Evidence for additive effects of virus infection and water availability on phytohormone induction in a staple crop
- 1Natural Resources Management and Environmental Sciences, California State Polytechnic University, San Luis Obispo, CA, USA
- 2Plant, Soil, and Entomological Sciences Department, University of Idaho, Moscow, ID, USA
Infection with phytoviruses influences plant responses to environmental stress, but the biochemical mechanisms underlying these interactions are unknown. Infection of wheat (Triticum aestivum) with a cereal virus (Barley yellow dwarf virus, BYDV) has context-dependent effects on plant productivity and survival conditional to water stress, and we hypothesized this was due to phytohormone induction resulting from virus infection. We tested whether BYDV infection and water availability interact to influence hormone profiles in wheat across multiple time periods. Wheat plants were inoculated with BYDV by exposing them to infectious aphids (Rhopalosiphum padi). Concentrations of five hormones [abscisic acid, jasmonic acid, methyl jasmonate, methyl salicylate (MS), and salicylic acid (SA)] in leaf tissues were compared to concentrations in plants exposed to noninfectious aphids (sham treatment) and nondamaged control plants for five time-since-infection periods (0, 8, 16, 24, and 32 d) and two levels of water availability (0.2 and 0.8 g H20/g soil). Three important findings emerged: (1) total hormone concentrations in BYDV-infected plants exceeded concentrations in sham-treated and control plants up to 16 d following infection, after which nondamaged plants exhibited the highest concentrations of hormones; compared with nondamaged and BYDV-infected plants, hormone levels were reduced in sham-treated plants; (2) inoculation treatment affected concentrations of MS and SA: SA concentrations were increased in BYDV-infected plants, but control plants exhibited higher MS concentrations than either BYDV-infected or sham-treated plants irrespective of watering treatments and across all time periods; and (3) correlation analysis revealed no evidence of hormonal cross-inhibition. This study provides the first evidence that BYDV infection elevates both total phytohormone levels and SA in wheat in a time-sensitive manner, suggesting a potential biochemical basis for virus-induced hormonal responses that alter plant responses to environmental stress.
Introduction
Infection with viruses can alter downstream plant responses to biotic and abiotic stressors. Plant viruses have historically been viewed as strictly pathogenic agents, causing plant injury and economic losses for producers (Malmstrom et al., 2011); yet, recent research indicates that virus infection is much more widespread in managed and nonmanaged systems than previously thought, and cause a variety of effects other than pathogenicity (Roossinck, 2013). In nonmanaged ecosystems, some authors estimate that natural infection rates often exceed 50%, with many infections causing no discernible symptoms (Roossinck, 2012b). Virus infection can also impart advantages to infected hosts. For example, genetic stock of certain crop species has been inadvertently selected by breeders for infection with vertically transmitted viruses, presumably because of yield advantages imparted by infection (e.g., Capsicum annuum L., Okada et al., 2011; Roossinck, 2012a). In beets (Beta vulgaris L.), infection with Cucumber mosaic virus (CMV) enhances plant thermal and water-stress tolerance (Xu et al., 2008). Similarly, in a tropical panic grass (Dichanthelium lanuginosum), association with specific virus-infected fungal endophytes imparts heat tolerance (Márquez et al., 2007). However, despite these apparently widespread and complex ecological associations, the underlying mechanisms driving differential plant responses to stress following virus infection are seldom explored (Xu et al., 2008).
One probable pathway by which viruses alter the capacity of plants to withstand environmental stressors is via induction of plant growth regulators, or “phytohormones.” Phytohormones are generally grouped into five major classes: abscisic acid (ABA), auxins, cytokinins, ethylene, and gibberellins; however, numerous other compounds such as brassinosteroids, jasmonates, salicylates, and polyamines are also important plant growth regulators (Tuteja and Sopory, 2008). Many of these compounds regulate plant responses to stress events, and their induction can lead to changes in plant physiological characters and growth to produce stress-tolerant phenotypes (Hale and Orcutt, 1987). For example, water stress-induced ABA translocation incites stomatal closure and gas exchange at the leaf surface, and heightened ABA concentrations affect newly developing leaves by reducing stomatal densities (Zhang et al., 1987; Frank and Farquhar, 2001). Similarly, induction of SA can reduce oxidative damage and promote photosynthesis under conditions of water and osmotic stress (Borsani et al., 2001).
Phytohormones are also involved in responses to biotic stresses including pathogen infection and herbivory (Bostock, 2005; Rostas and Turlings, 2008). The role of SA in promoting systemic acquired resistance in plants following challenge with pathogens is well established (Vasyukova and Ozeretskovkaya, 2007). Both SA and ABA were shown to be responsive to virus infection in multiple study systems (Whenham et al., 1986; Xu et al., 2008). The multiple roles of phytohormones suggest they are associated with or can account for virus infection effects on drought and stress tolerance. If plants infected with viruses exhibit increased concentrations of certain phytohormones, this could affect plant phenotypes and buffer plants against subsequent environmental stress (Márquez et al., 2007).
In previous experiments, wheat (Triticum aestivum L.) infected with Barley yellow dwarf virus (BYDV) exhibited enhanced tolerance to acute water stress but was damaged by BYDV infection under conditions of ample water (Davis et al., 2015), and it was hypothesized that this was due to induction of phytohormones following BYDV infection. Here, we investigate this hypothesis by evaluating the concentrations of five phytohormones (ABA; SA; JA, jasmonic acid; MJ, methyl jasmonate; and MS, methyl salicylate), which are linked to drought and thermal tolerance and systemic acquired resistance. Our specific objective was to track the concentrations of these hormones in wheat over time following short-term exposure to herbivory from BYDV-infected aphids (Rhopalosiphum padi L.), and under conditions of both water stress and ample water availability. Our analysis suggests that BYDV infection has stronger effects on hormone concentrations than water availability treatments, with infected and noninfected plants displaying complex but differing hormonal responses.
Methods and Materials
Aphid Colonies and Virus Inoculations
Viruliferous and nonviruliferous aphid colonies originated from a R. padi clone collected in the vicinity of Moscow, ID, USA (coordinates: 46.732°N, 116.999°W, 786 m elevation). Aphid colonies are maintained on barley (Hordeum vulgare L., “Sprinter”) in environmental growth chambers (Mod-1-36VLX, Percival Scientific, Perry, IA, USA) at 20 ± 1°C under a photoperiod of 16L/8D. Colony infection status is regularly tested using the enzyme-linked immunosorbent assay (ELISA) or RT-PCR to ensure that aphid colonies are either viruliferous or not. A Washington state isolate of BYDV (Padi-avenae virus; PAV) maintained by mass transfer of R. padi on barley was used to inoculate wheat plants in all experiments (Jiménez-Martínez and Bosque-Pérez, 2004).
We used methods described by Jiménez-Martínez and Bosque-Pérez (2004) to inoculate wheat plants with BYDV-PAV, which achieves a 100% success rate in virus transmission. Ten viruliferous aphids were contained on 15-d-old plants in dialysis tubes (3 cm long, 1 cm diameter, Spectrum Lab, Inc., Rancho Dominguez, CA, USA) stoppered with foam. Aphids were allowed to feed within tubes for 72 h, and were then carefully removed and euthanized in soapy water. As a control treatment, we exposed plants to herbivory from nonviruliferous aphids using the above technique (“sham” treatment). BYDV infection (or lack of infection) was confirmed for all experimental plants using ELISA following inoculations.
Experimental Design
We employed a factorial design to test how water availability and “time-since-infection” affect the concentrations of hormones in the leaf tissues of a soft white spring club wheat variety (T. aestivum ssp. compactum, cv. “JD”). A total of 90 plants were sown in 12 cm diameter pots containing 330 ± 7 g SD of soil (Sunshine mix no. 1; Lot no. S13-084; SunGro Horticulture, Agawam, MA, USA) in a greenhouse under the following environmental conditions: mean temperature: 24.0 ± 0.33°C SE; maximum temperature: 30.5 ± 1.1°C SE; minimum temperature: 16.6 ± 1.4°C SE; relative humidity: 60.5 ± 2.11% SE; and a photoperiod of 14L/10D.
At 15 d post sowing plants were exposed to infection treatments, and plants were inoculated with BYDV (n = 30) or sham-treated (n = 30) as described above, or left as nondamaged controls (n = 30). Watering treatments were applied on a gravimetric basis, and each infection status treatment was further subdivided such that half of the plants received a “low water” (0.2 g water/g soil) treatment and half the plants received a “high water” treatment (0.8 g water/g soil). Watering treatments were applied at the soil surface using a graduated cylinder every 48 h. The development of watering treatments is described in Davis et al. (2015). Plants were randomly arranged on greenhouse benches and three plants in each infection status × water treatment combination were randomly selected for destructive leaf tissue sampling every 8 days for five sample periods, beginning immediately after removal of aphids (i.e., samples taken at 0, 8, 16, 24, and 32 d post-inoculation). At each sampling period, the two most apical leaves on each selected plant were harvested into liquid nitrogen by excision with a scalpel at the auricle, and after leaf excision plants were removed from the experiment to ensure that all samples were independent. Flash-frozen leaf samples were immediately placed in a freezer at −20°C until analysis.
Extraction and Quantification of Phytohormones
We used the methods described in Forcat et al. (2008) to simultaneously characterize concentrations of ABA, SA, JA, MJ, and MS in each extract. Prior to analysis, flash-frozen leaf tissue samples were freeze dried and powdered tissue was weighed and placed into 2 ml centrifuge tubes and extracted with 400 μl of 10% methanol containing 1% acetic acid using a bead beater (Bullet Blender, Next Advance, Averill Park, NY) with two 3 mm tungsten beads at 25 Hz/s for 3 min. After beating, samples were placed on ice for 30 min, then centrifuged at 10,000 g for 10 min at room temperature. The resulting supernatant was decanted into new 2 ml centrifuge tubes. The remaining pellet was re-extracted using the same procedure and decanted into the first collection of supernatant, for a total of 800 μl of extract for each leaf tissue sample.
A 5 μl aliquot of each extract was analyzed for hormones. Analysis of samples was performed by high performance liquid chromatography (HPLC) using an Agilent 1200 Series HPLC system with a diode array detection (DAD) system coupled to an Agilent G1969A TOF-MS system equipped with an ESI source (Agilent, Santa Clara, CA, USA). The chromatographic separation of plant hormones was performed on a Zorbax XDB-C18, 50 mm × 4.6 mm, with a 1.8 μm i.d. (Agilent, Santa Clara, CA, USA) maintained at 30°C. The mobile phase consisted of 0.1% formic acid in water (solvent A) and 0.1% formic acid in methanol (solvent B). A linear gradient was used from 50 to 95% B in 5 min, followed by isocratic elution at 95% B for 2 min, and finally equilibrated at 50% B for 3 min. For the first 1 min of the analysis, the flow was diverted from the MS to prevent MS contamination and ion suppression with salts and other polar species. The flow rate through the column was 0.4 ml min−1 and spectra were recorded from 190 to 400 nm. Electrospray ionization was operated in negative mode for the first 4 min, and then switched to positive mode. In both modes the absolute values for electrospray ionization potential and collision-induced dissociation potential are 3500 and 125 V, respectively. Gas temperature was held at 350°C and drying gas (N2) was delivered at a flow rate of 12 L min−1 with nebulizer pressure maintained at 2.4 × 105 Pa. Quantification was performed in the reconstructed ion current mode using m/z of 263.13 (ABA), 137.02 (SA), 209.11 (JA), and 225.15 (MJ). MS was quantified by its absorbance at 300 nm. Calibration curves were constructed for remaining hormones of interest using authentic standards (Sigma-Aldrich), and limits of detection for these analytes were approximately 60 ppb.
Statistical Analysis
Our experiment was analyzed using a nested two-way ANOVA, with water availability (n = 2 water treatments), infection status (n = 3 infection treatments), and the interaction between water availability and infection status nested by day (n = 5 time periods) and treated as fixed effects on the response variables of concentrations (mg/g) of ABA, JA, MJ, MS, and SA, as well as the total sum of all hormones. We also analyzed the correlation structure of hormone concentrations by evaluating all pairwise correlation coefficients in hormone concentrations within each infection status treatment, and unscaled correlations were subjected to principal components analysis to visualize the multivariate relationships between hormone concentrations due to BYDV infection. All analyses were performed using the software package JMP 10.0 (SAS Institute, Cary, NC).
Results
Our statistical model revealed that water availability and BYDV infection explained a significant portion of the variance in total phytohormone concentrations in wheat leaf tissues [F(29, 58) = 3.076; P < 0.001; R2 = 0.606; Figure 1]. The main effect of water availability occurred at the 8, 24, and 32 d time periods, where plants watered at 0.8 g water/g soil exhibited 22, 69, and 59% higher total phytohormone concentrations than plants watered at the 0.2 g water/g soil level [F(5, 58) = 5.104, P < 0.001]. At 8 and 16 d following experimental inoculations, plants infected with BYDV exhibited 42 and 30% higher total phytohormone concentrations than nondamaged controls and 60 and 83% higher phytohormone concentration than sham-treated plants on average. At 24 and 32 d following experimental inoculations, nondamaged control plants had the highest average phytohormone concentrations with 35 and 66% higher total phytohormone concentrations than BYDV-infected plants, and 63 and 62% higher total phytohormone concentrations than sham-treated plants, respectively [F(10, 58) = 4.446, P < 0.001]. There was no statistical evidence that water × infection status interactions affected total phytohormone concentrations [F(10, 58) = 1.221, P = 0.297] or that total phytohormone concentrations increased over time [F(4, 58) = 3.956, P = 0.091].
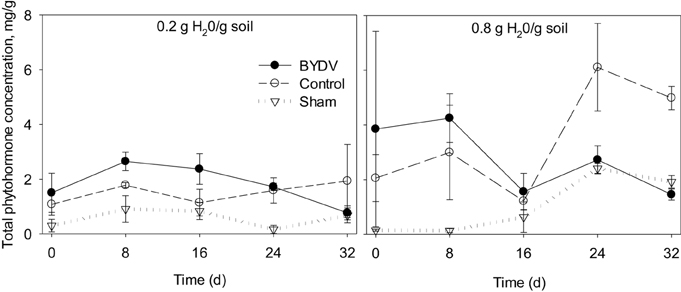
Figure 1. Time series tracking changes in the summed concentrations of five phytohormones (abscisic acid, jasmonic acid, methyl jasmonate, methyl salicylate, and salicylic acid) in wheat leaves in response to two watering treatments (0.2 and 0.8 g H20/g soil) and three virus infection treatments (BYDV, sham treatment, and nondamaged control). Bars show one standard error of the mean.
There was no evidence that concentrations of ABA were affected by water availability, BYDV infection, or an interaction between water availability and BYDV infection (Table 1A). ABA concentrations increased consistently over the course of the experiment, with average ABA leaf tissue concentrations of 0.329, 0.466, 1.167, 1.488, and 2.237 mg/g corresponding to 0, 8, 16, 24, and 32 d following the virus infection treatments (Figure 2A).
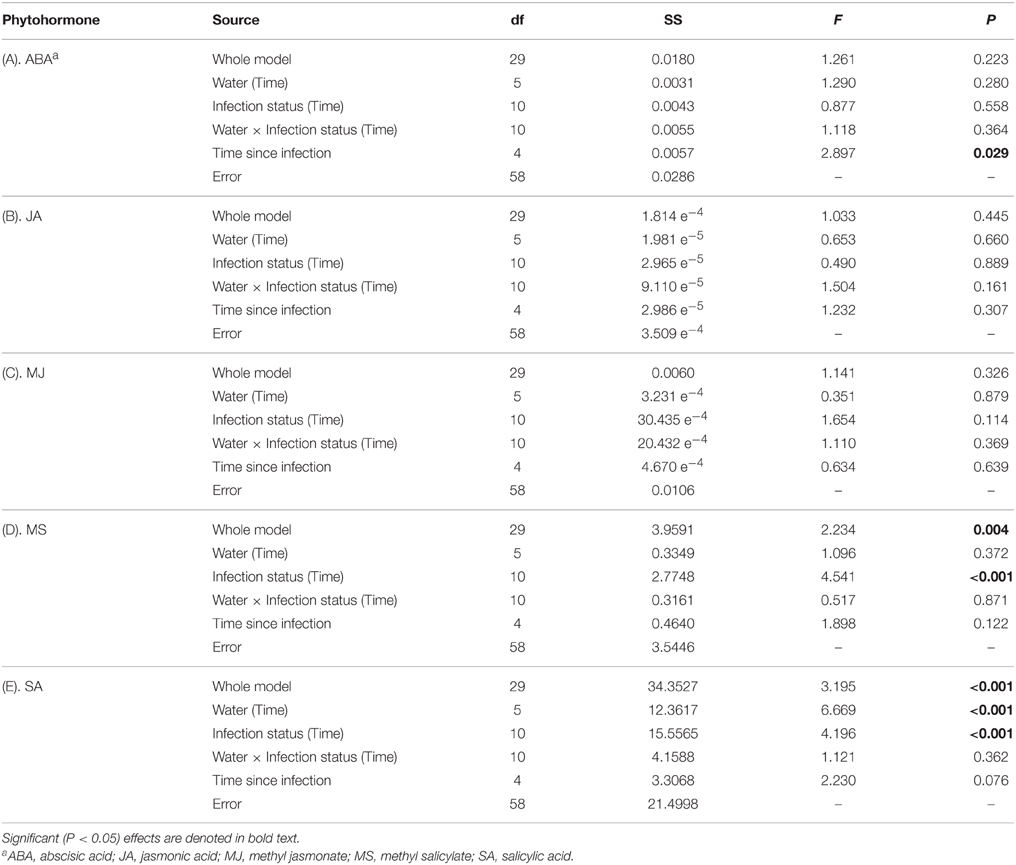
Table 1. Summary of ANOVA model for the effects of water availability and virus infection treatment, nested by time since infection, on concentrations (mg/g) of five phytohormones in wheat leaf tissues.
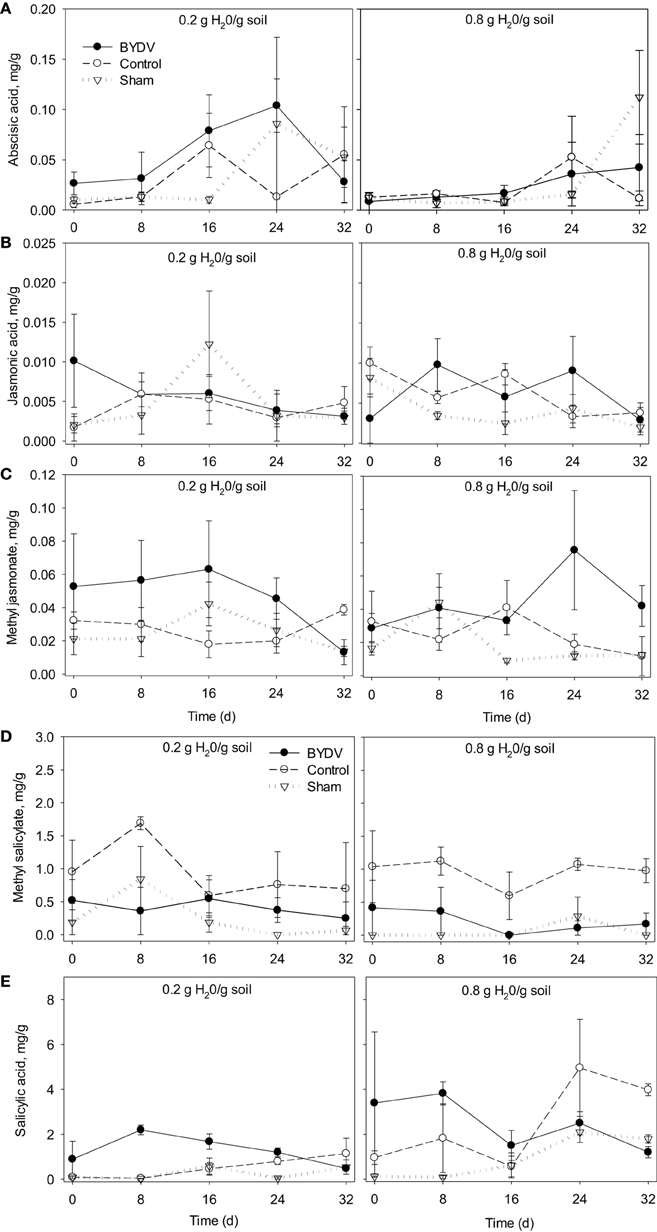
Figure 2. Time series showing changes in mean concentrations of (A) abscisic acid, (B) jasmonic acid, (C) methyl jasmonate, (D) methyl salicylate, and (E) salicylic acid in wheat leaf tissues in response to two watering treatments (0.2 and 0.8 g H20/g soil) and three virus infection treatments (BYDV, sham treatment, and nondamaged control). Bars show one standard error of the mean.
Concentrations of JA were not affected by any factors in our statistical model (Table 1B; Figure 2B). In contrast, MJ concentrations were marginally affected (P = 0.114) by BYDV infection, but not by water availability, the water availability × infection status interaction, or time since infection (Table 1C). Under conditions of low water availability (0.2 g water/g soil) mean MJ concentrations in leaf tissues of BYDV infected plants were higher than concentrations found in nondamaged control and sham-treated plants from 0 to 24 d. In contrast, under conditions of ample water (0.8 g water/g soil) MJ concentrations were similar across virus infection treatments until 24–32 d, at which point BYDV infected plants exhibited substantially higher concentrations of MJ than nondamaged control and sham-treated plants (Figure 2C).
Our statistical model also accounted for a substantial portion of the variance in average MS concentrations (R2 = 0.520), with short-term exposure to herbivory contributing the majority of the variance in MS concentration (Table 1D). Under conditions of both low and ample water, the leaves of nondamaged control plants had on average 58 and 79% higher MS concentrations than BYDV infected plants, and 76 and 92% higher MS concentrations than sham-treated plants, respectively (Figure 2D).
Similarly, our model accounted for a significant portion of the variance in SA concentrations in wheat leaf tissues (R2 = 0.694), though there was no evidence for a water availability × infection status interaction (Table 1E). Under conditions of low water availability, SA concentrations in the leaves of BYDV infected plants were on average 87, 98, and 71% higher than those of nondamaged control and sham-treated plants at 0, 8, and 16 d after inoculations. This effect declined over time and SA concentrations were highest in nondamaged control plants at 32 d. A similar pattern was observed under conditions of ample water, with average concentrations of SA in leaves of BYDV infected plants surpassing those of nondamaged control and sham-treated plants by 82, 78, and 67% at 0, 8, and 16 d after inoculation, respectively. As occurred under conditions of low water, the effect of infection on SA declined over time and nondamaged control plants had the highest mean SA concentrations at 32 d (Figure 2E).
Concentrations of individual phytohormones in leaf tissues were correlated (Table 2), but there was no evidence of cross-inhibition in hormone concentrations. In BYDV-infected plants, there was marginal statistical support for a positive correlation between concentrations of SA and MJ. In nondamaged control plants, SA and ABA concentrations were positively correlated, as were JA and MJ. Principal components analysis using concentrations of all phytohormones across all time periods explained a modest portion of the variance in the dataset (Figure 3), indicating a multivariate response of phytohormone concentrations to virus infection × water availability interactions.
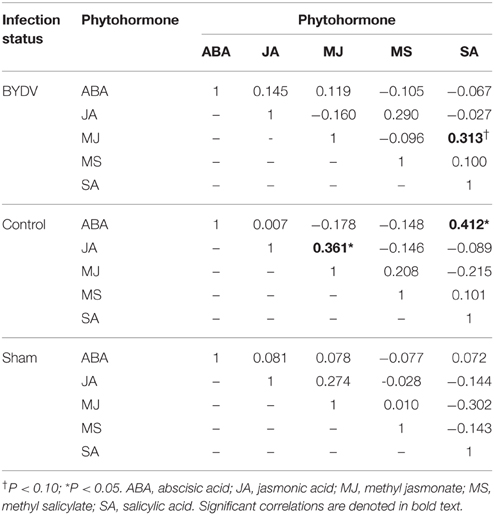
Table 2. Correlation coefficients (r) for pairwise comparisons of concentrations (mg/g) of five phytohormones in wheat leaf tissues, with correlations nested by virus infection treatment.
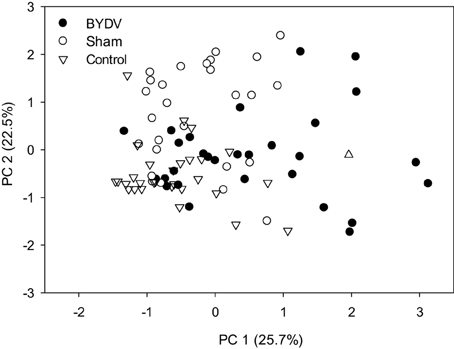
Figure 3. Principal components analysis comparing the multivariate structure in concentrations (mg/g) of five phytohormones: abscisic acid, jasmonic acid, methyl jasmonate, methyl salicylate, and salicylic acid due to the effect of three virus infection treatments (BYDV, sham treatment, and nondamaged control).
Discussion
Our experiment provides evidence that BYDV infection induces some phytohormones in a time-sensitive manner, potentially providing a biochemical explanation for previously described context-dependent effects of virus infection on subsequent plant responses to water availability (Davis et al., 2015). Water availability and BYDV infection had complex and variable effects on the induction of five phytohormones in wheat leaf tissues. Our experiment suggests that the effects of water availability and virus infection treatments on concentrations of ABA, JA, MJ, MS, and SA in wheat leaves can be treated additively, as no significant interaction between these two variables was observed for any measured phytohormones. The only hormone responsive to differences in water availability was SA, although total concentrations of hormones varied due to water inputs with well-watered plants exhibiting a higher capacity for induction. In contrast, MS and SA were both responsive to virus infection treatments, with BYDV-infected plants generally exhibiting higher concentrations of SA, and nondamaged control plants with higher concentrations of MS. The timing of MJ induction in response to BYDV infection was modulated by water availability, and although this response was only marginally statistically significant (Figure 2). These patterns suggest that the timing of pathogen-induced MJ responses depend on water availability, whereas SA induction appears to occur immediately after infection with BYDV but is reduced relative to nondamaged control plants after several weeks. Moreover, total hormone concentrations were reduced in sham-treated plants (as compared with nondamaged control plants) under both watering regimes but more strongly under low water conditions, suggesting a possible effect of aphid feeding and water availability on phytohormone induction in wheat plants that differs from the effects of BYDV infection.
The present study was designed to test the hypothesis that BYDV infection induces biochemical pathways that play a role in environmental stress tolerance (Davis et al., 2015). Hormone induction resulting from BYDV infection has not been previously linked with subsequent plant responses to abiotic stress, although virus-induced phytohormone production that influences plant stress tolerance is well-described in other pathosystems. For example, Whenham et al. (1986) demonstrated that concentrations of ABA increase dramatically in Nicotiana tabacum L. leaves systemically infected with the Tobacco mosaic virus (TMV). In wheat, ABA has been implicated in responses to drought stress. ABA plays a critical role in protecting plant cells from oxidative damage resulting from water stress, and moderately water stressed plants treated topically with ABA exhibit increases in leaf chlorophyll and carotenoids, as well as relative water content (Agarwal et al., 2005). We measured ABA at 8 day intervals, and did not detect a significant response of ABA to watering or infection status treatments. There were nonsignificant trends of increasing concentrations of ABA over time differing among water treatments (Figure 2A). However, when ABA measurements were performed at 48 h intervals as described by Vaseva et al. (2010), ABA concentrations showed a rapid increase over 7 days before plateauing, though Agarwal et al. (2005) and Vaseva et al. (2010) relied on water deprivation rather than watering at low levels as in the present study. Thus, rates of ABA induction may vary across the timescale of observation and depending on whether water stress is chronic or acute, accounting for patterns of plant resistance or tolerance to certain stress events.
In addition to ABA, other phytohormones could influence how cereal crops respond to abiotic stress events following challenge with viral pathogens. We found that SA concentrations in new wheat leaves were affected by both water availability and BYDV infection, suggesting that SA-dependent pathways are part of the plant's response to infection with BYDV. Induction of SA signaling pathways has been strongly linked with plant tolerance of abiotic stress (Horváth et al., 2007). For instance, Kang et al. (2013) demonstrated that exogenous application of SA, which is involved in systemic acquired resistance following exposure to pathogens, directly improved wheat drought tolerance in greenhouse studies. Similarly, Hamada (1998) found that directly applying SA to nongerminated wheat seeds alleviated the inhibitory growth effects of drought stress by enhancing photosynthesis and reducing nighttime respiration. Shakirova et al. (2003) showed that treatment of wheat seedlings with 0.05 mM SA reduced plant injury due to the effects of salinity, and also demonstrated that SA application was associated with a simultaneous accumulation of ABA and indoleacetic acid (IAA), suggesting dynamic interactions among induction pathways. However, comparisons between the effects of exogenous applications and pathogen induced SA accumulation are tenuous; for example, Shakirova et al. (2003) applied SA at a concentration that was roughly 100-fold what we detected in BYDV-induced leaf tissues.
Cross-inhibition of phytohormone induction pathways complicates the interpretation of experimental studies such as the one described here, as well as tests of hormonal interactions on plant performance. For example, a rise in ABA concentrations in plant tissues following viral infection can suppress SA induction. In Arabidopsis thaliana, application of ABA suppressed systemic acquired resistance resulting from chemical exposure by inhibiting the SA response (Yasuda et al., 2008). Similar inhibitory cross-talk has been demonstrated between ABA and jasmonate and ethylene pathways (Anderson et al., 2004), which are important for subsequent plant responses to herbivory, although the roles of these other phytohormones in environmental stress sensitivity have not been extensively tested. Likewise, induction of SA is inhibitory to JA in A. thaliana and interferes with systemic acquired resistance to herbivory from both generalist chewing herbivores (Cipollini et al., 2004) and sap-feeding herbivores (Zarate et al., 2007), though this effect is known to be herbivore-specific (Diezel et al., 2009). In the present study, a lack of negative correlations in the data suggests that cross-inhibition in hormonal induction pathways did not occur in appreciable magnitude. Alternatively, cross-hormonal inhibition may not occur in cultivated wheat to the same extent as has been documented in model species such as A. thaliana. This possibility is supported by Bandurska and Stroiṅski (2005), who found that concentrations of ABA in barley leaves were promoted rather than reduced by external application of SA, resulting in diminished plant injury under experimental drought. Similar findings were reported by Shakirova et al. (2003). Moreover, we showed that positive correlations between phytohormones were present in nondamaged wheat plants (Table 2), but these correlations were not detected in plants exposed to short-term (72 h) aphid herbivory (sham-treated plants), potentially indicating that aphid herbivory can decouple linked plant growth regulator pathways.
The present study is among the first investigations into phytohormone profiles in wheat following infection with a viral pathogen, and the first to consider how pathogen infection and environmental conditions act in tandem and over time to affect plant induction pathways. Here, we report on the concentrations of only five phytohormones, although many other biochemical pathways may be responsive to interactions between water availability and BYDV infection in wheat (Comeau and Haber, 2002). For instance, Wang et al. (2013) identified more than 30 gene transcripts related to hormone induction and signaling that were differentially expressed in wheat infected with BYDV, and this effect was largely genotype-dependent. Their data, similar to ours, demonstrated that plant biochemical shifts following BYDV infection are extremely complex and present significant interpretive challenges. Although focusing on individual signaling pathways in response to pathogen infection may simplify interpretations of biological relevance, it is a much more realistic approach to consider the induction of signaling molecules from a multivariate perspective; over time, meta-analytical studies can enhance the application of such data in both agriculture and ecology. From the present work, we conclude that pathogen (BYDV) infection and water availability are likely to act additively rather than interactively to influence phytohormone abundances, except in the case of MJ, which may respond to interactions between pathogen infection and environmental conditions. In general, pathogen infection had stronger effects on hormone concentrations than watering treatments, and differed from the effects of exposure to short-term herbivory. We found no evidence for cross-inhibition among the measured hormones, and moderate support for discriminant groupings based on multivariate signaling phenotypes. Overall, new leaves of BYDV-infected plants exhibited higher concentrations of SA than leaves from sham-treated and nondamaged control plants, and other studies suggest that the induction of these hormones (particularly SA) by BYDV may play a role in subsequent plant resistance to environmental stress (as in Davis et al., 2015).
Author Contributions
TD, NB, and SE designed the experiment. TD performed the experiment, analyzed the data, and wrote the first draft of the manuscript. IP performed phytohormone analyses. All authors contributed to revising the manuscript and approving the final version.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors are grateful to Lana Unger for providing access to aphid colonies and to Dr. Matthew Morra (University of Idaho) for committing technical support to analysis of hormone profiles. Dr. Arron Carter (University of Washington) provided wheat seed, and Dr. Robert Tripepi (University of Idaho) loaned laboratory equipment. We also appreciate the efforts of the referees who contributed their time and effort to improve this manuscript. This work was supported with funds from Award #2011-68002-30191 from the USDA National Institute of Food and Agriculture.
References
Agarwal, S., Sairam, R. K., Srivastava, G. C., and Meena, R. C. (2005). Changes in antioxidant enzymes activity and oxidative stress by abscisic acid and salicylic acid in wheat genotypes. Biol. Plantarum 49, 541–550. doi: 10.1007/s10535-005-0048-z
Anderson, J. P., Badruzsaufari, E., Schenk, P. M., Manners, J. M., Desmond, O. J., Ehlert, C., et al. (2004). Antagonistic interaction between abscisic acid and jasmonate-ethylene signaling pathways modulates defense gene expression and disease resistance in Arabidopsis. Plant Cell 16, 3460–3479. doi: 10.1105/tpc.104.025833
Bandurska, H., and Stroiṅski, A. (2005). The effect of salicylic acid on barley response to water deficit. Acta Physiol. Plant. 27, 379–386. doi: 10.1007/s11738-005-0015-5
Borsani, O., Valpuesta, V., and Botella, M. A. (2001). Evidence for a role of salicylic acid in the oxidative damage generated by NaCl and osmotic stress in Arabidopsis seedlings. Plant Physiol. 126, 1024–1030. doi: 10.1104/pp.126.3.1024
Bostock, R. M. (2005). Signal crosstalk and induced resistance: straddling the line between cost and benefit. Ann. Rev. Phytopathol. 43, 545–580. doi: 10.1146/annurev.phyto.41.052002.095505
Cipollini, D., Enright, S., Traw, M. B., and Bergelson, J. (2004). Salicylic acid inhibits jasmonic acid-induced resistance of Arabidopsis thaliana to Spodoptera exigua. Mol. Ecol. 13, 1643–1653. doi: 10.1111/j.1365-294X.2004.02161.x
Comeau, A., and Haber, S. (2002). “Breeding for BYDV tolerance in wheat as a basis for multiple stress tolerance strategy”, in Barley Yellow Dwarf Disease: Recent Advances and Future Strategies, eds M. Henry and A. McNAb (Mexico: CIMMYT), 82–92.
Davis, T. S., Bosque-Pérez, N. A., Foote, N. E., Magney, T., and Eigenbrode, S. D. (2015). Environmentally dependent host-pathogen and vector-pathogen interactions in the Barley yellow dwarf virus pathosystem. J. Appl. Ecol. 52, 1392–1401. doi: 10.1111/1365-2664.12484
Diezel, C., von Dahl, C. C., Gaquerel, E., and Baldwin, I. T. (2009). Different lepidopteran elicitors account for cross-talk in herbivory-induced phytohormone signaling. Plant Physiol. 150, 1576–1586. doi: 10.1104/pp.109.139550
Forcat, S., Bennett, M. H., Mansfield, J. W., and Grant, M. R. (2008). A rapid and robust method for simultaneously measuring changes in the phytohormones ABA, JA, and SA in plants following biotic and abiotic stress. Plant Methods 4:16. doi: 10.1186/1746-4811-4-16
Frank, P. J., and Farquhar, G. D. (2001). The effects of exogenous abscisic acid on stomatal development, stomatal mechanics, and leaf gas exchange in Tradescantia virginiana. Plant Physiol. 125, 935–942. doi: 10.1104/pp.125.2.935
Hale, M. G., and Orcutt, D. M. (1987). The Physiology of Plants Under Stress. Chichester: John Wiley & Sons.
Hamada, A. M. (1998). ”Effects of exogenously added ascorbic acid, thiamin, or aspirin on photosynthesis and some related activities of drought-stressed wheat plants”, in Photosynthesis: Mechanisms and Effects, ed G. Garab (Dordrecht: Kluwer Academic Publishing), 2581–2584.
Horváth, E., Szalai, G., and Janda, T. (2007). Induction of abiotic stress tolerance by salicylic acid signaling. J. Plant Growth Regul. 26, 290–300. doi: 10.1007/s00344-007-9017-4
Jiménez-Martínez, E. S., and Bosque-Pérez, N. A. (2004). Variation in barley yellow dwarf virus transmission efficiency by Rhopalosiphum padi after acquisition on transgenic and nontransformed wheat genotypes. J. Econ. Entomol. 97, 1790–1796. doi: 10.1093/jee/97.6.1790
Kang, G. Z., Li, G. Z., Liu, G. Q., Xu, W., Peng, X. Q., Wang, C. Y., et al. (2013). Exogenous salicylic acid enhances wheat drought tolerance by influence on the expression of genes related to ascorbate-glutathione cycle. Biol. Plant. 57, 718–724. doi: 10.1007/s10535-013-0335-z
Malmstrom, C. M., Melcher, U., and Bosque-Pérez, N. A. (2011). The expanding field of plant virus ecology: historical foundations, knowledge gaps, and research directions. Virus Res. 159, 84–94. doi: 10.1016/j.virusres.2011.05.010
Márquez, L. M., Redman, R. S., Rodriguez, R. J., and Roossinck, M. J. (2007). A virus in a fungus in a plant: three way symbiosis required for thermal tolerance. Science 315, 513–515. doi: 10.1126/science.1136237
Okada, R., Kiyota, E., Sabanadzovic, S., Moriyama, H., Fukuhara, T., Saha, P., et al. (2011). Bell pepper edornavirus: molecular and biological properties, and occurrence in the genus Capsicum. J. Gen. Virol. 92, 2664–2673. doi: 10.1099/vir.0.034686-0
Roossinck, M. J. (2012a). Persistent plant viruses: molecular hitchhikers or epigenetic elements, in Viruses: Essential Agents of Life, ed G. Witzany (New York, NY: Springer). doi: 10.1007/978-94-007-4899-6_8
Roossinck, M. J. (2012b). Plant virus metagenomics: biodiversity and ecology. Ann. Rev. Genet. 46, 357–367. doi: 10.1146/annurev-genet-110711-155600
Roossinck, M. J. (2013). Plant virus ecology. PLOS Pathog. 9:e1003304. doi: 10.1371/journal.ppat.1003304
Rostas, M., and Turlings, C. J. (2008). Induction of systemic acquired resistance in Zea mays also enhances the plant's attractiveness to parasitoids. Biol. Control 46, 178–186. doi: 10.1016/j.biocontrol.2008.04.012
Shakirova, F. M., Sakhabutdinova, A. R., Berukova, M., Fatkhutdinova, R. A., and Fatkhutdinova, D. R. (2003). Changes in the hormonal status of wheat seedlings induced by salicylic acid and salinity. Plant Sci. 164, 317–322. doi: 10.1016/S0168-9452(02)00415-6
Tuteja, N., and Sopory, S. K. (2008). Chemical signaling under abiotic stress environment in plants. Plant Signal. Behav. 3, 525–536. doi: 10.4161/psb.3.8.6186
Vaseva, I. I., Grigorova, B. S., Simova-Stoilova, L. P., Demirevska, K. N., and Feller, U. (2010). Abscisic acid and late embryogenesis abundant protein profile changes in winter wheat under progressive drought stress. Plant Biol. 12, 698–707. doi: 10.1111/j.1438-8677.2009.00269.x
Vasyukova, N. I., and Ozeretskovkaya, O. L. (2007). Induced plant resistance and salicylic acid: a review. Appl. Biochem. Microbiol. 43, 367–373. doi: 10.1134/S0003683807040011
Wang, X., Liu, Y., Chen, L., Zhao, D., Wang, X., and Zhang, Z. (2013). Wheat resistome in response to barley yellow dwarf virus infection. Funct. Integr. Genomics 13, 155–165. doi: 10.1007/s10142-013-0309-4
Whenham, R. J., Fraser, R. S. S., Brown, L. P., and Payne, J. A. (1986). Tobacco mosaic virus-induced increase in abscisic acid concentration in tobacco leaves. Planta 168, 592–598. doi: 10.1007/BF00392281
Xu, P., Chen, F., Mannas, J. P., Feldman, T., Sumner, L. W., and Roossinck, M. J. (2008). Virus infection improves drought tolerance. New Phytol. 80, 911–921. doi: 10.1111/j.1469-8137.2008.02627.x
Yasuda, M., Ishikawa, A., Jikumaru, Y., Seki, M., Umezawa, T., Asami, T., et al. (2008). Antagonistic interaction between systemic acquired resistance and the abscisic acid-mediated abiotic stress response in Arabidopsis. Plant Cell 20, 1678–1692. doi: 10.1105/tpc.107.054296
Zarate, S. I., Kempema, L. A., and Walling, L. L. (2007). Silverleaf whitefly induces salicylic acid defenses and suppresses effectual jasmonic acid defenses. Plant Physiol. 143, 866–875. doi: 10.1104/pp.106.090035
Keywords: aphid, drought, phytohormones, induction, systemic response, Barley yellow dwarf virus, Triticum aestivum
Citation: Davis TS, Bosque-Pérez NA, Popova I and Eigenbrode SD (2015) Evidence for additive effects of virus infection and water availability on phytohormone induction in a staple crop. Front. Ecol. Evol. 3:114. doi: 10.3389/fevo.2015.00114
Received: 06 June 2015; Accepted: 17 September 2015;
Published: 30 September 2015.
Edited by:
Astrid Eben, Julius Kühn-Institut, GermanyReviewed by:
Peter Witzgall, Swedish Agricultural University, SwedenVladimir Jakovljevic, Julius Kühn-Institut, Germany
Copyright © 2015 Davis, Bosque-Pérez, Popova and Eigenbrode. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Thomas S. Davis, Natural Resources Management and Environmental Sciences Department, California State Polytechnic University, 1 Grand Ave., San Luis Obispo, CA 93407, USA, tsdavis1@gmail.com
 Thomas S. Davis
Thomas S. Davis Nilsa A. Bosque-Pérez
Nilsa A. Bosque-Pérez Ina Popova2
Ina Popova2  Sanford D. Eigenbrode
Sanford D. Eigenbrode