- 1Biochemical Institute of the Medical Faculty of the Christian-Albrechts-University, Kiel, Germany
- 2Philipps-University of Marburg, Marburg, Germany
- 3Department of Medicine, The University of Alabama at Birmingham, Birmingham, USA
The diversity of the third complementarity determining region of the IgH chain is constrained by natural selection of immunoglobulin diversity (DH) sequence. To test the functional significance of this constraint in the context of thymus-dependent (TD) immune responses, we immunized BALB/c mice with WT or altered DH sequence with 2-phenyloxazolone-coupled chicken serum albumin (phOx-CSA). We chose this antigen because studies of the humoral immune response to the hapten phOx were instrumental in the development of the current theoretical framework on which our understanding of the forces driving TD responses is based. To allow direct comparison, we used the classic approach of generating monoclonal Ab (mAb) from various stages of the immune response to phOx to assess the effect of changing the sequence of the DH on clonal expansion, class switching, and affinity maturation, which are hallmarks of TD responses. Compared to WT, TD-induced humoral IgM as well as IgG antibody production in the D-altered ΔD-DμFS and ΔD-iD strains were significantly reduced. An increased prevalence of IgM-producing hybridomas from late primary, secondary, and tertiary memory responses suggested either impaired class switch recombination (CSR) or impaired clonal expansion of class switched B cells with phOx reactivity. Neither of the D-altered strains demonstrated the restriction in the VH/VL repertoire, the elimination of VH1 family-encoded antibodies, the focusing of the distribution of CDR-H3 lengths, or the selection for the normally dominant Ox1 clonotype, which all are hallmarks of the anti-phOx response in WT mice. These changes in clonal selection and expansion, as well as CSR indicate that the genetic constitution of the DH locus, which has been selected by evolution, can strongly influence the functional outcome of a TD humoral response.
Introduction
In immunoglobulins, juxtaposition of the three complementary determining regions (CDRs) of the L chain and the three of the H chain creates the site at which antigen binds (1, 2). While CDRs 1 and 2 are entirely of germline origin and CDR-L3 is largely so, CDR-H3 is the direct product of VDJ rearrangement and N nucleotide addition (3). This makes CDR-H3 the focus for pre-immune Ig diversity. In combination, this diversity and its physical location at the center of the antigen binding site tends to endow CDR-H3 with the ability to define the antigen binding specificity and affinity of the antibody.
Analyses of anti-hapten immune responses have been crucial for the dissection of the roles played by T cells in initiating and regulating humoral immune maturation. Immune maturation in the classic humoral immune response of BALB/c mice to the hapten 2-phenyloxazolone (phOx) (4) focuses on the clonal expansion and somatic hypermutation of Ig bearing the dominant Ox1 Id (IdOx1). While this Id is marked by the use of a combination of VHOx1 and VκOx1 variable genes, the presence of a short DRG peptide sequence in CDR-H3 is typically determinative (4, 5).
To test the role of natural selection of D gene segment sequence on humoral immune function, we previously created a panel of BALB/c-derived D-altered mutant mouse strains (6–8). ΔD-DμFS and ΔD-iD B cells produce two alternative, polyclonal Ig repertoires with a normal and intact set of VH, JH, and CH exons that are fully capable of undergoing somatic hypermutation and class switching (6, 8). The only change that has been made is the simplification of DH locus to contain only one D of alternative sequence. After VDJ rearrangement, even the loxP sites are deleted, leaving only the imprint of the three to seven amino acids encoded by the DH. The CDR-H3s that contain identifiable DH sequence create an antigen binding site repertoire that differs greatly in the pattern of amino acid use from WT. However, CDR-H3 sequences that lack identifiable DH sequence and are created by V, J, and N sequence alone appear indistinguishable from similar sequences created in wild-type (WT) mice (Figures S1 and S2 in Supplementary Material).
The DRG peptide sequence characteristic of the dominant Ox1 Id is an example of a CDR-H3 that can be easily created either with or without D gene segment sequence. The nine nucleotides used to encode DRG can include three to five nucleotides from 5 of the 13 DH gene segments. However, the DRG sequence can also be created by simply introducing five N nucleotides between VHOx1 and JH3. Our panel of D-altered mice thus provided us with the means to test the extent to which loss of the naturally selected D-dependent CDR-H3 repertoire would influence the development of a classic T dependent response to a defined hapten even when the loss of D sequence could be easily mitigated by N addition alone.
To allow direct comparisons to previous studies, we used the classic approach of generating monoclonal Ab (mAb) from various stages of the immune response to phOx. We found that changing conserved elements of the sequence of the diversity gene segment locus led to the failure to select for the use of VHOx1/VκOx1 gene combination, the failure to yield the normal focusing of CDR-H3 sequence, and thus the loss of IdOx1 dominance. Further, we observed an enhanced and persistent production of hybridomas secreting low affinity IgM indicating a profound failure to develop a fully mature, class switched IgG response. Together, these findings suggest that TD B cell responses can be heavily influenced by the effects of natural selection of DH content on CDR-H3 repertoire diversity.
Materials and Methods
Animals
Wild-type female BALB/c (H-2d) mice (Harlan-Winkelmann; Borchen, Germany) and BALB/c D-altered homozygous ΔD-DμFS (7) and ΔD-iD (6) mice were reared under clean conventional conditions the University of Kiel animal house. Immunizations and experimental procedures were approved by the “Ministry of Agriculture, Environment and Rural Areas” of the local government of Schleswig-Holstein, permission no. V 312-72241.121-3 (35-3/06).
Antigens, Immunizations, Antibody Titrations, and Production of Monoclonal Antibodies
BALB/c WT and D-altered ΔD-DμFS and ΔD-iD mice were immunized with the TD hapten–protein complex phOx-coupled chicken serum albumin (phOx-CSA) (molar ratio ~11) (9, 10). At 3–4 months of age, animals of all three strains received 80 μg of phOx-CSA adsorbed to Al(OH)3 as a primary intraperitoneal immunization. Venous blood was serially obtained by tail vein phlebotomy and serum antibody concentrations were determined after primary or secondary immunization. PhOx-binding IgM and IgG titers were determined with ELISA using class-specific secondary antisera. Spleen cells of phOx-CSA-immunized WT or D-altered mice were fused with the non-secretor Ag8.653 myeloma cells by means of the conventional PEG-mediated hybridization technique. Fusions were performed 7 or 14 days after primary immunization and 3 days after each secondary (memory) immunization. Resulting mAb reactive with phOx-BSA but not with BSA alone were selected for further study.
Determination of Relative Affinities of Anti-phOx Antibodies
The relative affinities of mAb antibodies to phOx were determined with a hapten-inhibition test as previously described (9). These affinities were assessed in comparison to that of two prototypic Ox1-idiotypic mAb: H11.5 (μ,κ) for IgM and NQ2/16.2 (γ,κ) for IgG Ab. Briefly, the binding of comparable amounts of anti-phOx mAb to surface-bound phOx-BSA was inhibited with graded concentrations of soluble phOx-caproic acid. Concentration values giving 50% inhibition were taken as relative affinity measures. An affinity factor was generated as the quotient of the relative affinity of H11.5 (μ,κ) for IgM and NQ2/16.2 (γ,κ) for IgG mAb divided by that of a particular mAb. MAb with higher affinity than the controls exhibited an affinity factor greater than 1, and those with a lower affinity exhibited an affinity factor of less than 1. Independent confirmation of these measures of affinity was obtained by fluorescence quenching (10).
Sequencing of Antibody V Region Genes
Sequence analysis of the variable regions of mAb was performed as previously described (11) with some modifications. Briefly, total RNA of anti-phOx Ab secreting hybrid cell lines was isolated with TRIZOL® (GIBCO-BRL, Eggenstein, Germany) and transcribed into cDNA with SuperScriptTM II RNAse H reverse transcriptase (GIBCO-BRL, Eggenstein, Germany) using pd(N)6 random and pd(T)12–18 primers. The VH and VL mRNA sequences were first amplified by PCR using 10 primer sets for each of the VH-regions, and 7 primer sets for each of the VL-regions and two forward primers specific for the 3′-end for the first domain of the VH and VL constant regions, respectively. A second semi-nested amplification at 3′-end was then performed with relevant primers coupled to M13 oligonucleotides. This amplimer product was then used for sequencing (MWG Biotech; Ebersberg, Germany). V region sequences were analyzed with the integrative database VBASE2 (http://www.vbase2.org/).
Statistical Analysis
One-way analysis of variance (ANOVA) followed by Dunnet’s multiple comparison post test, using Graphpad Prism for Windows (version 4.02, GraphPad Software Inc., San Diego, USA) was used for statistical comparisons of individual IgM and IgG end-point titers.
Accession Numbers
GenBank accession numbers for all antibodies are indicated in the supplementary table legends of each group of antibodies.
Results
Humoral Immune Response and Analysis of 2-Phenyl-5-Oxazolone-Specific Monoclonal Antibodies
The humoral Ab response to phOx in BALB/c WT mice was compared to that of homozygous D-altered ΔD-DμFS and ΔD-iD mutant mice. The DH locus in these strains was first simplified by deleting 12 of the 13 DH gene segments by means of cre/loxP gene-targeting (12); and then modifying the sequence of the remaining DH. In the ΔD-DμFS strain, the WT sequence of the DFL16.1 DH segment was modified by introducing two frame-shift mutations that flipped the normal preference for reading frame 1 (RF1), which encodes neutral tyrosine and glycine, to RF2, which encodes hydrophobic valine. In the ΔD-iD strain, the center of the DFL16.1 segment was replaced with the DSP2.2 DH gene segment in inverted form. This leads to CDR-H3 enriched for charged arginine, asparagine, and histidine in place of tyrosine and glycine (6, 8).
In both mutant strains, primary immunization with the TD Ag phOx-CSA-induced significantly lower IgM and IgG anti-phOx Ab titers than WT (Figures 1A,B). Following secondary (memory) immunization, the IgG response remained suppressed while IgM production was meager and indistinguishable between all three strains.
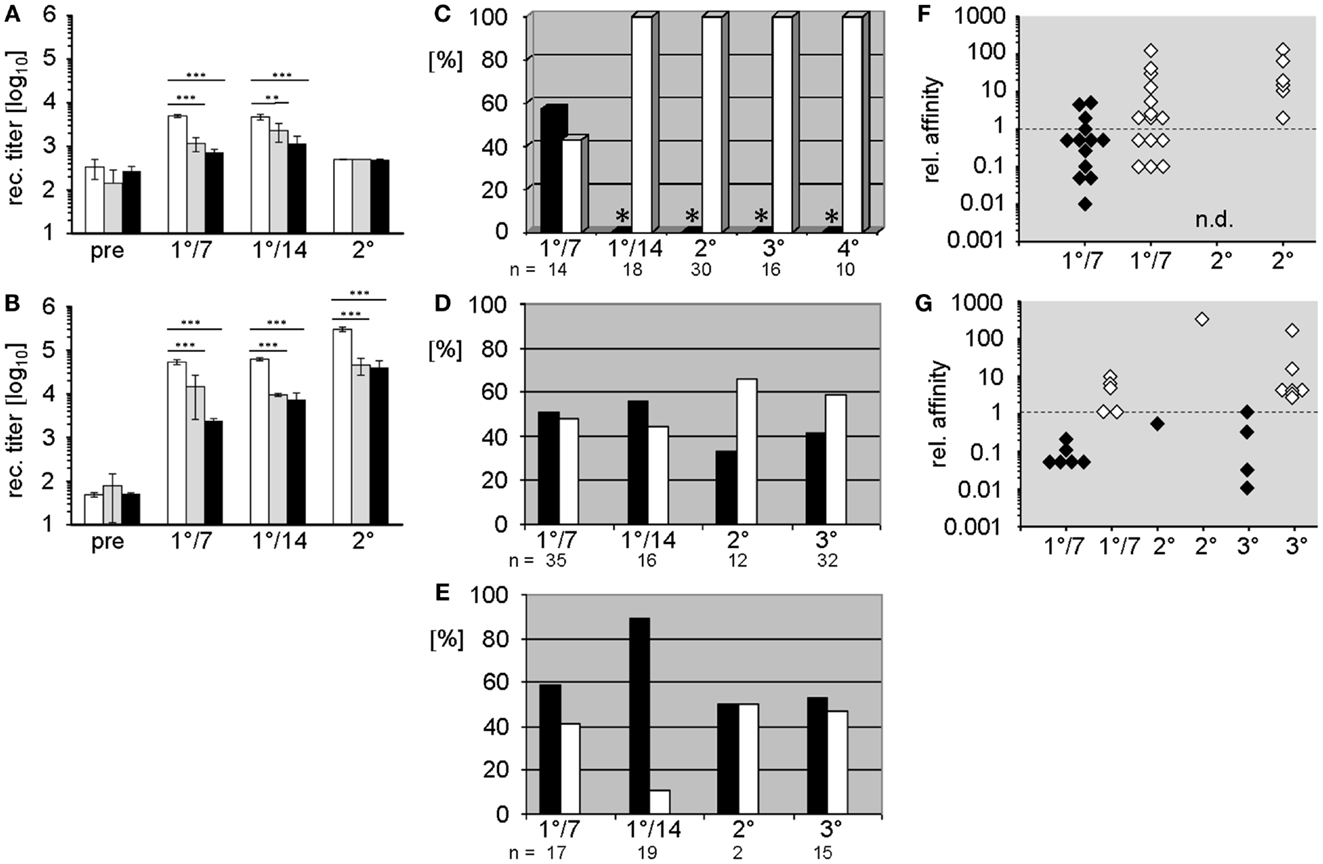
Figure 1. Comparison of humoral Ab responses to the hapten 2-phenyloxazolone in BALB/c wild-type and D-altered ΔD-DμFS and ΔD-iD mutant mice. (A,B) IgM (A) and IgG (B) anti-phOx Ab titers of primary and secondary thymus-dependent immune responses after immunization with phOx-CSA in BALB/c wild-type mice (white bars), ΔD-DμFS mice (gray bars), and ΔD-iD mice (black bars). The bars show the mean + SD of five mice per group. Pre – pre-immune sera; 1°/7 – primary day 7 and 1°/14 – primary day 14 responses. Secondary immunization followed 14 weeks after primary and Ab titers were determined after two more weeks. Significance is indicated at **p < 0.01; ***p < 0.001. (C–E) Monoclonal anti-phOx mAb were prepared from (C) BALB/c wild-type, (D) ΔD-DμFS, and (E) ΔD-iD mice on day 7 (1°/7) and day 14 (1°/14) after primary immunization with phOx-CSA and 3 days after secondary (2°), tertiary (3°), and from wild-type mice also after quaternary (4°) immunizations. For ΔD-DμFS and ΔD-iD mice, percentages of IgM and IgG mAb are indicated as black and white bars, respectively. However, because extremely low numbers of IgM mAb have been isolated from the late primary and memory responses of BALB/c wild-type mice (*), only IgG mAb have been produced. (F,G) The relative affinities of IgM (black diamonds) and IgG (white diamonds) anti-phOx mAb from different stages of the immune response of (F) ΔD-DμFS and (G) ΔD-iD mutant mice are compared either to the IgM IdOx1 mAb H11.5 (μ,κ) or to the IgG IdOx1-prototypic mAb NQ2/16.2 (γ,κ). Affinity factors of >l indicate higher while affinity factors of <l indicate lower affinities than H11.5 and NQ2/16.2, respectively.
Study of the molecular events during immune maturation of the TD anti-phOx response classically focused on analysis of mAb (9, 13, 14) secreted by hybridomas produced by fusion of permanently growing myeloma cells with immune splenocytes obtained after sequential primary and memory immunization (9, 13, 14). In order to directly compare the effect of altering DH locus content in the same classic context, we generated anti-phOx mAb-secreting hybridomas from immune splenocytes harvested after primary, secondary, and tertiary immunizations of D-altered ΔD-DμFS and ΔD-iD mutant mice.
The characteristic parameters of these mAb, including isotype, relative affinity, usage of VH/VL genes and DH and JH gene segments, amino acid sequence, and reading frame usage in CDR-H3, and correspondence with the normally dominating IdOx1 are summarized Tables S1 and S2 in Supplementary Material. A comparison of these mAb with those of previously published mAb (10) from WT mice revealed crucial differences induced by the change in DH sequence content. Unlike WT mice, where the majority of hybridomas ( >80%) isolated during late primary responses and on day 3 of secondary and tertiary responses produce IgG, and thus reflect T cell aided CSR (15); only half of the hybridomas obtained from the D-altered ΔD-DμFS and ΔD-iD mice produced IgG. This included both the hybridomas generated after the late primary response as well as hybridomas harvested from splenocytes 3 days after memory immunizations (Figures 1D,E). Thus, the normal pattern of favoring production of IgG secreting B cells failed to occur in mice lacking a WT DH locus, and therefore, a WT CDR-H3 repertoire. A full depiction of these antibodies is provided in Tables S1 and S2 in Supplementary Material.
To assess the quality of these mAb, we compared their relative affinities to those of prototypic IgM and IgG IdOx1 mAb. Early primary IgM mAb of both mutant strains, as well as those from secondary and tertiary responses, were mostly of rather low affinity (Figures 1F,G). However, three IgM mAb from ΔD-DμFS mice exhibited higher affinities than the IgM IdOx1 mAb H11.5 (Figure 1F), even though none of them included the IdOx1 V gene combination of VHOx1/VκOx1, which is designated VH171/Vκ072 in the integrative database VBASE2.
Early primary IgG mAb generally exhibited higher affinities than the IgM mAb, with some IgG mAb displaying even higher affinities than the IgG IdOx1 mAb NQ16.2. However, none of the mAb expressed the IdOx1 VHOx1/VκOx1 gene combination. IgG of higher affinity were found among secondary and tertiary response mAb. However, immune maturation did not progress in the steady way that characterizes the classic response of WT mice (see below).
In accordance with historical studies, in WT mice the anti-phOx response exhibited a drastic CSR-associated reduction in the variability of the VH and VL repertoires (10) (Figures 2A,D), especially in the class switched secondary response. No such reduction in VH or VL variability was observed in the D-altered ΔD-DμFS and ΔD-iD mice (Figures 2B,E and C,F). The classic secondary phOx response in WT mice is associated with a shift away from use of the VH1 family (10). However, in the D-altered mice hybridomas secreting VH1 family-encoded IgM and IgG mAb continued to be isolated at all phases of immune maturation (Tables S1 and S2 in Supplementary Material).
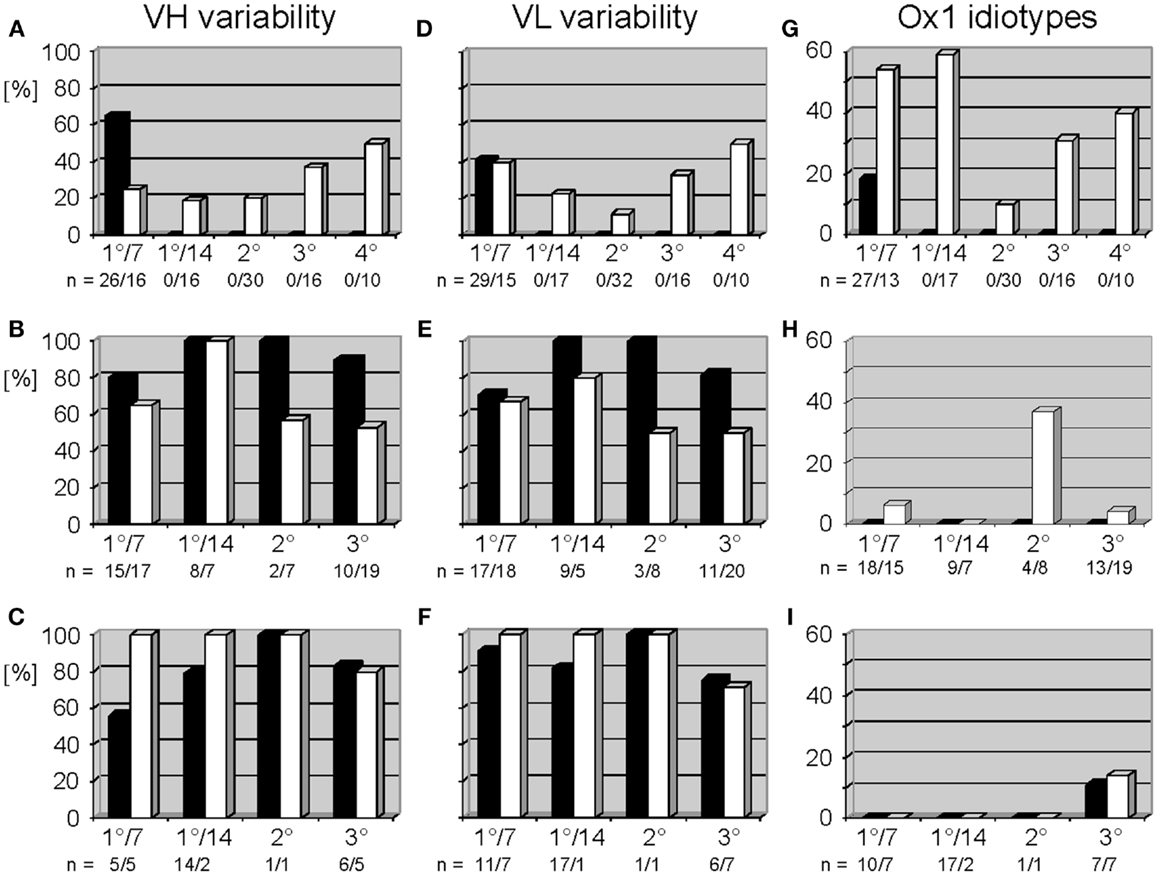
Figure 2. Variable gene usage of mAb of the thymus-dependent anti-phOx immune response in BALB/c wild-type and ΔD-DμFS and ΔD-iD DH mutant mice. Mice were immunized with the TD Ag phOx-CSA and mAb were prepared on day 7 (1°/7) and day 14 (1°/14°) after primary immunization and 3 days after secondary (2°), tertiary (3°), and quaternary (4°) immunizations, respectively. The characteristic attributes and utilized variable genes of these Ab are depicted in Tables S1 and S2 in Supplementary Material. Variability is calculated as the number of genes expressed by a group of antibodies divided by the number of monoclonal antibodies of this group and multiplied by 100. In BALB/c mice, IgM mAb (black bars) were only prepared early after primary immunization; see also legend to Figure 1. Data for IgG (white bars) are taken from two previous publications (9, 10). VH and VL variability is indicated for anti-phOx mAb of BALB/c wild-type mice (A,D), ΔD-DμFS mice (B,E), and ΔD-iD mice (C,F). (G–I) Expression of Ox1-idiotypic gene combination VH171/Vκ072 by monoclonal anti-phOx Ab from the TD response in BALB/c wild-type mice (G), ΔD-DμFS mice (H), and ΔD-iD mice (I). Data of BALB/c wild-type mice are taken from previous publications (5, 9, 10).
In WT mice, the TD response to phOx-CSA is dominated by VH171/Vκ072-encoded IdOx1 Ab (4) whose idiotypic determinant is located in CDR-H3 (5). A compilation of previous data (9, 10) shows that mAb with the gene combination VH171/Vκ072 (the “genetic” IdOx1) first dominate after CSR among IgG mAb (Figure 2G). This gene combination is counter-selected in secondary response mAb, but increases again in tertiary and quaternary responses. However, in both of the D-altered strains the IdOx1 gene combination VH171/Vκ072 only represented a minority of clones. Among ΔD-DμFS mAb, the VH171/Vκ072 gene combination was not identified among the IgM hybridomas. It was detected in IgG mAb drawn from the primary response, but at a low level. A plurality of the secondary IgG response used this combination, but this was again lost in the tertiary response (Figure 2H). In ΔD-iD mice, anti-phOx antibodies with the VH171/Vκ072 gene combination were not observed in either the primary or secondary responses. It was found among tertiary IgM and IgG, but again at a low level (Figure 2I).
Analysis of CDR-H3 of Monoclonal Anti-2-Phenyl-5-Oxazolone Antibodies
In WT mice, CSR and affinity maturation are strongly associated with a focusing of CDR-H3 content. For example, whereas the CDR-H3 of anti-phOx IgM hybridomas varied between 15 and 51 nucleotides, 70–100% of primary and memory IgG mAb exhibited a clearly dominating length of 21 nucleotides (10). This CSR-associated restriction of CDR-H3 lengths was not observed in D-altered ΔD-DμFS and ΔD-iD mice (Figure 3). CDR-H3 lengths with 21 and 36 nucleotides were slightly increased among ΔD-DμFS mAb (Figure 3A) and to an even lesser degree among ΔD-iD mAb (Figure 3B). Otherwise, IgM as well as IgG mAb from both strains of mice demonstrated a wide distribution of lengths.
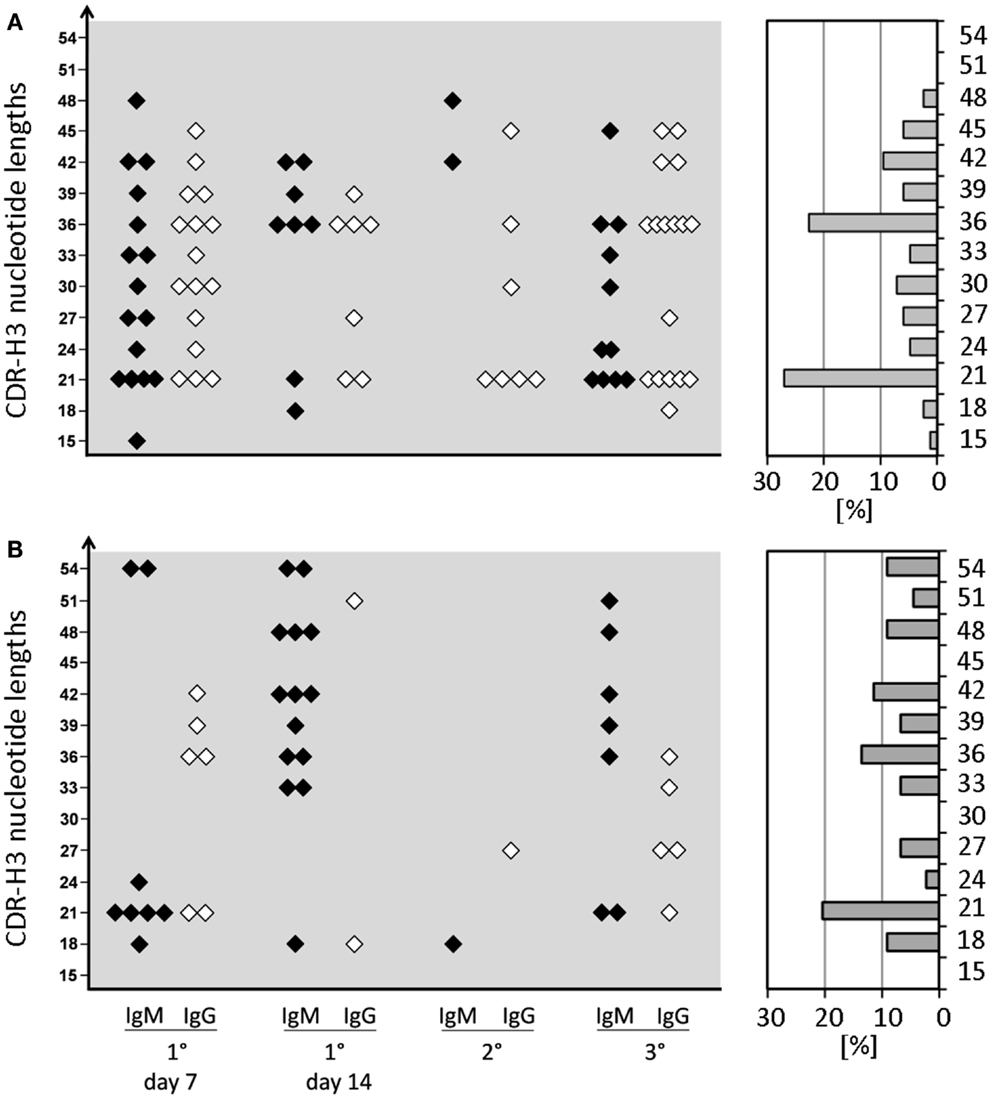
Figure 3. CDR-H3 nucleotide lengths of IgM and IgG mAb of the anti-phOx immune response of D-altered ΔD-DμFS and ΔD-iD mutant mice. Monoclonal Ab were prepared from mice that were immunized various times with the TD Ag phOx-CSA (see previous figures). The characteristic attributes of these Ab are depicted in Tables S1 and S2 in Supplementary Material. The CDR-H3 nucleotide lengths of IgM (black symbols) and IgG (white symbols) monoclonal anti-phOx Ab from different stages of the immune response of ΔD-DμFS mice are shown in (A) and those of ΔD-iD mice in (B). The percental contribution of each nucleotide length is indicated by gray bars at the right hand side.
The distribution of charged, neutral, and hydrophobic amino acids dictates the average hydropathicity of the CDR-H3 loop. During immune maturation among WT mice, highly hydrophobic amino acids found among IgM anti-phOx CDR-H3 are gradually supplanted by arginine, aspartic acid, glycine, and tryptophan (Figures S3A–H in Supplementary Material). In contrast, from the primary to tertiary responses in both ΔD-DμFS mice (Figure 4) as well as ΔD-iD mice (data not shown), hydrophobic amino acids were found to a similar extent in the CDR-H3 of both IgM and IgG anti-phOx antibodies. The content of hydrophobic amino acids in the CDR-H3 loops was also reflected in the corresponding hydrophobicity values. In anti-phOx mAb from WT mice, about 25% of IgM mAb exhibited CDR-H3 with positive average hydropathicity values, whereas only 6% of IgG antibodies (with the exception of secondary IgG) belonged to this category (Figures S4A–H in Supplementary Material). In contrast, IgM as well as IgG mAb produced by ΔD-DμFS B cells expressed similar proportions of CDR-H3 loops with positive average hydropathicity values (Figure 5). [The numbers of mAbs generated from the ΔD-iD mice were insufficient to allow a firm conclusion on whether average hydrophobicity was maintained or altered in this strain (data not shown).]
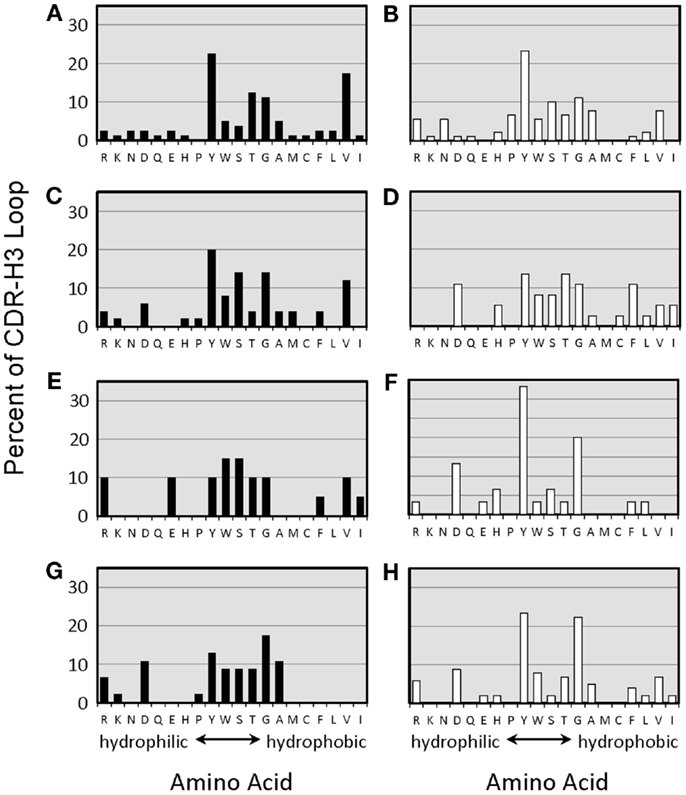
Figure 4. Amino acids of CDR-H3 loops of monoclonal anti-phOx Ab of D-altered ΔD-DμFS mice. Amino acids are indicated at the x-axis in the one-letter code. Hybridomas secreting anti-phOx antibodies (IgM black bars, IgG white bars) were generated after primary immunization on day 7 (A) IgM (n = 16 mAb), (B) IgG (n = 14 mAb), and on day 14 (C) IgM (n = 8 mAb), (D) IgG (n = 7 mAb), 3 days after secondary immunization (E) IgM (n = 2 mAb), (F) IgG (n = 7 mAb), 3 days after tertiary immunization, (G) IgM (n = 11 mAb), and (H) IgG (n = 17 mAb). See also Figure S3 in Supplementary Material.
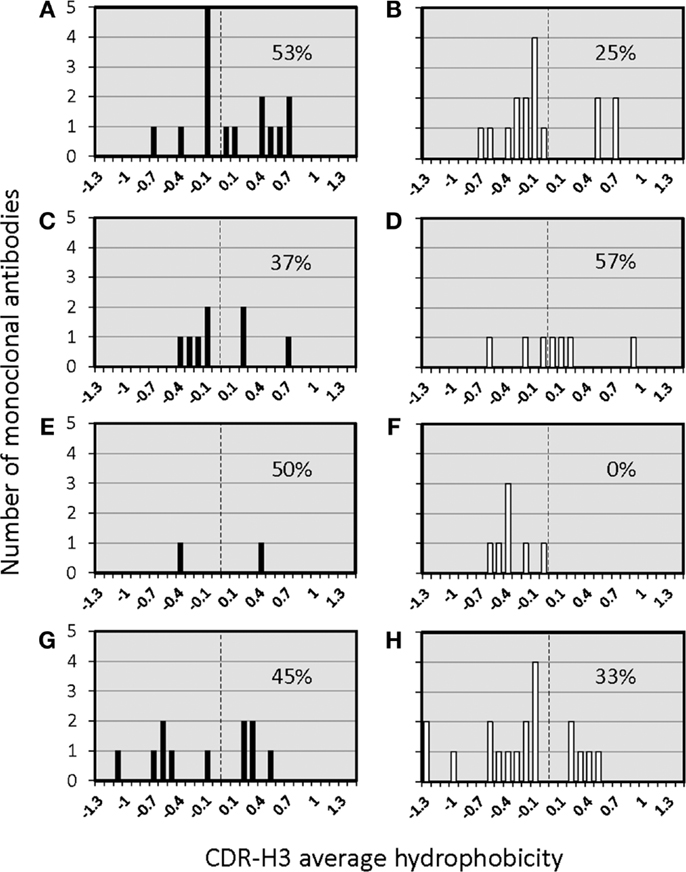
Figure 5. Distribution of average hydropathicity values of CDR-H3 loops of monoclonal anti-phOx Ab of D-altered ΔD-DμFS mutant mice. The average hydropathicity values of CDR-H3 loops were calculated with the normalized Kyte–Doolittle hydrophobicity scale (8). Anti-phOx antibody-secreting hybridomas (IgM black bars, IgG white bars) were generated after primary immunization on day 7 (A) IgM (n = 16 mAb), (B) IgG (n = 14 mAb), and on day 14 (C) IgM (n = 8 mAb), (D) IgG (n = 7 mAb), 3 days after secondary immunization (E) IgM (n = 2 mAb), (F) IgG (n = 7 mAb), 3 days after tertiary immunization, (G) IgM (n = 11 mAb), and (H) IgG (n = 17 mAb). See also Figure S3 in Supplementary Material.
Since idiotypic determinants defining IdOx1 antibodies are located in CDR-H3, we analyzed antibodies bearing this idiotype to assess, which DH gene segments could be used to generate the typical IdOx1 CDR-H3 sequence C_ARDRGAY. In solely phOx-CSA-induced mAb from WT mice (10), this sequence could be generated from the five DH gene segments DSP2.2, DSP2.9, DSP2.11, DST4, and DQ52 (Table 1). In contrast, after a primary immunization with the TI-2 Ag phOx-Ficoll and a subsequent TD immunization (primary to quaternary) with phOx-CSA, CDR-H3 sequences from anti-phOx mAb were drawn from a larger set of eight DH segments (DSP2.2, DSP2.3, DSP2.5, DSP2.6, DSP2.9, DSP2.10) plus two sequences from inverted DFL16.1 and DFL 16.2, respectively (9). Moreover, in half of the mAb, the DH segment could not be identified due to extensive nibbling (9). This raised the issue of whether ΔD-DμFS mutant mice bearing a frameshifted DFL16.1 DH segment or ΔD-iD mice with an inverted DSP2.2 gene sequence would be able to generate a typical IdOx1 CDR-H3 sequence in association with the VH171 gene.
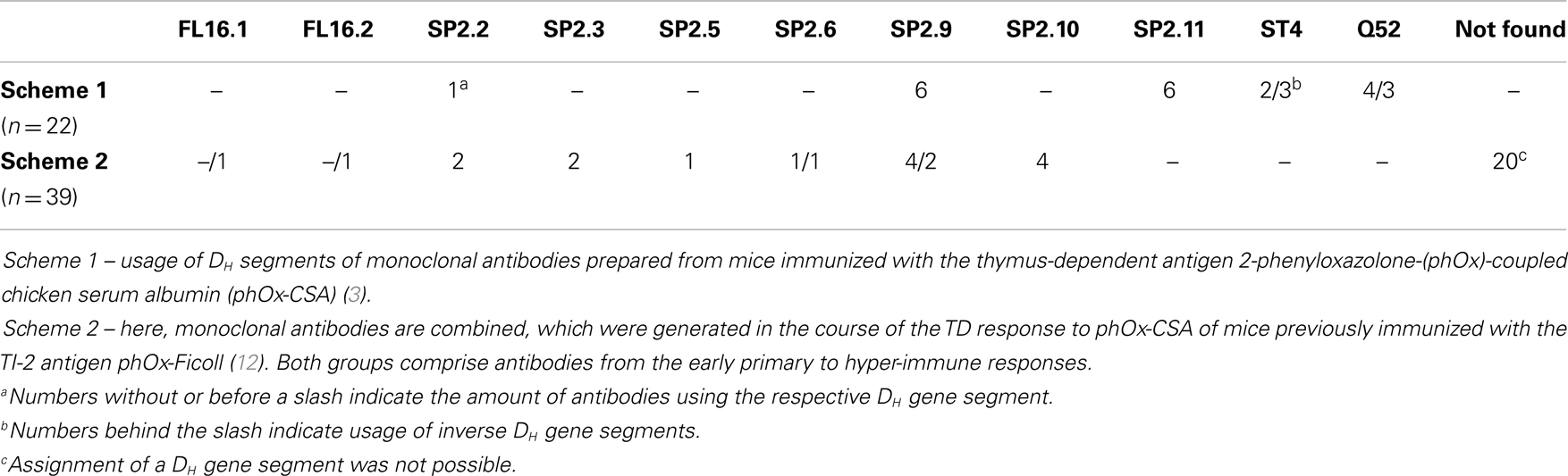
Table 1. DH segment usage of IGVH171-encoded monoclonal anti-2-phenyloxazolone antibodies from BALB/c wild-type mice immunized with two different immunization schemes.
C_ARDRGAY-related sequences were identified (Table 2A). The majority of these CDR-H3s lacked identifiable DH sequences and instead was largely the product of N and P nucleotide addition. Among the mAbs drawn from the ΔD-DμFS mice, two contained CDR-H3 amino acids coded by the frameshifted DFL16.1 gene segment. Five additional ΔD-DμFS mAb (FS1°14/05, FS3°/08, FS3°/09, FS3°/22, FS3°/18) also exhibited CDR-H3 sequences already found in IdOx1 antibodies from WT mice, but these mAbs used N and P nucleotides to recreate the sequence. Among the mAbs drawn from the ΔD-iD mice, one early primary mAb, iD1°7/06, exhibited the correct IdOx1 sequence in CDR-H3. These findings made it clear that both D-altered strains were able to generate IdOx1-typical or related CDR-H3 sequences in spite of the alteration in DH sequence content. Thus, differences in the CDR-H3 repertoire in these D-altered strains cannot be simply explained by an inability to generate the classic IdOx1 sequence.
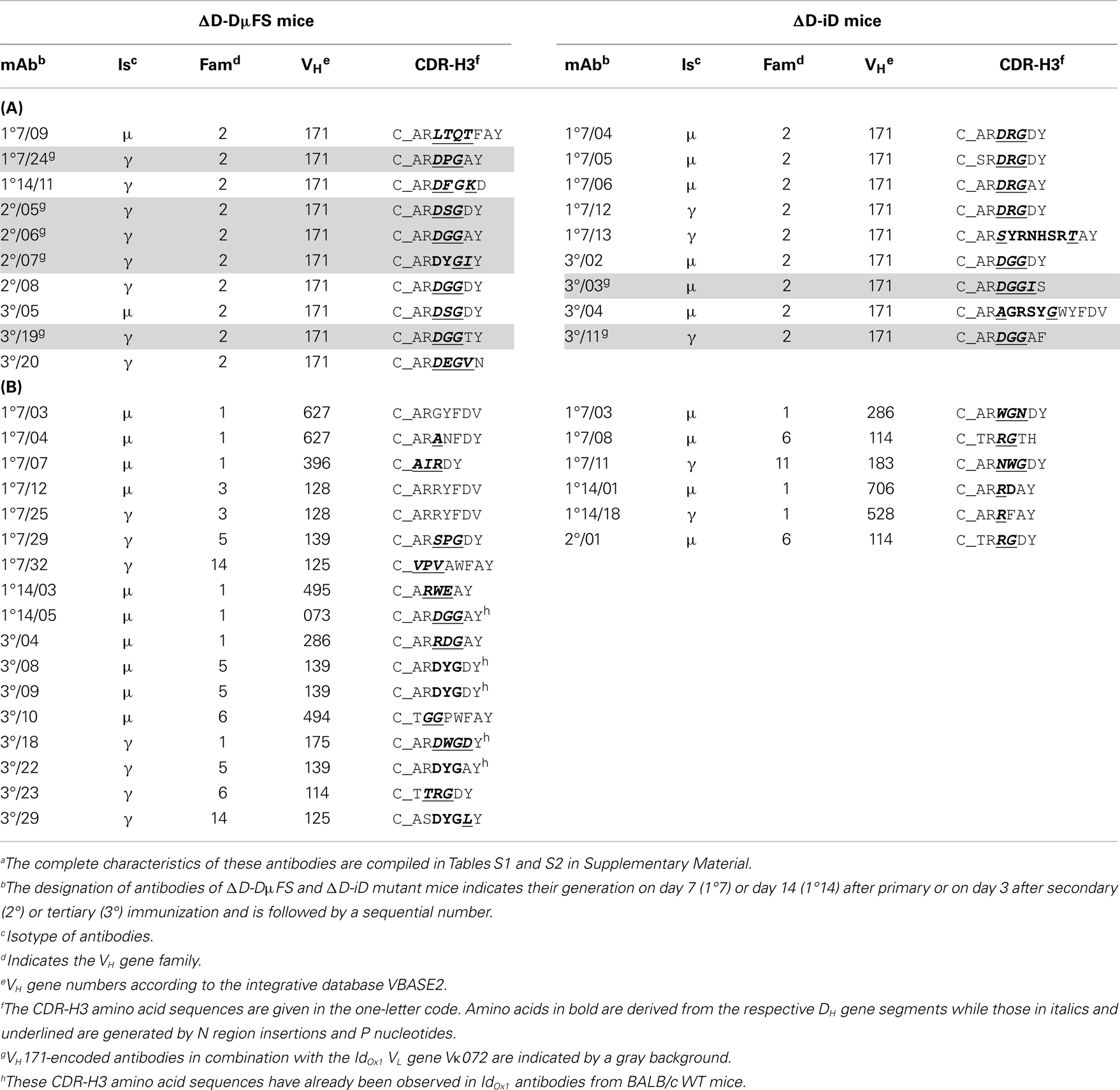
Table 2. CDR-H3 amino acid sequences of (A) VH171- and (B) non-VH171-encoded monoclonal anti-phOx antibodies from D-altered ΔD-DμFS and ΔD-iD mutant micea.
Short CDR-H3 with 6–8 amino acids (i.e., in the range of CDR-H3 of IdOx1 antibodies) were also found in association with VH genes other than VH171. These alternative VH belonged to non-VH2 families (Table 2B). Two of the VH171-encoded antibodies exhibited longer CDR-H3. Thus, the great majority of anti-phOx mAbs produced in these D-altered mice used H chains that differed greatly from the characteristic IdOx1 sequence, even though classic IdOx1-like CDR-H3s could be generated by both D-altered mice.
It has been argued that diversity at CDR-H3 is sufficient for the creation of Ag-specificity and that a particular specificity can be correlated with identical or very similar amino acids sequences in CDR-H3 (16). Our anti-phOx mAb from the two D-limited mouse strains offered the opportunity to check this hypothesis in another experimental system. A survey of all anti-phOx antibodies from ΔD-DμFS and ΔD-iD mice is depicted in Tables S3A,B in Supplementary Material, respectively. From the 83 mAb of ΔD-DμFS mice, 58 (70%) made use of varying lengths of the genomic DH sequence, 2 (2.4%) used inverted sequences, and in 23 (28%) the genomic DH sequence was not detectable. From the 44 mAb of ΔD-iD mice, 23 (52%) used varying lengths of the DH segment, 5 (11%) used variable lengths of the inverted sequence, and in 16 mAb (36%), no portion of the DH segment could be identified. This large variability of CDR-H3-coding sequences led to an equal variability of 0–13 amino acid sequences in CDR-H3 loops. The average hydropathicity values of CDR-H3 loops varied from strongly hydrophobic to strongly hydrophilic (Table 3). Similar findings were obtained in WT mice (data not shown). Thus, since common sequences or sequence motifs in CDR-H3 could not be detected, we conclude that Ag-specificity in this model, immune response is not limited by either this parameter or by other, readily apparent physicochemical properties of the CDR-H3 interval.
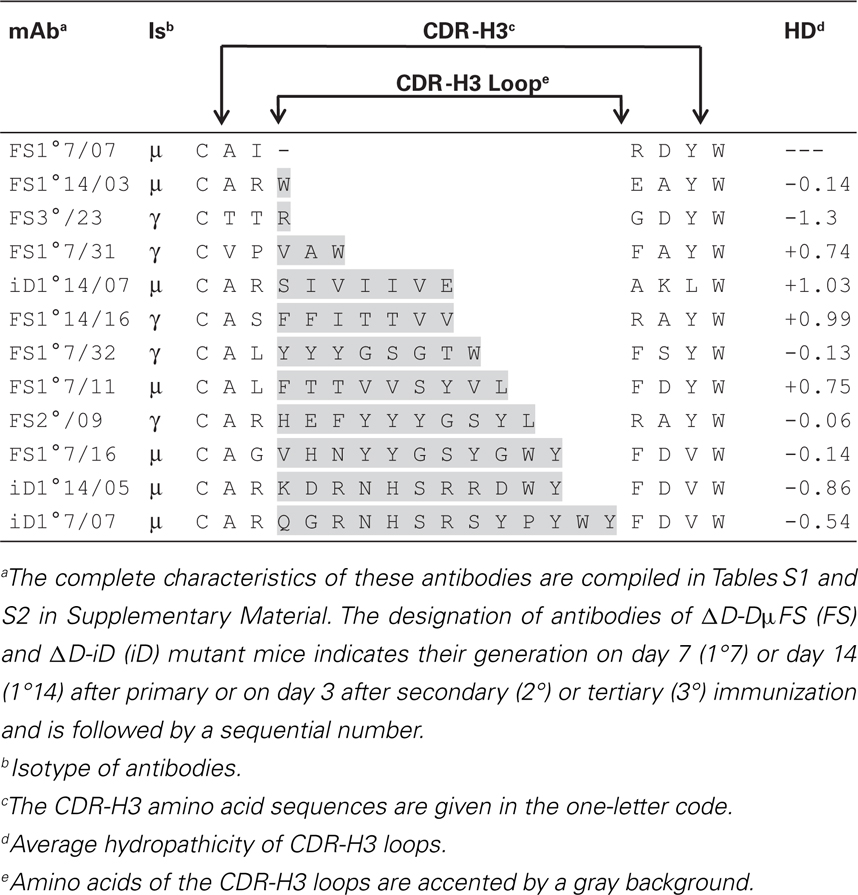
Table 3. Exemplary representation of the spectrum of CDR-H3 lengths and average hydropathicity values of CDR-H3 loops of selected anti-phOx antibodies from D-altered ΔD-DμFS and ΔD-iD mice.
Discussion
In WT BALB/c, the preference for IdOx1 anti-phOx Ab reflects both the failure of B cells producing phOx-binding IgM antibodies that use VH1-family genes to contribute to the IgG Ab repertoire and a focusing of CDR-H3 content (10). While the increase of Ab encoded by non-IdOx1 VH/VL gene combinations from later stages of the response may reflect enhanced Ka (17), the initial dominance of the IdOx1 has been attributed to its superior KD for the hapten phOx (14). This view has been challenged by the finding that the early primary IgM response contains a considerable number of anti-phOx Ab that demonstrate similar or even higher affinities than IdOx1 Ab (10). Because these high affinity IgM progenitors do not have counterparts among IgG Ab (10), these findings have raised questions about a strict attribution of higher affinity for Ag as the primary force behind the clonal selection and CSR exhibited by B cells that express Ig bearing the IdOx1 idiotype.
Attribution of the preference for the IdOx1 idiotype as reflective of higher Ag affinity carries with it the presumption that the choice of specific Ab reflects selection from a random repertoire of antigen binding sites. However, although VDJ rearrangement and N addition yields a tremendously diverse CDR-H3 repertoire, inspection of the actual composition of the antigen binding site repertoire reveals a major bias in CDR-H3 amino acid content. Tyrosine and glycine are preferred, whereas charged and hydrophobic amino acids are under-represented. This non-random pattern of amino acid usage reflects natural selection for a bias in D gene segment sequence by reading frame that is coupled to a bias in reading frame choice (8). Together, these conserved biases yield a greatly restricted repertoire with some types of antigen binding sites represented more frequently than random chance would allow, and others grossly under-represented.
To test the functional significance of constraints introduced by natural selection of the germline antibody repertoire on B cell development and antibody production, we previously altered the DH locus in BALB/c mice to force use of alternative DH sequence (8). Violation of naturally selected germline constraints on CDR-H3 content led to impairments in total serum IgG levels, in tetanus-specific IgG antibody production after tetanus toxoid vaccination, and to the ability to protect against re-infection with influenza virus of a different serotype, termed heterosubtypic immunity. All of these facets of antibody production and protection are T cell dependent.
In the present work, we have gone beyond global measurements of total immunoglobulin production or protection against infection with a pathogen to assess the effects on T dependent antibody production at the individual B cell sequence level in the classic phOx system, whose study yielded many of the paradigms still in common thought to this day.
Here, we report that changes in naturally selected DH sequence have not only yielded a decrease in serum immunoglobulin levels directed against yet another T cell dependent antigen; they have resulted in major changes at the molecular level to the nature of the antibody produced even though the simple addition of only five nucleotides of N addition can yield the classic I IdOx1 irrespective of the sequence of the D. In particular, we observe enhanced and persistent production of hybridomas secreting low affinity IgM after secondary and tertiary challenge, as well as a failure to develop a fully mature, class switched IgG response equivalent to that produced by WT mice.
Classically, anti-phOx hybridomas obtained on day 3 of memory responses predominantly produce IgG mAb (13, 15). Therefore, we anticipated that the D-altered mice would also primarily produce IgG mAb. The consistently high rescue of IgM-producing hybridomas from memory responses was thus unexpected.
Development of a fully mature IgG response in WT mice is marked by clonal expansion of B cells using the VHOx1/VκOx1 gene combination coupled with a focusing of CDR-H3 sequence with preference given to CDR-H3s encoded a short, DRG containing peptide sequence. In the D-altered mice, VH and VL variability persisted (Figure 2), VH1 family-encoded mAb continued to be produced, and no evidence of focusing of CDR-H3 sequence and structure was observed (Figures 2 and 3). This failure to clonally select for the various elements of the IdOx1 idiotype occurred in spite of the fact that both mutant strains were able to generate an anti-phOx antibodies containing both the IdOx1 VH171 H chain/Vκ072 L chain combination in conjunction with a CDR-H3 sequence similar or near identical to the classic idiotype (Table S3a in Supplementary Material).
Together, these findings suggest that the impairment in antibody production that we had observed in previous studies as a result of violation of evolutionarily conserved D gene sequence is accompanied by impairment of hallmarks of affinity maturation, such as clonal expansion and class switching. This occurs even when the classically preferred anti-phOx sequence can not only in theory be generated in the absence of D specific sequence, but is present in practice and thus available for clonal expansion and class switching.
The mechanism(s) that have led to the failure of antigen driven T cell dependent clonal expansion to produce the expected outcome are unclear. Two possible mechanisms involve B cells alone. First, the failure of the IdOx1 idiotype to dominate could reflect the reduced likelihood of creating the IdOx1 CDR-H3 sequence that might result from the loss of the DH gene segments that normally contribute to its generation. However, comparable IdOx1 CDR-H3 sequence was detected in the D-altered mice. Moreover, in WT mice the IdOx1+ Ab begins as a minority of the early primary IgM response. It only dominates with the help of T cells, since it occurs only after class switching (Figure 2). Thus, attributing the change in outcome to an absence or diminution in the initial prevalence of IdOx1 CDR-H3 is not compelling.
Second, the immunological imprinting that normally occurs during ontogeny might be altered as a result of the global change in the CDR-H3 repertoire (18, 19). The importance of controlling the B cell repertoire is underscored by the observation that neonatal injection or maternally derived anti-idiotypic antibodies may induce a drastic distortion of the adult B cell repertoire (20, 21) and maternal anti-idiotypes can even induce a long-lasting transgenerational suppression of IgE responsiveness (22). However, these types of early imprinted responses typically involve B cells expressing specificities directed against antigens encountered early in life. PhOx, however, is a foreign and manufactured antigen.
Alternatively, since the response that we have studied is T dependent, the mechanisms that have led to failure of clonal expansion and class switch recombination (CSR) could also reflect a DH sequence dependent effect on interactions between T cells and B cells. There are several possible mechanisms by which this could occur. First, in the D-altered mice, we have previously observed changes in the distribution of B cell subset numbers and in the repertoire expressed by these B cells (8). It has been suggested that TD Ag-activated IgG+ and IgM+ memory B cells form a whole spectrum of memory B cell populations (23), although this view is not undissented (24). Most memory B cells appear to differentiate as a result of germinal center reactions. However, they can also be generated in a GC-independent manner in the follicles or even outside follicles (25). Many GC-independent follicular memory B cells are of the IgM+-only type (25–27), contain few or no somatic mutations and have not undergone affinity maturation (28–30). These same attributes are found in our memory IgM anti-phOx mAb (Figure 1). It is thus possible that the memory IgM mAb in the D-altred mice derive from a GC-independent pathway.
A second possibility is that the change in the repertoire of D sequence-associated antigen binding sites alters the antigen presentation properties of B cells for the phOx bearing antigen. D-alteration shifts the distribution of antigen binding sites, including enrichment for sites that are normally rare and depletion of sites that are normally common. The production of novel immunoglobulins with high affinity for the hapten could lead to changes in the peptides derived from the carrier protein that are presented to T cells by B cells in their capacity as antigen presenting cells (31). A global altered pattern of epitope recognition by responding T cells could inhibit T cell helper driven clonal expansion and affinity maturation.
A third possible T dependent mechanism could reflect the role of CDR-H3 as a potential T cell epitope, and thus contribute to distortions in T cell–B cell interactions (32–35). For example, the response to the TI-2 antigen α (1–3) dextran can be influenced by CDR-H3-specific T cells, which inhibit CSR of the dominant J558 IgM idiotype (36). And, BCR-specific T cells have been shown to be capable of interrupting an ongoing GC reaction, favoring the differentiation of short-lived extrafollicular plasmablasts (37). This interaction could help explain the enhanced yield of IgM hybridomas that we observed during memory responses in the D-altered mice, as well as explaining the reduced humoral IgM titers at later times of these responses (Figure 1). We would note that none of these potential mechanisms are mutually exclusive. Thus, one or more could be contributing to the failure of this TD response. Studies to clarify the mechanisms by which this has occurred are ongoing in our laboratory.
In this manuscript, we report a test of the hypothesis that conservation of the sequence signature of H chain diversity gene segments, which reflects the effect of natural selection, can influence the outcome of a T dependent response to antigen at the Ab molecular level. We found that subverting the effects of natural selection on the B cell CDR-H3 repertoire led to an alteration of the pattern of T cell-dependent CSR-associated clonal progression in these mutant mice (no dominant idiotypes, no elimination of VH1 family-encoded antibodies, and no selection of homogeneous CDR-H3). Our finding that the sequence of the DH controls this specific TD response suggests that a full understanding of protective and non-protective responses to self or exogenous antigens, including vaccines, pathogens, and self antigens, will likely require clarification of the role of natural constraints on the antigen binding site repertoire. Vaccination strategies may need to be modified in order to take into account the constraints on humoral responses imposed by evolution.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Hybridoma cell lines H11.5 and NQ2/16.2 were kindly supplied by C. Berek, Deutsches Rheuma-Forschungszentrum Berlin. This work was supported by the Deutsche Forschungsgemeinschaft (Le328-7/2 to Hilmar Lemke, Ahmad Trad, and Radu Iulian Tanasa, SFB877 to Ahmad Trad, and SFBTR22 TPA17 to Michael Zemlin) and by the US National Institutes of Health (R01 AI090742, R21 AI088498, and R01 AI48115 to Harry W. Schroeder Jr.).
Supplementary Material
The Supplementary Material for this article can be found online at http://www.frontiersin.org/Journal/10.3389/fimmu.2014.00385/abstract
Abbreviations
CSA, chicken serum albumin; IdOx1, Ox1 idiotype; phOx, 2-phenyloxazolone.
References
1. Padlan EA. Anatomy of the antibody molecule. Mol Immunol (1994) 31(3):169–217. doi: 10.1016/0161-5890(94)90001-9
2. Kabat EA, Wu TT, Perry HM, Gottesman KS, Foeller C. Sequences of Proteins of Immunological Interest. Bethesda, Maryland: U.S. Department of Health and Human Services (1991).
3. Rolink A, Melchers F. Molecular and cellular origins of B lymphocyte diversity. Cell (1991) 66(6):1081–94. doi:10.1016/0092-8674(91)90032-T
4. Berek C, Milstein C. Mutation drift and repertoire shift in the maturation of the immune response. Immunol Rev (1987) 96:23–41. doi:10.1111/j.1600-065X.1987.tb00507.x
5. Lange H, Solterbeck M, Berek C, Lemke H. Correlation between immune maturation and idiotypic network recognition. Eur J Immunol (1996) 26(9):2234–42. doi:10.1002/eji.1830260940
6. Ippolito GC, Schelonka RL, Zemlin M, Ivanov II, Kobayashi R, Zemlin C, et al. Forced usage of positively charged amino acids in immunoglobulin CDR-H3 impairs B cell development and antibody production. J Exp Med (2006) 203(6):1567–78. doi:10.1084/jem.20052217
7. Schelonka RL, Zemlin M, Kobayashi R, Ippolito GC, Zhuang Y, Gartland GL, et al. Preferential use of DH reading frame 2 alters B cell development and antigen-specific antibody production. J Immunol (2008) 181(12):8409–15. doi:10.4049/jimmunol.181.12.8409
8. Schroeder HW Jr, Zemlin M, Khass M, Nguyen HH, Schelonka RL. Genetic control of DH reading frame and its effect on B-cell development and antigen-specifc antibody production. Crit Rev Immunol (2010) 30(4):327–44. doi:10.1615/CritRevImmunol.v30.i4.20
9. Lange H, Zemlin M, Tanasa RI, Trad A, Weiss T, Menning H, et al. Thymus-independent type 2 antigen induces a long-term IgG-related network memory. Mol Immunol (2008) 45(10):2847–60. doi:10.1016/j.molimm.2008.01.020
10. Lange H, Hecht O, Zemlin M, Trad A, Tanasa RI, Schroeder HW Jr., et al. Immunoglobulin class switching appears to be regulated by B-cell antigen receptor-specific T-cell action. Eur J Immunol (2012) 42(4):1016–29. doi:10.1002/eji.201141857
11. Lange H, Kobarg J, Yazynin S, Solterbeck M, Henningsen M, Hansen H, et al. Genetic analysis of the maternally induced affinity enhancement in the non-Ox1 idiotypic antibody repertoire of the primary immune response to 2-phenyloxazolone. Scand J Immunol (1999) 49(1):55–66. doi:10.1046/j.1365-3083.1999.00472.x
12. Schelonka RL, Ivanov II, Jung DH, Ippolito GC, Nitschke L, Zhuang Y, et al. A single DH gene segment creates its own unique CDR-H3 repertoire and is sufficient for B cell development and immune function. J Immunol (2005) 175(10):6624–32. doi:10.4049/jimmunol.175.10.6624
13. Berek C, Griffiths GM, Milstein C. Molecular events during maturation of the immune response to oxazolone. Nature (1985) 316(6027):412–8. doi:10.1038/316412a0
14. Berek C, Ziegner M. The maturation of the immune response. Immunol Today (1993) 14(8):400–4. doi:10.1016/0167-5699(93)90143-9
15. Berek C, Jarvis JM, Milstein C. Activation of memory and virgin B cell clones in hyperimmune animals. Eur J Immunol (1987) 17(8):1121–9. doi:10.1002/eji.1830170808
16. Xu JL, Davis MM. Diversity in the CDR3 region of V(H) is sufficient for most antibody specificities. Immunity (2000) 13(1):37–45. doi:10.1016/S1074-7613(00)00006-6
17. Foote J, Milstein C. Kinetic maturation of an immune response [see comments]. Nature (1991) 352(6335):530–2. doi:10.1038/352530a0
18. Lemke H, Lange H. Is there a maternally induced immunological imprinting phase a la Konrad Lorenz? Scand J Immunol (1999) 50(4):348–54. doi:10.1046/j.1365-3083.1999.00620.x
19. Lemke H, Coutinho A, Lange H. Lamarckian inheritance by somatically acquired maternal IgG phenotypes. Trends Immunol (2004) 25(4):180–6. doi:10.1016/j.it.2004.02.007
20. Vakil M, Sauter H, Paige C, Kearney JF. In vivo suppression of perinatal multispecific B cells results in a distortion of the adult B cell repertoire. Eur J Immunol (1986) 16(9):1159–65. doi:10.1002/eji.1830160921
21. Bernabe RR, Coutinho A, Cazenave PA, Forni L. Suppression of a "recurrent" idiotype results in profound alterations of the whole B-cell compartment. Proc Natl Acad Sci U S A. (1981) 78(10):6416–20. doi:10.1073/pnas.78.10.6416
22. Tanasa RI, Trad A, Lange H, Grotzinger J, Lemke H. Allergen IgE-isotype-specific suppression by maternally derived monoclonal anti-IgG-idiotype. Allergy (2010) 65(1):16–23. doi:10.1111/j.1398-9995.2009.02104.x
23. Tomayko MM, Steinel NC, Anderson SM, Shlomchik MJ. Cutting edge: Hierarchy of maturity of murine memory B cell subsets. J Immunol (2010) 185(12):7146–50. doi:10.4049/jimmunol.1002163
24. Tarlinton D. B-cell memory: are subsets necessary? Nat Rev Immunol (2006) 6(10):785–90. doi:10.1038/nri1938
25. Good-Jacobson KL, Tarlinton DM. Multiple routes to B-cell memory. Int Immunol (2012) 24(7):403–8. doi:10.1093/intimm/dxs050
26. Reynaud CA, Descatoire M, Dogan I, Huetz F, Weller S, Weill JC. IgM memory B cells: a mouse/human paradox. Cell Mol Life Sci (2012) 69(10):1625–34. doi:10.1007/s00018-012-0971-z
27. Taylor JJ, Jenkins MK, Pape KA. Heterogeneity in the differentiation and function of memory B cells. Trends Immunol (2012) 33(12):590–7. doi:10.1016/j.it.2012.07.005
28. Pape KA, Taylor JJ, Maul RW, Gearhart PJ, Jenkins MK. Different B cell populations mediate early and late memory during an endogenous immune response. Science (2011) 331(6021):1203–7. doi:10.1126/science.1201730
29. Dogan I, Bertocci B, Vilmont V, Delbos F, Megret J, Storck S, et al. Multiple layers of B cell memory with different effector functions. Nat Immunol (2009) 10(12):1292–9. doi:10.1038/ni.1814
30. Toyama H, Okada S, Hatano M, Takahashi Y, Takeda N, Ichii H, et al. Memory B cells without somatic hypermutation are generated from Bcl6- deficient B cells. Immunity (2002) 17(3):329–39. doi:10.1016/S1074-7613(02)00387-4
31. Watts C, Lanzavecchia A. Suppressive effect of antibody on processing of T cell epitopes. J Exp Med (1993) 178(4):1459–63. doi:10.1084/jem.178.4.1459
32. Woodland R, Cantor H. Idiotype-specific T helper cells are required to induce idiotype-positive B memory cells to secrete antibody. Eur J Immunol (1978) 8(8):600–6. doi:10.1002/eji.1830080812
33. L’Age-Stehr J. Priming of T helper cells by antigen-activated B cells. B cell-primed Lyt-1+ helper cells are restricted to cooperate with B cells expressing the IgvH phenotype of the priming B cells. J Exp Med (1981) 153(5):1236–45. doi:10.1084/jem.153.5.1236
34. Adorini L, Harvey M, Sercarz EE. The fine specificity of regulatory T cells. IV. Idiotypic complementarity and antigen-bridging interactions in the anti-lysozyme response. Eur J Immunol (1979) 9(11):906–9. doi:10.1002/eji.1830091113
35. Rosenberg YJ, Asofsky R. T cell regulation of isotype expression. The requirement for a second Ig-specific helper T cell population for the induction of IgG responses. Eur J Immunol (1981) 11(9):705–10. doi:10.1002/eji.1830110907
36. Rademaekers A, Specht C, Kolsch E. T-cell enforced invariance of the antibody repertoire in the immune response against a bacterial carbohydrate antigen. Scand J Immunol (2001) 53(3):240–4. doi:10.1046/j.1365-3083.2001.00864.x
Keywords: rodent, B cells, antibodies, class switch recombination, repertoire development
Citation: Trad A, Tanasa RI, Lange H, Zemlin M, Schroeder HW Jr and Lemke H (2014) Clonal progression during the T cell-dependent B cell antibody response depends on the immunoglobulin DH gene segment repertoire. Front. Immunol. 5:385. doi: 10.3389/fimmu.2014.00385
Received: 31 May 2014; Accepted: 28 July 2014;
Published online: 11 August 2014.
Edited by:
Paolo Casali, University of Texas School of Medicine, USAReviewed by:
Shiv Pillai, Harvard Medical School, USAJayanta Chaudhuri, Memorial Sloan Kettering Cancer Center, USA
Copyright: © 2014 Trad, Tanasa, Lange, Zemlin, Schroeder and Lemke. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hilmar Lemke, Biochemical Institute, Christian-Albrechts-University, Rudolf Höberstr. 1, Otto Meyerhof-Haus, Kiel D – 24098, Germany e-mail: hlemke@biochem.uni-kiel.de
†Present address: Radu Iulian Tanasa, Department of Research and Development, National Institute of Research–Development in Microbiology and Immunology “Cantacuzino”, Bucharest, Romania;
Hans Lange, Clinic of Applied Cellular Medicine, University Hospital Schleswig-Holstein, Kiel, Germany
 Ahmad Trad
Ahmad Trad Radu Iulian Tanasa
Radu Iulian Tanasa Hans Lange1†
Hans Lange1† Michael Zemlin
Michael Zemlin Harry W. Schroeder Jr.
Harry W. Schroeder Jr. Hilmar Lemke
Hilmar Lemke