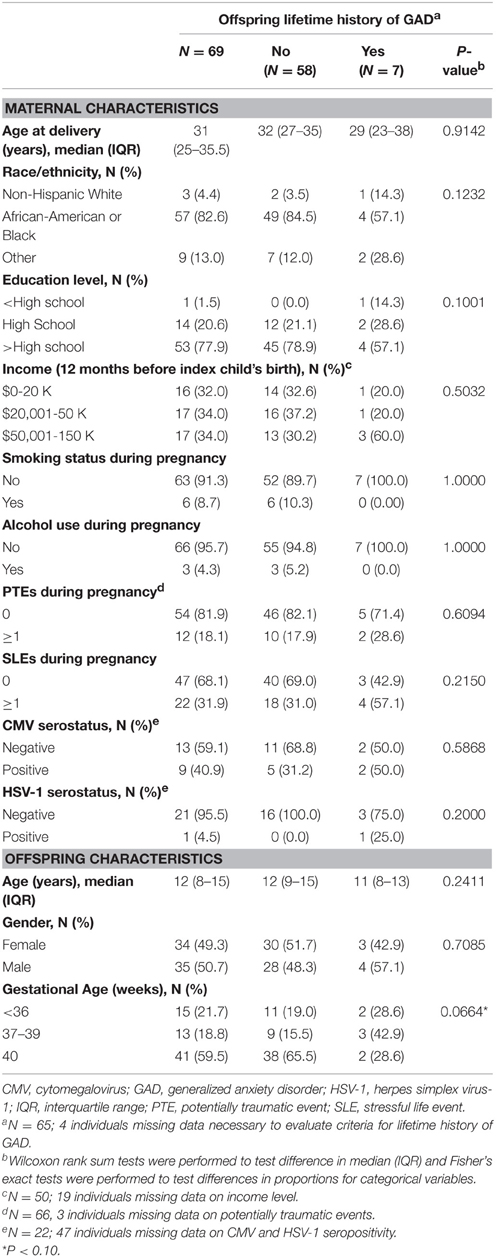
Table 1. Sociodemographic characteristics of a subset of women in the Detroit Neighborhood Health Study and their children.
The association between maternal experience of ≥1 PTE and SLE during pregnancy and offspring GAD, respectively, are shown in Table 2. We identified a positive, although not statistically significant association between maternal experience of ≥1 PTE or SLE during pregnancy and lifetime history of GAD in offspring [odds ratio (OR) 1.78, 95% confidence interval (CI): 0.25, 12.87, and OR 3.84, 95% CI: 0.68, 21.63, respectively], in models controlling for maternal age at time of offspring birth, race/ethnicity and current education level. A greater proportion of offspring who met the criteria for lifetime history of GAD were born to women seropositive for CMV and HSV-1, compared to those without lifetime history, but these differences were not statistically significant (see Table 1). Given the limited number of individuals for which samples were tested for maternal HSV-1 and CMV seropositivity and lack of statistically significant findings, we did not further examine the mediating role of herpesvirus seropositivity in the pathway between maternal PTEs or SLEs during pregnancy and lifetime history of GAD in offspring.
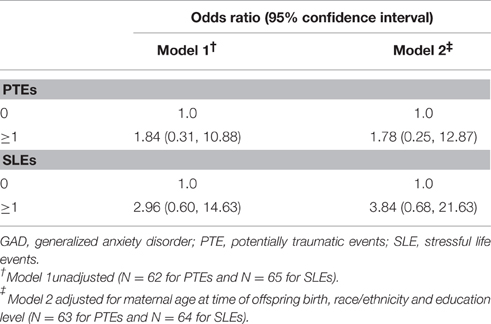
Table 2. Associations between maternal PTEs and SLEs during pregnancy and offspring lifetime history of GAD among a subset of women in the Detroit Neighborhood Health Study and their children.
Discussion
In this pilot study, we aimed to (1) re-contact women in DNHS with existing data on lifetime history of PTEs or SLEs and ascertain data on lifetime history of GAD in their youngest child between the ages of 6–17 born in Michigan (i.e., index child), (2) obtain maternal consent for retrieval and testing of neonatal bloodspot samples archived in the MNB corresponding to each index child and testing for markers of maternal CMV and HSV-1 seropositivity, and (3) examine whether maternal herpesvirus infection partially mediates the association between maternal PTEs or SLEs during pregnancy and offspring GAD.
Recent community-based surveys conducted across several cities in Michigan indicate that many individuals are not aware they or their children have archived bloodspot samples stored in the MNB and that there is lack of consensus among Michigan residents about use of these samples in research and ideal consent policies and practices (Duquette et al., 2012; Platt et al., 2014; Thiel et al., 2014). We took several measures to foster knowledge regarding the storage and use of neonatal bloodspots as well as options for consent among participants in our study. For example, we included an informational pamphlet, After Newborn Screening, provided by MDHHS, with the consent materials mailed to individuals in our study. We also conducted follow-up phone calls to study participants to answer questions and/or provide assistance with filling-out the consent materials and offered for study staff pick-up consent materials directly from participants. While 78.3% of women who completed the interview portion of the pilot study were willing to be mailed consent materials for participation in the bloodspot portion of our study, only 44.4% returned the consent form to study staff. It is possible that alternative processes for obtaining individual consent for use of bloodspot samples archived in the MNB, such as a recently proposed web-based portal (Thiel et al., 2015), could both serve as a more effective medium for providing participants information about consent options and increase participation rates in future studies by lowering participant burden.
Although participation in the bloodspot portion of our study was limited, we successfully tested retrieved samples that had been stored in temperature and humidity controlled conditions for maternal IgG antibodies specific for HSV-1 and CMV. Seroprevalence of HSV-1 and CMV among those tested in our sample was slightly lower than U.S. population-based estimates among women of childbearing age (Bate et al., 2010; Bradley et al., 2014). Lower than expected seroprevalence of these infections in our sample may be explained by self-selection bias as women who consented to retrieval and testing of their index child's bloodspot samples had characteristics associated with lower seroprevalence [i.e., were slightly younger and a greater proportion self-reported Non-Hispanic White race compared to those that did not provide consent (data not shown)]; (Schillinger et al., 2004; Dowd et al., 2009). While modified elution methods, reaction conditions, or sample volumes may serve to optimize results in future studies we have importantly established that samples stored in temperature and humidity controlled conditions in the MNB can reasonably be utilized to detect maternal IgG antibodies targeted against herpesviruses. This finding is important given the majority of available neonatal bloodspots (i.e., those obtained from those born before 2009) have been stored under these conditions.
The last aim of our study was to examine the mediating role of maternal herpesvirus seropositivity in the association between maternal PTEs and SLEs during pregnancy and offspring GAD. While we were underpowered to detect statistically significant associations between maternal experience of PTEs or SLEs during pregnancy and offspring GAD due to limited sample size, effect estimates observed in our study were in the expected direction. Indeed, several other studies that have identified a positive association between maternal trauma or stress during pregnancy and anxiety disorders in offspring during childhood (Najman et al., 2010; Davis and Sandman, 2012; Betts et al., 2014; Park et al., 2014). For example, Davis et al. recently examined the association between maternal perceived stress during pregnancy and offspring anxiety at ages 6 and 9 assessed via the Anxiety Problems subscale of the Child Behavior Checklist (CBC) among a longitudinal cohort of 178 mother-child pairs recruited from obstetric clinics in Southern California (Davis and Sandman, 2012). The authors found that higher average perceived stress during pregnancy (assessed at 19, 25, and 31 gestational weeks) was associated with higher anxiety score in offspring 6–9 years of age [beta = 0.20, F(4, 177) = 7.1, p < 0.05], adjusting for child's sex, gestational age at birth, maternal education level as well as maternal stress at time of assessment (Davis and Sandman, 2012). The authors also found that 20 weeks of gestation may be a particularly sensitive period during which fetal exposure to elevated maternal perceived stress increases risk for offspring anxiety (Davis and Sandman, 2012). In another study by Najman et al. the authors examined the association between maternal poverty during pregnancy and offspring anxiety assessed via the Youth Self-Report and Young Adult Self-Report questionnaires at age 14 and 21, respectively, among a longitudinal cohort of individuals born in Brisbane, Australia between 1981 and 1984 (Najman et al., 2010). The authors found that maternal poverty during pregnancy was associated with increased odds of anxiety reported at both ages (OR 1.7, 95% CI: 1.2, 1.5) (Najman et al., 2010). This association was, however, no longer statistically significant after adjusting for postnatal poverty and maternal distress (Najman et al., 2010). Taken together future studies which examine the association between a wider array of prenatal stressors and offspring anxiety disorders and also investigate whether prenatal exposure to maternal stress during particular periods of gestation and independent of postnatal stressors, poses increased risk for such disorders are warranted to clarify these associations.
We found that a greater proportion of offspring who met the criteria for lifetime history of GAD were born to women seropositive for CMV and HSV-1, compared to those without lifetime history. These associations were, however, not statistically significant and given limited participation in the bloodspot portion of the study, we did not further assess the mediating role of maternal herpesvirus infection in this pathway. While previous studies have examined the association between maternal herpesvirus infection and offspring mental health outcomes including schizophrenia (Buka et al., 2008) and mood disorders (Simanek and Meier, 2015), we are unaware of any studies that have assessed the role of such pathogens in the etiology of offspring anxiety disorders. A recent study by Betts et al. however, examined the association between self-reported genital infection among women during pregnancy and offspring anxiety disorders including GAD, social and specific phobias, panic disorders and PTSD among participants in the Mater University Study of pregnancy (Betts et al., 2015). The authors found that self-reported maternal genital infection during pregnancy was statistically significantly associated with offspring PTSD (OR 2.38, 95% CI: 1.14, 4.95) and social phobia (OR 1.93, 95% CI: 1.03, 3.61), but not GAD or panic disorders in models adjusting for antenatal maternal anxiety and depression, maternal age, smoking status, alcohol use, parity, and education level as well as offspring birth weight and gender (Betts et al., 2015). The association between maternal genital infection during pregnancy and PTSD was statistically significant and even stronger among male offspring, but not among females (Betts et al., 2015). These findings are consistent with some (Enayati et al., 2012), but not all (Babri et al., 2014), animal-based studies that have examined sex-specific effects of maternal immune activation during pregnancy and anxiety-like behaviors in offspring. Given the limited number of women that consented for retrieval and testing of their index child's bloodspots and lack of statistically significant findings, we did not further assess whether HSV-1 or CMV seropositivity mediated the association between maternal exposure to PTEs or SLEs during pregnancy and GAD in offspring. Future studies examining the association between maternal trauma and stress as well as infection with herpesviruses as well as other pathogens during pregnancy and a wider array of offspring anxiety disorders that are conducted among larger cohorts are warranted to clarify these relationships.
In addition to small sample size, there are a few other limitations to consider in the present pilot study. First, ascertainment of data on PTEs and SLEs among women during pregnancy as well as experiences of their children related to criteria for GAD could be subject to recall bias. Indeed, it has been demonstrated that memory impairment is a common sequelae of trauma exposure (Bremner, 2006). If women who experienced PTEs or SLEs during pregnancy were less likely to recall experiences of their child relevant to the GAD criteria, compared to women who did not, this could have served to bias our results toward the null. Another limitation of the present study was that psychometric performance of the “whole life” modules of the DISC-IV has not, to date, been evaluated (Shaffer et al., 2000). Furthermore, other mental health disorders and substance abuse disorders or medical conditions are not ruled out as part of the diagnostic criteria for GAD (Columbia University DISC Development Group, 2006a). Nonetheless, use of DISC-IV, parent version was advantageous as it is designed to be administered by interviewers with no formal clinical training (Columbia University DISC Development Group, 2006b) and we successfully adapted this tool be administered via a telephone interview.
The exact timing of acquisition of herpesvirus infection among women in our study could also not be determined and moreover, we did not assess herpesvirus infection in the index children of participants. For this reason questions remain regarding the role of timing of maternal infection and we cannot rule out the contribution of perinatal transmission of these infections in this pathway. We also cannot rule out the role of potentially heritable genetic polymorphisms that may both predispose women and their offspring to an exacerbated stress response (Ming et al., 2015), as an explanation for the associations observed. Last, while we adjusted for current maternal education level, we did not control for other postnatal experiences that may also increase risk for the onset of anxiety in offspring. For this reason, future studies should consider assessing markers of herpesvirus infection in offspring as well as women both before and during pregnancy and take into account genetic as well as postnatal risk factors for anxiety to clarify the role of these prenatal exposures of interest in the etiology of offspring anxiety disorders.
Overall, the growing body of human and animal-based research suggesting that maternal trauma and stress (Yehuda et al., 2001; Najman et al., 2010; Rice et al., 2010; Davis and Sandman, 2012) as well as infections (Enayati et al., 2012; Babri et al., 2014; Betts et al., 2015; Depino, 2015) during pregnancy may play a key role in the etiology of offspring anxiety disorders warrants further study of these relationships. If herpesviruses are identified in future studies as novel mediators in the pathway between maternal trauma or stress and offspring anxiety disorders, interventions aimed at infection prevention and/or improving stress-induced immune alterations during pregnancy may serve to decrease the onset of such conditions across generations. Importantly, neonatal bloodspot samples archived in biobanks such as the MNB, can serve as a novel alternative to more costly and invasive collection of maternal blood samples during pregnancy for studying the role of maternal infection in the etiology of offspring anxiety disorders as well as other neuropsychiatric outcomes.
Author Contributions
AS conceived the study aims and design, oversaw data collection, conducted the statistical analyses, interpreted the findings, and drafted the initial manuscript. AA contributed to conceptualization of the study aims and design and oversight of data collection, assisted with interpretation of findings, and provided critical review of the manuscript. MU contributed to conceptualization of the study aims and design, assisted with interpretation of findings, and provided critical review of the manuscript. RY oversaw sample testing, contributed to interpretation of study findings, and provided critical review of the manuscript. All authors have approved the manuscript in its final version.
Funding
We acknowledge funding from the Michigan Bloodspot Environmental Epidemiology Project (MI-BLEEP) [to AS], the Stanley Medical Research Institute [to AA and RY] and the National Institutes of health [grant numbers R01DA022720, R01DA022720-Revistion, R01DA022720-S1, and R01AG040115 to AA].
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the individuals in DNHS who participated in this pilot study.
References
American Psychiatric Association (1994). Diagnostic and Statistical Manual of Mental Disorders, 4th Edn. Washington, DC.
Ashford, J., van Lier, P. A., Timmermans, M., Cuijpers, P., and Koot, H. M. (2008). Prenatal smoking and internalizing and externalizing problems in children studied from childhood to late adolescence. J. Am. Acad. Child Adolesc. Psychiatry 47, 779–787. doi: 10.1097/CHI.0b013e318172eefb
Babri, S., Doosti, M. H., and Salari, A. A. (2014). Tumor necrosis factor-alpha during neonatal brain development affects anxiety- and depression-related behaviors in adult male and female mice. Behav. Brain Res. 261, 305–314. doi: 10.1016/j.bbr.2013.12.037
Bate, S. L., Dollard, S. C., and Cannon, M. J. (2010). Cytomegalovirus seroprevalence in the United States: the National Health and Nutrition Examination Surveys, 1988–2004. Clin. Infect. Dis. 50, 1439–1447. doi: 10.1086/652438
Bennett, J. M., Glaser, R., Malarkey, W. B., Beversdorf, D. Q., and Peng, J. Kiecolt-Glaser, J. K. (2012). Inflammation and reactivation of latent herpesviruses in older adults. Brain Behav. Immun. 26, 739–746. doi: 10.1016/j.bbi.2011.11.007
Betts, K. S., Salom, C. L., Williams, G. M., Najman, J. M., and Alati, R. (2015). Associations between self-reported symptoms of prenatal maternal infection and post-traumatic stress disorder in offspring: evidence from a prospective birth cohort study. J. Affect. Disord. 175, 241–247. doi: 10.1016/j.jad.2015.01.011
Betts, K. S., Williams, G. M., Najman, J. M., and Alati, R. (2014). Maternal depressive, anxious, and stress symptoms during pregnancy predict internalizing problems in adolescence. Depress. Anxiety 31, 9–18. doi: 10.1002/da.22210
Boardman, J. D. (2004). Stress and physical health: the role of neighborhoods as mediating and moderating mechanisms. Soc. Sci. Med. 58, 2473–2483. doi: 10.1016/j.socscimed.2003.09.029
Bradley, H., Markowitz, L. E., Gibson, T., and McQuillan, G. M. (2014). Seroprevalence of herpes simplex virus types 1 and 2–United States, 1999-2010. J. Infect. Dis. 209, 325–333. doi: 10.1093/infdis/jit458
Bremner, J. D. (2006). Traumatic stress: effects on the brain. Dialogues Clin. Neurosci. 8, 445–461.
Breslau, N., Kessler, R. C., Chilcoat, H. D., Schultz, L. R., Davis, G. C., and Andreski, P. (1998). Trauma and posttraumatic stress disorder in the community: the 1996 Detroit Area Survey of Trauma. Arch. Gen. Psychiatry 55, 626–632. doi: 10.1001/archpsyc.55.7.626
Buka, S. L., Cannon, T. D., Torrey, E. F., Yolken, R. H., and Collaborative Study Group on the Perinatal Origins of Severe Psychiatric Disorders (2008). Maternal exposure to herpes simplex virus and risk of psychosis among adult offspring. Biol. Psychiatry 63, 809–815. doi: 10.1016/j.biopsych.2007.09.022
Christian, L. M., Iams, J. D., Porter, K., and Glaser, R. (2012). Epstein-Barr virus reactivation during pregnancy and postpartum: effects of race and racial discrimination. Brain Behav. Immun. 26, 1280–1287. doi: 10.1016/j.bbi.2012.08.006
Dammann, O., and Leviton, A. (1997). Maternal intrauterine infection, cytokines, and brain damage in the preterm newborn. Pediatr. Res. 42, 1–8. doi: 10.1203/00006450-199707000-00001
Davis, E., Glynn, L., Waffarn, F., and Sandman, C. (2011). Prenatal maternal stress programs infant stress regulation. J. Child Psychol. Psychiatry 52, 119–129. doi: 10.1111/j.1469-7610.2010.02314.x
Davis, E. P., and Sandman, C. A. (2012). Prenatal psychobiological predictors of anxiety risk in preadolescent children. Psychoneuroendocrinology 37, 1224–1233. doi: 10.1016/j.psyneuen.2011.12.016
Depino, A. M. (2015). Early prenatal exposure to LPS results in anxiety- and depression-related behaviors in adulthood. Neuroscience 299, 56–65. doi: 10.1016/j.neuroscience.2015.04.065
Dowd, J. B., and Aiello, A. E. (2009). Socioeconomic differentials in immune response. Epidemiology 20, 902–908. doi: 10.1097/EDE.0b013e3181bb5302
Dowd, J. B., Aiello, A. E., and Alley, D. E. (2009). Socioeconomic disparities in the seroprevalence of cytomegalovirus infection in the US population: NHANES III. Epidemiol. Infect. 137, 58–65. doi: 10.1017/S0950268808000551
Dowd, J. B., Haan, M. N., Blythe, L., Moore, K., and Aiello, A. E. (2008). Socioeconomic gradients in immune response to latent infection. Am. J. Epidemiol. 167, 112–120. doi: 10.1093/aje/kwm247
Dowd, J. B., Palermo, T. M., and Aiello, A. E. (2012). Family poverty is associated with cytomegalovirus antibody titers in U.S. children. Health Psychol. 31, 5–10. doi: 10.1037/a0025337
Duquette, D., Langbo, C., Bach, J., and Kleyn, M. (2012). Michigan BioTrust for Health: public support for using residual dried blood spot samples for health research. Public Health Genomics 15, 146–155. doi: 10.1159/000336565
Ekblad, M., Gissler, M., Lehtonen, L., and Korkeila, J. (2010). Prenatal smoking exposure and the risk of psychiatric morbidity into young adulthood. Arch. Gen. Psychiatry 67, 841–849. doi: 10.1001/archgenpsychiatry.2010.92
Enayati, M., Solati, J., Hosseini, M. H., Shahi, H. R., Saki, G., and Salari, A. A. (2012). Maternal infection during late pregnancy increases anxiety- and depression-like behaviors with increasing age in male offspring. Brain Res. Bull. 87, 295–302. doi: 10.1016/j.brainresbull.2011.08.015
Glaser, R., and Kiecolt-Glaser, J. K. (1994). “Stress-associated immune modulation and its implications for reactivation of latent herpesviruses,” in Human Herpesvirus Infections, eds R. Glaser and J. Jones (New York, NY: Dekker), 245–270.
Glover, V., O'Connor, T. G., and O'Donnell, K. (2010). Prenatal stress and the programming of the HPA axis. Neurosci. Biobehav. Rev. 35, 17–22. doi: 10.1016/j.neubiorev.2009.11.008
Heim, C., and Nemeroff, C. B. (2001). The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol. Psychiatry 49, 1023–1039. doi: 10.1016/S0006-3223(01)01157-X
Hellemans, K. G., Sliwowska, J. H., Verma, P., and Weinberg, J. (2010). Prenatal alcohol exposure: fetal programming and later life vulnerability to stress, depression and anxiety disorders. Neurosci. Biobehav. Rev. 34, 791–807. doi: 10.1016/j.neubiorev.2009.06.004
Hovens, J. G., Giltay, E. J., Spinhoven, P., van Hemert, A. M., and Penninx, B. W. (2015). Impact of childhood life events and childhood trauma on the onset and recurrence of depressive and anxiety disorders. J. Clin. Psychiatry 76, 931–938. doi: 10.4088/JCP.14m09135
Hovens, J. G., Giltay, E. J., Wiersma, J. E., Spinhoven, P., Penninx, B. W., and Zitman, F.G. (2012). Impact of childhood life events and trauma on the course of depressive and anxiety disorders. Acta Psychiatr. Scand. 126, 198–207. doi: 10.1111/j.1600-0447.2011.01828.x
Hovens, J. G., Wiersma, J. E., Giltay, E. J., van Oppen, P., Spinhoven, P., Penninx, B. W., et al. (2010). Childhood life events and childhood trauma in adult patients with depressive, anxiety and comorbid disorders vs. controls. Acta Psychiatr. Scand. 122, 66–74. doi: 10.1111/j.1600-0447.2009.01491.x
Indredavik, M. S., Brubakk, A. M., Romundstad, P., and Vik, T. (2007). Prenatal smoking exposure and psychiatric symptoms in adolescence. Acta Paediatr. 96, 377–382. doi: 10.1111/j.1651-2227.2006.00148.x
Klauke, B., Deckert, J., Reif, A., Pauli, P., Zwanzger, P., Baumann, C., et al. (2011). Serotonin transporter gene and childhood trauma—a G × E effect on anxiety sensitivity. Depress. Anxiety 28, 1048–1057. doi: 10.1002/da.20840
Kuo, J. R., Goldin, P. R., Werner, K., Heimberg, R. G., and Gross, J. J. (2011). Childhood trauma and current psychological functioning in adults with social anxiety disorder. J. Anxiety Disord. 25, 467–473. doi: 10.1016/j.janxdis.2010.11.011
Lochner, C., Seedat, S., Allgulander, C., Kidd, M., Stein, D., and Gerdner, A. (2010). Childhood trauma in adults with social anxiety disorder and panic disorder: a cross-national study. Afr. J. Psychiatry (Johannesbg). 13, 376–381.
Machado, C. J., Whitaker, A. M., Smith, S. E., Patterson, P. H., and Bauman, M. D. (2015). Maternal immune activation in nonhuman primates alters social attention in juvenile offspring. Biol. Psychiatry 77, 823–832. doi: 10.1016/j.biopsych.2014.07.035
Marques, A. H., O'Connor, T. G., Roth, C., Susser, E., and Bjorke-Monsen, A. L. (2013). The influence of maternal prenatal and early childhood nutrition and maternal prenatal stress on offspring immune system development and neurodevelopmental disorders. Front. Neurosci. 7:120. doi: 10.3389/fnins.2013.00120
Merikangas, K. R., He, J. P., Burstein, M., Swanson, S. A., Avenevoli, S., Cui, L., et al. (2010). Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication–Adolescent Supplement (NCS-A). J. Am. Acad. Child Adolesc. Psychiatry 49, 980–989. doi: 10.1016/j.jaac.2010.05.017
Miguel-Hidalgo, J. J. (2013). Brain structural and functional changes in adolescents with psychiatric disorders. Int. J. Adolesc. Med. Health 25, 245–256. doi: 10.1515/ijamh-2013-0058
Ming, Q., Zhang, Y., Yi, J., Wang, X., Zhu, X., and Yao, S. (2015). Serotonin transporter gene polymorphism (5-HTTLPR) L allele interacts with stress to increase anxiety symptoms in Chinese adolescents: a multiwave longitudinal study. BMC Psychiatry 15:248. doi: 10.1186/s12888-015-0639-y
Mortensen, P. B., Norgaard-Pedersen, B., Waltoft, B. L., Sorensen, T. L., Hougaard, D., Torrey, E. F., et al. (2007). Toxoplasma gondii as a risk factor for early-onset schizophrenia: analysis of filter paper blood samples obtained at birth. Biol. Psychiatry 61, 688–693. doi: 10.1016/j.biopsych.2006.05.024
Moylan, S., Gustavson, K., Øverland, S., Karevold, E. B., Jacka, F. N., Pasco, J. A., and Berk, M. (2015). The impact of maternal smoking during pregnancy on depressive and anxiety behaviors in children: the Norwegian Mother and Child Cohort Study. BMC Med. 13, 1–12. doi: 10.1186/s12916-014-0257-4
Najman, J. M., Hayatbakhsh, M. R., Clavarino, A., Bor, W., O'Callaghan, M. J., and Williams, G. M. (2010). Family poverty over the early life course and recurrent adolescent and young adult anxiety and depression: a longitudinal study. Am. J. Public Health 100, 1719–1723. doi: 10.2105/AJPH.2009.180943
Nazmi, A., Diez-Roux, A. V., Jenny, N. S., Tsai, M. Y., Szklo, M., and Aiello, A. E. (2010). The influence of persistent pathogens on circulating levels of inflammatory markers: a cross-sectional analysis from the Multi-Ethnic Study of Atherosclerosis. BMC Public Health 10:706. doi: 10.1186/1471-2458-10-706
Park, S., Kim, B. N., Kim, J. W., Shin, M. S., Yoo, H. J., Lee, J., et al. (2014). Associations between maternal stress during pregnancy and offspring internalizing and externalizing problems in childhood. Int. J. Ment. Health Syst. 8:44. doi: 10.1186/1752-4458-8-44
Pedersen, A., Zachariae, R., and Bovbjerg, D. H. (2010). Influence of psychological stress on upper respiratory infection–a meta-analysis of prospective studies. Psychosom. Med. 72, 823–832. doi: 10.1097/PSY.0b013e3181f1d003
Perroud, N., Rutembesa, E., Paoloni-Giacobino, A., Mutabaruka, J., Mutesa, L., Stenz, L., et al. (2014). The Tutsi genocide and transgenerational transmission of maternal stress: epigenetics and biology of the HPA axis. World J. Biol. Psychiatry 15, 334–345. doi: 10.3109/15622975.2013.866693
Platt, T., Platt, J., Thiel, D. B., Fisher, N., and Kardia, S. L. (2014). ‘Cool! and creepy’: engaging with college student stakeholders in Michigan's biobank. J. Community Genet. 5, 349–362. doi: 10.1007/s12687-014-0190-4
Pynoos, R. S., Steinberg, A. M., and Piacentini, J. C. (1999). A developmental psychopathology model of childhood traumatic stress and intersection with anxiety disorders. Biol. Psychiatry 46, 1542–1554. doi: 10.1016/S0006-3223(99)00262-0
Radtke, K., Ruf, M., Gunter, H., Dohrmann, K., Schauer, M., Meyer, A., et al. (2011). Transgenerational impact of intimate partner violence on methylation in the promoter of the glucocorticoid receptor. Transl. Psychiatry 1:e21. doi: 10.1038/tp.2011.21
Rector, J. L., Dowd, J. B., Loerbroks, A., Burns, V. E., Moss, P. A., Jarczok, M. N., et al. (2014). Consistent associations between measures of psychological stress and CMV antibody levels in a large occupational sample. Brain Behav. Immun. 38, 133–141. doi: 10.1016/j.bbi.2014.01.012
Rice, F., Harold, G. T., Boivin, J., van den Bree, M., Hay, D. F., and Thapar, A. (2010). The links between prenatal stress and offspring development and psychopathology: disentangling environmental and inherited influences. Psychol. Med. 40, 335–345. doi: 10.1017/S0033291709005911
Schelonka, R. L., and Infante, A. J. (1998). Neonatal immunology. Semin. Perinatol. 22, 2–14. doi: 10.1016/S0146-0005(98)80003-7
Schillinger, J. A., Xu, F., Sternberg, M. R., Armstrong, G. L., Lee, F. K., Nahmias, A. J., et al. (2004). National seroprevalence and trends in herpes simplex virus type 1 in the United States, 1976-1994. Sex. Transm. Dis. 31, 753–760. doi: 10.1097/01.olq.0000145852.43262.c3
Segerstrom, S. C., and Miller, G. E. (2004). Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychol. Bull. 130, 601–630. doi: 10.1037/0033-2909.130.4.601
Shaffer, D., Fisher, P., Lucas, C. P., Dulcan, M. K., and Schwab-Stone, M. E. (2000). NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. J. Am. Acad. Child Adolesc. Psychiatry 39, 28–38. doi: 10.1097/00004583-200001000-00014
Simanek, A. M., and Meier, H. C. (2015). Association between prenatal exposure to maternal infection and offspring mood disorders: a review of the literature. Curr. Probl. Pediatr. Adolesc. Health Care 45, 325–364. doi: 10.1016/j.cppeds.2015.06.008
Simister, N. E. (2003). Placental transport of immunoglobulin G. Vaccine 21, 3365–3369. doi: 10.1016/S0264-410X(03)00334-7
Skinner, M. K., Anway, M. D., Savenkova, M. I., Gore, A. C., and Crews, D. (2008). Transgenerational epigenetic programming of the brain transcriptome and anxiety behavior. PLoS ONE 3:e3745. doi: 10.1371/journal.pone.0003745
Stein, M. B., Walker, J. R., Anderson, G., and Hazen, A. L. (1996). Childhood physical and sexual abuse in patients with anxiety disorders and in a community sample. Am. J. Psychiatry 153:275. doi: 10.1176/ajp.153.2.275
Thiel, D. B., Platt, J., Platt, T., King, S. B., Fisher, N., Shelton, R., et al. (2015). Testing an online, dynamic consent portal for large population biobank research. Public Health Genomics 18, 26–39. doi: 10.1159/000366128
Thiel, D. B., Platt, T., Platt, J., King, S. B., and Kardia, S. L. (2014). Community perspectives on public health biobanking: an analysis of community meetings on the Michigan BioTrust for Health. J. Community Genet. 5, 125–138. doi: 10.1007/s12687-013-0162-0
Uddin, M., Aiello, A. E., Wildman, D. E., Koenen, K. C., Pawelec, G., de Los Santos, R., et al. (2010). Epigenetic and immune function profiles associated with posttraumatic stress disorder. Proc. Natl. Acad. Sci. U.S.A. 107, 9470–9475. doi: 10.1073/pnas.0910794107
Weaver, I., Cervoni, N., Champagne, F., D'Alessio, A., Sharma, S., Seckl, J., et al. (2004). Epigenetic programming by maternal behavior. Nat. Neurosci. 7, 847–854. doi: 10.1038/nn1276
Yehuda, R., Halligan, S. L., and Bierer, L. M. (2001). Relationship of parental trauma exposure and PTSD to PTSD, depressive and anxiety disorders in offspring. J. Psychiatr. Res. 35, 261–270. doi: 10.1016/S0022-3956(01)00032-2
Zhou, H. (2015). Region specific effects of maternal immune activation on offspring neuroimmune function. Open J. Immunol. 5, 51–63. doi: 10.4236/oji.2015.52006
Keywords: prenatal, trauma, stress, herpesvirus, offspring, generalized anxiety disorder
Citation: Simanek AM, Uddin M, Yolken RH and Aiello AE (2016) Use of Archived Neonatal Bloodspots for Examining Associations between Prenatal Exposure to Potentially Traumatic or Stressful Life Events, Maternal Herpesvirus Infection and Lifetime History of Generalized Anxiety Disorder in Offspring. Front. Environ. Sci. 4:54. doi: 10.3389/fenvs.2016.00054
Received: 01 March 2016; Accepted: 02 August 2016;
Published: 23 August 2016.
Edited by:
Jeffrey Mark Craig, Murdoch Childrens Research Institute, AustraliaReviewed by:
Lei Lu, University of Chicago, USAElena E. Terenina, INRA - Centre Toulouse Midi-Pyrénées, France
Copyright © 2016 Simanek, Uddin, Yolken and Aiello. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amanda M. Simanek, simaneka@uwm.edu
 Amanda M. Simanek
Amanda M. Simanek Monica Uddin
Monica Uddin Robert H. Yolken3
Robert H. Yolken3