Pheromone races of Cydia splendana (Lepidoptera, Tortricidae) overlap in host plant association and geographic distribution
- 1Chemical Ecology Group, Department of Plant Protection Biology, Swedish University of Agricultural Sciences, Alnarp, Sweden
- 2SUAMME, Mas de Saporta, Lattes, France
- 3Plant Protection Institute MTA ATK, Budapest, Hungary
- 4Swiss Federal Research Station, Wädenswil, Switzerland
- 5Department of Chemistry and Biomedical Sciences, Faculty of Health and Life Sciences, Linnaeus University, Kalmar, Sweden
Identification of the sex pheromone of Cydia splendana (Lepidoptera, Tortricidae) by pheromone gland analysis followed by field trapping with synthetic compounds shows the occurrence of two pheromone races. Acorn moth females from Sweden, where oak Quercus robur is the only host plant, use a blend of the E,Z and E,E isomers of 8,10-dodecadien-1-yl acetate. In Central and Southern Europe, where C. splendana feeds on chestnut Castanea sativa and several species of oak, males respond to another isomer blend, E,E and Z,E. The distribution of the two pheromone races of C. splendana overlaps in Northern France, where they share oak as plant host. Differences in sex communication signals between these populations of C. splendana corroborate the role of specific mate recognition in speciation events.
Introduction
Sex pheromone communication in moths is the original paradigm in chemical ecology (Fabre, 1879; Butenandt et al., 1959). Lepidopteran sex pheromones encode species and mate recognition, and taxonomically closely related species often use specific blends of positional or geometric isomers of unsaturated straight-chain acetates (Arn et al., 1986; Wyatt, 2003; Johansson and Jones, 2007; El-Sayed, 2014).
The geometric isomers of 8,10-dodecadien-1-yl acetate (Δ8,Δ10-12Ac) are typical sex pheromone components in the tortricid subfamily Olethreutinae (Lepidoptera, Tortricidae). In species communicating with Δ8,Δ10-12Ac, each of these isomers is behaviorally active, either as main pheromone compound, attraction synergist or attraction inhibitor. A coincident and reciprocal, synergistic or antagonistic effect on male attraction enables specific communication and mate recognition with only four compounds, in 30 or more species of this subfamily (Witzgall et al., 1996, 2010; El-Sayed, 2014). Behavioral observations and field trapping studies in several species have shown that distinct pheromone communication channels result in specific mate recognition in tortricid moths (Witzgall et al., 1993, 1996, 2010) and most likely also in premating reproductive isolation, which may give rise to speciation. Indeed, distinct pheromone races, using specific isomer blends have been found in several of these species (Witzgall et al., 1996).
A reciprocal synergistic/antagonistic behavioral role of pheromone compounds may result from selection on pheromone blends for isolation function in populations or related species. An alternative explanation is that changes in pheromone blends, resulting from saltational changes in female pheromone biosynthesis, are tracked by the responding males, and thus lead to new pheromone communication channels (Phelan, 1992; Baker, 2002). This is in line with the specific mate recognition concept, according to which sexual selection drives the optimization of communication and mate recognition; diversification of pheromone blends between populations, as an initial step toward reproductive isolation, is regarded merely as a by-product of selection on pheromone blends for optimal communication (Paterson, 1985; Linn and Roelofs, 1995; Vrba, 1995; Mendelson and Shaw, 2012).
In nature, sex pheromone and host plant odors are perceived as an ensemble. That mating and habitat cues are coded as blends in the male antennal lobe, the olfactory center of the insect brain, highlights the role of plant signals in habitat selection and in premating sexual communication (Trona et al., 2010, 2013; Chaffiol et al., 2014; Deisig et al., 2014). Closely related tortricid moths are often associated with taxonomically related host plant species. However, species or pheromone races and plant hosts do not always overlap congruently. The European Fagaceae, oak Quercus spec., chestnut Castanea sativa and beech Fagus sylvatica, are hosts for the phylogenetically closely related acorn moth or chestnut tortrix Cydia splendana and the beech tortrix C. fagiglandana. North of the geographical distribution of chestnut, the larvae of C. fagiglandana feed on beech nuts and the larvae of C. splendana on acorns. In southern Europe, both species are also found on chestnut, where they cause significant damage (Bovey, 1966; Bradley et al., 1979). Pheromone-baited traps are increasingly important for monitoring the seasonal flight period and population density of these insects. Protecting chestnuts with insecticide sprays is technically difficult due to a large tree canopy, and few efficient insecticides are available (Avtzis et al., 2013).
The female sex pheromone of C. splendana feeding on acorns or oak nuts of Quercus robur in Sweden is a blend of (E,Z)- and (E,E)-8,10-dodecadienyl acetate (EZ and EE). The main compound EZ alone is highly attractive to male moths, and trap captures are further augmented by adding the EE isomer, which is a pheromone synergist (Witzgall et al., 1996). However, the EZ/EE-pheromone blend of C. splendana from Swedish oak forests did not attract males in chestnut plantations in France. Host races of several moths, including European corn borer, fall armyworm and larch budmoth are known to use different pheromone blends (Guerin et al., 1984; Leppik and Frerot, 2012; Unbehend et al., 2014). This led us to reinvestigate the sex pheromone of C. splendana from chestnut, showing that C. splendana uses two different pheromone blends that probably contribute to reproductive isolation.
Materials and Methods
Chemicals and Insects
(E,E)-8,10-dodecadien-1-yl acetate (E8,E10-12Ac) was purchased from S. Voerman (Institute for Pesticide Research, Wageningen, The Netherlands). The EZ, ZE, and ZZ isomers of 8,10-dodecadien-1-yl acetate were synthesized, and purified by high-pressure liquid chromatography (HPLC) (Witzgall et al., 1993). Chemical purity of the four isomers was ≥99.6%, isomeric purity was ≥99.3% by capillary gas chromatography (GC).
Insects were collected as last-instar larvae from acorns of Q. robur (Scania, Sweden) and chestnuts C. sativa (Hungary, Pilismarot). Larvae were overwintered under field conditions and eclosed the following summer.
Pheromone Gland Analysis
Pheromone gland analysis of C. splendana females is hampered by the difficulties to obtain calling females, i.e., females that visibly expose their pheromone glands. In codling moth C. pomonella, a closely related species, the female pheromone gland titre strongly increases after the onset of calling (Bäckman et al., 1997). Preliminary tests showed that extracts of 20 to 30 non-calling C. splendana females did not contain sufficiently large amounts of unsaturated acetates for GC-MS analysis.
In the laboratory, C. splendana females did not release pheromone, not even in the presence of freshly cut oak branches or green acorns. Reliable induction of female calling was obtained only in mesh cages suspended in oak or chestnut trees, during dusk. Larvae from acorns were collected in large numbers, and up to 80 calling females for gland extraction were obtained from cages containing up to ca. 200 females. In comparison, fewer chestnut insects from Hungary were available for pheromone gland analysis, and extracts were made of 20 calling females.
Pheromone gland extraction followed the protocol used with other tortricids (Witzgall et al., 2001, 2005). Pheromone glands of 2- to 3-day-old female moths, retrieved from field cages in oak and chestnut trees close to the laboratory, were extracted in batches of 20–80 (N = 7 and N = 3 for insects from Sweden and Hungary, respectively), 1–3 h after onset of the scotophase. Sex glands were detached with forceps from forcefully protruded ovipositors, and were kept in a 3-ml reaction vial held in liquid air, and then extracted with ca. 5 μ l redistilled heptane, containing heptyl acetate as internal standard, at ca. 20°C during 2 min. The extracts were stored in sealed glass capillaries at −19°C.
Compounds in gland extracts were identified on a coupled gas chromatography-mass spectrometer (GC-MS; 6890 GC and 5975 MS; Agilent Technologies, Palo Alto, CA, USA), operated in the electron impact ionization mode at 70 eV. The GC was equipped with fused silica capillary columns (30 m × 0.25 mm), DB-wax (J&W Scientific, Folsom, CA, USA) or HP-5MS (Agilent Technologies). Helium was used as the mobile phase at an average linear flow rate of 35 cm/s. Two μl of each sample were injected (splitless mode, injector temperature 225°C). The GC oven temperature for both columns was programmed from 60°C (2 min hold) at 10°C/min 21–100°C, at 1.5°C/min to 150°C, and at 10–230°C.
For electroantennographic detection (EAD) (Arn et al., 1975), the DB-wax column in a Hewlett-Packard 6890 GC was split between the flame ionization detector (FID) and an electroantennogram (EAG) apparatus (IDAC-2; Syntech, Kirchzarten, Germany). One arm of the split column led into a glass tube (Ø 8 mm), with a charcoal-filtered and humidified air stream (0.5 l/min). C. splendana male antennae were at 0.5 cm from the end of this glass tube, 30 cm from the EAD-outlet of the GC. The antennae were mounted between two glass pipette electrodes containing Ringer solution (Beadle-Ephrussi); one electrode was connected to ground and the other to an amplifier (Syntech). The GC was operated in splitless injection mode and the oven was programmed from 50°C (2 min hold) at 10°C/min to 230°C. Injector and EAD-outlet temperature was 220°C and the split ratio between FID and EAD was ca. 1:1.
Field Trapping
The EE, EZ, ZE, and ZZ isomers of 8,10-12Ac were tested alone and as 2-component blends in an oak (Quercus robur) forest in Scania, Sweden and Touraine, France. In subsequent tests, EE and EZ were tested alone and in different blends with the ZE and EE, respectively, in chestnut (Castanea sativa) groves and oak forests in several locations in Southern France (Ardèche, Cévennes) and in Switzerland (Tessin; Q. coccifera, Q. ilex, Q. pubescens, Q. robur). Other, minor gland compounds found in oak insects, were tested in oak forests in Scania, Sweden. Further tests, comparing traps placed at 2 and 4–5 m height in chestnut trees were conducted in Hungary. Tests were done during the seasonal flight period in June and July, from 1992 to 2012.
Compounds in heptane solution were formulated on red rubber septa (Merck ABS, Dietikon, Switzerland). Tetra traps (Arn et al., 1979) were hung on green branches, traps within one replicate were ca. 10 m apart, they were placed along tree rows, replicates were 30–100 m apart. The numbers of captured males were transformed to log(x + 1) and submitted to an analysis of variance, followed by a Tukey-test. Trapped insects were stored in Petri dishes for genital analysis (Danilevskij and Kuznetsov, 1968). The number of replicates refers to trap series, comprising between 4 and 10 traps.
Moths were taxonomically determined according to wing pattern; this was verified by genital preparations of all males captured in one trap test in Scania, Touraine, Cévennes and Tessin, respectively.
Results
GC-MS and GC-EAD Analysis of Pheromone Gland Extracts
Calling females of C. splendana collected from acorns in Sweden produced a range of pheromonal compounds, the most abundant unsaturated compound was E8,Z10-12Ac (Table 1). In extracts of up to 20 calling females collected from chestnut in Hungary, E8,E10-12Ac was the only unsaturated compound which could be identified by GC-MS. However, GC-EAD recordings showed an antennal response at the retention time of the ZE isomer (Table 1).
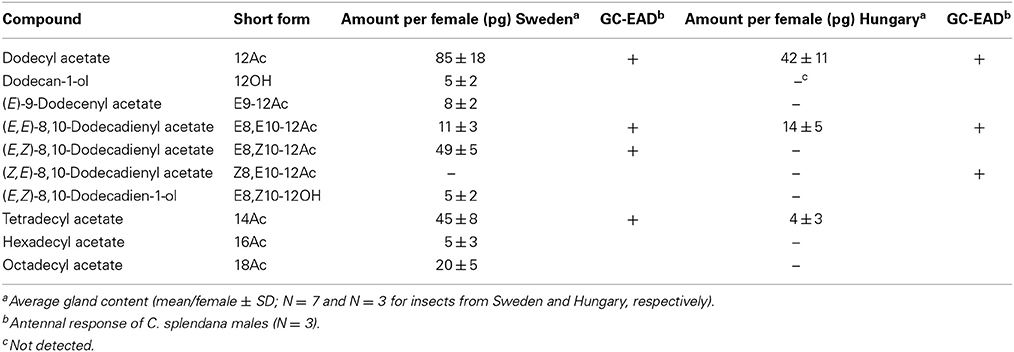
Table 1. Compounds identified from pheromone gland extracts of C. splendana females from oak Q. robur (Sweden) and chestnut C. sativa (Hungary), by GC-MS and GC-EAD.
Field Trapping
The field attraction of C. splendana males, in Scanian oak forests, to the isomers of Δ8,Δ10-12Ac and their blends is shown in Table 2. The main pheromone compound, E8,Z10-12Ac was attractive by itself, trap captures were augmented by admixture of the EE isomer, while a blend with the ZZ isomer captured fewer insects.
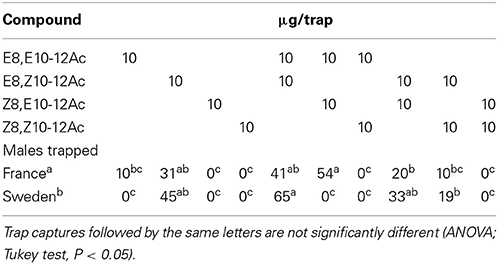
Table 2. Field attraction of C. splendana males to the geometric isomers of 8,10-12Ac and their blends in Touraine, France and Scania, Sweden.
The EZ/EE-blend ratio was optimized in other tests in Scanian oak forests, as shown in Table 3. A 10:2 EZ/EE blend captured significantly more males than a 2:10-blend or the EZ isomer alone (N = 10, Tukey test, P < 0.05). Two further compounds identified from female glands, the corresponding alcohol E8,Z10-12OH and the monounsaturated E9-12Ac (Table 1) did not increase male captures when added singly or together, at the ratio found in gland extracts, to a 10:2-blend of EZ and EE. The same was true for the saturated 12Ac and 14Ac (Scania; N = 10, Tukey test, P < 0.05; data not shown). Adding 5 and 20% of a third isomer, ZE and ZZ, to the 10:2 EZ/EE-blend in Swedish oak forests decreased captures, but not significantly (N = 10, Tukey test; data not shown).
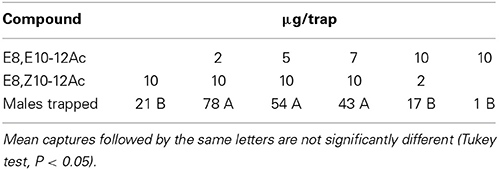
Table 3. Field attraction of C. splendana males to synthetic pheromone blends in an oak forest (Scania; N = 10).
In chestnut orchards in Southern France, a 10:2 EE/ZE blend was more attractive than a 2:10 blend, or the EE isomer by itself (Table 4). Tests in various other locations confirmed that the EE/ZE blend consistently captured more males both in chestnut orchards and oak forests in France and Switzerland, while the EZ/EE blend captured most insects in Sweden (Table 5). Both the “Swedish oak” (EZ/EE) and “French chestnut” (EE/ZE) blends captured males in an oak forest in Northern France (Table 2).
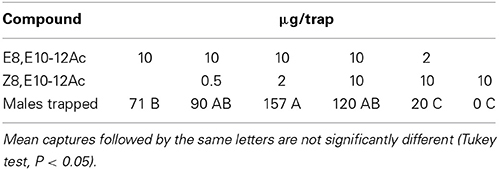
Table 4. Field attraction of C. splendana males to synthetic pheromone blends in a chestnut orchard (Cévennes; N = 10).
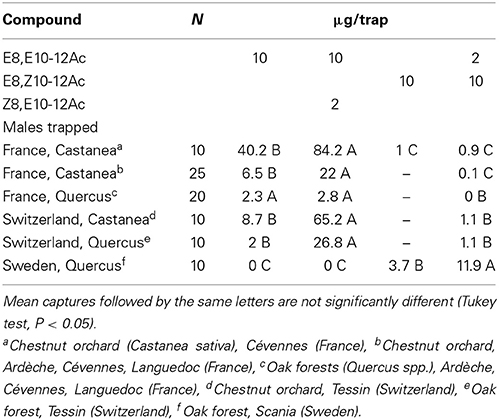
Table 5. Field attraction of C. splendana males to synthetic pheromone blends in Southern France, Switzerland, and Sweden.
The corresponding aldehyde E8,E10-12Al did not attract moths in Scania, Sweden and Cévennes, France (N = 10). Beech tortrix C. fagiglandana was attracted in most locations to some of the lures shown in Table 5; most males were trapped with EE alone (157) and the 10:2-blend of EE/ZE (102), but none were captured with a 2:10 EE/EZ blend (N = 22, Tukey test, P < 0.05).
Lures baited with 10 μg of the 10:2 EE/ZE blend were sufficient to attract moths throughout the season in chestnut orchards. Traps placed 4–5 m high in trees captured more moths than traps placed at 2 m, for example during the seasonal flight at Pilismarot, Hungary (63 and 27 moths, respectively; N = 10, Tukey test, P < 0.05). Increasing the amount of pheromone from 10:2 to 100:20 μg EE/ZE consistently led to a slight decrease in trap capture, which was, however, not significant (N = 10, Tukey test, P < 0.05; data not shown). The EE/ZE blend lures captured significant numbers of chestnut tortrix moths also in various other locations in Italy, Portugal, and Spain.
Discussion
Sex Pheromone Races of C. splendana and their Plant Hosts
Pheromone gland analysis by GC-MS and GC-EAD followed by field trapping with synthetic pheromone suggests the occurrence of two sex pheromone races in C. splendana. The pheromone of acorn moth females from Sweden, where Quercus robur is the only host plant, is a blend of E,Z- and E8,E10-12Ac (EZ/EE). In Southern France, Hungary and Switzerland, where C. splendana feeds on both chestnut Castanea sativa and several species of oak, males respond instead to a blend of E,E- and Z8,E10-12Ac (EE/ZE), corroborating a previous identification of the main compound E8,E10-12Ac (Frérot et al., 1995).
These two pheromone races may be reproductively isolated, since Swedish acorn moths were not at all attracted to the EE/ZE pheromone blend, which attracted moths from chestnut in Central and Southern Europe. Weak attraction to the “oak blend” in Southern France and Switzerland may be due to limited cross-attraction, or it may instead be due to an overlap in the geographical distribution of the two pheromone races. Captures with both EZ/EE and EE/ZE blend in an oak forest in Northern France indicate overlapping distribution of both races, at least in Northern France.
The EZ/EE pheromone race of C. splendana may be associated with oak only, while the EE/ZE race is found on oak and chestnut. A closely related North American species, filbertworm Cydia latiferreana, using the EZ/EE pheromone blend, is found on acorns, walnuts, and hazelnuts (Davis et al., 1984; Chambers et al., 2011). Pheromone races of the Artemisia tortrix Epiblema foenella are all associated with the same host plant, mugwort Artemisia vulgaris. Males were best attracted to a EE/ZZ-blend of Δ8,Δ10-12Ac in Sweden, a EZ/ZZ-blend in Hungary and to the ZE isomer alone in France (Witzgall et al., 1996).
Sex Pheromones and Chemosensory Reproductive Isolation
In several insect herbivores, mate recognition has been shown to involve two types of chemosensory cues—pheromones and host plant volatiles. Changes in either host or mate recognition may accordingly lead to premating isolation during chemosensory speciation (Smadja and Butlin, 2009). Populations of C. splendana using two entirely different pheromone blends are likely reproductively isolated. This illustrates how diverging mate recognition systems, which are under sexual selection, can contribute to premating isolation and speciation processes (Ritchie, 2007; M'Gonigle et al., 2012; Safran et al., 2013).
Premating isolation as a result of diverging pheromones is, however, not necessarily the result of selection for avoidance of cross-attraction and interbreeding. Lepidopteran pheromone races may instead be viewed as by-products of mutational changes in female pheromone biosynthesis in geographical or host plant populations; the ensueing adaptation of the male olfactory response maintains efficient mate communication. The species recognition concept postulates signal-response co-adaption as a result of fine-tuning of efficient mate recognition, rather than selection for reproductive isolation (Paterson, 1985; Lambert and Spencer, 1995).
Pheromone antagonists, on the other hand, are compounds producing a heterospecific behavioral response, between different pheromone races or species. The existence of pheromone antagonists could accordingly stem from selection for reproductive isolation and would thus contradict the species recognition concept (Linn and Roelofs, 1995; White et al., 1995). As long as we ignore the olfactory circuitry underlying the reciprocal synergistic/antagonistic effect of these 8,10-12Ac isomers, we cannot exclude, however, that antagonistic effects result instead from constraints imposed by the architecture of the olfactory apparatus. Switching on new synergists in the olfactory circuit of tortricid moths may turn the other isomers to inhibitors. For example, in the species using Δ8,Δ10-12Ac pheromones, synergism has been found only for any combination of 2 isomers, but not for blends of 3 isomers (Witzgall et al., 2010). This emphasizes the need for neuroethological data if we aim to decipher the role of pheromone communication in the phylogenetic diversification of these insects.
Interaction of Pheromones and Plant Volatiles
Plant volatile compounds play a key role in host choice and the colonization of new host plants in insect herbivores (Bengtsson et al., 2006; Bruce and Pickett, 2011; Cha et al., 2012) and host plant shifts have been associated with speciation events (Dres and Mallet, 2002; Servedio et al., 2011; Soto et al., 2012; Safran et al., 2013). Pheromone races in moths are sometimes associated with different host plants, for example in the European corn borer Ostrinia nubilalis (Leppik and Frerot, 2012; Unbehend et al., 2014). In comparison, pheromone races in C. splendana demonstrate that separate pheromone communication channels alone can account for premating isolation, despite overlapping host use and geographical distribution.
Nonetheless, host odor likely plays a role in C. splendana mate-finding, which has been recorded from Quercus and Castanea only (Bovey, 1966; Bradley et al., 1979; Den Otter et al., 1996). In codling moth C. pomonella, apple and pear odor strongly synergizes male attraction to sex pheromone (Yang et al., 2004; Trona et al., 2010, 2013). Codling moth females, which are approximately the same size as C. splendana, produce ca. 100 times more of the main pheromone compound than C. splendana females (Table 1; Bäckman et al., 1997) and codling moth is the only tortricid known to use E8,E10-12OH as the main pheromone compound. In comparison, several other tortricids, feeding on different plant hosts, use the same isomers of Δ8,Δ10-12Ac as C. splendana (Witzgall et al., 1996, 2010). This leads us to believe that production of small amounts of pheromone, in combination with host plant volatiles, contributes to specific premating communication in C. splendana, since it will reduce cross-attraction of other species using the same or similar pheromone blends.
A recent study on cotton leafworm males Spodoptera littoralis corroborates the close interaction of social and habitat olfactory cues. Male attraction to both larval host plant volatiles, signaling mating sites, and to female-produced sex pheromone is concurrently downregulated shortly after mating, while mated males are still attracted to floral cues for foraging (Kromann et al., submitted). Moreover, in C. pomonella, an olfactory receptor belonging to the clade of pheromone receptors has been shown to respond to a bisexual plant volatile attractant, pear ester, indicating a close phylogenetic relationship between the olfactory receptor genes tuned to sex pheromones and plant compounds (Bengtsson et al., 2014).
This study (Bengtsson et al., 2014) employed a new technique, the functional characterization of individual olfactory receptors, following heterologous expression in Drosophila empty neurons (Dobritsa et al., 2003; Hallem et al., 2004). This technique will enable a complete identification of behaviorally relevant odorants, sex pheromone components and plant volatile attractants, in C. splendana and other insects. Given the difficulties associated with the chemical and behavioral analysis of the very large number of volatiles released by plants and of sex pheromones produced in very small amounts, functional characterization of olfactory receptors is expected to greatly contribute to our understanding of the roles of semiochemicals in specific mate recognition and reproductive isolation.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was funded the Foundation for Strategic Environmental Research (MISTRA). Marie Bengtsson and Peter Witzgall are supported by the Linnaeus environment “Insect Chemical Ecology, Ethology, and Evolution” IC-E3 (Formas, SLU). We are indebted to Drs. Erzsebet Voigt (Budapest) and Jean-Pierre Chambon (Versailles) for help with field trapping experiments.
References
Arn, H., Rauscher, S., and Schmid, A. (1979). Sex attractant formulations and traps for the grape moth Eupoecilia ambiguella Hb. Mitt. Schweiz. Entomol. Ges. 52, 49–55.
Arn, H., Städler, E., and Rauscher, S. (1975). The electroantennographic detector - a selective and sensitive tool in the gas chromatographic analysis of insect pheromones. Z. Naturforsch. 30c, 722–725.
Arn, H., Tóth, M., and Priesner, E. (1986). List of Sex Pheromones of Lepidoptera and Related Attractants. Paris: OILB-SROP.
Avtzis, D. N., Perlerou, C., and Diamandis, S. (2013). Geographic distribution of chestnut feeding insects in Greece. J. Pest Sci. 86, 185–191. doi: 10.1007/s10340-012-0451-0
Bäckman, A.-C., Bengtsson, M., and Witzgall, P. (1997). Pheromone release by individual females of codling moth, Cydia pomonella L. (Lepidoptera: Tortricidae). J. Chem. Ecol. 23, 807–815.
Baker, T. C. (2002). Mechanism for saltational shifts in pheromone communication systems. Proc. Natl. Acad. Sci. U.S.A. 99, 13368–13370. doi: 10.1073/pnas.222539799
Bengtsson, J. M., Gonzalez, F., Cattaneo, A. M., Montagné, N., Walker, W. B., Bengtsson, M., et al. (2014). A predicted sex pheromone receptor of codling moth Cydia pomonella detects the plant volatile pear ester. Front. Ecol. Evol. 2:33. doi: 10.3389/fevo.2014.00033
Bengtsson, M., Jaastad, G., Knudsen, G., Kobro., S., Bäckman, A. C., Pettersson, E., et al. (2006). Plant volatiles mediate attraction to host and non-host plant in apple fruit moth, Argyresthia conjugella. Entomol. Exp. Appl. 118, 77–85. doi: 10.1111/j.1570-7458.2006.00359.x
Bovey, P. (1966). “Super–famille des Tortricoidea,” in Entomologie Appliquée à L'agriculture, Vol. II, ed A. S. Balachowsky (Paris: Masson), 456–893.
Bradley, J. D., Tremewan, W. G., and Smith, A. (1979). British tortricoid moths. Tortricidae: Olethreutinae. London: The Ray Society.
Bruce, T. J. A., and Pickett, J. A. (2011). Perception of plant volatile blends by herbivorous insects–finding the right mix. Phytochemistry 72, 1605–1611. doi: 10.1016/j.phytochem.2011.04.011
Butenandt, A., Beckmann, R., Stamm, D., and Hecker, E. (1959). Über den Sexual-Lockstoff des Seidensspinners Bombyx mori. Reindarstellung und Konstitution. Z. Naturforsch. B. 14, 283–284.
Cha, D. H., Yee, W. L., Goughnour, R. B., Sim, S. B., Powell, T. H. Q., Feder, J. L., et al. (2012). Identification of host fruit volatiles from domestic apple (Malus domestica), native black hawthorn (Crataegus douglasii) and introduced ornamental hawthorn (C. monogyna) attractive to Rhagoletis pomonella flies from the Western United States. J. Chem. Ecol. 38, 319–329. doi: 10.1007/s10886-012-0087-9
Chaffiol, A., Dupuy, F., Barrozo, R. B., Kropf, J., Renou, M., Rospars, J. P., et al. (2014). Pheromone modulates plant odor responses in the antennal lobe of a moth. Chem. Senses 39, 451–463. doi: 10.1093/chemse/bju017
Chambers, U., Walton, V. M., and Mehlenbacher, S. A. (2011). Susceptibility of hazelnut cultivars to filbertworm, Cydia latiferreana. Hortscience 46, 1377–1380.
Danilevskij, A. S., and Kuznetsov, V. I. (1968). Listovertki Tortricidae: Triba plodozhorki Laspeyresiini in Fauna SSSR, Nasekomye Tcheshuekrylye, Vol. 5. Leningrad: Nauka.
Davis, H. G., McDonough, L. M., Burditt, A. K., and Bierl-Leonhardt, B. A. (1984). Filbertworm sex pheromone. Identification and field tests of (E,E)- and (E,Z)-8,10-dodecadien-1-ol acetates. J. Chem. Ecol. 10, 53–61.
Deisig, N., Dupuy, F., Anton, S., and Renou, M. (2014). Responses to pheromones in a complex odor world: sensory processing and behavior. Insects 5, 399–422. doi: 10.3390/insects5020399
Den Otter, C. J., De Cristofaro, A., Voskamp, K. E., and Rotundo, G. (1996). Electrophysiological and behavioural responses of chestnut moth, Cydia fagiglandana and C. splendana (Lep., Tortricidae), to sex attractants and odours of host plants. J. Appl. Entomol. 120, 413–421.
Dobritsa, A. A., van der Goes van Naters, W., Warr, C. G., Steinbrecht, R. A., and Carlson, J. R. (2003). Integrating the molecular and cellular basis of odor coding in the Drosophila antenna. Neuron 37, 827–841. doi: 10.1016/S0896-6273(03)00094-1
Dres, M., and Mallet, J. (2002). Host races in plant-feeding insects and their importance in sympatric speciation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 357, 471–492. doi: 10.1098/rstb.2002.1059
El-Sayed, A. M. (2014). The pherobase: database of insect pheromones and semiochemicals. Available online at: www.pherobase.com
Frérot, B., Marro, J. P., and Malosse, C. (1995). In vitro incubation of sex pheromone gland and identification of pheromone components in Cydia splendana (Hb). C. R. Acad. Sci. Paris Life Sci. 318, 447–451.
Guerin, P. M., Baltensweiler, W., Arn, H., and Buser, H. R. (1984). Host race pheromone polymorphism in the larch budmoth. Cell. Mol. Life Sc. 40, 892–894.
Hallem, E. A., Ho, M. G., and Carlson, J. R. (2004). The molecular basis of odor coding in the Drosophila antenna. Cell 117, 965–979. doi: 10.1016/j.cell.2004.05.012
Johansson, B. G., and Jones, T. M. (2007). The role of chemical communication in mate choice. Biol. Rev. 82, 265–289. doi: 10.1111/j.1469-185X.2007.00009.x
Lambert, D. M., and Spencer, H. G. (1995). Speciation and the Recognition Concept: Theory and Application. Baltimore, MD: John Hopkins University.
Leppik, E., and Frerot, B. (2012). Volatile organic compounds and host-plant specialization in European corn borer E and Z pheromone races. Chemoecology 22, 119–129. doi: 10.1007/s00049-012-0104-z
Linn, C. E., and Roelofs, W. L. (1995). “Pheromone communication in moths and its role in the speciation process,” in Speciation and the Recognition Concept: Theory and Application, eds D. M. Lambert, and H. Spencer (Baltimore, MD: John Hopkins University Press,), 263–300.
Mendelson, T. C., and Shaw, K. L. (2012). The (mis)concept of species recognition. Trends Ecol. Evol. 27, 421–427. doi: 10.1016/j.tree.2012.04.001
M'Gonigle, L. K., Mazzucco, R., Otto, S. P., and Dieckmann, U. (2012). Sexual selection enables long-term coexistence despite ecological equivalence. Nature 484, 506–509. doi: 10.1038/nature10971
Paterson, H. E. H. (1985). “The recognition concept of species,” in Species and Speciation, ed E. S. Vrba (Pretoria: Transvaal Museum Monograph No. 4), 21–29.
Phelan, P. L. (1992). “Evolution of sex pheromones and the role of assymetric tracking,” in Insect Chemical Ecology: An Evolutionary Approach, eds B. D. Roitberg, and M. B. Isman (New York, NY: Chapman and Hall), 265–314.
Ritchie, M. G. (2007). Sexual selection and speciation. Annu. Rev. Ecol. Evol. Syst. 38, 79–102. doi: 10.1146/annurev.ecolsys.38.091206.095733
Safran, R. J., Scordato, E. S. C., Symes, L. B., Rodriguez, R. L., and Mendelson, T. C. (2013). Contributions of natural and sexual selection to the evolution of premating reproductive isolation: a research agenda. Trends Ecol. Evol. 28, 643–650. doi: 10.1016/j.tree.2013.08.004
Servedio, M. R., Van Doorn, G. S., Kopp, M., Frame, A. M., and Nosil, P. (2011). Magic traits in speciation: “magic” but not rare? Trends Ecol. Evol. 26, 389–397. doi: 10.1016/j.tree.2011.04.005
Smadja, C., and Butlin, R. K. (2009). On the scent of speciation: the chemosensory system and its role in premating isolation. Heredity 102, 77–97. doi: 10.1038/hdy.2008.55
Soto, E. M., Goenaga, J., Hurtado, J. P., and Hasson, E. (2012). Oviposition and performance in natural hosts in cactophilic Drosophila. Evol. Ecol. 26, 975–990. doi: 10.1007/s10682-011-9531-5
Trona, F., Anfora, G., Balkenius, A., Bengtsson, M., Tasin, M., Knight, A., et al. (2013). Neural coding merges sex and habitat chemosensory signals in an insect herbivore. Proc. Biol. Sci. 280:20130267. doi: 10.1098/rspb.2013.0267
Trona, F., Anfora, G., Bengtsson, M., Witzgall, P., and Ignell, R. (2010). Coding and interaction of sex pheromone and plant volatile signals in the antennal lobe of the codling moth Cydia pomonella. J. Exp. Biol. 213, 4291–4303. doi: 10.1242/jeb.047365.
Unbehend, M., Hänniger, S., Vásquez, G. M., Juárez, M. L., Reisig, D., McNeil, J. N., et al. (2014). Geographic variation in sexual attraction of Spodoptera frugiperda corn- and rice-strain males to pheromone lures. PLoS ONE 9:e89255. doi: 10.1371/journal.pone.0089255
Vrba, E. S. (1995). “Species as habitat-specific, complex systems,” in Speciation and the Recognition Concept: Theory and Application, eds D. M. Lambert and H. Spencer (Baltimore, MD: John Hopkins University Press), 3–44.
White, C. S., Lambert, D. M., and Foster, S. P. (1995). “Chemical signals and the recognition concept,” in Speciation and the Recognition Concept: Theory and Application, eds D. M. Lambert, and H. Spencer (Baltimore, MD: John Hopkins University Press), 301–326.
Witzgall, P., Bengtsson, M., Rauscher, S., Liblikas, I., Bäckman, A.-C., Coracini, M., et al. (2001). Identification of further sex pheromone synergists in the codling moth, Cydia pomonella. Entomol. Exp. Appl. 101, 131–141. doi: 10.1046/j.1570-7458.2001.00898.x
Witzgall, P., Bengtsson, M., Unelius, C. R., and Löfqvist, J. (1993). Attraction of pea moth Cydia nigricana F. (Lepidoptera: Tortricidae) to female sex pheromone (E,E)-8,10-dodecadien-1-yl acetate, is inhibited by geometric isomers (E,Z), (Z,E) and (Z,Z). J. Chem. Ecol. 19, 1917–1928.
Witzgall, P., Chambon, J.-P., Bengtsson, M., Unelius, C. R., Appelgren, M., Makranczy, G., et al. (1996). Sex pheromones and attractants in the Eucosmini and Grapholitini (Lepidoptera, Tortricidae). Chemoecology 7, 13–23. doi: 10.1007/BF01240633
Witzgall, P., Tasin, M., Buser, H.-R., Wegner-Kiß, G., Mancebón, V. S. M., Ioriatti, C., et al. (2005). New pheromone components of the grapevine moth Lobesia botrana. J. Chem. Ecol. 31, 2923–2932. doi: 10.1007/s10886-005-8404-1
Witzgall, P., Trematerra, P., Liblikas, I., Bengtsson, M., and Unelius, C. R. (2010). Pheromone communication channels in tortricid moths: lower specificity of alcohol vs. acetate geometric isomer blends. Bull. Entomol. Res. 100, 225–230. doi: 10.1017/S0007485309990186
Wyatt, T. D. (2003). Pheromones and Animal Behaviour: Communication by Smell and Taste. Cambridge: Cambridge University Press. doi: 10.1017/CBO9780511615061
Keywords: specific mate recognition, reproductive isolation, sibling species, host plant, Castanea, Quercus, Fagaceae
Citation: Bengtsson M, Boutitie A, Jósvai J, Toth M, Andreadis S, Rauscher S, Unelius CR and Witzgall P (2014) Pheromone races of Cydia splendana (Lepidoptera, Tortricidae) overlap in host plant association and geographic distribution. Front. Ecol. Evol. 2:46. doi: 10.3389/fevo.2014.00046
Received: 23 March 2014; Accepted: 21 July 2014;
Published online: 06 August 2014.
Edited by:
Stefano Colazza, University of Palermo, ItalyReviewed by:
Nick Bos, University of Helsinki, FinlandJoachim Ruther, University of Regensburg, Germany
Copyright © 2014 Bengtsson, Boutitie, Jósvai, Toth, Andreadis, Rauscher, Unelius and Witzgall. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peter Witzgall, Chemical Ecology Group, Department of Plant Protection Biology, Swedish University of Agricultural Sciences, SLU, Box 102, Växtskyddsvägen 3, 23053 Alnarp, Sweden e-mail: peter.witzgall@slu.se
 Marie Bengtsson
Marie Bengtsson Anne Boutitie
Anne Boutitie Julia Jósvai
Julia Jósvai Miklos Toth
Miklos Toth Stefanos Andreadis
Stefanos Andreadis Stefan Rauscher4
Stefan Rauscher4  C. Rikard Unelius
C. Rikard Unelius Peter Witzgall
Peter Witzgall