Combination of Electrochemical Processes with Membrane Bioreactors for Wastewater Treatment and Fouling Control: A Review
- 1Environmental Engineering Program, National Graduate School of Engineering, University of the Philippines, Quezon City, Philippines
- 2Sanitary Environmental Engineering Division, Department of Civil Engineering, University of Salerno, Fisciano, Italy
- 3Department of Chemical Engineering, University of the Philippines, Quezon City, Philippines
This paper provides a critical review about the integration of electrochemical processes into membrane bioreactors (MBR) in order to understand the influence of these processes on wastewater treatment performance and membrane fouling control. The integration can be realized either in an internal or an external configuration. Electrically enhanced membrane bioreactors or electro membrane bioreactors (eMBRs) combine biodegradation, electrochemical and membrane filtration processes into one system providing higher effluent quality as compared to conventional MBRs and activated sludge plants. Furthermore, electrochemical processes, such as electrocoagulation, electrophoresis, and electroosmosis, help to mitigate deposition of foulants into the membrane and enhance sludge dewaterability by controlling the morphological properties and mobility of the colloidal particles and bulk liquid. Intermittent application of minute electric field has proven to reduce energy consumption and operational cost as well as minimize the negative effect of direct current field on microbial activity which are some of the main concerns in eMBR technology. The present review discusses important design considerations of eMBR, its advantages as well as its applications to different types of wastewater. It also presents several challenges that need to be addressed for future development of this hybrid technology which include treatment of high strength industrial wastewater and removal of emerging contaminants, optimization study, cost benefit analysis and the possible combination with microbial electrolysis cell for biohydrogen production.
Introduction
Numerous water and wastewater treatment processes have been employed in order to minimize water pollution and to augment drinking water resources (de Luna et al., 2009; Naddeo et al., 2011; Cesaro et al., 2013; Secondes et al., 2014; Ballesteros et al., 2016). Among these techniques, conventional activated sludge (CAS) process is the most widely used method due to its effective mineralization of organic and inorganic compounds from various types of wastewater. However, CAS has been shown to have limited capability of degrading recalcitrant pollutants such as pharmaceuticals, flame retardants, persistent organic compounds, anionic surfactants, etc. (Bernhard et al., 2006; Singhal and Perez-Garcia, 2016). Besides, this treatment method also poses several disadvantages which include high footprint and reactor volume, the production of large amount of excess sludge, that requires further treatment and disposal, and the installation of the sedimentation tank for separation of the mixed liquor from the treated effluent. Furthermore, a final disinfection treatment (e.g., UV, ozone, chlorination, etc.) is required for the removal of pathogenic microorganisms.
A more promising alternative for wastewater remediation is the combination of the activated sludge treatment with membrane filtration process. This technology, also known as membrane bioreactor (MBR), differs from CAS since a membrane module is used instead of the secondary clarifier to separate activated sludge from the final effluent (Xing et al., 2000). MBR offers many advantages such as higher effluent quality, smaller footprint and reactor volume, lower energy consumption and less sludge production (Cicek, 2003; Yuniarto et al., 2008). As a result, MBR technology has experienced unprecedented growth in recent years. Its applications have extended widely from treatment of municipal wastewater (Weiss and Reemtsma, 2008; Ma et al., 2013; Zhou et al., 2015) to removal of toxic and hazardous compounds in landfill leachate (Ahmed and Lan, 2012), and industrial wastewaters (Artiga et al., 2005; Yigit et al., 2009; Palogos et al., 2014).
However, an inevitable fouling problem limits the application of this technology into full scale practical operation. The deposition of sludge particles and colloids onto and into the membrane leads to a severe decline of the permeate flux and affects stable operational performance (Kimura et al., 2005). There are several approaches that can reduce fouling in membranes (Naddeo et al., 2015a,b; Scannapieco et al., 2015) but the most common techniques used in MBR technology are the excess aeration and frequent membrane cleaning. However, these methods also result in an increase of energy demand and operating costs as well as reduction of membrane lifespan (Wei C. H. et al., 2011; Zhang et al., 2011; Shi et al., 2014).
Recent advances have demonstrated that the application of an electrical field to membrane bioreactors (electrically enhanced membrane bioreactors or electro membrane bioreactor, eMBR) can effectively reduce fouling (Chen et al., 2007; Akamatsu et al., 2010; Liu et al., 2012a). This method induces electrochemical mechanisms such as electrocoagulation, electroosmosis, and electrophoresis that help in degradation of pollutants and, at the same time, control the mobility, and deposition of foulants onto the membrane surface (Bani-Melhem and Elektorowicz, 2011). Electrocoagulation is the main mechanism influencing the removal of organic compounds with high fouling potential from wastewater. It involves the in situ generation of coagulants from the electrochemical dissolution of immersed sacrificial anodes (usually iron or aluminum) (Figure 1C). Depending on the pH of the solution, various metal species are produced during electrocoagulation which react with the pollutants leading to the destabilization and aggregation of suspended particles and the precipitation and adsorption of dissolved contaminants (Giwa et al., 2016). Moreover, the applied voltage also allows the negatively charged foulants such as activated sludge and secreted polymers to drift toward the oppositely charged electrode and away from the membrane via electrophoretic motion (Figure 1A) (Chen et al., 2007; Akamatsu et al., 2010, 2012). Consequently, electroosmotic force propels the removal of bound water from the microbial flocs electrical double layer which decreases the sludge specific resistance to filtration hence improving fouling control (Figure 1B) (Ibeid et al., 2013a).
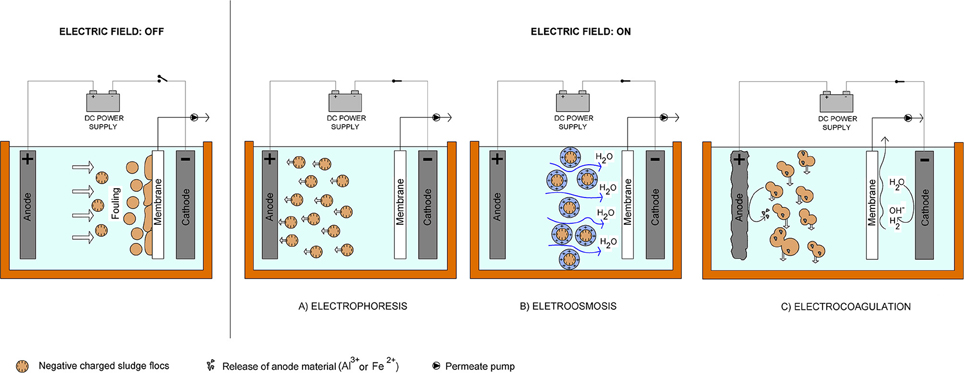
Figure 1. Electrochemical mechanisms occurring in the operation of the electro MBR (eMBR): (A) electrophoresis, (B) electroosmosis, (C) electrocoagulation.
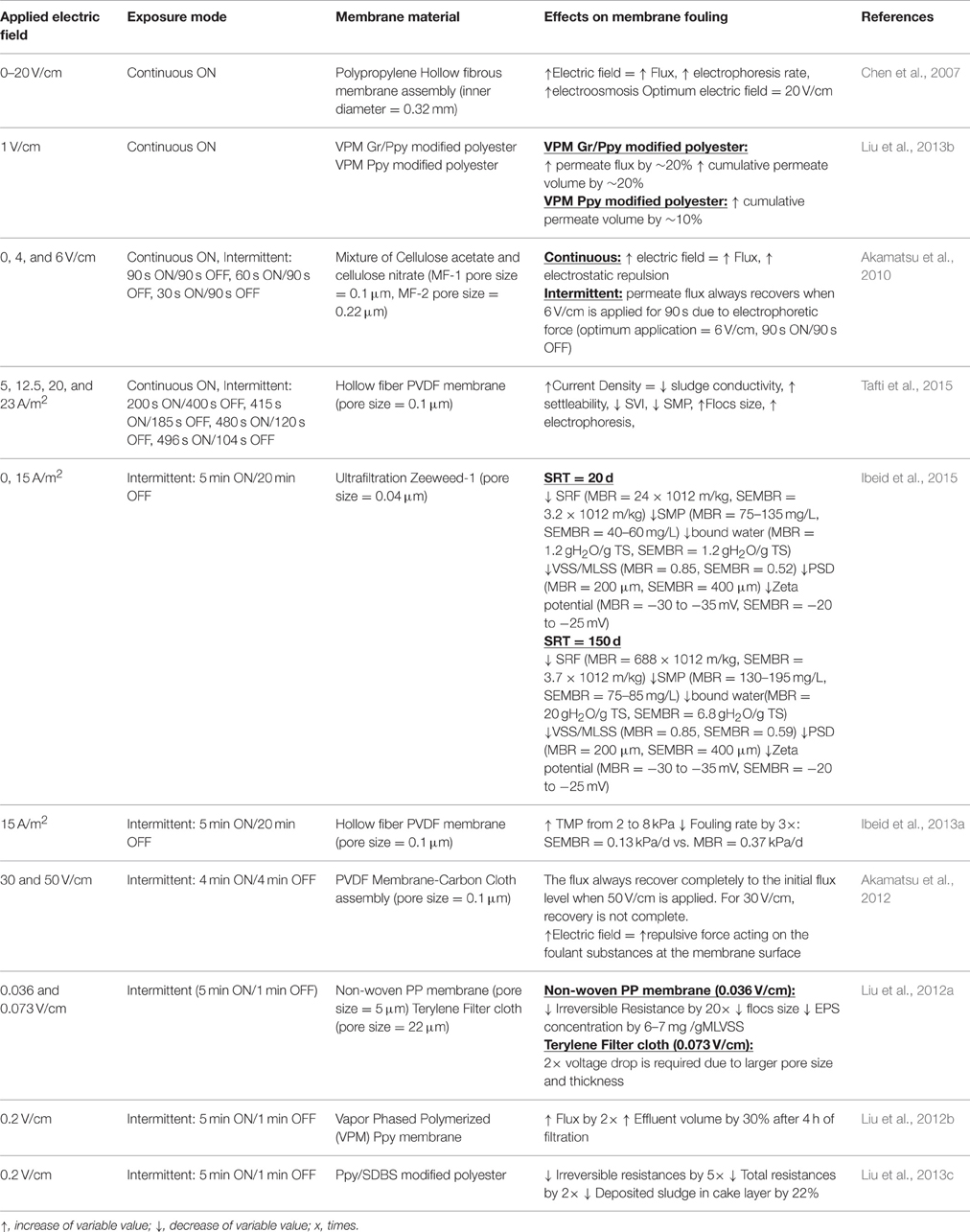
The application of a direct current (DC) field to MBRs has proven to be more effective compared to other fouling control methods, however, it is also associated with high energy consumption and affect bacterial lifespan (Wei V. et al., 2011; Giwa et al., 2015; Zeyoudi et al., 2015). New and cheaper developments suggest that intermittent application of minute electric field also enhances membrane filterability and is similarly effective in controlling fouling as that with continuous DC field (Akamatsu et al., 2010, 2012; Liu et al., 2012a,b). It also minimizes the direct exposure of bacteria to the electric field, hence, reducing the negative effects to microbial community (Akamatsu et al., 2010; Bani-Melhem and Elektorowicz, 2010; Liu et al., 2012a; Hasan et al., 2014). Some studies also focused on extracting internal energy contained in wastewater (e.g., 17.8–28.7 kJ/gCOD) (Heidrich et al., 2011) which can be used to offset the input of external DC field for fouling control. This can be achieved by combining bioelectrochemical systems (BES) with MBRs. Microbial fuel cells (MFCs) and microbial electrolysis cells (MECs) are two types of BES that use exoelectrogenic microbes to produce energy from wastewater by converting biodegradable organic matter directly into electricity, hydrogen, and other valuable products via biological and electrochemical processes (Zhang F. et al., 2014). They are innovative methods for simultaneous renewable energy production and wastewater treatment (Wang et al., 2015). Hybrid systems combining BES with MBR have also been recently investigated for cost-effective wastewater remediation and fouling control (Wang et al., 2012; Liu et al., 2013a, 2014; Li et al., 2014).
This paper critically reviews the combination of electrochemical processes with MBR. This is the first review paper that explores the advantages, the limitations, future challenges, and developments of this hybrid technology in wastewater treatment. The design considerations, applications to different types of wastewater and mechanisms of pollution removal and fouling control using electrically enhanced membrane bioreactor are also discussed.
Electrically Enhanced Membrane Bioreactor (eMBR)
This section is divided into three parts: the first part highlights three of the most important considerations for the design of eMBR which are the main configurations, materials and operating conditions; the second part enumerates the numerous applications of this technology on different types of wastewater and the third discusses the effects of appending electricity on pollutant removal, fouling control, and bacterial activity in an eMBR technology.
Design Considerations
Main Configurations
The main eMBR configurations applied in wastewater treatment can be generally classified into two: (1) External electrochemical unit (usually electrocoagulation unit) preceding a submerged membrane bioreactor (EC-SMBR) (Figure 2A) and (2) submerged membrane electro-bioreactor (SMEBR) (Figure 2B).
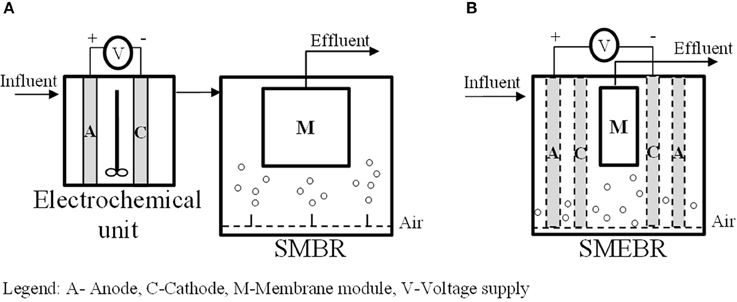
Figure 2. Main eMBR configurations applied to wastewater treatment: (A) external electrochemical unit (usually electrocoagulation unit) preceding a submerged membrane bioreactor (EC-SMBR), (B) submerged membrane electro-bioreactor (SMEBR).
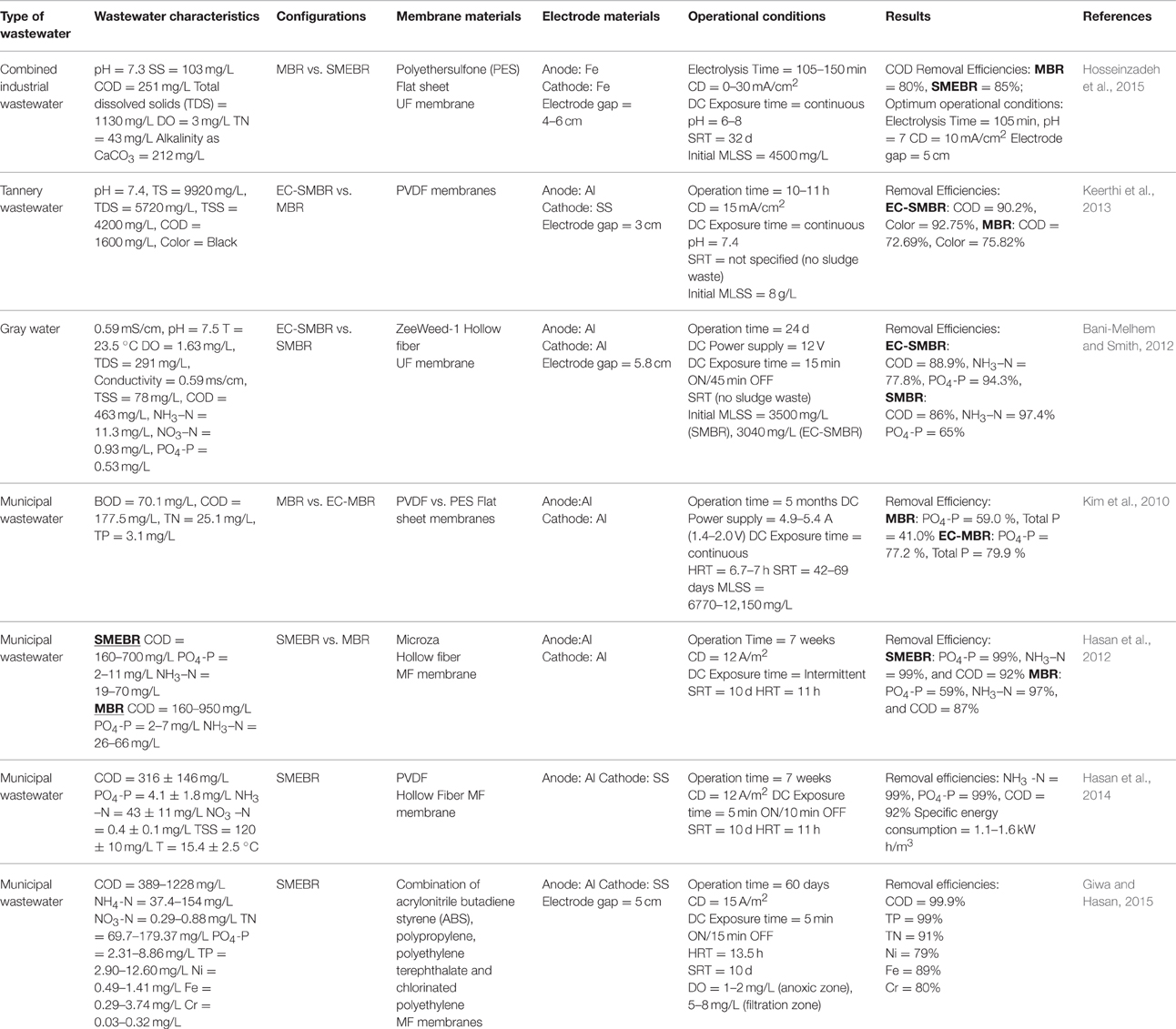
EC-SMBR was used by Kim et al. (2010), Bani-Melhem and Smith (2012), and Keerthi et al. (2013) to treat municipal wastewater, actual gray water, and tannery wastewater, respectively. Here, the influent is first treated via electrocoagulation process in a separate unit prior to biological degradation and membrane filtration in a conventional submerged membrane bioreactor (SMBR). This system was developed primarily to enhance the phosphorus removal from wastewater and prevent the applied DC field to have direct contact with the microbial community since it could have inhibitory effects on bacterial activity (Bani-Melhem and Smith, 2012). It was also proven to positively improve chemical oxygen demand (COD) and color removal from tannery wastewater (Keerthi et al., 2013).
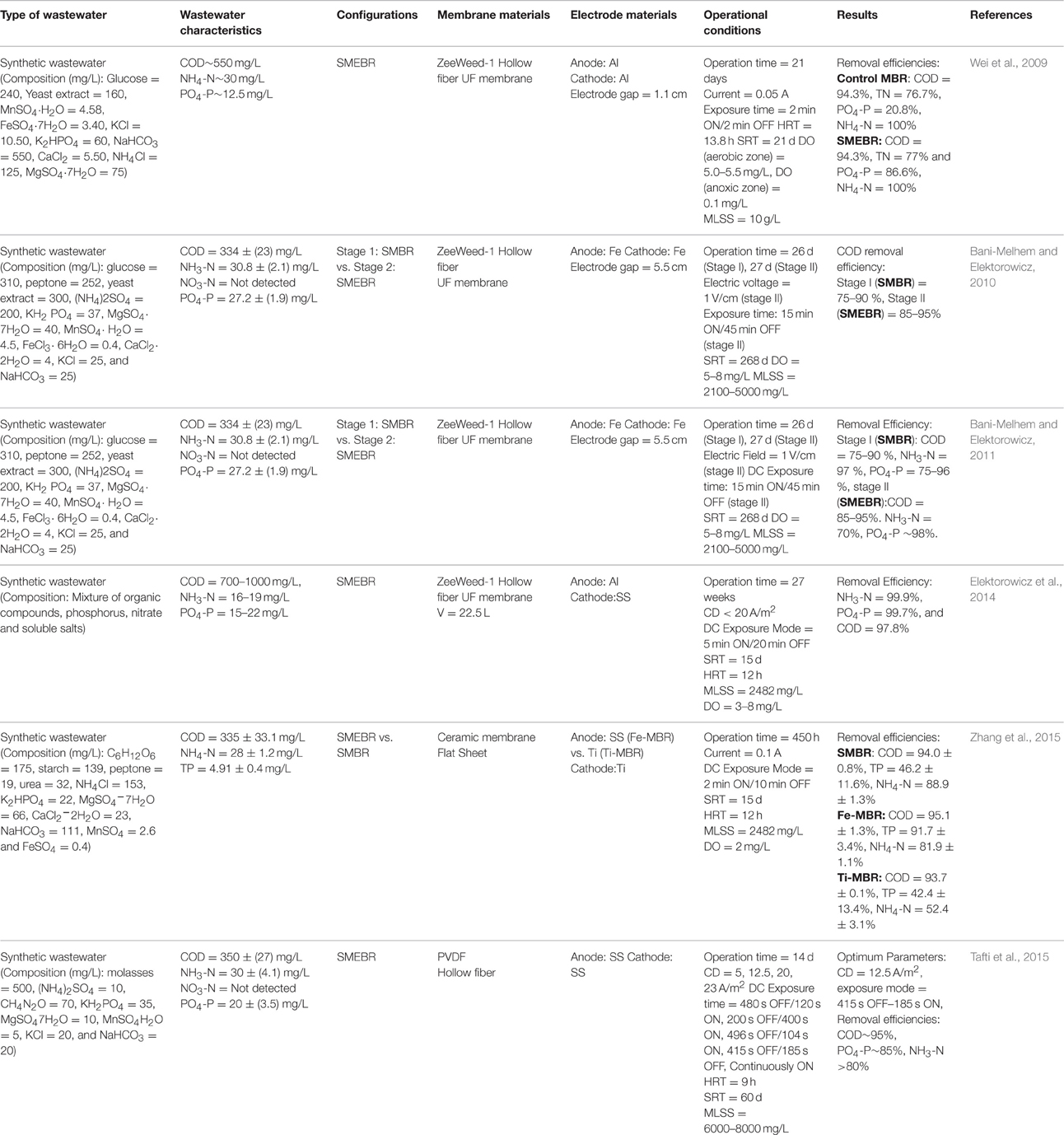
In SMEBR, on the other hand, electrochemical processes are integrated with membrane bioreactor by the addition of sacrificial electrodes inside the SMBR. The wastewater first passes through the biological treatment zone for biodegradation, that is between the reactor wall and the anode, then across the electrical zone (i.e., between the electrodes) to undergo some electrochemical processes and further degradation, and eventually is filtered out through the membrane module to remove the solids (Hasan et al., 2014) (Figure 1). In some SMEBR studies, the cathode is designed as part of the membrane module [e.g., carbon cloth electrode fabricated with polyvinylidene fluoride (PVDF) membrane or copper wire cathode inside a PP non-woven sheet] or an electrically conductive low- cost membrane is used as the cathode itself [e.g., terylene cloth modified with polypyrrole (Ppy), polyester filter cloth modified with PPy coated graphene (GR/Ppy), or with PPy coated graphene oxide (GO/Ppy)] (Akamatsu et al., 2012; Liu et al., 2012a,b, 2013b; Zhang et al., 2015). Details about membrane and electrode materials are discussed in Section Materials. The resulting effluent quality of eMBR is observed to be better than the EC-SMBR (Tables 1, 2). Moreover, fouling alleviation is further enhanced, as reported in the Section Membrane Fouling Reduction, in this configuration due to the combined efforts of electrocoagulation, electroosmosis, and electrophoresis inside the SMEBR system. A novel design of SMEBR was proposed and developed by Bani-Melhem and Elektorowicz (2010) which consists of cylindrical perforated electrodes immersed inside the bioreactor with a membrane module placed at the center.
Between the two configurations, SMEBR is increasingly becoming more popular and has been utilized in many studies (Bani-Melhem and Elektorowicz, 2010; Ibeid et al., 2013a; Hasan et al., 2014). Since it allows all the three fundamental operations (i.e., biodegradation, electrochemical processes, and membrane filtration) to take place in a single reactor vessel (smaller footprint) and it can be adapted as the most suitable design for large scale applications. Its compact characteristics and high treatment efficiency make SMEBR an economically attractive technology for wastewater treatment.
Materials
Membrane and electrode materials are equally important in designing eMBR as both can significantly affect the reactor's performance, pollutant removal, and fouling control. The membranes are responsible for the retention of microbes in the reactor which improves biological treatment and for further purification of the resulting effluent via filtration (Giwa et al., 2016). Among the numerous papers available in literature for eMBR, only few studies have been made comparing the different performances of membrane materials and these studies only focused on the effects of the membrane's pore size on fouling.Kim et al. (2010) used two plate type membranes: PVDF (mean pore size: 0.08 μm) and polyethersulfone (PES) (mean pore size: 0.2 μm) in an EC-SMBR system and observed that although both membranes maintained a stable transmembrane pressure (TMP) for a period of 5 months without chemical cleaning, PVDF has better performance, as shown in its lower average TMP (PVDF = 10 kPa vs. PES = 16 kPa) which was attributed to its smaller pore size. Apparently, membranes with large pore sizes are more susceptible to fouling as more smaller particles can migrate, attach and accumulate into the interior surfaces of the pores resulting in pore narrowing and pore blocking and eventually to irreversible fouling (Kim et al., 2010). The same conclusion was reached by Akamatsu et al. (2010) when they used MF-1 (0.1 μm) and MF-2 membranes (0.22 μm) in eMBR, both membranes were made up of a mixture of cellulose acetate and cellulose nitrate.
The high cost of membrane materials limits eMBR in large scale application. This brought the development of low cost alternatives such as non-wovens, meshes, and filter cloths (Liu et al., 2012a). Though cost efficient, these alternative filters have relatively large pore size and inadequate fouling control. Such constraint has been addressed by a group of researchers modifying low cost filters to improve its effectivity.Liu et al. (2012b) utilized polypyrrole (PPy) modified polyester filter cloth as both the cathode and filtration material in eMBR. Membrane modification was done by liquid and vapor phase polymerization using different concentrations of FeCl3·6H2O (10, 20, and 40%). Results showed that vapor phased PPy modification with 20% iron oxidants has substantially increased the membrane's electrical conductivity (lower specific resistances) and a DC field as low as 0.2 V/cm is sufficient enough to drive electrostatic repulsion between negatively charged foulants and membrane. This development also paved the way of addressing the issue on the effects of direct application of electric field on bacterial activity which is discussed in Section Microbial Activity. In another study, vapor modified PPy membranes (VPM) were coated by graphene (Gr/PPy) and graphene oxides (GO/PPy) to further enhance their conductivity (Liu et al., 2013b). Graphene and its oxides have unique electronic properties which can be enhanced by coating with polypyrrole (PPy). The electric resistance of GO/PPy modified membrane is 0.71 kΩ/cm while for Gr/PPy is 0.68 kΩ/cm. These values are significantly lower compared to 2.03 kΩ/cm for PPy modified filter cloth (Liu et al., 2013b). In terms of anti-fouling property, membrane with higher conductivity (i.e., lower electric resistance) shows improvement in permeate flux.Liu et al. (2013b) tested the antifouling performance of Gr/PPy and compared it with PPy modified filter cloth under 1.0 V/cm electric field. VPM modified Gr/PPy membrane can suppress the fouling more effectively than PPy by increasing the permeate flux and cumulative permeate volume by 20% (Table 3). PPy modified filter only increases 10% of cumulative permeate volume (Table 3). Above aforementioned studies have proven that using electrically conductive membranes as the cathode itself was possible and very effective in fouling suppression. Some low-cost membranes used in eMBR and their performances with applied electric field are included in Table 3.
Utilizing better configuration of electrode and membrane materials helps minimize electric energy consumption in fouling reduction.Akamatsu et al. (2012) fabricated a low cost membrane-carbon cloth assembly made of PVDF flat-sheet microfiltration (MF) and plain-woven carbon cloth as the membrane and electrodes, respectively. The study successfully demonstrated that upon application of electricity, either continuously or intermittently, the membrane flux always recovers to the initial flux level (Table 3).Liu et al. (2012a) placed copper wires inside the flat sheet membrane module and two stainless mesh anodes outside the module. This configuration ensures constant electrophoretic forces against the deposition of foulant on the membrane (Table 3). This suggests that inexpensive electrode materials can also be used in eMBR instead of high cost membranes (e.g., PVDF, PES, titanium) which can be a valuable consideration for full scale use.
Anode material is one of the most important parameters in studying eMBR since it significantly affects the current density which is one of the typical performance indicator in any electrochemical system. The two most commonly used anodes in eMBR are aluminum (Al) and iron (Fe) electrodes. Both of them are proven to be useful for pollution removal (Wei et al., 2009) as well as for membrane fouling reduction (Zhang J. et al., 2014). Most importantly, they also serve as micronutrient necessary for microbial growth (Bani-Melhem and Elektorowicz, 2010). However, higher concentrations of dissolved anode materials may create inhibitory effects on bacterial metabolism, hence, electrode impact should be tested according to the applied DC field prior to the eMBR design. There are other anode materials used in eMBR study but this paper focused only on these two sacrificial anodes as they have more benefits than other tested materials (Zhang et al., 2015). The mechanisms of pollutant removal and fouling suppression using these aluminum and iron electrodes are discussed in the succeeding Sections Pollutants Removal and Membrane Fouling Reduction. Aluminum electrodes are preferred compared to iron electrodes due to the higher surface area of aluminum hydroxides, generated from electrocoagulation process, which increases the adsorption capacity (Emamjomeh and Sivakumar, 2009) of soluble compounds and the entrapment of particulate materials.
Operating Conditions
Operating parameters such as aeration intensity, hydraulic retention time (HRT), sludge retention time (SRT), mixed liquor suspended solids (MLSS), current density (CD), and direct current (DC) exposure time play a vital role in determining both treatment performance and membrane fouling in eMBR. In conventional SMBR, surplus aeration is applied to aid in the scouring of flocs that have adhered on the membrane surfaces for fouling control (Rahimi et al., 2011). In eMBR, excess amount of oxygen is not needed since deposition of foulants onto the membrane surface is controlled by the applied DC field. Studies have shown that the running costs of eMBRs are much lower than MBRs using excess aeration for fouling control (Akamatsu et al., 2010). However, an optimum aeration condition in eMBR is not yet fully studied as it varies accordingly on the design and configuration of the bioreactor. In EC-SMBR, for example, 4.2 L/min of compressed air was considered adequate to supply oxygen for microbial incubation, to provide good mixing of the sludge suspension and to create a shear stress for effective scouring of membrane surfaces (Bani-Melhem and Smith, 2012).Zhang et al. (2015), on the other hand, applied an aeration intensity equal to 1 m3/h to maintain 2 ppm dissolved oxygen concentration in the SMEBR. Large aeration intensity, greater than 800 L/h, was observed to be harmful for microbial growth and may cause break down of floc's formation which promotes the release of colloids and solutes resulting in severe pore blocking (Meng et al., 2008).
The effects of HRT and sludge properties (SRT and MLSS) in wastewater treatment using eMBR were explored by Giwa and Hasan (2015). In their experiments, reduction of effluent characteristics [COD, total nitrogen (TN), and total phosphorus (TP)] were realized at increasing HRT due to more exposure time of reactor content to biodegradation and electrocoagulation. HRT was varied by increasing the influent flow rate from 20 to 50 L/d. COD and TP concentrations in the effluent were lower at shorter SRT due to the decline in the MLSS concentration, while high TN removal was observed when SRT was increased from 5 to 20 days due to the provision of ample time for the growth of nitrifying bacteria responsible for the biological nitrification processes (Giwa and Hasan, 2015). The values of HRT in some studies range from 6 to 14 h (Wei et al., 2009; Kim et al., 2010; Elektorowicz et al., 2014; Hasan et al., 2014) while, regarding SRT, it can last up to 268 days (Bani-Melhem and Elektorowicz, 2011) (Tables 1, 2). Longer SRT is one of the many advantages of eMBR over CAS process. Sludge wasting can be minimized, hence, eMBR has lower sludge production.
Meanwhile, the effect of MLSS on fouling control varies in some studies.Giwa et al. (2015) proved that increasing MLSS (1455–4111 mg/L) causes more severe fouling due to the increase in sludge viscosity leading to higher hydrodynamic force required to propel sludge circulation. Bani-Melhem and Elektorowicz (2011), on the other hand, showed that MLSS concentration within the range of 2100–5000 mg/L has less effect on membrane fouling. To date, there is no study yet on the optimum range of MLSS in eMBR that would give excellent pollutant removal and fouling minimization.
Current density (CD = current [A]/anode electrode area [m2]) is a crucial factor which affects the performance of the electrochemical treatment in eMBR. It controls the dosing rate of metal ions as well as the gas bubble density that are released in the solution via redox reactions of electrodes (Ibeid et al., 2013b). The higher the current density, the more coagulants are formed in a shorter period of time and the stronger electrokinetic forces are induced between foulants, bulk liquid, and membrane, hence, improving pollutant removal and fouling abatement. Excessive CD also results in waste of energy in the form of heat dissipation inside the reactor (Zhang et al., 2015). At the same time, DC exposure time is also important since both the intensity and application time can significantly influence bacterial community in the eMBR system (Giwa and Hasan, 2015). Optimization of these parameters is, therefore, a must.Tafti et al. (2015) studied the impact of CD ranging from 5 to 23 A/m2 in combination with different DC exposure modes as reported in Table 2 (480 s OFF/120 s ON, 200 s OFF/400 s ON, 496 s OFF/104 s ON, 415 s OFF/185 s OFF and continuously ON) on activated sludge properties and pollutant removal efficiencies using synthetic municipal wastewater. Results showed that at higher CD (>20 A/m2), eMBR exhibits higher COD, phosphate and ammonia removals as compared to conventional SMBR and that the microbial flocs were able to tolerate the electrical shocks at these values. The optimum conditions observed by Tafti et al. (2015) were CD = 12.5 A/m2 and exposure mode equal to 415 s OFF/185 s ON. Other papers employed CD intensities ranging from 10 to 12 A/m2 in their eMBR studies and obtained similar results (Hasan et al., 2012, 2014; Hosseinzadeh et al., 2015) (Table 1). Moreover, the amount of DC exposure time used in eMBR primarily depends on the current density and sludge properties (i.e., MLSS). At CD equals to 25 A/m2 and MLSS below 10,000 mg/L, shorter time-OFF was needed in each electrical cycle while at higher MLSS values, longer time-OFF is required (Ibeid et al., 2013b). The effects of electricity on pollutant removal, fouling control and microbial activity are further discussed in Section Effects of the Electric Field on eMBR Performance.
As mentioned, all of these parameters influence eMBR performance by directly affecting the sludge properties. Therefore, it is important to note that understanding the relationship between these parameters and identifying their appropriate combination, which will depend on the type of wastewater (low, medium, high-strength wastewater) being treated and configuration used, result in an enhancement of eMBR performance.
Application with Different Influent Wastewater
Due to numerous advantages that the technology provides, eMBR has been used in the treatment of different types of influent wastewater. It has been employed to remove organic substances (COD) and nutrients (nitrogen and phosphorous) as well as color from combined industrial wastewater (Hosseinzadeh et al., 2015), tannery wastewater (Keerthi et al., 2013), and municipal or domestic wastewater (Kim et al., 2010; Bani-Melhem and Smith, 2012; Hasan et al., 2012, 2014). Tables 1, 2 provide a comprehensive summary of the applications of electrically induced membrane bioreactor on real and synthetic wastewater, respectively.
Most investigations of this hybrid reactor were still limited to lab scale reactors fed with synthetic or low-to-medium strength wastewater and only few focuseson pilot scale application. The challenge, therefore, centers on scale-up of this system and its application to wastewater with high pollutant loading (e.g., landfill leachate, food processing wastewater, etc.). Moreover, eMBR could also provide a suitable environment for the biodegradation of emerging contaminants (i.e., endocrine disrupting substances, pharmaceutical products, flame retardants, and personal care products) from water and wastewater streams due to its high solid retention and use of diverse microbial culture.
Effects of the Electric Field on eMBR Performance
Pollutants Removal
Previous studies have reported high satisfactory results in eMBR performance. In fact, removal efficiencies of organic and inorganic compounds in wastewater using eMBR exceeded the performance of conventional MBR systems (Wei et al., 2009; Bani-Melhem and Elektorowicz, 2010, 2011; Kim et al., 2010; Bani-Melhem and Smith, 2012; Hasan et al., 2012; Zhang et al., 2015). Both MBR and eMBR systems exhibit excellent removal of physical impurities i.e., color (>91%), turbidity (>95%), and suspended solids (100%) due to the effective solid retention by the membrane module (Bani-Melhem and Smith, 2012). However, in terms of COD, eMBR gives higher removal efficiency (85–98%) compared to MBR (72–95%) (Tables 1, 2) which is attributed to the effects of electrochemical processes (i.e., electrocoagulation, electroosmosis, electrophoresis) in addition to biodegradation and membrane filtration (Hosseinzadeh et al., 2015). During electrocoagulation (Figure 1C), various monomeric and polymeric metal complexes are generated from the electrochemical dissolution (electro-oxidation) of sacrificial metal electrodes (aluminum or iron) (Wei et al., 2009). In the case of aluminum anode, Al3+ ions react with OH− ions to form amorphous Al(OH)3(s) while for iron, gelatinous iron hydroxide Fe(OH)2(s) and/or Fe(OH)3(s) is formed in the aqueous stream due to the reaction between Fe2+ and Fe3+ with OH− (Equations 1–8). These metal complexes react with contaminants in the wastewater to form flocs which can both destabilize and aggregate colloidal particles or precipitate and adsorb dissolved compounds (Elabbas et al., 2016). Oxidation and reduction of some pollutants may also be possible which lead to its deposition at the anode and cathode, respectively (Giwa et al., 2016).
For Aluminum:
For Iron:
Likewise, eMBR performs better in phosphorus removal than SMBR. EC-SMBR can remove as high as 94.3% P (compared to 65% in SMBR) (Bani-Melhem and Smith, 2012) while SMEBR can remove up to 99% (MBR = 59% removal) (Hasan et al., 2012) from both raw (Table 1) and synthetic wastewaters (Table 2). This is because generated Al and Fe coagulants facilitate adsorption of soluble phosphorus in the bioreactor (Kim et al., 2010; Bani-Melhem and Smith, 2012). Biodegradation alone does not adequately eliminate P from aqueous solution hence the low removal efficiency in conventional MBR (as low as 20.8%) (Wei et al., 2009). Using aluminum anode, cationic polymers such as Al3+ and [Al6(OH15)]3+ react with phosphate ions to precipitate AlPO4(s) and Al6(OH15)PO4(s), respectively, according to Equations (7), (8) (Bani-Melhem and Smith, 2012). In the case of sacrificial Fe anode, the iron hydroxides that are formed adsorb the soluble phosphorus and the excess Fe3+ combines with phosphorus ions to form FePO4(s) precipitates (Equation 9) (Bani-Melhem and Elektorowicz, 2011).
Depending on their net surface charge, the pollutants can also migrate and deposit on oppositely charged electrodes via electrophoretic motion upon DC application (Giwa et al., 2015). For example, the deposition of anions on the anode as represented by Equation (10) where M+ and A− correspond to metal ions and anion, respectively. In addition, electroosmosis mechanism (Figure 1B) also occurs within the system which enhances dewatering of the smaller floc particles and decreasing specific resistance to filtration (Ibeid et al., 2013a). Heavier flocs that are not adsorbed on the electrode surface settled at the bottom of the reactor as sludge due to gravity and to the gas produced at the electrodes (Giwa et al., 2015).
Treatment efficiencies of ammonia nitrogen (NH3-N) were consistently excellent in conventional MBR (~89% and up) but vary in electro-enhanced MBR (70–100%) (Tables 1, 2). There are two factors being considered for such results: (1) the application of high DC field and (2) excessive metal ion concentration (Bani-Melhem and Elektorowicz, 2011; Tafti et al., 2015). Nitrifying bacteria, which are responsible for the conversion of ammonia nitrogen (NH3-N) to nitrate nitrogen (NO3-N), are very much sensitive to high voltage. Direct exposure to applied DC >2.5 A/m2 was proved to have inhibitory effects in the metabolism of nitrifying bacteria (Li et al., 2001). Furthermore, accumulation of metal ion complexes as a result of electro-dissolution of sacrificial anodes is also detrimental to nitrifying bacteria. Iron complexes, in particular, form a barrier that hinders the transfer of enzymes and nutrients through the microbial cell membrane (Bani-Melhem and Elektorowicz, 2011). This is the reason why ammonia removal using iron and stainless steel anodes are relatively lower (70 and 81.9%, respectively) (Bani-Melhem and Elektorowicz, 2011; Zhang et al., 2015) as compared when using aluminum anode (98–100%) (Wei et al., 2009; Hasan et al., 2012; Giwa et al., 2016). Optimization of electrolysis conditions should, therefore, be done so as not to impede biological treatment.
Membrane Fouling Reduction
Investigation on the influence of appending electricity on fouling control in eMBR was pioneered by Chen et al. (2007). In this study, six different DC field strengths ranging from 0 to 20 V/cm were applied and the results showed that better filtrate flux recovery was obtained at higher DC intensity due to the increase in particle electrophoresis. In SMEBR, the movement of the suspended particles and the water during membrane filtration can be controlled by the applied electrical field. Major foulants in wastewater usually possess negative surface charge and upon DC application, electrophoresis force counteracts permeation-induced deposition of these foulants away from the membrane surface and toward the electrode of opposite charge (anode) (Figure 1A) (Wei et al., 2009). On the other hand, the bulk liquid which is commonly positively charged is drifted toward the cathode then flows to the membrane facilitating the filtration, a process called electroosmosis (Figure 1B). Electroosmosis, thus, removes bound water from the microbial flocs enhancing sludge dewaterability and decreasing the specific resistance to filtration (Ibeid et al., 2013a). Specific cake resistance to filtration and modified fouling index were observed to decrease by 82 and 81%, respectively, when 12 A/m2 current density was applied to eMBR in the treatment of raw municipal wastewater (Hasan et al., 2014).
Application of electric field for membrane fouling suppression does not only control the movement of charged particles and bulk liquid, it also modifies the sludge properties through the electrocoagulation process (Figure 1C) (Hasan et al., 2014). The use of sacrificial anodes releases metal ions which precipitate or adsorb negatively charged foulants [i.e., extracellular polymeric substances (EPS), soluble microbial products (SMP)] and facilitates formation of larger flocs which drives the particle back-transport from the membrane surface to the bulk solution (Ibeid et al., 2013a). In a study conducted by Zhang et al. (2015), two types of anode materials were used: stainless steel (Fe-MBR) and titanium (Ti-MBR). Between the two, Fe-MBR showed better fouling mitigation performance than the Ti-MBR due to the combined action of electric field force induced backwash (electrophoresis) and the formation of iron coagulants which reduced the SMP concentration (electrocoagulation). Titanium mesh anodes used in Ti-MBR were inactive metal hence it did not release any coagulant ions and fouling control relied mostly on the magnitude of electric field applied (Zhang et al., 2015). Moreover,Kim et al. (2010) proved that electrocoagulation does not only effectively removed phosphorus by precipitating AlPO4(s) crystals but these precipitates are also capable of adsorbing EPS.Hosseinzadeh et al. (2015) attributed the lower tansmembrane pressure (TMP) and sludge volume index (SVI) to electrocoagulation in SMEBR. In EC-SMBR, membrane fouling was reduced by 13% due to the electrocoagulation pre-treatment (Bani-Melhem and Smith, 2012).
Nevertheless, it should be noted that energy consumption and operational cost will obviously be affected by high electricity requirements and, therefore, must be reduced. Some researchers studied the use of intermittent application of minute electric field for effective fouling control and possible energy savings. Table 3 summarizes the different studies that investigates effects of continuous and intermittent application of electric field on membrane fouling.Akamatsu et al. (2010) developed a novel fouling suppression system in MBRs which controls the motion of activated sludge by applying electric current only when the permeate flux has drastically declined due to membrane fouling. By switching on and off every 90 s, electric field strength of 6 V/cm facilitates detachment of foulant activated sludge from the surface of the membrane thus significantly improved the average permeate flux (Figure 1A). Upon cost estimation, about half of the energy requirements were saved using this fouling suppression system. In another experiment, Akamatsu et al. (2012) investigated the performance of MBR on fouling suppression by applying 50 and 33 V/cm electric field at a 4 min ON/4 min OFF cycle directly on the membrane cathode made of carbon cloth. The results showed that the flux always recovered to the initial flux level when the electric field was ON and at voltage gradient applied equal to 50 V/cm. This proved that higher electric field intensity means stronger repulsive force between the foulants and the membrane surfaces enhancing the fouling control (Akamatsu et al., 2012). A minute electric field, 0.2 V (0.036 V/cm) and 0.4 V (0.073 V/cm) were applied by Liu et al. (2012a) using a low cost, micro-porous PP non-woven sheet and a terylene filter cloth. For both membranes, application of either 0.2 or 0.4 V electric field causes increase in permeate flux, enhancement of microbial growth and activity, decrease of filtration resistance and reduction of sludge EPS content which all contributed to fouling control.
Microbial Activity
Like any other biological wastewater treatment processes, eMBR relies mostly on the mixed microorganisms present in the system. The ability of bacterial community to effectively degrade organic matter (COD) and nutrients (N and P) from raw wastewater makes biological process the most widely used treatment method. Therefore, integrating electrochemical technologies with biological treatment should take into consideration the effects of electricity on bacterial viability.
Microbial analyses have revealed that a stressor such as strong electric current provides negative impact on the microbial properties (i.e., metabolism, physiology, shape, and mobility) (Wei V. et al., 2011). Increasing intensity of electric field (DC > 2.5 A/m2) decreases nitrification rate by 20% (Li et al., 2001). As a result, low ammonia removal is observed due to the inhibition of nitrobacteria and denitrifying bacteria by large voltage (Bani-Melhem and Elektorowicz, 2011; Bani-Melhem and Smith, 2012; Hasan et al., 2012). Moreover, at higher voltage, breakage of bacterial cells and production of SMP are observed to occur which increases fouling (Zhang et al., 2015). Wei V. et al. (2011) proved that increasing DC intensity (6.2, 12.3, and 24.7 A/m2) also increases pH (from nearly neutral to around pH 10) of electrolytic biomass fluid causing 10, 15, and 29% death percentage, respectively.
It should be noted that beneficial effects can also be achieved in applying minute electric field in eMBR system. Low voltage (0.036–0.073 V/cm) promotes the growth of filamentous bacteria which are proven to improve sludge filterability (Liu et al., 2012a). Intermittent application prevents prolonged direct contact between electric field and microbial community hence minimizing the negative effects of high DC field. To date, there are no studies yet on the optimal electric field suitable for enhancing bacterial activity in an eMBR application.
Conclusions
Membrane fouling is a major limitation in MBR operation that should be resolved. Among the different fouling control techniques, integration of electrochemical processes into MBR seems to be a promising method. Electrochemical phenomena such as electrocoagulation, electroosmosis and electrophoresis are the main mechanisms of membrane fouling control in eMBR. It also enhances pollutant removal. However, direct application of electric field to MBRs incurs high energy consumption and has negative effects on bacterial viability which is responsible for the biodegradation of the organic pollutants. Optimum configuration of electric field helps to reduce energy consumption while simultaneously reducing inadvertent interferences with eMBR performance. This can be done by using better configuration of electrode and membrane materials and intermittent application of low or moderate electric field.
The combination of electrochemical processes with MBR processes is still in its infancy stage, with a small number of scientific papers published during the last decade. This review focused only on those publications with international significance. The studies on eMBR are limited to bench or pilot scale reactor fed with real or synthetic wastewater. Large scale applications are not reported. Optimization study, modeling and cost benefit analysis between MBR and eMBR are, therefore, useful for scaling up the system. Moreover, treatment of high strength industrial wastewater and the removal of emerging contaminants through eMBRs have not been yet highly explored. There are numerous already published papers regarding the effective removal of these contaminants by electrochemical processes or MBR, but not by the combined process. Moreover, the potential of this combined system as an efficient technology for biohydrogen production, due to hydrogen ions reduction at the cathode side, has not been yet investigated. Therefore, further studied could be focused on the combination of MECs with MBR through the operation of the electro MBR at anaerobic conditions for the production of renewable energy.
Author Contributions
VN and VB developed the research idea and planned activities. BE and LB prepared the manuscript. VN, VB, MD and FB reviewed the final draft and supervised the work.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors gratefully thank the Department of Science and Technology-Engineering Research Development and Technology (ERDT-DOST), the University of the Philippines—Diliman, and the Sanitary Environmental Engineering Division (SEED) of the University of Salerno in Italy for the Ph.D. Sandwich program and research funds. Research activities were partially funded by the FARB project of the University of Salerno.
References
Ahmed, F. N., and Lan, C. Q. (2012). Treatment of landfill leachate using membrane bioreactors: a review. Desalination 287, 41–54. doi: 10.1016/j.desal.2011.12.012
Akamatsu, K., Lu, W., Sugawara, T., and Nakao, S. (2010). Development of a novel fouling suppression system in membrane bioreactors using an intermittent electric field. Water Res. 44, 825–830. doi: 10.1016/j.watres.2009.10.026
Akamatsu, K., Yoshida, Y., Suzaki, T., Sakai, Y., Nagamoto, H., and Nakao, S. (2012). Development of a membrane – carbon cloth assembly for submerged membrane bioreactors to apply an intermittent electric field for fouling suppression. Sep. Purif. Technol. 88, 202–207. doi: 10.1016/j.seppur.2011.12.031
Artiga, P., Ficara, E., Malpei, F., Garrido, J. M., and Méndez, R. (2005). Treatment of two industrial wastewaters in a submerged membrane bioreactor. Desalination 179, 161–169. doi: 10.1016/j.desal.2004.11.064
Ballesteros, F. Jr., Vuong, T. H., Secondes, M. F., and Tuan, P. D. (2016). Removal efficiencies of constructed wetland and efficacy of plant on treating benzene. Sustain. Environ. Res. 26, 93–96. doi: 10.1016/j.serj.2015.10.002
Bani-Melhem, K., and Elektorowicz, M. (2010). Development of a novel submerged membrane electro-bioreactor (SMEBR): performance for fouling reduction. Environ. Sci. Technol. 44, 3298–3304. doi: 10.1021/es902145g
Bani-Melhem, K., and Elektorowicz, M. (2011). Performance of the submerged membrane electro-bioreactor (SMEBR) with iron electrodes for wastewater treatment and fouling reduction. J. Membr. Sci. 379, 434–439. doi: 10.1016/j.memsci.2011.06.017
Bani-Melhem, K., and Smith, E. (2012). Grey water treatment by a continuous process of an electrocoagulation unit and a submerged membrane bioreactor system. Chem. Eng. J. 198–199, 201–210. doi: 10.1016/j.cej.2012.05.065
Bernhard, M., Müller, J., and Knepper, T. P. (2006). Biodegradation of persistent polar pollutants in wastewater: comparison of an optimised lab-scale membrane bioreactor and activated sludge treatment. Water Res. 40, 3419–3428. doi: 10.1016/j.watres.2006.07.011
Cesaro, A., Naddeo, V., and Belgiorno, V. (2013). Wastewater treatment by combination of advanced oxidation processes and conventional biological systems. J. Bioremed. Biodeg. 4:208. doi: 10.4172/2155-6199.1000208
Chen, J., Yang, C., Zhou, J., and Wang, X. (2007). Study of the influence of the electric field on membrane flux of a new type of membrane bioreactor. Chem. Eng. J. 128, 177–180. doi: 10.1016/j.cej.2006.10.010
Cicek, N. (2003). A review of membrane bioreactor and their potential application in the treatment of agricultural wastewater. Can. Biosyst. Eng. 45, 637–649.
de Luna, M. D. G., and Warmadewanthi Liu, J. C. (2009). Combined treatment of polishing wastewater and fluoride-containing wastewater from a semiconductor manufacturer. Colloids Surf. A 347, 64–68. doi: 10.1016/j.colsurfa.2008.12.006
Elabbas, S., Ouazzani, N., Mandi, L., Berrekhis, F., Perdicakis, M., Pontvianne, S., et al. (2016). Treatment of highly concentrated tannery wastewater using electrocoagulation: influence of the quality of aluminium used for the electrode. J. Hazard. Mater. 319, 69–77. doi: 10.1016/j.jhazmat.2015.12.067
Elektorowicz, M., Arian, Z., and Ibeid, S. (2014). “Submerged membrane electro-bioreactor for water recovery,” in Membranes and Membrane Processes in Environmental Protection, Vol. 119, eds M. Bodzek and J. Pelczar (Monographs of the Environmental Engineering Committee, Polish Academy of Sciences), 93–98.
Emamjomeh, M. M., and Sivakumar, M. (2009). Review of pollutants removed by electrocoagulation and electrocoagulation/flotation processes. J. Environ. Manage. 90, 1663–1679. doi: 10.1016/j.jenvman.2008.12.011
Giwa, A., Ahmed, I., and Hasan, S. W. (2015). Enhanced sludge properties and distribution study of sludge components in electrically-enhanced membrane bioreactor. J. Environ. Manage. 159, 78–85. doi: 10.1016/j.jenvman.2015.05.035
Giwa, A., Daer, S., Ahmed, I., Marpu, P. R., and Hasan, S. W. (2016). Experimental investigation and artificial neural networks ANNs modeling of electrically-enhanced membrane bioreactor for wastewater treatment. J. Water Process Eng. 11, 88–97. doi: 10.1016/j.jwpe.2016.03.011
Giwa, A., and Hasan, S. W. (2015). Theoretical investigation of the influence of operating conditions on the treatment performance of an electrically-induced membrane bioreactor. J. Water Process Eng. 6, 72–82. doi: 10.1016/j.jwpe.2015.03.004
Hasan, S. W., Elektorowicz, M., and Oleszkiewicz, J. A. (2012). Correlations between trans-membrane pressure (TMP) and sludge properties in submerged membrane electro-bioreactor (SMEBR) and conventional membrane bioreactor (MBR). Bioresour. Technol. 120, 199–205. doi: 10.1016/j.biortech.2012.06.043
Hasan, S. W., Elektorowicz, M., and Oleszkiewicz, J. A. (2014). Start-up period investigation of pilot-scale submerged membrane electro-bioreactor (SMEBR) treating raw municipal wastewater. Chemosphere 97, 71–77. doi: 10.1016/j.chemosphere.2013.11.009
Heidrich, E. S., Curtis, T. P., and Dolfing, J. (2011). Determination of the internal chemical energy of wastewater. Environ. Sci. Technol. 45, 827–832. doi: 10.1021/es103058w
Hosseinzadeh, M., Bidhendi, G. N., Torabian, A., Mehrdadi, N., and Pourabdullah, M. (2015). A new flat sheet membrane bioreactor hybrid system for advanced treatment of effluent, reverse osmosis pretreatment and fouling mitigation. Bioresour. Technol. 192, 177–184. doi: 10.1016/j.biortech.2015.05.066
Ibeid, S., Elektorowicz, M., and Oleszkiewicz, J. A. (2013a). Novel electrokinetic approach reduces membrane fouling. Water Res. 47, 6358–6366. doi: 10.1016/j.watres.2013.08.007
Ibeid, S., Elektorowicz, M., and Oleszkiewicz, J. A. (2013b). Modification of activated sludge properties caused by application of continuous and intermittent current. Water Res. 47, 903–910. doi: 10.1016/j.watres.2012.11.020
Ibeid, S., Elektorowicz, M., and Oleszkiewicz, J. A. (2015). Electro-conditioning of activated sludge in a membrane electro-bioreactor for improved dewatering and reduced membrane fouling. J. Membr. Sci. 494, 136–142. doi: 10.1016/j.memsci.2015.07.051
Keerthi, S. V, Mahalakshmi, M., and Balasubramanian, N. (2013). Development of hybrid membrane bioreactor for tannery effluent treatment. Desalination 309, 231–236. doi: 10.1016/j.desal.2012.10.014
Kim, H. G., Jang, H. N., Kim, H. M., Lee, D. S., and Chung, T. H. (2010). Effect of an electro phosphorous removal process on phosphorous removal and membrane permeability in a pilot-scale MBR. Desalination 250, 629–633. doi: 10.1016/j.desal.2009.09.038
Kimura, K., Yamato, N., Yamamura, H., and Watanabe, Y. (2005). Membrane fouling in pilot-scale membrane bioreactors (MBRs) treating municipal wastewater. Environ. Sci. Technol. 39, 6293–6299. doi: 10.1021/es0502425
Li, X. G., Cao, H. B., Wu, J. C., and Yu, K. T. (2001). Inhibition of the metabolism of nitrifying bacteria by direct electric current. Biotechnol. Lett. 23, 705–709. doi: 10.1023/A:1010346501857
Li, Y., Liu, L., Liu, J., Yang, F., and Ren, N. (2014). PPy/AQS (9, 10-anthraquinone-2-sulfonic acid) and PPy/ARS (Alizarin Red's) modified stainless steel mesh as cathode membrane in an integrated MBR/MFC system. Desalination 349, 94–101. doi: 10.1016/j.desal.2014.06.027
Liu, J., Liu, L., Gao, B., and Yang, F. (2013a). Integration of bio-electrochemical cell in membrane bioreactor for membrane cathode fouling reduction through electricity generation. J. Membr. Sci. 430, 196–202. doi: 10.1016/j.memsci.2012.11.046
Liu, J., Liu, L., Gao, B., Yang, F., Crittenden, J., and Ren, N. (2014). Integration of microbial fuel cell with independent membrane cathode bioreactor for power generation, membrane fouling mitigation and wastewater treatment. Int. J. Hydrog. Energy 39, 17865–17872. doi: 10.1016/j.ijhydene.2014.08.123
Liu, L., Liu, J., Bo, G., Yang, F., Crittenden, J., and Chen, Y. (2013c). Conductive and hydrophilic polypyrrole modified membrane cathodes and fouling reduction in MBR. J. Membr. Sci. 429, 252–258. doi: 10.1016/j.memsci.2012.11.066
Liu, L., Liu, J., Gao, B., and Yang, F. (2012a). Minute electric field reduced membrane fouling and improved performance of membrane bioreactor. Sep. Purif. Technol. 86, 106–112. doi: 10.1016/j.seppur.2011.10.030
Liu, L., Liu, J., Gao, B., Yang, F., and Chellam, S. (2012b). Fouling reductions in a membrane bioreactor using an intermittent electric field and cathodic membrane modified by vapor phase polymerized pyrrole. J. Membr. Sci. 394–395, 202–208. doi: 10.1016/j.memsci.2011.12.042
Liu, L., Zhao, F., Liu, J., and Yang, F. (2013b). Preparation of highly conductive cathodic membrane with graphene (oxide)/PPy and the membrane antifouling property in filtrating yeast suspensions in EMBR. J. Membr. Sci. 437, 99–107. doi: 10.1016/j.memsci.2013.02.045
Ma, D., Gao, B., Hou, D., Wang, Y., Yue, Q., and Li, Q. (2013). Evaluation of a submerged membrane bioreactor (SMBR) coupled with chlorine disinfection for municipal wastewater treatment and reuse. Desalination 313, 134–139. doi: 10.1016/j.desal.2012.12.016
Meng, F., Yang, F., Shi, B., and Zhang, H. (2008). A comprehensive study on membrane fouling in submerged membrane bioreactors operated under different aeration intensities. Sep. Purif. Technol. 59, 91–100. doi: 10.1016/j.seppur.2007.05.040
Naddeo, V., Belgiorno, V., Borea, L., Secondes, M. F. N., and Ballesteros, F. Jr. (2015a). Control of fouling formation in membrane ultrafiltration by ultrasound irradiation. Environ. Technol. 36, 1299–1307. doi: 10.1080/09593330.2014.985731
Naddeo, V., Borea, L., and Belgiorno, V. (2015b). Sonochemical control of fouling formation in membrane ultrafiltration of wastewater: Effect of ultrasonic frequency. J. Water Process Eng. 8, e92–e97. doi: 10.1016/j.jwpe.2014.12.005
Naddeo, V., Rizzo, L., and Belgiorno, V. (2011). Water, Wastewater and Soil Treatment by Advanced Oxidation Processes. Fisciano, SA. Available online at: www.Lulu.com
Palogos, I., Babatsouli, P., Costa, C., and Kalogerakis, N. (2014). Computer simulation of a submerged membrane bioreactor treating high COD industrial wastewater. Front. Environ. Sci. 2:30. doi: 10.3389/fenvs.2014.00030
Rahimi, Y., Torabian, A., Mehrdadi, N., Habibi-rezaie, M., Pezeshk, H., and Nabi-bidhendi, G. R. (2011). Optimizing aeration rates for minimizing membrane fouling and its effect on sludge characteristics in a moving bed membrane bioreactor. J. Hazard. Mater. 186, 1097–1102. doi: 10.1016/j.jhazmat.2010.11.117
Scannapieco, D., Naddeo, V., and Belgiorno, V. (2015). Control of fouling in MBRs through nanospheres addition. Desalin. Water Treat. 55, 702–711. doi: 10.1080/19443994.2014.942382
Secondes, M. F. N., Naddeo, V., Belgiorno, V., and Ballesteros, F. Jr. (2014). Removal of emerging contaminants by simultaneous application of membrane ultrafiltration, activated carbon adsorption, and ultrasound irradiation. J. Hazard. Mater. 264, 342–349. doi: 10.1016/j.jhazmat.2013.11.039
Shi, X., Tal, G., Hankins, N. P., and Gitis, V. (2014). Fouling and cleaning of ultrafiltration membranes: a review. J. Water Process Eng. 1, 121–138. doi: 10.1016/j.jwpe.2014.04.003
Singhal, N., and Perez-Garcia, O. (2016). Degrading organic micropollutants: the next challenge in the evolution of biological wastewater treatment processes. Front. Environ. Sci. 4:36. doi: 10.3389/fenvs.2016.00036
Tafti, A. D., Seyyed Mirzaii, S. M., Andalibi, M. R., and Vossoughi, M. (2015). Optimized coupling of an intermittent DC electric field with a membrane bioreactor for enhanced effluent quality and hindered membrane fouling. Sep. Purif. Technol. 152, 7–13. doi: 10.1016/j.seppur.2015.07.004
Wang, H., Luo, H., Fallgren, P. H., Jin, S., and Ren, Z. J. (2015). Bioelectrochemical system platform for sustainable environmental remediation and energy generation. Biotechnol. Adv. 33, 317–334. doi: 10.1016/j.biotechadv.2015.04.003
Wang, Y. P., Liu, X. W., Li, W. W., Li, F., Wang, Y. K., Sheng, G. P., et al. (2012). A microbial fuel cell-membrane bioreactor integrated system for cost-effective wastewater treatment. Appl. Energy 98, 230–235. doi: 10.1016/j.apenergy.2012.03.029
Wei, C. H., Huang, X., Ben Aim, R., Yamamoto, K., and Amy, G. (2011). Critical flux and chemical cleaning-in-place during the long-term operation of a pilot-scale submerged membrane bioreactor for municipal wastewater treatment. Water Res. 45, 863–871. doi: 10.1016/j.watres.2010.09.021
Wei, V., Elektorowicz, M., and Oleszkiewicz, J. A. (2011). Influence of electric current on bacterial viability in wastewater treatment. Water Res. 45, 5058–5062. doi: 10.1016/j.watres.2011.07.011
Wei, V., Oleszkiewicz, J. A., and Elektorowicz, M. (2009). Nutrient removal in an electrically enhanced membrane bioreactor. Water Sci. Technol. 60, 3159–3163. doi: 10.2166/wst.2009.625
Weiss, S., and Reemtsma, T. (2008). Membrane bioreactors for municipal wastewater treatment - A viable option to reduce the amount of polar pollutants discharged into surface waters? Water Res. 42, 3837–3847. doi: 10.1016/j.watres.2008.05.019
Xing, C., Tardieu, E., Qian, Y., and Wen, X. (2000). Ultrafiltration membrane bioreactor for urban wastewater reclamation. J. Membr. Sci. 177, 73–82. doi: 10.1016/S0376-7388(00)00452-X
Yigit, N. O., Uzal, N., Koseoglu, H., Harman, I., Yukseler, H., Yetis, U., et al. (2009). Treatment of a denim producing textile industry wastewater using pilot-scale membrane bioreactor. Desalination 240, 143–150. doi: 10.1016/j.desal.2007.11.071
Yuniarto, A., Ujang, Z., and Noor, Z. Z. (2008). Performance of bio-fouling reducers in aerobic submerged membrane bioreactor for palm oil mill effluent treatment performance of bio-fouling reducers in aerobic submerged membrane bioreactor for palm oil mill effluent treatment. Management 49, 555–566.
Zeyoudi, M., Altenaiji, E., Ozer, L. Y., Ahmed, I., Yousef, A. F., and Hasan, S. W. (2015). Impact of continuous and intermittent supply of electric field on the function and microbial community of wastewater treatment electro-bioreactors. Electrochim. Acta 181, 271–279. doi: 10.1016/j.electacta.2015.04.095
Zhang, F., Li, J., and He, Z. (2014). A new method for nutrients removal and recovery from wastewater using a bioelectrochemical system. Bioresour. Technol. 166, 630–634. doi: 10.1016/j.biortech.2014.05.105
Zhang, J., Satti, A., Chen, X., Xiao, K., Sun, J., Yan, X., et al. (2015). Low-voltage electric field applied into MBR for fouling suppression: performance and mechanisms. Chem. Eng. J. 273, 223–230. doi: 10.1016/j.cej.2015.03.044
Zhang, J., Xiao, K., Liang, P., Waite, T. D., and Huang, X. (2014). Electrically released iron for fouling control in membrane bioreactors: a double-edged sword? Desalination 347, 10–14. doi: 10.1016/j.desal.2014.05.018
Zhang, K., Wei, P., Yao, M., Field, R. W., and Cui, Z. (2011). Effect of the bubbling regimes on the performance and energy cost of flat sheet MBRs. Desalination 283, 221–226. doi: 10.1016/j.desal.2011.04.023
Keywords: electro membrane bioreactors (eMBRs), fouling precursors, bacterial activity, electrocoagulation, electroosmosis, electrophoresis, extracellular polymeric substances (EPS), soluble microbial products (SMP)
Citation: Ensano BMB, Borea L, Naddeo V, Belgiorno V, de Luna MDG and Ballesteros FC Jr (2016) Combination of Electrochemical Processes with Membrane Bioreactors for Wastewater Treatment and Fouling Control: A Review. Front. Environ. Sci. 4:57. doi: 10.3389/fenvs.2016.00057
Received: 20 May 2016; Accepted: 18 August 2016;
Published: 31 August 2016.
Edited by:
Vasudevan Subramanyan, Council of Scientific and Industrial Research-Central Electrochemical Research Institute, IndiaReviewed by:
Devendra P. Saroj, University of Surrey, UKHimabindu Vurimindi, Jawaharlal Nehru Technological University, India
Gomathi Priya Ponnaiah, Anna University, India
Doraiswamy Raju Mohan, Anna University, India
Motakatla Venkateswer Reddy, Muroran Institute of Technology, Japan
Lucinda Elizabeth Doyle, Singapore Centre for Environmental Life Sciences Engineering, Singapore
Copyright © 2016 Ensano, Borea, Naddeo, Belgiorno, de Luna and Ballesteros. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vincenzo Belgiorno, v.belgiorno@unisa.it
 Benny M. B. Ensano
Benny M. B. Ensano Laura Borea
Laura Borea Vincenzo Naddeo
Vincenzo Naddeo Vincenzo Belgiorno
Vincenzo Belgiorno Mark D. G. de Luna
Mark D. G. de Luna Florencio C. Ballesteros Jr.1,3
Florencio C. Ballesteros Jr.1,3