Color contrast and stability as key elements for effective warning signals
- 1Butterfly Genetics Group, Department of Zoology, University of Cambridge, Cambridge, UK
- 2Sensory Ecology Group, Centre for Ecology and Conservation, College of Life and Environmental Sciences, University of Exeter, Cornwall Campus, Penryn, UK
Vivid warning signals (aposematism) have evolved repeatedly throughout the animal kingdom. However, relatively few studies consider what makes an effective signal, in terms of preventing attack and promoting avoidance learning by predators. Signal form varies substantially among and sometimes within species, but there has also been apparent convergence on relatively few main color types. We aimed to determine why warning signals often combine red, orange, yellow, and black colors, and specifically to determine whether these colors provide highly salient and reliable visual signals under a range of environmental conditions. Using digital image analysis, we modeled ladybird (ladybug) coloration to an avian visual system. We calculated the contrast of several different ladybird species against an average green background, based on predicted opponent color channel responses in bird vision. Our results suggest that longwave colors (i.e., red, orange) are more contrasting than colors such as blue, against green natural backgrounds. Moreover, these colors yield relatively unchanging (stable) signals throughout the day and under different weather conditions. These analyses show how aposematic signals have evolved under selection to be more effective by being more conspicuous and reliable to the visual system of their potential avian predators.
Introduction
Adaptive coloration is widespread in nature and a key system to study evolution by natural and sexual selection. Animals often use coloration as a warning signal to defend themselves from predators, to advertise that they have distasteful or harmful chemicals, or are otherwise unprofitable (aposematism) (Wallace, 1889; Poulton, 1890; Cott, 1940; Ruxton et al., 2004). Although warning signals have been widely studied in terms of their initial evolution, comparatively less effort has been made to establish what makes an effective warning signal, and how this effectiveness can lead to potential trade-offs with other aspects of a species' ecology, such as its reproductive success or an effective foraging strategy (Mappes et al., 2005; Stevens, 2007; Nokelainen et al., 2012; Stevens and Ruxton, 2012).
Aposematic species use a wide range of patterns to advertise their unpalatability, aiding the process of aversion learning and initial avoidance by naïve predators (Roper and Redston, 1987; Roper, 1990; Brodie and Janzen, 1995; Marples et al., 1998). Interestingly, although the variation in signal form is in itself remarkable, many species have converged on the use of similar signals, with red, orange, yellow, and black colors being especially common, at least in terrestrial systems (Cott, 1940; Aronsson and Gamberalle-Stille, 2008; Stevens and Ruxton, 2012). One hypothesis for why these colors are so common is that they are both more stable under varying light conditions and highly contrasting against the background vegetation color (Endler, 1992; Aronsson and Gamberalle-Stille, 2008; Stevens and Ruxton, 2012). To be effective, warning signals need to be detectable and identifiable, and both biotic and abiotic factors are likely to influence how they are perceived (Sherratt, 2002; Stevens, 2007; Stevens and Ruxton, 2012). For instance, environmental light varies greatly from dawn to dusk, and depending on weather conditions (Endler, 1993; Nieves et al., 2012). Moreover, the signal may be intended to be perceived by multiple predators with different spectral sensitivities. This could interfere with the interpretation of the information that can be extracted from color signals. Warning coloration should, therefore, be easy to detect and identify, even in heterogeneous environments with variations in light conditions (Endler, 1992), especially given that predators will often be under time constraints to make quick foraging decisions.
However, color is not a physical property of an object, and as such, its perception depends on a number of neurophysiological mechanisms, including the presence of opponent color channels (Snowden et al., 2006). Studies have demonstrated that color opponency evolved as a mechanism to detect important components of a visual scene (Lovell et al., 2005). Such mechanisms can maximize the perception of color contrast, where achromatic information is unreliable due to spatial and temporal variation: for example ripe fruit against leafy backgrounds (Mollon, 1989; Maximov, 2000; Lovell et al., 2005). Color opponency is a mechanism of signal detection that involves antagonistic pairs of colors (Chatterjee and Callaway, 2003). In this process, different (opposing) neural pathways are either activated or inhibited depending on the type of stimuli reaching the eye (DeValois et al., 1966; Lythgoe, 1979; Kaiser and Boynton, 1996). Human color vision, for example, depends on the relative activation of three photoreceptor types (trichromatic), and two opponent mechanisms: one processing the differences between long (LW) and mediumwave (MW) stimuli (Red–Green system, henceforth RG), and a Blue–Yellow (hereafter BY) system that processes the difference between shortwave (SW) cones and a combined signal from the LW and MW cones (DeValois et al., 1966; Derrington et al., 1984). However, if the number of photoreceptor cell types is increased, the number of potential color opponent systems may be increased as well (Kelber et al., 2003). For instance birds, a major predator of aposematic insects (Cook et al., 1969) are likely to be tetrachromatic (Cuthill, 2006). Osorio et al. (1999a) found evidence for the existence of at least three opponent channels in domestic chicks (Gallus gallus), corresponding to MW vs. LW (red–green), SW vs. LW + MW (blue–yellow), and UV vs. SW systems. Moreover, the evolution of trichromatic vision in primates is thought to be convergent to that of birds for a fruit/leaf based diet (Mollon, 1989; Maximov, 2000; Osorio et al., 2004). As such, it seems likely that birds and humans share some analogous opponent channels, and past work has modeled how these channels may encode colors to birds and primates (Lovell et al., 2005). Additional opponent systems have also been described for tetrachromatic turtles that share similar visual systems to birds (Ammermüller et al., 1998; Ventura et al., 2001). In birds, differences in luminance (perceived lightness) are probably encoded by a fifth photoreceptor type, the double cones, with a broader spectrum (Osorio et al., 1999a; Osorio and Vorobyev, 2005).
Previous work has investigated how red and yellow fruit colors provide stable and high-contrast signals over the course of a day to opponent color channels. Lovell et al. (2005) compared the visual perception of humans (or old world primates with similar vision) with starlings, using ripe and unripe fruits photographed over a day. They found that red fruit is especially contrasting when processed by the RG opponent system. In addition, the RG response is more stable than the BY (primate only) system over the course of a day as light conditions change. They also suggested that the latter is less effective in phasing out shade from a given scene. Thus, we would expect avian predators to rely on highly detectable stable color signals to process information about prey under changing environments.
The aim of this study was to analyze the color properties and general background contrast of warning signals, using ladybird (ladybug) beetles (Coleoptera: Coccinellidae) as a study system. In particular, we tested whether warning colors were more conspicuous when encoded by opponent color channels against natural green backgrounds then colors that are not often used as aposematic signals. Ladybird coloration varies greatly across species, and there are also several cases of within-species color polymorphisms (Osawa and Nishida, 1992; de Jong and Brakefield, 1998). In addition, ladybirds have toxic chemicals that are produced endogenously (Dixon, 2000; Bezzerides et al., 2007; Blount et al., 2012). These chemicals are correlated with color properties in some species (Blount et al., 2012). Several studies have suggested that ladybird color patterns, and overall appearance, are important for predator detection (Marples et al., 1989, 1994; Dolenská et al., 2009). However, only recently Blount et al. (2012) considered the actual role of avian visual sensitivity in their results. Ladybird beetles are widely distributed and abundant in the United Kingdom, and the diversity of their coloration is impressive. Therefore, they serve as an ideal model group to study aposematic signal form.
In this study we analyzed the contrast of ladybird warning coloration of different species under a range of light and weather conditions. Using digital image analyses, we photographed ladybirds and mapped the images to bird color space (Stevens et al., 2007; Pike, 2011). We examined whether classic warning colors have greater contrast against green foliage than other colors that are not frequently used as aposematic signals, and if they transmit a consistent signal across a range of environmental conditions. This could facilitate the detection and recognition of unpalatable prey. Our prediction was that warning signals are often red, orange, or yellow, because these colors have higher contrasts than colors such as blue or white against green natural backgrounds, maximizing their conspicuousness (Endler, 1992; Endler and Mappes, 2004; Stevens and Ruxton, 2012). Illuminant spectra are known to vary with time of day and atmospheric conditions (Lovell et al., 2005; Nieves et al., 2012). Short wavelengths are expected to be less stable than longer wavelengths as atmospheric particles and cloud cover alter the contribution of Rayleigh-scattered sunlight. We analyzed the contrast of each color signal as a function of time, for different weather conditions. In particular, we calculated the absolute variation of the contrast of each of the warning signals, represented as the Standard Deviation (SD) of the contrast. We expected longwave colors to have smaller SD and higher contrasts. Furthermore, the mean contrast should be higher for colors such as red, orange, and yellow. We predicted these results would be especially true for the RG output, since this system has been proposed to detect the maximum contrast between objects like fruits against natural backgrounds (Maximov, 2000; Lovell et al., 2005). Stevens and Ruxton (2012) proposed that warning signals should not only be highly contrasting, but stable throughout a day and somewhat unchanging in different light conditions. To test this prediction we calculated the stability of the contrast, determined by the Coefficient of Variation (CV) of each color signal. We predicted that warning signals colors would have smaller CV values than colors that are not usually used in aposematic signaling.
Materials and Methods
Study Species and Sites
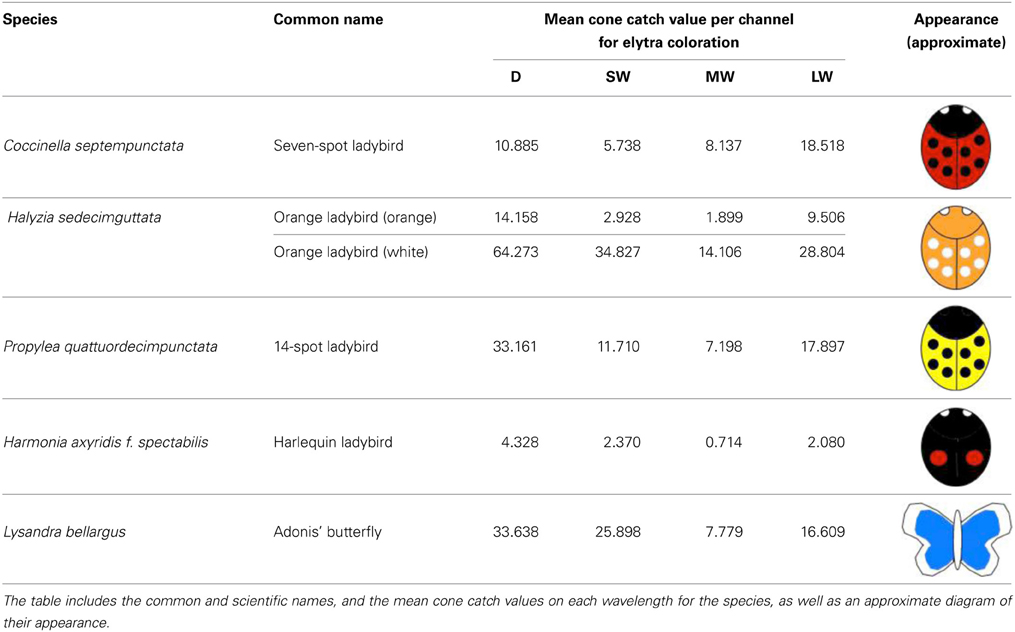
In order to examine the contrast of different ladybird warning signals under varying light conditions, we collected ladybird species broadly representing the main aposematic color types (Stevens and Ruxton, 2012). Experiments were carried out under the local ethical guidelines. All animals collected were euthanized as soon as possible by freezing them in a −80°C freezer, to preserve the specimens until the experiments were conducted. None of the species included in this study are currently endangered or protected by any conservation agency. The ladybird species collected were: (1) Seven-spot Coccinella septempunctata that have black spots on red elytra; (2) Fourteen-spots Propylea quattuordecimpunctata that have black spots on yellow elytra; (3) orange ladybirds, Halyzia sedecimguttata that have white spots on orange elytra (which we also used in our analyses as a “rare” aposematic signal), and (4) Harlequin ladybirds, Harmonia axyridis f. spectabilis that have red spots on black elytra. In order to be able to measure the contrast of colors that are rarely found in aposematic signals, such as blue, we photographed three museum specimens (using one specimen per day) of the Adonis' blue butterfly Lysandra bellargus. Table 1 summarizes the mean color values (mapped cone catch values) for each species.
To make the contrast calculations, we used three different leaves from plants where we have often found these ladybirds: common nettle (Urtica dioica L.), ground ivy (Glechoma hederacea L.), and dog's mercury (Mercurialis perennis L.). In addition we included a piece of Ash bark (Fraxinus oxycarpa) to calculate the contrast of the ladybird colors against brown backgrounds. We collected these species (except the butterflies, which were previously collected and mounted) in two main sites: (1) Madingley Woods, Cambridgeshire, UK (52°13′0.98″N, 0°3′2.93″E), and (2) the city of Cambridge, UK (52°12′19.21″N, 0°7′18.54″E). No specific permissions were required to work in these locations.
Image Collection and Photo Setup
Light conditions change rapidly at sunrise, and this coincides with increased foraging effort in the morning by birds (Bednekoff and Houston, 1994). Therefore, we were especially interested in analyzing how warning signals are perceived during the early hours of the morning. We started taking photographs at sunrise (4:30–5:45, depending on the date) at 15-min intervals, to be able to detect rapid changes in color contrasts during sunrise. For each day, we took 12 photographs in these 15-min intervals. After the morning period, we took photographs over 30 min intervals, since light conditions are less variable around the middle period of the day (Endler, 1993), and birds often forage at a lower intensity during the day until sunset (Bonter et al., 2013). This period went on until 15:00–16:00, and comprised 12 additional photographs, making a total of 24 data points for each day. The photographs were taken during the summer (early June through early September) of 2012.
To take the photographs we used a Nikon D90 digital SLR camera, which had undergone a quartz conversion to enable UV light to reach the CCD array of the camera, which is naturally highly sensitive to UV light (Advanced Camera Services, Norfolk, UK). The camera was fitted with an AF-S VR Micro-Nikkor 105 mm lens, sensitive to ultraviolet wavelengths. For the human visible light photos, a UV/infrared (IR) blocking filter was used which transmits wavelengths between 400 and 700 nm (Baader UV/IR Cut Filter). For the UV images, a UV pass and IR blocking filter was used (Baader U filter), which transmits between 300 and 400 nm. This yielded four images corresponding to different parts of the spectrum (UV, SW, MW, and LW). The sensitivity range and peaks of the camera set up for each of these channels, accounting for the camera sensitivity and the lens and filter transmission is: UV: 360–400 nm (peak 366 nm), SW: 400–550 nm (peak 465 nm), MW: 420–620 nm (peak 522 nm), LW: 560–700 nm (peak 667 nm). These spectral sensitivity calculations were undertaken using a new method developed in our laboratory (Troscianko and Stevens, in prep) involving placing a dispersing prism between the lens elements and camera sensor, combined with calibration of wavelength locations on the sensor using light sources of known emission spectra. This method has a close correspondence with other approaches based on quadratic programming procedures (Pike, 2011) and interference filters (see Stevens et al., 2014).
Preliminary measurements showed that ladybird elytra have very low reflectance in the UV spectrum. Furthermore, several studies have suggested that ultraviolet cues are unlikely to play an important role in aposematism (Lyytinen et al., 2001; Stevens and Ruxton, 2012). Lyytinen et al. (2001) found that even if aposematic prey have UV reflecting markings, bird predators did not learn to associate these with distastefulness, whereas the correct association was made with visible colors. Therefore, once it became clear from our initial analyses that the ladybirds only reflected about 5% UV light, combined with a lack of evidence for the importance of UV in aposematic prey, we concentrated on analyzing the color signals as processed by two visible opponent mechanisms described earlier (i.e., RG and BY), and that of the achromatic channel (luminance).
The photography setup used for the experiments consisted of a 15 × 10 cm sheet of black ethylene-vinyl acetate (EVA) used as a low-UV reflective background (having less than 5% reflectance). Every photo contained a 40% Spectralon gray standard (Labsphere Congleton, UK) used for calibration. Photos were taken under an arboreal canopy in Madingley Woods. The camera was fitted to a tripod and pointed toward the ground (90°) at a height of approximately 1 m. We conducted the experiments over a total of 9 days, three for each weather type, namely cloudy, part-cloudy, and sunny with a sample of different specimens, to account for variation in light conditions and individuals. Each photo setup consisted of three individuals of the seven-spot ladybird, three individuals of the fourteen-spot ladybird, one individual of the orange ladybird, and one individual of the Harlequin ladybird, plus one individual of the Adoni's butterfly and one leaf of each of the plant species described above and three pieces of bark. In previous experiments we established that the contrast measurements of ladybird coloration could only be made on freshly collected individuals (Arenas, unpublished). This is because sunlight and decomposition alter coloration after death. However, because the coloration of butterfly wings is structural, it does not change and thus, we were able to use preserved individuals. Thus, the differences in the numbers of individuals used throughout the experiment are attributed to the annual abundance patterns of each species that we could freshly collect. After a preliminary analysis, we averaged the three types of green background contrasts used, as there were no differences between them in the luminance (Lum) and RG channels, which are likely to be more informative than the BY system for our purposes (Lum: ANOVA N = 590; DF = 2; F = 1.102; p = 0.333. RG: ANOVA N = 590; DF = 2; F = 0.479; p = 0.620. BY: ANOVA N = 590; DF = 2; F = 7.128; p = 0.001). In addition, we found that the contrast against brown backgrounds was not different from the contrast against green backgrounds (RG: ANOVA N = 1164; DF = 4; F = 0.401; p = 0.808. BY: ANOVA N = 1161; DF = 4; F = 1.629; p = 0.164). Because the species we used ladybird species that are primarily found basking on green foliage, we concentrate on these results. However, we include the results of the contrast against brown background as supplementary information for this work (Supplementary Figures 3, 4). Each photograph then consisted of one blue butterfly, six-eight ladybirds (depending on each species' availability), three leaves and three bark pieces collected for a specific day. A total of 135 items were photographed during the experiment.
Image Calibration and Analyses
Because most cameras have non-linear responses to image values according to light levels that need to be corrected, we linearized each photograph to reflectance levels using a set of Spectralon gray standards varying from 2 to 99% reflectance (Westland and Ripamonti, 2004; Stevens et al., 2007; Garcia et al., 2013). The linearization process was made using camera-specific self-written plugins in Image J (Rasband, 1997–2013).
Our main interest was to measure how coloration changes with varying light conditions. However, to account for how visual processing of color may take place, we prepared two parallel sets of data. The first dataset was one that was not normalized (equalized) to the gray standard value, which is commonly done to specifically remove effects of illuminating conditions and convert data to reflectance (Stevens et al., 2007). The second dataset was one that included the normalization process. A normalized image's value would be an approximation to the idea of color constancy, a process whereby the visual system removes the effects of changes in the light conditions on color perception to when processing color information (Maloney and Wandell, 1986; Hulbert, 1999, 2007). Normalization works by equalizing the values of each images channel (SW, MW, LW) and removing variation in light conditions with regards to the 40% gray standard (Stevens et al., 2007). This process also converts each layer of the image into an 8 bit scale, such that a value of 255 equals 100% reflectance.
We aimed to analyze aposematic coloration from an avian predator's point of view. To do so, we used cone sensitivities for a model avian species, in this case the blue tit Cyanistes caeruleus (Hart et al., 2000), which is a commonly used species for modeling avian vision. We transformed our images (both normalized and non-normalized versions) to predicted avian cone catch values using a polynomial mapping technique using a D65 irradiance spectrum (Stevens et al., 2007; Pike, 2011). Compared to modeling predicted cone catch values with reflectance spectra, this mapping technique is highly accurate, with very low levels of potential error and R2 values for each channel from 0.96 to 0.98 between derived cone catch values based on spectrometry and cameras (Stevens and Cuthill, 2006; Pike, 2011; Stevens et al., 2014). Once the LW, MW and SW, UV and double cone images were obtained, we proceeded to measure the image values for each element (animals and background samples) in the photograph, for each of these the channels. Because the elytra of Coccinellidae are curved, all measurements were made using an area that did not have any specular reflectance. Following this, we standardized the images to control for variation in shutter speeds among photographs by dividing the cone catch values by the exposure time of each photograph (as did Lovell et al., 2005, pers. commun.).
Opponent channel processing is very important for the perception and accurate interpretation of a color signal. There is evidence that birds have opponent channels that are similar to those known for primates (including humans), including RG and BY systems (Osorio et al., 1999a; see above). Using the McCleod and Boynton (1979) formulas and using a ratio-based approach suggested for similar purposes by Lovell et al. (2005), we calculated the opponent channel responses for a RG, BY, and an achromatic channel as follows:
Where Lum corresponds to the activation of the Luminance channel and RG and BY correspond to the activations of the Red–Green and Blue–Yellow channels, respectively. The LW, MW, and SW terms in the equations correspond to the cone catch values in the long, medium, and short wavelengths. To examine whether warning colors have greater contrast against green background colors we calculated the Weber Contrast (Whittle, 1994), which takes into account the image value of the objects of interest as a fraction of the background appearance using the formula:
Where I(o) corresponds to the value of any one object (i.e., ladybird elytra) and I(b) corresponds to the value of the background color. This particular measure is suited to comparisons between small objects against larger backgrounds, such as a ladybird against a leaf or spots on the elytra. We plotted the mean absolute contrast of each color signal as a function of time and in relation to its SD. In addition, to examine the degree of stability of each color signal, we calculated the CV of the opponent outputs. The CV is an effective measurement to determine how relatively stable a measurement is around a mean value (Quinn and Keough, 2002). The data for the RG and BY contrast values were analyzed separately form the Luminance values, since they provide different types of visual information (Osorio and Vorobyev, 2005). Further, we divided the data set into four time periods (two in the morning and two for the afternoon) to establish if the time of day on where the signal is being analyzed plays a role in its stability or contrast against the background.
Statistical Analyses
We analyzed our data in terms of two separate dependent variables, namely absolute contrast and CV of each color using SPSS 20. After checking the distribution of our residual errors as well as the normality of the data using SPSS 20 we fit Analysis of Variance models (ANOVA) separately for each variable in a model that included the main effects of weather, time, and color as factors, and the interactions between these. Once the models were run, we discarded the non-significant interactions and ran the models again. Each ANOVA was followed by post-hoc tests (Tukey HSD) when relevant. We also ran the same statistical analyses for the dataset involving normalized images.
Results
Signal Contrast and Conspicuousness
Our results show that the different color signals analyzed have different contrasts in the RG and BY opponent systems, and that this contrast changes according to weather conditions (Weather*Color: DF = 10, F = 3.32, R2 = 0.801, p < 0.005) (Figures 1A,B). The contrast of each color under cloudy weather is different from both part-cloudy and sunny weathers (Tukey HSD P < 0.001). In addition, red, orange, yellow, and black colors themselves differ in their contrast even without the influence of weather conditions (ANOVA. DF = 5, F = 105.94, p < 0.001). Furthermore, blue and white signals do not differ from each other (Tukey HSD, p = 0.49). In the luminance channel (Figure 1C) the average color contrast against an average green foliage color is different under varying light conditions. (DF = 10, F = 6.19, adjusted R2 = 0.910, p < 0.001). However, colors such as white (Tukey HSD P < 0.001), and blue (Tukey HSD P < 0.001) are more contrasting than the other signals analyzed. Likewise, colors like orange and yellow have similar luminance contrasts (Tukey HSD, P = 0.72). In this channel red and black signals are both perceived as dark, and do not differ from each other (Tukey HSD, P = 0.91).
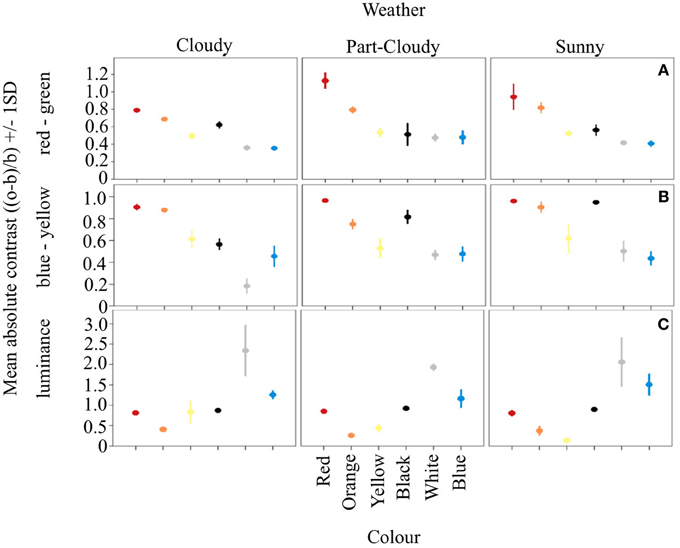
Figure 1. Mean absolute contrast and Standard Deviation (SD) of the six color signals analyzed. Vertical panels show the variation of these signals on the different weather conditions, while horizontal panels show the opponent channel activation of the three systems analyzed. Panel (A) shows how in the RG system the longwave colors are more contrasting. Similar results can be seen from (B), despite the fact that this system should be better at processing colors such as blue. Panel (C) shows the opponent activation on the luminance channel, where achromatic colorations tend to be either very stable (black) or highly variable (white), providing different types of information.
Figure 2 shows the overall mean contrast over time. It is clear that red colors have a very stable output throughout the day, yielding an overall constant signal (mean contrast (MC) = 0.977). Although orange signals are highly contrasting against green backgrounds, these are not as stable through time as red colors (Orange MC = 0.77, CV = 0.19). Similar results were found for the yellow signal, which has lower, rather unstable contrast values (MC 0.51, CV = 0.58). Black coloration is particularly variable over time, as can be seen by the CV calculations (MC = 0.80, CV = 0.94). The variation of white (MC = 0.41, CV = 1.05) and blue (MC = 0.42, CV = 0.24) signals is also considerable, and these colors also have overall lower contrast than the rest of the warning colors analyzed. With respect to the four time periods that we defined to test if the signals change over the course of a day, our results show that there are no differences in the contrast (ANOVA, DF = 3, F = 1.51, p = 0.21) or the stability (ANOVA, DF = 3, F = 1.49, p = 0.21) of the colors that are related to the time of day.
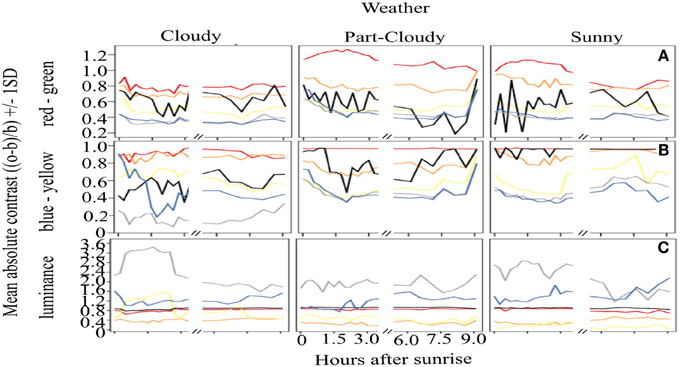
Figure 2. Absolute contrast of the different color signals analyzed over the course of a day. Vertical panels show the absolute contrast of the different colors on the three opponent channel activation systems analysed [(A) (RG), (B) (BY), (C), Lum] while horizontal panels show the contrast according to the different weather conditions. Despite the variations, red remains as the most contrasting color (A,B), and it is very stable in the BY opponent system (B), contrary to our predictions. Achromatic signals (black and white) (C) have great variation, indicating that these colors should not be considered a reliable warning signal.
The dataset that includes the normalization process shows that even when we try to take away the influence of the illuminant, the differences in weather conditions still have a significant effect over color contrast (Weather*Color: ANOVA, DF = 10, F = 2.05, p < 0.025) (Supplementary Figure 1). Furthermore, this interaction between color and weather is also significant when we analyzed the stability of the signal (ANOVA, DF = 10, F = 2.14, p < 0.04—Supplementary Figure 2).
Signal Stability
We calculated the amount of fluctuation of each color signal around a mean, over the course of a day, defined as the CV (Figure 3). Our results suggest that the fluctuation of the signals varies with each weather condition (ANOVA DF = 5, F = 18.97, p < 0.001) and variation in color stability depends on the different weather (and hence light) conditions (Color-Weather interaction: DF = 10, F = 2.79, p < 0.005). For both the RG and BY channels, the fluctuation of red signals tends to be lower (Tukey HSD, p < 0.005) than that of other colors. However, the fluctuation of orange, yellow, black, white, and blue signals does not differ significantly (Tukey HSD, P > 0.05) (Figures 3A,B). Figure 3C shows signal fluctuations (CV) in the luminance channel. In contrast to the results discussed above, these fluctuations differ only marginally under the different weather conditions. However, the luminance of the color signals is different from each other (ANOVA, DF = 5, F = 77.63, p < 0.005). Here, the contrast signal of darker colors such as red and black is indistinguishable from each other (Tukey HSD, p = 0.55), as well as the fluctuations between white and blue (Tukey HSD, p = 0.99). In addition, the amount of fluctuation of orange (Tukey HSD, p < 0.01) and yellow Tukey HSD, p < 0.05) are different from the other signals, and the highest in the luminance channel.
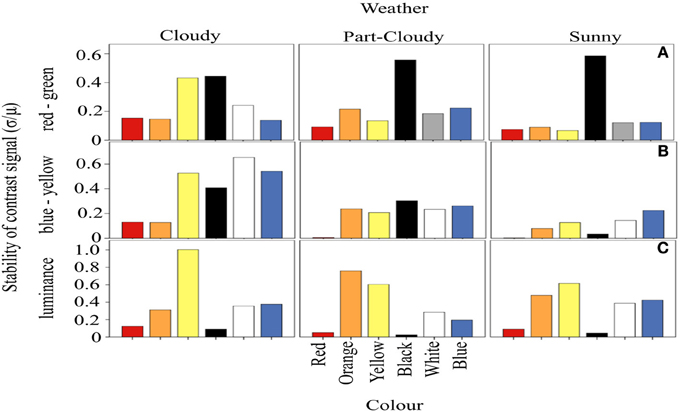
Figure 3. Coefficient of variation of the proportion of activation of each opponent system. The horizontal panels represent the three systems analyzed, while the vertical panels correspond to the different weather conditions. In (A), it is clear that red and orange signals generally vary less than the rest of the colors. The variation of the signals in the BY system are smaller than expected (B). Panel (C) shows the variations of each color in the luminance channel. These CV values are highly independent of the color and weather condition.
The normalized data set shows that for signal stability there are no significant effects of weather conditions on the different colors analyzed (ANOVA, DF = 2, F = 1.800, p = 0.170). However, the interaction between color and weather condition is still present (Weather*Color: ANOVA, DF = 10, F = 2.149, p < 0.025) (Supplementary Figure 2).
Discussion
This study aimed to determine which types of potential aposematic signal colors are most salient against natural green backgrounds. We photographed different ladybird species, and analyzed their elytra coloration, mapped to the visual sensitivities of a potential avian predator over 9 days, and under three different weather conditions. Our two main tests involved (1) analyzing the overall contrast of each color signal and how it changes over time, and (2) determining the stability of each contrast signal over time. Our results show that, despite some variation, longwave colors are not only more contrasting against green backgrounds to a bird's visual system, but in the case of red (and to a lesser extent the orange) signals, these also fluctuate less over time and across different light conditions (i.e., they are more stable and reliable signals).
Warning signals in terrestrial habitats are usually combinations of LW colors, such as red, orange, and yellow (Stevens and Ruxton, 2012). It has long been argued that the reason why these colors are widely represented in aposematic coloration is that they are highly conspicuous and have a greater contrast against natural backgrounds (Cott, 1940; Endler, 1992; Gamberalle-Stille, 2001; Stevens and Ruxton, 2012). Also, these colors may be more stable under a range of natural conditions and illuminations (Lovell et al., 2005; Stevens and Ruxton, 2012). However, these predictions have rarely been tested empirically, especially with regards to signal stability. In line with our predictions, ladybird red coloration is highly salient against an average green background. These results are consistent regardless of the weather conditions, and time of day. Furthermore, our hypothesis holds even when the colors are processed with different opponent channel mechanisms (i.e., RG and BY). Our study suggests that red signals may be commonly used as a warning color, because they are highly effective in stimulating avian opponent color channels. Furthermore these signals are highly conspicuous regardless of changes environmental light, possibly aiding with predator recognition and learning.
There is ample evidence that red (Roper, 1990), orange (Ritland, 1998), and yellow (Rowe and Guilford, 1999; Lindström et al., 2001) colors serve as warning signals to avian predators. In addition, several studies have found that predators avoid red and orange stimuli (Exnerová et al., 2006b). However, these studies do not determine why these associations are made with such color types (i.e., the underlying mechanism for what makes such colors effective signals). Our work suggests that the generalization of these two colors as an indication of the presence of toxic chemicals, and the general avoidance of LW colors may be related to their contrast against the background. Endler (1992) proposed that one of the ways that conspicuousness can be maximized is by displaying color patterns that are complementary to each other (e.g., red with green or yellow with blue). Ladybirds do not have such color patterns themselves, but they are often found basking on green vegetation. This then may be a signaling strategy that ensures opponent channel mechanisms can effectively decode the warning colors of these species. Our work demonstrates that warning signals composed of LW colors may have a twofold benefit. First, they provide a highly conspicuous signal when displayed next to green backgrounds, stimulating the RG system and maximizing their contrast (Wallace, 1889; Cott, 1940; Lythgoe, 1979; Hurvich, 1981). Second, our work also provides a new line of empirical evidence that these signals are highly reliable even under different light conditions. The latter finding may explain why these colors are better learnt by potential predators (Roper and Wistow, 1986; Roper, 1990; Exnerová et al., 2006a; Aronsson and Gamberalle-Stille, 2008) in comparison to other color combinations and achromatic signals (Osorio et al., 1999b).
Here, we focus on the contrast of aposematic signals against an average green background. It is worth noting that in the preliminary stages of this study we also measured the contrast of ladybird warning colors against an average of brown backgrounds, namely twigs and bark. We considered these additional backgrounds as ladybirds are sometimes found basking on the twigs rather than the leaves of a given plant. The results for the analyses of signal contrast and stability showed the same tendency as those presented in this study against green leaves, suggesting that the use of warning coloration is effective and stable on a variety of backgrounds and under different weather conditions.
Although Schuler and Hesse (1985) and Jones (1932) suggested that colors such as yellow and white may also serve as warning signals, our results show that the color contrast of these is not as high as that of red signals, and thus, predators (especially naïve ones) may be more prone to attack yellow or white prey (Lyytinen et al., 1999). Blount et al. (2012) found that aposematic signals serve not only to alert predators about the presence of secondary chemical defenses, but also give an indication of the strength of these defenses. Furthermore, they found that the concentration of carotenoids in the elytra of seven spot ladybirds (Coccinella septempunctata) was directly correlated to the amount of the toxin precoccinelline. Seven spot ladybirds exhibit bright red colors on their elytra (Roy et al., 2011). In accordance to our results, the honesty of a signal (i.e., a direct correlation between the concentration of carotenoids and chemicals) could also be interpreted as a highly contrasting signal over time. Nevertheless, studies on the honesty of aposematic signal of colors other than red would be needed to support this idea. Likewise, it is also important to note that this study is focused on the perception of warning colorations by avian predators. Yet, insect predators, including ladybirds themselves, have also been found to prey upon other warning colored prey (Dixon, 2000; Hodek et al., 2012). While birds have tetrachromatic vision, insect vision is variable (Stavenga, 1992; Chitka, 1996; Briscoe and Chitka, 2001). Further studies would benefit of including different visual systems when studying the perception of color signals.
In addition to color opponency, there are other post-receptoral processes that have been studied to achieve an accurate determination of a visual scene. These include color constancy, whereby the brain at least partly removes the effect of changes in environmental light on color perception (Maloney and Wandell, 1986; Hulbert, 1999; Foster, 2003). However, the mechanisms underlying color constancy are not fully understood (Hulbert, 2007). One way to approach this problem when working with digital photography is to normalize each image to a gray standard value (Stevens et al., 2007). Since we were interested on the effect of light conditions on the perception of color differences between a signal and the background, we did not undertake this step in the main part of our study (in line with Lovell et al., 2005). However, we did repeat our contrast calculations after normalizing the images to determine the effect of this. These parallel results showed that even when we remove the initial changes of the illuminant, the contrast of ladybird colors against green backgrounds is maintained. Moreover the stability analyses on these standardized signals also result in a significant interaction between the each color signal and the type of weather. Thus, even when correcting for lighting changes in the environment, aposematic colors, especially red and orange ones, are still more contrasting than other colors.
Warning signals usually have a black component in addition to long-wave colors (Schuler and Hesse, 1985; Komárek, 1998; Lindström et al., 2001; Rowe et al., 2004). However, Aronsson and Gamberalle-Stille (2008) showed that the pattern of a warning signal may not be as important as the color component. Moreover, a completely black prey may not elicit an avoidance response from an avian predator (Exnerová et al., 2006b). Our analyses show that black signals are highly variable both during the day and between weather conditions. A possible explanation for the use of black colors is that these enhance thermoregulatory processes in several insect species (True, 2003; Trullas et al., 2007), including ladybirds (de Jong et al., 1996). Despite the benefits of thermal melanism, the proportion of black coloration has also been found to affect predation rates (Hegna et al., 2013). Our results suggest that this might be due to the low contrast of black colors, which could potentially be interpreted as edible prey. Several studies on aposematic coloration in firebugs (Pyrrhocoris apterus: Heteroptera) have shown that predators pay more attention to the “main” color of the signal, rather than the patterns it may have (Exnerová et al., 2003, 2006b). Moreover, a few studies have discussed the maximization of conspicuousness by achieving the greatest mismatch to the background, rather than just color contrast (Poulton, 1890; Cott, 1940; Endler, 1988). Preliminary results for this study revealed that the “internal contrast” (i.e., contrast between the elytra background and its spots) is unstable over time, and color combinations with black are no different in contrast than combinations with colors such as white (Arenas, unpublished). Further work is needed to understand what drives the specific features and diversity in warning signal patterns.
A range of past work has discussed how the effectiveness of a warning signal will depend on its detectability and reliability. Here, we analyzed two opponent color pathways and an achromatic channel used to process a warning signal. Yet, the final decision on whether or not to attack prey should be the combination of all the information reaching a predator's brain. According to our results, the RG opponent system yields longwave colors as highly contrasting and stable. In addition, when we analyzed each color in the BY system, LW colorations also yielded very stable signals, although not as contrasting as in the RG opponent channel. Our results show that blue signals (which have shorter wavelengths) have low contrasts against the background. Likewise, white colors are very variable and yield low contrasts in both the RG and BY systems. These signals are less common in nature, and are often referred to as weak aposematic colors (Endler and Mappes, 2004; Stevens and Ruxton, 2012). Some studies that have suggested that these signals might not be as effective as those with longwave colors (Endler and Mappes, 2004; Speed and Ruxton, 2007; Ruxton et al., 2009). However, there is also evidence that weak aposematic prey often increase the production of bitter tasting and foul smelling substances to further deter predators (Nokelainen et al., 2012). It has also been suggested that luminance contrast (i.e., black and/or white colorations) may aid in the initial detection of the signal (Osorio et al., 1999b; Osorio and Vorobyev, 2005; Stevens and Ruxton, 2012). However, since the contrast of these colors changes throughout the day, they might not be contributing greatly to predator learning processes.
One limitation of our study is that we have sampled only three to nine individuals for each of the three weather conditions tested. It is worth noting, however, that the results across weather conditions are comparable. Given past studies on color processing in avian visual systems (Lovell et al., 2005; Osorio and Vorobyev, 2005) and the results that we present here, we believe that we present a reliable interpretation as to the efficacy ladybird color signals. Our work also provides similar overall conclusions to Lovell et al.'s (2005) analyses of fruit coloration, but with a greater sample size and studies of different weather conditions. Further studies on this subject should test how stability (reliability) of different warning signal colors under different conditions influences the rate and persistence of aversion learning in predators. Endler (1988) commented on the need to investigate which colors might be favored in different environments in terms of them mismatching against the background. We believe that the results we present here give evidence on how aposematic signals might be received in terrestrial environments and in varying light conditions. However, it is worth noting that the perception of coloration may vary in different environments. For example in clear water, longer wavelengths are often attenuated more quickly than shorter wavelengths, and this can affect signal form in animals (Stevens, 2013). Possibly for these reasons, aquatic organisms may use a wide range of different colors to advertise their toxicity, as is the case of marine nudibranchs (Cortesi and Cheney, 2010). Warning signals in the marine environment often seem to contain more blue components. Thus, our results primarily relate to the efficacy of aposematic signals in terrestrial environments, and further work should be conducted to how broadly our conclusions apply to in different conditions and environments, especially aquatic ones. In addition, not only does the environment can change a signal but also its perception may vary according to the predator's visual system (Endler, 1992; Stevens, 2013). Several studies have evaluated prey perception in species that have different numbers of photoreceptors. For instance, Smith et al. (2012) compared the prey detection and catching abilities of dichromat and trichromat tamarin monkeys (Saguinus spp.). They found that even though trichromat monkeys captured more prey than dichromat individuals, the latter caught a larger proportion of camouflaged prey. Thus, the perception of warning signals will depend on the visual system of the receiver (Stevens, 2007).
Our results may also be important when evaluating coloration from perspectives other than predator-prey interactions. Color signals are also widely involved in sexual selection and social signaling. We might expect that sexual and social signals would benefit from being reliable under a wide range of signaling conditions, especially if they provide information about quality or condition. Studies on species with aposematic coloration show that females of several species prefer to mate with brightly colored males (Summers et al., 1999; Maan and Cummings, 2009). In the butterfly genus Heliconius and the ladybird genus Adalia, females preferentially mate with males having their own color patterns (Muggleton, 1979; Majerus et al., 1982; Jiggins et al., 2001). Moreover, several authors suggest a trade-off between fitness and the color attributes displayed by males (Ruxton et al., 2009; Nokelainen et al., 2012). Our results provide insight into the use of aposematic coloration in predator prey interactions. However, if warning signals are being used to warn predators about unprofitable prey, and also to attract mates, then there is a chance that both are benefiting from the same components of the signal. If two opposing selection pressures may be acting on a species' ecology, then they should maintain a suitable balance between them (Endler, 1991, 1992). As a consequence, not only is it beneficial for an aposematic species to have colorations that are highly contrasting against the background and stable through time in terms of predator avoidance, but also to exploit the female's sensory system appropriately, and attract more mates. Further studies on the interactions of sexual and natural selection on warning coloration would improve our understanding of the trade-offs between natural and sexual selection and how these shape the evolution phenotypic characteristics in colored prey.
Author Contributions
Lina María Arenas and Martin Stevens conceived and designed the experiments; Lina María Arenas collected the data; Lina María Arenas and Jolyon Troscianko analyzed the data; Lina María Arenas and Martin Stevens wrote the paper.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank G. Ruxton, G. Lovell, N. Davies and H. Rowland, C. Pardo-Díaz, V. Crawford, and I. Medina for their valuable comments and discussions on this work. We also thank three referees for comments on the work and manuscript. This work was supported by the Biotechnology and Biological Sciences Research Council David Phillips Research Fellowship (BB/G022887/1 to Martin Stevens), Lina María Arenas was funded by Colciencias-Colombia; Colfuturo-Colombia; and the Amateur Enthomologists' Society.
Supplementary Material
The Supplementary Material for this article can be found online at: http://www.frontiersin.org/journal/10.3389/fevo.2014.00025/abstract
Supplementary Figure 1 | Color contrast of each warning signal analyzed under normalized conditions. The analyses show that even when the main effects of the illuminant are taken away, the contrast of longwave colors is influenced by the different weather conditions. Panel (A) in both figures refers to the RG system, which yields the highest values of contrast, especially for red signals. Panel (B) shows very low activation in the BY system. Panel (C) shows the luminance activation of each color signals. These contrast values are very low, compared to the outputs of the RG and BY systems, indicating that this channel is not very reliable.
Supplementary Figure 2 | Signal stability of each warning signal analyzed under normalized conditions. The graph shows the same tendency as Figure 2, where red varies less than the rest of the signals even in different light conditions (A). The BY opponent system (B) shows greater variation than the red–yellow system, since the former is less effective when processing longwave colors. The luminance channel shown in (C) shows low variation for dark colors such as red and black, and large variation for lighter, medium-shorter wave colors.
Supplementary Figure 3 | Color contrast of each warning signal against brown backgrounds. The graph shows the contrast of each color signal analyzed under the three opponent channels in the horizontal panels and the different weather conditions in the vertical panels. Similar to the situation presented with the green backgrounds, longwave colors have higher contrasts in both the RG and the BY channels. In the luminance channel, the white and blue signals have higher contrast, as it happens with the green backgrounds.
Supplementary Figure 4 | Absolute contrast of the different color signals analyzed over the course of a day under brown background colors. Vertical panels show the absolute contrast of the different signals on the three opponency channels, and horizontal panels compare how this signals change under different weather conditions. In the RG channel, longwave colors have higher contrasts, similar to the results presented for green backgrounds. In the BY channel the tendency remains the same, except for the case of the sunny weather, where black colors have higher contrasts. In the Luminance channel, the white signal has higher contrasts, and these results are comparable to those obtained for the green backgrounds.
Abbreviations
SW, Short wavelength; MW, Medium wavelength; LW, Long wavelength; UV, Ultra violet wavelength; RG, Red-Green Opponent system; BY, Blue-Yellow opponent system; SD, Standard deviation; CV, Coefficient of Variation; MC, Mean Contrast.
References
Ammermüller, J., Itzhaki, A., and Perlman, I. (1998). UV-Sensitive input to horizontal cells in the turtle retina. Eur. J. Neurosci. 10, 1544–1552. doi: 10.1046/j.1460-9568.1998.00160.x
Aronsson, M., and Gamberalle-Stille, G. (2008). Domestic chicks primarily attend to color, not pattern, when learning an aposematic coloration. Anim. Behav. 75, 417–423. doi: 10.1016/j.anbehav.2007.05.006
Bednekoff, P. A., and Houston, A. I. (1994). Avian daily foraging patterns: effects of digestive constraints and variability. Evol. Ecol. 8, 36–52. doi: 10.1007/BF01237664
Bezzerides, A. L., McGraw, K. J., Parker, R. S., and Husseini, J. (2007). Elytra color as a signal of chemical defense in the Asian ladybird beetle Harmonia axyridis. Behav. Ecol. Sociobiol. 61, 1401–1408. doi: 10.1007/s00265-007-0371-9
Blount, J. D., Rowland, H. M., Drijfhout, F. P., Endler, J. A., Inger, R., Sloggett, J. J., et al. (2012). How the ladybird got its spots: effects of resource limitation on the honesty of aposematic signals. Funct. Ecol. 26, 334–342. doi: 10.1111/j.1365-2435.2012.01961.x
Bonter, D. N., Zuckerberg, B., Sedgwick, C. W., and Hochachka, W. M. (2013). Daily foraging patterns in free-living birds: exploring the predation-starvation trade-off. Proc. R. Soc. Lond. Ser. B 280, 1471–2954. doi: 10.1098/rspb.2012.3087
Briscoe, A. D., and Chitka, L. (2001). The evolution of color vision in insects. Annu. Rev. Entomol. 46, 471–510. doi: 10.1146/annurev.ento.46.1.471
Brodie, III E. D., and Janzen, F. J. (1995). Experimental studies of coral snake mimicry: generalized avoidance of ringed snake patterns by free-ranging avian predators. Funct. Ecol. 9, 186–190. doi: 10.2307/2390563
Chatterjee, S., and Callaway, E. M. (2003). Parallel color-opponent pathways to primary visual cortex. Nature 426, 668–671. doi: 10.1038/nature02167
Chitka, L. (1996). Optimal sets of color receptors and opponent systems for coding of natural objects in insect vision. J. Theor. Biol. 181, 179–196. doi: 10.1006/jtbi.1996.0124
Cook, L. M., Brower, L. P., and Alcock, J. (1969). An attempt to verify mimetic advantage in a neotropical environment. Evolution 23, 339–345. doi: 10.2307/2406796
Cortesi, F., and Cheney, K. L. (2010). Conspicuousness is correlated with toxicity in marine opistobranchs. J. Evol. Biol. 23, 1509–1518. doi: 10.1111/j.1420-9101.2010.02018.x
Cuthill, I. (2006). “Color perception,” in Bird Coloration: Mechanism and Measurements, Vol. 1, eds G. E. Hill and K. J. McGraw (Cambridge, MA: Harvard University Press), 3–40.
de Jong, P. W., and Brakefield, P. M. (1998). Climate and change in clines for melanism in the two–spot ladybird, Adalia bipunctata (Coleoptera: Coccinellidae). Proc. R. Soc. Lond. Ser. B 265, 39–43. doi: 10.1098/rspb.1998.0261
de Jong, P. W., Gusselkoo, S. W. S., and Brakefield, P. M. (1996). Differences in thermal balance, body temperature and activity between non-melanic and melanic two-spot ladybird beetles (Adalia bipunctata) under controlled conditions. J. Exp. Biol. 199, 2655–2666.
Derrington, A. M., Krauskopf, J., and Lennie, P. (1984). Chromatic mechanisms in the lateral geniculate nucleus of macaque. J. Physiol. (Lond). 357, 241–265.
DeValois, R. L., Abramov, I., and Jacobs, G. H. (1966). Analysis of response patterns in LGN cells. J. Opt. Soc. Am. A 56, 966–977. doi: 10.1364/JOSA.56.000966
Dixon, A. F. G. (2000). Insect Predator-Prey Dynamics. Ladybird Beetles and Biological Control. Cambridge: Cambridge University Press
Dolenská, M., Nedvěd, O., Veselý, P., Tesařová, M., and Fuchs, R. (2009). What constitutes optical warning signals of ladybirds (Coleoptera: Coccinellidae) towards bird predators: color, pattern or general look? Biol. J. Linn. Soc. 98, 234–242. doi: 10.1111/j.1095-8312.2009.01277.x
Endler, J. A. (1988). Frequency dependent predation, crypsis and aposematic coloration. Philos. Trans. R. Soc. Lond. B Biol. Sci. 319, 505–523. doi: 10.1098/rstb.1988.0062
Endler, J. A. (1991). Variation in the appearance of guppy color patterns and their predatorsunder different visual conditions. Vis. Res. 31, 587–608. doi: 10.1016/0042-6989(91)90109-I
Endler, J. A. (1992). Signals, signal conditions, and the direction of evolution. Am. Nat. 139, S125–S153. doi: 10.1086/285308
Endler, J. A. (1993). The color of light in forests and its implications. Ecol. Monogr. 63, 2–27. doi: 10.2307/2937121
Endler, J. A., and Mappes, J. (2004). Predator mixes and the conspicuousness of aposematic signals. Am. Nat. 163, 532–547. doi: 10.1086/382662
Exnerová, A., Štys, P., Fucikova. E., Vesela, S., Svádova, K., Prokopova, M., et al. (2006a). Avoidance of aposematic prey in European tits (Paridae): learned or innate? Behav. Ecol. 18, 148–156. doi: 10.1093/beheco/arl061
Exnerová, A., Štys, P., Kittin, A., Volf, O., and Pudil, M. (2003). Birds as predators of true bugs (Heteroptera) in different habitats. Biologia (Bratislava) 57, 253–264. doi: 10.1046/j.0024-4066.2002.00161.x
Exnerová, A., Svádová, K., Štys, P., Barcalova, S., Landová, E., Prokopova, M., et al. (2006b). Importance of color in the reaction of passerine predators to aposematic prey: experiments with mutants of Pyrrhocoris apterus (Heteroptera). Biol. J. Linn. Soc. 88, 143–153. doi: 10.1111/j.1095-8312.2006.00611.x
Foster, D. H. (2003). Does color constancy exist? Trends Cogn. Neurosci. 7, 439–443. doi: 10.1016/j.tics.2003.08.002
Gamberalle-Stille, G. (2001). Benefit by contrast: an experiment with live aposematic prey. Behav. Ecol. 12, 768–772. doi: 10.1093/beheco/12.6.768
Garcia, J. E., Dyer, A. G., Greentree, A. D., Spring, G., and Wilksch, P. A. (2013). Linearisation of RGB camera responses for quantitative image analysis of visible and UV photography: a comparison of two techniques. PLoS ONE 8:e79534. doi: 10.1371/journal.pone.0079534
Hart, N. S., Partridge, J. C., Cuthill, I. C., and Bennett, A. T. D. (2000). Visual pigments, oil droplets, ocular media and cone photoreceptor distribution in two species of passerine bird: the blue tit (Parus caeruleus L.) and the blackbird (Turdus merula L). J. Comp. Physiol. A 186, 375–387. doi: 10.1007/s003590050437
Hegna, R. H., Nokelainen, O., Hegna, J. R., and Mappes, J. (2013). To quiver or to shiver: increased melanization benefits thermoregulation, but reduces warning signal efficacy in the wood tiger moth. Proc. R. Soc. Lond. Ser. B 280:20122812 doi: 10.1098/rspb.2012.2812
Hodek, H. F., Van Emden, H. F., and Honěk, A. (2012). Ecology and Behaviour of the Ladybird Beetles (Coccinellidae). Oxford: Blackwell Publishing
Hulbert, A. (1999). Color vision: is color constancy real? Curr. Biol. 9, R558–R561. doi: 10.1016/S0960-9822(99)80354-6
Jiggins, C. D., Naisbit, R. E., Coe, R. L., and Mallet, J. (2001). Reproductive isolation caused by color pattern mimicry. Nature 411, 302–305. doi: 10.1038/35077075
Jones, F. M. (1932). Insect coloration and the relative acceptability of insects to birds. Ecol. Entomol. 80, 345–371.
Kaiser, P. K., and Boynton, R. M. (1996). Human Color Vision. Washington, DC: The Optical Society of America.
Kelber, A., Vorobyev, M., and Osorio, D. (2003). Animal color vision – behavioural and physiological concepts. Biol. Rev. 78, 81–118. doi: 10.1017/S1464793102005985
Komárek, S. (1998). Mimicry, Aposematism, and Related Phenomena Mimetism in Nature and the History of its Study. Munchen: Lincom München
Lindström, L., Alatalo, R. V., Lyytinen, A., and Mappes, J. (2001). Predator experience on cryptic prey affects the survival of conspicuous aposematic prey. Proc. R. Soc. Lond. Ser. B 268, 357–361. doi: 10.1098/rspb.2000.1377
Lovell, P. G., Tolhurst, D. J., Párraga, C. A., Baddeley, R., Leonards, U., Troscianko, J., et al. (2005). Stability of the color-opponent signals under changes of illuminant in natural scenes. J. Opt. Soc. Am. A 22, 2060–2071. doi: 10.1364/JOSAA.22.002060
Lyytinen, A., Alatalo, R. V., Lindström, L., and Mappes, J. (1999). Are European white butterflies aposematic? Evol. Ecol. 13, 709–719. doi: 10.1023/A:1011081800202
Lyytinen, A., Alatalo, R. V., Lindström, L., and Mappes, J. (2001). Can ultraviolet cues function as aposematic signals? Behav. Ecol. 12, 65–70. doi: 10.1093/oxfordjournals.beheco.a000380
Maan, M., and Cummings, M. (2009). Sexual dimorphism and directional selection on aposematic signals in a poison frog. Proc. Natl. Acad. Sci. U.S.A. 106, 19072–19077. doi: 10.1073/pnas.0903327106
Majerus, M., O'Donald, P., and Weir, J. (1982). Evidence for preferential mating in Adalia bipunctata. Heredity 49, 37–49.
Maloney, L. T., and Wandell, B. A. (1986). Color constancy: a method for recovering surface spectral reflectance. J. Opt. Soc. Am. A 3, 29–33. doi: 10.1364/JOSAA.3.000029
Mappes, J., Marples, N., and Endler, J. A. (2005). The complex business of survival by aposematism. Trends Ecol. Evol. 20, 598–603. doi: 10.1016/j.tree.2005.07.011
Marples, N. M., Brakefield, P. M., and Cowie, R. J. (1989). Differences between the 7−spot and 2−spot ladybird beetles (Coccinellidae) in their toxic effects on a bird predator. Ecol. Entomol. 14, 79–84. doi: 10.1111/j.1365-2311.1989.tb00756.x
Marples, N. M., Roper, T. J., and Harper, D. G. C. (1998). Responses of wild birds to novel prey: evidence of dietary conservatism. Oikos 83, 161–165. doi: 10.2307/3546557
Marples, N. M., van Veelen, W., and Brakefield, P. M. (1994). The relative importance of color, taste and smell in the protection of an aposematic insect Coccinella septempunctata. Anim. Behav. 48, 967–974. doi: 10.1006/anbe.1994.1322
Maximov, V. (2000). Environmental factors which may have led to the appearance of color vision. Philos. Trans. R. Soc. Lond. Ser. B 355, 1239–1242. doi: 10.1098/rstb.2000.0675
McCleod, D. I. A., and Boynton, R. M. (1979). Chromacity diagram showing cone exitation by stimuli with equal luminance. J. Opt. Soc. Am. A 68, 1183–1187. doi: 10.1364/JOSA.69.001183
Mollon, J. D. (1989). “Tho' she kneel'd in that place where they grew…” The uses and origins of primate color vision. J. Exp. Biol. 146, 21–38.
Muggleton, J. (1979). Non-random mating in wild populations of polymorphic Adalia bipunctata. Heredity 42, 57–65. doi: 10.1038/hdy.1979.6
Nieves, J. L., Nascimento, S. M. C., and Romero, J. (2012). Contrast edge colors under different natural illuminations. J. Opt. Soc. Am. A 29, A240–A246. doi: 10.1364/JOSAA.29.00A240
Nokelainen, O., Hegna, R. H., Reudler, J. H., Lindstedt, C., and Mappes, J. (2012). Trade-off between warning signal efficacy and mating success in the wood tiger moth. Proc. R. Soc. Lond. Ser. B 279, 257–265. doi: 10.1098/rspb.2011.0880
Osawa, N., and Nishida, T. (1992). Seasonal variation in elytral color polymorphism in Harmonia axyridis (the ladybird beetle): the role of non-random mating. Heredity 69, 297–307. doi: 10.1038/hdy.1992.129
Osorio, D., Jones, C. D., and Vorobyev, M. (1999b). Accurate memory for color but not pattern contrast in chicks. Curr. Biol. 9, 199–202. doi: 10.1016/S0960-9822(99)80089-X
Osorio, D., Smith, A. C., Vorobyev, M., and Buchanan-Smith, H. M. (2004). Detection of fruit and the selection of primate visual pigments for color vision. Am. Nat. 164, 696–708. doi: 10.1086/425332
Osorio, D., and Vorobyev, M. (2005). Photoreceptor spectral sensitivities in terrestrial animals: adaptations for luminance and color vision. Proc. R. Soc. Lond. Ser. B 272, 1745–1752. doi: 10.1098/rspb.2005.3156
Osorio, D., Vorobyev, M., and Jones, C. D. (1999a). Color vision of domestic chicks. J. Exp. Biol. 202, 2951–2959.
Pike, T. W. (2011). Using digital cameras to investigate animal coloration: Estimating sensor sensitivity functions. Behav. Ecol. Sociobiol. 65, 849–858. doi: 10.1007/s00265-010-1097-7
Poulton, E. B. (1890). The Colours of Animals, Their Meaning and Use, Especially Considered in the Case of Insects. New York, NY: D. Appleton and Company.
Quinn, G. P., and Keough, M. J. (2002). Experimental Design and Data Analysis for Biologists. Cambridge: Cambridge University Press.
Rasband, W. S., (1997–2013). ImageJ. U.S. National Institutes of Health, Bethesda, MD. Available online at: imagej.nih.gov/ij/
Ritland, D. B. (1998). Mimicry-related predation on two viceroy butterfly (Limenitis archippus) phenotypes. Am. Midl. Nat. 140, 1–20. doi: 10.1674/0003-0031(1998)140[0001:MRPOTV]2.0.CO;2
Roper, T. J. (1990). Responses of domestic chicks to artificially colored insect prey: effects of previous experience and background color. Anim. Behav. 39, 466–473. doi: 10.1016/S0003-3472(05)80410-5
Roper, T. J., and Redston, S. (1987). Conspicuousness of distasteful prey affects the strength and durability of one-trial avoidance learning. Anim. Behav. 35, 739–747. doi: 10.1016/S0003-3472(87)80110-0
Roper, T. J., and Wistow, R. (1986). Aposematic coloration and avoidance learning in chicks. Q. J. Exp. Psychol. B 38, 141–149.
Rowe, C., and Guilford, T. (1999). The evolution of multimodal warning displays. Evol. Ecol. 13, 655–671. doi: 10.1023/A:1011021630244
Rowe, C., Lindstrom, L., and Lyytinen, A. (2004). The importance of pattern similarity between Mullerian mimics in predator avoidance learning. Proc. R. Soc. Lond. Ser. B 271, 407–413. doi: 10.1098/rspb.2003.2615
Roy, H., Brown, P., Frost, R., and Poland, R. (2011). Ladybirds (Coccinellidae) of Britain and Ireland. Field Studies Council on Behalf of the Centre for Ecology & Hydrology's Biological Records Centre. Shrewsbury: FSC Publications
Ruxton, G. D., Sherratt, T. N., and Speed, M. P. (2004). Avoiding Attack. Oxford: Oxford University Press.
Ruxton, G. D., Speed, M. P., and Broom, M. (2009). Identifying the ecological conditions that select for intermediate levels of aposematic signalling. Evol. Ecol. 23, 491–501. doi: 10.1007/s10682-008-9247-3
Schuler, W., and Hesse, E. (1985). On the function of warning coloration: a black and yellow pattern inhibits prey-attack by naive domestic chicks. Behav. Ecol. Sociobiol. 16, 249–255. doi: 10.1007/BF00310988
Sherratt, T. N. (2002). The coevolution of warning signals. Proc. R. Soc. Lond. Ser. B 269, 741–746. doi: 10.1098/rspb.2001.1944
Smith, A. C., Surridge, A. K., Prescott, M. J., Osorio, D., Mundy, N. I., and Buchanan-Smith, H. M. (2012). Effect of color vision status on insect prey capture efficiency of captive and wild tamarins (Saguinus spp.). Anim. Behav. 83, 479–486. doi: 10.1016/j.anbehav.2011.11.023
Snowden, R. J., Thompson, P., and Troscianko, T. (2006). Basic Vision: An Introduction to Visual Perception. Oxford, UK: Oxford University Press.
Speed, M. P., and Ruxton, G. D. (2007). How bright and how nasty: explaining diversity in warning signal strength. Evolution 61, 623–635. doi: 10.1111/j.1558-5646.2007.00054.x
Stavenga, D. G. (1992). Eye regionalization and spectral tuning of retinal pigments in insects. Trends Neurosci. 15, 213–218. doi: 10.1016/0166-2236(92)90038-A
Stevens, M. (2007). Predator perception and the different forms of protective coloration. Proc. R. Soc. Lond. Ser. B 274, 1457–1464. doi: 10.1098/rspb.2007.0220
Stevens, M., and Cuthill, I. C. (2006). Disruptive coloration, crypsis and edge detection in early visual processing. Proc. Biol. Sci. 273, 2141–2147. doi: 10.1098/rspb.2006.3556
Stevens, M., Lown, A. E., and Wood, L. E. (2014). Color change and camouflage in juvenile shore crabs Carcinus maenas. Front. Ecol. Evol. 2:14. doi: 10.3389/fevo.2014.00014
Stevens, M., Párraga, C. A., Cuthill, I. C., Partridge, J. C., and Troscianko, T. (2007). Using digital photography to study animal coloration. Biol. J. Linn. Soc. 90, 211–237. doi: 10.1111/j.1095-8312.2007.00725.x
Stevens, M., and Ruxton, G. D. (2012). Linking the evolution and form of warning coloration in nature. Proc. R. Soc. Lond. Ser. B 279, 417–442. doi: 10.1098/rspb.2011.1932
Summers, K., Symula, R., Clough, M., and Cronin, T. (1999). Visual mate choice in poison frogs. Proc. R. Soc. Lond. Ser. B 266, 2141–2145. doi: 10.1098/rspb.1999.0900
True, J. R. (2003). Insect melanism: the molecules matter. Trends Ecol. Evol. 18, 640–647. doi: 10.1016/j.tree.2003.09.006
Trullas, S. C., van Wyk, J. H., and Spotila, J. R. (2007). Thermal melanism in ectotherms. J. Therm. Biol. 32, 235–245 doi: 10.1016/j.jtherbio.2007.01.013
Ventura, D. F., Zana, Y., de Souza, J. M., and DeVoe, R. D. (2001). Ultraviolet color opponency in the turtle retina. J. Exp. Biol. 204, 2527–2534.
Wallace, A. R. (1889). Darwinism: An Exposition of the Theory of Natural Selection with Some of its Applications. London: MacMillan & Co.
Keywords: aposematism, coloration, ladybird, predation, vision, warning signals
Citation: Arenas LM, Troscianko J and Stevens M (2014) Color contrast and stability as key elements for effective warning signals. Front. Ecol. Evol. 2:25. doi: 10.3389/fevo.2014.00025
Received: 11 March 2014; Accepted: 27 May 2014;
Published online: 17 June 2014.
Edited by:
Sasha Raoul Xola Dall, University of Exeter, UKReviewed by:
Karen Cheney, The University of Queensland, AustraliaXimena J. Nelson, University of Canterbury, New Zealand
Julian Charles Partridge, University of Bristol, UK
Copyright © 2014 Arenas, Troscianko and Stevens. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lina María Arenas, Sensory Ecology Group, Centre for Ecology and Conservation, College of Life and Environmental Sciences, University of Exeter, Cornwall Campus, TR10 9EZ, Penryn, UK e-mail: lma38@cam.ac.uk
 Lina María Arenas
Lina María Arenas Jolyon Troscianko
Jolyon Troscianko Martin Stevens
Martin Stevens