- 1Department of Gene Regulation, Faculty of Pharmaceutical Sciences, Tokyo University of Science, Noda, Japan
- 2Department of Biochemistry, Faculty of Pharmaceutical Sciences, Tohoku Pharmaceutical University, Sendai, Japan
- 3GeneCare Research Institute Co., Ltd., Kamakura, Japan
Recent progress in pharmaceutical sciences has made it possible for us to live longer and longer. For example, antibiotics and vaccines have been developed that were successfully administered to patients with infectious diseases. A number of effective drugs for specific diseases could be purified from natural resources or created by chemical synthesis, and recent recombinant DNA technologies have brought about antibody-drugs. It seems increasingly possible that a treatment for every disease could be established in the near future. Nevertheless, prevention or remedies for inherited age-related diseases, including cancer, have not yet been completely established. However, recent progresses in human genetics and molecular biology revealed that premature aging is caused by mutations on DNA helicase encoding genes (Bernstein et al., 2010). These exciting findings have encouraged scientists to research mechanisms of the age-related diseases.
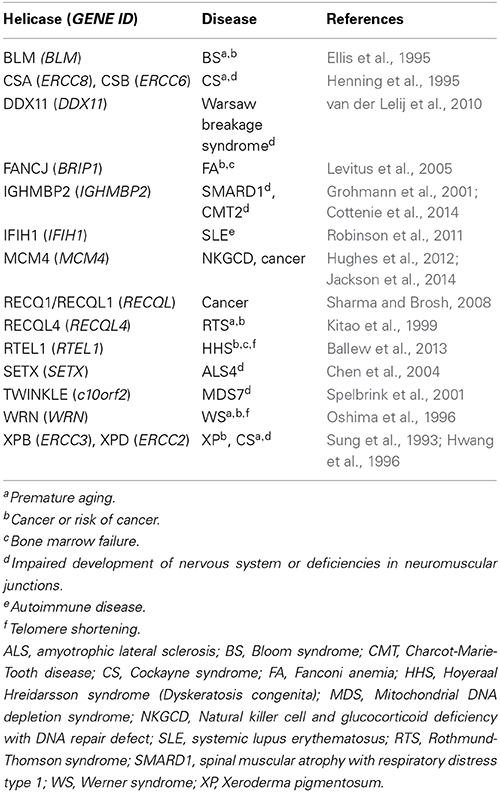
DNA/RNA helicases are enzymes that unwind DNA/DNA, DNA/RNA, and RNA/RNA duplexes to execute and regulate DNA replication, recombination, repair, and transcription (Patel and Donmez, 2006). To date, numerous genes have been identified to encode helicases. Importantly, genetic studies have revealed that mutations in some of these genes are associated with certain human diseases, including Xeroderma Pigmentosum (XP), Cockayne Syndrome (CS), and Werner Syndrome (WS) (Puzianowska-Kuznicka and Kuznicki, 2005). Given that helicases play an important role in the regulation and maintenance of chromosomal DNAs, it might not be so difficult to understand that their dysfunction leads to unfavorable states. Nuclear events, such as nucleotide excision repair (NER), transcription coupled repair (TCR), and telomere maintenance, are thought to be individually affected by XPB/XPD, CSA/CSB and WRN helicases, respectively (Table 1). Because epigenetic changes and disruption of chromosomal integrity have been strongly suggested to correlate with cellular senescence, these helicases may be important factors to regulate aging and age-related diseases.
Despite great efforts being made to elucidate the properties of helicases on a molecular and cellular level, it seems that the gap from molecule to patient is still distant. In this research topic, authors have described and discussed the forefront of the helicase studies. It is very important to establish a molecular model of how helicases interact with DNA repair machinery. In the research topic, the properties of the FANCJ (BRIP1) that affect cancer and Fanconi Anemia (FA) development have been summarized (Brosh and Cantor, 2014). In order to assess the mechanisms of diseases, including cancer, which are caused by dysfunctions of helicases, several approaches could be applied. Genetic and expression analyses of samples from patients will enable us to discuss the alterations in both the quality of DNA and the quantity of RNA. Therefore, diagnosis/prognosis of cancer or age-related diseases will be possible by analyzing the RECQ1 (RECQL) gene expression (Sharma, 2014). Based on the concept that helicases play important roles in the maintenance of chromosomal DNAs, novel therapeutics will be applicable for cancer therapy with siRNAs of the RECQL1 (RECQL) and WRN DNA helicase-encoding genes (Futami and Furuichi, 2015). The therapy is supported by experimental results showing that siRNA of the RECQL could be effectively applied for ovarian cancer treatment by inducing apoptosis (Matsushita et al., 2014). Structural analyses of the helicase protein molecules will provide their precise function in the process of DNA repair. The precise molecular structure models of the WRN and BLM helicases will contribute for a development of rational design of specific drugs to prevent aging and cancer (Kitano, 2014). Moreover, establishment of iPSCs from helicase deficient cells will contribute to the clinical tests to develop novel drugs that delay aging and age-related diseases (Shimamoto et al., 2015). Furthermore, studies on RNA helicases, especially those that are involved in immune responses, will contribute to developing strategies against viral infections. It was shown that DDX3 could be a novel therapeutic target for HIV-1 and HCV replication (Ariumi, 2014). Importantly, IFIH1, which controls anti-viral responses, will be a molecular target of diagnosis and treatment for systemic lupus erythematosus (SLE) (Oliveira et al., 2014). All these articles provide new insights into the molecular pathology of the helicase-associated diseases. Further studies on various helicases will not only contribute to diagnoses and treatment of specific diseases (Table 1) but also to prevention and next generation-therapeutics on cancer and age-related diseases.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Ariumi, Y. (2014). Multiple functions of DDX3 RNA helicase in gene regulation, tumoligenesis, and viral infection. Front. Genet. 5:423. doi: 10.3389/fgene.2014.00423
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ballew, B. J., Yeager, M., Jacobs, K., Giri, N., Boland, J., Burdett, L., et al. (2013). Germline mutations of regulator of telomere elongation helicase 1, RTEL1, in dyskeratosis congenita. Hum. Genet. 132, 473–480. doi: 10.1007/s00439-013-1265-8
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Bernstein, K. A., Gangloff, S., and Rothstein, R. (2010). The RecQ DNA helicases in DNA repair. Annu. Rev. Genet. 44, 393–417. doi: 10.1146/annurev-genet-102209-163602
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Brosh, R. M. Jr., and Cantor, S. B. (2014). Molecular and cellular functions of the FANCJ DNA helicase defective in cancer and in Fanconi anemia. Front. Genet. 5:372. doi: 10.3389/fgene.2014.00372
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Chen, Y. Z., Bennett, C. L., Huynh, H. M., Blair, I. P., Puls, I., Irobi, J., et al. (2004). DNA/RNA helicase gene mutations in a form of juvenile amyotrophic lateral sclerosis (ALS4). Am. J. Hum. Genet. 74, 1128–1135. doi: 10.1086/421054
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Cottenie, E., Kochanski, A., Jordanova, A., Bansagi, B., Zimon, M., Horga, A., et al. (2014). Truncating and missense mutations in IGHMBP2 cause Charcot-Marie tooth disease type 2. Am. J. Hum. Genet. 95, 590–601. doi: 10.1016/j.ajhg.2014.10.002
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ellis, N. A., Groden, J., Ye, T. Z., Straughen, J., Lennon, D. J., Ciocci, S., et al. (1995). The Bloom's syndrome gene product is homologous to RecQ helicases. Cell 83, 655–666. doi: 10.1016/0092-8674(95)90105-1
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Futami, K., and Furuichi, Y. (2015). RECQL1 and WRN DNA repair helicases: potential therapeutic targets and proliferative markers against cancers. Front. Genet. 5:441. doi: 10.3389/fgene.2014.00441
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Grohmann, K., Schuelke, M., Diers, A., Hoffmann, K., Lucke, B., Adams, C., et al. (2001). Mutations in the gene encoding immunoglobulin mu-binding protein 2 cause spinal muscular atrophy with respiratory distress type 1. Nat. Genet. 29, 75–77. doi: 10.1038/ng703
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Henning, K. A., Li, L., Iyer, N., McDaniel, L. D., Reagan, M. S., Legerski, R., et al. (1995). The Cockayne syndrome group A gene encodes a WD repeat protein that interacts with CSB protein and a subunit of RNA polymerase II TFIIH. Cell 82, 555–564. doi: 10.1016/0092-8674(95)90028-4
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Hughes, C. R., Guasti, L., Meimaridou, E., Chuang, C.-H., Schimenti, J. C., King, P. J., et al. (2012). MCM4 mutation causes adrenal failure, short stature, and natural killer cell deficiency in humans. J. Clin. Invest. 122, 814–820. doi: 10.1172/JCI60224
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Hwang, J. R., Moncollin, V., Vermeulen, W., Seroz, T., van Vuuren, H., Hoeijmakers, J. H., et al. (1996). A 3′ –> 5′ XPB helicase defect in repair/transcription factor TFIIH of xeroderma pigmentosum group B affects both DNA repair and transcription. J. Biol. Chem. 271, 15898–15904. doi: 10.1074/jbc.271.27.15898
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Jackson, A. P., Laskey, R. A., and Coleman, N. (2014). “Replication proteins and human disease,” in DNA Replication, eds S. D. Bell, M. Mechali, and M. L. DePamphilis (Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press), 327–342.
Kitano, K. (2014). Structural mechanisms of human RecQ helicases WRN and BLM. Front. Genet. 5:366. doi: 10.3389/fgene.2014.00366
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kitao, S., Shimamoto, A., Goto, M., Miller, R. W., Smithson, W. A., Lindor, N. M., et al. (1999). Mutations in RECQL4 cause a subset of cases of Rothmund-Thomson syndrome. Nat. Genet. 22, 82–84. doi: 10.1038/8788
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Levitus, M., Waisfisz, Q., Godthelp, B. C., de Vries, Y., Hussain, S., Wiegant, W. W., et al. (2005). The DNA helicase BRIP1 is defective in Fanconi anemia complementation group J. Nat. Genet. 37, 934–935. doi: 10.1038/ng1625
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Matsushita, Y., Yokoyama, Y., Yoshida, H., Osawa, Y., Mizunuma, M., Shigeto, T., et al. (2014). The level of RECQL1 expression is a prognostic factor for epithelial ovarian cancer. J. Ovarian. Res. 7:107. doi: 10.1186/s13048-014-0107-1
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Oliveira, L., Sinicato, N. A., Postal, M., Appenzeller, S., and Niewold, T. B. (2014). Dysregulation of antiviral helicase pathways in systemic lupus erythematosus. Front. Genet. 5:418. doi: 10.3389/fgene.2014.00418
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Oshima, J., Yu, C. E., Piussan, C., Klein, G., Jabkowski, J., Balci, S., et al. (1996). Homozygous and compound heterozygous mutations at the Werner syndrome locus. Hum. Mol. Genet. 5, 1909–1913. doi: 10.1093/hmg/5.12.1909
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Patel, S. S., and Donmez, I. (2006). Mechanisms of helicases. J. Biol. Chem. 281, 18265–18268. doi: 10.1074/jbc.R600008200
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Puzianowska-Kuznicka, M., and Kuznicki, J. (2005). Genetic alterations in accelerated ageing syndromes. Do they play a role in natural ageing? Int. J. Biochem. Cell Biol. 37, 947–960. doi: 10.1016/j.biocel.2004.10.011
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Robinson, T., Kariuki, S. N., Franek, B. S., Kumabe, M., Kumar, A. A., Badaracco, M., et al. (2011). Autoimmune disease risk variant of IFIH1 is associated with increased sensitivity to IFN-α and serologic autoimmunity in lupus patients. J. Immunol. 187, 1298–1303. doi: 10.4049/jimmunol.1100857
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Sharma, S. (2014). An appraisal of RECQ1 expression in cancer progression. Front. Genet. 5:426. doi: 10.3389/fgene.2014.00426
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Sharma, S., and Brosh, R. M. Jr. (2008). Unique and important consequences of RECQ1 deficiency in mammalian cells. Cell Cycle 7, 989–1000. doi: 10.4161/cc.7.8.5707
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Shimamoto, A., Yokote, K., and Tahara, H. (2015). Werner syndrome-specific induced pluripotent stem cells: recovery of telomere function by reprogramming. Front. Genet. 6:10. doi: 10.3389/fgene.2015.00010
Spelbrink, J. N., Li, F. Y., Tiranti, V., Nikali, K., Yuan, Q. P., Tariq, M., et al. (2001). Human mitochondrial DNA deletions associated with mutations in the gene encoding Twinkle, a phage T7 gene 4-like protein localized in mitochondria. Nat. Genet. 28, 223–231. doi: 10.1038/90058
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Sung, P., Bailly, V., Weber, C., Thompson, L. H., Prakash, L., and Prakash, S. (1993). Human xeroderma pigmentosum group D gene encodes a DNA helicase. Nature 365, 852–855. doi: 10.1038/365852a0
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
van der Lelij, P., Chrzanowska, K. H., Godthelp, B. C., Rooimans, M. A., Oostra, A. B., Stumm, M., et al. (2010). Warsaw breakage syndrome, a cohesinopathy associated with mutations in the XPD helicase family member DDX11/ChIR1. Am. J. Hum. Genet. 86, 262–266. doi: 10.1016/j.ajhg.2010.01.008
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Keywords: helicasees, genetic diseases, RecQ helicases, Fanconi Anemia, premature aging, cancer, RNA helicases
Citation: Uchiumi F, Seki M and Furuichi Y (2015) Helicases and human diseases. Front. Genet. 6:39. doi: 10.3389/fgene.2015.00039
Received: 22 January 2015; Accepted: 26 January 2015;
Published online: 12 February 2015.
Edited and reviewed by: Blanka Rogina, University of Connecticut Health Center, USA
Copyright © 2015 Uchiumi, Seki and Furuichi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: uchiumi@rs.noda.tus.ac.jp
 Fumiaki Uchiumi
Fumiaki Uchiumi Masayuki Seki
Masayuki Seki Yasuhiro Furuichi
Yasuhiro Furuichi