- 1Department of Epidemiology, University of Alabama at Birmingham, Birmingham, AL, USA
- 2Department of Biostatistics, University of Alabama at Birmingham, Birmingham, AL, USA
- 3Department of Biostatistics, Washington University in St. Louis, St. Louis, MO, USA
- 4Department of Internal Medicine, University of Utah, Salt Lake City, UT, USA
- 5Department of Medicine, Human and Molecular Genetics Center, Medical College of Wisconsin, Milwaukee, WI, USA
Proprotein convertase subtilisin/kexin type 9 (encoded by PCSK9) plays a well-known role in the regulation of low-density lipoprotein (LDL) receptors, and an inhibitor of this enzyme is a promising new therapeutic for hyperlipidemia. Recently, animal and human studies also implicate PCSK9 genetic variation in the regulation of blood pressure. The goal of this study was to examine if common and rare polymorphisms in PCSK9 are associated with blood pressure in an African-American population at high risk for cardiovascular disease. Using genomic data assayed on the Affymetrix 6.0 array (n = 1199) and the Illumina HumanExome Beadchip (n = 1966) from the Hypertension Genetic Epidemiology Network (HyperGEN), we tested the association of PCSK9 polymorphisms with blood pressure. We used linear mixed models and the sequence kernel association test (SKAT) to assess the association of 31 common and 19 rare variants with blood pressure. The models were adjusted for age, sex, center, smoking status, principal components for ancestry and diabetes as fixed effects and family as a random effect. The results showed a marginally significant effect of two genome-wide association study (GWAS) single-nucleotide polymorphisms (SNPs) (rs12048828: β = 1.8, P = 0.05 and rs9730100: β = 1.0, P = 0.05) with diastolic blood pressure (DBP); however these results were not significant after correction for multiple testing. Rare variants were cumulatively associated with DBP (P = 0.04), an effect that was strengthened by restriction to non-synonymous or stop-gain SNPs (P = 0.02). While gene-based results for DBP did not replicate (P = 0.36), we found an association with SBP (P = 0.04) in the Reasons for Geographic And Racial Differences in Stroke study (REGARDS). The findings here suggest rare variants in PCSK9 may influence blood pressure among African Americans, laying the ground work for further validation studies.
Introduction
Hypertension is currently a major health problem in African Americans (Kramer et al., 2004). Data from the National Health and Nutrition Examination Survey (NHANES) in 2008 showed that age-adjusted prevalence of hypertension was 40% in African Americans vs. 30% in Caucasians (Egan et al., 2010). Overall, African Americans are more prone to hypertension-related morbidity and mortality than Caucasians (Lackland, 2014). For instance stroke risk is twice as high and risk for end-stage renal disease is 4–5 times higher among African Americans (Go et al., 2014). The reason for this disparity is not completely understood, although differences in genetic background are hypothesized to play a role (Allison et al., 1994; Kato, 2012). Because dyslipidemia is linked to hypertension, genetic variants associated with lipid levels represent promising candidates for novel hypertension loci (Brown et al., 2000; Sesso et al., 2005; Halperin et al., 2006; Nguyen et al., 2008; Maharjan et al., 2012; Yin et al., 2012).
Proprotein convertase subtilisin/kexin type 9 (encoded by the PCSK9 gene) contributes to low-density lipoprotein (LDL) cholesterol levels by reducing cell surface expression of LDL receptors. Mutations in PCSK9 can cause hypercholesterolemia (gain of function) and hypocholesterolemia (loss of function), which has made it an attractive therapeutic target for the treatment of dyslipidemia (Horton et al., 2007; Abifadel et al., 2009). Recently, research in an animal model suggested a role for PCSK9 in the regulation of blood pressure (Sharotri et al., 2012), showing that the PCSK9 gene product decreases trafficking of the epithelial sodium channel (ENaC) protein to the cell surface in Xenopus oocytes by enhancing proteasomal degradation. ENaC forms a pathway for sodium to enter epithelial cells at the apical membrane, thus regulating sodium absorption in the kidney and contributing to blood pressure regulation. Therefore, PCSK9 activity could affect blood pressure by modulating sodium absorption. Because most previous studies of PCSK9 have focused on cholesterol metabolism, the role of PCSK9 in blood pressure is a novel area of investigation. To address this gap, we examined the association of common and rare variants in PCSK9 and blood pressure in a population of African Americans at high risk for cardiovascular disease.
Methods
Study Population
We used clinical and genetic data collected from African Americans participating in the Hypertension Genetic Epidemiology Network (HyperGEN) study. HyperGEN was designed to study genes promoting hypertension as part of the Family Blood Pressure Program funded by the National Heart, Lung and Blood Institute. African-American participants were recruited in Forsyth County, NC and Birmingham, AL from siblings who were diagnosed with hypertension before age 60 years and had a least one additional hypertensive sibling who agreed to participate. In the second phase of the study, the offspring of the original sib-pairs were recruited. The HyperGEN study was approved by Institutional Review Boards of the collaborating institutions. All participants gave written informed consent.
Participants with type 1 diabetes or advanced renal disease (defined as serum creatinine level > 2 mg/dL) were excluded from the parent HyperGEN study since these two conditions can cause secondary hypertension and the focus of HyperGEN was to identify novel essential hypertension loci. Patients taking lipid-lowering agents or missing lipid-lowering treatment data were excluded from the current analysis (n = 59 from the common variant analysis and 145 in the rare variant analysis).
Clinical Variables
Six consecutive BP measurements were made during the study visit, three on the left and three on the right arm. The participants rested for 5 min before measurement and for 30 s between measurements (Knox et al., 2010). An average sitting systolic blood pressure (SBP) and diastolic blood pressure (DBP) was calculated based on the second and third measurements in the second arm. Blood pressure measurements were obtained using automated Dinamap devices (model 1846 SX/P, Critikon, Tampa, FL) to promote comparable measurements among HyperGEN field centers. Hypertension was defined as average SBP ≥ 140 mm Hg or average DBP ≥ 90 mm Hg, or using at least one class of antihypertensive medication. Participants were categorized as treated if they were taking at least one antihypertensive medication according to the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) classifications (Chobanian et al., 2003). Participants were categorized as having diabetes if they were on anti-diabetic medications or insulin treatment, or had fasting glucose ≥126 mg/dl.
More details regarding study design and methodology of the HyperGEN study are available in previous publications (Williams et al., 2000).
Genotyping
Common Variants
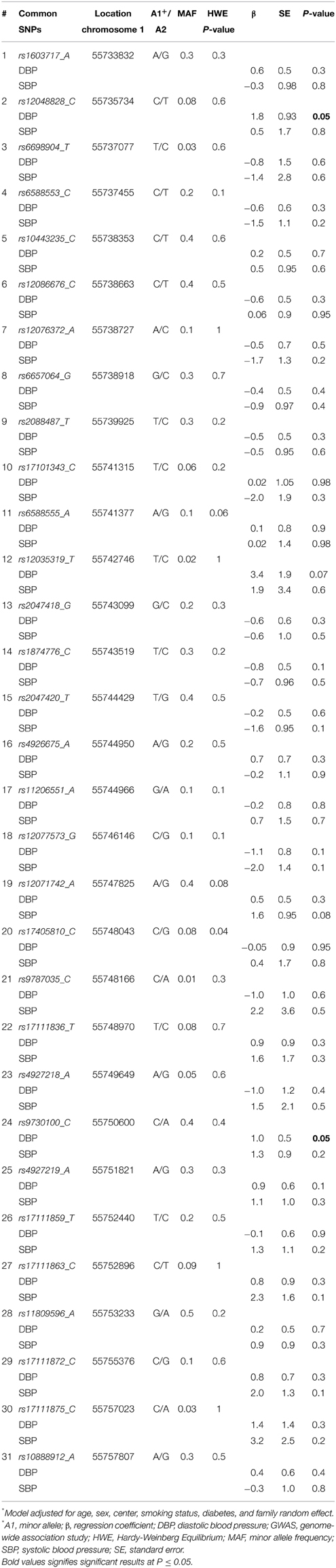
DNA extraction and purification from stored blood were performed using commercial Puregene reagents (Gentra System, Inc., Minneapolis, MN) as previously described (Williams et al., 2000). Genome-wide association study (GWAS) genotyping was performed using the Affymetrix Genome-Wide Human SNP 6.0 Array following the Affymetrix defined protocol (Arnett et al., 2011). Using a subset of 1258 subjects, approximately 3.01 million HapMap SNPs were imputed using MACH v. 1.0 with Human Genome Build 36 as the reference. Overlapping SNP genotypes were 99.5% concordant in the imputed dataset as compared to the genotype data set. SNPs which were not in HapMap (n = 94.337), monomorphic (n = 14.363), R2 < 0.3, minor allele frequency (MAF) < 1%, or Hardy-Weinberg equilibrium (HWE) P ≤ 10−6 (n = 150.096) were removed from the imputed dataset. The overall Mendelian error rate was 0.045%. A total of 50 common variants in PCSK9 were obtained from the imputed GWAS data in 1199 samples. Using Haploview (Barrett et al., 2005) with r2 threshold at 0.8 to examine linkage disequilibrium between the 50 common SNPs (Supplementary Material), we chose 31 independent tag SNPs in PCSK9 for our analysis. We additionally considered the associations of 3 SNPs located near PCSK9 that were reported in prior GWAS of lipid traits [rs11206510 (Schunkert et al., 2011), rs2479409 (Willer et al., 2013), and rs2495478 (Turnbull et al., 2012)] with both SBP and DBP.
Rare Variants
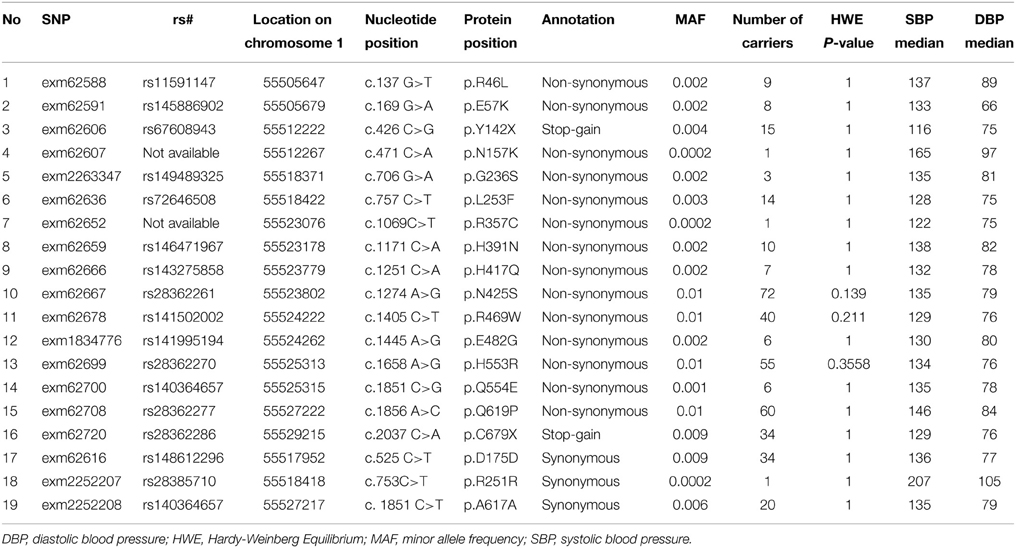
Study samples were processed on the HumanExome BeadChip v1.0 (Illumina, Inc., San Diego, CA) using manufacturer protocols for a total of 2111 samples. The total number of SNPs assayed was 242,901. Monomorphic SNPs (n = 105.723), SNPs with missing rate >5% (n = 36), and SNPs with Hardy-Weinberg equilibrium P < 10−6 (n = 175) were removed. The Mendelian error rate was 0.002%. A total of 19 rare variants (MAF < 0.01) in PCSK9 were included in this analysis from 1966 samples. There were 1131 samples that had both common and rare variant data available for PCSK9 from GWAS and exome chip assays, respectively.
Statistical Methods
To reduce bias and improve statistical power, the effect of antihypertensive treatment was controlled by adding 15 mm Hg to observed SBP and 10 mm Hg to DBP for those who reported taking medications (Tobin et al., 2005). To control for population substructure 10 principal components were generated in Eigenstrat (Price et al., 2006).
For common PCSK9 variants, we used linear mixed models adjusted for age, sex, center, smoking status, principal components, diabetes (fixed effects), and family (random effect) to evaluate main genetic effects. For rare variants, we used the sequence kernel association test (SKAT) (Wu et al., 2011) adjusting for the same covariates. We also examined the joint effect of common and rare variants in PCSK9 with blood pressure among the subset with both GWAS and exome chip data (n = 1131) using SKAT. Common variants that achieved nominal statistical significance (P < 0.05) in the mixed models were selected to test the joint effect.
A P-value of 0.05 was considered as suggestive evidence for an association. A Bonferroni correction was used to correct for multiple tests of common variants, where the type 1 error rate was equal to 0.05/31 = 0.0016. R version 3.0.2 was used for SKAT and SAS version 9.3 (SAS Institute Inc., Cary, NC, USA) was used for all other analyses.
Replication
Results from HyperGEN reaching at least marginal significance (P ≤ 0.05) were tested in The REasons for Geographic And Racial Differences in Stroke (REGARDS) Study. REGARDS is a national, population-based, longitudinal study of incident stroke and associated risk factors among 30,239 African-American and European-American adults aged ≥ 45 years. Participants were randomly sampled and contacted by mail, then telephone, and enrolled between 2003 and 2007. A total of 7700 randomly selected African Americans were recently genotyped with the same Illumina exome chip as HyperGEN. Similar QC as in HyperGEN was carried out (Howard et al., 2005). There was a total of 1502 participants with GWAS data and 4960 participants with exome chip data after we excluded those with missing information on blood pressure or antihypertensive treatment and those on lipid-lowering drugs. We used similar statistical methodology and adjustment for covariates including ancestry to examine the association of selected variants with blood pressure in REGARDS. The REGARDS study was approved by Institutional Review Boards of the collaborating institutions and all participants gave written informed consent.
Results
Table 1 shows the baseline characteristics of HyperGEN participants with PCSK9 variant data available from GWAS, the exome chip, and both assays. The majority of participants were middle-aged and female, as well as obese (BMI ≥ 30 kg/m2) and hypertensive. Diabetes prevalence did not exceed 17% in any subset. The REGARDS population was also mostly female (64%), but was, on average, older (mean age 63.9 ± 9) with a higher prevalence of diabetes (24.5%).
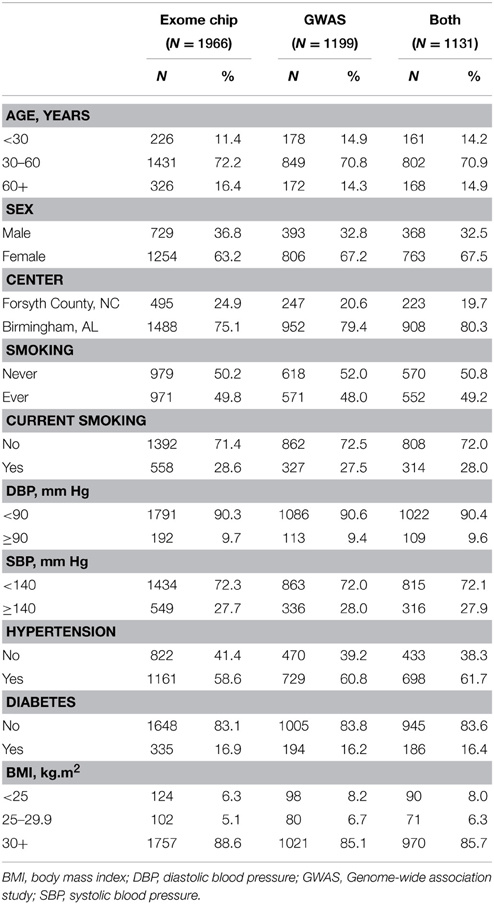
Table 1. Baseline characteristics for African-American participants with exome chip data, GWAS data, and both data sets.
All SNPs were in HWE except for rs17405810 (HWE P = 0.04). Table 2 shows the association of the 31 common SNPs with blood pressure. The SNPs rs12048828 and rs9730100 were both marginally associated with DBP (both P = 0.05). There were no significant associations observed with SBP. The two GWAS SNPs did not show significant association with DBP (P = 0.44 for rs4927219 and 0.89 for rs12048828) or SBP (P = 0.19 and 0.42, respectively) in the REGARDS population (rs4927219 was used as a proxy for rs9730100) (Ward and Kellis, 2012). Our look-up of 3 known lipid loci near PCSK9 from prior GWAS showed no significant association with DBP or SBP [rs11206510 (P = 0.3 for DBP and 0.8 for SBP), rs2479409 (P = 0.1 for DBP and 0.4 for SBP), and rs2495478 (P = 0.7 for DBP and 0.6 for SBP)]. Table 3 annotates 19 rare variants in PCSK9 (MAF ≤ 0.01) included in this study. Overall, we observed a higher median SBP and DBP for carriers of non-synonymous SNPs (Table 3) compared to non-carriers (median SBP and DBP for non-carriers is 127 mm Hg and 73 mm Hg, respectively). Association analysis demonstrated a significant cumulative effect of rare variants with DBP (P = 0.04) but not with SBP (P = 0.14) in HyperGEN. We separately tested the association of 16 non-synonymous or stop-gain SNPs and 3 synonymous SNPs with DBP where there was a significant cumulative effect of non-synonymous or stop-gain SNPs (P = 0.02) but not synonymous SNPs (P = 0.73). The joint effect of rs12048828, rs9730100, and 19 rare variants was not statistically significantly associated with either DBP (P = 0.07) or SBP (P = 0.53). However, the joint effect of the 2 GWAS SNPs and 16 non-synonymous SNPs was significant for DBP (P = 0.03) but not for SBP (P = 0.41).
In REGARDS data, 18 of 19 PCSK9 SNPs from exome chip data (SNP “exm62667” did not pass QC in the REGARDS dataset) were available for our analysis. PCSK9 rare variants had a cumulative significant association with SBP (P = 0.04) but not with DBP (P = 0.36). The results did not change when restricted to 15 non-synonymous SNPs (P = 0.04 for SBP and P = 0.40 for DBP).
Discussion
Using existing exome chip and GWAS data from HyperGEN, we tested the association of PCSK9 polymorphisms with blood pressure. We found a marginal effect of two GWAS SNPs (rs12048828 and rs9730100) and a significant cumulative effect of all PCSK9 rare variants, driven by non-synonymous SNPs, on DBP. Though our results for the two GWAS SNPs and for DBP did not replicate, we found a cumulative association with SBP in REGARDS, suggesting that rare variants in PCSK9 may be important for blood pressure regulation. Overall, the median blood pressure was higher by rare-variant status in our study suggesting that these variants may downregulate function (i.e., less degradation of ENaC); however we cannot draw conclusions based on our research and future studies are required to understand the functional impact of these variants.
PCSK9 is located on chromosome 1p32.3. It has 12 exons and encodes a 692 amino acid glycoprotein belonging to the family of protein convertases (Benjannet et al., 2004). More than 53 non-synonymous variants and 17 synonymous variants associated with cholesterol metabolism have been identified (Abifadel et al., 2009). Additionally, the rs11206510 (Schunkert et al., 2011), rs2479409 (Willer et al., 2013), and rs2495478 (Turnbull et al., 2012) variants have been highlighted by previous GWAS of lipid traits. Despite the plethora of studies on PCSK9 mutations in cholesterol metabolism (Abifadel et al., 2003), its role in the etiology of hypertension remains poorly understood. A recent animal study (Sharotri et al., 2012) showed that PCSK9 could alter blood pressure by modulating sodium absorption through ENaC. In that study PCSK9 degraded ENaC during trafficking to the cell membrane, an effect linked to decreased blood pressure. PCSK9 degradation of LDL-R at the cell membrane surface increases cholesterol. However, the mechanism of action of PCSK9 on degration of ENaC and LDL-R (during trafficking to the cell membrane vs. at the cell membrane, respectively) is different, and teasing out how these polymorphisms affect the function of PCSK9 in relation to blood cholesterol and blood pressure was beyond the scope of this study. Recent evidence from a mouse model suggests that knocking out the PCSK9 gene does not change BP or sodium balance in mice (Berger et al., 2015). Our findings are clearly discrepant in comparison to that report, which may be due to insufficient homology between humans and mice, statistical power, or chance.
In addition to animal studies, a recent report examined the association of PCSK9 and eight other lipid-related gene polymorphisms with blood pressure in a Chinese population stratified by gender (Yin et al., 2012). They tested only one variant of PCSK9, rs505151 (E670G), and reported a correlation with DBP in both male and female hypertensive groups. However, rs505151 did not have significant effect on DBP in the HyperGEN sample (P = 0.37). To our knowledge, our study is the first to investigate the association of multiple common and rare PCSK9 variants with blood pressure. Interestingly in our study the association of PCSK9 was observed only with DBP in the HyperGEN cohort and only with systolic blood pressure in the REGARDS cohort. This discrepancy could be due to differences between the REGARDS and HyperGEN populations. REGARDS participants are on average older than HyperGEN participants 64 ± 9 vs. 46 ± 13. Mean SBP (131 ± 17 in REGARDS vs. 129 ± 22 in HyperGEN) and DBP (79 ± 10 in REGARDS and 74 ± 12 in HyperGEN) are slightly lower in HyperGEN and there is a higher prevalence of antihypertensive treatment in REGARDS (68% vs. 57%). Importantly, hypertension at younger ages is characterized by increased DBP. As the arteries age and become more atherosclerotic, SBP becomes the dominant hypertension trait (Chrysant, 2013). If PCSK9 is truly associated with hypertension, then it is reasonable to speculate it would associate with DBP at younger ages and SBP at older ages.
The strength of our study is the inclusion of African Americans, a historically under-represented population in genomic studies. Moreover, the majority of our population is also obese and, thus, at high risk for cardiovascular disease. Also, our study is the first to examine the association of multiple variants in PCSK9 with blood pressure. Our findings must also be interpreted in context of some limitations. First, our study does not capture all common and rare variants in PCSK9. Future sequencing studies of African-American populations are necessary to fully cover the gene. Second, as PCSK9 variants are unequally distributed in different populations (Abifadel et al., 2009), our findings might not generalize to other ethnic groups.
On balance, our results support the emerging hypothesis that PCSK9 rare variants may have an involvement in the regulation of blood pressure. Upon further validation, our results may provide additional evidence of the pleiotropic function of PCSK9. Future studies should capitalize on sequencing data to further support or refute these hypotheses.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This study was supported by grant NIH R01-HL055673 (Arnett), U01 NS041588 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Department of Health and Human Service, and 5U54RR026137.
Supplementary Material
The Supplementary Material for this article can be found online at: http://www.frontiersin.org/journal/10.3389/fgene.2015.00136/abstract
References
Abifadel, M., Rabes, J. P., Devillers, M., Munnich, A., Erlich, D., Junien, C., et al. (2009). Mutations and polymorphisms in the proprotein convertase subtilisin kexin 9 (PCSK9) gene in cholesterol metabolism and disease. Hum. Mutat. 30, 520–529. doi: 10.1002/humu.20882
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Abifadel, M., Varret, M., Rabes, J. P., Allard, D., Ouguerram, K., Devillers, M., et al. (2003). Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat. Genet. 34, 154–156. doi: 10.1038/ng1161
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Allison, D. B., Heshka, S., Neale, M. C., and Heymsfield, S. B. (1994). Race effects in the genetics of adolescents' body mass index. Int. J. Obes. Relat. Metab. Disord. 18, 363–368.
Arnett, D. K., Meyers, K. J., Devereux, R. B., Tiwari, H. K., Gu, C. C., Vaughan, L. K., et al. (2011). Genetic variation in NCAM1 contributes to left ventricular wall thickness in hypertensive families. Circ. Res. 108, 279–283. doi: 10.1161/circresaha.110.239210
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Barrett, J. C., Fry, B., Maller, J., and Daly, M. J. (2005). Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 21, 263–265. doi: 10.1093/bioinformatics/bth457
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Benjannet, S., Rhainds, D., Essalmani, R., Mayne, J., Wickham, L., Jin, W., et al. (2004). NARC-1/PCSK9 and its natural mutants: zymogen cleavage and effects on the low density lipoprotein (LDL) receptor and LDL cholesterol. J. Biol. Chem. 279, 48865–48875. doi: 10.1074/jbc.M409699200
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Berger, J. M., Vaillant, N., Le May, C., Calderon, C., Bregeon, J., Prieur, X., et al. (2015). PCSK9-deficiency does not alter blood pressure and sodium balance in mouse models of hypertension. Atherosclerosis 239, 252–259. doi: 10.1016/j.atherosclerosis.2015.01.012
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Brown, C. D., Higgins, M., Donato, K. A., Rohde, F. C., Garrison, R., Obarzanek, E., et al. (2000). Body mass index and the prevalence of hypertension and dyslipidemia. Obes. Res. 8, 605–619. doi: 10.1038/oby.2000.79
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Chobanian, A. V., Bakris, G. L., Black, H. R., Cushman, W. C., Green, L. A., Izzo, J. L. Jr., et al. (2003). Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension 42, 1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Chrysant, S. G. (2013). Treating blood pressure to prevent strokes: the age factor. World J. Cardiol. 5, 22–27. doi: 10.4330/wjc.v5.i3.22
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Egan, B. M., Zhao, Y., and Axon, R. N. (2010). US trends in prevalence, awareness, treatment, and control of hypertension, 1988-2008. JAMA 303, 2043–2050. doi: 10.1001/jama.2010.650
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Go, A. S., Mozaffarian, D., Roger, V. L., Benjamin, E. J., Berry, J. D., Blaha, M. J., et al. (2014). Executive summary: heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circulation 129, 399–410. doi: 10.1161/01.cir.0000442015.53336.12
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Halperin, R. O., Sesso, H. D., Ma, J., Buring, J. E., Stampfer, M. J., and Gaziano, J. M. (2006). Dyslipidemia and the risk of incident hypertension in men. Hypertension 47, 45–50. doi: 10.1161/01.HYP.0000196306.42418.0e
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Horton, J. D., Cohen, J. C., and Hobbs, H. H. (2007). Molecular biology of PCSK9: its role in LDL metabolism. Trends Biochem. Sci. 32, 71–77. doi: 10.1016/j.tibs.2006.12.008
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Howard, V. J., Cushman, M., Pulley, L., Gomez, C. R., Go, R. C., Prineas, R. J., et al. (2005). The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology 25, 135–143. doi: 10.1159/000086678
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kato, N. (2012). Ethnic differences in genetic predisposition to hypertension. Hypertens. Res. 35, 574–581. doi: 10.1038/hr.2012.44
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Knox, S. S., Guo, X., Zhang, Y., Weidner, G., Williams, S., and Ellison, R. C. (2010). AGT M235T genotype/anxiety interaction and gender in the HyperGEN study. PLoS ONE 5:e13353. doi: 10.1371/journal.pone.0013353
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kramer, H., Han, C., Post, W., Goff, D., Diez-Roux, A., Cooper, R., et al. (2004). Racial/ethnic differences in hypertension and hypertension treatment and control in the multi-ethnic study of atherosclerosis (MESA). Am. J. Hypertens. 17, 963–970. doi: 10.1016/j.amjhyper.2004.06.001
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lackland, D. T. (2014). Racial differences in hypertension: implications for high blood pressure management. Am. J. Med. Sci. 348, 135–138. doi: 10.1097/maj.0000000000000308
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Maharjan, B. R., Bhandary, S., Sunuwar, L., Shrestha, A., and Ranjitkar, N. (2012). Association of hypertension with microalbuminuria and lipid profile in the local population of Patan. Nepal Med. Coll. J. 14, 157–162.
Nguyen, N. T., Magno, C. P., Lane, K. T., Hinojosa, M. W., and Lane, J. S. (2008). Association of hypertension, diabetes, dyslipidemia, and metabolic syndrome with obesity: findings from the National Health and Nutrition Examination Survey, 1999 to 2004. J. Am. Coll. Surg. 207, 928–934. doi: 10.1016/j.jamcollsurg.2008.08.022
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Price, A. L., Patterson, N. J., Plenge, R. M., Weinblatt, M. E., Shadick, N. A., and Reich, D. (2006). Principal components analysis corrects for stratification in genome-wide association studies. Nat. Genet. 38, 904–909. doi: 10.1038/ng1847
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Schunkert, H., Konig, I. R., Kathiresan, S., Reilly, M. P., Assimes, T. L., Holm, H., et al. (2011). Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nat. Genet. 43, 333–338. doi: 10.1038/ng.784
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sesso, H. D., Buring, J. E., Chown, M. J., Ridker, P. M., and Gaziano, J. M. (2005). A prospective study of plasma lipid levels and hypertension in women. Arch. Intern. Med. 165, 2420–2427. doi: 10.1001/archinte.165.20.2420
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sharotri, V., Collier, D. M., Olson, D. R., Zhou, R., and Snyder, P. M. (2012). Regulation of epithelial sodium channel trafficking by proprotein convertase subtilisin/kexin type 9 (PCSK9). J. Biol. Chem. 287, 19266–19274. doi: 10.1074/jbc.M112.363382
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Tobin, M. D., Sheehan, N. A., Scurrah, K. J., and Burton, P. R. (2005). Adjusting for treatment effects in studies of quantitative traits: antihypertensive therapy and systolic blood pressure. Stat. Med. 24, 2911–2935. doi: 10.1002/sim.2165
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Turnbull, C., Perdeaux, E. R., Pernet, D., Naranjo, A., Renwick, A., Seal, S., et al. (2012). A genome-wide association study identifies susceptibility loci for Wilms tumor. Nat. Genet. 44, 681–684. doi: 10.1038/ng.2251
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Ward, L. D., and Kellis, M. (2012). HaploReg: a resource for exploring chromatin states, conservation, and regulatory motif alterations within sets of genetically linked variants. Nucleic Acids Res. 40, D930–D934. doi: 10.1093/nar/gkr917
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Willer, C. J., Schmidt, E. M., Sengupta, S., Peloso, G. M., Gustafsson, S., Kanoni, S., et al. (2013). Discovery and refinement of loci associated with lipid levels. Nat. Genet. 45, 1274–1283. doi: 10.1038/ng.2797
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Williams, R. R., Rao, D. C., Ellison, R. C., Arnett, D. K., Heiss, G., Oberman, A., et al. (2000). NHLBI family blood pressure program: methodology and recruitment in the HyperGEN network. Hypertension genetic epidemiology network. Ann. Epidemiol. 10, 389–400.
Wu, M. C., Lee, S., Cai, T., Li, Y., Boehnke, M., and Lin, X. (2011). Rare-variant association testing for sequencing data with the sequence kernel association test. Am. J. Hum. Genet. 89, 82–93. doi: 10.1016/j.ajhg.2011.05.029
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Yin, R. X., Wu, J. Z., Liu, W. Y., Wu, D. F., Cao, X. L., Miao, L., et al. (2012). Association of several lipid-related gene polymorphisms and blood pressure variation in the Bai Ku Yao population. Am. J. Hypertens. 25, 927–936. doi: 10.1038/ajh.2012.55
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Keywords: PCSK9, blood pressure, hypertension, dyslipidemia, low-density lipoprotein cholesterol
Citation: Tran NT, Aslibekyan S, Tiwari HK, Zhi D, Sung YJ, Hunt SC, Rao DC, Broeckel U, Judd SE, Muntner P, Kent ST, Arnett DK and Irvin MR (2015) PCSK9 variation and association with blood pressure in African Americans: preliminary findings from the HyperGEN and REGARDS studies. Front. Genet. 6:136. doi: 10.3389/fgene.2015.00136
Received: 26 January 2015; Accepted: 20 March 2015;
Published: 08 April 2015.
Edited by:
Amit V. Pandey, University of Bern, SwitzerlandReviewed by:
Krishna Rani Kalari, Mayo Clinic, USAJohanna Sistonen, Bern University Hospital, Switzerland
Copyright © 2015 Tran, Aslibekyan, Tiwari, Zhi, Sung, Hunt, Rao, Broeckel, Judd, Muntner, Kent, Arnett and Irvin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marguerite R. Irvin, Department of Epidemiology, University of Alabama at Birmingham, 1665 University Blvd, RPHB Room 230P, Birmingham, AL 35294-0022, USA irvinr@uab.edu
 Ngan T. Tran
Ngan T. Tran Stella Aslibekyan
Stella Aslibekyan Hemant K. Tiwari
Hemant K. Tiwari Degui Zhi
Degui Zhi Yun Ju Sung
Yun Ju Sung Steven C. Hunt4
Steven C. Hunt4 Suzanne E. Judd
Suzanne E. Judd Shia T. Kent
Shia T. Kent Donna K. Arnett
Donna K. Arnett Marguerite R. Irvin
Marguerite R. Irvin