- 1Laboratory of Immune Regulation, WPI-Immunology Frontier Research Center, Osaka University, Suita, Japan
- 2National Key Laboratory of Gene Technology, Institute of Biotechnology, Vietnam Academy of Science and Technology, Hanoi, Vietnam
- 3Department of RNA Biology and Neuroscience, Graduate School of Medicine Osaka University, Suita, Japan
Aryl hydrocarbon receptor (AHR) is thought to be a crucial factor in the regulation of immune responses. Many AHR-mediated immunoregulatory mechanisms have been discovered, and this knowledge may enhance our understanding of the molecular pathogenesis of autoimmune inflammatory syndromes such as collagen-induced arthritis, experimental autoimmune encephalomyelitis, and experimental colitis. Recent findings have elucidated the critical link between AHR and indoleamine 2,3-dioxygenase (IDO) in the development of regulatory T cells and Th17 cells, which are key factors in a variety of human autoimmune diseases. Induction of IDO and IDO-mediated tryptophan catabolism, together with its downstream products such as kynurenine, is an important immunoregulatory mechanism underlying immunosuppression, tolerance, and immunity. Recent studies revealed that induction of IDO depends on AHR expression. This review summarizes the most current findings regarding the functions of AHR and IDO in immune cells as they relate to the pathogenesis of autoimmune diseases in response to various stimuli. We also discuss the potential link between AHR and IDO/tryptophan metabolites, and the involvement of several novel related factors (such as microRNA) in the development of autoimmune diseases. These novel factors represent potential therapeutic targets for the treatment of autoimmune disorders.
Roles of AHR in the Immune System
Aryl hydrocarbon receptor (AHR) is a ligand-activated member of the Per–Arnt–Sim (PAS) family of basic helix–loop–helix (HLH) transcription factors. AHR mediates cellular responses to toxins or its ligands, including TCDD, 6-formylindolo[3,2-b]carbazole (FICZ), kynurenine, and 2-(1′H-indole-3′-carbonyl)-thiazole-4-carboxylic acid methyl ester (ITE) (1–4). AHR forms an active complex in the cytoplasm with chaperone proteins such as heat shock protein 90 (HSP90), AHR-interacting protein (AIP), and p23 (5–7). Once bound to its ligands, the AHR complex translocates to the nucleus and binds AHR nuclear translocator (Arnt). The resultant AHR–Arnt heterodimers bind specific motifs, called dioxin-responsive elements (DREs), in the promoter region of target genes. These targets, the so-called AHR battery genes, include CYP1A1, CYP1A2, CYP1B1, and other members of cytochrome P450 family (8–11). Several pathways are involved in the regulation of AHR, including proteasomal degradation of AHR, ligand metabolism by CYP1A1, and formation of the AHR–Arnt complex (12, 13). One of these pathways involves an inhibitory peptide. Mimura et al. isolated a cDNA clone that encode a polypeptide with high similarity to the sequence of the bHLH/PAS of AHR (14). This polypeptide can repress the transcriptional activity of AHR by competing with AHR for binding to Arnt and by binding to the enhancer sequence XRE, upstream of the AHRR gene; therefore, this peptide is designated AHR repressor or AHRR. Expression of AHRR is induced by the AHR/Arnt heterodimer through binding to XRE, resulting in feedback inhibition of AHR. In addition, several transcription factors can interact and regulate AHR signaling; these include STAT-1, STAT3, STAT5, Pai-2, Sp1, c-maf, and Bach2 in certain cell types (15–24). AHR is activated in many immune cell types, including T cells, B cells, NK cells, macrophages, and dendritic cells (DCs), as well as in epithelial cells, Langerhans cells, innate lymphoid cells, intraepithelial lymphocytes, and microglia (15, 16, 20, 21, 25–40). Depending on the presence of specific ligands, AHR activation may suppress or exacerbate experimental autoimmune diseases. For examples, TCDD and ITE can suppress experimental autoimmune encephalomyelitis (EAE), a model of multiple sclerosis (MS), whereas FICZ exacerbates disease development (17, 41–43). Differences in the stability and structure of these ligands, as well as their affinity for AHR, should be taken into account when considering their mode of action in the activation of AHR. In addition, AHR seems to be regulated by unraveled factors such as transcription factors, tryptophan metabolites, feedback regulation of the cytokine network, and microRNA (miR). Below, we will discuss in detail the factors that may interact with AHR to modulate immune responses.
IDO and Tryptophan Metabolites
Indoleamine 2,3-dioxygenase (IDO) is the rate-limiting enzyme in extrahepatic catabolism of the essential amino acid tryptophan via the kynurenine pathway (44). IDO is constitutively expressed in placenta, but is also highly expressed in epididymis, gut, lymph nodes, spleen, thymus, and lung (45). IDO converts tryptophan into kynurenine and other downstream metabolites, some with neuroactive properties, such as kynurenic acid, 3-hydroxykynurenine, quinolinic acid (QUIN), and serotonin. Deficiency or overexpression of IDO can result in changes in the levels of these neuroactive IDO-mediated metabolites, ultimately leading to neuronal disorders. For instance, elevated levels of QUIN, a potently neurotoxic N-methyl-D-aspartate (NMDA) receptor agonist, can cause not only neurodegenerative conditions such as Huntington’s disease (46) and Parkinson’s disease (47), but also infections of the central nervous system and psychiatric diseases such as depression (48). On the other hand, in pregnant mice, IDO may play a role in preventing the rejection of allogeneic fetuses (49). In addition, blocking IDO activity with the competitive inhibitor 1-methyl tryptophan (1-MT) selectively disrupts the maintenance of pregnancy in mice (49, 50). Consistent with the observations that IDO induces tolerance in allogeneic fetuses, IDO expression, especially in DCs, suppresses the T cell response (49, 51, 52). Various mediators can induce IDO expression and activity, including IFN-γ, TNF-α, IL-1β, and IL-6 (53–58). Recent work showed that the induction of IDO depends on AHR (31, 59, 60). Furthermore, kynurenine has been identified as a potent AHR agonist (2, 61, 62).
Immunoregulatory IDO activity and tryptophan metabolites participate in the regulation of many cell types, including T cells, DCs, monocytes, macrophages, and microglia, that play specific roles in immune responses and regulate the development of immune-mediated inflammatory diseases (62–66). Alberati-Giani et al. showed that IFN-γ induced the expression of IDO activity in immortalized murine macrophages (MT2) and microglial (N11) cells. IFN-γ-treated MT2 cells, but not N11 cells, were able to produce detectable amounts of radiolabeled 3-hydroxyanthranilic and QUIN from L-[5-3H] tryptophan. In addition, Heyes et al. demonstrated that increased activities of IDO, kynurenine-3-hydroxylase, and kynureninase in infiltrating primary macrophages may accelerate the synthesis of QUIN, L-kynurenine, and kynurenic acid in conditions of brain inflammation (48). Taken together, these findings suggested that infiltrating macrophages may contribute high amount of cerebral QUIN in brain inflammation (48, 67). Besides, IDO has been demonstrated to be constitutively expressed in DCs (68). Particularly, CD8α+DC exhibited high functional activity of IDO while CD8− fraction of DC did not exhibit significant enzyme activity (68). Moreover, it has been shown that the expression of IDO makes CD8+DC treated with IFN-γ capable of affecting apoptosis of T helper type 1 (Th1) cells (68, 69). Fallarino et al. also found that tryptophan metabolites such as 3-hydroxyanthranilic and QUIN induce the selective apoptosis in vitro of murine Th1 cells at relatively low concentrations of kynurenines (70). Together, IDO and kynurenines metabolites are able to be induced in various cell types under different stimuli regulating many immune responses. Modulation of IDO activity and/or kynurenine pathway may develop therapeutics for inflammatory diseases. In addition to IDO, tryptophan 2,3-dioxygenase (TDO) originated from liver and neuron, is also an important rate-limiting enzyme in the tryptophan metabolism. TDO plays a pivotal role in tumor development and various diseases in the brain (61, 71–73).
The transcriptional regulation of IDO is mediated by two main pathways, IFN-γ-dependent and -independent, which involve transcription factors such as NF-κB, STAT-1, and IRF-1 (74). We will discuss the translational regulation of IDO by AHR in the following section.
Translational Regulation of IDO
Although the regulatory roles of IDO in tryptophan metabolism in immune regulation have been extensively studied, the mechanisms by which IDO is controlled at the pre-, co-, and post-translational levels are poorly understood. Fujigaki et al. demonstrated that IDO activity is regulated by post-translational modification: specifically, IDO activity is inhibited by peroxynitrite due to the nitration of tyrosine resides (Tyr15, Tyr345, and Tyr353) (75). The same group also demonstrated that an N-terminal alanine of IDO is acetylated in IFN-γ-stimulated THP-1 cells, but the biological significance of this modification has not been fully investigated (76, 77). In addition, another group found that Tyr115 and Tyr253 in mouse IDO can be phosphorylated, and these phosphorylations are required for formation of the IDO/SOCS3 complex (78). This complex is ubiquitinated and subsequent proteasomally degraded in DCs. These studies indicate that post-translational modifications such as nitration and phosphorylation of tyrosine in IDO may affect IDO/kynurenine-mediated immune regulation. Recently, we showed that the miR-132/212 cluster participates in AHR-dependent generation of Th17 cells (79). miRs, 20–22-nucleotide non-coding RNAs, are a new class of regulators of gene expression at the posttranscriptional level. miRs binding to the 3′UTR of target mRNAs, leading to translational inhibition and/or degradation of the targets (80). Although numerous immunoregulatory genes are controlled by miRs, to date no miRs targeting IDO have been identified. According to a widely used computational miR target prediction tool, microRNA.org, several miRs potentially regulate IDO mRNA (http://www.microrna.org/microrna/home.do). For example, miR-203 has a putative binding site in the 3′UTR of IDO in mouse. Interestingly, this miR is induced by AHR ligands such as TCDD and BaP, and it negatively affects AHR expression in human cancer cell lines (81). In addition, it has been reported that miR-203 in macrophage RAW264.7 cells negatively regulates LPS-induced IL-6 and TNF-α by targeting MyD88 (82). Considering that AHR inhibits pro-inflammatory cytokines production such as IL-6 and TNF-α in LPS-stimulated macrophages as similarly to miR-203, miR-203 may also be involved in AHR-mediated regulation of inflammatory responses in macrophages. Although experimental verification is necessary in order to confirm this possible relationship between miR and IDO mRNA, identification of miR-mediated regulation of the IDO pathway may shed light on novel mechanisms of immune regulation.
AHR/IDO Axis in Pathogenesis in Autoimmune Disease
For several decades, AHR has been studied as an important transcription factor involved in regulation of a large superfamily of genes encoding cytochrome p450 proteins, which are xenobiotic metabolizing enzymes. Two independent groups demonstrated that AHR controls the generation of regulatory T (Treg) cells and/or IL-17-producing T helper (Th17) cells in EAE, a mouse model of MS (17, 41). Because the balance between Treg and Th17 cells is now considered to be more important than the Th1/Th2 balance in regard to the onset of autoimmunity, AHR has attracted increased attention in the context of immunology. To investigate the pathophysiological roles of AHR in autoimmunity, several groups have studied mouse models of autoimmune disease, such as colitis and rheumatoid arthritis, using AHR-KO mice and/or AHR ligands (83–85). Meanwhile, the roles of AHR in various immune cells such as DCs and macrophages have also been investigated (16, 25, 60). In DCs, AHR positively regulates IDO expression and subsequent kynurenine production (60). In addition, Kimura et al. observed reduced phosphorylation of STAT-1 in AHR-deficient macrophages; by contrast, AHR acts as a negative regulator of STAT-1 activation in T cells under Th17-polarizing conditions (15, 16). Thus, the effect of AHR on STAT-1 status is complex, and may be cell type- or stimulus-dependent. Given that expression of IDO is predominantly controlled by the IFN-γ/STAT-1 axis, AHR may positively control STAT-1 activation and subsequent IDO expression in DCs. More importantly, kynurenine is an agonist of AHR, and may participate in a positive-feedback loop in AHR signaling. In both plasma and CSF from MS patients, tryptophan levels were significantly reduced (86). In addition, IFN-β treatment, a first-line immunomodulatory treatment for MS, causes elevation of IDO mRNA and plasma or serum kynurenine (87, 88). As well as the observation in MS patients, EAE induction leads to alteration of the ratio of kynurenine and tryptophan or IDO expression in brain and spinal cord (89, 90). On the other hand, in spite of evidence that IDO is induced by IFN-γ, IDO expression is negatively correlated with IFN-γ mRNA levels in brain and spinal cord. This observation suggests that IDO activity inhibits the generation of IFN-γ-producing Th1 cells, a primary subset of pathogenic T cells (89). Consistent with these findings, deletion of IDO in mice and treatment with the IDO inhibitor 1-MT result in exacerbated disease symptoms in association with elevated levels of Th1 cells (89–91). We previously showed that LPS- or CpG-stimulated AHR-deficient BMDCs co-cultured with naïve T cells contain a smaller proportion of Treg cells and elevated levels of Th1 and Th17 (60). Furthermore, the aberrant generation of each Th-cell subset can be rescued by kynurenine. These observations indicate that kynurenine generates a tolerogenic condition by controlling not only Th1, but also Th17 and Treg cells. In addition, it has been reported that AHR participates in type 1 regulatory T (Tr1) cell generation in vitro and in vivo (18, 92, 93). Thus, we cannot exclude the possibility that not only TCDD and FICZ, but also kynurenine regulates Tr1 cell induction. Taken together, further investigation of the roles of kynurenine in T cell differentiation may reveal potential therapeutic strategies for MS. Thus, further investigation of the roles of kynurenine in T cell differentiation may reveal potential therapeutic strategies for MS. Immune regulation through the AHR/IDO axis is summarized in Figure 1.
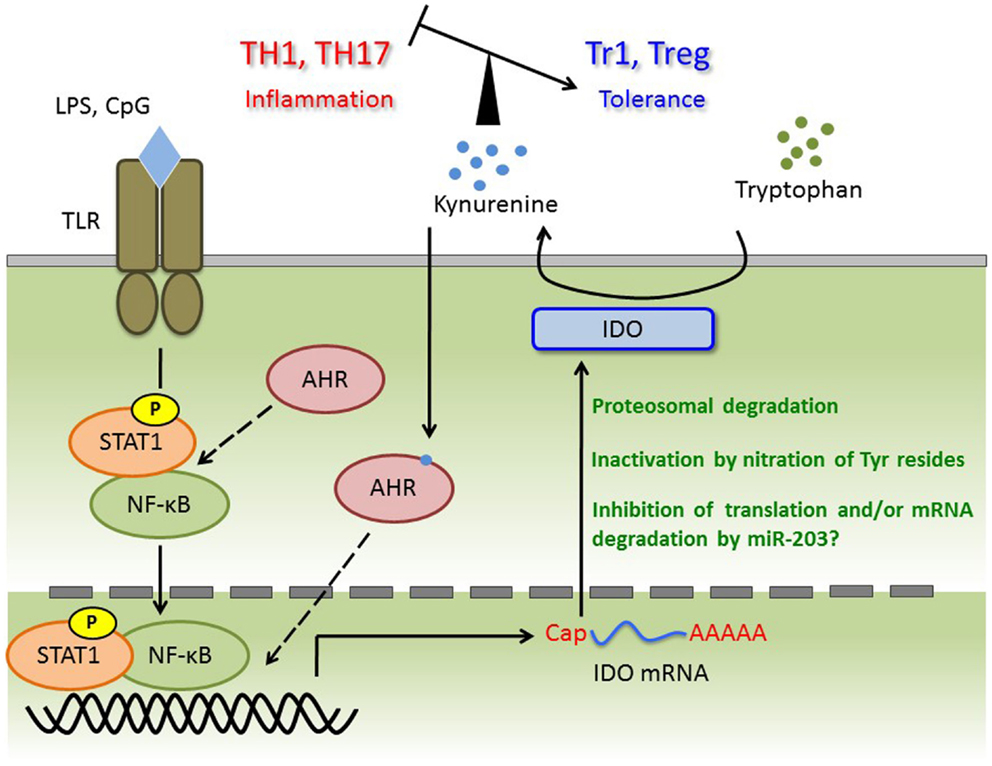
Figure 1. TLR ligands trigger transcriptional activation of STAT-1 and NF-κB, and then induce IDO mRNA. Although AHR forms complex with STAT-1 and NF-κB in macrophages under pro-inflammatory cytokines production, whether this complex is appeared in DC or required for IDO expression is not known. Induced IDO mRNA may be controlled by miR-203 (not investigated), and the activity or amount of IDO protein is regulated at post-translational modification such as nitration of Tyr and ubiquitin ligation. Kynurenine catalyzed by IDO induces tolerance via regulating the balance of TH1, TH17, Tr1, and Treg. Kynurenine may activate the AHR for IDO induction with autocrine manner, and form AHR/Kynurenine positive-feedback loop.
Perspectives of AHR/IDO Axis: From Bench to Bedside
Aryl hydrocarbon receptor and tryptophan metabolites participate in experimental models of autoimmune diseases (4, 17, 26, 41, 42, 61, 85, 94–96). However, the possible role of AHR in dioxin-exposed people is still unknown, particularly in autoimmune disorders. Therefore, it will be interesting to examine the expression of AHR and AHR-related genes in dioxin-affected people with the aim of identifying the potential link between AHR induction and dioxin-related autoimmune diseases. Future investigations should focus on determining whether activation of AHR leads to stimulation of IDO expression, and consequently promotes production of tryptophan metabolites such as kynurenine, which potentially mediate the neurological disorders and/or autoimmune diseases in dioxin-exposed people. Promising therapeutics based on intervention in the AHR/IDO axis may help to improve the health outcomes of dioxin exposure.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by the Kishimoto Foundation and Project CSK14-02 (for Nam Trung Nguyen) from the Institute of Biotechnology, Vietnam Academy of Science and Technology.
References
1. Nguyen LP, Bradfield CA. The search for endogenous activators of the aryl hydrocarbon receptor. Chem Res Toxicol (2008) 21:102–16. doi: 10.1021/tx7001965
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
2. DiNatale BC, Murray IA, Schroeder JC, Flaveny CA, Lahoti TS, Laurenzana EM, et al. Kynurenic acid is a potent endogenous aryl hydrocarbon receptor ligand that synergistically induces interleukin-6 in the presence of inflammatory signaling. Toxicol Sci (2010) 115:89–97. doi:10.1093/toxsci/kfq024
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
3. Kerkvliet NI. TCDD: an environmental immunotoxicant reveals a novel pathway of immunoregulation – a 30-year odyssey. Toxicol Pathol (2012) 40:138–42. doi:10.1177/0192623311427710
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
4. Opitz CA, Wick W, Steinman L, Platten M. Tryptophan degradation in autoimmune diseases. Cell Mol Life Sci (2007) 64:2542–63. doi:10.1007/s00018-007-7140-9
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
5. Perdew GH. Association of the Ah receptor with the 90-kDa heat shock protein. J Biochem (1988) 263:13802–5.
6. Kazlauskas A, Poellinger L, Pongratz I. Evidence that the co-chaperone p23 regulates ligand responsiveness of the dioxin (aryl hydrocarbon) receptor. J Biochem (1999) 274:13519–24.
7. Ma Q, Whitlock JP Jr. A novel cytoplasmic protein that interacts with the Ah receptor, contains tetratricopeptide repeat motifs, and augments the transcriptional response to 2,3,7,8-tetrachlorodibenzo-p-dioxin. J Biochem (1997) 272:8878–84.
8. Sogawa K, Fujii-Kuriyama Y. Ah receptor, a novel ligand-activated transcription factor. J Biochem (1997) 122:1075–9. doi:10.1093/oxfordjournals.jbchem.a021864
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
9. Burbach KM, Poland A, Bradfield CA. Cloning of the Ah-receptor cDNA reveals a distinctive ligand-activated transcription factor. Proc Natl Acad Sci USA (1992) 89:8185–9. doi:10.1073/pnas.89.17.8185
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
10. Schrenk D. Impact of dioxin-type induction of drug-metabolizing enzymes on the metabolism of endo- and xenobiotics. Biochem Pharmacol (1998) 55:1155–62.
11. Tomita S, Jiang HB, Ueno T, Takagi S, Tohi K, Maekawa S, et al. T cell-specific disruption of arylhydrocarbon receptor nuclear translocator (Arnt) gene causes resistance to 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced thymic involution. J Immunol (2003) 171:4113–20. doi:10.4049/jimmunol.171.8.4113
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
12. Esser C, Rannug A, Stockinger B. The aryl hydrocarbon receptor in immunity. Trends Immunol (2009) 30:447–54. doi:10.1016/j.it.2009.06.005
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
13. Stockinger B, Di Meglio P, Gialitakis M, Duarte JH. The aryl hydrocarbon receptor: multitasking in the immune system. Annu Rev Immunol (2014) 32:403–32. doi:10.1146/annurev-immunol-032713-120245
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
14. Mimura J, Ema M, Sogawa K, Fujii-Kuriyama Y. Identification of a novel mechanism of regulation of Ah (dioxin) receptor function. Genes Dev (1998) 13:20–5. doi:10.1101/gad.13.1.20
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
15. Kimura A, Naka T, Nohara K, Fujii-Kuriyama Y, Kishimoto T. Aryl hydrocarbon receptor regulates Stat1 activation and participates in the development of Th17 cells. Proc Natl Acad Sci USA (2008) 105:9721–6. doi:10.1073/pnas.0804231105
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
16. Kimura A, Naka T, Nakahama T, Chinen I, Masuda K, Nohara K, et al. Aryl hydrocarbon receptor in combination with Stat1 regulates LPS-induced inflammatory responses. J Exp Med (2009) 206:2027–35. doi:10.1084/jem.20090560
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
17. Veldhoen M, Hirota K, Christensen J, O’Garra A, Stockinger B. The aryl hydrocarbon receptor links Th17-cell-mediated autoimmunity to environmental toxins. Nature (2008) 453:106–9. doi:10.1038/nature06881
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
18. Apetoh L, Quintana FJ, Pot C, Joller N, Xiao S, Kumar D, et al. The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat Immunol (2010) 11:854–61. doi:10.1038/ni.1912
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
19. Cui G, Qin X, Wu L, Zhang Y, Sheng X, Yu Q, et al. Liver X receptor (LXR) mediates negative regulation of mouse and human Th17 differentiation. J Clin Invest (2011) 121:658–70. doi:10.1172/JCI42974
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
20. Masuda K, Kimura A, Hanieh H, Nguyen NT, Nakahama T, Chinen I, et al. Aryl hydrocarbon receptor negatively regulates LPS-induced IL-6 production through suppression of histamine production in macrophages. Int Immunol (2011) 23:637–45. doi:10.1093/intimm/dxr072
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
21. Monteiro P, Gilot D, Le Ferrec E, Lecureur V, N’diaye M, Le Vee M, et al. AhR- and c-maf-dependent induction of beta7-integrin expression in human macrophages in response to environmental polycyclic aromatic hydrocarbons. Biochem Biophys Res Commun (2007) 358:442–8. doi:10.1016/j.bbrc.2007.04.111
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
22. De Abrew KN, Phadnis AS, Crawford RB, Kaminski NE, Thomas RS. Regulation of Bach2 by the aryl hydrocarbon receptor as a mechanism for suppression of B-cell differentiation by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Appl Pharmacol (2011) 252:150–8. doi:10.1016/j.taap.2011.01.020
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
23. Nakajima K, Maekawa Y, Kataoka K, Ishifune C, Nishida J, Arimochi H, et al. The ARNT-STAT3 axis regulates the differentiation of intestinal intraepithelial TCRαβ?CD8αα? cells. Nat Commun (2013) 4:2112. doi:10.1038/ncomms3112
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
24. Litzenburger UM, Opitz CA, Sahm F, Rauschenbach KJ, Trump S, Winter M, et al. Constitutive IDO expression in human cancer is sustained by an autocrine signaling loop involving IL-6, STAT3 and the AHR. Oncotarget (2014) 5:1038–51.
25. Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol (2010) 185:3190–8. doi:10.4049/jimmunol.0903670
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
26. Monteleone I, Rizzo A, Sarra M, Sica G, Sileri P, Biancone L, et al. Aryl hydrocarbon receptor-induced signals up-regulate IL-22 production and inhibit inflammation in the gastrointestinal tract. Gastroenterology (2011) 141:237–48. doi:10.1053/j.gastro.2011.04.007
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
27. Sekine H, Mimura J, Oshima M, Okawa H, Kanno J, Igarashi K, et al. Hypersensitivity of aryl hydrocarbon receptor-deficient mice to lipopolysaccharide-induced septic shock. Mol Cell Biol (2009) 29:6391–400. doi:10.1128/MCB.00337-09
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
28. Matsunawa M, Amano Y, Endo K, Uno S, Sakaki T, Yamada S, et al. The aryl hydrocarbon receptor activator benzo[a]pyrene enhances vitamin D3 catabolism in macrophages. Toxicol Sci (2009) 109:50–8. doi:10.1093/toxsci/kfp044
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
29. Wu D, Li W, Lok P, Matsumura F, Vogel CF. AhR deficiency impairs expression of LPS-induced inflammatory genes in mice. Biochem Biophys Res Commun (2011) 410:358–66. doi:10.1016/j.bbrc.2011.06.018
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
30. Thurmond TS, Staples JE, Silverstone AE, Gasiewicz TA. The aryl hydrocarbon receptor has a role in the in vivo maturation of murine bone marrow B lymphocytes and their response to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Appl Pharmacol (2000) 165:227–36. doi:10.1006/taap.2000.8942
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
31. Jux B, Kadow S, Esser C. Langerhans cell maturation and contact hypersensitivity are impaired in aryl hydrocarbon receptor-null mice. J Immunol (2009) 182:6709–17. doi:10.4049/jimmunol.0713344
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
32. Kadow S, Jux B, Zahner SP, Wingerath B, Chmill S, Clausen BE, et al. Aryl hydrocarbon receptor is critical for homeostasis of invariant gammadelta T cells in the murine epidermis. J Immunol (2011) 187:3104–10. doi:10.4049/jimmunol.1100912
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
33. Lee JS, Cella M, McDonald KG, Garlanda C, Kennedy GD, Nukaya M, et al. AHR drives the development of gut ILC22 cells and postnatal lymphoid tissues via pathways dependent on and independent of Notch. Nat Immunol (2011) 13:144–51. doi:10.1038/ni.2187
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
34. Lee JS, Cella M, Colonna M. AHR and the transcriptional regulation of type-17/22 ILC. Front Immunol (2012) 3:1. doi:10.3389/fimmu.2012.00010
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
35. Kiss EA, Diefenbach A. Role of the aryl hydrocarbon receptor in controlling maintenance and functional programs of RORγt(+) innate lymphoid cells and intraepithelial lymphocytes. Front Immunol (2012) 3:124. doi:10.3389/fimmu.2012.00124
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
36. Qiu J, Heller JJ, Guo X. The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells. Immunity (2012) 36:92–104. doi:10.1016/j.immuni.2011.11.011
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
37. Li Y, Innocentin S, Withers DR, Roberts NA, Gallagher AR, Grigorieva EF, et al. Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell (2011) 147:629–40. doi:10.1016/j.cell.2011.09.025
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
38. Stevens EA, Mezrich JD, Bradfield CA. The aryl hydrocarbon receptor: a perspective on potential roles in the immune system. Immunology (2009) 127:299–311. doi:10.1111/j.1365-2567.2009.03054.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
39. Pot C. Aryl hydrocarbon receptor controls regulatory CD4 + T cell function. Swiss Med Wkly (2012) 142:w13592. doi:10.4414/smw.2012.13592
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
40. Wagage S, John B, Krock BL, Hall AO, Randall LM, Karp CL, et al. The aryl hydrocarbon receptor promotes IL-10 production by NK cells. J Immunol (2014) 192:1661–70. doi:10.4049/jimmunol.1300497
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
41. Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, et al. Control of Treg and Th17 cell differentiation by the aryl hydrocarbon receptor. Nature (2008) 453:65–71. doi:10.1038/nature06880
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
42. Quintana FJ, Murugaiyan G, Farez MF, Mitsdoerffer M, Tukpah AM, Burns EJ, et al. An endogenous aryl hydrocarbon receptor ligand acts on dendritic cells and T cells to suppress experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA (2010) 107:20768–73. doi:10.1073/pnas.1009201107
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
43. Duarte JH, Di Meglio P, Hirota K, Ahlfors H, Stockinger B. Differential influences of the aryl hydrocarbon receptor on Th17 mediated responses in vitro and in vivo. PLoS One (2013) 8:e79819. doi:10.1371/journal.pone.0079819
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
44. Mellor AL, Munn DH. Tryptophan catabolism and T-cell tolerance: immunosuppression by starvation? Immunol Today (1999) 20:469–73. doi:10.1016/S0167-5699(99)01520-0
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
45. Takikawa O, Yoshida R, Kido R, Hayaishi O. Tryptophan degradation in mice initiated by indoleamine 2,3-dioxygenase. J Biol Chem (1986) 261:3648–53.
46. Reynolds GP, Pearson SJ. Increased brain 3-hydroxykynurenine in Huntington’s disease. Lancet (1989) 2:979–80. doi:10.1016/S0140-6736(89)90987-2
47. Ogawa T, Matson WR, Beal MF, Myers RH, Bird ED, Milbury P, et al. Kynurenine pathway abnormalities in Parkinson’s disease. Neurology (1992) 42:1702–6. doi:10.1212/WNL.42.9.1702
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
48. Heyes MP, Saito K, Major EO, Milstien S, Markey SP, Vickers JH. A mechanism of quinolinic acid formation by brain in inflammatory neurological disease. Attenuation of synthesis from L-tryptophan by 6- chlorotryptophan and 4-chloro-3-hydroxyanthranilate. Brain (1993) 116:1425–50. doi:10.1093/brain/116.6.1425
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
49. Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science (1998) 281:1191–3. doi:10.1126/science.281.5380.1191
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
50. Mellor AL, Sivakumar J, Chandler P, Smith K, Molina H, Mao D, et al. Prevention of T cell-driven complement activation and inflammation by tryptophan catabolism during pregnancy. Nat Immunol (2001) 2:64–8. doi:10.1038/83183
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
51. Mellor AL, Keskin DB, Johnson T, Chandler P, Munn DH. Cells expressing indoleamine 2,3-dioxygenase inhibit T cell responses. J Immunol (2002) 168:3771–6. doi:10.4049/jimmunol.168.8.3771
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
52. Terness P, Bauer TM, Röse L, Dufter C, Watzlik A, Simon H, et al. Inhibition of allogeneic T cell proliferation by indoleamine 2,3-dioxygenase-expressing dendritic cells: mediation of suppression by tryptophan metabolites. J Exp Med (2002) 196:447–57. doi:10.1084/jem.20020052
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
53. Musso T, Gusella GL, Brooks A, Longo DL, Varesio L. Interleukin-4 inhibits indoleamine 2,3-dioxygenase expression in human monocytes. Blood (1994) 83:1408–11.
54. Babcock TA, Carlin JM. Transcriptional activation of indoleamine dioxygenase by interleukin 1 and tumor necrosis factor alpha in interferon-treated epithelial cells. Cytokine (2000) 12:588–94. doi:10.1006/cyto.1999.0661
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
55. Robinson CM, Shirey KA, Carlin JM. Synergistic transcriptional activation of indoleamine dioxygenase by IFN-gamma and tumor necrosis factor-alpha. J Interferon Cytokine Res (2003) 23:413–21. doi:10.1089/107999003322277829
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
56. Moreau M, Lestage J, Verrier D, Mormede C, Kelley KW, Dantzer R, et al. Bacille Calmette-Guérin inoculation induces chronic activation of peripheral and brain indoleamine 2,3-dioxygenase in mice. J Infect Dis (2005) 192:537–44. doi:10.1086/431603
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
57. Connor TJ, Starr N, O’Sullivan JB, Harkin A. Induction of indolamine 2,3-dioxygenase and kynurenine 3-monooxygenase in rat brain following a systemic inflammatory challenge: a role for IFN-gamma? Neurosci Lett (2008) 441:29–34. doi:10.1016/j.neulet.2008.06.007
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
58. Fujigaki H, Saito K, Fujigaki S, Takemura M, Sudo K, Ishiguro H, et al. The signal transducer and activator of transcription 1alpha and interferon regulatory factor 1 are not essential for the induction of indoleamine 2,3-dioxygenase by lipopolysaccharide: involvement of p38 mitogen-activated protein kinase and nuclear factor-kappaB pathways, and synergistic effect of several proinflammatory cytokines. J Biochem (2006) 139:655–62. doi:10.1093/jb/mvj072
59. Vogel CFA, Goth SR, Dong B, Pessah IN, Matsumura F. Aryl hydrocarbon receptor signaling mediates expression of indoleamine 2,3-dioxygenase. Biochem Biophys Res Commun (2008) 375:331–5. doi:10.1016/j.bbrc.2008.07.156
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
60. Nguyen NT, Kimura A, Nakahama T, Chinen I, Masuda K, Nohara K, et al. Aryl hydrocarbon receptor negatively regulates dendritic cell immunogenicity via a kynurenine-dependent mechanism. Proc Natl Acad Sci USA (2010) 107:19961–6. doi:10.1073/pnas.1014465107
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
61. Opitz CA, Litzenburger UM, Sahm F, Ott M, Tritschler I, Trump S, et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature (2011) 478:197–203. doi:10.1038/nature10491
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
62. Bessede A, Gargaro M, Pallotta MT, Matino D, Servillo G, Brunacci C, et al. Aryl hydrocarbon receptor control of a disease tolerance defence pathway. Nature (2014) 511:184–90. doi:10.1038/nature13323
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
63. Guillemin GJ, Kerr SJ, Pemberton LA, Smith DG, Smythe GA, Armati PJ, et al. IFN-beta1b induces kynurenine pathway metabolism in human macrophages: potential implications for multiple sclerosis treatment. J Interferon Cytokine Res (2001) 21(12):1097–101. doi:10.1089/107999001317205231
64. Jung ID, Lee CM, Jeong YI, Lee JS, Park WS, Han J, et al. Differential regulation of indoleamine 2,3-dioxygenase by lipopolysaccharide and interferon gamma in murine bone marrow derived dendritic cells. FEBS Lett (2007) 581:1449–56. doi:10.1016/j.febslet.2007.02.073
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
65. O’Connor JC, Lawson MA, André C, Moreau M, Lestage J, Castanon N, et al. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry (2009) 14:511–22. doi:10.1038/sj.mp.4002148
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
66. Pallotta MT, Orabona C, Volpi C, Vacca C, Belladonna ML, Bianchi R, et al. Indoleamine 2,3-dioxygenase is a signaling protein in long-term tolerance by dendritic cells. Nat Immunol (2011) 12:870–8. doi:10.1038/ni.2077
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
67. Alberati-Giani D, Ricciardi-Castagnoli P, Köhler C, Cesura AM. Regulation of the kynurenine metabolic pathway by interferon-gamma in murine cloned macrophages and microglial cells. J Neurochem (1996) 66:996–1004. doi:10.1046/j.1471-4159.1996.66030996.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
68. Fallarino F, Vacca C, Orabona C, Belladonna ML, Bianchi R, Marshall B, et al. Functional expression of indoleamine 2,3-dioxygenase by murine CD8 alpha(+) dendritic cells. Int Immunol (2002) 14:65–8. doi:10.1093/intimm/14.1.65
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
69. Grohmann U, Fallarino F, Bianchi R, Belladonna ML, Vacca C, Orabona C, et al. IL-6 inhibits the tolerogenic function of CD8 alpha + dendritic cells expressing indoleamine 2,3-dioxygenase. J Immunol (2001) 167:708–14. doi:10.4049/jimmunol.167.2.708
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
70. Fallarino F, Grohmann U, Vacca C, Orabona C, Spreca A, Fioretti MC, et al. T cell apoptosis by kynurenines. Adv Exp Med Biol (2003) 527:183–90. doi:10.1007/978-1-4615-0135-0_21
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
71. Kanai M, Funakoshi H, Takahashi H, Hayakawa T, Mizuno S, Matsumoto K, et al. Tryptophan 2,3-dioxygenase is a key modulator of physiological neurogenesis and anxiety-related behavior in mice. Mol Brain (2009) 2:8. doi:10.1186/1756-6606-2-8
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
72. Pilotte L, Larrieu P, Stroobant V, Colau D, Dolusic E, Frédérick R, et al. Reversal of tumoral immune resistance by inhibition of tryptophan 2,3-dioxygenase. Proc Natl Acad Sci USA (2012) 109:2497–502. doi:10.1073/pnas.1113873109
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
73. Adams S, Braidy N, Bessede A, Brew BJ, Grant R, Teo C, et al. The kynurenine pathway in brain tumor pathogenesis. Cancer Res (2012) 72:5649–57. doi:10.1158/0008-5472.CAN-12-0549
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
74. Campbell BM, Charych E, Lee AW, Möller T. Kynurenines in CNS disease: regulation by inflammatory cytokines. Front Neurosci (2014) 8:12. doi:10.3389/fnins.2014.00012
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
75. Fujigaki H, Saito K, Lin F, Fujigaki S, Takahashi K, Martin BM, et al. Nitration and inactivation of IDO by peroxynitrite. J Immunol (2006) 176:372–9. doi:10.4049/jimmunol.176.1.372
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
76. Fujigaki H, Takahashi K, Fujigaki S, Masuda J, Takikawa O, Markey SP, et al. Post-translational modification of indoleamine 2,3-dioxygenase: N-terminal modification and nitration. Int Congr Ser (2007) 1304:41–5. doi:10.1007/s00216-012-5946-2
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
77. Fujigaki H, Seishima M, Saito K. Posttranslational modification of indoleamine 2,3-dioxygenase. Anal Bioanal Chem (2012) 403:1777–82. doi:10.1007/s00216-012-5946-2
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
78. Orabona C, Pallotta MT, Volpi C, Fallarino F, Vacca C, Bianchi R, et al. SOCS3 drives proteasomal degradation of indoleamine 2,3-dioxygenase (IDO) and antagonizes IDO-dependent tolerogenesis. Proc. Natl Acad Sci USA (2008) 105:20828–33. doi:10.1073/pnas.0810278105
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
79. Nakahama T, Hanieh H, Nguyen NT, Chinen I, Ripley B, Millrine D, et al. Aryl hydrocarbon receptor-mediated induction of the microRNA-132/212 cluster promotes interleukin-17-producing T-helper cell differentiation. Proc Natl Acad Sci U S A (2013) 110:11964–9. doi:10.1073/pnas.1311087110
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
80. Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell (2009) 136:215–33. doi:10.1016/j.cell.2009.01.002
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
81. Li D, Liu C, Yu H, Zeng X, Xing X, Chen L, et al. AhR is negatively regulated by miR-203 in response to TCDD or BaP treatment. Toxicol Res (2014) 3:142–51. doi:10.1039/c3tx50083g
82. Wei J, Huang X, Zhang Z, Jia W, Zhao Z, Zhang Y, et al. MyD88 as a target of microRNA-203 in regulation of lipopolysaccharide or Bacille Calmette-Guerin induced inflammatory response of macrophage RAW264.7 cells. Mol Immunol (2013) 55:303–9. doi:10.1016/j.molimm.2013.03.004
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
83. Takamura T, Harama D, Matsuoka S, Shimokawa N, Nakamura Y, Okumura K, et al. Activation of the aryl hydrocarbon receptor pathway may ameliorate dextran sodium sulfate-induced colitis in mice. Immunol Cell Biol (2010) 88:685–9. doi:10.1038/icb.2010.35
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
84. Furumatsu K, Nishiumi S, Kawano Y, Ooi M, Yoshie T, Shiomi Y, et al. A role of the aryl hydrocarbon receptor in attenuation of colitis. Dig Dis Sci (2011) 56:2532–44. doi:10.1007/s10620-011-1643-9
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
85. Nakahama T, Kimura A, Nguyen NT, Chinen I, Hanieh H, Nohara K, et al. Aryl hydrocarbon receptor deficiency in T cells suppresses the development of collagen-induced arthritis. Proc Natl Acad Sci USA (2011) 108:14222–7. doi:10.1073/pnas.1111786108
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
86. Monaco F, Fumero S, Mondino A, Mutani R. Plasma and cerebrospinal fluid tryptophan in multiple sclerosis and degenerative diseases. J Neurol Neurosurg Psychiatry (1979) 42:640–1. doi:10.1136/jnnp.42.7.640
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
87. Amirkhani A, Rajda C, Arvidsson B, Bencsik K, Boda K, Seres E, et al. Interferon-beta affects the tryptophan metabolism in multiple sclerosis patients. Eur J Neurol (2005) 12:625–31. doi:10.1111/j.1468-1331.2005.01041.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
88. Durastanti V, Lugaresi A, Bramanti P, Amato M, Bellantonio P, De Luca G, et al. Neopterin production and tryptophan degradation during 24-months therapy with interferon beta-1a in multiple sclerosis patients. J Transl Med (2011) 9:42. doi:10.1186/1479-5876-9-42
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
89. Sakurai K, Zou JP, Tschetter JR, Ward JM, Shearer GM. Effect of indoleamine 2,3-dioxygenase on induction of experimental autoimmune encephalomyelitis. J Neuroimmunol (2002) 129:186–96. doi:10.1016/S0165-5728(02)00176-5
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
90. Kwidzinski E, Bunse J, Aktas O, Richter D, Mutlu L, Zipp F, et al. Indolamine 2,3-dioxygenase is expressed in the CNS and down-regulates autoimmune inflammation. FASEB J (2005) 19:1347–9. doi:10.1096/fj.04-3228fje
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
91. Yan Y, Zhang GX, Gran B, Fallarino F, Yu S, Li H, et al. IDO upregulates regulatory T cells via tryptophan catabolite and suppresses encephalitogenic T cell responses in experimental autoimmune encephalomyelitis. J Immunol (2010) 185:5953–61. doi:10.4049/jimmunol.1001628
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
92. Wu HY, Quintana FJ, da Cunha AP, Dake BT, Koeglsperger T, Starossom SC, et al. In vivo induction of Tr1 cells via mucosal dendritic cells and AHR signaling. PLoS One (2011) 6:e23618. doi:10.1371/journal.pone.0023618
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
93. Gandhi R, Kumar D, Burns EJ, Nadeau M, Dake B, Laroni A, et al. Activation of the aryl hydrocarbon receptor induces human type 1 regulatory T cell-like and Foxp3(+) regulatory T cells. Nat Immunol (2010) 11:846–53. doi:10.1038/ni.1915
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
94. Platten M, Ho PP, Youssef S, Fontoura P, Garren H, Hur EM, et al. Treatment of autoimmune neuroinflammation with a synthetic tryptophan metabolite. Science (2005) 310:850–5. doi:10.1126/science.1117634
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
95. Nguyen NT, Hanieh H, Nakahama T, Kishimoto T. The roles of aryl hydrocarbon receptor in immune responses. Int Immunol (2013) 25:335–43. doi:10.1093/intimm/dxt011
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
96. Nguyen NT, Nakahama T, Kishimoto T. Aryl hydrocarbon receptor and experimental autoimmune arthritis. Semin Immunopathol (2013) 35:637–44. doi:10.1007/s00281-013-0392-6
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Keywords: dioxin receptor, indoleamine 2,3-dioxygenase, transcription factor, autoimmunity, immune regulation
Citation: Nguyen NT, Nakahama T, Le DH, Van Son L, Chu HH and Kishimoto T (2014) Aryl hydrocarbon receptor and kynurenine: recent advances in autoimmune disease research. Front. Immunol. 5:551. doi: 10.3389/fimmu.2014.00551
Received: 30 July 2014; Paper pending published: 05 September 2014;
Accepted: 16 October 2014; Published online: 29 October 2014.
Edited by:
Paolo Puccetti, University of Perugia, ItalyReviewed by:
Teresa Zelante, University of Perugia, ItalyPaolo Puccetti, University of Perugia, Italy
Francisco Javier Quintana, Harvard Medical School, USA
Copyright: © 2014 Nguyen, Nakahama, Le, Van Son, Chu and Kishimoto. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tadamitsu Kishimoto, Laboratory of Immune Regulation, WPI-Immunology Frontier Research Center, Osaka University, 3-1 Yamada-oka, Suita, Osaka 565-0871, Japan e-mail: kishimoto@ifrec.osaka-u.ac.jp
†Nam Trung Nguyen and Taisuke Nakahama have contributed equally to this work.
 Nam Trung Nguyen
Nam Trung Nguyen Taisuke Nakahama
Taisuke Nakahama Duc Hoang Le
Duc Hoang Le Le Van Son
Le Van Son Ha Hoang Chu2
Ha Hoang Chu2 Tadamitsu Kishimoto
Tadamitsu Kishimoto