The color of plant reproduction: macroecological trade-offs between biotic signaling and abiotic tolerance
- School of Biological Sciences, Victoria University of Wellington, Wellington, New Zealand
Flowers and fruits are often vividly colored. An obvious explanation for fruit and flower pigmentation is that it serves to attract animal mutualists. However, decades of research has produced equivocal support for the hypothesis that animals are the primary selection pressure acting on the color of plant reproductive structures. Exciting new research into geographic variation in flower colors suggests an alternative explanation—flower pigments protect gametes against the damaging effects of solar radiation. Here, I present new evidence suggesting that a similar explanation might apply to Rubus spectabilis, a much studied but poorly understood bird-dispersed plant species. These and other recent results provide a new perspective on the color of plant reproduction. In addition to signaling to animals, fruit, and flower colors might often play vital roles in protecting plants against the harmful effects of solar radiation.
Fruits and flowers come in a bewildering array of colors. At first glance, an explanation for the colors associated with plant reproduction seems obvious. Because plants often utilize animals as vectors for successful pollination and seed dispersal, conspicuously colored flowers and fruits could act as mutualistic signals (see Schaefer and Ruxton, 2011). However, several decades of research have failed to provide consistent support for this as the sole explanation for flower and fruit hues (see Burns et al., 2009; Cazetta et al., 2012; Schaefer et al., 2014).
Exciting new work on geographic variation in flower colors suggests a potential solution to the problem. Koski and Ashman (2015) recently documented that UV-absorbing pigments in the flowers of Argentina anserina increases toward the tropics. Although these floral pigments could be signals to insect pollinators, they might also help to protect gametes against the harmful effects of UV light, which increases at lower latitudes. These and several other recent studies across large spatial scales (Amico et al., 2011; Arista et al., 2013; Stournaras et al., 2013) provide a fresh perspective on plant color polymorphisms by offering an alternative explanation for plant color polymorphisms—that some colors of flowers and fruits represent adaptations to abiotic, rather than biotic, factors.
Research on the colors associated with plant reproduction has focused heavily on flowers. Nevertheless, fleshy-fruits are often highly pigmented as well, yet far fewer studies have focused on the adaptive significance of fruit colors. A sizable fraction of the previous work on fruit colors has focused on a single species, salmonberry (Rubus spectabilis, Traveset and Willson, 1998; Gervais et al., 1999; Burns and Dalen, 2002; Burns, 2005a,b).
Salmonberries are color-polymorphic (see Figure 1). Plants produce either red or orange fruits on separate plants, for reasons that remain stubbornly illusive. Previous research has shown that birds consistently prefer red salmonberries over orange ones (Gervais et al., 1999; Burns, 2005a). So while this work has identified the adaptive significance of the red morph, the adaptive significance of the orange morph has yet to be identified and the maintenance of the polymorphism remains a mystery.
Red or orange salmonberries are identical in most respects (see Traveset and Willson, 1998; Gervais et al., 1999). However, several notable differences have been observed between the two fruit color morphs. First, the orange morph is more abundant at lower latitudes (Oregon), while the red morph is more abundant at higher latitudes (Gervais et al., 1999). Second, they differ in size, with red fruits being slightly smaller than orange fruits (Traveset and Willson, 1998). Although these two attributes might be key to understanding the maintenance of the polymorphism, they have yet to be explained.
To test whether the fruit color polymorphism in R. spectabilis might be maintained by a combination of biotic and abiotic factors, I conducted two new analyses. First, I performed spectrographic analyses to test whether orange fruits reflect greater amounts of solar radiation than red fruits. Second, I conducted a simple fruit desiccation experiment to test whether orange fruits lose water less rapidly when exposed to sunlight. Results are then used to test the hypothesis that the fruit color polymorphism in R. spectabilis is maintained by a trade-off between their resistance to desiccation and attractiveness to birds.
Both analyses were conducted on the west coast of Vancouver Island, British Columbia, Canada (48°50.1′N, 125°08.1′W). I collected 30 fruits of each color morph and the closest undamaged leaf from 30 separate plants inhabiting old-growth conifer forest. Twenty fruits of each color were then randomly selected and subjected to spectrographic analyses. Immediately afterwards they were included in the fruit desiccation experiment.
Spectrographic Analyses
Spectrographic analyses were conducted using a USB Ocean Optics 2000 spectroradiometer and Xenon Pulse X2 lamp (Ocean Optics) light source. Fruit reflectance properties were measured as the proportion of a diffuse reflectance standard (white standard). The fiber optics probe was mounted inside a matte black plastic tube to exclude ambient light and standardize the distance between each fruit and the probe at 1 cm. The angle of illumination and reflection was also fixed at 45° to minimize glare. Spectra were calculated at 5 nm intervals from 300 to 700 nm with SpectraSuite software. Irradiance was measured with a cosine corrected sensor and a D65 (normal daylight) light bulb as a reference.
I assessed fruit conspicuousness according to avian vision because birds are the primary seed dispersers of R. spectabilis (see Burns, 2005a, 2006). Moreover, avian vision is comparatively well known facilitating the use of a well-developed eye model based on the spectral sensitivities and the receptor noise of the four cone types that determine avian color discrimination (Vorobyev and Osorio, 1998). Based on an analytical approximation of cone visual pigments and oil droplet spectra, the model calculates cone excitation values for each spectra under standard D65 illumination. We used cone excitation values to calculate the coordinates of each fruit and background spectrum in the color space of birds, which has the shape of a tetrahedron (Goldsmith, 1990; Neumeyer, 1991). In general, the photoreceptors of birds are remarkably similar among even distantly related taxa, with variation occurring mainly in the UV sensitive cone (Hart, 2001). We based our model on the well-known spectral sensitivities of the blue tit (Parus caeruleus), which is a typical passerine bird with a UVS cone type (Hart et al., 2000). However, these results are also representative for birds with different short-wave sensitivities [visible-sensitive (VS) cone] under typical daylight viewing conditions (Martin Schaefer et al., 2007).
Endler and Mielke (2005) derived a method to evaluate ecological color patterns based on compositional analyses (see also Aitchison, 2003). Following Burns et al. (2009), I used a slightly modified version of their technique to quantify fruit conspicuousness, which was defined specifically as the contrast in color (i.e., reflected wavelengths as perceived by birds) between red and orange fruits and their natural backgrounds (leaves). First, the four cone output values (U, S, M, and L) were each divided by the sum of all cone outputs to obtain relative cone outputs (Goldsmith, 1990). Relative cone output values (u, s, m, and l) were then transformed into three new variables:
which can then be plotted in three dimensional, “tetrahedral color space,” where x, y, and z are Cartesian coordinates within a tetrahedron with a height = 1.
Fruit-background spectral contrasts (C) were measured as the distance between each fruit (represented by the coordinates x, y, z) and their associated background (represented by the coordinates xb, yb, zb):
Greater Euclidian distances between fruits and backgrounds in tetrahedral color space represent higher fruit-background color contrasts and greater fruit conspicuousness.
Results showed that the average Euclidean distance between red fruits and their leaf backgrounds in tetrahedral color space was higher than the average fruit-leaf Euclidean distance in orange fruits (t = 2.61, P = 0.016). Therefore, red fruits had greater visual contrasts with their natural backgrounds than orange fruits, indicating they are more conspicuous to birds (Figures 2, 3).
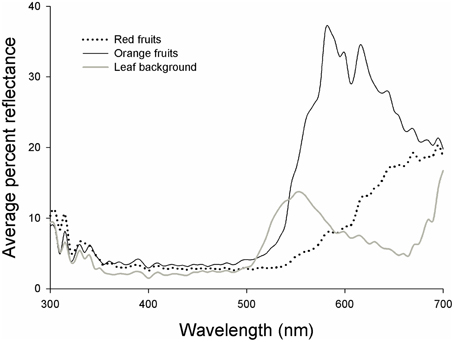
Figure 2. Reflectance curves for red fruits (dashed line), orange fruits (black line), and leaf backgrounds (gray line) of Rubus spectabilis.
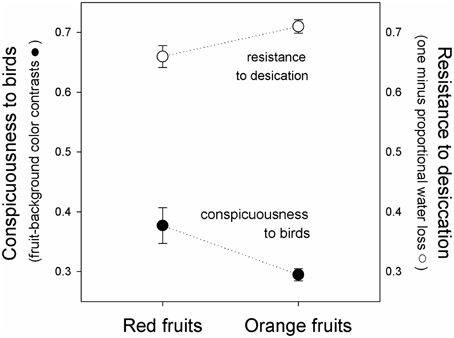
Figure 3. Fruit conspicuousness (Euclidian distance between fruits and leaf backgrounds in tetrahedral color space, black symbols, ± se), and resistance to desiccation (one minus the proportion of water lost after 24 h of exposure to sunlight, white symbols), of red (left) and orange (right) fruits of Rubus spectabilis.
Fruit Desiccation Experiment
After spectrographic analyses, fruits were subject to a simple experiment that tested whether the two color morphs desiccated at different rates when exposed to full sunlight. At the start of the experiment, each fruit was marked individually with a small piece of tape on its peduncle and weighed to the nearest 0.01 g with an electronic balance. Fruits were then placed in a glass aquarium with an open top that was situated in a sunny location on a clear, cloudless day. Fruits were then re-weighed 24 h later. This length of time was chosen because preliminary trials indicated that after 48 h fruits were desiccated to such an extent that animals would no longer view them as a viable food source. Repeated measures ANOVA was used to test whether the two color morphs desiccated at different rates. Time (before and after exposure) was used as the repeated measure, color morph (red and orange) was treated as a fixed effect, and the interaction between them was used to test whether orange fruits are more tolerant to desiccation than red fruits.
While this experimental design provides a general reflection of how fast R. spectabilis fruits loose water in direct sunlight, it does not establish how fast fruits might desiccate in indirect light. Furthermore, because fruits were removed from parent plants prior to the start of the experiment, experimental desiccation rates are likely to be higher than under natural conditions, when fruits are still attached to parent plants by their peduncles. It also does not provide information on how differences in pigmentation between color morphs might interact with other morphological and physiological factors (e.g., cuticle thickness) to determine fruit desiccation rates in the field.
Results showed a significant effect of time [F(1, 38) = 310.44, P < 0.001], indicating that the weight of both color morphs declined during the course of the experiment (Figure 2). The fixed-effect was insignificant [F(1, 38) = 0.14, P = 0.714], indicating that both color morphs had similar weights overall. However, a significant interaction was observed [F(1, 38) = 5.03, P = 0.031], which resulted from greater water loss in red fruits (Figure 3), indicating red morph is more susceptible to desiccation than the orange morph.
Implications
When viewed in light of previous work on salmonberry colors, results reported here suggest that the fruit color polymorphism is maintained by a trade-off between adaptations to biotic and abiotic factors. The red morph is more conspicuous to the avian eye than the orange morph, supporting previous work indicating that avian fruit consumers consistently select for the red color morph. Although the orange morph tends to be removed more slowly by birds, they reflect greater quantities of visible light and loose water less rapidly when exposed to direct sunlight, suggesting that they persist for longer periods on parent plants prior to dispersal. Therefore, the polymorphism might be maintained by a trade-off between conspicuousness to avian seed dispersers and susceptibility to desiccation.
These results can explain why the orange morph is more common at lower latitudes (Oregon) than at higher latitudes (Gervais et al., 1999). If the red morph is more susceptible to desiccation, it may be selected against in warmer, dryer sites relative to the orange morph, whose pigmentation better enables it to avoid water stress. They can also explain previous work indicating that orange fruits are longer than red fruits (Traveset and Willson, 1998). If red fruits are more prone to desiccation, then a reduction in their size relative to orange fruits would reduce the surface area exposed to sunlight, slowing rates of desiccation.
Similar biogeographic variation has been observed in the relative abundance of red and orange fruits produced by other polymorphic fruit species. For example, Whitney and Lister (2004) found that the red arils produced by an Australian acacia (Acacia ligulata) were more common at cooler, wetter sites, whereas orange arils were more prevalent in hotter, dryer sites. Therefore, trade-offs between conspicuousness and desiccation might occur in other animal-dispersed fruit species. Similar mechanisms might also shape flower color polymorphisms. For example, Arista et al. (2013) found that pleiotrophic effects explain the maintenance of the flower color polymorphism in scarlet pimpernel (Lysimachia arvensis), with red-flowered individuals performing better as seeds and seedlings in wetter, cooler environments.
However, other recent work suggests that geographic variation in the distribution of animal mutualists might also drive macroecological variation in the color of plant reproductive structures. For example, Amico et al. (2011) recently documented variation in the relative abundance of green and yellow fruit morphs in a South American mistletoe (Tristerix corymbosus) across nearly 15° in latitude. They found that in high latitude forests, green-fruited plants predominate and are dispersed primarily by small marsupial mammals, but in low-latitude scrublands, yellow fruits prevail and are dispersed primarily by birds. Therefore, biotic processes might can also explain macroecological variation in color polymorphisms, so future work on the color of plant reproduction would benefit from considering both biotic and abiotic factors.
A key, unifying feature of recent work on the color of plant reproduction is their geographic scope (e.g., Amico et al., 2011; Arista et al., 2013; Stournaras et al., 2013; Koski and Ashman, 2015). A growing body of literature across macroecological spatial scales illustrates that variation fruit and flower colors often matches geographic variation in both biotic and abiotic factors. If this work were restricted to single geographic locales, it would fail to resolve the conditions under which different colors are selectively advantageous. Hopefully a macroecological perspective will ultimately provide a solution to the mysteries surrounding the color of plant reproduction.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Amico, G. C., Rodriguez-Cabal, M. A., and Aizen, M. A. (2011). Geographic variation in fruit colour is associated with contrasting seed disperser assemblages in a south-Andean mistletoe. Ecography 34, 318–326. doi: 10.1111/j.1600-0587.2010.06459.x
Arista, M., Talavera, M., Berjano, R., and Ortiz, P. L. (2013). Abiotic factors may explain the geographical distribution of flower colour morphs and the maintenance of colour polymorphism in the scarlet pimpernel. J. Ecol. 101, 1613–1622. doi: 10.1111/1365-2745.12151
Burns, K. C. (2005b). Effects of bi-colored displays on avian fruit color preferences in a color polymorphic plant. J. Torrey Bot. Soc. 132, 505–509. doi: 10.3159/1095-5674(2005)132[505:EOBDOA]2.0.CO;2
Burns, K. C. (2006). A simple null model predicts bird-fruit interactions in a temperate rainforest. Oikos 115, 427–432. doi: 10.1111/j.2006.0030-1299.15068.x
Burns, K. C., and Dalen, J. L. (2002). Foliage color contrasts and adaptive fruit color variation in a bird-dispersed plant community. Oikos 96, 463–469. doi: 10.1034/j.1600-0706.2002.960308.x
Burns, K. C., Cazetta, E., Galetti, M., Valido, A., and Schaefer, H. M. (2009). Geographic patterns in fruit colour diversity—do leaves add colour to fleshy fruits? Oecologia 159, 337–343. doi: 10.1007/s00442-008-1227-3
Cazetta, E., Galetti, M., Rezende, E. L., and Schaefer, H. M. (2012). On the reliability of visual communication in vertebrate-dispersed fruits. J. Ecol. 100, 277–286. doi: 10.1111/j.1365-2745.2011.01901.x
Endler, J. A., and Mielke, P. W. (2005). Comparing entire colour patterns as birds see them. Biol J. Linn. Soc. 86, 405–431. doi: 10.1111/j.1095-8312.2005.00540.x
Gervais, J. A., Noon, B. R., and Willson, M. F. (1999). Avian selection of the color-dimorphic fruits of salmonberry, Rubus spectabilis: a field experiment. Oikos 84, 77–86. doi: 10.2307/3546868
Goldsmith, T. H. (1990). Optimization, constraint, and history in the evolution of eyes. Q. Rev. Biol. 65, 281–322. doi: 10.1086/416840
Hart, N. S. (2001). The visual ecology of avian photoreceptors. Prog. Retinal Eye Res. 20, 675–703. doi: 10.1016/S1350-9462(01)00009-X
Hart, N. S., Partridge, J. C., Cuthill, I. C., and Bennett, A. T. D. (2000). Visual pigments, oil droplets, ocular media and cone photoreceptor distribution in two species of passerine bird: the blue tit (Parus caeruleus L.) and the blackbird (Turdus merula L.). J. Comp. Phys. A 186, 375–387. doi: 10.1007/s003590050437
Koski, M. H., and Ashman, T.-L. (2015). Floral pigmentation patterns provide an example of Gloger's rule in plants. Nat. Plants 1, 1–5. doi: 10.1038/nplants.2014.7
Martin Schaefer, H., Schaefer, V., and Vorobyev, M. (2007). Are fruit colors adapted to consumer vision and birds equally efficient in detecting colorful signals? Am. Nat. 169, S159–S169. doi: 10.1086/510097
Neumeyer, C. (1991). “Evolution of colour vision,” in Vision and Visual Dysfunction, eds J. R. Cronley-Dillon and R. L. Gregory (London: Macmillan), 284–305.
Schaefer, H. M., and Ruxton, G. (2011). Plant-animal Communication. New York, NY: Oxford University Press.
Schaefer, H. M., Valido, A., and Jordano, P. (2014). Birds see and use the true colours of fruits to live off the fat of the land. Proc. R. Soc. Lond. Ser. B 281:20132516. doi: 10.1098/rspb.2013.2516
Stournaras, K. E., Lo, E., Böhning-Gaese, K., Cazetta, E., Matthias Dehling, D., Schleuning, M., et al. (2013). How colorful are fruits? Limited color diversity in fleshy fruits on local and global scales. New Phytol. 198, 617–629. doi: 10.1111/nph.12157
Traveset, A., and Willson, M. F. (1998). Ecology of the fruit-colour polymorphism in Rubus spectabilis. Evol. Ecol. 12, 331–345. doi: 10.1023/A:1006504317585
Vorobyev, M., and Osorio, D. (1998). Receptor noise as a determinant of colour thresholds. Proc. R. Soc. Lond. B 265, 351–358. doi: 10.1098/rspb.1998.0302
Keywords: color, flower, fruit, plant, polymorphism, reflectance, seed dispersal
Citation: Burns KC (2015) The color of plant reproduction: macroecological trade-offs between biotic signaling and abiotic tolerance. Front. Ecol. Evol. 3:118. doi: 10.3389/fevo.2015.00118
Received: 23 July 2015; Accepted: 06 October 2015;
Published: 26 October 2015.
Edited by:
Marco A. Molina-Montenegro, University of Talca, ChileReviewed by:
Jofre Carnicer, University of Groningen, NetherlandsCristian Torres, Universidad del Bío-Bío, Chile
Copyright © 2015 Burns. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kevin C. Burns, kevin.burns@vuw.ac.nz
 Kevin C. Burns
Kevin C. Burns