Functions and Therapeutic Roles of Exosomes in Cancer
- 1Susan Lehman Cullman Laboratory for Cancer Research, Department of Chemical Biology, Ernest Mario School of Pharmacy, Rutgers the State University, Piscataway, NJ, USA
- 2Joint Graduate Program in Toxicology, Environmental and Occupational Health Sciences Institute, Rutgers the State University, Piscataway, NJ, USA
- 3Rutgers Cancer Institute of New Jersey, New Brunswick, NJ, USA
The activation of G-Protein Coupled Receptors (GPCRs) by their respective ligands initiates a cascade of multiple signaling processes within the cell, regulating growth, metabolism and other essential cellular functions. Dysregulation and aberrant expression of these GPCRs and their subsequent signaling cascades are associated with many different types of pathologies, including cancer. The main life threatening complication in patients diagnosed with cancer is the dissemination of cells from the primary tumor to distant vital organs within the body, metastasis. Communication between the primary tumor, immune system, and the site of future metastasis are some of the key events in the early stages of metastasis. It has been postulated that the communication is mediated by nanovesicles that, under non-pathological conditions, are released by normal cells to relay signals to other cells in the body. These nanovesicles are called exosomes, and are utilized by the tumor cell to influence changes within the recipient cell, such as bone marrow progenitor cells, and cells within the site of future metastatic growth, in order to prepare the site for colonization. Tumor cells have been shown to release an increased number of exosomes when compared to their normal cell counterpart. Exosome production and release are regulated by proteins involved in localization, degradation and size of the multivesicular body, whose function may be altered within cancer cells, resulting in the release of an increased number of these vesicles. This review investigates the possibility of GPCR signaling cascades acting as the upstream activator of proteins involved in exosome production and release, linking a commonly targeted trans-membrane protein class with cellular communication utilized by tumor cells in early stages of metastasis.
Introduction
Increasing evidence links the aberrant protein expression of G-protein coupled receptors with numerous pathologies, including cancer. Exosomes are membrane-bound nanovesicles that have been implicated as an important component in preparing distal organs for tumor cell metastasis. This review intends to explore and speculate about G-protein coupled receptors and their links to cancer, exosomes, and the involvement in cancer metastasis.
G-Protein Coupled Receptors
Guanine nucleotide binding-protein coupled receptors (GPCRs) make up the largest family of proteins found within the mammalian genome (Lander et al., 2001; Venter et al., 2001). The GPCR superfamily contains over 800 different seven trans-membrane receptors. Two requirements must be met in order to be classified as a GPCR; the first is that the receptor contains seven stretches of about 30 highly hydrophobic residues that represent trans-membrane locations, which provide the protein with both intracellular domains and an extracellular domain that has the ability to interact with its ligand. The second requirement that defines a GPCR is interaction with guanine nucleotide binding proteins (G-proteins). GPCR classification within the superfamily is based on how the ligand binds to the receptor, physiological, and structural features of the receptor, as well as phylogenetics. The most frequently used classification system is A, B, C, D, E, and F (Attwood and Findlay, 1994; Kolakowski, 1994) which represent GPCRs from all living beings from humans to bacteria. The majority of human GPCRs are separated into 5 different families; glutamate, rhodopsin, adhesion, frizzled/taste2, and secretin (GRAFS nomenclature; Fredriksson et al., 2003; Lagerstrom and Schioth, 2008).
The natural ligands for GPCRs vary from ions, proteins, lipids, hormones, neurotransmitters, amines, nucleotides, odorant molecules to photons. GPCRs are associated with heterotrimeric G-protein subunits consisting of Gα, Gβ, and Gγ, that function as dimers at the intracellular domain of the GPCR. Once the ligand binds to the receptor, it causes a conformational change, activating the receptor and initiating an intracellular cascade. The inactive form of the receptor is bound to guanine diphosphate (GDP), and this conformational change results in the exchange of GDP with guanine triphosphate (GTP) of the associated G-protein within the intracellular domain of the GPCR. This phosphate exchange alters the affinity of the G-protein with the GPCR and results in the dissociation of that G-protein (Hamm, 1998; Bunemann et al., 2003), GPCRs can then interact with a multitude of different targets including ion channels, tyrosine kinases, adenylyl cyclases, phosphodiesterases, and others (Lee et al., 2008; Lappano and Maggiolini, 2011). Disruption in the function of GPCRs are known to be responsible for many prevalent human diseases such as nephrogenic diabetes insipidus (Spiegel, 1996a), cardiovascular disease (Hata and Koch, 2003), endocrine diseases (Spiegel, 1996b; Lee et al., 2008; Lappano and Maggiolini, 2011), and others.
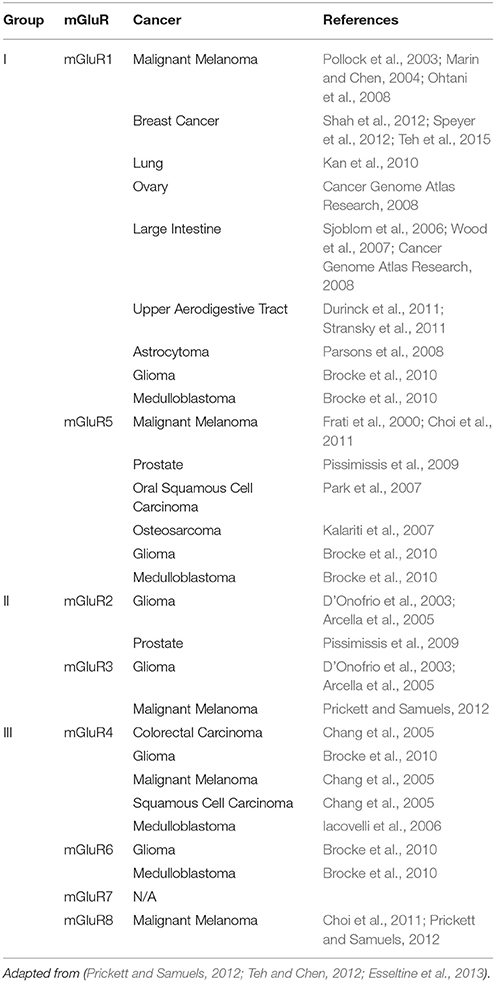
The GPCRs whose natural ligands are neurotransmitters, specifically glutamate, are classified under class C receptors (Bjarnadottir et al., 2005), and are broken down into metabotropic glutamate receptors (mGluR), GABA receptors, calcium sensing receptors, taste receptors, and some orphan receptors (Wu et al., 2014). The remainder of this review focuses on the metabotropic glutamate receptors (mGluRs) particularly mGluR1. The mGluRs can be broken down into groups I through III, based on their sequence homology, pharmacologic responses, and intracellular second messengers. Group I consists of mGluR1 and mGluR5, group II contains mGluR2 and mGluR3, and group III contains mGluR4, mGluR6, mGluR7, and mGluR8 (Nakanishi, 1992). Binding of the ligand, glutamate, to group I mGluRs resulted in exchange of GTP for GDP on Gα. Specifically, groups II and III mGluRs are coupled to Gαi/o. Group I mGluR activation results in the stimulation of phospholipase C β (PLCβ) which cleaves phosphatidylinositol 4,5-bisphosphate (PIP2) into two second messengers: inositol triphosphate (IP3), which are released into the cytoplasm, and diacylgycerol (DAG), which remains associated with the plasma membrane. Discharged IP3 initiates the activation of protein kinase C (PKC), which is involved in phosphorylation of various proteins to participate in numerous cellular functions. The hydrolysis to the second messenger IP3 results in the mobilization of calcium from the endoplasmic reticulum, and the subsequent activation of various calcium dependent kinases (Marin and Chen, 2004). The group II and III mGluRs associated Gαi/o, once activated, prevent the formation of cAMP by inhibiting adenylyl cyclase activity.
GPCRs and Cancer
The first report identifying a GPCR as an oncogene was in 1986 by Wigler and co-workers when they demonstrated the transforming activity of a rat protein, MAS (Young et al., 1986). Unlike most oncogenes identified at that time, MAS did not have activating mutations. Subsequent studies showed that the ability of GPCRs to possess oncogenic potential is by either aberrant protein expression or the excessive local production of ligands by tumor cells themselves (autocrine) or stromal counterparts (paracrine) and increasing the available ligand and subsequent receptor activation (Young et al., 1986). Mutations have also been detected in GPCRs, including a gain of function mutation causing amino-acid changes in G-proteins where GTP is bound. These mutations can initiate signaling cascades independent of GPCR activation (Van Raamsdonk et al., 2004).
Our laboratory was the first to suggest the role of dysregulated glutamatergic signaling in melanoma pathogenesis, which was subsequently confirmed by other investigators. It was discovered that a gain-of-function of the murine form of a neuronal receptor, metabotropic glutamate receptor 1 (gene: GRM1, protein: mGluR1), when ectopically expressed in melanocytes, was sufficient to induce in vitro melanocytic transformation and spontaneous malignant melanoma development in vivo in a transgenic mouse model, TG-3 (Pollock et al., 2003; Ohtani et al., 2008; Shin et al., 2008). Subsequent investigation revealed mGluR1 expression was also detected in 80% of human melanoma cell lines and 65% of human melanoma biopsy samples at levels of protein and mRNA (Pollock et al., 2003). Earlier studies showed the aberrant protein expression of GPCRs and the availability of abundant ligand in the surrounding environment are involved in cell transformation (Julius et al., 1989). We assessed levels of extra-cellular glutamate in several melanoma cell lines. We found elevated glutamate levels only in mGluR1-expressing melanoma cells (Namkoong et al., 2007). We also demonstrated stimulation of mGluR1 by its ligand, glutamate as well as other agonists, led to formation of two second messengers, DAG and IP3, as described in the central nervous system (CNS; Hermans and Challiss, 2001). DAG remains bound to the cell membrane, and activates PKC (Newton, 2001). PKC then activates the MAPK signaling cascade responsible for cell proliferation and inhibits apoptosis (Marin and Chen, 2004). PKC also activates the PI3K/AKT pathway (Spiegel, 1996a; Lappano and Maggiolini, 2011), which is involved in tumor cell survival, epithelial-mesenchymal transition and angiogenesis (Marin et al., 2006; Stepulak et al., 2009). Unlike many mouse models of cancer, TG-3 displays metastasis to several distal organs as the disease progresses (Zhu et al., 1998). Consequently, activation of ectopically expressed GRM1 initiates signaling cascades important for melanoma pathogenesis, which could include activation of the exosomal production pathway, paving the way for metastasis. In addition to mGluR1, other mGluRs have been implicated in numerous cancers. Table 1 summarizes various cancers associated with mGluR misregulation.
Exosomes
Exosomes are small membrane-bound nanovesicles with the characteristic size of 30–120 nm in diameter, derived from endosomal origins, generated constitutively, and released by various cell types and more frequently by tumor cells (Thery et al., 2002). Exosomes can be found in the blood (Taylor and Gercel-Taylor, 2008), urine (Pisitkun et al., 2004), saliva (Gonzalez-Begne et al., 2009), plasma (Caby et al., 2005), breast milk (Admyre et al., 2007) as well as other bodily fluids (Andre et al., 2002; Gatti et al., 2005; Keller et al., 2007; da Silveira et al., 2012). Exosomes are actively secreted from cells by an exocytosis pathway used for receptor removal and crosstalk between cells (Stoorvogel et al., 2002; Thery et al., 2002; Valenti et al., 2007). Exosomes are shed from the surface of healthy cells, and take with them membrane proteins and cytoplasm contents of the cells they are released from including miRNAs, mRNAs, siRNAs, and proteins (Thery et al., 2002). Studies of exosomes from various cell types show several common proteins contained in all exosomes (Raposo et al., 1996; Escola et al., 1998; Thery et al., 1999, 2001; van Niel et al., 2001).
Composition of Exosomes
Exosomes contain a unique composition of proteins and nucleic acids that can vary depending on the cell type they originated from. Studies of exosomes from immature dendritic cells (DCs; Thery et al., 1999, 2001), B lymphocytes (Raposo et al., 1996; Escola et al., 1998), intestinal epithelial cells (van Niel et al., 2001), and other cell types show that there are common, as well as cell-type specific proteins residing within exosomes. Cell-type specific proteins within exosomes include Major Histocompatibility Complex (MHC) class I and II proteins, which have been detected in B lymphocyte, DCs, mast cells and intestinal epithelial cell exosomes. Von Willebrand factor (Heijnen et al., 1999), perforin and granzymes (Peters et al., 1991) were found in platelet and cytotoxic T cell exosomes, respectively. The proteins that were found to be consistent across exosome types include chaperones (Hsc73 and Hsc90), subunits of trimeric G proteins, Tsg101, cytoskeletal proteins and tetraspanins such as CD9, CD63, CD81, and CD82 (Thery et al., 1999, 2001; van Niel et al., 2001). Kahlert et al. identified double stranded genomic DNA present within exosomes (Kahlert et al., 2014).
Formation of Exosomes
One of the defining characteristics of exosomes is the endocytic origin, which sets it apart from other cellular vesicles such as apoptotic bodies that are budded off of the plasma membrane. The initial step in the formation of exosomes is endocytosis. Invagination of the plasma membrane is initiated by the deformation of the lipid bilayer, which can be influenced extrinsically or by internal membrane structural modification. Specific membrane manipulating proteins interact with and bend the membrane surface to initiate tubulation. Membranes that are tubulated experience an external force, which causes the inward curvature, or invagination of the membrane (Lipowsky, 2013). The proteins involved in this process include endocytosis proteins such as epsin (Ford et al., 2002), N-BAR proteins, such as amphiphysin (Takei et al., 1999; Peter et al., 2004) and endophilin, (Farsad et al., 2001) or F-BAR proteins, such as syndapins (Wang et al., 2009) and its associated protein, dynamin. Dynamin is a GTPase that connects with both actin and F-BAR to successfully form and cut membrane tubules to create a successful invagination of the membrane. (Reviewed by Lipowsky, 2013). Once the invaginated membrane forms and becomes severed from the plasma membrane, it is released into the cytosol of the cell as an endosome.
The Endosomal Sorting Complex Required for Transport (ESCRT) functions on the newly formed endosome to initiate the internal budding of the multivesicular bodies (MVB) membrane to form smaller intraluminal vesicles within the MVB, these vesicles are exosomes. Ceramide, a sphingolipid, was found to trigger budding of exosome vesicles into the multivesicular body (Trajkovic et al., 2008). ESCRT is made up of four different complexes (ESCRT-0, -I, -II, and -III) and associated accessory proteins. The primary function of the ESCRT proteins is to constrict the membrane, create budding within the endosome and cause severing of the budded vesicle neck to separate the vesicle from the MVB membrane. The precise mechanism of the severing is unknown. (Hurley and Hanson, 2010; Peel et al., 2011; Henne et al., 2013; McCullough et al., 2013). The proteins in the ESCRT pathway are broken up into four different complexes: ESCRT-0, -I, -II, and -III. ESCRT-0 is involved in collecting ubiquitinated proteins on the membrane of the endosome. ESCRT-I and -II initiate the inward budding of the endosomal membrane and ESCRT-III severs the budding membrane from the endosome, creating a separate smaller vesicle within the endosome; an exosome (Reviewed by Hurley and Odorizzi, 2012). ESCRTIII is recruited for scission by ALIX. Syndecans are proteins involved in sulfate-presentation on the membrane surface, and are found on exosomes. These proteins are sorted into exosomes by an adapter protein, syntenin, which binds to ALIX, recruiting ESCRTIII to finalize the formation of the exosome (Baietti et al., 2012; Hurley and Odorizzi, 2012).
The specificity of cargo sorting into these exosome vesicles is still unclear. However, it has been shown that ubiquitination serves as a signal for sorting cargo into the vesicles formed within the MVB. Additionally, evidence has shown that ESCRT-I recognizes ubiquitinated cargo, suggesting that this protein and its associated protein, Vps23, initiate MVB sorting by binding cargo and directing it to MVB for loading in a ubiquitin-binding manner (Katzmann et al., 2001).
Exosome Release
Once the MVB is formed and contains exosomes within its membrane, it has one of two fates; targeted degradation by the lysosome or plasma membrane fusion resulting in exosome release.
If the MVB is targeted for lysosomal degradation, it fuses with the lysosome and results in the release of the internal exosomes and the macromolecules contained within them, into the lumen of the lysosome. These components are then exposed to the hydrolytic enzymes within the lumen of the lysosome and are degraded (Futter et al., 1996).
Alternatively, the MVB will travel to the plasma membrane. In this case, a GTPase, RAL-1, has recently been identified to mediate the fusion of the MVB membrane with the plasma membrane of the cell to allow the release of the exosomes into the extracellular space. Syx-5 is a t-SNARE that is recruited by RAL-1 to the plasma membrane to stimulate MVB fusion. Hyenne et al., showed that without Syx-5, the MVB is unable to fuse with the plasma membrane (Hyenne et al., 2015). Ostrowski et al., identified Rab27a, Rab27b, and their effectors (SYTL4 and Slac2b, respectively) to be involved in the exosomal pathway in HeLa cells (Ostrowski et al., 2010). Specifically, Rab27a was shown to be involved in the size of the MVE, while Rab27b regulated localization of the MVB to the plasma membrane. Another Rab-GTPase, Rab35, was identified as a regulator in the docking or tethering of the MVB to the plasma membrane (Hsu et al., 2010). In addition to enzymatic involvement of exosome regulation, intracellular levels of Ca2+ have been shown to be proportional to exosome release (Savina et al., 2003), in addition, low pH within the microenvironment influences the release of exosomes as well as the uptake (Parolini et al., 2009).
In cancer, oncogenes have been shown to play a role in exosome secretion, including a p53-regulated pathway, TSAP6, both in-vitro (Yu et al., 2006) and in-vivo using a TSAP/Steap3-null mouse (Lespagnol et al., 2008). As tumors become more aggressive, the expression and activation of the enzyme heparanase becomes upregulated. The activation of heparanase increases the release of exosomes, as well as the cargo levels found within the exosomes (Thompson et al., 2013).
Exosome Uptake
Once the exosomes are released from the plasma membrane, they have the ability to travel to distant sites of the body, and/or interact with the cells in the surrounding microenvironment. Exosomes involved in intracellular communication contain phosphatidylserine on their outer membrane and interacts with T-cell immunoglobulin and mucin-domain-containing molecule 1 (Tim1), a transmembrane protein present on recipient cells (Thery et al., 2002). This interaction initiates the engulfment of exosomes by the recipient cell (Miyanishi et al., 2007). In ovarian cancer cells, exosome uptake was shown by clathrin-dependent endocytosis. Both proteins and specific glycoproteins present on exosomes and the cell surface were shown to be important for exosome uptake (Escrevente et al., 2011). The transfer of major histocompatibility complex (MHC)-peptide complexes between dendritic cells was shown to be dependent on the presence of intercellular adhesion molecule 1 (ICAM-1) on exosomes. Exosomes from immature dendritic cells (DCs) were unable to transfer MHC to other DCs, however, exosomes from mature DCs contained ICAM-1 on the surface of the exosomes, and resulted in transfer of MHC from the exosomes (Segura et al., 2005). Additionally, heparin sulfate proteoglycans (HSPGs) have been shown to act as receptors of tumor derived exosomes (Christianson et al., 2013). Parolini et al., were the first to show that endocytosis is not the sole route of exosome uptake. Under certain conditions, exosomes will undergo lipid-dependent membrane fusion with the recipient cell independent of energy-dependent exocytosis and protein-protein interaction (Parolini et al., 2009).
Once the exosomes enter the recipient cell, the cargo has the potential to interact and alter the physiology of the cell. Exosomes are also known to modulate gene expression as Valadi and colleagues demonstrated that RNAs in mast cell exosomes could be delivered to human and mouse mast cells leading to new protein production in recipient cells (Valadi et al., 2007).
Exosomes in Cancer
Circulating tumor cells (CTCs) are potential biomarkers for cancer; however, depending on the stage of cancer, there can be as few as 1-10 CTCs per mL of blood. Exosomes, however, are found in abundance within the blood, typically, 1 × 1012 exosomes per mL of blood, making them a non-invasive and ideal screening method for diagnostics, cancer progression and targeted therapy (Hyun et al., 2015). Fujita et al., suggested that exosomes have the potential to be used as biomarkers for asthma (Fujita et al., 2014). In addition to a minimally invasive biomarker, there have been efforts in using exosomes to develop a new method of drug delivery to target drug-resistant cancer. Exosome-encapsulated Paclitaxel (exoPTX) increases the cytotoxic effects on prostate cancer cells when compared to drug alone, and holds significant potential for the delivery of various chemotherapeutics to treat cancers that have became resistant to the regimen (Saari et al., 2015). In addition to drug delivery, dendritic cell-derived exosomes are being explored for their potential in cancer immunotherapy (Viaud et al., 2010). Increased exosome plasma levels are observed only in patients with advanced stage diseases (Logozzi et al., 2009; Peinado et al., 2012). Recently, an assay was developed to detect a proteoglycan molecule, glypican-1 (GPC1) found on extracellular vesicles in patients with late-stage pancreatic cancer with 100% confidence. This method is more reliable than a more common assay looking for a tumor antigen in whole blood (Thery, 2015).
Along with the potential in using these vesicles to diagnose and treat cancers, tumor exosomes have been shown to play a role in the aggressiveness of cancer. These microvesicles are more frequently released by tumor cells and may facilitate communication within the local microenvironment and the primary tumor (Baj-Krzyworzeka et al., 2006; Valadi et al., 2007; Huber et al., 2008; Iero et al., 2008). Patient-derived cancer-associated fibroblast exosomes have been shown to alter the cellular metabolism of prostate and pancreatic tumor cells in vitro, redirecting from oxidative phosphorylation to a glycolysis and glutamine-dependent reductive carboxylation (Zhao et al., 2016). This study indicates the impact exosomes released by cells within the tumor microenvironment have on the cellular function of the tumor cells. Communication between the tumor microenvironment and the cancer cells supports tumor cell dissemination and early events in metastasis (Hood et al., 2009, 2011). Exosomes may have the ability to promote metastasis via the horizontal transfer of proteins, miRNAs and other molecules to recipient cells (Ratajczak et al., 2006; Aliotta et al., 2010; Balaj et al., 2011; Peinado et al., 2012). Exosomes containing the RNA-binding protein LIN28 (which is a known marker of poor outcome for ovarian cancer) were shown to be taken up by recipient cells and significantly increase transcription of genes involved in Epithelial-to-Mesenchymal Transition (EMT), cell migration and invasion in the recipient cells (Enriquez et al., 2015).
Metastasis
Metastasis is the major cause of cancer-related death (Mehlen and Puisieux, 2006) that occurs in a stepwise fashion relying on a number of host-tumor interactions (Fidler and Hart, 1982; Pauli et al., 1983). In order for a metastatic tumor to form, a cell from the primary tumor must have the ability to survive on its own, dissociate from the tumor, occupy the surrounding tissue (Liotta and Stetler-Stevenson, 1991), enter circulation, survive the environment of the circulatory system, invade the distant parenchyma and proliferate on its own (Liotta and Stetler-Stevenson, 1991). Circulating tumor cells (CTCs) can be found in the vasculature of various organs but only in some organs where a secondary tumor will survive and develop into sites of metastasis (Poste and Fidler, 1980). It has been noted that primary tumors preferentially home to particular organs. For example, melanoma preferentially metastasizes to the lung and brain (Fidler, 2003), therefore, successful metastatic growth is dependent on a microenvironment that is receptive of that particular cancer cell type (Fidler, 2003). Aberrant expression of GPCR proteins has been suggested to play a role in the organ-specific metastasis of cancer cells by way of enhancing mobilization, promoting angiogenesis and proliferation (Lee et al., 2008). To develop therapies focused on treating metastatic diseases, understanding the molecular mechanisms of metastasis is vital. Although the disseminated primary tumor cells are essential to metastasis, the cells from the surrounding tumor microenvironment are equally critical in prompting metastatic ability.
Formation of the Pre-Metastatic Niche
The formation of the pre-metastatic niche is an essential step in successful metastatic growth. The primary tumor initiates this formation by releasing factors into circulation and exosomes released from the tumor have been implicated in this process. Peinedo et al., described the involvement of exosomes in tumor progression and in the preparation of the pre-metastatic niche of future secondary tumor sites in a melanoma model system (Peinado et al., 2012). They provided evidence that exosomes are released by the primary tumor into the circulation, which results in the leakiness of the vasculature, as well as recruitment of immune cells, both events are involved in pre-metastatic niche formation (Peinado et al., 2012).
Changes within the Pre-Metastatic Environment
Exosomes released by tumor cells contain factors such as macrophage migration inhibitory factor (MIF) that influence the physiology of the recipient cells. The engulfment of MIF-containing exosomes promotes the release of transforming growth factor beta (TGFβ) by Kupffer cells, which then initiates the production of fibronectin by the hepatic stellate cells (hStCs; Costa-Silva et al., 2015). Resident fibroblasts and cells from the primary tumor stimulate fibronectin deposition (Kaplan et al., 2005; Erler et al., 2009). The deposition of fibronectin within the organs determines the location of the metastatic niche formation (Kaplan et al., 2005). Fibronectin deposited within the tissue causes the arrest of bone marrow derived cells (BMDC), specifically macrophages and neutrophils, within the deposits (Erler et al., 2009).
In addition to fibronectin, fibroblasts express Tenascin-C (TN-C) glycoprotein, within the premetastatic site, which may protect the cancer cells from apoptosis (O'Connell et al., 2011). Several cytokines, as well as Wnt and Ras/MAPK signaling, could induce TN-C glycoprotein expression. TN-C is not found in normal tissues, however, under pathological conditions, such as inflammation and cancer, its protein expression is strikingly increased and induces the production of angiogenic protein factors such as MMP-9. TN-C also has been implicated to affect steps in cancer progression including proliferation, migration, invasion and angiogenesis. Reviewed by Tse and Kalluri (2007).
Periostin is a secretory protein also deposited within the extracellular matrix (ECM) by fibroblasts, which acts as a bridge that binds to TN-C as well as fibronectin and collagen (Kii et al., 2010; Wang and Ouyang, 2012). Studies showed that periostin did not have a direct effect on the growth of tumor cells, however, knocking out periostin leads to a significant reduction in the metastatic potential (Wang and Ouyang, 2012). Versican is an extracellular matrix (ECM) proteoglycan that is expressed by myeloid cells present in the pre-metastatic niche. It is involved in mesenchymal to epithelial transition by decreasing phospho-Smad2 levels, which increases proliferation and metastasis, but does not play a role in the recruitment of immune cells or the manipulation of the immune environment (Gao et al., 2012).
In addition to remodeling the extracellular matrix to create greater permeability within the surrounding vasculature, which is necessary in forming a pre-metastatic niche that is receptive of CTCs, the vasculature is manipulated as well. Vascular remodeling occurs to allow for the extravasation of CTCs out of circulation, into the pre-metastatic environment. This process is dependent on angiopoietin 2 (Angpt2), matrix metalloproteinase 3 (MMP-3), and MMP-10. Huang et al., showed that knocking down these proteins reduces the vascular permeability and decreases the infiltration of myeloid cells and inhibits spontaneous lung metastasis in an in-vivo model (Huang et al., 2009).
In a breast cancer exosome model, the macrophages within the lung and brain both phagocytose exosomes, which results in the activation of NF-kB and subsequent release of pro-inflammatory cytokines such as IL-6, TNFα, GCSF, and CCL2, which promote metastasis development (Chow et al., 2014). Hypoxic breast cancer cells release an amine oxidase, lysyl oxidase (LOX) that accumulates at sites of pre-metastatic niche formation. LOX co-localizes with metastases and crosslinks collagen within the basement membrane and is essential for the recruitment and adherence of myeloid cells. This crosslinking is critical for CD11b+ myeloid cell recruitment, which led to interactions with the collagen and production of MMP-2, breaking down collagen into peptides that act as chemoattractants for bone marrow derived cells (BMDCs) and circulating tumor cells (CTCs; Erler et al., 2009).
Recruitment of Immune Cells
Exosomes have the ability to “educate” bone progenitor cells to be receptive of and support tumor cell growth and metastasis (Peinado et al., 2012). BMDCs express vascular endothelial growth receptor 1 (VEGFR1), which may be responsible for the homing of tumor cells to the pre-metastatic niche. Erler et al., showed accumulation of VEGFR1+ BMDCs in common sites of metastasis in the lung, within 9 days post-accumulation, micrometastases formed and BMDCs remained within the site (Erler et al., 2009). As described earlier, fibronectin deposition within the pre-metastatic environment will result in the arrest of bone marrow derived cells. When the BMDCs arrive, they form clusters of cells in the tissue parenchyma at common sites of metastasis before evidence of tumor cells (Kaplan et al., 2006). VEGFR1+ hematopoietic cells (HPCs) express VLA-4, which allows them to adhere to the newly synthesized fibronectin to initiate the cellular clustering (Kaplan et al., 2006). Interaction of VLA-4 with fibronectin is responsible for the ability of HPCs to move within the bone marrow (Burger et al., 2003). After fibronectin binding in HPCs, MMP protein expression is enhanced with the presence of integrin signaling (Huhtala et al., 1995; Yakubenko et al., 2000). MMP-9 functions to breakdown basement membranes and the release of Kit-ligand and VEGF-A, presumably to support bone marrow migrating cells that express c-Kit (Bergers et al., 2000; Heissig et al., 2002).
Myeloid cell recruitment is influenced by the protein expression of several inflammatory chemoattractants, which are influenced by the primary tumor. These chemoattractants recruit Mac1+ (macrophage antigen 1) myeloid cells to the lung. Furthermore, Hiratsuka et al., found these chemoattractants were involved in the ability of the tumor cells to migrate, using pseudopodia for invasion. When the protein expression of these inflammatory chemoattractants was abolished, migration of both tumor cells and Mac1+ myeloid cells was prevented (Hiratsuka et al., 2006).
GPCRs and Exosomes
A potential relationship between GPCRs and MVB formation, exosome endocytosis, or exosome release has been suggested. For example, the G protein-coupled pheromone receptor, Ste2, is downregulated after activation by the transfer of the receptor to the lumen of the vacuole by way of MVB sorting (Odorizzi et al., 1998). However, Myers et al., showed that activation of GPCRs result in growth factor shedding by way of proteolytic cleavage, and not by exosome release (Myers et al., 2009). Therefore, certain GPCRs, but not all, may play a role in the MVB exocytosis. Some GPCRs, specifically A2A receptors, have been shown to have the ability to be transferred by exosomes from a source cell expressing these receptors to a target cell that does not. Upon incubation with an A2A receptor agonist, the target cells produced an increased amount of cAMP, suggesting that the transferred receptor was then shown to be functionally active within the target cell (Guescini et al., 2012). Additionally, under cellular stress responses to neurohormonal stimulation, cardiomyocytes are stimulated to release exosomes containing an endogenous functional GPCR, AT1R, which, upon activation with an AT1R agonist, results in phosphorylated-ERK (Pironti et al., 2015). These studies suggest that functioning GPCRs can be transferred by exosomes, influencing physiological changes within the recipient cell. Locke et al., identified the relationship between the activation of GPR143 by its natural ligand, L-DOPA, in retinal pigment epithelial cells, and the release of exosomes for intercellular communication in the eye (Locke et al., 2014). Downstream exosome release is dependent on the interaction of L-DOPA with the receptor, which activates Gαq, initiating the release of calcium storage from the cell. Calcium mobilization has been suggested to play a role in the release of exosomes (Savina et al., 2003; Pant et al., 2012).
Given the examples of GPCR activation resulting in exosome formation, release, and uptake, it seems logical to suggest a potential role of GPCRs in exosome biogenesis and function. Furthermore, activated group I mGluRs promote the release of calcium from the endoplasmic reticulum by the second messenger, IP3, and increased intracellular calcium levels have been suggested to result in the release of exosomes (Savina et al., 2003; Pant et al., 2012). Interestingly, activated phospholipase C (PLC) that hydrolyzes PIP2 for IP3 formation was detected within exosomes of a leukemia cell line, suggesting that exosomes may carry functional phospholipases to recipient cells (Subra et al., 2010). Modulation of calcium concentration may be a potential link between group I mGluR activation and exosome release as depicted in Figure 1. This association between mGluRs and exosome release may provide hints to elucidate the aggressive nature of cancers that ectopically express mGluRs, and the role exosomes play in the metastatic potential of the tumor, and formation of the pre-metastatic niche.
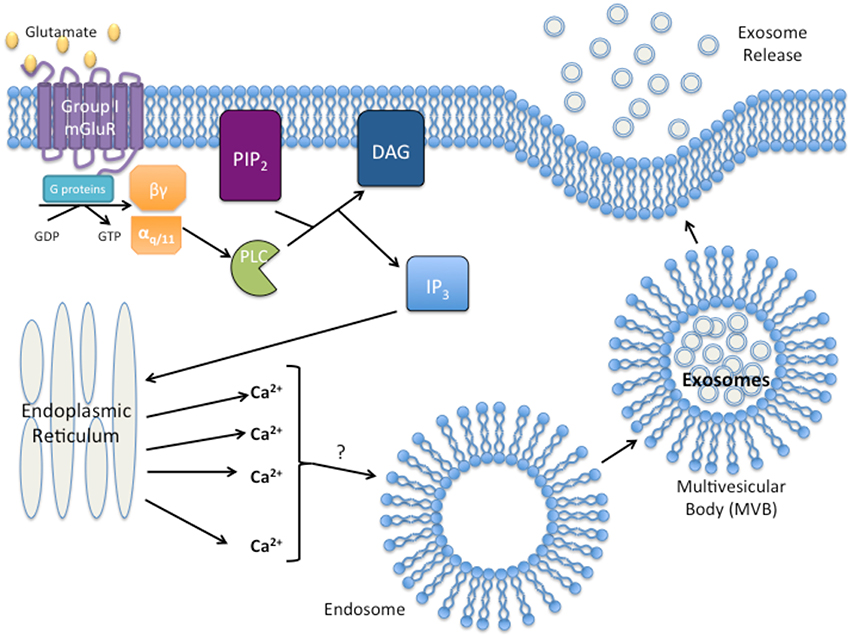
Figure 1. Proposed model of group I Metabotropic glutamate receptor (mGluR) activation and exosome release. Activation of group I mGluR by glutamate results in the intracellular G-protein exchange of guanine diphosphate (GDP with guanine triphosphate (GTP). Exchange results in the activation of the αq∕11 subunit and activation of phospholipase C (PLC). PLC then cleaves phosphatidylinositol 4,5-bisphosphate (PIP2) into diacylgycerol (DAG) and inositol triphosphate (IP3). IP3 initiates release of Ca2+ from the endoplasmic reticulum. Excess intracellular Ca2+ initiates exosome formation/release through an unknown mechanism.
Conclusions
Taken together, the aggressiveness and malignancy exhibited by cancers aberrantly expressing GPCRs could be explained by the release of a high volume of exosomes not only manipulating the surrounding stromal of the tumor, but also preparing the sites of future metastasis for the arrival of a circulating tumor cell. We hypothesize that stimulation of GPCR by it ligand/agonist initiates signaling cascades, activating a multitude of different downstream effectors that may regulate exosomal secretion and/or production. The precise mechanisms remain unknown. Calcium has been proposed as one of the “factors” involved, for example, stimulated group I mGluRs activate PLC and promote hydrolytic cleavage of PIP2 for the formation of two second messengers, IP3 and DAG. IP3 brings about the release of calcium from the endoplasmic reticulum, which initiates multiple diverse physiological alterations within the cell; one of them could be exosome release. Therefore, it is plausible that GPCR signaling may participate in exosome production or secretion by tumor cells.
Author Contributions
This review was written by AI, who works under the guidance of SC, the principle investigator.
Funding
This review was funded by the National Institute of Health (R21CA185835) and The Bristol-Myers Squibb Graduate Research Fellowship in Toxicology.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Admyre, C., Johansson, S. M., Qazi, K. R., Filen, J. J., Lahesmaa, R., Norman, M., et al. (2007). Exosomes with immune modulatory features are present in human breast milk. J. Immunol. 179, 1969–1978. doi: 10.4049/jimmunol.179.3.1969
Aliotta, J. M., Pereira, M., Johnson, K. W., de Paz, N., Dooner, M. S., Puente, N., et al. (2010). Microvesicle entry into marrow cells mediates tissue-specific changes in mRNA by direct delivery of mRNA and induction of transcription. Exp. Hematol. 38, 233–245. doi: 10.1016/j.exphem.2010.01.002
Andre, F., Schartz, N. E., Chaput, N., Flament, C., Raposo, G., Amigorena, S., et al. (2002). Tumor-derived exosomes: a new source of tumor rejection antigens. Vaccine 20(Suppl. 4), A28–A31. doi: 10.1016/s0264-410x(02)00384-5
Arcella, A., Carpinelli, G., Battaglia, G., D'Onofrio, M., Santoro, F., Ngomba, R. T., et al. (2005). Pharmacological blockade of group II metabotropic glutamate receptors reduces the growth of glioma cells in vivo. Neuro Oncol. 7, 236–245. doi: 10.1215/S1152851704000961
Attwood, T. K., and Findlay, J. B. (1994). Fingerprinting G-protein-coupled receptors. Protein Eng. 7, 195–203. doi: 10.1093/protein/7.2.195
Baietti, M. F., Zhang, Z., Mortier, E., Melchior, A., Degeest, G., Geeraerts, A., et al. (2012). Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat. Cell Biol. 14, 677–685. doi: 10.1038/ncb2502
Baj-Krzyworzeka, M., Szatanek, R., Weglarczyk, K., Baran, J., Urbanowicz, B., Branski, P., et al. (2006). Tumour-derived microvesicles carry several surface determinants and mRNA of tumour cells and transfer some of these determinants to monocytes. Cancer Immunol. Immunother. 55, 808–818. doi: 10.1007/s00262-005-0075-9
Balaj, L., Lessard, R., Dai, L., Cho, Y. J., Pomeroy, S. L., Breakefield, X. O., et al. (2011). Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nat. Commun. 2:180. doi: 10.1038/ncomms1180
Bergers, G., Brekken, R., McMahon, G., Vu, T. H., Itoh, T., Tamaki, K., et al. (2000). Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat. Cell Biol. 2, 737–744. doi: 10.1038/35036374
Bjarnadottir, T. K., Fredriksson, R., and Schioth, H. B. (2005). The gene repertoire and the common evolutionary history of glutamate, pheromone (V2R), taste(1) and other related G protein-coupled receptors. Gene 362, 70–84. doi: 10.1016/j.gene.2005.07.029
Brocke, K. S., Staufner, C., Luksch, H., Geiger, K. D., Stepulak, A., Marzahn, J., et al. (2010). Glutamate receptors in pediatric tumors of the central nervous system. Cancer Biol. Ther. 9, 455–468. doi: 10.4161/cbt.9.6.10898
Bunemann, M., Frank, M., and Lohse, M. J. (2003). Gi protein activation in intact cells involves subunit rearrangement rather than dissociation. Proc. Natl. Acad. Sci. U.S.A. 100, 16077–16082. doi: 10.1073/pnas.2536719100
Burger, J. A., Spoo, A., Dwenger, A., Burger, M., and Behringer, D. (2003). CXCR4 chemokine receptors (CD184) and alpha4beta1 integrins mediate spontaneous migration of human CD34+ progenitors and acute myeloid leukaemia cells beneath marrow stromal cells (pseudoemperipolesis). Br. J. Haematol. 122, 579–589. doi: 10.1046/j.1365-2141.2003.04466.x
Caby, M. P., Lankar, D., Vincendeau-Scherrer, C., Raposo, G., and Bonnerot, C. (2005). Exosomal-like vesicles are present in human blood plasma. Int. Immunol. 17, 879–887. doi: 10.1093/intimm/dxh267
Cancer Genome Atlas Research, N. (2008). Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 455, 1061–1068. doi: 10.1038/nature07385
Chang, H. J., Yoo, B. C., Lim, S. B., Jeong, S. Y., Kim, W. H., and Park, J. G. (2005). Metabotropic glutamate receptor 4 expression in colorectal carcinoma and its prognostic significance. Clin. Cancer Res. 11, 3288–3295. doi: 10.1158/1078-0432.CCR-04-1912
Choi, K. Y., Chang, K., Pickel, J. M., Badger, J. D. II., and Roche, K. W. (2011). Expression of the metabotropic glutamate receptor 5 (mGluR5) induces melanoma in transgenic mice. Proc. Natl. Acad. Sci. U.S.A. 108, 15219–15224. doi: 10.1073/pnas.1107304108
Chow, A., Zhou, W., Liu, L., Fong, M. Y., Champer, J., Van Haute, D., et al. (2014). Macrophage immunomodulation by breast cancer-derived exosomes requires Toll-like receptor 2-mediated activation of NF-kappaB. Sci. Rep. 4:5750. doi: 10.1038/srep05750
Christianson, H. C., Svensson, K. J., van Kuppevelt, T. H., Li, J. P., and Belting, M. (2013). Cancer cell exosomes depend on cell-surface heparan sulfate proteoglycans for their internalization and functional activity. Proc. Natl. Acad. Sci. U.S.A. 110, 17380–17385. doi: 10.1073/pnas.1304266110
Costa-Silva, B., Aiello, N. M., Ocean, A. J., Singh, S., Zhang, H., Thakur, B. K., et al. (2015). Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat. Cell Biol. 17, 816–826. doi: 10.1038/ncb3169
da Silveira, J. C., Veeramachaneni, D. N., Winger, Q. A., Carnevale, E. M., and Bouma, G. J. (2012). Cell-secreted vesicles in equine ovarian follicular fluid contain miRNAs and proteins: a possible new form of cell communication within the ovarian follicle. Biol. Reprod. 86:71. doi: 10.1095/biolreprod.111.093252
D'Onofrio, M., Arcella, A., Bruno, V., Ngomba, R. T., Battaglia, G., Lombari, V., et al. (2003). Pharmacological blockade of mGlu2/3 metabotropic glutamate receptors reduces cell proliferation in cultured human glioma cells. J. Neurochem. 84, 1288–1295. doi: 10.1046/j.1471-4159.2003.01633.x
Durinck, S., Ho, C., Wang, N. J., Liao, W., Jakkula, L. R., Collisson, E. A., et al. (2011). Temporal dissection of tumorigenesis in primary cancers. Cancer Discov. 1, 137–143. doi: 10.1158/2159-8290.CD-11-0028
Enriquez, V. A., Cleys, E. R., Da Silveira, J. C., Spillman, M. A., Winger, Q. A., and Bouma, G. J. (2015). High LIN28A expressing ovarian cancer cells secrete exosomes that induce invasion and migration in HEK293 Cells. Biomed Res. Int. 2015:701390. doi: 10.1155/2015/701390
Erler, J. T., Bennewith, K. L., Cox, T. R., Lang, G., Bird, D., Koong, A., et al. (2009). Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell 15, 35–44. doi: 10.1016/j.ccr.2008.11.012
Escola, J. M., Kleijmeer, M. J., Stoorvogel, W., Griffith, J. M., Yoshie, O., and Geuze, H. J. (1998). Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B-lymphocytes. J. Biol. Chem. 273, 20121–20127. doi: 10.1074/jbc.273.32.20121
Escrevente, C., Keller, S., Altevogt, P., and Costa, J. (2011). Interaction and uptake of exosomes by ovarian cancer cells. BMC Cancer 11:108. doi: 10.1186/1471-2407-11-108
Esseltine, J. L., Willard, M. D., Wulur, I. H., Lajiness, M. E., Barber, T. D., and Ferguson, S. S. (2013). Somatic mutations in GRM1 in cancer alter metabotropic glutamate receptor 1 intracellular localization and signaling. Mol. Pharmacol. 83, 770–780. doi: 10.1124/mol.112.081695
Farsad, K., Ringstad, N., Takei, K., Floyd, S. R., Rose, K., and De Camilli, P. (2001). Generation of high curvature membranes mediated by direct endophilin bilayer interactions. J. Cell Biol. 155, 193–200. doi: 10.1083/jcb.200107075
Fidler, I. J. (2003). The pathogenesis of cancer metastasis: the “seed and soil” hypothesis revisited. Nat. Rev. Cancer 3, 453–458. doi: 10.1038/nrc1098
Fidler, I. J., and Hart, I. R. (1982). Biological diversity in metastatic neoplasms: origins and implications. Science 217, 998–1003. doi: 10.1126/science.7112116
Ford, M. G., Mills, I. G., Peter, B. J., Vallis, Y., Praefcke, G. J., Evans, P. R., et al. (2002). Curvature of clathrin-coated pits driven by epsin. Nature 419, 361–366. doi: 10.1038/nature01020
Frati, C., Marchese, C., Fisichella, G., Copani, A., Nasca, M. R., Storto, M., et al. (2000). Expression of functional mGlu5 metabotropic glutamate receptors in human melanocytes. J. Cell. Physiol. 183, 364–372. doi: 10.1002/(SICI)1097-4652(200006)183:3<364::AID-JCP9>3.0.CO;2-X
Fredriksson, R., Lagerstrom, M. C., Lundin, L. G., and Schioth, H. B. (2003). The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol. Pharmacol. 63, 1256–1272. doi: 10.1124/mol.63.6.1256
Fujita, Y., Yoshioka, Y., Ito, S., Araya, J., Kuwano, K., and Ochiya, T. (2014). Intercellular communication by extracellular vesicles and their microRNAs in asthma. Clin. Ther. 36, 873–881. doi: 10.1016/j.clinthera.2014.05.006
Futter, C. E., Pearse, A., Hewlett, L. J., and Hopkins, C. R. (1996). Multivesicular endosomes containing internalized EGF-EGF receptor complexes mature and then fuse directly with lysosomes. J. Cell Biol. 132, 1011–1023. doi: 10.1083/jcb.132.6.1011
Gao, D., Joshi, N., Choi, H., Ryu, S., Hahn, M., Catena, R., et al. (2012). Myeloid progenitor cells in the premetastatic lung promote metastases by inducing mesenchymal to epithelial transition. Cancer Res. 72, 1384–1394. doi: 10.1158/0008-5472.CAN-11-2905
Gatti, J. L., Metayer, S., Belghazi, M., Dacheux, F., and Dacheux, J. L. (2005). Identification, proteomic profiling, and origin of ram epididymal fluid exosome-like vesicles. Biol. Reprod. 72, 1452–1465. doi: 10.1095/biolreprod.104.036426
Gonzalez-Begne, M., Lu, B., Han, X., Hagen, F. K., Hand, A. R., Melvin, J. E., et al. (2009). Proteomic analysis of human parotid gland exosomes by multidimensional protein identification technology (MudPIT). J. Proteome Res. 8, 1304–1314. doi: 10.1021/pr800658c
Guescini, M., Leo, G., Genedani, S., Carone, C., Pederzoli, F., Ciruela, F., et al. (2012). Microvesicle and tunneling nanotube mediated intercellular transfer of g-protein coupled receptors in cell cultures. Exp. Cell Res. 318, 603–613. doi: 10.1016/j.yexcr.2012.01.005
Hamm, H. E. (1998). The many faces of G protein signaling. J. Biol. Chem. 273, 669–672. doi: 10.1074/jbc.273.2.669
Hata, J. A., and Koch, W. J. (2003). Phosphorylation of G protein-coupled receptors: GPCR kinases in heart disease. Mol. Interv. 3, 264–272. doi: 10.1124/mi.3.5.264
Heijnen, H. F., Schiel, A. E., Fijnheer, R., Geuze, H. J., and Sixma, J. J. (1999). Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood 94, 3791–3799.
Heissig, B., Hattori, K., Dias, S., Friedrich, M., Ferris, B., Hackett, N. R., et al. (2002). Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell 109, 625–637. doi: 10.1016/S0092-8674(02)00754-7
Henne, W. M., Stenmark, H., and Emr, S. D. (2013). Molecular mechanisms of the membrane sculpting ESCRT pathway. Cold Spring Harb Perspect Biol 5:a016766. doi: 10.1101/cshperspect.a016766.
Hermans, E., and Challiss, R. A. (2001). Structural, signalling and regulatory properties of the group I metabotropic glutamate receptors: prototypic family C G-protein-coupled receptors. Biochem. J. 359, 465–484. doi: 10.1042/bj3590465
Hiratsuka, S., Watanabe, A., Aburatani, H., and Maru, Y. (2006). Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat. Cell Biol. 8, 1369–1375. doi: 10.1038/ncb1507
Hood, J. L., Pan, H., Lanza, G. M., Wickline, S. A., Consortium for Translational Research in Advanced I. and Nanomedicine (2009). Paracrine induction of endothelium by tumor exosomes. Lab. Invest. 89, 1317–1328. doi: 10.1038/labinvest.2009.94
Hood, J. L., San, R. S., and Wickline, S. A. (2011). Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis. Cancer Res. 71, 3792–3801. doi: 10.1158/0008-5472.CAN-10-4455
Hsu, C., Morohashi, Y., Yoshimura, S., Manrique-Hoyos, N., Jung, S., Lauterbach, M. A., et al. (2010). Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A-C. J. Cell Biol. 189, 223–232. doi: 10.1083/jcb.200911018
Huang, Y., Song, N., Ding, Y., Yuan, S., Li, X., Cai, H., et al. (2009). Pulmonary vascular destabilization in the premetastatic phase facilitates lung metastasis. Cancer Res. 69, 7529–7537. doi: 10.1158/0008-5472.CAN-08-4382
Huber, V., Filipazzi, P., Iero, M., Fais, S., and Rivoltini, L. (2008). More insights into the immunosuppressive potential of tumor exosomes. J. Transl. Med. 6, 63. doi: 10.1186/1479-5876-6-63
Huhtala, P., Humphries, M. J., McCarthy, J. B., Tremble, P. M., Werb, Z., and Damsky, C. H. (1995). Cooperative signaling by alpha 5 beta 1 and alpha 4 beta 1 integrins regulates metalloproteinase gene expression in fibroblasts adhering to fibronectin. J. Cell Biol. 129, 867–879. doi: 10.1083/jcb.129.3.867
Hurley, J. H., and Hanson, P. I. (2010). Membrane budding and scission by the ESCRT machinery: it's all in the neck. Nat. Rev. Mol. Cell Biol. 11, 556–566. doi: 10.1038/nrm2937
Hurley, J. H., and Odorizzi, G. (2012). Get on the exosome bus with ALIX. Nat. Cell Biol. 14, 654–655. doi: 10.1038/ncb2530
Hyenne, V., Apaydin, A., Rodriguez, D., Spiegelhalter, C., Hoff-Yoessle, S., Diem, M., et al. (2015). RAL-1 controls multivesicular body biogenesis and exosome secretion. J. Cell Biol. 211, 27–37. doi: 10.1083/jcb.201504136
Hyun, K. A., Kim, J., Gwak, H., and Jung, H. I. (2015). Isolation and enrichment of circulating biomarkers for cancer screening, detection, and diagnostics. Analyst. 141, 382–392. doi: 10.1039/C5AN01762A
Iacovelli, L., Arcella, A., Battaglia, G., Pazzaglia, S., Aronica, E., Spinsanti, P., et al. (2006). Pharmacological activation of mGlu4 metabotropic glutamate receptors inhibits the growth of medulloblastomas. J. Neurosci. 26, 8388–8397. doi: 10.1523/JNEUROSCI.2285-06.2006
Iero, M., Valenti, R., Huber, V., Filipazzi, P., Parmiani, G., Fais, S., et al. (2008). Tumour-released exosomes and their implications in cancer immunity. Cell Death Differ. 15, 80–88. doi: 10.1038/sj.cdd.4402237
Julius, D., Livelli, T. J., Jessell, T. M., and Axel, R. (1989). Ectopic expression of the serotonin 1c receptor and the triggering of malignant transformation. Science 244, 1057–1062. doi: 10.1126/science.2727693
Kahlert, C., Melo, S. A., Protopopov, A., Tang, J., Seth, S., Koch, M., et al. (2014). Identification of double-stranded genomic DNA spanning all chromosomes with mutated KRAS and p53 DNA in the serum exosomes of patients with pancreatic cancer. J. Biol. Chem. 289, 3869–3875. doi: 10.1074/jbc.C113.532267
Kalariti, N., Lembessis, P., Papageorgiou, E., Pissimissis, N., and Koutsilieris, M. (2007). Regulation of the mGluR5, EAAT1 and GS expression by glucocorticoids in MG-63 osteoblast-like osteosarcoma cells. J. Musculoskelet. Neuronal Interact. 7, 113–118.
Kan, Z., Jaiswal, B. S., Stinson, J., Janakiraman, V., Bhatt, D., Stern, H. M., et al. (2010). Diverse somatic mutation patterns and pathway alterations in human cancers. Nature 466, 869–873. doi: 10.1038/nature09208
Kaplan, R. N., Rafii, S., and Lyden, D. (2006). Preparing the “soil”: the premetastatic niche. Cancer Res. 66, 11089–11093. doi: 10.1158/0008-5472.CAN-06-2407
Kaplan, R. N., Riba, R. D., Zacharoulis, S., Bramley, A. H., Vincent, L., Costa, C., et al. (2005). VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438, 820–827. doi: 10.1038/nature04186
Katzmann, D. J., Babst, M., and Emr, S. D. (2001). Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, ESCRT-I. Cell 106, 145–155. doi: 10.1016/S0092-8674(01)00434-2
Keller, S., Rupp, C., Stoeck, A., Runz, S., Fogel, M., Lugert, S., et al. (2007). CD24 is a marker of exosomes secreted into urine and amniotic fluid. Kidney Int. 72, 1095–1102. doi: 10.1038/sj.ki.5002486
Kii, I., Nishiyama, T., Li, M., Matsumoto, K., Saito, M., Amizuka, N., et al. (2010). Incorporation of tenascin-C into the extracellular matrix by periostin underlies an extracellular meshwork architecture. J. Biol. Chem. 285, 2028–2039. doi: 10.1074/jbc.M109.051961
Kolakowski, L. F. Jr. (1994). GCRDb: a G-protein-coupled receptor database. Recept. Channels 2, 1–7.
Lagerstrom, M. C., and Schioth, H. B. (2008). Structural diversity of G protein-coupled receptors and significance for drug discovery. Nat. Rev. Drug Discov. 7, 339–357. doi: 10.1038/nrd2518
Lander, E. S., Linton, L. M., Birren, B., Nusbaum, C., Zody, M. C., Baldwin, J., et al. (2001). Initial sequencing and analysis of the human genome. Nature 409, 860–921. doi: 10.1038/35057062
Lappano, R., and Maggiolini, M. (2011). G protein-coupled receptors: novel targets for drug discovery in cancer. Nat. Rev. Drug Discov. 10, 47–60. doi: 10.1038/nrd3320
Lee, H. J., Wall, B., and Chen, S. (2008). G-protein-coupled receptors and melanoma. Pigment Cell Melanoma Res. 21, 415–428. doi: 10.1111/j.1755-148X.2008.00478.x
Lespagnol, A., Duflaut, D., Beekman, C., Blanc, L., Fiucci, G., Marine, J. C., et al. (2008). Exosome secretion, including the DNA damage-induced p53-dependent secretory pathway, is severely compromised in TSAP6/Steap3-null mice. Cell Death Differ. 15, 1723–1733. doi: 10.1038/cdd.2008.104
Liotta, L. A., and Stetler-Stevenson, W. G. (1991). Tumor invasion and metastasis: an imbalance of positive and negative regulation. Cancer Res. 51, 5054s–5059s.
Lipowsky, R. (2013). Spontaneous tubulation of membranes and vesicles reveals membrane tension generated by spontaneous curvature. Faraday Discuss. 161, 305–331; discussion 419–359. doi: 10.1039/C2FD20105D
Locke, C. J., Congrove, N. R., Dismuke, W. M., Bowen, T. J., Stamer, W. D., and McKay, B. S. (2014). Controlled exosome release from the retinal pigment epithelium in situ. Exp. Eye Res. 129, 1–4. doi: 10.1016/j.exer.2014.10.010
Logozzi, M., De Milito, A., Lugini, L., Borghi, M., Calabro, L., Spada, M., et al. (2009). High levels of exosomes expressing CD63 and caveolin-1 in plasma of melanoma patients. PLoS ONE 4:e5219. doi: 10.1371/journal.pone.0005219
Marin, Y. E., and Chen, S. (2004). Involvement of metabotropic glutamate receptor 1, a G protein coupled receptor, in melanoma development. J. Mol. Med. 82, 735–749. doi: 10.1007/s00109-004-0566-8
Marin, Y. E., Namkoong, J., Cohen-Solal, K., Shin, S. S., Martino, J. J., Oka, M., et al. (2006). Stimulation of oncogenic metabotropic glutamate receptor 1 in melanoma cells activates ERK1/2 via PKCepsilon. Cell. Signal. 18, 1279–1286. doi: 10.1016/j.cellsig.2005.10.012
McCullough, J., Colf, L. A., and Sundquist, W. I. (2013). Membrane fission reactions of the mammalian ESCRT pathway. Annu. Rev. Biochem. 82, 663–692. doi: 10.1146/annurev-biochem-072909-101058
Mehlen, P., and Puisieux, A. (2006). Metastasis: a question of life or death. Nat. Rev. Cancer 6, 449–458. doi: 10.1038/nrc1886
Miyanishi, M., Tada, K., Koike, M., Uchiyama, Y., Kitamura, T., and Nagata, S. (2007). Identification of Tim4 as a phosphatidylserine receptor. Nature 450, 435–439. doi: 10.1038/nature06307
Myers, T. J., Brennaman, L. H., Stevenson, M., Higashiyama, S., Russell, W. E., Lee, D. C., et al. (2009). Mitochondrial reactive oxygen species mediate GPCR-induced TACE/ADAM17-dependent transforming growth factor-alpha shedding. Mol. Biol. Cell 20, 5236–5249. doi: 10.1091/mbc.E08-12-1256
Nakanishi, S. (1992). Molecular diversity of glutamate receptors and implications for brain function. Science 258, 597–603. doi: 10.1126/science.1329206
Namkoong, J., Shin, S. S., Lee, H. J., Marin, Y. E., Wall, B. A., Goydos, J. S., et al. (2007). Metabotropic glutamate receptor 1 and glutamate signaling in human melanoma. Cancer Res. 67, 2298–2305. doi: 10.1158/0008-5472.CAN-06-3665
Newton, A. C. (2001). Protein kinase C: structural and spatial regulation by phosphorylation, cofactors, and macromolecular interactions. Chem. Rev. 101, 2353–2364. doi: 10.1021/cr0002801
O'Connell, J. T., Sugimoto, H., Cooke, V. G., MacDonald, B. A., Mehta, A. I., LeBleu, V. S., et al. (2011). VEGF-A and Tenascin-C produced by S100A4+ stromal cells are important for metastatic colonization. Proc. Natl. Acad. Sci. U.S.A. 108, 16002–16007. doi: 10.1073/pnas.1109493108
Odorizzi, G., Babst, M., and Emr, S. D. (1998). Fab1p PtdIns(3)P 5-kinase function essential for protein sorting in the multivesicular body. Cell 95, 847–858. doi: 10.1016/S0092-8674(00)81707-9
Ohtani, Y., Harada, T., Funasaka, Y., Nakao, K., Takahara, C., Abdel-Daim, M., et al. (2008). Metabotropic glutamate receptor subtype-1 is essential for in vivo growth of melanoma. Oncogene 27, 7162–7170. doi: 10.1038/onc.2008.329
Ostrowski, M., Carmo, N. B., Krumeich, S., Fanget, I., Raposo, G., Savina, A., et al. (2010). Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol 12, 19–30; sup pp 11–13. doi: 10.1038/ncb2000
Pant, S., Hilton, H., and Burczynski, M. E. (2012). The multifaceted exosome: biogenesis, role in normal and aberrant cellular function, and frontiers for pharmacological and biomarker opportunities. Biochem. Pharmacol. 83, 1484–1494. doi: 10.1016/j.bcp.2011.12.037
Park, S. Y., Lee, S. A., Han, I. H., Yoo, B. C., Lee, S. H., Park, J. Y., et al. (2007). Clinical significance of metabotropic glutamate receptor 5 expression in oral squamous cell carcinoma. Oncol. Rep. 17, 81–87. doi: 10.3892/or.17.1.81
Parolini, I., Federici, C., Raggi, C., Lugini, L., Palleschi, S., De Milito, A., et al. (2009). Microenvironmental pH is a key factor for exosome traffic in tumor cells. J. Biol. Chem. 284, 34211–34222. doi: 10.1074/jbc.M109.041152
Parsons, D. W., Jones, S., Zhang, X., Lin, J. C., Leary, R. J., Angenendt, P., et al. (2008). An integrated genomic analysis of human glioblastoma multiforme. Science 321, 1807–1812. doi: 10.1126/science.1164382
Pauli, B. U., Schwartz, D. E., Thonar, E. J., and Kuettner, K. E. (1983). Tumor invasion and host extracellular matrix. Cancer Metastasis Rev. 2, 129–152. doi: 10.1007/BF00048966
Peel, S., Macheboeuf, P., Martinelli, N., and Weissenhorn, W. (2011). Divergent pathways lead to ESCRT-III-catalyzed membrane fission. Trends Biochem. Sci. 36, 199–210. doi: 10.1016/j.tibs.2010.09.004
Peinado, H., Aleckovic, M., Lavotshkin, S., Matei, I., Costa-Silva, B., Moreno-Bueno, G., et al. (2012). Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med. 18, 883–891. doi: 10.1038/nm.2753
Peter, B. J., Kent, H. M., Mills, I. G., Vallis, Y., Butler, P. J., Evans, P. R., et al. (2004). BAR domains as sensors of membrane curvature: the amphiphysin BAR structure. Science 303, 495–499. doi: 10.1126/science.1092586
Peters, P. J., Borst, J., Oorschot, V., Fukuda, M., Krahenbuhl, O., Tschopp, J., et al. (1991). Cytotoxic T lymphocyte granules are secretory lysosomes, containing both perforin and granzymes. J. Exp. Med. 173, 1099–1109. doi: 10.1084/jem.173.5.1099
Pironti, G., Strachan, R. T., Abraham, D., Mon-Wei Yu, S., Chen, M., Chen, W., et al. (2015). Circulating exosomes induced by cardiac pressure overload contain functional angiotensin II type 1 receptors. Circulation 131, 2120–2130. doi: 10.1161/CIRCULATIONAHA.115.015687
Pisitkun, T., Shen, R. F., and Knepper, M. A. (2004). Identification and proteomic profiling of exosomes in human urine. Proc. Natl. Acad. Sci. U.S.A. 101, 13368–13373. doi: 10.1073/pnas.0403453101
Pissimissis, N., Papageorgiou, E., Lembessis, P., Armakolas, A., and Koutsilieris, M. (2009). The glutamatergic system expression in human PC-3 and LNCaP prostate cancer cells. Anticancer Res. 29, 371–377.
Pollock, P. M., Cohen-Solal, K., Sood, R., Namkoong, J., Martino, J. J., Koganti, A., et al. (2003). Melanoma mouse model implicates metabotropic glutamate signaling in melanocytic neoplasia. Nat. Genet. 34, 108–112. doi: 10.1038/ng1148
Poste, G., and Fidler, I. J. (1980). The pathogenesis of cancer metastasis. Nature 283, 139–146. doi: 10.1038/283139a0
Prickett, T. D., and Samuels, Y. (2012). Molecular pathways: dysregulated glutamatergic signaling pathways in cancer. Clin. Cancer Res. 18, 4240–4246. doi: 10.1158/1078-0432.CCR-11-1217
Raposo, G., Nijman, H. W., Stoorvogel, W., Liejendekker, R., Harding, C. V., Melief, C. J., et al. (1996). B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med. 183, 1161–1172. doi: 10.1084/jem.183.3.1161
Ratajczak, J., Wysoczynski, M., Hayek, F., Janowska-Wieczorek, A., and Ratajczak, M. Z. (2006). Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia 20, 1487–1495. doi: 10.1038/sj.leu.2404296
Saari, H., Lazaro-Ibanez, E., Viitala, T., Vuorimaa-Laukkanen, E., Siljander, P., and Yliperttula, M. (2015). Microvesicle- and exosome-mediated drug delivery enhances the cytotoxicity of Paclitaxel in autologous prostate cancer cells. J. Control. Release. 220(Pt B): 727–737. doi: 10.1016/j.jconrel.2015.09.031
Savina, A., Furlan, M., Vidal, M., and Colombo, M. I. (2003). Exosome release is regulated by a calcium-dependent mechanism in K562 cells. J. Biol. Chem. 278, 20083–20090. doi: 10.1074/jbc.M301642200
Segura, E., Amigorena, S., and Thery, C. (2005). Mature dendritic cells secrete exosomes with strong ability to induce antigen-specific effector immune responses. Blood Cells Mol. Dis. 35, 89–93. doi: 10.1016/j.bcmd.2005.05.003
Shah, S. P., Roth, A., Goya, R., Oloumi, A., Ha, G., Zhao, Y., et al. (2012). The clonal and mutational evolution spectrum of primary triple-negative breast cancers. Nature 486, 395–399. doi: 10.1038/nature10933
Shin, S. S., Namkoong, J., Wall, B. A., Gleason, R., Lee, H. J., and Chen, S. (2008). Oncogenic activities of metabotropic glutamate receptor 1 (Grm1) in melanocyte transformation. Pigment Cell Melanoma Res. 21, 368–378. doi: 10.1111/j.1755-148X.2008.00452.x
Sjoblom, T., Jones, S., Wood, L. D., Parsons, D. W., Lin, J., Barber, T. D., et al. (2006). The consensus coding sequences of human breast and colorectal cancers. Science 314, 268–274. doi: 10.1126/science.1133427
Speyer, C. L., Smith, J. S., Banda, M., DeVries, J. A., Mekani, T., and Gorski, D. H. (2012). Metabotropic glutamate receptor-1: a potential therapeutic target for the treatment of breast cancer. Breast Cancer Res. Treat. 132, 565–573. doi: 10.1007/s10549-011-1624-x
Spiegel, A. M. (1996a). Defects in G protein-coupled signal transduction in human disease. Annu. Rev. Physiol. 58, 143–170. doi: 10.1146/annurev.ph.58.030196.001043
Spiegel, A. M. (1996b). Mutations in G proteins and G protein-coupled receptors in endocrine disease. J. Clin. Endocrinol. Metab. 81, 2434–2442. doi: 10.1210/jcem.81.7.8675557
Stepulak, A., Luksch, H., Gebhardt, C., Uckermann, O., Marzahn, J., Sifringer, M., et al. (2009). Expression of glutamate receptor subunits in human cancers. Histochem. Cell Biol. 132, 435–445. doi: 10.1007/s00418-009-0613-1
Stoorvogel, W., Kleijmeer, M. J., Geuze, H. J., and Raposo, G. (2002). The biogenesis and functions of exosomes. Traffic 3, 321–330. doi: 10.1034/j.1600-0854.2002.30502.x
Stransky, N., Egloff, A. M., Tward, A. D., Kostic, A. D., Cibulskis, K., Sivachenko, A., et al. (2011). The mutational landscape of head and neck squamous cell carcinoma. Science 333, 1157–1160. doi: 10.1126/science.1208130
Subra, C., Grand, D., Laulagnier, K., Stella, A., Lambeau, G., Paillasse, M., et al. (2010). Exosomes account for vesicle-mediated transcellular transport of activatable phospholipases and prostaglandins. J. Lipid Res. 51, 2105–2120. doi: 10.1194/jlr.M003657
Takei, K., Slepnev, V. I., Haucke, V., and De Camilli, P. (1999). Functional partnership between amphiphysin and dynamin in clathrin-mediated endocytosis. Nat. Cell Biol. 1, 33–39. doi: 10.1038/9004
Taylor, D. D., and Gercel-Taylor, C. (2008). MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol. Oncol. 110, 13–21. doi: 10.1016/j.ygyno.2008.04.033
Teh, J. L., and Chen, S. (2012). Glutamatergic signaling in cellular transformation. Pigment Cell Melanoma Res. 25, 331–342. doi: 10.1111/j.1755-148X.2012.00983.x
Teh, J. L., Shah, R., La Cava, S., Dolfi, S. C., Mehta, M. S., Kongara, S., et al. (2015). Metabotropic glutamate receptor 1 disrupts mammary acinar architecture and initiates malignant transformation of mammary epithelial cells. Breast Cancer Res. Treat. 151, 57–73. doi: 10.1007/s10549-015-3365-8
Thery, C. (2015). Cancer: diagnosis by extracellular vesicles. Nature 523, 161–162. doi: 10.1038/nature14626
Thery, C., Boussac, M., Veron, P., Ricciardi-Castagnoli, P., Raposo, G., Garin, J., et al. (2001). Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J. Immunol. 166, 7309–7318. doi: 10.4049/jimmunol.166.12.7309
Thery, C., Regnault, A., Garin, J., Wolfers, J., Zitvogel, L., Ricciardi-Castagnoli, P., et al. (1999). Molecular characterization of dendritic cell-derived exosomes. Selective accumulation of the heat shock protein hsc73. J. Cell Biol. 147, 599–610. doi: 10.1083/jcb.147.3.599
Thery, C., Zitvogel, L., and Amigorena, S. (2002). Exosomes: composition, biogenesis and function. Nat. Rev. Immunol. 2, 569–579. doi: 10.1038/nri855.
Thompson, C. A., Purushothaman, A., Ramani, V. C., Vlodavsky, I., and Sanderson, R. D. (2013). Heparanase regulates secretion, composition, and function of tumor cell-derived exosomes. J. Biol. Chem. 288, 10093–10099. doi: 10.1074/jbc.C112.444562
Trajkovic, K., Hsu, C., Chiantia, S., Rajendran, L., Wenzel, D., Wieland, F., et al. (2008). Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 319, 1244–1247. doi: 10.1126/science.1153124
Tse, J. C., and Kalluri, R. (2007). Mechanisms of metastasis: epithelial-to-mesenchymal transition and contribution of tumor microenvironment. J. Cell. Biochem. 101, 816–829. doi: 10.1002/jcb.21215
Valadi, H., Ekstrom, K., Bossios, A., Sjostrand, M., Lee, J. J., and Lotvall, J. O. (2007). Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 9, 654–659. doi: 10.1038/ncb1596
Valenti, R., Huber, V., Iero, M., Filipazzi, P., Parmiani, G., and Rivoltini, L. (2007). Tumor-released microvesicles as vehicles of immunosuppression. Cancer Res. 67, 2912–2915. doi: 10.1158/0008-5472.CAN-07-0520
van Niel, G., Raposo, G., Candalh, C., Boussac, M., Hershberg, R., Cerf-Bensussan, N., et al. (2001). Intestinal epithelial cells secrete exosome-like vesicles. Gastroenterology 121, 337–349. doi: 10.1053/gast.2001.26263
Van Raamsdonk, C. D., Fitch, K. R., Fuchs, H., de Angelis, M. H., and Barsh, G. S. (2004). Effects of G-protein mutations on skin color. Nat. Genet. 36, 961–968. doi: 10.1038/ng1412
Venter, J. C., Adams, M. D., Myers, E. W., Li, P. W., Mural, R. J., Sutton, G. G., et al. (2001). The sequence of the human genome. Science 291, 1304–1351. doi: 10.1126/science.1058040
Viaud, S., Thery, C., Ploix, S., Tursz, T., Lapierre, V., Lantz, O., et al. (2010). Dendritic cell-derived exosomes for cancer immunotherapy: what's next? Cancer Res. 70, 1281–1285. doi: 10.1158/0008-5472.CAN-09-3276
Wang, Q., Navarro, M. V., Peng, G., Molinelli, E., Goh, S. L., Judson, B. L., et al. (2009). Molecular mechanism of membrane constriction and tubulation mediated by the F-BAR protein Pacsin/Syndapin. Proc. Natl. Acad. Sci. U.S.A. 106, 12700–12705. doi: 10.1073/pnas.0902974106
Wang, Z., and Ouyang, G. (2012). Periostin: a bridge between cancer stem cells and their metastatic niche. Cell Stem Cell 10, 111–112. doi: 10.1016/j.stem.2012.01.002
Wood, L. D., Parsons, D. W., Jones, S., Lin, J., Sjoblom, T., Leary, R. J., et al. (2007). The genomic landscapes of human breast and colorectal cancers. Science 318, 1108–1113. doi: 10.1126/science.1145720
Wu, H., Wang, C., Gregory, K. J., Han, G. W., Cho, H. P., Xia, Y., et al. (2014). Structure of a class C GPCR metabotropic glutamate receptor 1 bound to an allosteric modulator. Science 344, 58–64. doi: 10.1126/science.1249489
Yakubenko, V. P., Lobb, R. R., Plow, E. F., and Ugarova, T. P. (2000). Differential induction of gelatinase B (MMP-9) and gelatinase A (MMP-2) in T lymphocytes upon alpha(4)beta(1)-mediated adhesion to VCAM-1 and the CS-1 peptide of fibronectin. Exp. Cell Res. 260, 73–84. doi: 10.1006/excr.2000.5002
Young, D., Waitches, G., Birchmeier, C., Fasano, O., and Wigler, M. (1986). Isolation and characterization of a new cellular oncogene encoding a protein with multiple potential transmembrane domains. Cell 45, 711–719. doi: 10.1016/0092-8674(86)90785-3
Yu, X., Harris, S. L., and Levine, A. J. (2006). The regulation of exosome secretion: a novel function of the p53 protein. Cancer Res. 66, 4795–4801. doi: 10.1158/0008-5472.CAN-05-4579
Zhao, H., Yang, L., Baddour, J., Achreja, A., Bernard, V., Moss, T., et al. (2016). Tumor microenvironment derived exosomes pleiotropically modulate cancer cell metabolism. Elife 5:e10250. doi: 10.7554/eLife.10250. [Epub ahead of print].
Keywords: GPCR, exosome, pre-metastatic niche, cancer, mGluR
Citation: Isola AL and Chen S (2016) Exosomes: The Link between GPCR Activation and Metastatic Potential? Front. Genet. 7:56. doi: 10.3389/fgene.2016.00056
Received: 10 February 2016; Accepted: 22 March 2016;
Published: 08 April 2016.
Edited by:
Ashani Weeraratna, The Wistar Institute, USAReviewed by:
Parvin Mehdipour, Tehran University of Medical Sciences, IranBryan Raymond George Williams, Monash Institite of Medical Research, Australia
Copyright © 2016 Isola and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Suzie Chen, suziec@pharmacy.rutgers.edu
 Allison L. Isola
Allison L. Isola Suzie Chen
Suzie Chen