Niche separation of pollen beetle parasitoids
- 1Soil Ecology Group, Department of Biology, Lund University, Lund, Sweden
- 2Chemical Ecology Group, Department of Plant Protection Biology, Swedish University of Agricultural Sciences, Alnarp, Sweden
Species with similar resource requirements are commonly assumed to competitively exclude each other, unless they differentiate their ecological niches. Hence, parasitoid wasps that use the same host species need to find some way to avoid competition. The aim of this study was to identify the role of volatile cues from oilseed rape plants and the larval host in niche separation between three coexisting parasitoid species. We examined how Phradis interstitialis, Phradis morionellus and Tersilochus heterocerus, sympatric parasitoids of Brassicogethes aeneus, differ in their abundances, distribution on buds and flowers, and oviposition behavior in the field. Furthermore, we tested their preferences for odors from uninfested and infested oilseed rape plants in the bud and flowering stage, and their preferences for odors from three developmental stages of pollen beetle larvae in a two-choice olfactometer bioassay. P. interstitialis was active in the field early in the season, preferred odors of infested buds vs. uninfested, and oviposited into buds which contained only pollen beetle eggs, while P. morionellus was active late in the season, preferred odors of infested buds as well as odors of infested flowers over uninfested, and oviposited into buds which contained only larvae. T. heterocerus was active throughout the season, and preferred odors of infested flowers over uninfested. Neither Phradis species were attracted to larval odors, whereas T. heterocerus was attracted to odors from first-instar pollen beetle larvae both in the absence of plant odors, and when presented simultaneously with uninfested plant odor. This suggests that the two Phradis species are separated on a temporal scale and that they parasitize different host stages, while the larval parasitoids P. morionellus and T. heterocerus are separated by choice of microhabitat. The former oviposits into larvae in buds, and the latter in flowers.
Introduction
Strategies used by hymenopterous parasitoids for the location of herbivorous hosts vary largely and depend on e.g., host life stage attacked and host life style (Vet et al., 1995; Vinson, 1998). Generally, host selection behavior of hymenopterous parasitoids can be structured into habitat location, host location, host recognition and host acceptance, ultimately leading to oviposition (Vinson, 1976). Parasitoids are known to employ all their senses in this process, using highly volatile chemicals as long-distance attractants, and further olfactory, gustatory, visual and vibrational cues as short distance stimulants (Vinson, 1998). Most strategies involve odors from plants attacked by their host (Fatouros et al., 2012; Reddy, 2012; Wäschke et al., 2013). Many plants change their odor emission after damage and release a number of volatiles that are induced specifically in response to herbivore oviposition (Dicke and Baldwin, 2010; Hilker and Meiners, 2011) and larval feeding (Poelman et al., 2009). These compounds are reliable cues since they indicate immediate host presence, and are thus important in attraction of natural enemies to arthropod herbivores (De Moraes et al., 1998; Dicke et al., 2003; Turlings and Wäckers, 2004; Reddy, 2012). Natural selection should therefore, favor the ability of parasitoids to differentiate between infested and uninfested plant parts by odors.
As classical ecological theory predicts that two or more species cannot coexist when competing for a single limited resource, it is commonly assumed that species with similar resource requirements need to differentiate their use of resources (Hardin, 1960; Meszéna et al., 2006; Kalmykov and Kalmykov, 2013). Parasitoid wasps that attack and develop in the same host can achieve this e.g., by temporal and spatial separation (Comins and Hassel, 1996; Hackett-Jones et al., 2009; Sachet et al., 2009), specialization on different developmental stages of the host (Briggs et al., 1993; Yamamoto et al., 2007), specialization on different microhabitats (Fleury et al., 2009), differential host detection behavior (van Dijken and van Alphen, 1998), or wasp life history parameters (Bonsall et al., 2002).
Oilseed rape (OSR), Brassica napus L. (Brassicaceae), is commonly infested by the pollen beetle, Brassicogethes aeneus (syn. Meligethes aeneus), which oviposits only in buds of crucifers and whose two larval instars (Osborne, 1965; Nilsson, 1988) and adult beetles feed on pollen in the buds and flowers. The immature stages of the pollen beetle are attacked by at least nine species of parasitoid Hymenoptera, out of which Tersilochus heterocerus, Phradis interstitialis and Phradis morionellus (Ichneumonidae: Tersilochinae) are the most abundant and common in many parts of northern and central Europe (Nilsson, 2003). These three are koinobiont endoparasitoids, i.e., they don't hatch from their egg until shortly before their host has finished feeding and is ready to pupate (Nilsson, 2003). It is likely that they are competitors, since multiparasitism is frequently reported (Osborne, 1960; Nilsson and Andreasson, 1987; Williams, 2006), i.e., more than one parasitoid species oviposits into the same host larva, but only one of them will finally develop and emerge from the host pupa. There is thus potential for both intrinsic competition (i.e., competition among parasitoid larvae for host resources) and extrinsic competition (i.e., competition among adult parasitoid females) (Harvey et al., 2013).
The three closely related parasitoids of the pollen beetle have been reported to occur together in winter OSR in Southern Sweden (Nilsson, 1985; Jönsson et al., 2004). Their host stage preferences are not fully understood, though P. interstitialis has a long and slender ovipositor and has been suggested to oviposit in eggs and first instar larvae (Osborne, 1960), while T. heterocerus females are frequently found in flowering OSR and are assumed to prefer to oviposit in large second-instar larvae (Nilsson and Andreasson, 1987). The host-stage preference of P. morionellus has not been studied, but based on their high abundance during OSR flowering, it is generally assumed that they have similar preferences to T. heterocerus and attack large second-instar larvae in the flowers (Nilsson, 2003).
There is evidence that P. interstitialis locates its host habitat by using upwind anemotaxis, probably responding to odor cues that are released from OSR plants and carried downwind from the crop (Williams et al., 2007), and this might be true for all three species. But after finding the host habitat and landing on the host plant, a parasitoid female must still locate its host. While earlier literature suggested that this happens by random walking, host finding can be better understood by the search for physical and chemical cues of host presence, under the assumption that a parasitoid using such cues would have a reproductive advantage over parasitoids that search randomly (Vinson, 1976; Casas, 2000). For pollen beetle parasitoids, there is evidence for the use of OSR odors and visual stimuli: Jönsson et al. (2005) found that P. morionellus avoided odors of flowering OSR and was attracted to bud odors similarly to P. interstitialis, while T. heterocerus was attracted to flower stimuli. This suggests that P. morionellus and T. heterocerus differ more than was supposed by earlier workers, and that the host and plant stage preferences of these three related species deserve closer attention.
The aim of this study was to identify the role of volatile cues from OSR and the larval host in niche separation between these three coexisting parasitoid species. We investigated (1) whether they have differential phenologies during the field season, (2) whether they show selective oviposition behavior on buds and flowers in the field, (3) whether they have differential microhabitat preferences, reflected by olfactory discrimination of infested and uninfested buds and flowers in outdoor conditions, (4) whether they have differential host stage preferences, reflected by olfactory discrimination of isolated living immature stages of the pollen beetle under laboratory conditions, and (5) whether the host stage preference for isolated living immature stages under laboratory conditions is affected by OSR as a background odor.
Material and Methods
Insects
Adult pollen beetles and parasitoids were collected with sweepnet and aspirator in unsprayed OSR fields at Lönnstorp Experimental Farm owned by the Swedish Agriculture University Alnarp in southern Sweden (N55°40.15′ E13°06.51′). Parasitoids collected for outdoor bioassays were tested within 4 h after collection; parasitoids collected for laboratory bioassays were kept indoors at 12°C in ventilated plastic boxes containing wet tissue paper. A freshly cut OSR inflorescence and fresh honey ad libitum was added twice a week. The animals were tested within 2 weeks. After each experiment, they were stored in Eppendorf cups filled with 70% ethanol for later identification to species according to taxonomic literature (Horstmann, 1971, 1981).
The parasitoids were collected to perform three types of bioassays: (1) Outdoor bioassays testing odors from infested and uninfested OSR plants, (2) laboratory bioassays testing odors from isolated pollen beetle larvae and eggs, and (3) laboratory bioassays presenting pollen beetle larvae together with uninfested OSR plants.
Pollen beetle eggs and larvae for bioassays 2 and 3 were collected in the field from OSR plants. Eggs and first instar larvae were obtained by dissection of buds. Second instar larvae were swept from flowers.
Plants
For plant odor bioassays, spring-sown double-low OSR plants (cv. Maskot, Svalöf Weibull) were individually grown from seed in 3-liter pots. The soil was fertilized at the time of planting with a 3- to 4-month formulation of a controlled-release NPK-fertilizer (Osmocote®, Scotts Europe, Bayer Home & Garden, Lomma, Sweden). The pots were kept in a greenhouse chamber at 20°C during daytime (9 a.m. to 19 p.m.) and 15°C during night-time, and at 70 ± 10% r.h. In addition to natural light, artificial light (Philips, SGR 050-400, T400W) was provided between 6 a.m. and 10 p.m. Plants were infested at the bud stage by keeping them in fine mesh cages together with 50–100 pollen beetles for 2 days. Control plants were kept in cages without beetles. The 20 cm upper part of one plant in either bud stage 55–59 or in mid-flowering stage 65–67 according to the BBCH scale (Lancashire et al., 1991) were used. Plants in the bud stage were used on the same day the infestation period ended. The adult beetles were taken off the plants immediately before the start of experiments. After the experiments, buds of the infested plants used were dissected to confirm the presence of pollen beetle eggs and/or larvae. The flowering plants were used 6–10 days after infestation with adult beetles and infested plants had 3–8 visible pollen beetle larvae. The flowering plants had approximately 15 flowers and 10 buds. The number of flowers of plant pairs (infested/uninfested) compared in bioassays never differed by more than two.
For the larval stage bioassays, winter OSR plants were collected from the field, prior to the arrival of pollen beetles: In March 2012, young plants which had not yet reached bud stage (BBCH 30–33) were transplanted from the Lönnstorp field to 4-liter pots and subsequently grown in the greenhouse of Lund University. The pots were kept at 16°C during daytime (6 a.m. to 10 p.m.) and 14°C during night time. In addition to natural light, artificial light was provided during daytime. Plants were grown in the greenhouse until they reached flowering stage. Their uppermost 15 cm were selected for an equal ratio of buds and flowers, and used as background odor source.
Phenology
To quantify the phenologies of three parasitoid species, their abundances in sweepnet catches were compared for every week between 4 May and 21 June 2011; during this time span, winter OSR developed from bud stage to seedpod maturation. The sweepnetting was done by the same operator, using three tractor tracks as transect lines, and taking 10 semicircular sweeps in each.
Field Observations
Ovipositing behavior of female parasitoids was observed in winter OSR and in summer OSR. The first period was 6 days during 3–11 June, 2004 in a late developed part of a flowering winter OSR field (BBCH 65–68) at Lönnstorp. The second observation period was during 1–7 July, 2004 at two summer OSR fields in the same growth stages as the early period: For 2 days at Lönnstorp and for another 2 days at a farm in Dalby (N55°40.54′ E13°20.68′). During both periods, the observations were made either 9 a.m. to 12 a.m. or 1 p.m. to 5 p.m., with equal numbers of observations undertaken in the morning and the afternoon.
Two categories of female parasitoids were collected, namely those observed to insert or probe with their ovipositor in buds and all female parasitoids observed on flowers, independent of their activity. Females observed to be inserting their ovipositor into buds were collected and stored for later identification, together with the bud they penetrated. These buds were later dissected under the microscope and the number of pollen beetle eggs and larvae counted. The majority of these eggs were placed on glass micro-slides and colored using green food coloring containing quinoline yellow and patent blue (Ekströms®, Procordia Food AB, Eslöv, Sweden). When the dye had passed the pollen beetle egg chorion and colored the interior green, the preparations were examined under the microscope. Parasitoid eggs were identified as brighter structures within the pollen beetle eggs.
Bioassays
Several olfactory bioassays were performed using Y-shaped glass olfactometers (see below). In all bioassays, the parasitoids not making a choice within 5 min were counted as non-responding. Experimental days with more than 50% non-responding parasitoids were excluded from the dataset. The bottles used for stimuli sources, the tubing and the Y-tube were rinsed with ethanol after each session. In addition, glassware was heated to 350°C between experimental days. To avoid the effects of positional preferences, the position of odor sources was shifted between sessions. To minimize effects of day-to-day variation in parasitoid behavior, the experiments were performed on several days.
Odors from Infested and Uninfested Plants
Odors from infested and uninfested OSR plants were assayed in a mobile out-door Y-tube olfactometer similar to that described by Jönsson et al. (2005). This consisted of a Y-shaped glass tube, with 22 cm long arms and 1.5 cm inner diameter. The Y-tube was fixed and positioned with the side arms slightly upwards (15° angle). To avoid visual distraction, the Y-tube was placed inside a box of white fabric (40 × 40 × 50 cm) and the whole set-up was placed on a trolley and regularly moved to assure symmetrical sunlight from behind the trolley. Pieces of black tubing (2 cm long) were placed around each of the three arms at the Y-junction. Air was pumped (Micro pump NMP 30 KNDC, 12 V, KNF Neuberger) into a 250 ml gas wash bottle with activated charcoal and thereafter divided into two lines of Teflon tubing via two flow meters (BA-4AR, Kytölä, Finland) leading to the two gas wash bottles (250 ml) containing either the uppermost 20 cm of three plants or no plants. Both gas wash bottles received circa 10 ml of water. From the two gas wash bottles the air was delivered at a rate of 0.3 l/min to each arm of the olfactometer. The bioassays ended within 4 h after plants had been cut. Following stimuli combinations were tested: (1) Infested OSR in the bud stage vs. uninfested OSR in the bud stage (all three parasitoid species.) (2) Infested OSR in the flowering stage vs. uninfested OSR in the flowering stage (P. morionellus and T. heterocerus). Female parasitoids were released individually in the central arm and allowed to walk up and choose one arm. Parasitoids that walked 7 cm up into one of the arms were considered to have made a choice.
Immature Stages of the Host
Odors from immature stages of the pollen beetle were assayed in a laboratory Y-shaped olfactometer similar to that described by Bengtsson et al. (1991). The Y-shaped glass tube had 15 mm diameter, 4 cm long arms (angle of 45°) and a 2 cm base. A release chamber of 2 cm length was connected with a metal clamp to the base of the Y-tube and its other end was connected by a Teflon tubing to a flowmeter and suction pump (Piab Labvac 32.16.009). Both arms of the Y-tube were connected by metal clamps to terminal chambers of 2 cm length. The terminal chambers were either left empty (control) or contained immature stages of the pollen beetle (stimulus). Each terminal chamber was sealed by a fine-meshed piece of polyamid fabric, to prevent physical contact between the parasitoids and the stimulus. The other end of each terminal chamber was connected by Teflon tubing to an air regulator tube to maintain constant air flow, and to a glass tube filled with activated charcoal to provide clean air. Experiments were performed in a laboratory at room temperature.
The Y-tube was positioned horizontally inside a white plastic box to avoid visual distraction. The following procedure was used for all laboratory bioassays: Locomotory active female parasitoid wasps were sucked up by an aspirator from their container, transferred to empty Petri dishes and allowed to acclimatize in the laboratory before the start of the experiments. When in the Petri dish, each wasp was covered by an upright standing release chamber and placed under a light source, until the parasitoid walked up into the chamber. The release chamber was then connected with the Y-tube and the parasitoid was allowed to walk in and choose an arm. A parasitoid which reached the terminal chamber at the end of an arm within 5 min was counted as having made a decision.
Twenty freshly isolated pollen beetle eggs, or 10 pollen beetle larvae, were used as stimuli. Only P. interstitialis, the suspected egg-larval parasitoid, was assayed for egg odors vs. purified air. The other two species were assayed for larval odors vs. purified air. Larvae were separated into three size categories: Smallest larvae (young first instar, 1–1.5 mm length), intermediate-sized larvae (older first instar, 2–2.5 mm length), and large larvae (second instar, 3–5 mm). When a preference for a particular larval size category against purified air was found in a parasitoid species, then this species was subjected to further experiments: All three larval stages were assayed vs. each other with this parasitoid species. In an additional experiment, a possible effect of footprints or feces left by larvae in the stimulus chamber was tested: Larvae were removed from the chamber after having been inside for at least 3 h and the emptied chamber was assayed against an unused acetone-rinsed chamber, both receiving only purified air.
Immature Stages of the Host + Uninfested Plants
As immature stage odors always cooccur with plant odors in nature, we further investigated host stage preference for living immature stages of the pollen beetle in the presence of a background odor from uninfested OSR as additional second stimulus. The same setup was used as in the previous bioassay, with pollen beetle larvae as primary stimulus and empty chamber as control. Both primary stimulus and control were enriched by a background odor from uninfested OSR plants grown in the greenhouse: A 0.5 liter glass bottle was added between terminal chamber and flowmeter, containing the uppermost 15 cm of a flowering OSR plant and 10 ml water. A ratio of approximately 10 buds and 10 flowers was prepared for every session. Each bioassay session was terminated within 4 h after the plants had been cut.
Statistical Analysis
The parasitoid preferences in the Y-tube bioassays were tested with the G-test of goodness-of-fit against an expected 50:50 ratio (McDonald, 2014a). The calculation was made using McDonald's G-test spreadsheet (McDonald, 2014b).
Differences in oviposition activity between species were calculated with the two-tailed binomial test (Sokal and Rohlf, 1995).
Results
Phenology
Abundance data from May and June 2011 show differential phenologies among the three parasitoids (Figure 1) P. interstitialis females were most abundant in early May, while P. morionellus females had their abundance peak in the first week of June. T. heterocerus females had their highest abundancies between 11 May and 7 June. All three species were found to co-occur in early June.
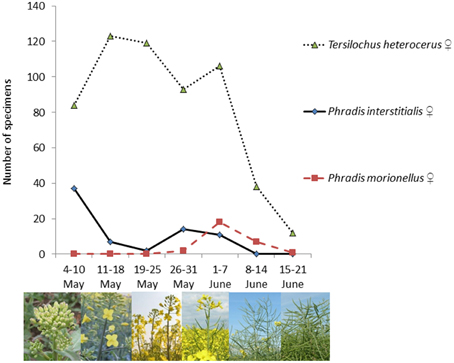
Figure 1. Phenology of three pollen beetle parasitoid species collected by sweepnetting at Lönnstorp, southern Sweden, in 2011.
Oviposition Behavior
During the field observations in June 2004 (winter OSR), P. interstitialis was the most abundant (69%) of all females collected (n = 160) of the three species, T. heterocerus the second most abundant species (29%), and P. morionellus was the least abundant (2%). P. interstitialis was the only species observed to probe with or insert its ovipositor into buds (n = 100) (Figure 2A). None of these buds had turned yellow and no fully hatched pollen beetle larvae were found in the buds, although both first and second instar pollen beetle larvae were frequent in the crop. Buds that were observed to be penetrated contained between 1 and 7 pollen beetle eggs (n = 15). Out of these eggs, 29 were examined and four had parasitoid eggs (0.2–0.3 mm long). All three species were collected from flowers (n = 60), and the most abundant was T. heterocerus (Figure 2C).
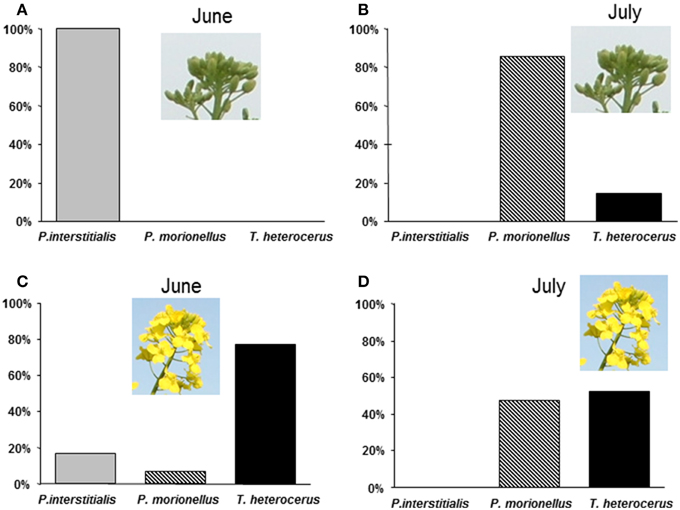
Figure 2. Percentages of three pollen beetle parasitoids observed either on buds probing with ovipositor or observed in flowers independently of activity. (A) Females collected from buds, June 3–11 (n = 100) (B) Females collected from buds in July 1–7 (n = 14) (C) Females collected from flowers, June 3–11 (n = 60) (D) Females collected from flowers, July 1–7 (n = 42).
During the second period of field observations in July 2004 (summer OSR), P. morionellus was the most frequent species (60%) of all females collected (n = 56), and relatively many T. heterocerus were found (40%), but no P. interstitialis. Among the females collected from flowers (n = 42), T. heterocerus and P. morionellus were equally abundant (Figure 2D). The number of P. morionellus found on buds was significantly higher than that of T. heterocerus found on buds (two-tailed Binomial test, p < 0.05, n = 14) (Figure 2B). In all buds except one, we found 1–5 pollen beetle larvae but no eggs. The buds that T. heterocerus was observed to probe had started to shift color from green to yellow, while P. morionellus probed both green and yellow buds. We could not quantify the parasitations on flowers as these can take place inside the flower and are difficult to observe.
Olfactory Discrimination
The two Phradis species significantly preferred odor of infested against uninfested OSR in the bud stage (Figure 3A). A similar preference was not observed for T. heterocerus. However, a statistically significant difference in preference between the three species was not found (G = 1.46; df = 2; p > 0.05). Both P. morionellus and T. heterocerus significantly preferred odors from infested flowering OSR over odors from uninfested flowering OSR (Figure 3B).
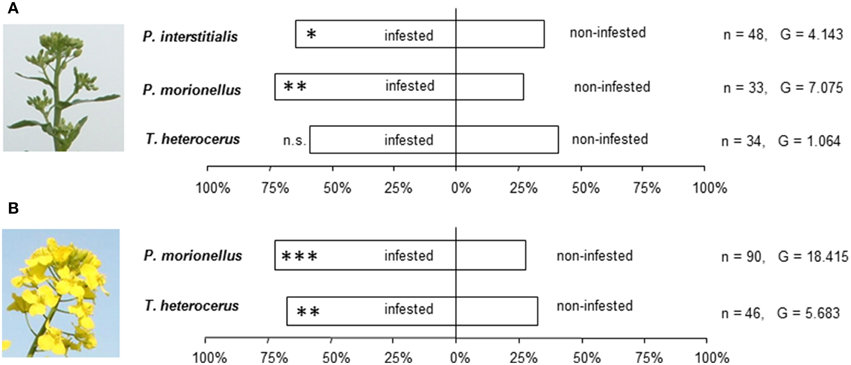
Figure 3. The response of pollen beetle parasitoids in Y-tube experiments to odors from infested and non-infested oilseed rape. (A) Bud stage. (B) Flowering stage. Asterisks indicate statistically significant differences in the number of females making a choice in a likelihood ratio test (n.s., not significant; *P < 0.05; **P < 0.01; ***P < 0.001). The number of responding females (n) and the G-values of the likelihood ratio test are given.
P. interstitialis females did not discriminate between odors from isolated pollen beetle eggs vs. purified air (n = 50; G = 2.908; df = 1; p = 0.088), small (n = 21; G = 0.43; df = 1; p = 0.512), or intermediate pollen beetle larvae (n = 26; G = 1.007; df = 1; p = 0.316). Furthermore, P. morionellus did not discriminate between purified air and intermediate-sized larvae (n = 23; G = 0.043; df = 1; p = 0.835) nor large larvae (n = 24; G = 0.167; df = 1; p = 0.683).
T. heterocerus females preferred the odor of intermediate-sized (older first instar) pollen beetle larvae against purified air (n = 73; G = 13.592; df = 1; p < 0.001), while no significant preference was found neither for the smallest larvae (n = 74; G = 1.355; df = 1; p = 0.244) nor for the largest larvae (n = 59; G = 2.063; p = 0.151) (Figure 4A). When larvae of different sizes were compared against each other, T. heterocerus preferred both the smallest larvae (n = 39; G = 5.921; df = 1; p = 0.015) and the intermediate larvae (n = 42; G = 6.252; df = 1; p = 0.012) against largest larvae (Figure 4B). No preference was found when comparing the smallest with intermediate larvae (n = 30; G = 0.535; df = 1; p = 0.465). We found no preference when comparing an empty acetone-rinsed chamber with an empty chamber containing larval footprints, feces or other larval secretions (n = 37; G = 1.332; df = 1; p = 0.248).
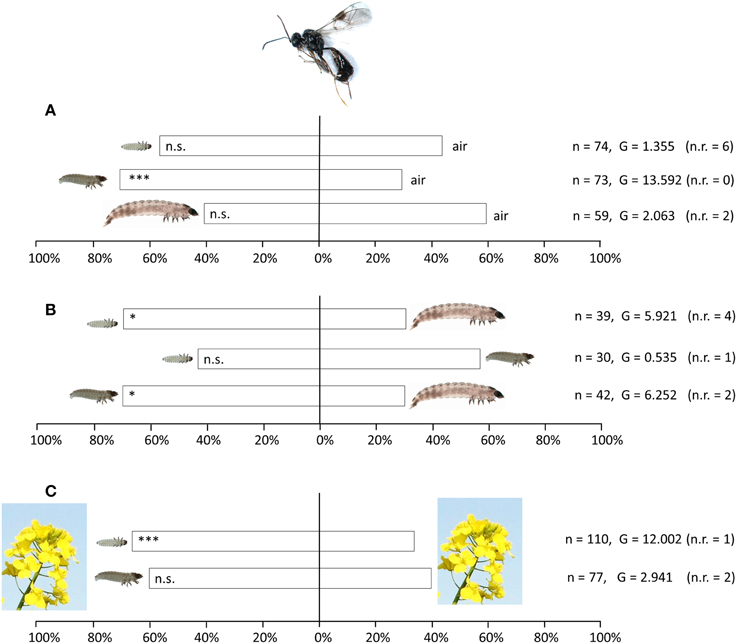
Figure 4. Response of Tersilochus heterocerus females in Y-tube experiments to odors from pollen beetle larvae in three size categories. (A) Larvae tested against purified air, (B) Larval size categories tested against each other, (C) Larvae and flowering oilseed rape tested against flowering oilseed rape only. Asterisks indicate statistically significant differences in a likelihood ratio test (n.s., not significant; *P < 0.05; ***P < 0.001). The number of responding females (n) and the G-values of the likelihood ratio test are given. To the right the number of not responding females (n.r.) is given in parentheses.
When flowering uninfested OSR was added as a background odor to the stimulus and the control (Figure 4C), T. heterocerus showed a highly significant preference for the odor of smallest larvae against plant odor without larvae (n = 110; G = 12.002; df = 1; p < 0.001). P. interstitialis did not discriminate between these two odor stimuli (n = 25; G = 1.986; df = 1; p = 0.159), and neither did P. morionellus (n = 25; G = 1.986; df = 1; p = 0.159). Furthermore, when the intermediate larval stage and plant odor were tested against plant odor only, no significant differences were detected in T. heterocerus (n = 77; G = 2.941; df = 1; p = 0.086), P. interstitialis (n = 16; G = 2.306; df = 1; p = 0.126) nor in P. morionellus (n = 32; G = 0; df = 1; p = 1).
Discussion
In this study we found interspecific differences in temporal activity peaks, plant part preferences and host stage preferences of the three pollen beetle parasitoids. These results indicate that coexistence between these species is based on temporal and behavioral niche separation.
Between the Phradis species, the niche separation was primarily found to be temporal, as there was overlap in their activity only during a short period in early June. P. interstitialis is most frequent early in the season when the buds are green, while P. morionellus is more frequent in late season when the buds are yellow and flowering. T. heterocerus was abundant throughout the entire season except very early and very late, which was in accordance with other studies (Jönsson et al., 2004; Klukowski, 2008; Williams and Ferguson, 2010).
The difference in activity period was also reflected by their distribution on different plant development stages, indicating also a spatial separation between the species. T. heterocerus was mostly observed in flowers and was never found to search for hosts on green buds, and only a few specimens were observed on yellow buds, while both Phradis species were observed ovipositing into buds. This suggests that both Phradis species are specialized on host search in buds while T. heterocerus is not. This agrees with the findings of Jönsson et al. (2005) that T. heterocerus responds positively to odors of flowering OSR, while Phradis species avoid it.
We observed P. interstitialis females frequently probing on green buds, and because dissection of these buds revealed almost exclusively pollen beetle eggs, this confirms that this species oviposits into pollen beetle eggs in buds which are still green (Williams and Cook, 2010). This species has previously been recognized as a larval parasitoid (Winfield, 1963; Nilsson and Andreasson, 1987), although Osborne (1960) also suggested an ability of this species to oviposit in pollen beetle eggs. Fully developed P. interstitialis larvae can be found inside pollen beetle larvae (Osborne, 1960), hence the term egg-larval parasitoid (Godfray, 1994) is probably more accurate to describe this species, as oviposition is made in the egg but the host is not killed before the larval stage.
Our results also confirm that P. morionellus is a larval parasitoid, since it was found probing in both green and yellow buds and dissection of these buds revealed only pollen beetle larvae but no eggs. This supports the hypothesis that P. interstitialis and P. morionellus differ in host stage preference. In a phylogenetic perspective, P. interstitialis and P. morionellus are closer related to each other than to T. heterocerus. The close relationship of the two Phradis species is paralleled by their shared preference for host search in OSR buds. It is possible that both Phradis species have coevolved under pressure of competition, and partitioned their niche on the temporal axis and microhabitat axis, separating into early season (P. interstitialis: eggs in buds) and later season (P. morionellus: larvae in buds). There is no knowledge whether the ancestral state was larval or egg-larval parasitism before the assumed character displacement, and it cannot be extrapolated from other Palearctic Phradis species, since the biology of most of them is unknown (Khalaim et al., 2009). If we assume larval parasitism, which is the common strategy in the subfamily Tersilochinae, as ancestral state, then P. interstitialis could have been pushed into exploitation of pollen beetle eggs if P. morionellus is a superior competitor for larvae in buds. By emerging earlier and arriving in the OSR field before superior competitors for larvae arrive, P. interstitialis might have shifted to oviposition into host eggs since eggs are more common in green buds in the early OSR season, thereby getting an advantage over P. morionellus.
The attraction to volatile cues from OSR was linked to the observed behavior and the larval stage attacked by the different species. All parasitoids were attracted by volatile cues from the infested plant, while only T. heterocerus responded to volatiles from isolated pollen beetle larvae. Both Phradis species were attracted to volatiles from the infested OSR plants in the bud stage, which releases a volatile blend dominated by terpenoids and two green leaf volatiles (GLVs), (Z)-3-hexenyl acetate and (Z)-3-hexenol (Jönsson et al., 2005). These two GLVs are released at increasing rates from the wound caused by oviposting pollen beetles (Borg and Borg-Karlsson, 1996). These volatiles are possibly detected at distance, but could also be important during the observed parasitoid antennation and involved in locating the oviposition hole, through which pollen beetle eggs are probably accessed. There is evidence that herbivore egg deposition can induce biochemical changes in the plant surface which attract and arrest egg parasitoids (Hilker and Meiners, 2011). Host finding in P. morionellus might be further mediated by an interaction between larval feeding and the plant tissue of the bud. Attraction to odors of plants infested by feeding larvae has been found in other larval parasitoids that attack hosts feeding on Brassicaceae (Agelopoulos and Keller, 1994a; Mattiacci et al., 1994; Potting et al., 1999). These studies were made with leaf-feeding larvae, but our study shows that a similar response takes place when reproductive plant parts are under attack. In fact, many herbivore-induced volatiles are known to be released from both vegetative and reproductive plant parts (Dudareva et al., 2004, 2006). Increased rates of volatile release have been found in flowering OSR infested by pollen beetles (Jönsson and Anderson, 2008) and other Brassicaceae under attack by feeding larvae (Agelopoulos and Keller, 1994b; Mattiacci et al., 1994). From the parasitoid's point of view, it seems most reliable to exploit cues that lead directly to the immature stage. Chemicals induced by larval feeding should therefore be relevant to P. morionellus and T. heterocerus, that both oviposit in larvae; but for P. interstitialis that needs to find eggs, every clue should be of crucial importance that indicates fresh oviposition sites. Hence, particularly the latter species might benefit from chemical traces left by adult ovipositing pollen beetle females. Also, the adult beetle female bites a hole into the bud prior to oviposition—hence, there could be some chemicals left by, or induced through an interaction of the beetle mandibles and/or the beetle ovipositor with the damaged plant tissue. Also, epideictic (spacing) pheromones, carried by adult beetle females wherever they go, might play a role here. There is some laboratory evidence that female pollen beetles avoid odors from large numbers of conspecific females (Ruther and Thiemann, 1997; Cook et al., 2006) and it could be interesting to look whether such chemical information can be perceived by pollen beetle parasitoids too.
There is electrophysiological evidence that the antennae of P. morionellus respond to several components of a volatile blend collected from pollen-beetle infested flowering OSR (Jönsson and Anderson, 2008). The same substances might be relevant for T. heterocerus as well. However, a partial explanation for flower visiting in T. heterocerus and P. morionellus may be feeding on carbohydrates: It is known that hymenopteran parasitoids, including Tersilochinae, visit flowers to feed on nectar, pollen, and honeydew (Jervis et al., 1993). Increased parasitoid fecundity in the presence of nectar has been demonstrated numerous times in laboratories (Jervis et al., 1996), and there is physiological evidence for the acquisition of sugars in the field by T. heterocerus during the flowering season (Rusch et al., 2012).
In the absence of plant odors, T. heterocerus preferred the odor of isolated intermediate-sized larvae over purified air, while we found no evidence for a preference of large second instar larvae in this species, as is often claimed in recent reviews (Nilsson, 2003; Williams and Cook, 2010). From the observed preference of T. heterocerus for smaller larval stage odors and avoidance of large larvae, we conclude that pollen beetle larvae probably get parasitized earlier than at the time when they are big enough to actively drop from the plant.
The attraction of T. heterocerus females to host stage odors was also found to be context dependent. In the absence of plant odors, T. heterocerus preferred late first instar larvae against a control, but in the presence of uninfested OSR with an equal ratio of buds and flowers, T. heterocerus preferred the odor of the smallest stage (early first instar) over plant odor alone. Background odors are always present in nature and may affect the use of volatiles by parasitoids in various ways (Hilker and McNeil, 2008; Schröder and Hilker, 2008). It is difficult to explain why uninfested plant odor decreases the attractivity of late first instar larvae while it enhances the attractivity of early first instar larvae, but it is possible that flower volatiles, indicating floral nectar and pollen, may play some confounding role here. Nevertheless, these results show that first instar larvae should not be neglected as potential host of pollen beetle parasitoids. A black egg, typical for T. heterocerus, can often be seen through the cuticle of late first-instar larvae (J.Berger, pers.observation), a hint that this parasitoid species oviposits into its host at an earlier stage than commonly assumed. Hitherto, only second instar larvae have been thought to be the preferred host of T. heterocerus and P. morionellus, but Jönsson and Anderson (2008) found no response of P. morionellus antennae to volatiles identified from large second instar pollen beetle larvae. For future studies, it might be worth to isolate and identify the volatiles from all larval stages, and to measure the electrophysiological response of T. heterocerus, to identify which compounds cause the differential preference of larval stages.
The niche separation of these three species might further be studied on developmental level, particularly by taking into account super- and multiparasitism by these species. Nitzsche (1998) found that pollen beetle larvae are commonly superparasitized by T. heterocerus, but not by P. interstitialis. T. heterocerus has probably evolved superparasitism to increase the survival of its eggs by overcoming host defense (Jourdheuil, 1960; van Alphen and Visser, 1990), while P. interstitialis is apparently more egg-limited, since Fritzsche (1957) reported that T. heterocerus laid nearly twice as many eggs as P. interstitialis. All three species are known to delay their development until the host has reached the prepupal stage, and their first-instar larvae possess heavily sclerotised sickle-shaped mandibles (Osborne, 1960). This suggests that their larvae have the ability to bite each other in intrinsic competition if they hatch inside the same host (Harvey et al., 2013). When the host defense ability is lowered by multiparasitism through both P. interstitialis and T. heterocerus, P. interstitialis was suggested to have a competitive advantage (Nilsson, 2003); this could be due to an earlier hatching and a subsequent superiority in larval combat. Similar competition may happen between the larvae of P. morionellus and T. heterocerus, although it was speculated by Nilsson (2003) that in multiparasitism by the latter two species, it is a random event which one survives.
Conclusions
The observed temporal, spatial and behavioral differences between species can partly explain how the host resource is explored and are necessary for these parasitoids to coexist. All three species use odors to locate infested plants, but since they differ in host stage preference and host microhabitat preference, they are most likely attracted to different plant odor blends. This study is also the first evidence for an attraction of T. heterocerus to smaller larval stages than what has hitherto been thought. Since this species is numerically of highest relevance in biocontrol of the pollen beetle, improving our knowledge of its biology might be beneficial when considering organic management of oilseed rape crops.
Author Contributions
JB and MJ did the field work and the bioassays, analyzed the data, and wrote the first versions of the manuscript. PA and KH contributed substantially to the conception and interpretation of the work and revised the manuscript critically for important intellectual content. JB, MJ, KH, and PA have approved the final version to be published, and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Nanna Hacker, Ulf Nilsson and Britt Åhman for technical help and Olle Anderbrant and Christer Nilsson for valuable comments on the manuscript. This research was supported financially by Formas (the Swedish Research Council for Environment, Agricultural Sciences, and Spatial Planning), the Swedish Research Council VR (Linnaeus grant 217-2006-1750), and the Magnus Bergvall Foundation.
References
Agelopoulos, N. G., and Keller, M. A. (1994a). Plant-natural enemy association in the tritrophic system Cotesia rubecula - Pieris rapae - Brassicaceae (Cruciferae): II. Preference of C. rubecula for landing and searching. J. Chem. Ecol. 20, 1735–1748. doi: 10.1007/BF02059895
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Agelopoulos, N. G., and Keller, M. A. (1994b). Plant-natural enemy association in the tritrophic system Cotesia rubecula - Pieris rapae - Brassicaceae (Cruciferae). III. Collection and identification of plant and frass volatiles. J. Chem. Ecol. 20, 1955–1967. doi: 10.1007/BF02066236
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Bengtsson, G., Hedlund, K., and Rundgren, S. (1991). Selective odour perception in the soil Collembola Onychiurus armatus. J. Chem. Ecol. 17, 2113–2125. doi: 10.1007/BF00987995
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Bonsall, M. A., Hassell, M. P., and Asefa, G. (2002). Ecological trade-offs, resource partitioning, and coexistence in a host-parasitoid assemblage. Ecology 83, 925–934. doi: 10.2307/3071902
Borg, A., and Borg-Karlsson, A. K. (1996). “Differences in female behaviour in two Meligethes species on buds of Brassica napus, B. juncea and Sinapis alba,” in Oviposition Behaviour of Two Pollen Beetles (Meligethes aeneus and M. viridescens) on Different Host Plants, ed A. Borg (Uppsala: Swedish University of Agricultural Sciences, Ph.D. thesis).
Briggs, C. J., Nisbet, R. M., and Murdoch, W. W. (1993). Coexistence of competing parasitoid species on a host with a variable life-cycle. Theor. Popul. Biol. 44, 341–373. doi: 10.1006/tpbi.1993.1032
Casas, J. (2000). “Host location and selection in the field,” in Parasitoid Population Biology, ed M. E. Hochberg and A. R. Ives (Princeton; Oxford: Princeton University Press), 17–26.
Comins, H. N., and Hassel, M. P. (1996). Persistence of multispecies host-parasitoid interactions in spatially distributed models with local dispersal. J. Theor. Biol. 183, 19–28. doi: 10.1006/jtbi.1996.0197
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Cook, S. M., Murray, D. A., Watts, N. P., and Williams, I. H. (2006). Responses of pollen beetles (Meligethes aeneus) to conspecific odours. IOBC/WPRS Bull. 29, 143–150. Available online at: http://www.iobc-wprs.org/pub/bulletins/iobc-wprs_bulletin_2006_29_07.pdf#page=161 (Accessed April 23, 2015).
De Moraes, C. M., Lewis, W. J., Paré, P. W., Alborn, H. T., and Tumlinson, J. H. (1998). Herbivore-infested plants selectively attract parasitoids. Nature 393, 570–573. doi: 10.1038/31219
Dicke, M., and Baldwin, I. T. (2010). The evolutionary context for herbivore-induced plant volatiles: beyond the ‘cry for help’. Trends Plant Sci. 15, 167–175. doi: 10.1016/j.tplants.2009.12.002
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Dicke, M., van Poecke, R. M. P., and de Boer, J. G. (2003). Inducible direct defence of plants: from mechanisms to ecological functions. Basic Appl. Ecol. 4, 27–42. doi: 10.1078/1439-1791-00131
Dudareva, N., Negre, F., Nagegowda, D. A., and Orlova, I. (2006). Plant volatiles: recent advances and future perspectives. Crit. Rev. Plant Sci. 25, 417–440. doi: 10.1080/07352680600899973
Dudareva, N., Pichersky, E., and Gershenzon, J. (2004). Biochemistry of plant volatiles. Plant Physiol. 135, 1893–1902. doi: 10.1104/pp.104.049981
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Fatouros, N. E., Lucas-Barbosa, D., Weldegergis, B. T., Pashalidou, F. G., van Loon, J. J. A., Dicke, M., et al. (2012). Plant volatiles induced by herbivore egg deposition affect insects of different trophic levels. PLOS ONE 7:e43607. doi: 10.1371/journal.pone.0043607
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Fleury, F., Gibert, P., Ris, N., and Allemand, R. (2009). Ecology and life history evolution of frugivorous Drosophila parasitoids. Adv. Parasit. 70, 3–44. doi: 10.1016/S0065-308X(09)70001-6
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Fritzsche, R. (1957). “Beeinflussung der Populationsdichte verschiedener Meligethes-Arten von gleichen Wirtspflanzen durch Parasiten,” in Bericht über die Hundertjahrfeier der Deutschen Entomologischen Gesellschaft, 30. September bis 5. Oktober 1956, ed H. J. Hannemann (Berlin: Akademie Verlag), 141–145.
Godfray, H. C. J. (1994). Parasitoids: Behavioural and Evolutionary Ecology. Princeton, NJ: Princeton University Press.
Hackett-Jones, E., Cobbold, C., and White, A. (2009). Coexistence of multiple parasitoids on a single host due to differences in parasitoid phenology. Theor. Ecol. 2, 19–31. doi: 10.1007/s12080-008-0025-1
Hardin, G. (1960). Competitive exclusion principle. Science 131, 1292–1297. doi: 10.1126/science.131.3409.1292
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Harvey, J. A., Poelman, E. H., and Tanaka, T. (2013). Intrinsic inter- and intraspecific competition in parasitoid wasps. Annu. Rev. Entomol. 58, 333–351. doi: 10.1146/annurev-ento-120811-153622
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Hilker, M., and McNeil, J. (2008). “Chemical and behavioral ecology in insect parasitoids: how to behave optimally in a complex odorous environment,” in Behavioral Ecology of Insect Parasitoids: from Theoretical Approaches to Field Applications, eds E. Wajnberg, C. Bernstein, and J. van Alphen (Oxford: Blackwell Publishing), 92–112.
Hilker, M., and Meiners, T. (2011). Plants and insect eggs: how do they affect each other? Phytochemistry 72, 1612–1623. doi: 10.1016/j.phytochem.2011.02.018
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Horstmann, K. (1971). Revision der europäischen Tersilochinen 1 (Hymenoptera, Ichneumonidae). Veröff. Zool. Staatssamml. Muench. 15, 45–138.
Horstmann, K. (1981). Revision der europäischen Tersilochinae 2 (Hymenoptera, Ichneumonidae). Spixiana Suppl. 4, 1–76.
Jervis, M. A., Kidd, N. A. C., Fitton, M. G., Huddleston, T., and Dawah, H. A. (1993). Flower-visiting by hymenopteran parasitoids. J. Nat. Hist. 27, 67–105. doi: 10.1080/00222939300770051
Jervis, M. A., Kidd, N. A. C., and Heimpel, G. E. (1996). Parasitoid adult feeding behaviour and biocontrol - a review. Biocontrol News Inf. 17, 11–26.
Jönsson, M., and Anderson, P. (2008). Emission of oilseed rape volatiles after pollen beetle infestation; behavioural and electrophysiological responses in the parasitoid Phradis morionellus. Chemoecology 17, 201–207. doi: 10.1007/s00049-007-0379-7
Jönsson, M., Lindkvist, A., and Anderson, P. (2005). Behavioural responses in three ichneumonid pollen beetle parasitoids to volatiles emitted from different phenological stages of oilseed rape. Entomol. Exp. Appl. 115, 365–369. doi: 10.1111/j.1570-7458.2005.00271.x
Jönsson, M., Nilsson, C., and Anderson, P. (2004). Occurence of pollen beetle parasitoids in the south of Sweden. IOBC/WPRS Bull. 27, 239–242. Available online at: http://www.iobc-wprs.org/pub/bulletins/iobc-wprs_bulletin_2004_27_10.pdf#page=251(Accessed April 23, 2015).
Jourdheuil, P. (1960). Influence de quelques facteurs écologiques sur les fluctuations de population d'une biocénose parasitaire: étude relative à quelques hyménoptères (Ophininae, Diospilinae, Euphorinae) parasites de divers coléoptères inféodés aux crucifères. Ann. Épiphyties 11, 445–658.
Kalmykov, L. V., and Kalmykov, V. L. (2013). Verification and reformulation of the competitive exclusion principle. Chaos Soliton Fract. 56, 124–131. doi: 10.1016/j.chaos.2013.07.006
Khalaim, A. I., Bordera, S., and Rodríguez-Berrío, A. (2009). A review of the European species of Phradis (Hymenoptera: Ichneumonidae: Tersilochinae), with a description of a new species from Spain. Eur. J. Entomol. 106, 107–118. doi: 10.14411/eje.2009.015
Klukowski, Z. (2008). Chronologia i dynamika wystêpowania parazytoidów (Tersilochinae, Ichneumonidae) wa?‘niejszych skodników rzepaku ozimego. Prog. Plant Prot./Post. Ochr. Roœlin 48, 1299–1303.
Lancashire, P. D., Bleiholder, H., van den Boom, T., Langelüddeke, P., Stauss, R., Weber, E., et al. (1991). A uniform decimal code for growth stages of crops and weeds. Ann. Appl. Biol. 119, 561–601. doi: 10.1111/j.1744-7348.1991.tb04895.x
Mattiacci, L., Dicke, M., and Posthumus, M. A. (1994). Induction of parasitoid attracting synomone in Brussels sprouts plants by feeding of Pieris brassicae larvae: role of mechanical damage and herbivore elicitor. J. Chem. Ecol. 20, 2229–2247. doi: 10.1007/BF02033199
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
McDonald, J. H. (2014a). Handbook of Biological Statistics, 3rd Edn. Baltimore, MD: Sparky House Publishing
McDonald, J. H. (2014b). Spreadsheet for the Calculation of the G-test of Goodness-of-fit. Available online at: http://www.biostathandbook.com/gtestgof.html (Accessed December 11, 2014).
Meszéna, G., Gyllenberg, M., Pásztor, L., and Metz, J. A. J. (2006). Competitive exclusion and limiting similarity: a unified theory. Theor. Popul. Biol. 69, 68–87. doi: 10.1016/j.tpb.2005.07.001
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Nilsson, C. (1985). Impact of ploughing on emergence of pollen beetle parasitoids after hibernation. Z. Angew. Entomol. 100, 302–308. doi: 10.1111/j.1439-0418.1985.tb02783.x
Nilsson, C. (1988). The number of larval instars of Meligethes aeneus (F.) in southern Sweden. Växtskyddsnotiser 52, 151–152.
Nilsson, C. (2003). “Parasitoids of pollen beetles,” in Biocontrol of Oilseed Rape Pests, ed D. V. Alford (Oxford: Blackwell), 74–86. doi: 10.1002/9780470750988.ch4
Nilsson, C., and Andreasson, B. (1987). Parasitoids and predators attacking pollen beetles (Meligethes aeneus F.) in spring and winter rape in southern Sweden. IOBC/WPRS Bull. 10, 64–73.
Nitzsche, O. (1998). Auftreten und Effizienz von Parasitoiden als natürliche Gegenspieler von Schadinsekten im Winterraps unter besonderer Berücksichtigung unterschiedlicher Bodenbearbeitungsmaßnahmen nach Winterraps. Doctoral dissertation, Göttingen University.
Osborne, P. (1960). Observations on the natural enemies of Meligethes aeneus (F.) and M. viridescens (F.) [Coleoptera: Nitidulidae]. Parasitology 50, 91–110. doi: 10.1017/S0031182000025233
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Osborne, P. (1965). Morphology of the immature stages of Meligethes aeneus (F.) and M. viridescens (F.) (Coleoptera, Nitidulidae). Bull. Entomol. Res. 55, 747–759. doi: 10.1017/S0007485300049853
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Poelman, E. H., Oduor, A. M. O., Broekgaarden, C., Hordijk, C. A., Jansen, J. J., van Loon, J. J. A., et al. (2009). Field parasitism rates of caterpillars on Brassica oleracea plants are reliably predicted by differential attraction of Cotesia parasitoids. Funct. Ecol. 23, 951–962. doi: 10.1111/j.1365-2435.2009.01570.x
Potting, R. P. J., Poppy, G. M., and Schuler, T. H. (1999). The role of volatiles from cruciferous plants and pre-flight experience in the foraging behaviour of the specialist parasitoid Cotesia plutellae. Entomol. Exp. Appl. 93, 87–95. doi: 10.1046/j.1570-7458.1999.00565.x
Reddy, G. V. P. (2012). “Recent trends in the olfactory responses of insect natural enemies to plant volatiles,” in Biocommunication of Plants. Signaling and communication in plants 12, eds G. Witzany and F. Baluška (Berlin; Heidelberg: Springer), 281–301. doi: 10.1007/978-3-642-23524-5_15
Rusch, A., Suchail, S., Valantin-Morison, M., Sarthou, J. P., and Roger-Estrade, J. (2012). Nutritional state of the pollen beetle parasitoid Tersilochus heterocerus foraging in the field. BioControl 58, 17–26. doi: 10.1007/s10526-012-9463-1
Ruther, J., and Thiemann, K. (1997). Response of the pollen beetle Meligethes aeneus to volatiles emitted by intact plants and conspecifics. Entomol. Exp. Appl. 84, 183–188. doi: 10.1046/j.1570-7458.1997.00213.x
Sachet, J. M., Poncet, B., Roques, A., and Desprès, L. (2009). Adaptive radiation through phenological shift: the importance of the temporal niche in species diversification. Ecol. Entomol. 34, 81–89. doi: 10.1111/j.1365-2311.2008.01045.x
Schröder, R., and Hilker, M. (2008). The relevance of background odour in resource location by insects: a behavioral approach. BioScience 58, 308–316. doi: 10.1641/B580406
Sokal, R. R., and Rohlf, F. J. (1995). Biometry: The Principles and Practice of Statistics in Biological Research, 3rd Edn. New York, NY: W. H. Freeman.
Turlings, T. C. J., and Wäckers, F. (2004). “Recruitment of predators and parasitoids by herbivore-injured plants,” in Advances in Insect Chemical Ecology, ed. R. T. Cardé and J. G. Millar (Cambridge, Cambridge University Press), 21–75. doi: 10.1017/CBO9780511542664.003
van Alphen, J. J. M., and Visser, M. E. (1990). Superparasitism as an adaptive stragegy for insect parasitoids. Annu. Rev. Entomol. 35, 59–79. doi: 10.1146/annurev.en.35.010190.000423
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
van Dijken, M. J., and van Alphen, J. J. M. (1998). The ecological significance of differences in host detection behaviour in coexisting parasitoid species. Ecol. Entomol. 23, 265–270. doi: 10.1046/j.1365-2311.1998.00130.x
Vet, L. E. M., Lewis, W. J., and Cardé, R. T. (1995). “Parasitoids foraging and learning,” in Chemical Ecology of Insects 2, eds R. T. Cardé and W. J. Bell (New York: Chapman & Hall), 65–104. doi: 10.1007/978-1-4615-1765-8_3
Vinson, S. B. (1976). Host selection by insect parasitoids. Annu. Rev. Entomol. 21, 109–133. doi: 10.1146/annurev.en.21.010176.000545
Vinson, S. B. (1998). The general host selection behavior of parasitoid Hymenoptera and a comparison of initial strategies utilized by larvaphagous and oophagous species. Biol. Control 11, 79–96. doi: 10.1006/bcon.1997.0601
Wäschke, N., Meiners, T., and Rostás, M. (2013). “Foraging strategies of parasitoids in complex chemical environments,” in Chemical Ecology of Insect Parasitoids, eds E. Wajnberg and S. Colazza (Chichester: Wiley), 37–63. doi: 10.1002/9781118409589.ch3
Williams, I. H. (2006). Integrating parasitoids into management of pollen beetle on oilseed rape. Agron. Res. 4, 465–470. Available online at: http://agronomy.emu.ee/vol04Spec/p4S70.pdf (Accessed April 23, 2015).
Williams, I. H., and Cook, S. M. (2010). “Crop location by oilseed rape pests and host location by their parasitoids,” in Biocontrol-Based Integrated Management of Oilseed Rape Pests, ed I. H. Williams (Dordrecht: Springer), 215–244. doi: 10.1007/978-90-481-3983-5_7
Williams, I. H., and Ferguson, A. W. (2010). “Spatio-temporal distributions of pests and their parasitoids on the oilseed rape crop,” in Biocontrol-Based Integrated Management of Oilseed Rape Pests, ed I. H. Williams (Dordrecht: Springer), 245–271. doi: 10.1007/978-90-481-3983-5_8
Williams, I. H., Frearson, D. J. T., Barari, H., and McCartney, A. (2007). First field evidence that parasitoids use upwind anemotaxis for host habitat location. Entomol. Exp. Appl. 123, 299–307. doi: 10.1111/j.1570-7458.2007.00551.x
Winfield, A. L. (1963). A study on the effects of insecticides on parasites of larvae of blossom beetles (Meligethes aeneus F., Coleoptera: Nitidulidae). Entomol. Exp. Appl. 6, 309–318. doi: 10.1111/j.1570-7458.1963.tb00630.x
Keywords: niche separation, Tersilochus heterocerus, Phradis interstitialis, Phradis morionellus, pollen beetle
Citation: Berger J, Jönsson M, Hedlund K and Anderson P (2015) Niche separation of pollen beetle parasitoids. Front. Ecol. Evol. 3:45. doi: 10.3389/fevo.2015.00045
Received: 06 March 2015; Accepted: 17 April 2015;
Published: 01 May 2015.
Edited by:
Peter Schausberger, University of Natural Resources and Life Sciences Vienna, AustriaReviewed by:
Stefano Colazza, University of Palermo, ItalyTorsten Meiners, Freie Universität Berlin, Germany
Copyright © 2015 Berger, Jönsson, Hedlund and Anderson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Josef Berger, Soil Ecology Group, Department of Biology, Lund University, Sölvegatan 37, Lund 223 62, Sweden, josef.berger@biol.lu.se
 Josef Berger
Josef Berger Martin Jönsson
Martin Jönsson Katarina Hedlund
Katarina Hedlund Peter Anderson
Peter Anderson