The influence of environmental conditions on the age pattern in breeding performance in a transequatorial migratory seabird
- Population Ecology Group, Department of Biodiversity and Conservation, Mediterranean Institute of Advanced Studies (CSIC-UIB), Esporles, Spain
Several studies of marine top predators, above all of seabirds, have analyzed the effects of either individual age or environmental fluctuations on reproduction; nevertheless, little is known about the age patterns in breeding performance in a variable environment. To investigate the simultaneous influence of age and environmental conditions on laying dates and egg volumes, we tested different climate and food availability indices in a transequatorial migratory seabird using female data from a 23-year study. Our results show an improvement in breeding parameters with age (i.e., earlier laying dates and greater egg volumes) but no pattern of senescence in older age groups. The best models showed an interaction of time and age in breeding performance, i.e., the age pattern of breeding performance changed each year likely as a result of environmental variability. Nevertheless, climatic indexes used here explained part of that annual variability: NAO and SOI index accounted for 24 and 20% of deviances in laying dates and egg volume, respectively. Part of that unexplained variability might be related to other processes such as intermittent breeding and the individual quality of breeders, which were not assessed in our study.
Introduction
In marine ecosystems, climatic fluctuations and other physical oceanographic variables affect the population dynamics of organisms by exerting an influence on their vital rates. This influence is mediated by drivers such as food availability and environmental conditions during the breeding period or migration (Duffy-Anderson et al., 2005; Lee, 2011). For example, large-scale climatic phenomena and oceanographic processes produce changes in water temperatures and currents that generate spatio-temporal variation in the production, distribution, and abundance of the prey consumed by marine predators such as seabirds (Durant et al., 2004). Consequently, these processes may affect the foraging ecology of seabirds (Navarro and Gonzalez-Solís, 2009; Weimerskirch et al., 2012) and influence their survival, reproduction and population dynamics (Jenouvrier et al., 2005; Genovart et al., 2013).
Like environmental variability, individual covariates such as age and sex also influence vital rates (Stearns, 1992). Age-specific differences in breeding performance have been observed in several seabirds (Nevoux et al., 2007; Vieyra et al., 2009; Pardo et al., 2013). In most cases young individuals perform less well than older individuals (Martin, 1995), thereby indicating improvement in performance with age leading to a peak and then decline (i.e., senescence) (Clutton-Brock, 1988). Several non-exclusive hypotheses have been proposed to explain these age-related variations in breeding performance: a progressive appearance or disappearance of phenotypes, age-related improvements in the skills needed for reproducing, and an optimization of reproductive effort (Forslund and Pärt, 1995).
Demographic parameters in wild populations are thus expected to be shaped by both individual covariates and environmental variability (e.g., Stearns, 2000; Nevoux et al., 2007; Oro et al., 2010). Despite this, few studies have ever examined at the same time how environmental conditions affect age patterns in breeding performance (Bunce et al., 2005; Pardo et al., 2013; Oro et al., 2014). Depending on environmental conditions, different age patterns can exist (see Oro et al., 2014): in most studies, either (a) differences among age classes in breeding performance were found to decrease under better environmental conditions (Sydeman et al., 1991; Laaksonen et al., 2002; Barbraud and Weimerskirch, 2005; Bunce et al., 2005) or (b) environmental conditions had no clear influence on breeding performance (Nevoux et al., 2007; Vieyra et al., 2009; Lee, 2011; Pardo et al., 2013); just one study found that differences between age-classes were greater under favorable environmental conditions (Oro et al., 2014).
Here we analyze 23 years of data from a population of Scopoli's Shearwater (Calonectris diomedea), a long-lived and highly monogamous seabird with extreme life-history traits that include high adult survival rates, low fecundity (females lay a single egg), intermittent breeding, and delayed sexual maturity (Thibault, 1994; Thibault et al., 1997). Shearwaters breed in the Mediterranean and, after reproducing, perform long trips to their Atlantic wintering grounds where there are important upwellings that provide abundant food resources (González-Solís et al., 2007). We tested the influence of environmental conditions at different spatial scales on two breeding parameters, laying date and egg volume, in individuals of different age classes. Our main aim was to test (a) whether breeding performance increased with age and (b) whether or not differences between age classes depend on environmental conditions at different spatial scales.
Materials and Methods
Ethics Statement
All animals were handled in strict accordance with good animal practice as defined by the current European legislation and the animal work was approved by the regional committee (Govern Balear, Balearic Is., Spain).
Population Monitoring
The study was conducted in the Scopoli's Shearwater colony on Pantaleu Islet in the Balearic Archipelago (30° 34'N, 2° 21' E, Spain), where ca. 200 pairs breed in burrows under boulders or vegetation. In the period 1985–2010, a total of 1043 chicks were ringed with stainless-steel rings bearing unique numeric codes that allow individuals to be identified and aged. In 2001–2013, accessible nests (ca. 150 each year) were marked and visited during the breeding period (May–September); nests with breeders of known ages were examined to record laying dates (with a maximum of 72 h between visits), and the size of the single egg (with the use of a digital caliper). Both breeding parameters have been shown to be related to fitness components as chick survival, or juvenile survival (see review in Krist, 2011). Birds were sexed when first captured using morphometric measures and calls. We gathered data from 87 different breeders and the total number of monitored nests during the study was 302 (annual median number of nests = 24: 11–32 nests; median of age = 12-year old, range: 5–23-year old) of which 98 cases are females of known age (annual median number of nests = 8, range: 212 nests; median of age = 11-year old, range: 5–23-year old). As in many cases we only had the age of one member of the pair, we first assessed the association between the ages of the two members in nine pairs for which the two members of the pair were of known age. In these nine breeding pairs, assortative mating was low (n = 9, Spearman correlation rs = 0.068, p = 0.431). Given that both laying dates and egg volumes in this species mainly depend on female body condition and their breeding capabilities (Jouanin et al., 2001), we only used data from females to be conservative. Nevertheless, since sample size of females was much lower than for males, and we could not confirm assortative mating, we also ran all models taking into account both sexes (Appendix A in Supplementary Material) to increase power by increasing sample size and check if the best model was the same as when taking only female data into account.
Environmental Variables
Previous studies have shown that Scopoli's Shearwaters basically feed on fish (90%) during the breeding season (Granadeiro et al., 1998b). Like many other seabirds that breed in the study area, this species exploits fishing discards from the trawling fleet which fishes on the continental shelf off the Ebro Delta (Martínez-Abraín et al., 2002a). Thus, as an annual proxy of food availability, we used the total amount of trawling discards produced in March (when birds store energetic reserves before breeding) derived from statistics for trawling catches by a fishing guild in the Ebro Delta (Oro and Ruiz, 1997; Louzao et al., 2011). To obtain a proxy of food per-capita (i.e., correcting for density-dependence), we divided the total amount of available food by the total population size of all the scavenging seabirds—Audouin's gulls (Larus audouinii), Yellow-legged gulls (L. michahellis), and Scopoli's Shearwaters—that forage in the area (Oro and Ruiz, 1997).
As a proxy of an oceanic large-scale climatic index we used the Southern Oscillation Index (SOI), associated with Atlantic hurricane activity, which registers greater hurricane activity during positive phases (i.e., La Niña) and less in negative phases (i.e., El Niño) (Knutson et al., 2008). We used annual mean SOI values from January to December, obtained from http://www.cru.uea.ac.uk/cru/data/soi/soi.dat with a 1-year time lag to test the effect of environmental conditions in the Atlantic where birds winter before returning to their breeding areas.
To test the effect of local conditions during breeding we used another large-scale index, the North Atlantic Oscillation Index (NAO). This index affects water column hydrodynamics and may have a cascade effect at higher trophic levels (Gordo et al., 2011), thereby influencing the production, distribution and abundance of the organisms upon which birds feed (Durant et al., 2004). To test this effect we selected the extended annual winter NAO (i.e., December–March; NAOw) with positive values related to windy and warmer conditions, and negative values to colder air and wetter conditions in the Mediterranean (Hurrell, 1995). We also checked to see if there was any effect of the previous year's NAO value (NAOa) since some fish have temperature-dependent gonadal development (Ware and Tanasichuk, 1989) and may spawn and migrate earlier in warmer years (Sims et al., 2004). Data were obtained from https://climatedataguide.ucar.edu/climate-data/hurrell-north-atlantic-oscillation-nao-index-station-based.
Data Analysis
To analyze the influence of environmental variability on age patterns in different breeding parameters, we built linear mixed models (LMMs) with the package lme4 and function lmer (Bates, 2010) using R software (http://www.R-project.org). We used laying date and egg volumes as dependent, normally distributed, variables. We included year as a fixed effect and female age as a continuous explanatory variable, either as a linear, quadratic or logarithmic function, and environmental variables as covariates of the year-dependent variations (see previous section). We also considered models including the statistical interactions between these effects. In all models we included individual identity as random effect (random intercept model) to avoid pseudo-replication and account for differences across individuals (see Bolker et al., 2009).
We began by assessing the influence of age and determine the age pattern for each breeding parameter (i.e., linear, logarithmic or quadratic). Then we tested the influence of each environmental covariate (food availability, SOI, NAOw, NAOa) and progressively added complexity into the models, by including interactions term between the effects. We ranked the set of models using Akaike's information criterion with correction for small sample size (AICc; Anderson and Burnham, 2002) and selected the model with the lowest AICc value as the most parsimonious one, i.e., the best model for explaining our data using fewest parameters (Burnham and Anderson, 2002). When the difference between two models (i.e., ΔAICc) was less than 2 we considered that the models explained the data equally well (Lebreton et al., 1992; Burnham and Anderson, 2002). We also computed the Akaike weights (wi; Burnham and Anderson, 2002), which provides a measure of the relative likelihood of a particular model given the models considered (Anderson et al., 2000). To assess the fit of the model, we estimated the coefficient of determination (R2) between the fitted and observed values on selected models (Cameron and Windmeijer, 1996). We also estimated the R2 using the method proposed by Nakagawa and Schielzeth (2013; r.squaredGLMM function in the MuMIn package for R), which gives the marginal R2m representing the variance explained by fixed factors, and the conditional R2c as the variance explained by both fixed and random factors (i.e., the entire model). Additionally, we also estimated the percentage of variation explained by each covariate (r2) when tested alone as:
where Dev(Mcnt) is the estimated deviance of the constant model, i.e., no effects considered, Dev(Mcov) is the deviance of the model with the climatic covariate considered and Dev(Mt) is the deviance of the time-dependent model.
To obtain the χ2-values and the significance level of the different covariates, we performed a likelihood ratio test (LRT) comparing the null model with the different covariates for each breeding parameter (see Table 2). As random structure remained unchanged in all models tested, we can compare the LRT values of each case although the refitting model was run with ML and not with REML (see Zuur et al., 2009).
Results
We found a positive effect of age (log-transformed) on the breeding parameters analyzed (Table 1). Older individuals tend to lay earlier and to produce larger eggs than young birds (Table 1, Figure 1, and Appendix B in Supplementary Material). We found a large temporal variability in both parameters. In both cases, the retained model, i.e., the one with the lowest AICc value, assumed an effect of age, year and their statistical interaction, indicating that the strength of the relationship between breeding performance and age was changing across the years (Models 1 and 1; Table 1). The coefficient of determination (R2) between the fitted and observed values of the selected models was 0.74 and 0.67 for laying date and egg volume analysis, respectively. Another important finding was that in both analyses the parameter accounting for across-individual variation was large. When we estimated the variance explained by Nakagawa and Schielzeth's (2013) method, we obtained R2m = 0.39 and R2c = 0.64 for laying date (Model 1) and R2m = 0.28 and R2c = 0.53 for egg volume analysis (Model 1). Thus, the variance explained by the random effect (i.e., the breeder) was 0.24 and 0.25 in laying date and egg volume, respectively. Environmental covariates were negatively associated with the parameters considered, but although this association was statistically significant, the covariates explained a relatively small part of the total temporal variability (NAO winter index explained about 24% of variance in laying date and SOI index 20% in egg volume; Table 2). For this reason none of the models that included climatic indexes were retained by the model selection procedure. Similarly, fishing discards taken as a proxy of food availability had a positive effect on both parameters, but the variance explained was low and this effect cannot be considered an important predictor of laying dates and egg volume. The year-by-age interaction term is indicating that differences between age classes were more marked under good environmental conditions (negative values of the climatic indexes; Figure 1).
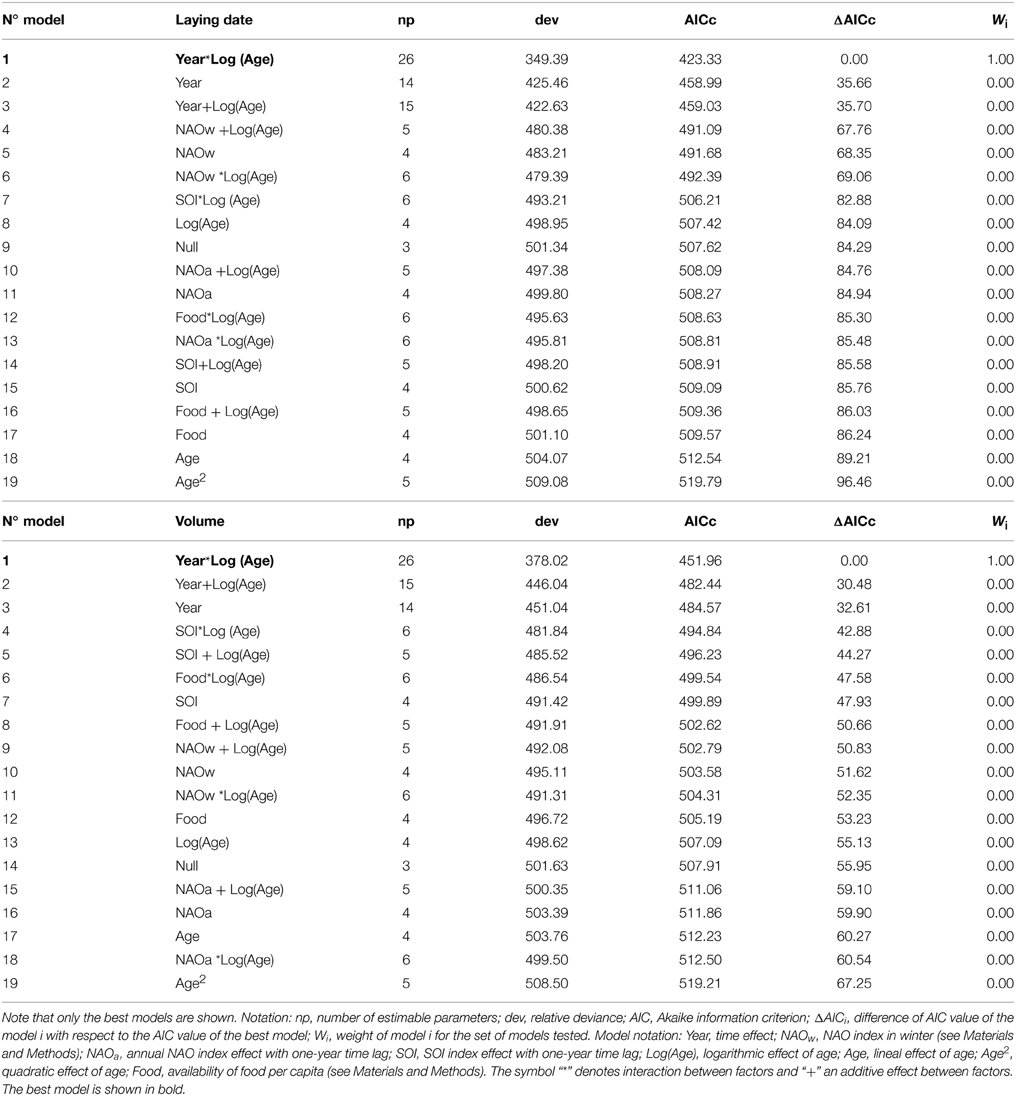
Table 1. Modeling the effects of age and environmental conditions on the breeding parameters of Scopoli's Shearwater's using generalized linear mixed models.
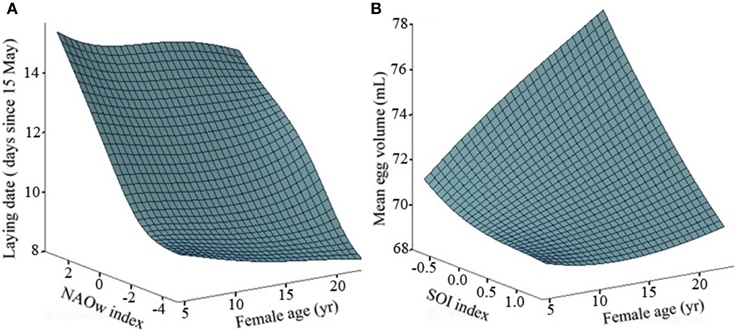
Figure 1. Smoothing regression surfaces taking into account the effects of age and the climatic index that explain the greatest amount of variance for each breeding parameter. (A) NAOw index for laying date, (B) SOI index for egg volume.
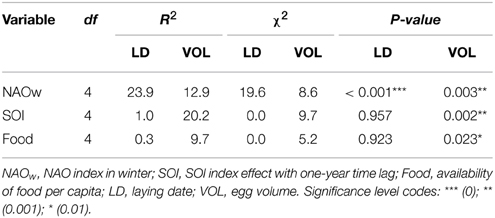
Table 2. Percentages of variance explained by each environmental covariable for each breeding parameter and likelihood ratio test values (see Materials and Methods).
Discussion
As has been previously reported in other long-lived animals (Clutton-Brock, 1988; Sæther, 1990; Forslund and Pärt, 1995) breeding performance in Scopoli's Shearwater increases with age; younger breeders have later laying dates and smaller egg volumes than older breeders. Since egg size and laying dates are positively associated with breeding success in several bird species and also in Scopoli's Shearwaters (e.g., Ramos et al., 2003), it is expected that fertility also increases with age. This increase in reproductive performance could be mediated by a gain in breeding skills over the years, which would enhance reproduction, or alternatively by the existence of a selection filter (the so-called “selection hypothesis”), whereby older age classes include a higher percentage of good breeders than younger age classes (Curio, 1983). In Storm petrel Hydrobates pelagicus, for example, individuals that survive their first breeding attempt should have a greater likelihood of breeding successfully the following year, probably due to the progressive selection of higher-quality individuals (e.g., Sanz-Aguilar et al., 2008). Furthermore, a previous study of Scopoli's Shearwater in our study colony (Genovart et al., 2013) detected that a large number first-time breeders do not return to the colony, either because they die (i.e., indicating thus the inherent costs of first reproduction) or permanently disperse to other breeding sites. Aside from the increase in breeding performance with age, several studies of long-lived animals have reported a decline in performance as a result of senescence (Vieyra et al., 2009; Pardo et al., 2013; Oro et al., 2014). We did not find a similar pattern, however the lack of senescence in our study may either be due to the small sample size (i.e., a lack of statistical power owing that there were only 4 individuals older than 20 years of age), to an increase of skip breeding with age, or to within-cohort phenotypic selection hiding senescence patterns (Cam et al., 2002).
Beside the clear and positive effect of age, we found that breeding performance varied across individuals and over the study period, the last probably reflecting environmental variability. We investigated whether the temporal variability was mirroring the fluctuations of large-scale climatic indices (namely NAOw and SOI) or the amount of fishing discards (a proxy for food availability just before the breeding season). NAOw and SOI are expected to influence breeding success of Scopoli's shearwater indirectly through their effects on marine productivity and oceanic conditions at the wintering grounds. Their influence on breeding performances, however, can be complex as climatic conditions might affect birds' energetic reserves through for example adverse flying conditions (Knutson et al., 2008; Arizmendi-Mejía et al., 2013) or alternatively they might influence them indirectly by changing the availability of prey, i.e., water column mixing, increasing river run off, and primary productivity (Lloret et al., 2001; Ottersen et al., 2001). This complexity is reflected in the fact that environmental covariates alone, despite being significant, explained only up to 24% of the yearly variability in breeding performances. A stronger effect of environmental covariates has been found for other seabirds (e.g., Frederiksen et al., 2004; Tavecchia et al., 2007; Dunn and Winkler, 2010; Barbraud et al., 2011). Genovart et al. (2013) found that the NAO winter index explained up to 40% of the temporal variance in breeding success in Scopoli's shearwater, but they included birds of unknown age and also nests monitored in an additional colony of the western Mediterranean (Chafarinas Island). Here we have restricted the analysis to birds of known age because we were interested in investigating the age-dependent pattern of breeding performance and whether environmental variability might change that pattern each year. It is possible that the statistical signal is lost in a smaller data set.
Our results showed an important heterogeneity among individuals in both, egg volume and laying dates (approximately 25% of the total variance of the retained model). At present we do not know the reason for such heterogeneity. Human disturbance and terrestrial predators are known to trigger dispersal and determined breeding success in seabirds (Cam et al., 2004; Fernández-Chacón et al., 2013). The studied colony however is located in a protected site with no human disturbances or alien predators. We can thus exclude the influence of these factors as a potential cause for nest quality heterogeneity. Differences among individuals might be due to difference in body size, individual investment, or individual ability in exploiting anthropogenic food resources (i.e., fishing discards, Votier et al., 2010). This individual heterogeneity might explain part of the differences between age classes, a pattern that was more marked under good environmental conditions than during harsh years, as it has been recorded for Audouin's gull (Oro et al., 2014). Other process that might be involved in the differences between the two patterns (good vs. bad years) is intermittent breeding, which has been commonly found in the study species (Mougin et al., 1997; Genovart et al., 2013). Previous studies on other seabirds found that during harsh years, individuals in bad condition may skip breeding (Cam and Monnat, 2000; Pyle et al., 2001; Sanz-Aguilar et al., 2008) and therefore the differences between age classes in breeding performance might be more marked in good years. In Scopoli's shearwater, males do not feed females during courtship as other seabirds (Jouanin et al., 2001), therefore male quality does not affect females' body condition and can be ruled out as a possible explanation. However, female reproductive investment might depend on male quality (Cunningham and Russell, 2000), but we have not enough data to assess whether shearwaters exhibit assortative mating. Out of nine cases in which both members of the pair were of known age, only in one case, female and male had the same age. Nevertheless, when males were included in the data set, results did not change.
Our results show clearly that the interplay of environmental conditions and individual age influences the observed breeding performance of Scopoli's shearwaters. Neither a proxy of the local food availability nor the large-scale climatic indexes can explain the temporal variability in the age pattern of breeding performance. This complex pattern is further complicated by the potential effects of individual quality. Future research should focus on the role of local climatic indexes and direct measures of food availability as predictors of breeding performances in long-lived birds.
Funding
Funds were provided by the Balearic Government (FEDER program) and the Spanish Ministry of Economy and Competitiveness (ref. CGL2013-42203-R).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to all the people who have participated in the fieldwork over the years. Permits were provided by the Balearic Regional Government. Giacomo Tavecchia and Isabel Palomera gently helped with data analysis and landing statistics, respectively; Michael Lockwood and Meschiya Lake improved the English. Daniel Barton, Joacim Näslund, an anonymous reviewer and Karin Charlotta Harding provided very helpful comments that improved the manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/article/10.3389/fevo.2015.00069
References
Anderson, D. R., and Burnham, K. P. (2002). Avoiding pitfalls when using information-theoretic methods. J. Wildl. Manage. 66, 912–918. doi: 10.2307/3803155
Anderson, D. R., Burnham, K. P., and Thompson, W. L. (2000). Null hypothesis testing: problems, prevalence, and an alternative. J. Wildl. Manage. 64, 912–923. doi: 10.2307/3803199
Arizmendi-Mejía, R., Militão, T., Viscor, G., and González-Solís, J. (2013). Pre-breeding ecophysiology of a long-distance migratory seabird. J. Exp. Mar. Biol. Ecol. 443, 162–168. doi: 10.1016/j.jembe.2013.02.047
Barbraud, C., and Weimerskirch, H. (2005). Environmental conditions and breeding experience affect costs of reproduction in Blue Petrels. Ecology 86, 682–692. doi: 10.1890/04-0075
Barbraud, C., Rivalan, P., Inchausti, P., Nevoux, M., Rolland, V., and Weimerskirch, H. (2011). Contrasted demographic responses facing future climate change in Southern Ocean seabirds. J. Anim. Ecol. 80, 89–100. doi: 10.1111/j.1365-2656.2010.01752.x
Bates, D. M. (2010). lme4: Mixed-effects Modeling with R. Available online at: http://lme4.r-forge.r-project.org/book
Bolker, B. M., Brooks, M. E., Clark, C. J., Geange, S. W., Poulsen, J. R., Stevens, M. H., et al. (2009). Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol. Evol. 24, 127–135. doi: 10.1016/j.tree.2008.10.008
Bunce, A., Ward, S. J., and Norman, F. I. (2005). Are age-related variations in breeding performance greatest when food availability is limited? J. Zool. 266, 163–169. doi: 10.1017/S0952836905006734
Burnham, K. P., and Anderson, D. R. (2002). Model Selection and Multi-model Inference: A Practical Information-theoretic Approach, 2nd Edn. New York, NY: Springer.
Cam, E., and Monnat, J. Y. (2000). Apparent inferiority of first-time breeders in the kittiwake: the role of heterogeneity among age classes. J. Anim. Ecol. 69, 380–394. doi: 10.1046/j.1365-2656.2000.00400.x
Cam, E., Link, W. A., Cooch, E. G., Monnat, J. Y., and Danchin, E. (2002). Individual covariation in life-history traits:seeing the trees despite the forest. Am. Nat. 159, 96–105. doi: 10.1086/324126
Cam, E., Oro, D., Pradel, R., and Jimenez, J. (2004). Assessment of hypotheses about dispersal in a long-lived seabird using multistate capture-recapture models. J. Anim. Ecol. 73, 723–736. doi: 10.1111/j.0021-8790.2004.00848.x
Cameron, A. C., and Windmeijer, F. A. G. (1996). R-Squared measures for count data regression models with applications to health-care utilization. J. Bus. Econ. Stat. 14, 209–220.
Clutton-Brock, T. H. (1988). Reproductive Success: Studies of Individual Variation in Contrasting Breeding Systems. Chicago, IL: University of Chicago Press.
Cunningham, E. J., and Russell, A. F. (2000). Egg investment is influenced by male attractiveness in the mallard. Nature 404, 74–77. doi: 10.1038/35003565
Curio, E. (1983). Why de young birds reproduce less well? Ibis 125, 400–404. doi: 10.1111/j.1474-919X.1983.tb03130.x
Duffy-Anderson, J. T., Bailey, K., Ciannelli, L., Cury, P., Belgrano, A., and Stenseth, N. C. (2005). Phase transitions in marine fish recruitment processes. Ecol. Complex 2, 205–218. doi: 10.1016/j.ecocom.2004.12.002
Dunn, P. O., and Winkler, D. W. (2010). “Effects of climate change on timing of breeding and reproductive success in birds,” in Effects of Climate Change on Birds, eds A. P. Møller, W. Fiedler, and P. Berthold (Oxford: Oxford University Press), 113–128.
Durant, J. M., Stenseth, N. C., Anker-Nilssen, T., Harris, M. P., Thompson, P., and Wanless, S. (2004). “Marine birds and climate fluctuation in North Atlantic,” in Marine Ecosystems and Climate Variation: The North Atlantic, eds N. C. Stenseth, G. Ottersen, J. W. Hurrell, and A. Belgrano (Oxford: Oxford University Press), 95–105.
Fernández-Chacón, A., Genovart, M., Pradel, R., Tavecchia, G., Bertolero, A., Piccardo, J., et al. (2013). When to stay, when to disperse and where to go: survival and dispersal patterns in a spatially structured seabird population. Ecography 36, 1117–1126. doi: 10.1111/j.1600-0587.2013.00246.x
Forslund, P., and Pärt, T. (1995). Age and reproduction in birds - hypotheses and tests. Trends Ecol. Evol. 10, 374–378. doi: 10.1016/S0169-5347(00)89141-7
Frederiksen, M., Harris, M. P., Daunt, F., Rothery, P., and Wanless, S. (2004). Scale-dependent climate signals drive breeding phenology of three seabird species. Glob. Chang. Biol. 10, 1214–1221. doi: 10.1111/j.1529-8817.2003.00794.x
Genovart, M., Sanz-Aguilar, A., Fernández-Chacón, A., Igual, J. M., Pradel, R., Forero, M. G., et al. (2013). Contrasting effects of climatic variability on the demography of a trans-equatorial migratory seabird. J. Anim. Ecol. 82, 121–130. doi: 10.1111/j.1365-2656.2012.02015.x
González-Solís, J., Croxall, J. P., Oro, D., and Ruiz, X. (2007). Trans-equatorial migration and mixing in the wintering areas of a pelagic seabird. Front. Ecol. Environ. 5, 297–301. doi: 10.1890/1540-9295(2007)5[297:TMAMIT]2.0.CO;2
Gordo, O., Barriocanal, C., and Robson, D. (2011). “Ecological impacts of the North Atlantic Oscillation (NAO) in mediterranean ecosystems,” in Hydrological, Socioeconomic and Ecological Impacts of the North Atlantic Oscillation in the Mediterranean Region, eds S. M. Vicente-Serrano and R. M. Trigo (Dordrecht: Springer), 153–170. doi: 10.1007/978-94-007-1372-7_11
Granadeiro, J. P., Monteiro, L. R., and Furness, R. W. (1998b). Diet and feeding ecology of Cory's shearwater Calonectris diomedea in the Azores, north-east Atlantic. Mar. Ecol. Prog. Ser. 166, 267–276. doi: 10.3354/meps166267
Hurrell, J. W. (1995). Decadal trends in the North Atlantic oscillation. Science 269, 676–679. doi: 10.1126/science.269.5224.676
Jenouvrier, S., Barbraud, C., Cazelles, B., and Weimerskirch, H. (2005). Modelling population dynamics of seabirds: importance of the effects of climate fluctuations on breeding proportions. Oikos 108, 511–522. doi: 10.1111/j.0030-1299.2005.13351.x
Jouanin, C., Mougin, J. L., and Stahl, J. (2001). Prelaying exodus of Cory's shearwaters (Calonectris diomedea borealis) on Selvagem Grande. J. Ornithol. 147, 212–217. doi: 10.1007/BF01651789
Knutson, T. R., Sirutis, J. J., Garner, S. T., Vecchi, G. A., and Held, I. M. (2008). Simulated reduction in Atlantic hurricane frequency under twenty-first-century warming conditions. Nat. Geosci. 1, 359–364. doi: 10.1038/ngeo202
Krist, M. (2011). Egg size and offspring quality: a meta-analysis in birds. Biol. Rev. 86, 692–716. doi: 10.1111/j.1469-185X.2010.00166.x
Laaksonen, T., Korpimäki, E., and Hakkarainen, H. (2002). Interactive effects of parental age and environmental variation on the breeding performance of Tengmalm's owls. J. Anim. Ecol. 71, 23–31. doi: 10.1046/j.0021-8790.2001.00570.x
Lebreton, J. D., Burnham, K. P., Clobert, J., and Anderson, D. R. (1992). Modeling survival and testing biological hypotheses using marked animals: a unified approach with case studies. Ecol. Monogr. 62, 67–118. doi: 10.2307/2937171
Lee, D. E. (2011). Effects of environmental variability and breeding experience on northern elephant seal demography. J. Mammal. 92, 517–526. doi: 10.1644/10-MAMM-A-042.1
Lloret, J., Lleonart, J., Solé, I., and Fromentin, J. M. (2001). Fluctuations of landings and environmental conditions in the north-western Mediterranean Sea. Fish. Oceanogr. 10, 33–50. doi: 10.1046/j.1365-2419.2001.00151.x
Louzao, M., Arcos, J. M., Guijarro, B., Valls, M., and Oro, D. (2011). Seabird-trawling interactions: from species-specific to global community approach. Fish. Oceanogr. 20, 263–277. doi: 10.1111/j.1365-2419.2011.00579.x
Martin, K. (1995). Patterns and mechanisms for age-dependent reproduction and survival in birds. Am. Zool. 35, 340–348. doi: 10.1093/icb/35.4.340
Martínez-Abraín, A., Maestre, R., and Oro, D. (2002a). Demersal trawling waste as a food source for Western Mediterranean seabirds during the summer. ICES J. Mar. Sci. 59, 529–537. doi: 10.1006/jmsc.2001.1175
Mougin, J. L., Jouanin, C. H. R., and Roux, F. (1997). Intermittent breeding in Cory's Shearwater Calonectris diomedea of Selvagem Grande, North Atlantic. Ibis 139, 40–44. doi: 10.1111/j.1474-919X.1997.tb04502.x
Nakagawa, S., and Schielzeth, H. (2013). A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 4, 133–142. doi: 10.1111/j.2041-210x.2012.00261.x
Navarro, J., and Gonzalez-Solís, J. (2009). Environmental determinants of foraging strategies in Cory's shearwaters Calonectris diomedea. Mar. Ecol. Prog. Ser. 378, 259–267. doi: 10.3354/meps07880
Nevoux, M., Weimerskirch, H., and Barbraud, C. (2007). Environmental variation and experience-related differences in the demography of the long-lived black-browed albatross. J. Anim. Ecol. 76, 159–167. doi: 10.1111/j.1365-2656.2006.01191.x
Oro, D., and Ruiz, X. (1997). Exploitation of trawler discards by breeding seabirds in the north-western Mediterranean: differences between the Ebro Delta and the Balearic Islands areas. ICES J. Mar. Sci. 54, 695–707. doi: 10.1006/jmsc.1997.0246
Oro, D., Hernández, N., Jover, L., and Genovart, M. (2014). From recruitment to senescence: food shapes the age-dependent pattern of breeding performance in a long-lived bird. Ecology 95, 446–457. doi: 10.1890/13-0331.1
Oro, D., Torres, R., Rodríguez, C., and Drummond, H. (2010). Climatic influence on demographic parameters of a tropical seabird varies with age and sex. Ecology 91, 1205–1214. doi: 10.1890/09-0939.1
Ottersen, G., Planque, B., Belgrano, A., Post, E., Reid, P. C., and Stenseth, N. C. (2001). Ecological effects of the North Atlantic Oscillation. Oecologia 128, 1–14. doi: 10.1007/s004420100655
Pardo, D., Barbraud, C., Authier, M., and Weimerskirch, H. (2013). Evidence for an age-dependent influence of environmental variations on a long-lived seabird's life-history traits. Ecology 94, 208–220. doi: 10.1890/12-0215.1
Pyle, P., Sydeman, W. J., and Hester, M. (2001). Effects of age, breeding experience, mate fidelity and site fidelity on breeding performance in a declining population of Cassin's auklets. J. Anim. Ecol. 70, 1088–1097. doi: 10.1046/j.0021-8790.2001.00567.x
Ramos, J. A., Moniz, Z., Solá, E., and Monteiro, L. R. (2003). Reproductive measures and chick provisioning of Cory's Shearwarter Calonectris diomedea borealis in the Azores. Bird Study 50, 47–54. doi: 10.1080/00063650309461289
Sæther, B. E. (1990). Age-specific variation in reproductive performance of birds. Curr. Ornithol. 7, 251–283.
Sanz-Aguilar, A., Tavecchia, G., Pradel, R., Mínguez, E., and Oro, D. (2008). The cost of reproduction and experience-dependent vital rates in a small petrel. Ecology 89, 3195–3203. doi: 10.1890/08-0431.1
Sims, D. W., Wearmouth, V. J., Genner, M. J., Southward, A. J., and Hawkins, S. J. (2004). Low-temperature-driven early spawning migration of a temperate marine fish. J. Anim. Ecol. 73, 333–341. doi: 10.1111/j.0021-8790.2004.00810.x
Stearns, S. C. (2000). Life history evolution: successes, limitations, and prospects. Naturwissenschaften 87, 476–486. doi: 10.1007/s001140050763
Sydeman, W. J., Penniman, J. F., Penniman, T. M., Pyle, P., and Ainley, D. G. (1991). Breeding performance in the western gull: effects of parental age, timing of breeding and year in relation to food availability. J. Anim. Ecol. 60, 135–149. doi: 10.2307/5450
Tavecchia, G., Pradel, R., Genovart, M., and Oro, D. (2007). Density−dependent parameters and demographic equilibrium in open populations. Oikos 116, 1481–1492. doi: 10.1111/j.0030-1299.2007.15791.x
Thibault, J. C. (1994). Nest-site tenacity and mate fidelity in relation to breeding success in Cory's shearwater Calonectris diomedea. Bird Study 41, 25–28. doi: 10.1080/00063659409477193
Thibault, J. C., Bretagnolle, V., and Rabouam, C. (1997). The Cory's shearwater (Calonectris diomedea). BWP Update 1, 75–98.
Vieyra, L., Velarde, E., and Ezcurra, E. (2009). Effects of parental age and food availability on the reproductive success of Heermann's gulls in the Gulf of California. Ecology 90, 1084–1094. doi: 10.1890/07-2009.1
Votier, S. C., Bearhop, S., Witt, M. J., Inger, R., Thompson, D., and Newton, J. (2010). Individual responses of seabirds to commercial fisheries revealed using GPS tracking, stable isotopes and vessel monitoring systems. J. Appl. Ecol. 47, 487–497. doi: 10.1111/j.1365-2664.2010.01790.x
Ware, D. M., and Tanasichuk, R. W. (1989). Biological basis of maturation and spawning waves in Pacific Herring (Clupea harengus pallasi). Can. J. Fish. Aquat. Sci. 46, 1776–1784. doi: 10.1139/f89-225
Weimerskirch, H., Louzao, M., Grissac, S., and Delord, K. (2012). Changes in wind pattern alter albatross distribution and life-history traits. Science 335, 211–214. doi: 10.1126/science.1210270
Keywords: age pattern, breeding performance, Scopoli's Shearwaters, climatic index, food availability, migratory seabird
Citation: Hernández N, Genovart M, Igual JM and Oro D (2015) The influence of environmental conditions on the age pattern in breeding performance in a transequatorial migratory seabird. Front. Ecol. Evol. 3:69. doi: 10.3389/fevo.2015.00069
Received: 08 October 2014; Accepted: 15 June 2015;
Published: 26 June 2015.
Edited by:
Karin Charlotta Harding, University of Gothenburg, SwedenReviewed by:
Daniel Croft Barton, Humboldt State University, USAJoacim Näslund, University of Gothenburg, Sweden
Copyright © 2015 Hernández, Genovart, Igual and Oro. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Noelia Hernández, Population Ecology Group, Department of Biodiversity and Conservation, Mediterranean Institute of Advanced Studies (CSIC-UIB), Miquel Marquès 21, 07190, Esporles, Mallorca, Spain, nhernandez@imedea.uib-csic.es
 Noelia Hernández
Noelia Hernández Meritxell Genovart
Meritxell Genovart José M. Igual
José M. Igual Daniel Oro
Daniel Oro