- University of Connecticut Health Center, Farmington, CT, USA
Pathogen-specific CD8 T cells provide a mechanism for selectively eliminating host cells that are harboring intracellular pathogens. The pathogens are killed when lytic molecules are injected into the cytoplasm of the infected cells and begin an apoptotic cascade. Activated CD8 T cells also release large quantities of pro-inflammatory cytokines that stimulate other immune cells in the local vicinity. As the alveoli are extraordinarily sensitive to cytokine induced damage, multiple layers of immune regulation limit the activities of immune cells that enter the lungs. These mechanisms include receptor-mediated signaling pathways in CD8 T cells that respond to peptide antigens and transforming growth factor β. Both pathways influence the functional and phenotypic properties of long-lived CD8 T cells populations in peripheral and lymphoid tissues.
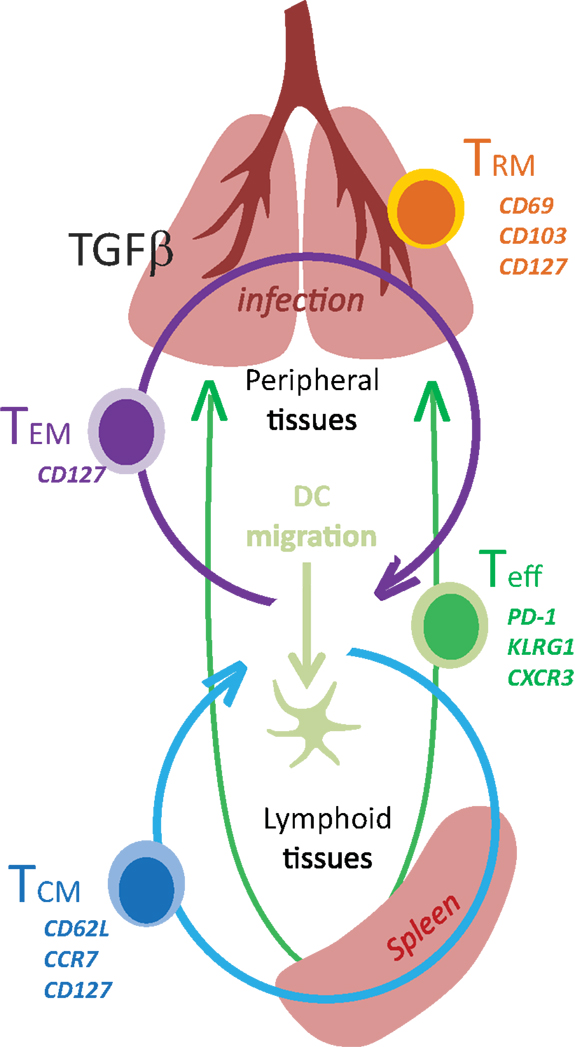
Adaptive immune responses to new pathogens begin after naïve T cells encounter mature DC with their cognate antigen in the secondary lymphoid organs. Extensive phenotypic and functional changes occur as the T cells progress along a complex differentiation pathway (Figure 1). Some of the earliest changes include the loss of homing receptors that are required to enter the encapsulated lymph nodes, which are replaced by other molecules that guide activated T cells into infected tissues. Many functional properties are also modulated during exposure to antigen or environmental stimuli, leading to the acquisition of new effector functions and altered capacity for long term survival (1). The enduring characteristics of the surviving memory T cells sometimes reflect partial progression along a chosen differentiation pathway after weak antigen stimulation, insufficient costimulation, or limited inflammation (2).
The Phenotypic Characteristics of Naïve CD8 T Cells
The secondary lymphoid organs serve as centralized sites of immune activation and accommodate large numbers migratory DC which carry microbial products from infected tissues (3, 4). Rare antigen-specific T cells provide comprehensive immune surveillance by moving sequentially between different lymphoid tissues until they encounter antigen presenting cells (APCs) with their cognate antigen. Some circulating lymphocytes (including naïve CD8 T cells) enter encapsulated lymph nodes by squeezing between cuboidal endothelial cells that line wide vessels known as high endothelial venules (HEV) (5). The migrating cells express L-selectin (CD62L) which interacts with peripheral lymph node addressins (pNAD) causing the T cells to begin rolling over the surface of the endothelial cells (6, 7). The rolling T cells constitutively express CC chemokine-receptor 7 (CCR7) and respond to chemokines ccl19 and ccl21 (8, 9) which promote a conformational change in the structure of an integrin known as leukocyte adhesion molecule-1 (LFA-1) (10–12). Tight interactions between activated LFA-1 and Intercellular Adhesion Molecule-1 (i.e., ICAM-1) are essential for diapedesis (13, 14). After crossing the endothelial layer, naive T cells use conduits of reticular cells which are coated with ccl19 and ccl21 to search for DC with their cognate antigen (9, 15, 16).
Antigen Stimulation Leads to Extensive Phenotypic and Functional Changes
All nucleated cells can assemble MHCI molecules using peptides from self-derived proteins however the mechanisms that are used to produce antigenic peptides from foreign proteins are not identical for all cell types (17, 18). Infected cells produce defective ribosomal products which are directed to the proteasomes for degradation and are pumped from the cytosol into the endoplasmic reticulum by the Transporter for Antigen Presentation (TAP) where the complete peptide/MHCI complexes are assembled. Other APCs (i.e., some DCs and macrophages) acquire foreign proteins from cells in the surrounding tissues and produce immunogenic peptides without infection which are used for cross-presentation to CD8 T cells (19, 20). The preferential use of a specific peptide processing pathway can influence the specificity of the CD8 T cell response and alter the pattern of epitope dominance during some infections (21).
At least two subsets of migratory DC carry microbial products into encapsulated lymph nodes and other lymphoid tissues (22). Other DCs are permanent residents of the lymph nodes and acquire antigens from neighboring cells (20, 23). These DC express a variety of coreceptors that exert positive or negative effects on T cells during antigen stimulation, but play little or no role in the immune response unless the TCR is engaged. The coreceptors that augment Teff functions, including cytokine production and lytic activity, are known as costimulatory molecules while inhibitory receptors suppress functional activities and cell cycle progression (24). Some important costimulatory signals are delivered through CD28 which interacts with CD80 and CD86 during the formation of the immunological synapse (25, 26). Clonal diversity can be increased by costimulation through CD27, which promotes cell survival during responses to low affinity antigens (27, 28). Other coreceptors are induced by TCR derived signals and modulate the properties of responding T cells as the infection progresses (29). Costimulation through 4-1BB, OX40, or CD27 leads to increased expression of anti-apoptotic molecules such as BCL-2 and BCL-XL and prolonged T cell survival (30), while CD30 has pleiotropic effects on T cell activation, apoptosis, and effector function.
Antigen stimulation causes many external changes as naïve CD8 T cells become Teff cells. Some permanent changes include increased CD44 and LFA-1 (CD11a) expression, which are required for activated T cells to enter peripheral tissues (11, 31–33). Other surface molecules are reversibly induced during antigen stimulation including chemokine receptors which control the distribution of antigen-specific CD8 T cells in inflamed tissues, such as CXCR3 (34, 35). Some activated T cells leave the blood vessels using chemokine-dependent mechanisms, however a recent study has shown that cognate antigen can induce transendothelial migration in vascularized transplants by a mechanism that is independent of Gαi-signaling (36). Other surface molecules are down regulated during antigen stimulation including CCR7 and CD62L which can be cleaved from the cell surface by metalloproteases (37). Foxo-1 plays a role in the transcriptional control of CCR7 and CD62L expression in T cells (38).
The functional characteristics of CD8 T cell populations are modified by cell-fate decisions during memory development. Some experiments indicate that asymmetric cell division determines the ratios of Teff cells and memory cells (39). Others suggest that the strength of the TcR signal determines whether CD8 T cells undergo symmetric or asymmetric cell division and thus controls the phenotype of the daughter cells (40, 41). This idea was not supported by transfer studies with individual OTI cells which express a high-affinity TcR and produced heterogeneous progeny after infection (41–43). Some experiments suggest that naïve CD8 T cells become TCM precursors (Tcmp), before becoming TEM precursors (Temp) and finally Teff cells (43). This linear differentiation model is supported by the finding that Tcmp proliferate slower than the Temp or Teff cells (43). The model can be reconciled with data which show that recurrent antigen stimulation or inflammation increases the percentages of short-lived Teff cells within the population, while virus-specific CD8 T cells that are activated later in the response may receive less stimulation and preferentially differentiate into the Tcm phenotype (44). The disparate fates of progeny cells from individual parent T cells underscore the importance of extrinsic signals during memory differentiation, which can come from a variety of sources including the APCs, costimulatory molecules, or cytokines.
Cytokines Contribute to the Heterogeneity of Activated T Cell Populations
Recent studies have shown that IL-1 is not only critical for the activation of DCs (45), but also significantly increases clonal expansion and augments the effector functions of virus-specific CTL (46). During the expansion phase of the infection, autocrine IL-2 production is essential for Teff cell differentiation and survival. The IL-2 derived signals promote sustained Blimp-1 expression and repress Bcl-6 (47) which sustains mTOR activity and glycolysis via the PI3K-Akt pathway (48). Some Teff cells maintain CD25 expression (i.e., the high-affinity IL-2 receptor) and undergo extensive proliferation before becoming terminally differentiated Teff cells, while other cells lose CD25 and maintain the capacity to become memory cells (49). Large numbers of Teff cells that express the Killer cell lectin-like receptor G1 (KLRG1) but not CD127, die during contraction of the CTL response and are known as short-lived effector cells (SLECs) (31). Other cells which lack KLRG1 and re-express CD127 before the contraction begins, are known as memory precursor effector cells (MPECs) because they are more resistant to apoptosis. KLRG1 is a useful phenotypic marker however expression is not required for Teff differentiation or development of robust effector functions (50). Two inhibitor of DNA binding proteins (Id2 and Id3) influence memory CD8 T cell development before the phenotypic markers of MPECs and SLECs change. Both proteins inhibit E-protein transcription factors but they promote CD8 T cell survival by different mechanisms (51–54). Specifically Id2 supports the survival of Teff cells by inducing anti-apoptotic molecules such as Bcl-2, while reducing the expression of pro-apoptotic molecules such as Bim (51, 52). In contrast Id3 prolongs the survival of memory cells by regulating key genes that are essential for genomic stability (53, 54).
The milieu of pro-inflammatory cytokines that are produced upon innate immune recognition of pathogen-associated molecular patterns (PAMPs) can also influence the functional properties of developing CD8+ Teff cells. For example, IL-12 or type I interferon (IFN-I) can lead to STAT4 phosphorylation and T-bet expression which promotes terminal differentiation of SLECs (55). In addition, IL-12 activates the PI3K-Akt-mTOR pathway which drives rapid proliferation of Teff cells and promotes degradation of Foxo-1, which in turn leads to the down regulation of Eomesodermin (Eomes) and loss of CD127, CD62L, and CCR7 (56). The T-bet and Eomes transcription factors also regulate CD8 T cell effector functions, as shown by high IL-17 expression and excessive leukocyte infiltration when these molecules are not expressed (57). As the levels of pro-inflammatory cytokines decline, IL-10 and IL-21 activate STAT3 to promote memory development by inducing Bcl-6, Eomes, and suppressor of cytokine signaling 3 (SOCS3) (58). SOCS3 expression may be essential for preserving memory potential by dampening the IL-12 response and shifting their metabolic state back to oxidative phosphorylation as the activated CD8 T cells become quiescent.
Most newly activated Teff cells are capable of immediate lytic activity and cytokine expression, but have a very short life span. Members of the common γ-chain cytokine family play a complex role in CD8 T cell survival and elicit responses that can be modulated through changing receptor expression. The loss of CD127 expression on naive CD8 T cells is partly controlled by the Foxo-1 transcription factor, which can be inactivated via the PI3K-Akt-mTOR signaling pathway (50). Some activated T cells re-express CD127 before the peak of the CTL response and have an enhanced capacity to become long-lived memory cells (59). Re-expression of CD127 is controlled by the transcription factor GA binding protein α (GABPα) which is responsible for hyperacetylation of the promoter, while growth factor independence 1 (Gfi-1) is an antagonist that suppresses CD127 expression on late Teff cells by recruiting histone deacetylase 1 (60). The upstream signaling molecules that regulate GABPα and Gfi-1 expression have not been clearly defined.
Multiple mechanisms contribute to the contraction of Teff response, including the withdrawal of essential growth factors such as IL-2 (48) and perforin or TGFβ induced apoptosis (61, 62). Only small percentages of Teff cells have the capacity to survive through the contraction and become long-lived memory cells. Cell survival is determined by a delicate balance between of pro-survival molecules such as Bcl-2 or Mcl-1, with pro-apoptotic molecules such as Bim or Noxa, which can be regulated by external signals in the tissues (63–65). A recent study has shown that some pro-apoptotic signals are induced by TGFβ, but can be antagonized by the pro-survival properties of IL-7 and IL-15 (62). Forced CD127 expression does not prevent contraction of the Teff population (66) which indicates that terminally differentiated SLECs have an intrinsic defect in their response to IL-7 signaling, as suggested by high expression of the cell cycle inhibitor p27Kip (43). Consequently IL-7 in combination with IL-15 promotes the survival of MPECs, while SLECs are critically dependent on the stimulation through the IL-2/IL-15 receptor (67).
Chronic Antigen Stimulation Promotes Phenotypic and Functional Heterogeneity in CD8 T Cells
CD69 and PD-1 are surface proteins that are transiently induced on activated CD8 T cells soon after TcR stimulation (68, 69). The function of CD69 is not known, but some studies suggest that interactions between CD69 and the sphingosine-1-phosphate receptor-1 (S1P1) facilitate efficient migration of activated CD8 T cells into the bloodstream (70). CD8 T cells transiently express CD69 in infected tissues when IFN-I is present, however expression levels quickly decline when the cytokine is removed (71). PD-1 is also expressed on activated T cells during antigen stimulation but expression cannot be induced by IFN-I. PD-1 disappears when the antigen is removed and is thus a reliable indicator of persisting peptide/MHC complexes.
When CD8 T cells are exposed to a continuous supply of antigen during chronic infections or inside tumors they adopt an altered phenotype which is characterized by high level PD-1 expression together with other inhibitory coreceptors such as TIM3, CTLA4, BTLA, CD160, LAG3, and 2B4 (72). The responses of CD8 T cells that express one or more of these inhibitory receptors are attenuated as shown by reduced proliferative capacity and tempered effector functions, which led to the term “exhausted” T cells (73). Interactions with PD-1 ligands can impair CD8 T cell functions through multiple mechanisms, including reduced mobility (74). The symptoms of exhausted CD8 T cells were reversed in some studies, using combinations of antibodies to block interactions with PD-1 and other inhibitors such as TIM3, CTLA4, and/or LAG3 (72). Large numbers of exhausted CD8 T cells are often accompanied by depleted populations of memory CD8 T cells, which suggests that they may be the product of chronically stimulated Teff cells. Evidence that specific APCs play a role in the development of exhausted CTL has not been reported but since the cells do not express KLRG1 suboptimal differentiation may play a role (75). Indeed, network analysis recently revealed fewer transcriptional modules of quiescence in exhausted CD8 T cells, as compare to functional memory cells (76). In contrast to memory CD8 T cells, exhausted CTL are maintained in an antigen-dependent manner and gradually disappear when they are transferred to infection-free mice (77). Most functional studies have focused on the properties of exhausted CD8+ T cells however there is evidence that CD4+ T cells can exhibit symptoms of exhaustion in some situations (26).
The Phenotypic Properties of Long Live Memory CD8 T Cells
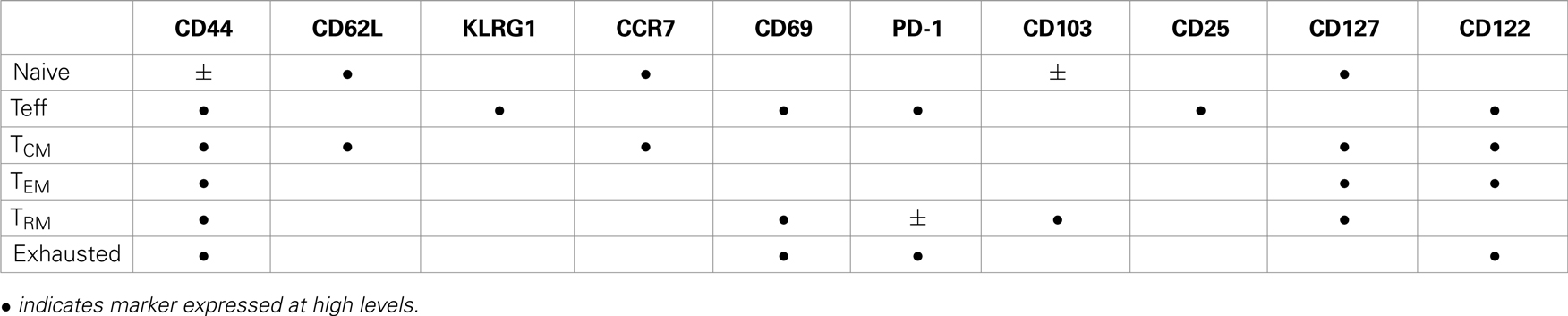
Two major subsets circulating memory CD8 T cells survive the contraction of the Teff response and can be distinguished using reciprocal CD62L and CCR7 expression (78). Central memory (TCM) CD8 T cells are CD62L+CCR7+ cells that can access secondary lymphoid organs via HEV and have a similar tissue distribution as naive CD8 T cells (Table 1). Since effector memory CD8 T cells (TEM) lack CD62L and CCR7 expression they cannot access encapsulated lymph nodes under steady state conditions, however some activated CTL can access inflamed lymph nodes during infection by a mechanism that requires CXCR3, but not CD62L (79). Recent studies have shown that a third major subset of memory CD8 T cells resides in selected peripheral tissues after local infections and does not return to circulation after inflammation subsides (80). The highest concentrations of these tissue-resident memory (TRM) CD8 T cells are typically found in tissues with an epithelial layer, during the recovery from a recent infection (81–84). Some studies indicate that recent exposure to cognate antigens plays a role in the long term retention of CD8+ TRM cells in tissues such as the lungs and CNS, where some KLRG1-negative CTL express CD103 (αeβ7 integrin) when activated TGFβ is present (81, 85, 86). Other studies indicate that sustained antigen exposure is dispensable for maintenance of TRM cell in the gastrointestinal tract (87). The influence of pathogen-derived peptides on lymphocyte migration is controversial since several viruses which were previously thought to induce “acute infections” leave residual peptides that persist in vivo for weeks or months after inoculation (88–90). Additional peptides may persist longer but are below the level of detection. Although the reasons for the heterogeneous characteristics of pathogen-specific memory CD8 T cells in vivo have not been clearly defined, the duration of the infection and the pathogen’s capacity to elicit specific cytokines can have a dramatic influence on the enduring characteristics of the response.
Stable CD69 and CD103 expression are hallmarks of TRM cells that can be found in the skin, gastrointestinal tract, and lungs (80, 91). Some studies suggest that epithelial cells provide signals for sustained CD69 expression, which does not require chronic antigen stimulation (87, 92). Whether CD69 influences the distribution of TRM cells in peripheral tissues such as the lungs (81) through interactions with the sphingosine-1-phosphate (S1P) receptor-1 remains to be determined (93). Others found that an ongoing response to antigen stimulation was required for TRM cells to maintain stable CD103 expression in the lungs (81) and CNS (85). Additional evidence of a prolonged response to antigen stimulation by TRM cells in the lungs includes low level expression of PD-1 (94) and interferon-induced transmembrane protein 3 (IFITM3) (95), while CD103 expression declined when antigen-specific antibodies were used to block TcR interactions with peptide/MHC complexes (81). TRM cells in the brain also expressed CD103 only after intracerebral inoculation with Vesicular stomatitis virus (VSV) (81, 95).
Transforming Growth Factor-β and Heterogeneity of CD8 T Cells in Mucosal Tissues
Transforming growth factor-β1 (TGFβ1) is pleiotropic cytokine that plays a central role in immune homeostasis. The regulatory properties of TGFβ include potent anti-proliferative and pro-apoptotic effects on virus-specific CD8 T cells, which contribute to the contraction of the Teff response during some infections (62). Teff cells are resistant to apoptosis during clonal expansion, but become highly vulnerable to deletion after KLRG1 is upregulated (62). Very few KLRG1+ CD8 T cells survive in the lungs during infections with some strains of influenza and other respiratory viruses that make enzymes which can activate TGF-β (96–99). Paradoxically, exposure to activated TGFβ leads to αEβ (7) integrin (CD103) expression on long-lived CD8+ TRM cells, which often reside near epithelial cells that express E-cadherin (81, 100).
The reasons why individual subsets of CD8 T cells respond to TGFβ in different ways is not known, but multiple different signaling pathways may play a role (79, 80). The apoptotic effects of TGFβ on Teff cells can be overcome by IL-2 and partially inhibited by IL-7, but IL-15 has no protective value (62). This reason why TGFβ exerts its pro-apoptotic role after the peak of the Teff response may be due to the presence of IL-2R (CD25) at earlier time points. This may also explain why SLECs are particularly sensitive to TGFβ-induced apoptosis, as this subset lacks CD127 and depends on IL-15 for survival. The ability of γc cytokines to antagonize the apoptotic effects of TGFβ signaling may be determined by their ability to activate the PI3K pathway, which interacts with TGFβ-induced Smad proteins in a complex manner. Activated Akt can directly associate with Smad3 and inhibit phosphorylation by TGFβRI, which prevents translocation into nucleus. Also, p15Ink4b and p21Cip1 are inhibitors of cyclin-dependent kinases, which can be induced by TGFβ and are required the formation of a transcription complex that is composed of Smad3, Smad4, and the Foxo transcription factors. The PI3K-Akt pathway can induce phosphorylation and degradation of Foxo proteins, and thus antagonize the inhibitory effect of TGFβ during cell cycle progression. On the other hand, TGFβ signaling can dampen the PI3K pathway through the induction of lipid phosphatase SHIP. TGFβ signaling can also dephosphorylate S6K downstream of PI3K-Akt-mTOR pathway via the induction of protein phosphatase 2A (PP2A) (83).
The signaling pathways that are activated during TGFβ regulation are more clearly defined for CD4 than CD8 T cells. Studies have shown that TGFβ induces Sma and Mad-related (SMAD) transcription factors to repress Id3 and enhance binding of E2A in CD4 T cells, which is crucial for the induction of the forkhead box p3 (Foxp3) gene (101) and inhibits the development of Th1 cells (102). Other signaling pathways include the MAP kinase (MAPK), Rho-like GTPase, and phosphatidylinositol-3-kinase (PI3K) pathways (103). A master transcription factor RORγt can be induced in CD4 T cells from mice that lack either Smad4, or Smad2 and Smad3 expression (102). TGFβ also promotes Th17 development by suppressing Eomes via the c-Jun N-terminal kinase (JNK)-c-Jun signaling pathway (104). Since some pathogens elicit robust TGFβ responses it is likely that these signaling pathways have a dramatic influence on the activities of pathogen-specific CD8 T cells during infection, which play a critical role immunity in mucosal tissues.
Summary
Together the current data show that the cytokine milieu and prolonged presence of foreign antigens are responsible for extensive heterogeneity in long-lived CD8 T cell populations. This heterogeneity is reflected by a broad tissue distribution and diverse functional properties which are absolutely essential to combat an enormous variety of different pathogens.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Sprent J, Surh CD. Normal T cell homeostasis: the conversion of naive cells into memory-phenotype cells. Nat Immunol (2011) 12(6):478–84. doi:10.1038/ni.2018
2. Cui W, Joshi NS, Jiang A, Kaech SM. Effects of Signal 3 during CD8 T cell priming: bystander production of IL-12 enhances effector T cell expansion but promotes terminal differentiation. Vaccine (2009) 27(15):2177–87. doi:10.1016/j.vaccine.2009.01.088
3. Kim TS, Braciale TJ. Respiratory dendritic cell subsets differ in their capacity to support the induction of virus-specific cytotoxic CD8+ T cell responses. PLoS ONE (2009) 4(1):e4204. doi:10.1371/journal.pone.0004204
4. Martin-Fontecha A, Sebastiani S, Hopken UE, Uguccioni M, Lipp M, Lanzavecchia A, et al. Regulation of dendritic cell migration to the draining lymph node: impact on T lymphocyte traffic and priming. J Exp Med (2003) 198(4):615–21. doi:10.1084/jem.20030448
5. Steeber DA, Tang ML, Zhang XQ, Muller W, Wagner N, Tedder TF. Efficient lymphocyte migration across high endothelial venules of mouse Peyer’s patches requires overlapping expression of L-selectin and beta7 integrin. J Immunol (1998) 161(12):6638–47.
6. Subramanian H, Grailer JJ, Ohlrich KC, Rymaszewski AL, Loppnow JJ, Kodera M, et al. Signaling through L-selectin mediates enhanced chemotaxis of lymphocyte subsets to secondary lymphoid tissue chemokine. J Immunol (2012) 188(7):3223–36. doi:10.4049/jimmunol.1101032
7. Tedder TF, Steeber DA, Chen A, Engel P. The selectins: vascular adhesion molecules. FASEB J (1995) 9(10):866–73.
8. Hickman HD, Li L, Reynoso GV, Rubin EJ, Skon CN, Mays JW, et al. Chemokines control naive CD8+ T cell selection of optimal lymph node antigen presenting cells. J Exp Med (2011) 208(12):2511–24. doi:10.1084/jem.20102545
9. Okada T, Cyster JG. CC chemokine receptor 7 contributes to Gi-dependent T cell motility in the lymph node. J Immunol (2007) 178(5):2973–8.
10. Faveeuw C, Di Mauro ME, Price AA, Ager A. Roles of alpha(4) integrins/VCAM-1 and LFA-1/ICAM-1 in the binding and transendothelial migration of T lymphocytes and T lymphoblasts across high endothelial venules. Int Immunol (2000) 12(3):241–51. doi:10.1093/intimm/12.3.241
11. Hogg N, Smith A, McDowall A, Giles K, Stanley P, Laschinger M, et al. How T cells use LFA-1 to attach and migrate. Immunol Lett (2004) 92(1-2):51–4. doi:10.1016/j.imlet.2003.10.014
12. Kunkel EJ, Ramos CL, Steeber DA, Muller W, Wagner N, Tedder TF, et al. The roles of L-selectin, beta 7 integrins, and P-selectin in leukocyte rolling and adhesion in high endothelial venules of Peyer’s patches. J Immunol (1998) 161(5):2449–56.
13. Hamann A, Jablonski-Westrich D, Duijvestijn A, Butcher EC, Baisch H, Harder R, et al. Evidence for an accessory role of LFA-1 in lymphocyte-high endothelium interaction during homing. J Immunol (1988) 140(3):693–9.
14. Lehmann JC, Jablonski-Westrich D, Haubold U, Gutierrez-Ramos JC, Springer T, Hamann A. Overlapping and selective roles of endothelial intercellular adhesion molecule-1 (ICAM-1) and ICAM-2 in lymphocyte trafficking. J Immunol (2003) 171(5):2588–93.
15. Forster R, Schubel A, Breitfeld D, Kremmer E, Renner-Muller I, Wolf E, et al. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell (1999) 99(1):23–33. doi:10.1016/S0092-8674(00)80059-8
16. Bajenoff M, Egen JG, Koo LY, Laugier JP, Brau F, Glaichenhaus N, et al. Stromal cell networks regulate lymphocyte entry, migration, and territoriality in lymph nodes. Immunity (2006) 25(6):989–1001. doi:10.1016/j.immuni.2006.10.011
17. Dudziak D, Kamphorst AO, Heidkamp GF, Buchholz VR, Trumpfheller C, Yamazaki S, et al. Differential antigen processing by dendritic cell subsets in vivo. Science (2007) 315(5808):107–11. doi:10.1126/science.1136080
18. Liu K, Nussenzweig MC. Origin and development of dendritic cells. Immunol Rev (2010) 234(1):45–54. doi:10.1111/j.0105-2896.2009.00879.x
19. Guermonprez P, Valladeau J, Zitvogel L, Thery C, Amigorena S. Antigen presentation and T cell stimulation by dendritic cells. Annu Rev Immunol (2002) 20:621–67. doi:10.1146/annurev.immunol.20.100301.064828
20. Allan RS, Waithman J, Bedoui S, Jones CM, Villadangos JA, Zhan Y, et al. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity (2006) 25(1):153–62. doi:10.1016/j.immuni.2006.04.017
21. Suarez-Ramirez JE, Wu T, Lee YT, Aguila CC, Bouchard KR, Cauley LS. Division of labor between subsets of lymph node dendritic cells determines the specificity of the CD8 recall response to influenza infection. Eur J Immunol (2011) 41:2632–41. doi:10.1002/eji.201141546
22. Ginhoux F, Liu K, Helft J, Bogunovic M, Greter M, Hashimoto D, et al. The origin and development of nonlymphoid tissue CD103+ DCs. J Exp Med (2009) 206(13):3115–30. doi:10.1084/jem.20091756
23. Allenspach EJ, Lemos MP, Porrett PM, Turka LA, Laufer TM. Migratory and lymphoid-resident dendritic cells cooperate to efficiently prime naive CD4 T cells. Immunity (2008) 29(5):795–806. doi:10.1016/j.immuni.2008.08.013
24. Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol (2013) 13(4):227–42. doi:10.1038/nri3405
25. Fuse S, Zhang W, Usherwood EJ. Control of memory CD8+ T cell differentiation by CD80/CD86-CD28 costimulation and restoration by IL-2 during the recall response. J Immunol (2008) 180(2):1148–57.
26. Sharpe AH. Mechanisms of costimulation. Immunol Rev (2009) 229(1):5–11. doi:10.1111/j.1600-065X.2009.00784.x
27. Hendriks J, Xiao Y, Borst J. CD27 promotes survival of activated T cells and complements CD28 in generation and establishment of the effector T cell pool. J Exp Med (2003) 198(9):1369–80. doi:10.1084/jem.20030916
28. van Gisbergen KP, Klarenbeek PL, Kragten NA, Unger PP, Nieuwenhuis MB, Wensveen FM, et al. The costimulatory molecule CD27 maintains clonally diverse CD8(+) T cell responses of low antigen affinity to protect against viral variants. Immunity (2011) 35(1):97–108. doi:10.1016/j.immuni.2011.04.020
29. Lee SJ, Myers L, Muralimohan G, Dai J, Qiao Y, Li Z, et al. 4-1BB and OX40 dual costimulation synergistically stimulate primary specific CD8 T cells for robust effector function. J Immunol (2004) 173(5):3002–12.
30. Cannons JL, Lau P, Ghumman B, DeBenedette MA, Yagita H, Okumura K, et al. 4-1BB ligand induces cell division, sustains survival, and enhances effector function of CD4 and CD8 T cells with similar efficacy. J Immunol (2001) 167(3):1313–24.
31. DeGrendele HC, Estess P, Siegelman MH. Requirement for CD44 in activated T cell extravasation into an inflammatory site. Science (1997) 278(5338):672–5. doi:10.1126/science.278.5338.672
32. Thatte J, Dabak V, Williams MB, Braciale TJ, Ley K. LFA-1 is required for retention of effector CD8 T cells in mouse lungs. Blood (2003) 101(12):4916–22. doi:10.1182/blood-2002-10-3159
33. Berlin-Rufenach C, Otto F, Mathies M, Westermann J, Owen MJ, Hamann A, et al. Lymphocyte migration in lymphocyte function-associated antigen (LFA)-1-deficient mice. J Exp Med (1999) 189(9):1467–78. doi:10.1084/jem.189.9.1467
34. Groom JR, Luster AD. CXCR3 ligands: redundant, collaborative and antagonistic functions. Immunol Cell Biol (2011) 89(2):207–15. doi:10.1038/icb.2010.158
35. Groom JR, Luster AD. CXCR3 in T cell function. Exp Cell Res (2011) 317(5):620–31. doi:10.1016/j.yexcr.2010.12.017
36. Walch JM, Zeng Q, Li Q, Oberbarnscheidt MH, Hoffman RA, Williams AL, et al. Cognate antigen directs CD8+ T cell migration to vascularized transplants. J Clin Invest (2013) 123(6):2663–71. doi:10.1172/JCI66722
37. Venturi GM, Tu L, Kadono T, Khan AI, Fujimoto Y, Oshel P, et al. Leukocyte migration is regulated by L-selectin endoproteolytic release. Immunity (2003) 19(5):713–24. doi:10.1016/S1074-7613(03)00295-4
38. Kerdiles YM, Stone EL, Beisner DR, McGargill MA, Ch’en IL, Stockmann C, et al. Foxo transcription factors control regulatory T cell development and function. Immunity (2010) 33(6):890–904. doi:10.1016/j.immuni.2010.12.002
39. Chang JT, Palanivel VR, Kinjyo I, Schambach F, Intlekofer AM, Banerjee A, et al. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science (2007) 315(5819):1687–91. doi:10.1126/science.1139393
40. King CG, Koehli S, Hausmann B, Schmaler M, Zehn D, Palmer E. T cell affinity regulates asymmetric division, effector cell differentiation, and tissue pathology. Immunity (2012) 37(4):709–20. doi:10.1016/j.immuni.2012.06.021
41. Gerlach C, Rohr JC, Perie L, van RN, van Heijst JW, Velds A, et al. Heterogeneous differentiation patterns of individual CD8+ T cells. Science (2013) 340:635–9. doi:10.1126/science.1235487
42. Gerlach C, van Heijst JW, Swart E, Sie D, Armstrong N, Kerkhoven RM, et al. One naive T cell, multiple fates in CD8+ T cell differentiation. J Exp Med (2010) 207(6):1235–46. doi:10.1084/jem.20091175
43. Buchholz VR, Flossdorf M, Hensel I, Kretschmer L, Weissbrich B, Graf P, et al. Disparate individual fates compose robust CD8+ T cell immunity. Science (2013) 340:630–5. doi:10.1126/science.1235454
44. van FH, Saldanha M, Gilbertson D, Dudani R, Krishnan L, Sad S. Reducing the stimulation of CD8+ T cells during infection with intracellular bacteria promotes differentiation primarily into a central (CD62LhighCD44high) subset. J Immunol (2005) 174(9):5341–50.
45. Pang IK, Ichinohe T, Iwasaki A. IL-1R signaling in dendritic cells replaces pattern-recognition receptors in promoting CD8(+) T cell responses to influenza A virus. Nat Immunol (2013) 14(3):246–53. doi:10.1038/ni.2514
46. Ben-Sasson SZ, Hogg A, Hu-Li J, Wingfield P, Chen X, Crank M, et al. IL-1 enhances expansion, effector function, tissue localization, and memory response of antigen-specific CD8 T cells. J Exp Med (2013) 210(3):491–502. doi:10.1084/jem.20122006
47. Pipkin ME, Sacks JA, Cruz-Guilloty F, Lichtenheld MG, Bevan MJ, Rao A. Interleukin-2 and inflammation induce distinct transcriptional programs that promote the differentiation of effector cytolytic T cells. Immunity (2010) 32(1):79–90. doi:10.1016/j.immuni.2009.11.012
48. van der Windt GJ, Everts B, Chang CH, Curtis JD, Freitas TC, Amiel E, et al. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity (2012) 36(1):68–78. doi:10.1016/j.immuni.2011.12.007
49. Kalia V, Sarkar S, Subramaniam S, Haining WN, Smith KA, Ahmed R. Prolonged interleukin-2Ralpha expression on virus-specific CD8+ T cells favors terminal-effector differentiation in vivo. Immunity (2010) 32(1):91–103. doi:10.1016/j.immuni.2009.11.010
50. Grundemann C, Schwartzkopff S, Koschella M, Schweier O, Peters C, Voehringer D, et al. The NK receptor KLRG1 is dispensable for virus-induced NK and CD8+ T-cell differentiation and function in vivo. Eur J Immunol (2010) 40(5):1303–14. doi:10.1002/eji.200939771
51. Ji Y, Pos Z, Rao M, Klebanoff CA, Yu Z, Sukumar M, et al. Repression of the DNA-binding inhibitor Id3 by Blimp-1 limits the formation of memory CD8(+) T cells. Nat Immunol (2011) 12(12):1230–7. doi:10.1038/ni.2153
52. Yang CY, Best JA, Knell J, Yang E, Sheridan AD, Jesionek AK, et al. The transcriptional regulators Id2 and Id3 control the formation of distinct memory CD8+ T cell subsets. Nat Immunol (2011) 12(12):1221–9. doi:10.1038/ni.2158
53. Cannarile MA, Lind NA, Rivera R, Sheridan AD, Camfield KA, Wu BB, et al. Transcriptional regulator Id2 mediates CD8+ T cell immunity. Nat Immunol (2006) 7(12):1317–25. doi:10.1038/ni1403
54. Knell J, Best JA, Lind NA, Yang E, D’Cruz LM, Goldrath AW. Id2 influences differentiation of killer cell lectin-like receptor G1(hi) short-lived CD8+ effector T cells. J Immunol (2013) 190(4):1501–9. doi:10.4049/jimmunol.1200750
55. Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, et al. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity (2007) 27(2):281–95. doi:10.1016/j.immuni.2007.07.010
56. Rao RR, Li Q, Gubbels Bupp MR, Shrikant PA. Transcription factor Foxo1 represses T-bet-mediated effector functions and promotes memory CD8(+) T cell differentiation. Immunity (2012) 36(3):374–87. doi:10.1016/j.immuni
57. Intlekofer AM, Banerjee A, Takemoto N, Gordon SM, Dejong CS, Shin H, et al. Anomalous type 17 response to viral infection by CD8+ T cells lacking T-bet and eomesodermin. Science (2008) 321(5887):408–11. doi:10.1126/science.1159806
58. Cui W, Liu Y, Weinstein JS, Craft J, Kaech SM. An interleukin-21-interleukin-10-STAT3 pathway is critical for functional maturation of memory CD8+ T cells. Immunity (2011) 35(5):792–805. doi:10.1016/j.immuni.2011.09.017
59. Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol (2003) 4(12):1191–8. doi:10.1038/ni1009
60. Chandele A, Joshi NS, Zhu J, Paul WE, Leonard WJ, Kaech SM. Formation of IL-7Ralphahigh and IL-7Ralphalow CD8 T cells during infection is regulated by the opposing functions of GABPalpha and Gfi-1. J Immunol (2008) 180(8):5309–19.
61. Schmidt NW, Khanolkar A, Hancox L, Heusel JW, Harty JT. Perforin plays an unexpected role in regulating T-cell contraction during prolonged Listeria monocytogenes infection. Eur J Immunol (2011) 42:629–40. doi:10.1002/eji.201141902
62. Sanjabi S, Mosaheb MM, Flavell RA. Opposing effects of TGF-beta and IL-15 cytokines control the number of short-lived effector CD8+ T cells. Immunity (2009) 31(1):131–44. doi:10.1016/j.immuni.2009.04.020
63. Kurtulus S, Tripathi P, Moreno-Fernandez ME, Sholl A, Katz JD, Grimes HL, et al. Bcl-2 allows effector and memory CD8+ T cells to tolerate higher expression of Bim. J Immunol (2011) 186(10):5729–37. doi:10.4049/jimmunol.1100102
64. Weant AE, Michalek RD, Khan IU, Holbrook BC, Willingham MC, Grayson JM. Apoptosis regulators Bim and Fas function concurrently to control autoimmunity and CD8+ T cell contraction. Immunity (2008) 28(2):218–30. doi:10.1016/j.immuni.2007.12.014
65. Wensveen FM, Klarenbeek PL, van Gisbergen KP, Pascutti MF, Derks IA, van Schaik BD, et al. Pro-apoptotic protein Noxa regulates memory T cell population size and protects against lethal immunopathology. J Immunol (2013) 190(3):1180–91. doi:10.4049/jimmunol.1202304
66. Hand TW, Cui W, Jung YW, Sefik E, Joshi NS, Chandele A, et al. Differential effects of STAT5 and PI3K/AKT signaling on effector and memory CD8 T-cell survival. Proc Natl Acad Sci U S A (2010) 107(38): 16601–6. doi:10.1073/pnas.1003457107
67. Rubinstein MP, Lind NA, Purton JF, Filippou P, Best JA, McGhee PA, et al. IL-7 and IL-15 differentially regulate CD8+ T-cell subsets during contraction of the immune response. Blood (2008) 112(9):3704–12. doi:10.1182/blood-2008-06-160945
68. Craston R, Koh M, Mc DA, Ray N, Prentice HG, Lowdell MW. Temporal dynamics of CD69 expression on lymphoid cells. J Immunol Methods (1997) 209(1): 37–45. doi:10.1016/S0022-1759(97)00143-9
69. Sancho D, Gomez M, Sanchez-Madrid F. CD69 is an immunoregulatory molecule induced following activation. Trends Immunol (2005) 26(3):136–40. doi:10.1016/j.it.2004.12.006
70. Shiow LR, Rosen DB, Brdickova N, Xu Y, An J, Lanier LL, et al. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature (2006) 440(7083):540–4. doi:10.1038/nature04606
71. Deblandre GA, Leo O, Huez GA, Wathelet MG. CD69 is expressed on Daudi cells in response to interferon-alpha. Cytokine (1992) 4(1):36–43. doi:10.1016/1043-4666(92)90034-O
72. Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature (2006) 439(7077):682–7. doi:10.1038/nature04444
73. Yang TC, Millar J, Groves T, Grinshtein N, Parsons R, Takenaka S, et al. The CD8+ T cell population elicited by recombinant adenovirus displays a novel partially exhausted phenotype associated with prolonged antigen presentation that nonetheless provides long-term immunity. J Immunol (2006) 176(1):200–10.
74. Zinselmeyer BH, Heydari S, Sacristan C, Nayak D, Cammer M, Herz J, et al. PD-1 promotes immune exhaustion by inducing antiviral T cell motility paralysis. J Exp Med (2013) 210:757–74. doi:10.1084/jem.20121416
75. Angelosanto JM, Blackburn SD, Crawford A, Wherry EJ. Progressive loss of memory T cell potential and commitment to exhaustion during chronic viral infection. J Virol (2012) 86(15):8161–70. doi:10.1128/JVI.00889-12
76. Doering TA, Crawford A, Angelosanto JM, Paley MA, Ziegler CG, Wherry EJ. Network analysis reveals centrally connected genes and pathways involved in CD8+ T cell exhaustion versus memory. Immunity (2012) 37(6):1130–44. doi:10.1016/j.immuni.2012.08.021
77. Shin H, Blackburn SD, Blattman JN, Wherry EJ. Viral antigen and extensive division maintain virus-specific CD8 T cells during chronic infection. J Exp Med (2007) 204(4):941–9. doi:10.1084/jem.20061937
78. Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol (2004) 22:745–63. doi:10.1146/annurev.immunol.22.012703.104702
79. Guarda G, Hons M, Soriano SF, Huang AY, Polley R, Martin-Fontecha A, et al. L-selectin-negative CCR7- effector and memory CD8+ T cells enter reactive lymph nodes and kill dendritic cells. Nat Immunol (2007) 8(7):743–52. doi:10.1038/ni1469
80. Cauley LS, Lefrancois L. Guarding the perimeter: protection of the mucosa by tissue-resident memory T cells. Mucosal Immunol (2013) 6(1):14–23. doi:10.1038/mi.2012.96
81. Lee YT, Suarez-Ramirez JE, Wu T, Redman JM, Bouchard K, Hadley GA, et al. Environmental and antigen-receptor derived signals support sustained surveillance of the lungs by pathogen-specific CTL. J Virol (2011) 85:4085–94. doi:10.1128/JVI.02493-10
82. Hogan RJ, Usherwood EJ, Zhong W, Roberts AA, Dutton RW, Harmsen AG, et al. Activated antigen-specific CD8+ T cells persist in the lungs following recovery from respiratory virus infections. J Immunol (2001) 166(3):1813–22.
83. Khanna KM, Bonneau RH, Kinchington PR, Hendricks RL. Herpes simplex virus-specific memory CD8+ T cells are selectively activated and retained in latently infected sensory ganglia. Immunity (2003) 18(5):593–603. doi:10.1016/S1074-7613(03)00112-2
84. Wang HC, Zhou Q, Dragoo J, Klein JR. Most murine CD8+ intestinal intraepithelial lymphocytes are partially but not fully activated T cells. J Immunol (2002) 169(9):4717–22.
85. Wakim LM, Woodward-Davis A, Bevan MJ. Memory T cells persisting within the brain after local infection show functional adaptations to their tissue of residence. Proc Natl Acad Sci U S A (2010) 107(42):17872–9. doi:10.1073/pnas.1010201107
86. Kim TS, Hufford MM, Sun J, Fu YX, Braciale TJ. Antigen persistence and the control of local T cell memory by migrant respiratory dendritic cells after acute virus infection. J Exp Med (2010) 207(6):1161–72. doi:10.1084/jem.20092017
87. Casey KA, Fraser KA, Schenkel JM, Moran A, Abt MC, Beura LK, et al. Antigen-independent differentiation and maintenance of effector-like resident memory T cells in tissues. J Immunol (2012) 188:4866–75. doi:10.4049/jimmunol.1200402
88. Zammit DJ, Turner DL, Klonowski KD, Lefrancois L, Cauley LS. Residual antigen presentation after influenza virus infection affects CD8 T cell activation and migration. Immunity (2006) 24(4):439–49. doi:10.1016/j.immuni.2006.01.015
89. Jelley-Gibbs DM, Brown DM, Dibble JP, Haynes L, Eaton SM, Swain SL. Unexpected prolonged presentation of influenza antigens promotes CD4 T cell memory generation. J Exp Med (2005) 202(5):697–706. doi:10.1084/jem.20050227
90. Turner DL, Cauley LS, Khanna KM, Lefrancois L. Persistent antigen presentation after acute vesicular stomatitis virus infection. J Virol (2007) 81(4):2039–46. doi:10.1128/JVI.02167-06
91. Sheridan BS, Lefrancois L. Regional and mucosal memory T cells. Nat Immunol (2011) 12(6):485–91. doi:10.1038/ni.2029
92. Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol (2009) 10(5):524–30. doi:10.1038/ni.1718
93. Bankovich AJ, Shiow LR, Cyster JG. CD69 suppresses sphingosine 1-phosophate receptor-1 (S1P1) function through interaction with membrane helix 4. J Biol Chem (2010) 285(29):22328–37. doi:10.1074/jbc.M110.123299
94. Khanna KM, Aguila CC, Redman JM, Suarez-Ramirez JE, Lefrancois L, Cauley LS. In situ imaging reveals different responses by naive and memory CD8 T cells to late antigen presentation by lymph node DC after influenza virus infection. Eur J Immunol (2008) 38(12):3304–15. doi:10.1002/eji.200838602
95. Wakim LM, Gupta N, Mintern JD, Villadangos JA. Enhanced survival of lung tissue-resident memory CD8(+) T cells during infection with influenza virus due to selective expression of IFITM3. Nat Immunol (2013) 14(3):238–45. doi:10.1038/ni.2525
96. Carlson CM, Turpin EA, Moser LA, O’Brien KB, Cline TD, Jones JC, et al. Transforming growth factor-beta: activation by neuraminidase and role in highly pathogenic H5N1 influenza pathogenesis. PLoS Pathog (2010) 6(10):e1001136. doi:10.1371/journal.ppat.1001136
97. Schultz-Cherry S, Hinshaw VS. Influenza virus neuraminidase activates latent transforming growth factor beta. J Virol (1996) 70(12):8624–9.
98. Uhl EW, Castleman WL, Sorkness RL, Busse WW, Lemanske RF Jr., McAllister PK. Parainfluenza virus-induced persistence of airway inflammation, fibrosis, and dysfunction associated with TGF-beta 1 expression in brown Norway rats. Am J Respir Crit Care Med (1996) 154(6 Pt 1):1834–42. doi:10.1164/ajrccm.154.6.8970378
99. Croom HA, Denton AE, Valkenburg SA, Swan NG, Olson MR, Turner SJ, et al. Memory precursor phenotype of CD8+ T cells reflects early antigenic experience rather than memory numbers in a model of localized acute influenza infection. Eur J Immunol (2011) 41(3):682–93. doi:10.1002/eji.201040625
100. El Asady R, Yuan R, Liu K, Wang D, Gress RE, Lucas PJ, et al. TGF-{beta}-dependent CD103 expression by CD8(+) T cells promotes selective destruction of the host intestinal epithelium during graft-versus-host disease. J Exp Med (2005) 201(10):1647–57. doi:10.1084/jem.20041044
101. Maruyama T, Li J, Vaque JP, Konkel JE, Wang W, Zhang B, et al. Control of the differentiation of regulatory T cells and T(H)17 cells by the DNA-binding inhibitor Id3. Nat Immunol (2011) 12(1):86–95. doi:10.1038/ni.1965
102. Takimoto T, Wakabayashi Y, Sekiya T, Inoue N, Morita R, Ichiyama K, et al. Smad2 and Smad3 are redundantly essential for the TGF-beta-mediated regulation of regulatory T plasticity and Th1 development. J Immunol (2010) 185(2):842–55. doi:10.4049/jimmunol.0904100
103. Zhang YE. Non-Smad pathways in TGF-beta signaling. Cell Res (2009) 19(1):128–39. doi:10.1038/cr.2008.328
Keywords: transforming growth factor beta, CD8 T cells, homing receptors, prolonged antigen presentation, tissue-resident memory cells, migration
Citation: Hu Y and Cauley L (2013) Antigen and transforming growth factor beta receptors contribute to long term functional and phenotypic heterogeneity of memory CD8 T cells. Front. Immunol. 4:227. doi: 10.3389/fimmu.2013.00227
Received: 07 April 2013; Accepted: 18 July 2013;
Published online: 12 August 2013.
Edited by:
Susan Swain, University of Massachusetts Medical School, USAReviewed by:
David Hildeman, Cincinnati Children’s Hospital, USAShahram Salek-Ardakani, University of Florida, USA
Copyright: © 2013 Hu and Cauley. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Linda Cauley, University of Connecticut Health Center, 263 Farmington Avenue, MC1319 Farmington, CT 06032, USA e-mail: lcauley@uchc.edu
 Yinghong Hu
Yinghong Hu