Peripubertal exposure to male chemosignals accelerates vaginal opening and induces male-directed odor preference in female mice
- 1Laboratoire de Physiologie de la Reproduction et des Comportements, UMR 7247 INRA/CNRS/Université de Tours, INRA Val de Loire, Nouzilly, France
- 2Program for Drug Discovery and Medical Technology Platforms, RIKEN Research Cluster for Innovation, Saitama, Japan
- 3Centre d'Ecologie Fonctionnelle and Evolutive, UMR 5175 CNRS/Université Montpellier/SupAgro, Montpellier, France
- 4Institut des Sciences de l'Evolution, UMR 5554 Université Montpellier/CNRS/IRD, Montpellier, France
Reproductive physiology in female mouse is profoundly affected by male odor. A well-known effect of male odor is the acceleration of puberty onset in prepubertal female mice exposed to male urine. Whether peripubertal exposure to male odor also influences female sexual behavior in adulthood is poorly known. Recently, we reported that female mice exposed to male-soiled bedding showed advanced vaginal opening associated with early expression of male-directed odor preference in adulthood. The aim of the present study is to determine whether peripubertal exposure to male urinary chemosignals affects both occurrence of vaginal opening and attraction to male odor at older age in female mice. Therefore, we exposed female mice to (1R, 5S, 7R)-3,4-dehydro-exo-brevicomin (DHB), 6-hydroxy-6-methyl-3-heptanone (HMH) and (S)-2-sec-butyl-4,5-dihydrothiazole (SBT), individually or in mixture, from postnatal day (PD) 21 to PD38 and monitored the occurrence of vaginal opening. We measured then the time that the female mice spent sniffing male and female mouse urinary volatiles at PD45. As expected, peripubertal exposure to DHB, HMH, or SBT accelerated vaginal opening in female mice. In addition, we showed that exposure to a mixture of these three compounds induced expression of male-directed odor preference at PD45, contrary to the single exposure to each of these molecules. In conclusion, the volatile compounds DHB, HMH and SBT in urine of male mice influence both occurrence of vaginal opening and adult expression of male-directed odor preference in female mice.
Introduction
Rodents communicate extensively through odors released in urine, feces, and scent glands. Urine of the house mouse (Mus musculus) contains a complex mixture of volatile odorants and nonvolatile proteins which conveys information about sex, reproductive and social status, individual identity (Hurst, 1990; Hurst et al., 2001). In male mice, many urinary compounds are regulated by androgens (Schwende et al., 1986) which profoundly alter reproductive physiology in female mice (Whitten, 1956; Bruce, 1959). A well-known endocrine effect of male odor is the acceleration of puberty onset in female mice (Vandenbergh, 1967, 1969). Peripubertal exposure to male urine or soiled bedding accelerated vaginal opening, uterine growth, first estrus and ovulation (Vandenbergh, 1969; Colby and Vandenbergh, 1974; Bronson, 1975; Vandenbergh et al., 1975); but urine or soiled bedding from castrated male had no effect (Lombardi et al., 1976; Jouhanneau et al., 2014). To date, the following androgen-dependent volatile compounds are reported to increase uterine weight in peripubertal mice: (1R, 5S, 7R)-3,4-dehydro-exo-brevicomin (DHB), 6-hydroxy-6-methyl-3-heptanone (HMH), (S)-2-sec-butyl-4,5-dihydrothiazole (SBT) and β-farnesene (Novotny et al., 1999a,b). Additionally, the contribution of nonvolatile major urinary proteins (MUPs), which bind DHB, HMH, SBT and β-farnesene, in the Vandenbergh effect cannot be excluded (Bacchini et al., 1992; Robertson et al., 1993; Mucignat-Caretta et al., 1995; Novotny et al., 1999b; Sharrow et al., 2002).
In addition to accelerating vaginal opening, we reported recently that peripubertal exposure to male-soiled bedding induced expression of male-directed odor preference in female mice when they were adults (Jouhanneau et al., 2014). At postnatal day (PD) 45, female mice exposed to male-soiled bedding for 18 days from weaning (i.e., from PD21 to PD38) investigated longer the odor of male-soiled bedding than that of female-soiled bedding, whereas females exposed to clean bedding did so only at PD60. Exposure to castrated male-soiled bedding also triggered an early attraction to intact male odor but without effect on vaginal opening, suggesting that the physiological and behavioral effects of male chemosignals in female mice are supported by different mechanisms. Additionally, we reported that female mice exposed to male-soiled bedding during the peripubertal period also preferred intact male odors over castrated male odors at PD45. Taken together, this suggested that female mice were attracted to androgen-dependent volatile compounds of male-soiled bedding previously learned during the peripubertal period. Our characterization of volatile compounds in intact male-soiled and castrated male-soiled beddings by gas chromatography coupled to mass spectrometry revealed that the major androgen-dependent volatile compounds present in intact male-soiled bedding are DHB, HMH and SBT (Jouhanneau, 2014). These three compounds have also been detected in urine of male mice (Liebich et al., 1977; Novotny et al., 1984, 1999a; Schwende et al., 1986; Flanagan et al., 2011; Supplementary Figure 1 and Table 1).
Taken together, these results suggest that the urinary compounds DHB, HMH and SBT present in male-soiled bedding, in addition to accelerating vaginal opening, could also be responsible for the early expression of male-directed odor preference in female mice. A study by Jemiolo et al. (1985) further supports this hypothesis by showing that a mixture of DHB+SBT is attractive to female mice in adulthood. Therefore, the present study explored whether peripubertal exposure to DHB, HMH and SBT accelerated vaginal opening and induced adult expression of male-directed odor preference in female mice.
Materials and Methods
Animals
The experiment was conducted on Swiss mice, purchased from R. Janvier breeding center (Le Genest Saint-Isle, France) and bred in our animal facility. A total of 100 female mice coming from 30 litters were used. 1 week before birth, pregnant females were housed in a clean room deprived of any male odors. At postnatal day (PD) 2–3, newborn pups were sexed and litters were equalized to five females and five males. At PD21, female mice were weaned and housed in individual cages (23 × 16 × 14 cm). Animals were kept under a constant 12:12 h light:dark cycle and ambient air temperature was maintained at 20 ± 1°C. Water and pellet food (Safe, Augy, France) were provided ad libitum. Data were collected during four sessions from January to June 2013 (due to technical constraints, several treatments (but not all) were conducted in each sessions). All the procedures were conducted in accordance with the European directive 2010/63/EU on the protection of animals used for scientific purposes and approved by an ethics committee for animal experimentation (CEEA Val de Loire, France, n°2012-10-2).
Odor Stimuli
DHB, HMH and SBT were synthesized following the procedures reported by Tashiro and Mori (1999) and Tashiro et al. (2008). The compounds were diluted in distilled water to their corresponding concentration found in male mouse urine (i.e., 1.3 ppm for DHB and SBT, 2000 ppm for HMH; Novotny et al., 1985, 1999a). Urine samples were obtained from several adult male and female mice by a gentle pressure on the belly and were pooled. All samples were aliquoted and frozen at −80°C until the experiment.
Exposure to Male Chemosignals and Detection of Vaginal Opening
The exposure procedure was adapted from Novotny et al. (1999b) and Jouhanneau et al. (2014). From PD21 to PD38, peripubertal female mice were exposed twice daily to DHB (n = 17), HMH (n = 15), SBT (n = 15) or a mixture of DHB+HMH+SBT (n = 17) (Figure 1). Females exposed to water (n = 19) or male urine (n = 17) were used as negative and positive control groups respectively. The appropriate solution (20 μL) was directly applied with a micropipette on the oronasal groove between 9:00 and 11:00 and between 16:00 and 18:00 during the light phase. During odor stimulation, females were restrained in the supine position. To prevent volatile odor contamination, females were housed in separate rooms according to their group for the whole period of stimulation.
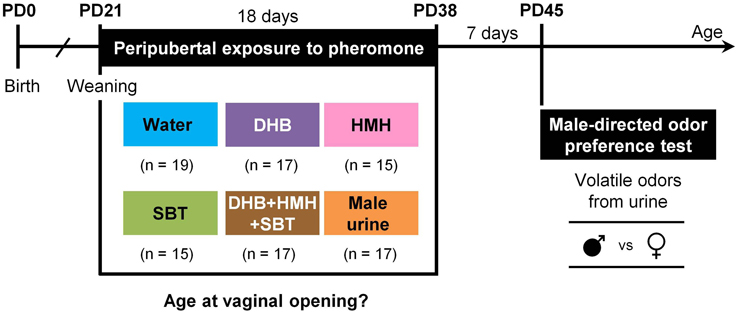
Figure 1. Experimental design. Female Swiss mice were exposed to water, (1R, 5S, 7R)-3,4-dehydro-exo-brevicomin (DHB), 6-hydroxy-6-methyl-3-heptanone (HMH), (S)-2-sec-butyl-4,5-dihydrothiazole (SBT), a mixture of DHB+HMH+SBT or male urine from postnatal day (PD) 21 to PD38. The age at vaginal opening was monitored during odor exposure. At PD45, the time that females spent sniffing volatile odorants from male and female urine in a Y-maze apparatus was measured.
During odor exposure, the age at vaginal opening was evaluated through daily visual examination, added with gentle tactile inspection with a Pasteur pipette in doubtful cases (experimenter was not blind as to the treatment when he judged vaginal opening). In Swiss mice, under our experimental conditions, vaginal opening appears 1 day before the first estrus and it is associated with uterine hypertrophy (Jouhanneau, 2014). At PD39, all females were housed in the same room until male-directed odor preference test was performed.
Male-Directed Odor Preference Test
Male-directed odor preference test was performed at PD45, i.e., 7 days after the last exposure to male chemosignals (Figure 1) (for a full description of the behavioral test, refer to Keller et al., 2006b or Jouhanneau et al., 2014). Briefly, each female mouse was tested for its preference to investigate the odor of male urine over the odor of female urine in a Y-maze. Three days before the behavioral test (i.e., at PD42), females were accustomed to the Y-maze for 5 min in the absence of any odor stimulus. On the day of the test, filter papers scented with 20 μL of male or female urine were placed on petri dishes in one of the two goal boxes of the Y-maze behind a door with holes. The female was first isolated in the start box of the Y-maze to adapt for 1 min. Then, the mouse could move freely in the apparatus for 5 min. The amount of time the animal spent sniffing each odor stimulus (i.e., poking its nose through the holes of the door and actively sniffing the door of the goal box) was recorded. After each test, odorized filter papers were changed and the maze was cleaned with 70% ethanol. The order of stimuli presentation was randomized across subject. The stage of the estrous cycle was determined by vaginal smear collected at the end of the behavioral test. No behavioral test was performed at PD60.
Statistical Analysis
Statistical differences in the mean age at vaginal opening between experimental groups were determined by the One-Way analysis of variance (ANOVA) followed by the Fisher's LSD post-hoc test. Mean time spent sniffing male odor vs. female odor during the preference test was compared using the paired t-test within experimental groups. The threshold for significant difference was set at p < 0.05. All analyses were conducted using the software Statistica 10 (StatSoft, Tulsa, OK, USA).
Results
Exposure to DHB, HMH, SBT, a mixture of DHB+HMH+SBT or male urine from PD21 to PD38 advanced vaginal opening in comparison to water exposure. Half of the animals exposed to a chemosignal (i.e., DHB or HMH or SBT), a mixture of the three chemosignals or male urine showed vaginal opening between PD24 and PD27 whereas half of the animals exposed to water showed vaginal opening on PD30 (Figure 2A). One-Way ANOVA revealed significant differences in the mean age at vaginal opening [F(5, 94) = 3.81, p = 0.003] (Figure 2B). Indeed, females exposed to a chemosignal, a mixture of the three chemosignals or male urine had vaginal opening earlier than females exposed to water (DHB vs. water groups: p < 0.001; HMH vs. water groups: p = 0.045; SBT vs. water groups: p = 0.016; DHB+HMH+SBT vs. water groups: p = 0.043; male urine vs. water groups: p < 0.001). No differences in the mean age at vaginal opening between females treated with chemosignal(s) or urine were observed.
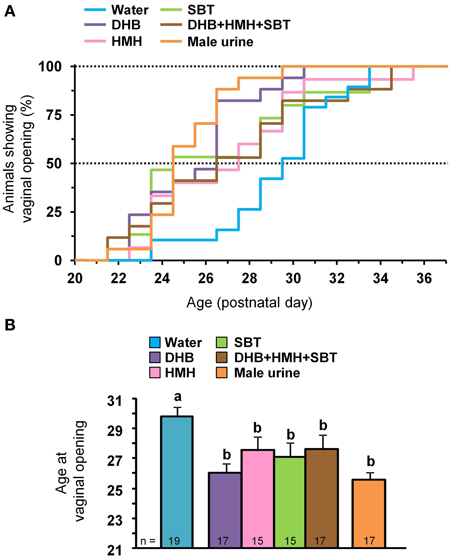
Figure 2. Vaginal opening during exposure to male chemosignals. (A) Cumulative percentage of female Swiss mice showing vaginal opening according to age and exposure to water, DHB, HMH, SBT, a mixture of DHB+HMH+SBT or male urine. (B) Mean age (±SE) at vaginal opening according to odor exposure. Dissimilar letters indicate significant differences, p < 0.05 using post-hoc Fisher's LSD test.
Exposure to a mixture of DHB+HMH+SBT or male urine from PD21 to PD38 triggered male-directed odor preference in female mice at PD45. Indeed, females exposed to a mixture of DHB+HMH+SBT or male urine investigated longer the odor of male urine than the odor of female urine [t(16) = 2.18, p = 0.044; t(16) = 3.15, p = 0.006 respectively] (Figure 3). By contrast, no differences in the time investigating male vs. female odor were observed for female mice exposed to DHB [t(16) = 0.30, p = 0.77], HMH [t(14) = 0.62, p = 0.54], SBT [t(14) = 1.68, p = 0.12] or water [t(18) = 1.51, p = 0.15] (Figure 3). Individual data showed that estrous cycle stage was distributed randomly in each group and therefore does probably not influence expression of male-directed odor preference (Figure 4).
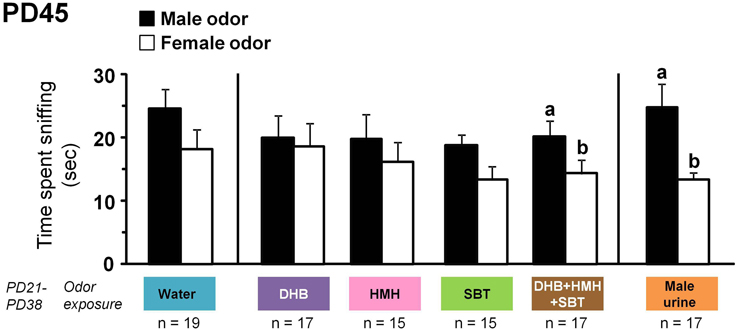
Figure 3. Male-directed odor preference after exposure to male chemosignals. Mean time (±SE) spent sniffing volatile odorants from male urine (black bar) and female urine (white bar) in a Y-maze apparatus at PD45 by female Swiss mice exposed to water, DHB, HMH, SBT, a mixture of DHB+HMH+SBT or male urine from PD21 to PD38. Dissimilar letters indicate significant differences, p < 0.05 using paired t-test.
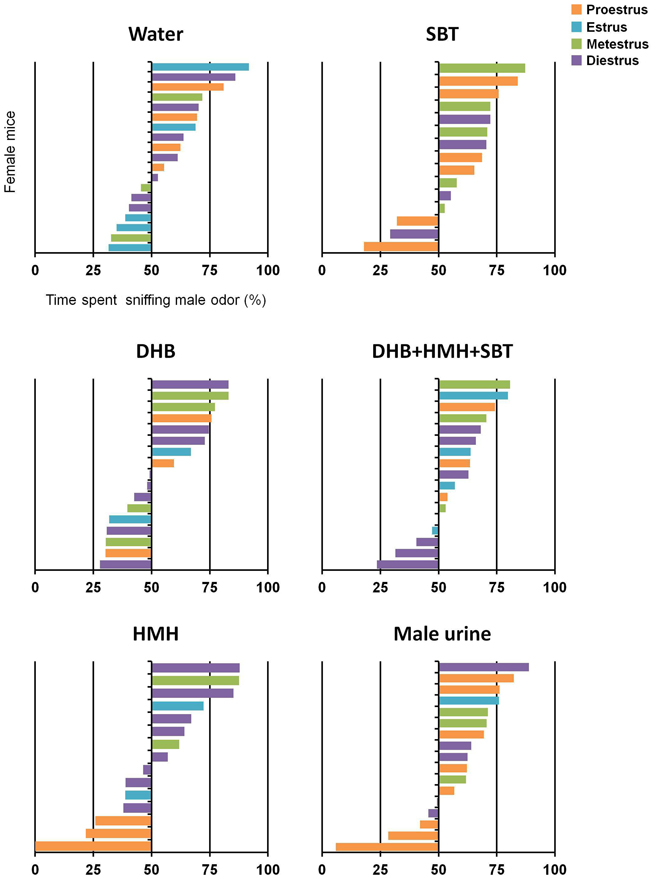
Figure 4. Male-directed odor preference according estrous cycle stage. Percentage of time spent sniffing male odor compared to total time spent sniffing both male and female odors at PD45 by each female Swiss mice in each group (water, DHB, HMH, SBT, DHB+HMH+SBT or male urine). Orange bars represent mice in proestrus. Blue bars represent mice in estrus. Green bars represent mice in metestrus. Purple bars represent mice in diestrus.
Discussion
The present study demonstrates that the combined peripubertal exposure to DHB, HMH and SBT, in addition to accelerating vaginal opening, also induced expression of male-directed odor preference in female mice in adulthood. Interestingly, each of these three compounds accelerated vaginal opening but was inefficient in stimulating male-directed odor preference. In accordance with our previous study (Jouhanneau et al., 2014), these present results confirmed that female attraction to male odor is not the consequence of the acceleration of vaginal opening by male chemosignals.
Early studies suggested that the male chemosignal responsible for puberty acceleration was a peptide bound to a protein (Vandenbergh et al., 1975, 1976). Then, volatile nature of chemosignal was considered when Novotny et al. (1984) isolated the MUP ligand DHB in the fraction active for puberty acceleration. DHB and also HMH and SBT accelerated uterine growth in prepubertal female ICR(CD-1) mice to a level comparable to the effect of male urine (Novotny et al., 1999b). HMH also increased uterine weight in (SJL/J × SWR/J) F1 hybrid mice (Novotny et al., 1999a). Here, we showed that DHB, HMH and SBT accelerated vaginal opening in another mouse strain derived from the Swiss lineage (Lynch, 1969; Beck et al., 2000; Yalcin et al., 2010). In Swiss mice, each compound was as efficient as male urine to accelerate vaginal opening and the mixture of the three compounds had no synergistic effect. However, no effect of MUP ligands on uterine weight has been reported in Swiss mice (Mucignat-Caretta et al., 1995). Future research will need to determine the reasons for this discrepancy. Moreover, the effect of MUP ligands on puberty acceleration appears to be lineage-specific. Indeed, in contrast to male urine, DHB and SBT did not accelerate uterine growth in female BALB/cJ mice (Flanagan et al., 2011), which are derived from the Castle lineage (Beck et al., 2000). According to Flanagan et al. (2011), an unknown nonvolatile compound present in male urine would participate in the acceleration of puberty onset in female mice.
Additionally, the present study reported that peripubertal exposure to male urine also induced a preference for male over female odors in female mice when they were adults. In contrast, female mice exposed to water did not show attraction to male odor. These results confirmed those of our previous study showing that female mice expressed a male-directed odor preference at PD45 after peripubertal exposure to male-soiled bedding whereas females exposed to clean bedding did not (Jouhanneau et al., 2014). Furthermore, the present findings revealed that DHB, HMH and SBT are sufficiently responsible for the expression of male-directed odor preference when used in combination. Our chemical analyses showed that SBT is exclusively produced in urine of male Swiss mice and DHB and HMH levels are higher in male urine than in female urine. Hence, female mice exposed to a mixture DHB+HMH+SBT were attracted to male odor probably because male urine is the only stimulus which releases these three compounds. Similarly, female mice exposed to male urine were attracted to male odor because they probably perceived the attractive mixture DHB+HMH+SBT in male urine. As female mice exposed to water did not display male-directed odor preference, we hypothesized that the attraction to the mixture DHB+HMH+SBT was learned during the peripubertal exposure to male odor. An alternative hypothesis would be that groups exposed to the mixture or to male urine expressed a preference for male odor because their hormonal status was different than those of control group. However, analysis of individual data revealed that estrous cycle stage had no effect on expression of male-directed odor preference. In agreement with this finding, Moncho-Bogani et al. (2004) demonstrated the hormonal independence of male-directed odor preference. Indeed, female mice still preferred investigating the odor of male mouse rather than the odor of female mouse following ovariectomy.
Moreover, our findings showed that exposure to each individual compound of the mixture (i.e., DHB or HMH or SBT) were not enough to induce female attraction to male odor. It suggests that at least two compounds in the mixture DHB+HMH+SBT act together to release female attraction to male odor. Interestingly, Jemiolo et al. (1985) reported that DHB and SBT released no behavioral response when they were presented separately to adult female mice but were attractive when they mixed together; therefore the combined exposure to DHB+SBT in the mixture could be responsible for the induction of male-directed odor preference observed in the present study.
The induction of male-directed odor preference after prior exposure to some volatile compounds of male urine reported in the present study contradicts previous findings (Ramm et al., 2008; Roberts et al., 2010). Indeed, these authors showed a lack of female attraction to male odor after prior exposure to the volatile fraction of male urine. The differences in methods of exposure to the odorant stimulus could explain the discrepancies in results. Indeed, in the present study, female mice had physical contact with the odorant stimulus since it was applied on their oronasal groove. In contrast, in previous studies, volatile components of male urine freely diffused into the cage. Allowing female mice to have direct physical contact with male-soiled bedding activated the vomeronasal organ (VNO), whereas preventing direct contact to male-soiled bedding did not (Kimoto et al., 2005). This suggests that airborne molecules from male-soiled bedding are not detected by the VNO in female mice. In the light of these findings, we hypothesized that the activation of the accessory olfactory system through physical contact with the liquid mixture DHB+HMH+SBT during peripubertal exposure was probably responsible for the acquisition of an attraction response to these three compounds in female mice. To support this hypothesis, it has been previously reported in female mice that 1/DHB, HMH and SBT individually activated VNO neurons in vitro (Moss et al., 1997; Zhou and Moss, 1997; Leinders-Zufall et al., 2000; Del Punta et al., 2002); 2/the mixture DHB+SBT applied on the oronasal groove induced Egr-1 expression in the accessory olfactory bulb (AOB) (Brennan et al., 1999) and 3/bilateral lesion of the AOB before exposure to male-soiled bedding in adulthood disrupted subsequent expression of male-directed odor preference (Martínez-Ricós et al., 2008).
However, the contribution of the main olfactory system in induction of male-directed odor preference cannot be excluded. Indeed, destruction of the main olfactory epithelium (MOE) within 1 week before the behavioral test abolished expression of male-directed odor preference in female mice (Keller et al., 2006a) whereas ablation of the VNO did not (Keller et al., 2006b). Interestingly, airborne urinary volatiles from male mice activated the AOB in female mice through the MOE-main olfactory bulb-medial amygdala-AOB pathway (Martel and Baum, 2007, 2009; Kang et al., 2009). Identity of the volatiles that activate this pathway has not yet been determined but it was previously reported that DHB, HMH and SBT, in addition to activating VNO neurons, also activated MOE neurons in vitro (Ziesmann et al., 2002). Hence, after acquisition of attraction response to the odor mixture by VNO activation during odor exposure, DHB, HMH and SBT emanating from male urine probably induced appetitive memory recall through MOE activation during the behavioral test; which resulted in induction of male-directed odor preference. Destruction of the MOE or the VNO in female mice before peripubertal exposure to a mixture of DHB+HMH+SBT or before the behavioral test will provide insight into the role of each olfactory sense organ in mediating the effect of MUP ligands on male-directed odor preference.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Claude Cahier, Marine Cirot and Deborah Crespin for taking good care of the animals. This work was supported by French National Research Agency (ANR) grant ANR-PHEROSEX; Mélanie Jouhanneau is a Ph.D. student supported by INRA PHASE Department and Région Centre. Dr. Matthieu Keller is a CNRS permanent research fellow.
Supplementary Material
The Supplementary Material for this article can be found online at: http://www.frontiersin.org/journal/10.3389/fevo.2015.00034/abstract
Abbreviations
AOB, accessory olfactory bulb; DHB, (1R, 5S, 7R)-3,4-dehydro-exo-brevicomin; HMH, 6-hydroxy-6-methyl-3-heptanone; MOE, main olfactory epithelium; PD, postnatal day; SBT, (S)-2-sec-butyl-4,5-dihydrothiazole; VNO, vomeronasal organ.
References
Bacchini, A., Gaetani, E., and Cavaggioni, A. (1992). Pheromone binding proteins of the mouse, Mus musculus. Experientia 48, 419–421. doi: 10.1007/BF01923448
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Beck, J. A., Lloyd, S., Hafezparast, M., Lennon-Pierce, M., Eppig, J. T., Festing, M. F., et al. (2000). Genealogies of mouse inbred strains. Nat. Genet. 24, 23–25. doi: 10.1038/71641
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Brennan, P. A., Schellinck, H. M., and Keverne, E. B. (1999). Patterns of expression of the immediate-early gene egr-1 in the accessory olfactory bulb of female mice exposed to pheromonal constituents of male urine. Neuroscience 90, 1463–1470. doi: 10.1016/S0306-4522(98)00556-9
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Bronson, F. H. (1975). Male-induced precocial puberty in female mice: confirmation of the role of estrogen. Endocrinology 96, 511–514. doi: 10.1210/endo-96-2-511
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Bruce, H. M. (1959). An exteroceptive block to pregnancy in the mouse. Nature 184, 105. doi: 10.1038/184105a0
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Colby, D. R., and Vandenbergh, J. G. (1974). Regulatory effects of urinary pheromones on puberty in the mouse. Biol. Reprod. 11, 268–279. doi: 10.1095/biolreprod11.3.268
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Del Punta, K., Leinders-Zufall, T., Rodriguez, I., Jukam, D., Wysocki, C. J., Ogawa, S., et al. (2002). Deficient pheromone responses in mice lacking a cluster of vomeronasal receptor genes. Nature 419, 70–74. doi: 10.1038/nature00955
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Flanagan, K. A., Webb, W., and Stowers, L. (2011). Analysis of male pheromones that accelerate female reproductive organ development. PLoS ONE 6:e16660. doi: 10.1371/journal.pone.0016660
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Hurst, J. L. (1990). Urine marking in populations of wild house mice Mus domesticus Rutty. III. Communication between the sexes. Anim. Behav. 40, 233–243. doi: 10.1016/S0003-3472(05)80918-2
Hurst, J. L., Payne, C. E., Nevison, C. M., Marie, A. D., Humphries, R. E., Robertson, D. H., et al. (2001). Individual recognition in mice mediated by major urinary proteins. Nature 414, 631–634. doi: 10.1038/414631a
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Jemiolo, B., Alberts, J., Sochinski-Wiggins, S., Harvey, S., and Novotny, M. (1985). Behavioural and endocrine responses of female mice to synthetic analogues of volatile compounds in male urine. Anim. Behav. 33, 1114–1118. doi: 10.1016/S0003-3472(85)80170-6
Jouhanneau, M. (2014). Accélération de la Puberté par Les Phéromones Mâles chez la Souris Femelle: Régulation des Neurones à Kisspeptine et Conséquences à Long terme sur le Comportement Sexuel. Doctoral dissertation, Université François-Rabelais, Tours, France.
Jouhanneau, M., Cornilleau, F., and Keller, M. (2014). Peripubertal exposure to male odors influences female puberty and adult expression of male-directed odor preference in mice. Horm. Behav. 65, 128–133. doi: 10.1016/j.yhbeh.2013.12.006
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kang, N., Baum, M. J., and Cherry, J. A. (2009). A direct main olfactory bulb projection to the ‘vomeronasal’ amygdala in female mice selectively responds to volatile pheromones from males. Eur. J. Neurosci. 29, 624–634. doi: 10.1111/j.1460-9568.2009.06638.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Keller, M., Douhard, Q., Baum, M. J., and Bakker, J. (2006a). Destruction of the main olfactory epithelium reduces female sexual behavior and olfactory investigation in female mice. Chem. Senses 31, 315–323. doi: 10.1093/chemse/bjj035
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Keller, M., Pierman, S., Douhard, Q., Baum, M. J., and Bakker, J. (2006b). The vomeronasal organ is required for the expression of lordosis behaviour, but not sex discrimination in female mice. Eur. J. Neurosci. 23, 521–530. doi: 10.1111/j.1460-9568.2005.04589.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kimoto, H., Haga, S., Sato, K., and Touhara, K. (2005). Sex-specific peptides from exocrine glands stimulate mouse vomeronasal sensory neurons. Nature 437, 898–901. doi: 10.1038/nature04033
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Leinders-Zufall, T., Lane, A. P., Puche, A. C., Ma, W., Novotny, M. V., Shipley, M. T., et al. (2000). Ultrasensitive pheromone detection by mammalian vomeronasal neurons. Nature 405, 792–796. doi: 10.1038/35015572
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Liebich, H. M., Zlatkis, A., Bertsch, W., Van Dahm, R., and Whitten, W. K. (1977). Identification of dihydrothiazoles in urine of male mice. Biomed. Mass Spectrom. 4, 69–72. doi: 10.1002/bms.1200040202
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lombardi, J. R., Vandenbergh, J. G., and Whitsett, J. M. (1976). Androgen control of the sexual maturation pheromone in house mouse urine. Biol. Reprod. 15, 179–186. doi: 10.1095/biolreprod15.2.179
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Martel, K. L., and Baum, M. J. (2007). Sexually dimorphic activation of the accessory, but not the main, olfactory bulb in mice by urinary volatiles. Eur. J. Neurosci. 26, 463–475. doi: 10.1111/j.1460-9568.2007.05651.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Martel, K. L., and Baum, M. J. (2009). A centrifugal pathway to the mouse accessory olfactory bulb from the medial amygdala conveys gender-specific volatile pheromonal signals. Eur. J. Neurosci. 29, 368–376. doi: 10.1111/j.1460-9568.2008.06564.x
Martínez-Ricós, J., Agustín-Pavón, C., Lanuza, E., and Martínez-García, F. (2008). Role of the vomeronasal system in intersexual attraction in female mice. Neuroscience 153, 383–395. doi: 10.1016/j.neuroscience.2008.02.002
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Moncho-Bogani, J., Lanuza, E., Lorente, M. J., and Martínez-García, F. (2004). Attraction to male pheromones and sexual behaviour show different regulatory mechanisms in female mice. Physiol. Behav. 81, 427–434. doi: 10.1016/j.physbeh.2004.01.014
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Moss, R. L., Flynn, R. E., Shen, X. M., Dudley, C., Shi, J., and Novotny, M. (1997). Urine-derived compound evokes membrane responses in mouse vomeronasal receptor neurons. J. Neurosci. 77, 2856–2862.
Mucignat-Caretta, C., Caretta, A., and Cavaggioni, A. (1995). Acceleration of puberty onset in female mice by male urinary proteins. J. Physiol. 486, 517–522. doi: 10.1113/jphysiol.1995.sp020830
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Novotny, M., Harvey, S., Jemiolo, B., and Alberts, J. (1985). Synthetic pheromones that promote inter-male aggression in mice. Proc. Natl. Acad. Sci. U.S.A. 82, 2059–2061. doi: 10.1073/pnas.82.7.2059
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Novotny, M., Schwende, F. J., Wiesler, D., Jorgenson, J. W., and Carmack, M. (1984). Identification of a testosterone-dependent unique volatile constituent of male mouse urine: 7-exo-ethyl-5-methyl-6,8-dioxabicyclo[3.2.1]-3-octene. Experientia 40, 217–219. doi: 10.1007/BF01963608
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Novotny, M. V., Jemiolo, B., Wiesler, D., Ma, W., Harvey, S., Xu, F., et al. (1999a). A unique urinary constituent, 6-hydroxy-6-methyl-3-heptanone, is a pheromone that accelerates puberty in female mice. Chem. Biol. 6, 377–383. doi: 10.1016/S1074-5521(99)80049-0
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Novotny, M. V., Ma, W., Wiesler, D., and Zidek, L. (1999b). Positive identification of the puberty-accelerating pheromone of the house mouse: the volatile ligands associating with the major urinary protein. Proc. R. Soc. B 266, 2017–2022. doi: 10.1098/rspb.1999.0880
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Ramm, S. A., Cheetham, S. A., and Hurst, J. L. (2008). Encoding choosiness: female attraction requires prior physical contact with individual male scents in mice. Proc. R. Soc. B 275, 1727–1735. doi: 10.1098/rspb.2008.0302
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Roberts, S. A., Simpson, D. M., Armstrong, S. D., Davidson, A. J., Robertson, D. H., McLean, L., et al. (2010). Darcin: a male pheromone that stimulates female memory and sexual attraction to an individual male's odour. BMC Biol. 8:75. doi: 10.1186/1741-7007-8-75
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Robertson, D. H. L., Beynon, R. J., and Evershed, R. P. (1993). Extraction, characterization, and binding analysis of two pheromonally active ligands associated with major urinary protein of house mouse (Mus Musculus) J. Chem. Ecol. 19, 1405–1416. doi: 10.1007/BF00984885
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Schwende, F. J., Wiesler, D., Jorgenson, J. W., Carmack, M., and Novotny, M. (1986). Urinary volatile constituents of the house mouse, Mus musculus, and their endocrine dependency. J. Chem. Ecol. 12, 277–296. doi: 10.1007/BF01045611
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sharrow, S. D., Vaughn, J. L., Zidek, L., Novotny, M. V., and Stone, M. J. (2002). Pheromone binding by polymorphic mouse major urinary proteins. Protein Sci. 11, 2247–2256. doi: 10.1110/ps.0204202
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Tashiro, T., and Mori, K. (1999). Synthesis of the Enantiomers of 2-sec-Butyl-4,5-dihydrothiazole and (1R,5S,7R)-3,4-Dehydro-exo-brevicomin, Pheromone components of the male mouse, Mus musculus. European J. Org. Chem. 1999, 2167–2173.
Tashiro, T., Osada, K., and Mori, K. (2008). Syntheses of 2-Isopropyl-4,5-dihydrothiazole and 6-Hydroxy-6-methyl-3-heptanone, pheromone components of the male mouse, Mus musculus. Biosci. Biotechnol. Biochem. 72, 2398–2402. doi: 10.1271/bbb.80293
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Vandenbergh, J. G. (1967). Effect of the presence of a male on the sexual maturation of female mice. Endocrinology 81, 345–349. doi: 10.1210/endo-81-2-345
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Vandenbergh, J. G. (1969). Male odor accelerates female sexual maturation in mice. Endocrinology 84, 658–660. doi: 10.1210/endo-84-3-658
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Vandenbergh, J. G., Finlayson, J. S., Dobrogosz, W. J., Dills, S. S., and Kost, T. A. (1976). Chromatographic separation of puberty accelerating pheromone from male mouse urine. Biol. Reprod. 15, 260–265. doi: 10.1095/biolreprod15.2.260
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Vandenbergh, J. G., Whitsett, J. M., and Lombardi, J. R. (1975). Partial isolation of a pheromone accelerating puberty in female mice. J. Reprod. Fertil. 43, 515–523. doi: 10.1530/jrf.0.0430515
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Whitten, W. K. (1956). Modification of the oestrous cycle of the mouse by external stimuli associated with the male. J. Endocrinol. 13, 399–404. doi: 10.1677/joe.0.0130399
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Yalcin, B., Nicod, J., Bhomra, A., Davidson, S., Cleak, J., Farinelli, L., et al. (2010). Commercially available outbred mice for genome-wide association studies. PLoS Genet. 6:e1001085. doi: 10.1371/journal.pgen.1001085
Zhou, A., and Moss, R. L. (1997). Effect of urine-derived compounds on cAMP accumulation in mouse vomeronasal cells. Neuroreport 8, 2173–2177. doi: 10.1097/00001756-199707070-00017
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Keywords: puberty, sexual behavior, male-female interaction, chemical communication, 3,4-dehydro-exo-brevicomin, 6-hydroxy-6-methyl-3-heptanone, 2-sec-butyl-4,5-dihydrothiazole
Citation: Jouhanneau M, Goudet C, Moussu C, Tashiro T, Buatois B, Mori K, Ganem G and Keller M (2015) Peripubertal exposure to male chemosignals accelerates vaginal opening and induces male-directed odor preference in female mice. Front. Ecol. Evol. 3:34. doi: 10.3389/fevo.2015.00034
Received: 28 October 2014; Accepted: 12 March 2015;
Published: 30 March 2015.
Edited by:
Juergen Gross, Julius Kühn-Institut, GermanyReviewed by:
Michael Baum, Boston University, USAKevin R. Theis, Michigan State University, USA
Carla Mucignat, University of Padova, Italy
Copyright © 2015 Jouhanneau, Goudet, Moussu, Tashiro, Buatois, Mori, Ganem and Keller. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Matthieu Keller, INRA Val de Loire, UMR Physiologie de la Reproduction et des Comportements, Equipe Neuroendocrinologie des Interactions et Comportements Sexuels, 37380 Nouzilly, France mkeller@tours.inra.fr
 Mélanie Jouhanneau
Mélanie Jouhanneau Camille Goudet
Camille Goudet Chantal Moussu1
Chantal Moussu1  Takuya Tashiro
Takuya Tashiro Matthieu Keller
Matthieu Keller