- 1Virology Laboratory, Department of Surgery, Basil Hetzel Institute, University of Adelaide, Adelaide, SA, Australia
- 2Molecular Mucosal Vaccine Immunology Group, The John Curtin School of Medical Research, The Australian National University, Canberra, ACT, Australia
- 3Lung and Allergy Research Centre, Translational Research Institute, UQ School of Medicine, The University of Queensland, Woolloongabba, QLD, Australia
It has been well-established that type I interferons (IFN-Is) have pleiotropic effects and play an early central role in the control of many acute viral infections. However, their pleiotropic effects are not always beneficial to the host and in fact several reports suggest that the induction of IFN-Is exacerbate disease outcomes against some bacterial and chronic viral infections. In this brief review, we probe into this mystery and try to develop answers based on past and recent studies evaluating the roles of IFN-Is in infection and immunity as this is vital for developing effective IFN-Is based therapeutics and vaccines. We also discuss the biological roles of an emerging IFN-I, namely IFN-ε, and discuss its potential use as a mucosal therapeutic and/or vaccine adjuvant. Overall, we anticipate the discussions generated in this review will provide new insights for better exploiting the biological functions of IFN-Is in developing efficacious therapeutics and vaccines in the future.
Introduction
Since the initial discovery of type I interferons (IFN-Is) as anti-viral agents (1), these cytokines have been extensively studied for their anti-microbial and immune regulatory properties. IFN-I family comprises 13 IFN-α subunits, IFN-β, IFN-ω, IFN-ε, IFN-κ, IFN-τ, and IFN-δ (in mice only) (2–8). All IFN-Is signal through the IFN-α receptor (IFN-AR) complex to induce synthesis and secretion of IFN-inducible genes or effector proteins with anti-viral, pro-apoptotic, and ubiquitination-modifying properties (9–11). The signaling pathways that IFN-Is utilize to exert various biological effects have been comprehensively reviewed elsewhere and will not be reviewed here [see Ref. (12)]. Numerous cell types produce IFN-Is (e.g., macrophages, myeloid dendritic cells (DCs), fibroblasts, and epithelial cells), but plasmacytoid DCs (pDCs) appear to be the most prolific producers of IFN-Is (13, 14). The production of these cytokines tends to be beneficial to the host particularly against acute viral infections, but there are considerable evidences to suggest that IFN-Is play detrimental roles in autoimmune diseases (15), bacterial and persistent viral infections. Herein, we review how IFN-Is could play beneficial or detrimental roles in pathogen control predominantly with respect to viral infections and discuss how they could be used as therapeutics and vaccine adjuvants. Furthermore, the importance of considering the emerging IFN-ε in immunity and vaccine development will be discussed.
The Benefits and Detriments of IFN-Is in the Control of Pathogens
The importance of IFN-Is in protecting hosts against pathogens has been demonstrated in several contexts. Firstly, IFN-AR deficient mice tend to be more susceptible to infection with viruses (particularly acute viral infections) compared to wild-type mice. Some examples include Henipavirus (16), acute Friend virus (17), encephalitic flavivirus (18), lymphocytic choriomeningitis virus (LCMV) Armstrong (19), Hazara virus (20), Dengue virus (21), Respiratory Syncytial Virus (22), and numerous other viral infections (23). Secondly, systemic exhaustion of IFN-Is following a primary viral infection has been shown to increase the host susceptibility to secondary unrelated viral infections in mice (24). Thirdly, therapeutic administration of IFN-Is can reduce viral loads in individuals infected with chronic viruses and promote cancer regression (see below Section “The Use of IFN-Is as Therapeutics and Adjuvants”). Finally, pathogens can attenuate IFN-I responses to promote immune evasion. For instance, human immunodeficiency virus (HIV)-1 can reduce the capacity of IFN producing cells to produce IFN-Is (25–27), induce cytopathic effects on these cells (28–32), and/or block IFN-I mediated intracellular signaling events (33) to help establish a chronic phase infection. Similarly, cancer immune evasion and development could also involve attenuation of IFN-I responses. In agreement with this, Critchley-Thorne et al. (34) have shown that various cancer patients have significantly attenuated expression of interferon stimulate genes in lymphocytes compared to healthy controls.
The benefits of IFN-Is in conferring protection against microbes have been mostly demonstrated using acute viral infection models, but several studies suggest that IFN-Is can also assist in the control of bacterial infections. This was first demonstrated in vitro where De la Maza and colleagues (35) showed that IFN-I inhibit Chlamydia trachomatis infectivity of human and mouse cell lines. Several subsequent studies have shown that IFN-I could indeed play important roles for inhibiting various stages of bacterial infections. Some examples include replication of Chlamydophila pneumoniae (36), recruitment of Myobacterium tuberculosis target cells into the lung during early infection (37), and invasion and transmigration of Streptococcus pneumoniae in the lungs (38). However, IFN-Is do not always appear to render beneficial outcomes in anti-bacterial immunity. Several studies have reported that IFN-AR deficient mice are better protected than WT controls following bacterial infections such as Ehrlichia muris (39), Chlamydia muridarum (40), Listeria monocytogenes (41, 42), Myobacterium species (43, 44), and Francisella tularensis (45). Furthermore, induction of IFN-Is following virus infections could make hosts more susceptible to secondary bacterial infections (46–48). The mechanisms as to how IFN-Is exacerbate or make hosts more susceptible to bacterial disease may vary depending on the infection. For instance, IFN-I mediated disease exacerbation has been linked to reduction of interleukin (IL)-17 expressing γδ T cells, increased expression of IL-10 or reduction in cell-mediate immune responses following F. tularensis, M. Leprae, or L. monocytogenes, respectively (42, 44, 45).
Several reports suggest that the detrimental effects of IFN-Is could also support the establishment of persistent viral infections depending on the quantities and duration of IFN-I induction. IFN-Is have been shown to play significant roles in inhibiting various stages (e.g., replication, virus assembly, protein trafficking, and transcription) of HIV-1 life cycle (49–53). However, sustained unlike transient production of IFN-Is resulting from chronic stimulation of pDCs has been proposed to facilitate HIV-1 persistence (54). Similarly following clone 13 LCMV infection transient (within 24 h) hyper-induction of IFN-α and -β has been reported to exacerbate virus pathogenesis and promote viral persistence (19). However, in the same study IFN-Is were crucial for the control of acute Armstrong LCMV infection, which was likely due to lower IFN-I induction following Armstrong compared to clone 13 LCMV infection. In chronic simian immunodeficiency virus (SIV) infection studies, disease free phenotypes of sooty mangabeys have been associated with the abolishment of interferon stimulated gene expression during chronic, but not in acute phase infection (55). Overall, it can be speculated that early, transient yet non-excessive induction of IFN-Is (at least α and β species) are important in the control of acute viral infections. On the contrary, chronic and/or hyper-induction of IFN-Is could provide an environment for enhanced persistence and/or pathogenesis of chronic viral infections.
IFN-Is and Regulation of Adaptive Immunity
Apart from their most celebrated role as direct anti-viral agents, IFN-Is have also been increasingly recognized as potent regulators of cellular immune responses. Of particular interest to vaccine development has been the ability of these cytokines to regulate adaptive immune responses and this aspect is discussed here.
Dendritic cells are often crucial for initiating adaptive immune responses and serve as important targets for IFN-Is to regulate adaptive immunity. Exposure of IFN-Is facilitates maturation of DCs via increasing the expression of DC-associated chemokine receptors, co-stimulatory molecules, and major histocompatibility complex class I and class II antigen presentation (56–60). Consequently, DCs that mature following IFN-I exposure can effectively prime protective T cell responses (61). A caveat here is that IFN-I responses could operate in a threshold dependent manner where excessive responsiveness is inhibitory to the ability of DCs to prime T cell responses. For instance, following LCMV infection higher induction of IFN-Is has been associated with heightened expression of programed death-ligand 1 (PD-L1) on DCs and PD-L1 interaction with programed death 1 (PD-1) on T cells can inhibit T cell activation (19, 62).
IFN-Is could also act directly on lymphocytes to alter adaptive immune outcomes. Naïve B cells up-regulate the expression of activation markers CD69, CD86, and CD25 following IFN-I exposure in vitro (63), but in vivo IFN-Is only up-regulate CD69 and CD86 expression on naïve B and T cells (64). The consequences of up-regulating these activation markers are not clear, but in vitro studies suggest it could serve to reduce the activation thresholds of naïve B cells unlike T cells (63, 65). Alternatively, CD69 expression resulting from IFN-I exposure can down-regulate sphigosine-1 phosphate receptor-1 on naïve lymphocytes to retain these cells in secondary lymphoid organs (66). This retention mechanism could facilitate a more durable interaction between naïve lymphocytes and DCs for efficient lymphocyte activation to occur. IFN-Is have been reported to represent a distinct third signal for naïve T cell activation to occur and prevent the expansion of regulatory T cells that can inhibit T cell activation (67–69). Furthermore, IFN-Is regulate the functions of lymphocytes even after naïve lymphocyte activation or effector/memory differentiation. Some examples of this include IFN-I mediated enhancement in cell division (63, 70), survival (71, 72), interferon-γ secretion (73), cytotoxicity (74), germinal center formation, and antibody isotype switching (75).
Despite the many studies demonstrating that IFN-Is are capable of boosting adaptive immunity; there have also been several studies in bacterial and chronic viral infection settings suggesting that IFN-I signaling leads to IL-10 production (19, 44, 76, 77). IL-10 is thought to be detrimental to the clearance of these pathogens as has been demonstrated with HIV-1 (78). It is likely that IFN-Is up-regulate PD-1 expression (e.g., on regulatory T cells) and PD-L1 (e.g., on DCs) on cells resulting in a milieu where PD-1/PD-L1 interactions occur; this could facilitate IL-10 production and exhaustion of T cell function during chronic viral infections (19, 76–80). A caveat here is that IFN-Is in some instances can also inhibit IL-10 production and IL-10 production can occur independently of IFN-I signaling (76, 81). Furthermore, IFN-Is up-regulate pro-apoptotic molecules such as Bak on T cells to induce apoptosis independently of T cell exhaustion (82).
Overall, IFN-Is play pivotal roles in boosting adaptive immunity, but the switch from becoming a booster to an inhibitor of adaptive immunity may reflect on how much apoptosis, PD-1/PD-L1 interactions and IL-10 signaling are induced on immune cells due to IFN-Is.
The Use of IFN-Is as Therapeutics and Adjuvants
The development of efficient methods to purify IFN-I and subsequent high yield purification of IFN-α2 during the late 1970s paved way for the first IFN-I based human clinical trial in 1986 where IFN-α2 was used for treating hairy cell leukemia (83, 84). Since then the therapeutic use of IFN-Is have shown promising outcomes for treatment of several cancers and viral infections. Therapeutic administration of pegylated IFN-α2 have rendered potent anti-viral and immune enhancing effects against hepatitis B virus infection (85, 86). A recent clinical trial has shown that similar outcomes could be achieved even when pegylated IFN-α2 is administered to HIV-infected patients (87). Systemic administration of IFN-α and/or IFN-β has also been reported to reduce viral growth and clinical manifestations of herpes zoster, herpes simplex virus, and cytomegalovirus (CMV) infections (88–91). Furthermore, systemic or intralesional administration of IFN-α and/or IFN-β has been shown to induce a regression of skin-associated wart infections following papilloma virus infections (92–98). IFN-Is have also been used in synergic regimens where administration of IFN-α2 or -β2 and anti-viral drugs (e.g., ribavirin and faldaprevir) could effectively reduce viral loads of certain hepatitis C virus (HCV) genotypes and is currently the best treatment for HCV-infected patients (99–102). A caveat here is that these regimens have also been reported to cause adverse side-effects (103). Apart from treatment of pathogen infections, IFN-Is especially IFN-α2, have also been used for treatment and regression of various cancers (e.g., leukemia, prostrate cancer, and cervical intraepithelial neoplasia) (104–106).
Studies in pre-clinical models suggest that IFN-Is could also be potent vaccine adjuvants for inducing adaptive immune responses. Some examples include when an influenza vaccine adjuvanted with IFN-α/β administered mucosally induced significantly higher IgG2a and IgA antibody responses and protection compared to non-adjuvanted vaccines (107, 108). Interestingly, the species of IFN-Is used as immune adjuvants could have different immune outcomes in terms of enhancing adaptive immunity. Studies in our laboratory suggest that recombinant pox viral vectors encoding IFN-β compared to those encoding IFN-α4 or IFN-ε significantly enhanced systemic T cell immunity against co-encoded antigens in prime-boost vaccination settings (109). However, Xi et al. (110) using similar prime-boost vaccination settings demonstrated that the use of IFN-ε was much more efficient in inducing T cell immunity in mucosal compartments (e.g., lung and gut) compared to IFN-α4 and IFN-β when used as vaccine adjuvants. Another important consideration here is that the vaccine vectors (i.e., pox viruses) used in our studies are acute attenuated viruses and do not chronically induce IFN-Is as is usually the case with persistent virus infections.
There are several confounding factors that could dictate the use of IFN-I in therapy and as vaccine adjuvants. Firstly, unique biological effects have been reported with different members of the IFN-I family and subtypes of IFN-α. Thus, the choice of IFN-I species (e.g., IFN-α2 or IFN-β) could dictate the success of IFN-I treatment or IFN-I based vaccine formulations. Secondly, members of the IFN-I family have different binding affinities and kinetics to the IFN-AR subunits with current comparative studies suggesting that IFN-β has the highest affinity to IFN-AR and anti-viral capacity (111–113). A caveat with these studies is that not all members of the IFN-I family were compared. Thirdly, IFN-Is can cause numerous adverse side-effects and induce autoimmunity (e.g., lupus, thyroiditis, diabetes, dermatitis, Sjogren’s syndrome, and arthritis) especially in patients with a history of autoimmune manifestations (114). The autoimmune outcomes in these settings are thought to be a combination of tolerogenic immune function failures and IFN-I mediated maturation of DCs that present autoantigens to activate autoreactive T cells and B cells that make autoantibodies (115).
Collectively, IFN-Is have shown considerable promise for the treatment of cancers and pathogen infections (e.g., chronic viruses) in some clinical settings. IFN-Is are also promising for use as vaccine adjuvants, but the species of IFN-Is used for this purpose could have a significant bearing on adaptive immunity generated at certain immune compartments. For instance, IFN-β could be used to effectively enhance systemic T cell immune responses, whereas IFN-ε is more promising as an adjuvant to enhance mucosal T cell immunity in the lung and the gut mucosae.
Importance of IFN-ε in Immunity and Vaccine Development
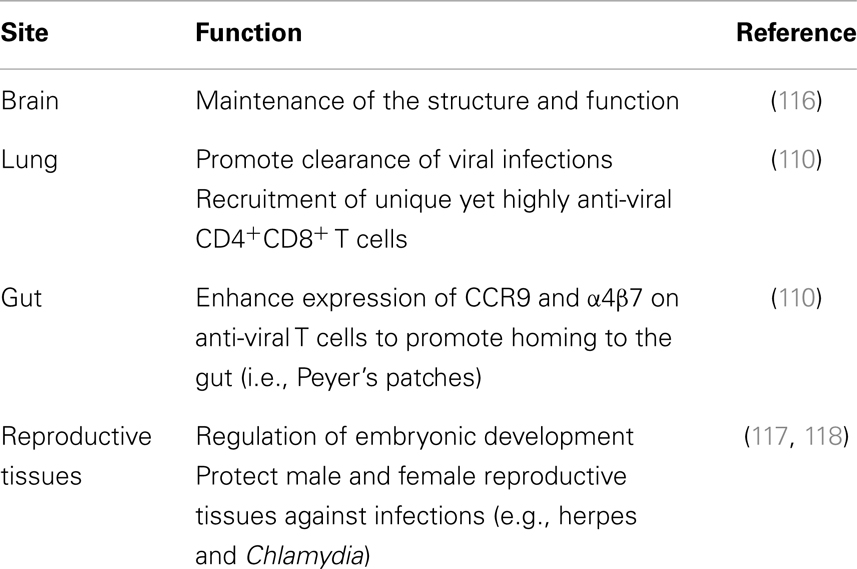
Most studies investigating the roles of IFN-Is have done so mainly analyzing the roles of IFN-α and -β. However, investigating the roles of other IFN-I family members is beneficial for effective therapeutic and vaccine development strategies especially given that higher induction of IFN-α and -β could be detrimental to the host as discussed previously. For this purpose, it is indeed intriguing to evaluate the roles of IFN-ε, which unlike other IFN-Is is constitutively expressed and plays various protective roles in reproductive tissues, gut, lung, and the brain (Table 1). Since our initial studies characterizing the roles of IFN-ε in inducing anti-viral states on cells (109), we have found that this cytokine also possesses potent immune regulatory capacity. Our recent studies indicated that, intranasal immunization of mice with vaccinia virus (VV) encoding murine IFN-ε (VV-HIV-IFN-ε) unlike IFN-α (VV-HIV-IFN-α4) or IFN-β (VV-HIV-IFN-β) could induce rapid clearance of VV in the lung (110). Viral clearance in this instance correlated with several immune outcomes: (i) elevated lung VV-specific CD8+CD107a+IFN-γ+ cell population expressing activation markers CD69/CD103, (ii) enhanced lymphocyte recruitment to lung alveoli with reduced inflammation, and (iii) highly functional CD8+CD4+ double positive T cell subset [CD3highC–C chemokine receptor (CCR)7highCD62Llow] in lung lymph nodes (110). Next when IFN-ε was used in an intranasal/intramuscular heterologous HIV-1 prime-boost vaccination regimen, elevated HIV-specific effector, but not memory CD8+ T cells responses were detected in spleen, genito-rectal nodes, and Peyer’s patches. Furthermore, homing marker α4β7 and CCR9 analysis showed that unlike other IFN-Is, IFN-ε promoted the migration of antigen-specific CD8+ T cells to the gut mucosae (110). These results for the first time established that unlike other IFN-Is, IFN-ε played a unique role at the mucosae. Another recent study has also further substantiated our findings demonstrating that IFN-ε deficient mice were more susceptible to intra-vaginal herpes simplex virus 2 and Chlamydia muridarum infections compared to wild-type mice (117). This suggests that IFN-ε could also be beneficial for the control of certain bacterial infections. A caveat here is that it is unknown whether IFN-ε could cause adverse side-effects in humans as it has not yet been used for treatment or vaccination purposes in humans.
Overall, IFN-ε has great potential to be used as a topical microbicide or a therapeutic to control local lung/gut infections or modulate tissue-specific immunity at sites where pathogens are initially encountered (i.e., mucosal surfaces). Specifically, IFN-ε’s ability to enhance CD8+ T cell homing to the gut [gut is the primary site of HIV virus replication and CD4+ T-cell depletion (119)] and also its ability to control infections at the lung mucosae suggest that administration of pegylated forms of IFN-ε or vaccines encoding IFN-ε could be effective for controlling mucosal pathogens such as HIV-1.
Concluding Remarks
The dual roles of IFN-Is in providing beneficial and detrimental effects to the host in pathogen control is intriguing for developing IFN-I based vaccines and therapies. Lessons learned from acute viral infection models and studies comparing acute versus chronic infection states suggest that transient, but not sustained and/or excessive induction of IFN-Is is likely to confer protective outcomes. IFN-Is have also proven to be promising therapeutic agents against various pathogens and cancers and could also be used as vaccine adjuvants. The caveat here is that the vaccine vector used should ideally not chronically stimulate the production of IFN-Is, which is expected to be detrimental for the generation of robust adaptive immune responses. Our laboratory and others have demonstrated that IFN-ε has great potential to provide protective outcomes against not only mucosal viral infections, but also certain mucosal bacterial infections. Keeping this in mind, more studies need to evaluate the contribution of the different species of IFN-Is not just IFN-α and -β in immunity against infections. These studies are expected to pave way for the development of novel and effective IFN-I based vaccines/therapies against chronic pathogens and cancers.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by Australian National Health and Medical Research Council project grant award 525431 (Charani Ranasinghe) and ACH2 EOI grants (Charani Ranasinghe).
References
1. Isaacs A, Lindenmann J. Virus interference. I. The interferon. Proc R Soc Lond B Biol Sci (1957) 147(927):258–67. doi: 10.1098/rspb.1957.0049
3. Foster GR, Finter NB. Are all type I human interferons equivalent? J Viral Hepat (1998) 5(3):143–52. doi:10.1046/j.1365-2893.1998.00103.x
4. Kawamoto S, Oritani K, Asada H, Takahashi I, Ishikawa J, Yoshida H, et al. Antiviral activity of limitin against encephalomyocarditis virus, herpes simplex virus, and mouse hepatitis virus: diverse requirements by limitin and alpha interferon for interferon regulatory factor 1. J Virol (2003) 77(17):9622–31. doi:10.1128/JVI.77.17.9622-9631.2003
5. Martal JL, Chene NM, Huynh LP, L’Haridon RM, Reinaud PB, Guillomot MW, et al. IFN-tau: a novel subtype I IFN1. Structural characteristics, non-ubiquitous expression, structure-function relationships, a pregnancy hormonal embryonic signal and cross-species therapeutic potentialities. Biochimie (1998) 80(8–9):755–77. doi:10.1016/S0300-9084(99)80029-7
6. LaFleur DW, Nardelli B, Tsareva T, Mather D, Feng P, Semenuk M, et al. Interferon-kappa, a novel type I interferon expressed in human keratinocytes. J Biol Chem (2001) 276(43):39765–71. doi:10.1074/jbc.M102502200
7. Oritani K, Medina KL, Tomiyama Y, Ishikawa J, Okajima Y, Ogawa M, et al. Limitin: an interferon-like cytokine that preferentially influences B-lymphocyte precursors. Nat Med (2000) 6(6):659–66. doi:10.1038/76233
8. Pestka S, Langer JA, Zoon KC, Samuel CE. Interferons and their actions. Annu Rev Biochem (1987) 56:727–77. doi:10.1146/annurev.bi.56.070187.003455
9. Stark GR, Kerr IM, Williams BR, Silverman RH, Schreiber RD. How cells respond to interferons. Annu Rev Biochem (1998) 67:227–64. doi:10.1146/annurev.biochem.67.1.227
10. Sen GC. Viruses and interferons. Annu Rev Microbiol (2001) 55:255–81. doi:10.1146/annurev.micro.55.1.255
11. Karpov AV. Endogenous and exogenous interferons in HIV-infection. Eur J Med Res (2001) 6(12):507–24.
12. Ivashkiv LB, Donlin LT. Regulation of type I interferon responses. Nat Rev Immunol (2014) 14(1):36–49. doi:10.1038/nri3581
13. Siegal FP, Kadowaki N, Shodell M, Fitzgerald-Bocarsly PA, Shah K, Ho S, et al. The nature of the principal type 1 interferon-producing cells in human blood. Science (1999) 284(5421):1835–7. doi:10.1126/science.284.5421.1835
14. Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nat Immunol (2004) 5(12):1219–26. doi:10.1038/ni1141
15. Hall JC, Rosen A. Type I interferons: crucial participants in disease amplification in autoimmunity. Nat Rev Rheumatol (2010) 6(1):40–9. doi:10.1038/nrrheum.2009.237
16. Dhondt KP, Mathieu C, Chalons M, Reynaud JM, Vallve A, Raoul H, et al. Type I interferon signaling protects mice from lethal henipavirus infection. J Infect Dis (2013) 207(1):142–51. doi:10.1093/infdis/jis653
17. Gerlach N, Schimmer S, Weiss S, Kalinke U, Dittmer U. Effects of type I interferons on Friend retrovirus infection. J Virol (2006) 80(7):3438–44. doi:10.1128/JVI.80.7.3438-3444.2006
18. Lobigs M, Mullbacher A, Wang Y, Pavy M, Lee E. Role of type I and type II interferon responses in recovery from infection with an encephalitic flavivirus. J Gen Virol (2003) 84(Pt 3):567–72. doi:10.1099/vir.0.18654-0
19. Teijaro JR, Ng C, Lee AM, Sullivan BM, Sheehan KC, Welch M, et al. Persistent LCMV infection is controlled by blockade of type I interferon signaling. Science (2013) 340(6129):207–11. doi:10.1126/science.1235214
20. Dowall SD, Findlay-Wilson S, Rayner E, Pearson G, Pickersgill J, Rule A, et al. Hazara virus infection is lethal for adult type I interferon receptor-knockout mice and may act as a surrogate for infection with the human-pathogenic Crimean-Congo hemorrhagic fever virus. J Gen Virol (2012) 93(Pt 3):560–4. doi:10.1099/vir.0.038455-0
21. Zust R, Toh YX, Valdes I, Cerny D, Heinrich J, Hermida L, et al. Type I IFN signals in macrophages and dendritic cells control dengue virus infection: implications for a new mouse model to test dengue vaccines. J Virol (2014) 88(13):7276–85. doi:10.1128/JVI.03827-13
22. Goritzka M, Durant LR, Pereira C, Salek-Ardakani S, Openshaw PJ, Johansson C. Interferon-α/β receptor signaling amplifies early pro-inflammatory 2 cytokine production in the lung during Respiratory Syncytial Virus 3 infection. J Virol (2014) 88(11):6128–36. doi:10.1128/JVI.00333-14
23. Carrero JA. Confounding roles for type I interferons during bacterial and viral pathogenesis. Int Immunol (2013) 25(12):663–9. doi:10.1093/intimm/dxt050
24. Alsharifi M, Regner M, Blanden R, Lobigs M, Lee E, Koskinen A, et al. Exhaustion of type I interferon response following an acute viral infection. J Immunol (2006) 177(5):3235–41. doi:10.4049/jimmunol.177.5.3235
25. Kamga I, Kahi S, Develioglu L, Lichtner M, Maranon C, Deveau C, et al. Type I interferon production is profoundly and transiently impaired in primary HIV-1 infection. J Infect Dis (2005) 192(2):303–10. doi:10.1086/430931
26. Martinelli E, Cicala C, Van Ryk D, Goode DJ, Macleod K, Arthos J, et al. HIV-1 gp120 inhibits TLR9-mediated activation and IFN-{alpha} secretion in plasmacytoid dendritic cells. Proc Natl Acad Sci U S A (2007) 104(9):3396–401. doi:10.1073/pnas.0611353104
27. Rasaiyaah J, Tan CP, Fletcher AJ, Price AJ, Blondeau C, Hilditch L, et al. HIV-1 evades innate immune recognition through specific cofactor recruitment. Nature (2013) 503(7476):402–5. doi:10.1038/nature12769
28. Fitzgerald-Bocarsly P, Jacobs ES. Plasmacytoid dendritic cells in HIV infection: striking a delicate balance. J Leukoc Biol (2010) 87(4):609–20. doi:10.1189/jlb.0909635
29. Soumelis V, Scott I, Gheyas F, Bouhour D, Cozon G, Cotte L, et al. Depletion of circulating natural type 1 interferon-producing cells in HIV-infected AIDS patients. Blood (2001) 98(4):906–12. doi:10.1182/blood.V98.4.906
30. Pacanowski J, Kahi S, Baillet M, Lebon P, Deveau C, Goujard C, et al. Reduced blood CD123+ (lymphoid) and CD11c+ (myeloid) dendritic cell numbers in primary HIV-1 infection. Blood (2001) 98(10):3016–21. doi:10.1182/blood.V98.10.3016
31. Schmidt B, Scott I, Whitmore RG, Foster H, Fujimura S, Schmitz J, et al. Low-level HIV infection of plasmacytoid dendritic cells: onset of cytopathic effects and cell death after PDC maturation. Virology (2004) 329(2):280–8. doi:10.1016/j.virol.2004.08.016
32. Bruel T, Dupuy S, Demoulins T, Rogez-Kreuz C, Dutrieux J, Corneau A, et al. Plasmacytoid dendritic cell dynamics tune interferon-alfa production in SIV-infected cynomolgus macaques. PLoS Pathog (2014) 10(1):e1003915. doi:10.1371/journal.ppat.1003915
33. Guha D, Ayyavoo V. Innate immune evasion strategies by human immunodeficiency virus type 1. ISRN AIDS (2013) 2013:954806. doi:10.1155/2013/954806
34. Critchley-Thorne RJ, Simons DL, Yan N, Miyahira AK, Dirbas FM, Johnson DL, et al. Impaired interferon signaling is a common immune defect in human cancer. Proc Natl Acad Sci U S A (2009) 106(22):9010–5. doi:10.1073/pnas.0901329106
35. de la Maza LM, Peterson EM, Goebel JM, Fennie CW, Czarniecki CW. Interferon-induced inhibition of Chlamydia trachomatis: dissociation from antiviral and antiproliferative effects. Infect Immun (1985) 47:719–22.
36. Buss C, Opitz B, Hocke AC, Lippmann J, van Laak V, Hippenstiel S, et al. Essential role of mitochondrial antiviral signaling, IFN regulatory factor (IRF)3, and IRF7 in Chlamydophila pneumoniae-mediated IFN-beta response and control of bacterial replication in human endothelial cells. J Immunol (2010) 184:3072–8. doi:10.4049/jimmunol.0902947
37. Desvignes L, Wolf AJ, Ernst JD. Dynamic roles of type I and type II IFNs in early infection with Mycobacterium tuberculosis. J Immunol (2012) 188:6205–15. doi:10.4049/jimmunol.1200255
38. LeMessurier KS, Hacker H, Chi L, Tuomanen E, Redecke V. Type I interferon protects against pneumococcal invasive disease by inhibiting bacterial transmigration across the lung. PLoS Pathog (2013) 9:e1003727. doi:10.1371/journal.ppat.1003727
39. Zhang Y, Thai V, McCabe A, Jones M, MacNamara KC. Type I interferons promote severe disease in a mouse model of lethal ehrlichiosis. Infect Immun (2014) 82(4):1698–709. doi:10.1128/IAI.01564-13
40. Nagarajan UM, Prantner D, Sikes JD, Andrews CW Jr., Goodwin AM, Nagarajan S, et al. Type I interferon signaling exacerbates Chlamydia muridarum genital infection in a murine model. Infect Immun (2008) 76(10):4642–8. doi:10.1128/IAI.00629-08
41. Auerbuch V, Brockstedt DG, Meyer-Morse N, O’Riordan M, Portnoy DA. Mice lacking the type I interferon receptor are resistant to Listeria monocytogenes. J Exp Med (2004) 200(4):527–33. doi:10.1084/jem.20040976
42. Archer KA, Durack J, Portnoy DA. STING-dependent type I IFN production inhibits cell-mediated immunity to Listeria monocytogenes. PLoS Pathog (2014) 10(1):e1003861. doi:10.1371/journal.ppat.1003861
43. Manca C, Tsenova L, Freeman S, Barczak AK, Tovey M, Murray PJ, et al. Hypervirulent M. tuberculosis W/Beijing strains upregulate type I IFNs and increase expression of negative regulators of the Jak-Stat pathway. J Interferon Cytokine Res (2005) 25(11):694–701. doi:10.1089/jir.2005.25.694
44. Teles RM, Graeber TG, Krutzik SR, Montoya D, Schenk M, Lee DJ, et al. Type I interferon suppresses type II interferon-triggered human anti-mycobacterial responses. Science (2013) 339(6126):1448–53. doi:10.1126/science.1233665
45. Henry T, Kirimanjeswara GS, Ruby T, Jones JW, Peng K, Perret M, et al. Type I IFN signaling constrains IL-17A/F secretion by gammadelta T cells during bacterial infections. J Immunol (2010) 184(7):3755–67. doi:10.4049/jimmunol.0902065
46. Navarini AA, Recher M, Lang KS, Georgiev P, Meury S, Bergthaler A, et al. Increased susceptibility to bacterial superinfection as a consequence of innate antiviral responses. Proc Natl Acad Sci U S A (2006) 103(42):15535–9. doi:10.1073/pnas.0607325103
47. Li W, Moltedo B, Moran TM. Type I interferon induction during influenza virus infection increases susceptibility to secondary Streptococcus pneumoniae infection by negative regulation of gammadelta T cells. J Virol (2012) 86(22):12304–12. doi:10.1128/JVI.01269-12
48. Trinchieri G. Type I interferon: friend or foe? J Exp Med (2010) 207(10):2053–63. doi:10.1084/jem.20101664
49. Barr SD, Smiley JR, Bushman FD. The interferon response inhibits HIV particle production by induction of TRIM22. PLoS Pathog (2008) 4(2):e1000007. doi:10.1371/journal.ppat.1000007
50. Sakuma R, Noser JA, Ohmine S, Ikeda Y. Rhesus monkey TRIM5alpha restricts HIV-1 production through rapid degradation of viral Gag polyproteins. Nat Med (2007) 13(5):631–5. doi:10.1038/nm1562
51. Sokolskaja E, Luban J. Cyclophilin, TRIM5, and innate immunity to HIV-1. Curr Opin Microbiol (2006) 9(4):404–8. doi:10.1016/j.mib.2006.06.011
52. Nisole S, Stoye JP, Saib A. TRIM family proteins: retroviral restriction and antiviral defence. Nat Rev Microbiol (2005) 3(10):799–808. doi:10.1038/nrmicro1248
53. Okumura A, Lu G, Pitha-Rowe I, Pitha PM. Innate antiviral response targets HIV-1 release by the induction of ubiquitin-like protein ISG15. Proc Natl Acad Sci U S A (2006) 103(5):1440–5. doi:10.1073/pnas.0510518103
54. Boasso A, Royle CM, Doumazos S, Aquino VN, Biasin M, Piacentini L, et al. Overactivation of plasmacytoid dendritic cells inhibits antiviral T-cell responses: a model for HIV immunopathogenesis. Blood (2011) 118(19):5152–62. doi:10.1182/blood-2011-03-344218
55. Bosinger SE, Li Q, Gordon SN, Klatt NR, Duan L, Xu L, et al. Global genomic analysis reveals rapid control of a robust innate response in SIV-infected sooty mangabeys. J Clin Invest (2009) 119(12):3556–72. doi:10.1172/JCI40115
56. Cella M, Salio M, Sakakibara Y, Langen H, Julkunen I, Lanzavecchia A. Maturation, activation, and protection of dendritic cells induced by double-stranded RNA. J Exp Med (1999) 189(5):821–9. doi:10.1084/jem.189.5.821
57. Parlato S, Santini SM, Lapenta C, Di Pucchio T, Logozzi M, Spada M, et al. Expression of CCR-7, MIP-3beta, and Th-1 chemokines in type I IFN-induced monocyte-derived dendritic cells: importance for the rapid acquisition of potent migratory and functional activities. Blood (2001) 98(10):3022–9. doi:10.1182/blood.V98.10.3022
58. Montoya M, Schiavoni G, Mattei F, Gresser I, Belardelli F, Borrow P, et al. Type I interferons produced by dendritic cells promote their phenotypic and functional activation. Blood (2002) 99(9):3263–71. doi:10.1182/blood.V99.9.3263
59. Welsh RM, Bahl K, Marshall HD, Urban SL. Type 1 interferons and antiviral CD8 T-cell responses. PLoS Pathog (2012) 8(1):e1002352. doi:10.1371/journal.ppat.1002352
60. Simmons DP, Wearsch PA, Canaday DH, Meyerson HJ, Liu YC, Wang Y, et al. Type I IFN drives a distinctive dendritic cell maturation phenotype that allows continued class II MHC synthesis and antigen processing. J Immunol (2012) 188(7):3116–26. doi:10.4049/jimmunol.1101313
61. Diamond MS, Kinder M, Matsushita H, Mashayekhi M, Dunn GP, Archambault JM, et al. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J Exp Med (2011) 208(10):1989–2003. doi:10.1084/jem.20101158
62. Cai G, Karni A, Oliveira EM, Weiner HL, Hafler DA, Freeman GJ. PD-1 ligands, negative regulators for activation of naive, memory, and recently activated human CD4+ T cells. Cell Immunol (2004) 230(2):89–98. doi:10.1016/j.cellimm.2004.09.004
63. Braun D, Caramalho I, Demengeot J. IFN-alpha/beta enhances BCR-dependent B cell responses. Int Immunol (2002) 14(4):411–9. doi:10.1093/intimm/14.4.411
64. Alsharifi M, Lobigs M, Regner M, Lee E, Koskinen A, Mullbacher A. Type I interferons trigger systemic, partial lymphocyte activation in response to viral infection. J Immunol (2005) 175(7):4635–40. doi:10.4049/jimmunol.175.7.4635
65. Wijesundara DK, Kumar S, Alsharifi M, Mullbacher A, Regner M. Antigen-specific activation thresholds of CD8+ T cells are independent of IFN-I-mediated partial lymphocyte activation. Int Immunol (2010) 22(9):757–67. doi:10.1093/intimm/dxq064
66. Shiow LR, Rosen DB, Brdickova N, Xu Y, An J, Lanier LL, et al. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature (2006) 440(7083):540–4. doi:10.1038/nature04606
67. Curtsinger JM, Schmidt CS, Mondino A, Lins DC, Kedl RM, Jenkins MK, et al. Inflammatory cytokines provide a third signal for activation of naive CD4+ and CD8+ T cells. J Immunol (1999) 162(6):3256–62.
68. Curtsinger JM, Gerner MY, Lins DC, Mescher MF. Signal 3 availability limits the CD8 T cell response to a solid tumor. J Immunol (2007) 178(11):6752–60. doi:10.4049/jimmunol.178.11.6752
69. Srivastava S, Koch MA, Pepper M, Campbell DJ. Type I interferons directly inhibit regulatory T cells to allow optimal antiviral T cell responses during acute LCMV infection. J Exp Med (2014) 211(5):961–74. doi:10.1084/jem.20131556
70. Starbeck-Miller GR, Xue HH, Harty JT. IL-12 and type I interferon prolong the division of activated CD8 T cells by maintaining high-affinity IL-2 signaling in vivo. J Exp Med (2014) 211(1):105–20. doi:10.1084/jem.20130901
71. Marrack P, Kappler J, Mitchell T. Type I interferons keep activated T cells alive. J Exp Med (1999) 189(3):521–30. doi:10.1084/jem.189.3.521
72. Kolumam GA, Thomas S, Thompson LJ, Sprent J, Murali-Krishna K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J Exp Med (2005) 202(5):637–50. doi:10.1084/jem.20050821
73. Brinkmann V, Geiger T, Alkan S, Heusser CH. Interferon alpha increases the frequency of interferon gamma-producing human CD4+ T cells. J Exp Med (1993) 178(5):1655–63. doi:10.1084/jem.178.5.1655
74. Kohlmeier JE, Cookenham T, Roberts AD, Miller SC, Woodland DL. Type I interferons regulate cytolytic activity of memory CD8(+) T cells in the lung airways during respiratory virus challenge. Immunity (2010) 33(1):96–105. doi:10.1016/j.immuni.2010.06.016
75. Le Bon A, Thompson C, Kamphuis E, Durand V, Rossmann C, Kalinke U, et al. Cutting edge: enhancement of antibody responses through direct stimulation of B and T cells by type I IFN. J Immunol (2006) 176(4):2074–8. doi:10.4049/jimmunol.176.8.4682
76. Zhang L, Yuan S, Cheng G, Guo B. Type I IFN promotes IL-10 production from T cells to suppress Th17 cells and Th17-associated autoimmune inflammation. PLoS One (2011) 6(12):e28432. doi:10.1371/journal.pone.0028432
77. Stewart CA, Metheny H, Iida N, Smith L, Hanson M, Steinhagen F, et al. Interferon-dependent IL-10 production by Tregs limits tumor Th17 inflammation. J Clin Invest (2013) 123(11):4859–74. doi:10.1172/JCI65180
78. Said EA, Dupuy FP, Trautmann L, Zhang Y, Shi Y, El-Far M, et al. Programmed death-1-induced interleukin-10 production by monocytes impairs CD4+ T cell activation during HIV infection. Nat Med (2010) 16(4):452–9. doi:10.1038/nm.2106
79. Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature (2006) 439(7077):682–7. doi:10.1038/nature04444
80. Terawaki S, Chikuma S, Shibayama S, Hayashi T, Yoshida T, Okazaki T, et al. IFN-alpha directly promotes programmed cell death-1 transcription and limits the duration of T cell-mediated immunity. J Immunol (2011) 186(5):2772–9. doi:10.4049/jimmunol.1003208
81. Lin L, Hou J, Ma F, Wang P, Liu X, Li N, et al. Type I IFN inhibits innate IL-10 production in macrophages through histone deacetylase 11 by downregulating microRNA-145. J Immunol (2013) 191(7):3896–904. doi:10.4049/jimmunol.1203450
82. Fraietta JA, Mueller YM, Yang G, Boesteanu AC, Gracias DT, Do DH, et al. Type I interferon upregulates Bak and contributes to T cell loss during human immunodeficiency virus (HIV) infection. PLoS Pathog (2013) 9(10):e1003658. doi:10.1371/journal.ppat.1003658
83. Rubinstein M, Rubinstein S, Familletti PC, Gross MS, Miller RS, Waldman AA, et al. Human leukocyte interferon purified to homogeneity. Science (1978) 202:1289–90. doi:10.1126/science.725605
84. Pestka S. The interferons: 50 years after their discovery, there is much more to learn. J Biol Chem (2007) 282:20047–51. doi:10.1074/jbc.R700004200
85. Lau GK, Piratvisuth T, Luo KX, Marcellin P, Thongsawat S, Cooksley G, et al. Peginterferon Alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med (2005) 352(26):2682–95. doi:10.1056/NEJMoa043470
86. Marcellin P, Lau GK, Bonino F, Farci P, Hadziyannis S, Jin R, et al. Peginterferon alfa-2a alone, lamivudine alone, and the two in combination in patients with HBeAg-negative chronic hepatitis B. N Engl J Med (2004) 351(12):1206–17. doi:10.1056/NEJMoa040431
87. Azzoni L, Foulkes AS, Papasavvas E, Mexas AM, Lynn KM, Mounzer K, et al. Pegylated Interferon alfa-2a monotherapy results in suppression of HIV type 1 replication and decreased cell-associated HIV DNA integration. J Infect Dis (2013) 207(2):213–22. doi:10.1093/infdis/jis663
88. Jones BR, Coster DJ, Falcon MG, Cantell K. Topical therapy of ulcerative herpetic keratitis with human interferon. Lancet (1976) 2(7977):128. doi:10.1016/S0140-6736(76)92850-6
89. Lui SF, Ali AA, Grundy JE, Fernando ON, Griffiths PD, Sweny P. Double-blind, placebo-controlled trial of human lymphoblastoid interferon prophylaxis of cytomegalovirus infection in renal transplant recipients. Nephrol Dial Transplant (1992) 7(12):1230–7.
90. Merigan TC, Rand KH, Pollard RB, Abdallah PS, Jordan GW, Fried RP. Human leukocyte interferon for the treatment of herpes zoster in patients with cancer. N Engl J Med (1978) 298(18):981–7. doi:10.1056/NEJM197805042981801
91. Pazin GJ, Armstrong JA, Lam MT, Tarr GC, Jannetta PJ, Ho M. Prevention of reactivated herpes simplex infection by human leukocyte interferon after operation on the trigeminal root. N Engl J Med (1979) 301(5):225–30. doi:10.1056/NEJM197908023010501
92. Deunas L, Alcantud V, Alvarez F, Arteaga J, Benitez A, Bopuza M, et al. Use of interferon-alpha in laryngeal papillomatosis: eight years of the Cuban national programme. J Laryngol Otol (1997) 111(2):134–40. doi:10.1017/S0022215100136667
93. Eron LJ, Judson F, Tucker S, Prawer S, Mills J, Murphy K, et al. Interferon therapy for condylomata acuminata. N Engl J Med (1986) 315(17):1059–64. doi:10.1056/NEJM198610233151704
94. Haglund S, Lundquist PG, Cantell K, Strander H. Interferon therapy in juvenile laryngeal papillomatosis. Arch Otolaryngol (1981) 107(6):327–32. doi:10.1001/archotol.1981.00790420001001
95. Healy GB, Gelber RD, Trowbridge AL, Grundfast KM, Ruben RJ, Price KN. Treatment of recurrent respiratory papillomatosis with human leukocyte interferon. Results of a multicenter randomized clinical trial. N Engl J Med (1988) 319(7):401–7. doi:10.1056/NEJM198808183190704
96. Lace MJ, Anson JR, Klingelhutz AJ, Harada H, Taniguchi T, Bossler AD, et al. Interferon-beta treatment increases human papillomavirus early gene transcription and viral plasmid genome replication by activating interferon regulatory factor (IRF)-1. Carcinogenesis (2009) 30(8):1336–44. doi:10.1093/carcin/bgp150
97. Pazin GJ, Ho M, Haverkos HW, Armstrong JA, Breinig MC, Wechsler HL, et al. Effects of interferon-alpha on human warts. J Interferon Res (1982) 2(2):235–43. doi:10.1089/jir.1982.2.235
98. Weck PK, Buddin DA, Whisnant JK. Interferons in the treatment of genital human papillomavirus infections. Am J Med (1988) 85(2A):159–64.
99. McHutchison JG, Gordon SC, Schiff ER, Shiffman ML, Lee WM, Rustgi VK, et al. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. Hepatitis Interventional Therapy Group. N Engl J Med (1998) 339(21):1485–92. doi:10.1056/NEJM199811193392101
100. Poynard T, Marcellin P, Lee SS, Niederau C, Minuk GS, Ideo G, et al. Randomised trial of interferon alpha2b plus ribavirin for 48 weeks or for 24 weeks versus interferon alpha2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. International Hepatitis Interventional Therapy Group (IHIT). Lancet (1998) 352(9138):1426–32. doi:10.1016/S0140-6736(98)07124-4
101. Baker DE. Pegylated interferon plus ribavirin for the treatment of chronic hepatitis C. Rev Gastroenterol Disord (2003) 3(2):93–109.
102. Sulkowski MS, Asselah T, Lalezari J, Ferenci P, Fainboim H, Leggett B, et al. Faldaprevir combined with pegylated interferon alfa-2a and ribavirin in treatment-naive patients with chronic genotype 1 HCV: SILEN-C1 trial. Hepatology (2013) 57(6):2143–54. doi:10.1002/hep.26276
103. Takagi H, Hoshino T, Naganuma A, Koitabashi E, Uehara S, Sakamoto N, et al. Drug induced hypersensitivity syndrome by triple therapy of peginterferon alpha2b, ribavirin and telaprevir in patient with double positive for HBV and HCV. Hepatogastroenterology (2013) 60(127):1557–60.
104. Bonifazi F, de Vivo A, Rosti G, Guilhot F, Guilhot J, Trabacchi E, et al. Chronic myeloid leukemia and interferon-alpha: a study of complete cytogenetic responders. Blood (2001) 98(10):3074–81. doi:10.1182/blood.V98.10.3074
105. Li YF, Wang QZ, Zhang TT, Li L, Wang JP, Ding GF, et al. Low dose of interferon-alpha improves the clinical outcomes of docetaxel in patients with castration-resistant prostate cancer: a pilot study. Oncol Lett (2014) 7(1):125–30. doi:10.3892/ol.2013.1653
106. Machado FA, Abdalla DR, Montes L, Etchebehere RM, Michelin MA, Murta EF. An evaluation of immune system cell infiltrate in the cervical stroma of patients with grade III cervical intraepithelial neoplasia after treatment with intralesional alpha-2B interferon. Eur J Gynaecol Oncol (2014) 35(1):20–5.
107. Bracci L, Canini I, Venditti M, Spada M, Puzelli S, Donatelli I, et al. Type I IFN as a vaccine adjuvant for both systemic and mucosal vaccination against influenza virus. Vaccine (2006) 24(Suppl 2):S2–56. doi:10.1016/j.vaccine.2005.01.121
108. Proietti E, Bracci L, Puzelli S, Di Pucchio T, Sestili P, De Vincenzi E, et al. Type I IFN as a natural adjuvant for a protective immune response: lessons from the influenza vaccine model. J Immunol (2002) 169(1):375–83. doi:10.4049/jimmunol.169.1.375
109. Day SL, Ramshaw IA, Ramsay AJ, Ranasinghe C. Differential effects of the type I interferons alpha4, beta, and epsilon on antiviral activity and vaccine efficacy. J Immunol (2008) 180(11):7158–66. doi:10.4049/jimmunol.180.11.7158
110. Xi Y, Day SL, Jackson RJ, Ranasinghe C. Role of novel type I interferon epsilon in viral infection and mucosal immunity. Mucosal Immunol (2012) 5(6):610–22. doi:10.1038/mi.2012.35
111. Roisman LC, Piehler J, Trosset JY, Scheraga HA, Schreiber G. Structure of the interferon-receptor complex determined by distance constraints from double-mutant cycles and flexible docking. Proc Natl Acad Sci U S A (2001) 98:13231–6. doi:10.1073/pnas.221290398
112. Lamken P, Lata S, Gavutis M, Piehler J. Ligand-induced assembling of the type I interferon receptor on supported lipid bilayers. J Mol Biol (2004) 341:303–18. doi:10.1016/j.jmb.2004.05.059
113. Jaks E, Gavutis M, Uze G, Martal J, Piehler J. Differential receptor subunit affinities of type I interferons govern differential signal activation. J Mol Biol (2007) 366:525–39. doi:10.1016/j.jmb.2006.11.053
114. Banchereau J, Pascual V. Type I interferon in systemic lupus erythematosus and other autoimmune diseases. Immunity (2006) 25:383–92. doi:10.1016/j.immuni.2006.08.010
115. Ronnblom L. The type I interferon system in the etiopathogenesis of autoimmune diseases. Ups J Med Sci (2011) 116:227–37. doi:10.3109/03009734.2011.624649
116. Peng G, Lei KJ, Jin W, Greenwell-Wild T, Wahl SM. Induction of APOBEC3 family proteins, a defensive maneuver underlying interferon-induced anti-HIV-1 activity. J Exp Med (2006) 203(1):41–6. doi:10.1084/jem.20051512
117. Fung KY, Mangan NE, Cumming H, Horvat JC, Mayall JR, Stifter SA, et al. Interferon-epsilon protects the female reproductive tract from viral and bacterial infection. Science (2013) 339(6123):1088–92. doi:10.1126/science.1233321
118. Hermant P, Francius C, Clotman F, Michiels T. IFN-epsilon is constitutively expressed by cells of the reproductive tract and is inefficiently secreted by fibroblasts and cell lines. PLoS One (2013) 8(8):e71320. doi:10.1371/journal.pone.0071320
Keywords: type I interferons, human immunodeficiency virus, IFN-ε, vaccine adjuvants, interferon immunity
Citation: Wijesundara DK, Xi Y and Ranasinghe C (2014) Unraveling the convoluted biological roles of type I interferons in infection and immunity: a way forward for therapeutics and vaccine design. Front. Immunol. 5:412. doi: 10.3389/fimmu.2014.00412
Received: 30 May 2014; Accepted: 13 August 2014;
Published online: 29 August 2014.
Edited by:
Christine Anne Biron, Brown University, USACopyright: © 2014 Wijesundara, Xi and Ranasinghe. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Danushka Kumara Wijesundara, Virology Laboratory, Department of Surgery, Basil Hetzel Institute, 37a Woodville Road, Woodville, SA 5011, Australia e-mail: danushka.wijesundara@adelaide.edu.au
†Danushka Kumara Wijesundara and Yang Xi have contributed equally to this work.
 Danushka Kumara Wijesundara
Danushka Kumara Wijesundara Yang Xi
Yang Xi Charani Ranasinghe
Charani Ranasinghe