Serendipitous, cross familial discovery of the first long-range chemical attractants for antlions (Neuroptera: Myrmeleontidae): (1R,2S,5R,8R)-iridodial and Z,E-nepetalactol
- 1Sterling International Inc., Spokane, WA, USA
- 2Invasive Insect Biocontrol and Behavior Laboratory, United States Department of Agriculture - Agricultural Research Service, Beltsville, MD, USA
- 3Jeffrey R. Aldrich Consulting, LLC, Santa Cruz, CA, USA
- 4Department of Entomology, University of California, Davis, CA, USA
Synthetic (1R,2S,5R,8R)-iridodial, the key pheromone component of many green lacewings in the genus Chrysopa, strongly attracted adult males and females of the North American antlion, Dendroleon speciosus Banks. In addition, one of the common sex pheromone components of many aphids to which Chrysopa spp. are weakly attracted, Z,E-nepetalactol, was also weakly attractive to D. speciosus adults. Iridodial and Z,E-nepetalactol also elicited strong and weak electroantennogram detector (EAD) responses, respectively, in D. speciosus adults. Previously reported semiochemicals from European and Israeli antlion species did not elicit EAD or behavioral responses from D. speciosus adults. The earlier studied antlions release volatile chemicals from male-specific metathoracic glands associated with structures on the hind wings (Eltringham's organs) that are thought to enhance evaporation of the secretion. Although D. speciosus males have Eltringham's organs similar to those of other antlion species, we discovered that D. speciosus males have a pair of white tubular glands that extend posteriorly into the abdomen, opening in the resting pits of the Eltringham's organs. Further gas chromatograph (GC)-EAD analysis of another commercially available antlion species, Myrmeleon crudelis Walker, showed that this species did not respond to the lacewing or aphid related volatile compounds, but strongly responded to the reported antlion semiochemicals, namely, nerol, 10-homonerol, and nerol oxide. The male-specific abdominal glands of D. speciosus are presumably pheromone glands, but this hypothesis requires verification in the future.
Introduction
With more than 1500 described species worldwide (Strange, 2004), the Myrmeleontidae (called antlions) is the largest family in the order Neuroptera. Antlions exploit a range of habitats; their larvae are sedentary predators living in tree holes, on the soil surface, and in specialized pits they create in sandy soil (Mansell, 1999). In contrast to the rich knowledge of larval behaviors (Lambert et al., 2011; Scharf et al., 2011), due to the short life span and nocturnal activity of adults their behavior is less well known (Yasseri and Parzefall, 1996; Güsten, 2002; Szentkirályi and Kazinczy, 2002; Penny et al., 2007).
In particular, semiochemicals mediating the behavior of mymeliontids are poorly known. Indeed, semiochemicals have been identified from only three sympatric European antlion species [Eurleon nostras (Fourc.), Grocus bore Tjed. [now Myrmeleon bore (Tjed.)], and Myrmeleon formicarius L.], and two sympatric species in Israel (Synclysis baetica Rambur, and Acanthacilicis occitanica Villers) (Bergström, 2008). These antlion species belong to four different genera. In the antlion species studied thus far, volatile chemicals are produced in paired male-specific thoracic glands, from which a total of six structurally similar compounds have been identified: two monoterpene alcohols (nerol and 10-homonerol); their corresponding oxides (nerol oxide and 10-homonerol oxide), and two mono-unsaturated secondary alcohols [(R)-(Z)-6-tridecen-2-ol and (R)-(Z)-6-undecen-2-ol]. Males of each species produce specific two-component blends thought to function in reproductive isolation (Elofsson and Löfqvist, 1974; Löfqvist and Bergström, 1980; Baeckström et al., 1989; Bergström et al., 1992). The roles of male-specific volatiles for the three European species were further tested by gas chromatography-electroantennogram detector (GC-EAD) and Y-tube bioassay experiments, which suggested that these secretions are sex or aggregation pheromones (Yasseri et al., 1996, 1997, 1998; Yasseri and Parzefall, 1996; Bergström, 2008). However, no long-range sex or aggregation attractants for any antlions have been positively elucidated.
In the course of testing synthetic pheromone candidates for the green lacewing, Chrysopa nigricornis Burmeister (Zhang et al., 2006a), an interesting discovery was made, namely, that both males and females of the antlion, Dendroleon speciosus Banks (Myrmeleontidae: Dendroleontinae) were significantly attracted to several of the lacewing treatments tested during late July and mid-August 2004. D. speciosus was previously unknown in the state of Washington (Penny, personal communication).
In the current paper, we report (1) the identity of two long-range attractants for D. speciosus, (2) electrophysiological responses of D. speciosus adults to reported lacewing and antlion semiochemicals, (3) behavioral responses of D. speciosus adults to reported lacewing and antlion semiochemicals in the field, and (4) the discovery of a unique orientation of male-specific glands in D. speciosus. In addition, the electrophysiological responses to previously known lacewing and antlion semiochemicals were tested on a common commercially available antlion species, Myrmeleon crudelis Walker.
Materials and Methods
Insects and Chemicals
Live adult D. speciosus males and females for GC-EAD studies, and dead specimens for gland dissections, were collected from sticky traps baited with green lacewing pheromone (Zhang et al., 2004), whereas M. crudelis adults for GC-EAD recordings were reared from commercially available larvae (http://www.antlionfarms.com/).
Chemical standards were obtained commercially or synthesized. (1R,2S,5R,8R)-Iridodial [80%; with 20% of (1R,2S,5R,8S)-iridodial as an impurity] was synthesized as previously described (Chauhan et al., 2004). Z,E-Nepetalactone (98%) and E,Z-nepetalactone (96%) were isolated from commercially available catnip oil via a pH-sensitive chemical separation technique (Chauhan and Zhang, 2008). Z,E-Nepetalactol (~90%) was prepared by NaBH4 reduction of Z,E-nepetalactone as previously described (Chauhan et al., 2004). Nerol (>97%) and methyl salicylate (99%) were from Sigma-Aldrich, St. Louis, MO, USA, and nerol oxide (>95%) was purchased from Bedoukian Research Inc., Danbury, CT, USA. 10-Homonerol (98%) and 10-homonerol oxide (97%) were synthesized as described by Baeckstrom et al. (1982) and Cahiez et al. (1976), respectively. (Z)-6-Tridecen-2-ol (90%) and (Z)-6-undecen-2-ol (90%) were synthesized according to Clososki et al. (2004). The Benallure™ dispensers were purchased from Gardens Alive, Inc., Lawrenceburg, IN, USA.
GC-EAD Analysis
Electrophysiological responses by antennae of three D. speciosus (both sexes) and three M. crudelis (females only) to a synthetic mixture including several green lacewing and antlion semiochemicals (100 ng/μl each in hexane) were recorded in splitless mode using a Varian CP-3800 GC equipped with a polar column (HP-INNOWAX; 30 m × 0.53 mm × 1.0 μm; Agilent Technologies, Wilmington, DE, USA), using a 1:1 effluent splitter that allowed simultaneous flame ionization detection (FID) and EAD of the separated volatile compounds. Helium was used as the carrier gas, and the injector temperature was 220°C. Column temperature was programmed from 50°C for 2 min, rising to 240°C at 10°C/min, then held for 10 min. The outlet for the EAD was held in a humidified 0.5-m/s air stream over an antlion antennal preparation. EAD recordings were made using silver wire-glass capillary electrodes filled with Beadle–Ephrussi Ringer solution (Zhang et al., 2000) on freshly cut antennae. Antennal signals were stored and analyzed on a PC equipped with a serial IDAC interface box, using an EAD ver. 2.5 Program (Ockenfels SYNTECH GmbH, Kirchzarten, Germany).
Dissections of Exocrine Glands
D. speciosus adults (both sexes) were carefully removed from the sticky traps, and kept in 70% ethanol. Dissections of these antlion specimens in search of exocrine glands were conducted under a stereomicroscope (Fisher Scientific), in a small glass Petri dish filled with tap water. Photos of these dissections were taken using an iPhone 5 camera through one of the microscope oculars.
Field Trapping
Three field-trapping experiments were carried out from late July through mid-September 2004, 2006, and 2010, either in a home garden or small orchards in and around Spokane, WA, USA. Pherocon VI traps with removable sticky inserts (Trécé Inc., Adair, OK, USA) were hung 1.5–2.0 m above ground on either garden stakes, fence posts or the branches of cherry or apple trees, ca. 5–10 m apart within each trap line. For each experiment, two or three sets of traps (each set contained all the tested treatments) were deployed, and the initial trap positions were randomized within a set. Traps were visited once a week, at which time the sticky inserts were replaced, and trap positions were re-randomized. The sticky inserts with captured insects were taken to the laboratory for recording of the species and gender of trapped specimens.
Experiment 1 (with two sets of sticky traps), conducted at Strawberry Hill Farm from 28 July to 24 August 2004, compared the lacewing pheromone [(1R,2S,5R,8R)-iridodial] with Z,E-nepetalactone, Z,E-nepetalactol, and the combination of iridodial with methyl salicylate; 5 mg of each compound in 50 μl hexane was loaded onto rubber septa, each of which were inserted into a 2-ml open plastic centrifuge-tube. A commercial beneficial insect attractant, Benallure™ (Gardens Alive Inc.), was also included in each trap line for comparison. In Experiment 2, three sets of sticky traps were deployed at three locations (Prestini, Arbor Crest, and Morgan) from 1 to 29 August 2006, to test different dosages of iridodial (0, 0.1, 1, 2, 5, 10 mg, each in 100 μl hexane) applied to 2.5 × 4.5 × 0.4 cm pieces of felt each sealed in a 0.15 mm polyethylene (PE) bag (3 × 5 cm). In Experiment 3, three sets of sticky traps were deployed at two locations (two sets at Mt. St. Michaels, and one set at Morgan) from 28 July through mid-September 2010, to test the responses of the native antlion species, D. speciosus. Lures tested in Experiment 3 included reported potential antlion pheromone candidates in binary blends (neat compounds loaded onto rubber septa inserted into a 2-ml open plastic centrifuge-tube), and the green lacewing pheromone, iridodial (2 mg in 100 μl hexane loaded onto rubber septa; inserted into a 2-ml open plastic centrifuge-tube).
Statistical Analysis
Because of heterogeneity of variances among treatments, trap catch data (number of antlions or lacewings/trap) were analyzed using the nonparametric Kruskal–Wallis ANOVA on rank test, followed by the Student–Newman–Keuls all pairwise comparison to separate means (Zar, 1984).
Results
Field Trapping
The results of Experiment 1 on green lacewings were reported in Zhang et al. (2006a). In this experiment, iridodial resulted in significantly higher trap catches of males for both green lacewing species (C. nigricornis and C. oculata) than did Z,E-nepetalactol, whereas addition of methyl salicylate to iridodial significantly increased trap catches for C. nigricornis but not for C. oculata (see Figure 4 in Zhang et al., 2006a). Surprisingly, significant numbers of male and female D. speciosus adults were also captured in traps baited with iridodial and Z,E-nepetalactol during late July and mid-August 2004, with iridodial being significantly more attractive than Z,E-nepetalactol (Figure 1). Addition of methyl salicylate to iridodial significantly decreased the antlion trap catches to levels not different from the unbaited blank control traps (Figure 1). Z,E-nepetalactone and the commercial beneficial attractant, Benallure™, were both inactive.
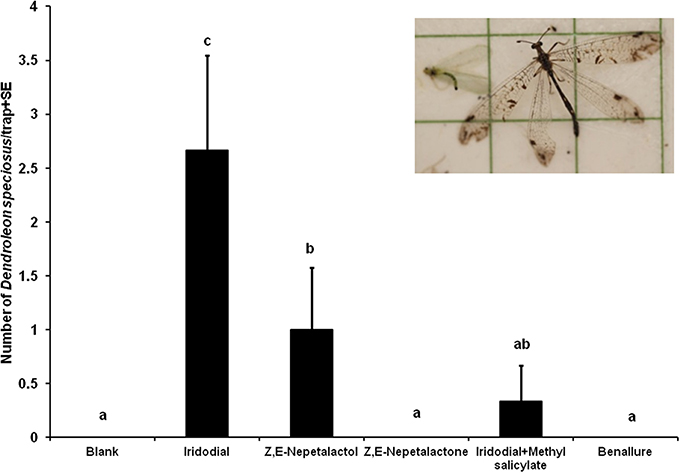
Figure 1. Field response of Dendroleon speciosus females and males to a pheromone compound (iridodial) of green lacewings (Neuroptera: Chrysopidae: Chrysopa spp.), two pheromone components of aphids (Z,E-nepetalactone and Z,E-nepetalactol), a commercial lure for lacewings and other beneficial insects (Benallure™), and unbaited control traps (N = 3; treatments loaded on rubber septa as described in test; 28 July to 4 August 2004, Spokane, WA, USA). Bars with the same letter were not statistically different (P > 0.05) by the Student–Newman–Keuls all pairwise comparisons after the nonparametric Kruskal–Wallis ANOVA on rank test.
In Experiment 2, D. speciosus adults were significantly attracted to sticky traps baited with PE-bag dispensers contaning ≥ 1-mg of iridodial. The 2-mg dose was the most attractive, followed by the 5-mg loading; further increase in dosage (e.g., 10-mg) resulted in significant decreases in trap catch (Figure 2). The lowest dosage loaded, 0.1-mg, was inactive for D. speciosus. In the same experiment, significant numbers of the golden-eyed lacewing (C. oculata) males were also captured in traps baited with ≥1-mg of iridodial, with no differences in trap catches among the 1 to 10-mg dosages (Figure 2).
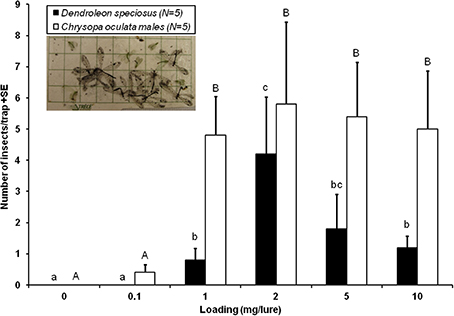
Figure 2. Dose-response attraction of Dendroleon speciosus antlions and Chrysopa oculata golden-eyed lacewings to (1R,2S,5R,8R)-iridodial (N = 5; PE-bag dispensers; 1–11 August 2006, Spokane, WA, USA). Bars with the same letter within the same species were not statistically different (P > 0.05) by the Student–Newman–Keuls all pairwise comparisons after the nonparametric Kruskal–Wallis ANOVA on rank test.
In Experiment 3, none of the reported potential antlion pheromone binary blends were attractive to D. speciosus or to green lacewings (Table 1). However, traps baited with the lacewing pheromone, iridodial (2-mg loading), captured significant numbers of antlions (both sexes) and green lacewings (males) (Table 1). The overall sex-ratio of captured antlions was 1:1.3 (♀:♂), whereas only males of the green lacewings were captured.
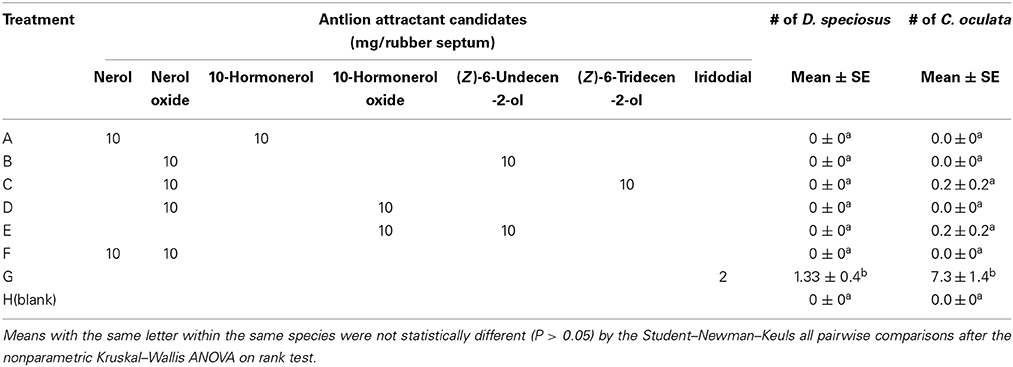
Table 1. Mean trap catches (#/trap/collection; N = 9) of the antlion, Dendroleon speciosus, and the golden-eyed lacewing, Chrysopa oculata, to previously reported antlion semiochemical candidates and the green lacewing pheromone, iridodial; July 28th to September 16th, 2010, Spokane, WA, USA.
GC-EAD
Antennae of D. speciosus (from both males and females) strongly responded to the green lacewing pheromone component, iridodial, and weakly (but consistently) to Z,E-nepetalactol (Figure 3; upper and middle EAD traces). No antennal responses were elicited from males or females of D. specious to nepetalactones, or to any of the three reported antlion candidate pheromone components, nerol, 10-homonerol, and nerol oxide. In contrast to D. speciosus, the commercially available M. crudelis females showed no antennal activities to any of the green lacewing attractants, but demonstrated strong EAD responses to all three reported antlion pheromone candidates (Figure 3, lower EAD trace).
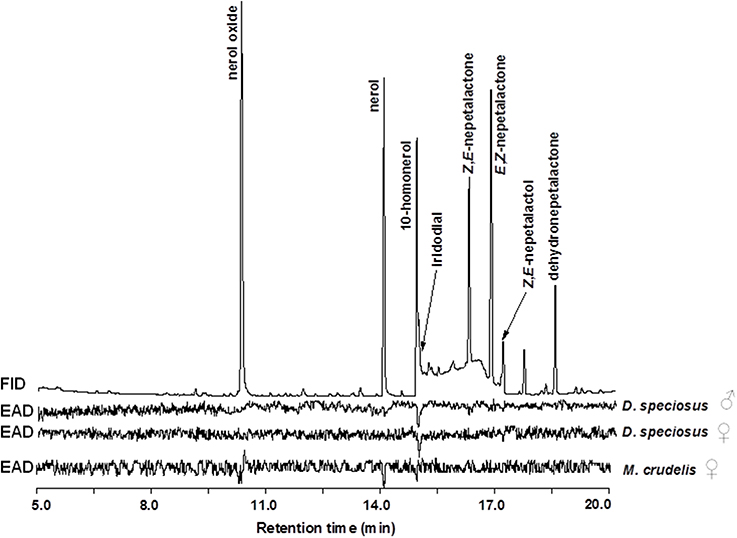
Figure 3. Gas chromatography-electroantennogram detector (GC-EAD) responses of Dendroleon speciosus and Myrmeleon crudelis to a mixture of synthetic green lacewing related compounds, and three volatile compounds previously reported for other antlion species (200 ng/μl each in hexane; see text for details).
Dissections of Exocrine Glands
Adult D. speciosus males possess yellowish-colored, paired Eltringham's organs (EO) (Eltringham, 1926) (also called axilular pilaris; Yasseri et al., 1998) at the base of their hindwings (EO; Figure 4A). Dissection of males revealed a pair of 8–10 mm long white tubular glands extending from the resting pits for Eltringham's organs in the first abdominal segment posteriorly nearly 1/2 length of the abdomen (Figure 4C). Eltringham's organs and the abdominal glands (AGs) are absent in D. speciosus females (Figures 4B,D).
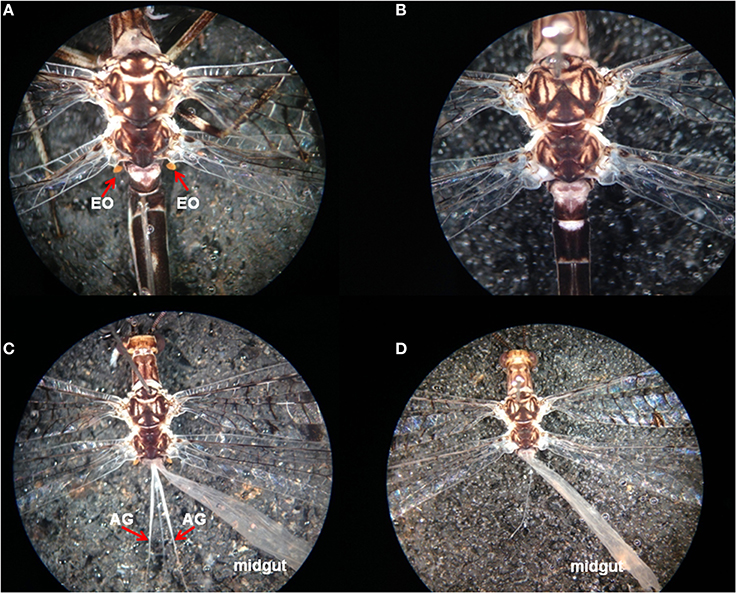
Figure 4. (A) Male-specific Eltringham's organs (EOs; red arrows) of Dendroleon speciosus; (B) Shows that the EOs are missing from D. speciosus females; (C) Shows the male-specific abdominal glands (AGs; red arrows) opening at the base of the resting pits for the EOs, extending posteriorly along the midgut; (D) Show that the AGs are missing from D. speciosus females.
Discussions
This is the first demonstration of long-range attractants for any antlion species. Our results clearly show that the male-produced green lacewing pheromone, (1R,2S,5R,8R)-iridodial (Chauhan et al., 2004, 2007; Zhang et al., 2004, 2006a,b), is also a strong attractant for both sexes of the antlion, D. speciosus. Z,E-Nepetalactol, which is a known sex pheromone component for many aphids (Pickett et al., 2013; Han et al., 2014), is also a weak attractant for D. speciosus adults. Iridodial and Z,E-nepetalactol also elicited a strong and a weak antennal electrical response, respectively, from D. speciosus adults indicating the existence of olfactory receptor neurons on the antlion antennae for these two volatile compounds. This finding revealed an interesting cross-attraction between species in different families within the same order. No fresh live D. speciosus adults were available for volatile collections and chemical analyses; the only live D. speciosus adults, which were not suitable for pheromone collection, were used for GC-EAD study after being taken from the sticky traps baited with the lacewing pheromone. Whether or not D. speciosus use the same or similar volatile compounds as its pheromone component(s) or use the lacewing pheromone as a kairomone, remains unknown.
There are about 20 described species of Dendroleon in the world, with nine species in China, four species in Australia, three species in North America, and one species each in Europe, Madagascar, Japan, and Java (Strange, 2004; Stange, 2008; Wang and Wang, 2008; Zhan et al., 2012). Only two Dendroleon species occur in the USA; D. speciosus in the west (west of 100° Meridian), and its allopatric counterpart, D. obsoletus (Say), in the eastern USA (east of 100° Meridian) (Stange, 2008). Larvae of D. speciosus have been found in northern California living in tree holes of pine and oak at low elevations (<600 m), and on rain and snow protected rock shelves at higher elevations (Stange, 2008). The larvae have also been found in houses near suitable forest habitats. According to Miller (1990) (cited in Stange, 2008, but not listed in the reference list) adult females of D. speciosus appear to be unique in this family in that they die shortly after laying their eggs instead of continuing to feed and developing more eggs. Based on our trapping results in three different years, adults of D. speciosus fly mainly during late July to mid-August in the Spokane area of Washington, which is in agreement with collection dates from western states in USA (listed in Stange, 2008), and new distributional records in British Columbia, Canada (Meinander et al., 2009).
As mentioned in the Introduction herein, antlions studied in Europe and Israel produce volatiles in a pair of male-specific thoracic glands (Elofsson and Löfqvist, 1974; Löfqvist and Bergström, 1980; Bergström et al., 1992; Bergström, 2008). These glands open ventrally at the junction of the thorax and abdomen. In E. nostras and M. bore, but not from M. formicarius, males have club-like projections (brush-like tufts; called Eltringham's organs) (Eltringham, 1926) from the posterior margin of the hind-wings. Eltringham's organs have been considered to be the dispersing organs for substances from the thoracic glands (Elofsson and Löfqvist, 1974; Löfqvist and Bergström, 1980). However, a morphological study by Güsten (1998) indicated that antlion thoracic glands are much more variable than thought from the few species of Myrmeleontini and Acanthaclisini initially investigated; species have now been found in the tribes Myrmecaelurini and Nemoleontini in which the thoracic glands are equally developed in both sexes.
The male-specific thoracic gland volatiles identified from three European species were also tested electrophysiologically and behaviorally in the laboratory (Yasseri et al., 1996, 1997, 1998). In female choice tests using a Y-tube olfactometer, solitary males were not able to attract females; however, females were strongly attracted by groups of males, indicating that male-produced volatiles might act as aggregation pheromones as part of lek formation. In the initial experiment, an occasional GC-EAD response by antenna of one M. bore female to conspecific male-specific thoracic gland volatiles [10-homonerol and (Z)-6-undecen-2-ol] was observed, but was not reproducible; likely this was due to the short life of antlion antennal contact gel electrode preparations (Yasseri et al., 1998). A previously undiscovered additional gland, opening between the meta- and mesothorax, was found in three antlion species (both sexes): E. nostras, M. bore, and M. formicarius. Analysis of the secretions of M. bore and E. nostras showed a lower concentration and different chemical compositions (dodecyl acetate and acetogenine in M. bore females; and hexadecane acid in E. nostras females) compared to compounds identified from the male-specific metathoracic glands (Yasseri et al., 1998). It is not known if these new chemical compounds are behaviorally active alone or with the two component blends identified earlier (Löfqvist and Bergström, 1980; Bergström et al., 1992). Thus far, there is no behavioral data on the function of male-produced binary blends in the two Israeli antlion species (S. baetica and A. occitanica) that have been investigated (Bergström, 2008).
In D. speciosus males, we not only observed the yellowish, paired Eltringham's organs at the base of the hindwings (EO; Figure 4A), but we also found a pair of long white tubular glands (Figure 4C) opening in the resting pits of the Eltringham's organs. Unlike the thoracic glands reported earlier for the European and Israeli antlion species (extending anteriorly into the thorax), the paired tubular glands in D. speciosus males, which we are designating abdominal glands (AGs), extend posteriorly into the abdomen (Figure 4C). Both the Eltringham's organs and the AGs are absent in D. speciosus females (Figures 4B,D). It is likely that these highly developed male-specific glands in D. speciosus males are the site of pheromone production; confirmation of this supposition is needed. Furthermore, the Eltringham's organs might function as the dispersers for substances released from the AGs. A similar male-specific AG system (with four narrow tubular glands extending posteriorly into the abdomen) was found earlier in Brachynenmurus longicaudus (Burmeister) of the tribe Brachynemurini (Güsten, 1998). The green lacewing (Chrysopa spp.) pheromone, (1R,2S,5R,8R)-iridodial, is produced from male-specific abdominal glands, but these pheromone glands are elliptical epidermal glands that are abundantly distributed on the third–eighth abdominal sternites (Zhang et al., 2004). Chrysopa and other genera of green lacewings also possess thoracic glands in both sexes that produce compounds, some of which are obnoxious to the human nose, presumably for defense (Aldrich et al., 2009). No long-range pheromones or attractants have yet been reported for other Neuropteran families.
In summary, our GC-EAD experiments showed that D. speciosus antlions do not have olfactory receptors on their antennae for nerol, 10-homonerol and nerol oxide, three antlion pheromone candidates known from some European and Israeli antlion species representing four genera from two subfamilies (Myrmeleontinae and Acanthaclisinae). On the other hand, antennae of female adults of the Nearctic M. crudelis (Myrmeleontinae) responded strongly to the three reported antlion pheromone candidates (nerol, 10-homonerol and nerol oxide), indicating that this species might use the same or similar volatile compounds for intra- and/or inter-specific chemical communication. The fact that D. speciosus larvae do not make sand pits to trap ants, as do the previously studied antlion species, and that adults of this species exhibit a strong interfamilial attraction to a key pheromone component of certain green lacewings rather than the known congeneric semiochemicals, suggests that dendroleontine antlions are not closely aligned with antlions whose chemical communication was studied earlier.
Author Contributions
Designed research: Qing-He Zhang and Rodney G. Schneidmiller. Performed research: Qing-He Zhang, Guiji Zhou, Doreen R. Hoover, Neil J. Michaelson, Paul Bryant, Armenak Margaryan, Kamlesh Chauhan, Jeffrey R. Aldrich, and Rodney G. Schneidmiller. Analyzed data: Qing-He Zhang, Guiji Zhou, Doreen R. Hoover, Neil J. Michaelson, Paul Bryant, Armenak Margaryan, Kamlesh Chauhan, Jeffrey R. Aldrich, and Rodney G. Schneidmiller. Statistical analyses: Qing-He Zhang and Doreen R. Hoover. Wrote the paper: Qing-He Zhang and Jeffrey R. Aldrich.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We sincerely thank Dr. Norman D. Penny, Department of Entomology, California Academy of Sciences, for identification of antlions, and Prof. Wittko Francke, Department of Organic Chemistry, University of Hamburg, Germany for obtaining several less commonly available, but critical literatures.
References
Aldrich, J. R., Le, T. C., Zhang, Q.-H., Torres, J., Winterton, S. L., Han, B., et al. (2009). Prothoracic gland semiochemicals of green lacewings. J. Chem. Ecol. 35, 1181–1187. doi: 10.1007/s10886-009-9701-x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Baeckström, P., Bergström, G., Björkling, F., Hui-Zhu, H., Högberg, H.-E., Jacobsson, U., et al. (1989). Structures, absolute configurations, and syntheses of volatile signals from three sympatric ant-lion species, Euroleon nostras, Grocus bore, and Myrmeleon formicarius (Neuroptera: Myrmeleontidae). J. Chem. Ecol. 15, 61–80. doi: 10.1007/BF02027774
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Baeckstrom, P., Okecha, S., Desilva, N., Wijekoon, D., and Norin, T. (1982). Photo-oxidation with simultaneous reduction of hydroperoxides with tetrabutylammonium borohydride-synthesis of perillenal from myrcene. Acta Chem. Scand. B 36, 31–36. doi: 10.3891/acta.chem.scand.36b-0031
Bergström, G., Wassgren, A.-B., Högberg, H.-E., Hedenström, E., Hefetz, A., Simon, D., et al. (1992). Species-specific, two-component, volatile signals in two sympatric ant-lion species: Synclysis baetica and Acanthaclisis occitanica (Neuroptera, Myrmeleontidae). J. Chem. Ecol. 18, 1177–1188. doi: 10.1007/BF00980072
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Bergström, L. G. W. (2008). Chemical communication by behaviour-guiding olfactory signals. Chem. Commun. 34, 3959–3979. doi: 10.1039/b712681f
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Cahiez, G., Bernard, D., and Normant, J. (1976). Stereospecific syntheses of alkenyllithium reagents from alkenyl iodides. Synthesis 1976, 245–248. doi: 10.1055/s-1976-25384
Chauhan, K. R., Levi, V., Zhang, Q.-H., and Aldrich, J. R. (2007). Female goldeneyed lacewings (Neuroptera: Chrysopidae) approach but seldom enter traps baited with the male-produced compound iridodial. J. Econ. Entomol. 100, 1751–1755. doi: 10.1603/0022-0493(2007)100[1751:FGLNCA]2.0.CO;2
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Chauhan, K. R., and Zhang, A. (2008). Methods of separating ZE-nepetalactone and EZ-nepetalactone from catnip oil. U.S. Patent 7,375,239.
Chauhan, K. R., Zhang, Q.-H., and Aldrich, J. R. (2004). Iridodials: enantiospecific synthesis and stereochemical assignment of the pheromone for the golden-eyed lacewing, Chrysopa oculata. Tetrahedron Lett. 45, 3339–3340. doi: 10.1016/j.tetlet.2004.03.034
Clososki, G. C., Ricci, L. C., Costa, C. E., and Comasseto, J. V. (2004). A short and efficient enantioselective synthesis of (+) and (–)-(Z)-7, 15-hexadecadien-4-olide: the sex pheromone of the yellowish elongate chafer, Heptophylla picea. J. Braz. Chem. Soc. 15, 809–812. doi: 10.1590/S0103-50532004000600004
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Elofsson, R., and Löfqvist, J. (1974). The Eltringham organ and a new thoracic gland: ultrastructure and presumed pheromone function (Insecta, Myrmeleontidae). Zool. Scr. 3, 31–40. doi: 10.1111/j.1463-6409.1974.tb00801.x
Eltringham, H. (1926). On the structure of an organ in the hind-wing of Myrmeleon nostras Fourc. Trans. R. Entomol. Soc. Lond. 74, 267–268. doi: 10.1111/j.1365-2311.1926.tb02237.x
Güsten, R. (1998). The morphology of the metathoracic gland system in the Myrmeleontidae (Neuroptera): a preliminary overview. Acta Zool. Fenn. 209, 121–128.
Güsten, R. (2002). Antlion assemblages (Neuroptera: Myrmeleontidae) of two arid habitats in Tunisia. Acta Zool. Acad. Sci. Hung. 48, 99–120.
Han, B.-Y., Wang, M.-X., Zheng, Y.-C., Niu, Y.-Q., Pan, C., Cui, L., et al. (2014). Sex pheromone of the tea aphid, Toxoptera aurantii (Boyer de Fonscolombe) (Hemiptera: Aphididae). Chemoecology 24, 179–187. doi: 10.1007/s00049-014-0161-6
Lambert, E. P., Motta, P. J., and Lowry, D. (2011). Modulation in the feeding prey capture of the ant-lion, Myrmeleon crudelis. J. Exp. Zool. A Ecol. Genet. Physiol. 315, 602–609. doi: 10.1002/jez.709
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Löfqvist, J., and Bergström, G. (1980). Nerol-derived volatile signals as a biochemical basis for reproductive isolation between sympatric populations of three species of ant-lions (Neuroptera: Myrmeleontidae). Insect Biochem. 10, 1–10. doi: 10.1016/0020-1790(80)90032-3
Mansell, M. W. (1999). Evolution and success of antlions (Neuropterida: Neuroptera, Myrmeleontidae). Stapfia 60, 49–58.
Meinander, M., Klimaszewski, J., and Scudder, G. (2009). New distributional records for some Canadian Neuropterida (Insecta: Neuroptera, Megaloptera). J. Entomol. Soc. Br. Columbia 106, 11–15.
Penny, N. D., Arias, J. R., and Armistead, J. S. (2007). Seasonal Emergence of Neuroptera in Fairfax County, Virginia. Proc. Calif. Acad. Sci. 58, 7–19.
Pickett, J. A., Allemann, R. K., and Birkett, M. A. (2013). The semiochemistry of aphids. Nat. Prod. Rep. 30, 1277–1283. doi: 10.1039/c3np70036d
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Scharf, I., Lubin, Y., and Ovadia, O. (2011). Foraging decisions and behavioural flexibility in trap−building predators: a review. Biol. Rev. 86, 626–639. doi: 10.1111/j.1469-185X.2010.00163.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Stange, L. A. (2008). A new species of the genus Dendroleon Brauer from Mexico (Neuroptera: Myrmeleontidae). Insecta Mundi 0054, 1–9.
Strange, L. (2004). A Systematic Catalog, Bibliography and Classification of the World Antlions (Insecta: Neuroptera: Myrmeleontidae). Vol. 74. Gainesville, FL: American Entomological Institute.
Szentkirályi, F., and Kazinczy, L. (2002). Seasonal flight patterns of antlions (Neuroptera, Myrmeleontidae) monitored by the Hungarian light trap network. Acta Zool. Sci. Hung. 48, 311–328.
Wang, Z.-L., and Wang, X.-L. (2008). A catalogue of Dendroleon Brauer, 1866 (Neuroptera, Myrmeleontidae) from China, with description of a new species. Acta Zootaxon. Sin. 33, 42–45.
Yasseri, A. M., Bergstrøm, G., W., F., and Wassgren, A.-B. (1996). “Laboratory studies on the role of volatile compounds in mating of the antlion Euroleon nostras (Geoffroy in Fourcroy, 1785): behavioural and chemical aspects (Insecta: Neuroptera: Myrmeleontidae),” in Pure and Applied Research in Neuropterology: Proceedings of the Fifth International Symposium on Neuropterology, eds M. Canard, H. Aspöck, and M. W. Mansell (Toulouse: Association Mondiale Nevropteristes), 289–297.
Yasseri, A. M., and Parzefall, J. (1996). “Life cycle and reproductive behaviour of the antlion Euroleon nostras (Geoffroy in Fourcroy, 1785) in northern Germany (Insecta: Neuroptera: Myrmeleontidae),” in Pure and Applied Research in Neuropterology, eds M. Canard, H. Aspöck, and M. W. Mansell (Toulouse: Association Mondiale Nevropteristes), 269–288.
Yasseri, A. M., Parzefall, J., and Francke, W. (1997). New aspects of chemical communication in antlions Myrmeleontidae. Mitt. Dtsch. Ges. Allg. Angew. Entomol. 11, 899–904.
Yasseri, A. M., Parzefall, J., Rietdorf, M., and Francke, W. (1998). New studies on the role of volatile compounds in antlions (Neuroptera, Myrmeleontidae). Acta Zool. Fenn. 209, 277–284.
Zhan, Q., Wang, Z., Abraham, L., and Wang, X. (2012). A new species of Dendroleon Brauer, 1866 (Neuroptera, Myrmeleontidae) from China. Zootaxa 3547, 64–70.
Zhang, Q.-H., Chauhan, K. R., Erbe, E. F., Vellore, A. R., and Aldrich, J. R. (2004). Semiochemistry of the goldeneyed lacewing Chrysopa oculata: attraction of males to a male-produced pheromone. J. Chem. Ecol. 30, 1849–1870. doi: 10.1023/B:JOEC.0000042406.76705.ab
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Zhang, Q.-H., Schlyter, F., and Birgersson, G. (2000). Bark volatiles from nonhost angiosperm trees of spruce bark beetle, Ips typographus (L.) (Coleoptera: Scolytidae): chemical and electrophysiological analysis. Chemoecology 10, 69–80. doi: 10.1007/s000490050010
Zhang, Q.-H., Schneidmiller, R. G., Hoover, D. R., Young, K., Welshons, D. O., Margaryan, A., et al. (2006a). Male-produced pheromone of the green lacewing, Chrysopa nigricornis. J. Chem. Ecol. 32, 2163–2176. doi: 10.1007/s10886-006-9137-5
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Zhang, Q.-H., Sheng, M., Chen, G., Aldrich, J. R., and Chauhan, K. R. (2006b). Iridodial: a powerful attractant for the green lacewing, Chrysopa septempunctata (Neuroptera: Chrysopidae). Naturwissenschaften 93, 461–465. doi: 10.1007/s00114-006-0132-z
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Keywords: antlion, lacewing, pheromone, attractant, iridodial, nepetalactol, trap, GC-EAD
Citation: Zhang Q-H, Zhou G, Hoover DR, Michaelson NJ, Bryant P, Margaryan A, Chauhan KR, Aldrich JR and Schneidmiller RG (2015) Serendipitous, cross familial discovery of the first long-range chemical attractants for antlions (Neuroptera: Myrmeleontidae): (1R,2S,5R,8R)-iridodial and Z,E-nepetalactol. Front. Ecol. Evol. 2:80. doi: 10.3389/fevo.2014.00080
Received: 18 September 2014; Accepted: 22 November 2014;
Published online: 06 January 2015.
Edited by:
Li Chen, The Chinese Academy of Sciences, ChinaReviewed by:
Carmen Quero, Consejo Superior de Investigaciones Cientificas, SpainLi Chen, The Chinese Academy of Sciences, China
John Allen Byers, United States Department of Agriculture - Agricultural Research Service, USA
Copyright © 2015 Zhang, Zhou, Hoover, Michaelson, Bryant, Margaryan, Chauhan, Aldrich and Schneidmiller. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qing-He Zhang, Sterling International Inc., 3808 N. Sullivan Rd., Bldg. 16, Spokane, WA 99216, USA e-mail: qing-he@rescue.com
 Qing-He Zhang
Qing-He Zhang Guiji Zhou1
Guiji Zhou1  Kamlesh R. Chauhan
Kamlesh R. Chauhan Jeffrey R. Aldrich
Jeffrey R. Aldrich