- 1Departamento de Biomedicina Molecular, Centro de Investigación y Estudios Avanzados del IPN, México City, DF, Mexico
- 2Department of Microbiology and Molecular Genetics, Irvine School of Medicine, University of California, Irvine, CA, USA
- 3Instituto Nacional de Salud Pública, Secretaría de Salud y Asistencia, Cuernavaca, Morelos CP, Mexico
Although B cells and antibodies are the central effectors of humoral immunity, B cells can also produce and secrete cytokines and present antigen to helper T cells. The uptake of antigen is mainly mediated by endocytosis; thus, antigens are often presented by MHC-II molecules. However, it is unclear if B cells can present these same antigens via MHC-I molecules. Recently, Salmonella bacteria were found to infect B cells, allowing possible antigen cross-processing that could generate bacterial peptides for antigen presentation via MHC-I molecules. Here, we will discuss available knowledge regarding Salmonella antigen presentation by infected B cell MHC-I molecules and subsequent inhibitory effects on CD8+ T cells for bacterial evasion of cell-mediated immunity.
Introduction
Salmonella typhi is the causative agent of typhoid fever in human beings, while infection with Salmonella enterica serovar Typhimurium (Salmonella typhimurium) produces a systemic illness in mice similar to that in human beings (1). In susceptible mice, the bacteria reside inside Salmonella-containing vacuoles (SCVs) of neutrophils, macrophages, and dendritic cells, in which they replicate, resist killing, and induce systemic disease (2–5). Uptake of Salmonella is mediated by the coordinated action of several virulence proteins translocated through the type III secretion system (T3SS), encoded by genes of Salmonella pathogenicity islands (SPIs) (6). While SPI-1 genes encode T3SS translocated proteins essential during bacterial invasion, T3SS SPI-2 genes are expressed once the bacteria are within the phagosome (7).
The bacteria exploit several types of immune cells for long-term survival (8–10). To survive within these cells and promote colonization, the bacteria release several virulence proteins that alter host cell functions, such as cytoskeletal architecture, membrane trafficking, signal transduction, cell death, cell trafficking, and cytokine gene expression (5, 6). This review focuses on the role of B cells during Salmonella infection, specifically as a niche from which the bacteria can evade immune responses and survive long-term within the host.
General Antigen Processing and Presentation
Antigen location influences its proteolytic processing pathway and its access to different classes of MHC molecules. Subsequent presentation of these antigens by MHC-I or MHC-II molecules is necessary to induce a T cell immune response. Extracellular antigens are captured by antigen-presenting cells (APCs) through phagocytosis, macropinocytosis, or endocytosis. Newly formed phagosomes containing antigen undergo progressive trafficking characterized by acquiring or losing endosomal markers to generate a mature phagosome. Finally, their fusion with lysosomes allows complete degradation of their cargo due mainly to serine proteases (cathepsins) (11). Assembly of peptide/MHC-II complexes takes place in a multilamellar endosomic compartment that contains newly synthesized MHC-II molecules bound with invariant chain-peptide (CLIP) and machinery necessary for efficient peptide loading. The acidic environment facilitates the exchange of CLIP for antigenic peptide, catalyzed by H-2M in mice or HLA-DM in human beings. Recycled MHC-II molecules from the cell surface can also be used to form peptide-MHC-II complexes. Then, the peptide-MHC-II complexes newly formed are transported to the plasma membrane. Finally, effective MHC-II presentation requires clustered peptide/MHC-II complexes at the APC surface that can subsequently interact with the T cell receptor (TCR) and CD4 co-receptor (11, 12).
Alternately, intracellular antigens in the majority of cells are processed within the cytosol by proteosomal degradation. The peptide fragments are then translocated to the endoplasmic reticulum (ER) lumen by the transporter associated with presentation. Nascent MHC-I molecules and β2-microglobulin associate with the ER proteins tapasin, calreticulin, and Erap57, which allows glycosylation of MHC-I and optimal folding necessary after peptide binding. Then newly peptide/MHC-I complexes are transported to the cell surface (12, 13). Stable heterotrimeric complexes are necessary to engage the TCR and CD8 co-receptor. However, extracellular antigens localized in vesicular compartments of APCs can also be efficiently presented by MHC-I molecules (14), a process known as cross-presentation or cross-priming. At least four routes for cross-priming have been described (15): (1) the cytosolic route requires peptide translocation from the phagosomes to the cytosol for their proteosomal processing and subsequent ER translocation (16); (2) the vacuolar route involves peptides generated within the phagosome be loaded in intravacuolar-recycled MHC-I molecules (17); (3) the antigen is cross-processed through a phagosome-cytosol-phagosome alternating pathway (18); and (4) peptides are processed in a previously non-characterized endocytic compartment, secreted into the cytosol, and loaded onto empty MHC-I molecules on the surfaces of macrophages and bystander cells (19, 20).
Salmonella Interferes with Antigen-Processing Mechanisms
Salmonella evade acquired immune responses to establish a chronic infection (21, 22). T cell responses can be inhibited by impaired APC antigen processing and presentation caused by bacterial proteins encoded by SPI-2 genes. As mentioned previously, Salmonella interferes with normal cell trafficking; for example, Salmonella protein SpiC inhibits maturation of Salmonella-containing phagosomes into phagolysosomes in macrophages and dendritic cells (2, 23–25). In addition, the phosphoinositide phosphatase SopB modulates vesicular trafficking (26). This virulence protein manipulates membrane surface charges of nascent SCVs by reducing levels of the negatively charged lipids phosphatidylinositol-4-5-biphosphate and phosphatidylserine, thus resulting in SCV maturation (27). Inhibition of phagosome acidification has been observed in macrophage cell lines (e.g., IC21) and may impede the proteolitic activity of cathepsins residing in late-endosomal compartments; this mechanism could also modify peptide processing prior to presentation (28). The integrity of the SCV, attributed to SifA, is also crucial for its resistance to oxidative killing mediated by the phagocyte oxidase phox (2). Salmonella mutants defective in SPI-1 and SPI-2 genes show reduced proliferation within macrophages, indicating these gene products could limit the source of peptides for antigen presentation, resulting in delayed T cell responses (29, 30). In support of this finding, Helaine et al. recently used fluorescent dilution to study intracellular replication of bacteria to determine the vacuolar environment induces phenotypic heterogeneity, thereby explaining the presence of non-replicating, yet persistent, Salmonella that could provide a reservoir for relapsing infection (31). Additionally, studies in human beings reveal the bacteria can control surface MHC-II expression through ubiquitination (32). Thus, Salmonella can impair antigen processing and presentation steps at multiple levels to prevent activation of T cell responses.
Salmonella Evade T Cell Responses
An immunosuppressive effect on T cells, both dependent and independent of bacteria, has been observed during Salmonella infection. Basel Al-Ramaldi first noted this effect in macrophages infected with an attenuated strain of Salmonella cultured with splenocytes in transwell plates. Soluble factors mediated T cell suppression, but the exact nature of the factor(s) was not determined at that time (33). Later, T cell proliferation assays were performed in the presence or absence of the inducible nitric oxide synthase (iNOS) inhibitor l-NMMA, which showed the suppression is also mediated by dendritic cells and is dependent on NOS induction (34). When nitric oxide was blocked with aminoguanidine, the inhibition of T cell suppression, macrophage activity, and polymorphonuclear leukocyte influx was observed (35). Thus, nitric oxide may play multiple biological roles during Salmonella infection. Other studies employing the human-restricted strain S. typhi showed the polysaccharide Vi, released from Salmonella, leads to an impairment of IL-2 production in T cells by interacting with the membrane prohibitin complex (36). Other transwell assays with CD8+ T cells and dendritic cells infected with Salmonella deficient in the proteins SPI-1, SPI-2, phoP, and sti or carrying virulence plasmids demonstrated priming can be inhibited by direct contact with the bacteria (37). Moreover, exposure to LPS during priming in Salmonella-infected mice suppressed IL-2 and TNF-α production of flagellin-specific CD4+ T cells, resulting in exacerbation of murine typhoid (38).
Other mechanisms for T cell evasion Salmonella infection have been described. Experiments involving adoptive transfer of CD4+ T cells from TCR-transgenic mice into Salmonella-infected mice showed the bacteria induce a progressive culling of newly activated, high-avidity, antigen-specific CD4+ T cells that express higher levels of programed death-ligand 1 (PD-L1) in an SPI-2 dependent manner (39, 40). This mechanism reshapes the repertoire of antigen-specific T cells after Salmonella infection. Furthermore, several groups have found the bacteria are able to reach the thymus (41, 42). We have observed that infections of the thymus cause Vβ chain rearrangements of TCRs in single-positive CD8+ T cells, possibly leading to a biased selection of certain types of clonal cell populations (unpublished data). Salmonella can downregulate TCR expression by reducing the amount of both surface and intracellular TCR-β chain in T cells co-cultured with S. typhimurium (43). However, it is unknown if the bacteria could trigger or produce crosstalk between signaling pathways that would lead to this phenotype.
Because regulatory T (Treg) cells mediate immune suppression, these cells can play both detrimental and protective roles in host defense against infection. Johanns et al. has shown that suppressive capacity of Treg coincide with a delay of elicing protective response during early Salmonella infection, contrary during late infection Treg suppressive potency diminish (44). Moreover, peritoneal NK1.1 αβ T cells reduced IL-12 production in macrophages by secretion of IL-4 upon TCR activation, during the early phase of Salmonella infection (45). Thus, Salmonella employ several strategies to overcome acquired immunity in order to persist and produce a chronic infection in the host.
B Cells as APCs in T Cell Priming
The introduction of fluorescence-activated cell sorting (FACS) revolutionized the study of B cells, allowing the classification of B cells from lymph nodes, the spleen, and more recently, from the liver (46) into phenotypically and functionally distinct populations, denoted B1 and B2. The B2 lymphocytes are further subdivided into marginal zone B (MZ-B) and follicular B (FO-B) cells, while B1 lymphocytes are grouped as B1a or B1b cells. All subsets differ in their development, location, function, and most importantly, their ability to present antigens to T cells. For B cells to become competent APCs, they first must receive signals either from the B cell receptor (BCR) or Toll-like receptors (TLRs) for activation. This feature allows enhanced B cells uptake of both soluble and particulate (phagocytosed) antigens, followed by the expression of co-stimulatory molecules and the subsequent processing and presentation of antigens with MHC-I or MHC-II molecules (47).
Marginal zone B cells are strategically located in the bloodstream for easy activation and to intercept and react to blood-borne antigens (48, 49). Antigens captured by MZ-B cells are delivered to follicular dendritic cells through shuttling dependent on the CXCR5-S1P1-S1P3 axis (50). However, MZ-B cells can also initiate a rapid first line of defense, demonstrated by Olivier et al., in which they express higher basal levels of co-stimulatory molecules CD80 and CD86, which are rapidly upregulated within 6–24 h after LPS exposure or BCR signaling. In fact, LPS-stimulated MZ-B cells induced a vigorous proliferation of alloreactive T cells in vitro, in contrast with LPS-stimulated FO-B cells, which then developed into mature plasma cells (51). In another set of experiments, Attanavanich et al. demonstrated that in vivo hen egg lysozyme (HEL)-specific MZ-B cells are more potent activators of naïve TCR-transgenic CD4+ T cells than HEL-specific FO-B cells. The MZ-B cells likely have better access to the antigen and can rapidly migrate toward the T cell area, followed by plasma cell differentiation (52). Together, these experiments highlight the role of MZ-B cells to provide a bridge between innate and adaptive immune responses.
Similarly, B1 cells express higher basal levels of CD80 and CD86, suggesting their potential role in rapidly initiating a T cell response (53). The capability of peritoneal cavity B1 cells to phagocytose, process, and present particulate antigens, such as OVA bound to latex beads (1 μM) (54). Interestingly, MZ-B cells, in conjunction with B1 cells from either the spleen or peritoneal cavity, participate in the response against blood-borne antigens (55). In addition to MZ-B and B1 cells, parabiosis studies suggest that mature B cells located in the perisinusoidal niche of bone marrow, which have access to the circulatory system and can freely enter and exit the bone marrow, are also specialized for T cell-independent responses to blood-borne antigens (56). Previous paradigms describing B cell antigen presentation have changed, further supported by recent findings involving phagocytic IgM+ cells from teleost fish and amphibians that indicate an evolutionary relationship between B cells and macrophages (57). This theory suggests B cells may have evolved from ancient phagocytic cells to macrophage-like cells to B cells that maintained their ability to phagocytose. Therefore, when B cells are activated, they become potent APCs when they encounter specific antigens, leading to cognate T-B cell interactions, T cell activation, and germinal center (GC) reactions. The amount of antigen captured and presented by GC B cells to follicular helper T (Tfh) cells is proportional to cell division and hypermutation rates because GC B cells with the highest affinity for antigens are selectively expanded and diversified (58).
In addition to priming T cells, APCs can also provide signals that instruct T cells to enter into effector/memory differentiation programs. Soo Choi et al. found that Tfh differentiation is mediated by two key players; during priming, dendritic cells induce Bcl6 expression in Tfh cells, while the stable commitment to this differentiation program requires interaction with FO-B cells (59). This mechanism was explored by experiments in which antigen-specific T cells from MD4/μMT B cell-deficient mice showed reduced levels of Bcl6 expression at day 7 post-immunization against lymphocytic choriomeningitis virus (LCMV). Experiments using B cell/dendritic cell MHC-II-deficient mice reinforced the role of MHC-II in antigen presentation by FO-B cells in cooperation with Tfh differentiation (60). This model suggested B cells participate in the initiation, maintenance, and full polarization of Tfh differentiation (61). Regarding Th1 differentiation, Barr et al. have shown that an antigen-specific IgG2c primary response is absolutely dependent on MyD88 signaling to B cells in mice immunized with T cell-dependent antigen or in mice infected with Salmonella (62). They also found that B cell-intrinsic MyD88 signaling is required for primary effector Th1 cell development, whereas antigen-specific BCR-mediated presentation is necessary for the development of Th1 memory cells against Salmonella (63). In addition, MZ-B cells participate in Th1 cell differentiation, and Attanavanich et al. found that, when cultured in vivo, HEL-primed MZ-B cells from MD4 mice with naïve CD4+ T cells produce large amounts of Th1-like cytokines and IFN-γ but low levels of IL-4, IL-5, and IL-10. This expression pattern suggests MZ-B cells also provide signals for Th1 cell development during the primary immune response (52). These findings emphasize the non-redundant role of B cells as programmers of CD4+ T cell differentiation.
The ability of B cells to process and present viral antigens to CD8+ T cells via MHC-I molecules was first explored by Ciavarra et al. with proliferation and cytotoxicity assays using [3H]thymidine and 51Cr release, respectively. These experiments highlighted the efficacy of mitogen (LPS)-activated B cells in displaying target antigens on their cell surface membranes, which are efficiently recognized in a MHC-I-dependent manner by vesicular stomatitis virus-specific cytotoxic T cells (CTLs) (64). Other experiments employing mice infected with LCMV-Clone 13, a strain that causes persistent infections, showed that neutralizing antibodies are induced unless CD8+ T cells were depleted. This result suggests B cells might be actively infected and capable of presenting viral peptides on MHC-I molecules; thus, they may become targets for LCMV-specific CTLs (65). Subsequent studies by the same group used 51Cr release assays with splenocytes from LCMV-infected BALB/c (H-2d) mice and as target, LCMV-infected, neutralizing antibody-secreting hybridomas. Showed that CTLs lysed the infected hybridomas, because LCMV was endocytosed through the membrane-anchored neutralizing antibody receptor and are later eliminated by virus-specific CTLs (66). These results reinforced the role of B-cell as APC. Although not absolutely required, they do play a role in T cell priming, thus positively impacting the function of CD8+ T cells. Multiple cytokines, such as IL-2, IL-12, IL-21, IL-27, and IL-33, which are produced by CD4+ T cells, APCs, and non-hematopoietic cells from the T cell zone, participate in promoting effector T cell differentiation (67, 68). Liu et al. first explored B cells’ potential “helper role” for CD8+ T cells by evaluating the anti-influenza cytolytic activity of CD8+ T cells. They demonstrated that soluble factors released by B cells could replace the CD4+ T cell requirement to induce cytotoxic responses to influenza virus (69). These previous studies changed our view of B cells as APCs and showed they strongly influence an effective CD8+ T cell response against pathogens localized in the cytoplasm, such as viruses.
Evidence that B cells possess machinery to perform alternative pathways for antigen processing for CD8+ T cell priming has been presented in studies related to vaccine development and bacterial infections. For example, the Mycobacterium tuberculosis heat shock protein 70 (HSP70) is endocytosed, subjected to vacuolar processing, and forms highly immunogenic complexes with chaperoned peptides that are presented on MHC-I molecules to elicit a CD8+ T cell response (70). In one experiment, CpG oligodeoxynucleotides-activated B cells could uptake OVA-associated HSP70, in a CD91-dependent manner, process the fusion protein by vacuolar mechanisms and prime OVA-specific CD8+ T cells. In another study involving immune-stimulating complexes (ISCOMS) that induce strong MHC-I-restricted responses, HEL-specific B cells could uptake OVA-HEL-ISCOMS and then stimulate OVA-specific CD8+ T cell responses. This cross-presentation required endosomal acidification, proteosomal processing, and classical MHC-I/peptide transport (71). During bacterial infections, BCR-mediated internalization of Salmonella led to efficient antigen delivery to MHC-II antigen-loading compartments; however, when the proteosome was inhibited with MG-132, only a partial dependence on this protease was observed (72, 73). These data indicate B cells may have machinery to employ the phagosome-cytosol antigen presentation pathway. In addition, our group has shown that Salmonella-infected B cells cannot use the vacuolar alternative pathway that involves antigen processing in a vacuolar compartment, which is often followed by secretion and loading of antigenic peptides to MHC-I molecules on the surface of B cells and bystander cells (28). In sum, these studies show that B cells possess machinery necessary to induce a CD8+ T cell response against intracellular pathogens localized in vacuolar compartments.
We have thus far reviewed evidence that portrays B cells as highly competent APCs that positively impact T cell functions; however, B cells are also negative regulators of T cell responses therefore denoted as Breg cells. Their inhibitory function has been associated mainly with IL-10 because this B cell derived-cytokine can protect against autoimmunity, yet increase the host’s susceptibility to infection (74). Recently, two separate studies identified an additional soluble factor that mediates regulatory functions in B cells. One study found IL-35 can induce the conversion of typical B cells into an IL-35-producing Breg cell population dependent on STAT1 and STAT3, which are induced through signaling by IL-12Rβ2 and IL-27Rα. In addition, induced B cells exerted a suppressive influence on pathogenic Th17 and Th1 cells from experimental autoimmune uveitis-induced mice (75). The second study revealed that B cells, through activation of TLR4 and CD40, secrete IL-35. This study focused on B35 cell-deficient mice and found B cell-derived IL-35 is necessary for pathogenic Th17 and Th1 cell suppression in an autoimmune encephalomyelitis model. Moreover, a lack of IL-35 production by B cells led to increased activation of macrophages and CD4+ Th1 cells and favored B cells as APCs in a Salmonella infection model (76).
B Cells during Salmonella Infection
T cells, particularly Th1 cells, are crucial for Salmonella infection control due to their IFN-γ secretion (62, 63, 77–81), while Salmonella-specific antibodies are required to resist secondary infection. The role of B cells as antibody-producing cells has been demonstrated using B cell-deficient mice (Igh-6−/− or Igμ−/−) (82, 83) that were immunized with an attenuated strain of Salmonella and then challenged with a virulent strain; these mice could not resist the infection (84). In addition, transfer of immune serum to immunized B cell-deficient mice (Igμ−/−) 1 day prior to challenge with virulent Salmonella effectively reconstituted their immunity (83). Thus, antibody-producing B cells are key players during secondary bacterial infections. Beyond this well-known role, another study suggests B cells are required for priming T cell responses during bacterial infections. Notably, Ugrinovic et al. found a reduced frequency of both IFN-γ-producing CD4+ and CD8+ T cells in immunized, gene-targeted, B cell-deficient Igh-6−/− mice. When primary B cells infected in vitro were cultured with Salmonella-specific CD4+ T cells from immunized mice, they induced modest proliferation compared to in vitro-infected bone marrow-derived macrophages (85). More recently, Nanton et al. evaluated T cell responses against Salmonella using B cell-deficient JhD mice, transgenic mice with B cells that could not class switch or secrete antibodies, and mice with B cells that could not class switch but were able to secrete IgM. They observed a decrease of both IFN-γ +CD8+ or CD4+ T cells, which suggested antibodies are not required for an optimal Salmonella-induced Th1 response (86). Collectively, these studies show that in vitro Salmonella-infected B cells can moderately prime CD4+ T cells and somehow participate in the activation of T cell responses. Recent findings from Barr et al. using a Salmonella infection model demonstrated intrinsic MyD88-derived B cell signals play a role in effector Th1 cell differentiation (62, 63). Our group, along with others, has shown that in vitro-infected B cells produce IL-6 (87), which contributes to the early multistages of Tfh cell differentiation. Therefore, it is not surprising that B cells can also act as T cell programmers, although it is unknown if B cell infection may impair the multistage and multifactorial Tfh cell differentiation program.
B Cells Prime CD8+ T Cells Responses during Salmonella Infection
Although CD4+ T cells are known as key players during Salmonella infection, the role of CD8+ T cell responses is less clear. Interestingly, CD8+ T cells participate in the eradication of bacteria during secondary Salmonella infections, but their role in primary infection seems contradictory (80, 88, 89). Previous evidence demonstrated a null to modest participation of CTLs in mice deficient of β2m or depleted of CD8+ T cells (80, 89). More recent reports focused on MHC-Ia-deficient mice (KbDb) demonstrated the role of CD8+ T cells during the later stages of a primary infection (88). In addition, the involvement of non-polymorphic MHC-Ib (Qa-1, HLA-E, H2-M3) during the response against Salmonella has recently gained attention due to their role as presentation molecules for Salmonella antigens (90, 91).
When acting as APCs, B cells that express a Salmonella-specific BCR can, after bacterial internalization, reactivate human memory CD8+ T cells via cross-presentation, leading to a CTL response (72). However, it is unclear if primary B cells infected with Salmonella by the natural entry pathway could cross-process and present Salmonella antigens via MHC-I. By using a Salmonella strain (S-OVA) that expresses the OVA peptide (OVAp) fusioned with the curli protein (Crl), we evaluate whether MHC-I present Salmonella antigens after infection. We found that in vitro and in vivo S-OVA-infected B cells express Kb-OVAp complexes. This presentation diminished by using inhibitors of the components of the classical (brefeldin and lactacystin) or vacuolar (leupeptin and ammonium chloride) pathways suggesting that processing of Salmonella antigens might involve the translocation of partial processed antigens from the SVC to the cytosol, followed by their proteosomal degradation and subsequent ER translocation (Figure 1) (unpublished data). In agreement with our results, a previous study demonstrated that, in human-specific B cells, cross-presentation of Salmonella antigens is partly proteosome-dependent (72). It is also likely that peptides generated within the SCV might load onto recycling MHC-I molecules (Figure 1). On the other hand, Salmonella infection also promotes B cell activation since expression of co-stimulatory molecules such as CD40, CD80, and CD86 within Salmonella-infected B cells is observed (unpublished data). In sum, these results suggest that infected B cells are capable of cross-processing and presenting Salmonella antigens and can express co-stimulatory molecules to become professional APCs that prime and sustain a CD8+ T cell response. Similarly, other studies employing antigen-specific B cells in Salmonella infection or diabetes type-1 models further support the capability of B cells to process and present exogenous antigens (72, 92).
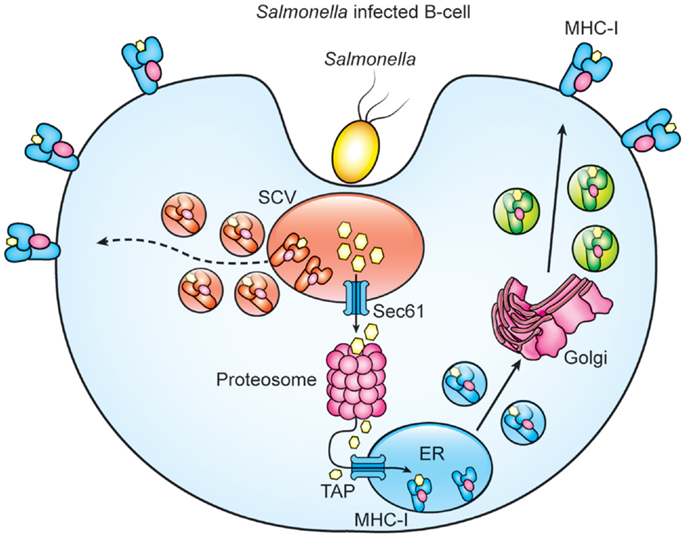
Figure 1. Proteasome and paghosomal degradation are involved for cross-processing of Salmonella antigens by B cells. Model of cross-priming in Salmonella-infected B cells. Salmonella infection generates antigens that are translocated to the cytosol for proteosomal processing and subsequent translocation of Salmonella peptides by TAPs to ER for loading MHC-I. Degradation of Salmonella proteins with the SCV generate peptides that load recycled MHC-I molecules and the resulting MHC-I/peptide complexes are then transported to the B cell surface.
B Cells as Trojan Horses during Salmonella Infection
We and other groups have demonstrated that S. typhimurium infects and persists long-term in splenic and lymph node macrophages, splenic dendritic cells, splenic B cells, bone marrow B cell precursors, and plasma cells. Studies in human beings have shown that S. typhi can be isolated from bone marrow cultures, regardless of disease stage or type of pharmacological treatment (93). Bone marrow B cells present a safe niche for Salmonella because they cannot enter peripheral circulation until they fully mature. In human beings and mice, most Salmonella infections occur in the ileum, spleen, and liver (94). More recently, gallstone biofilms and the gallbladder epithelium were demonstrated niches for chronic Salmonella infections; however, only 3–5% of S. typhi-infected individuals develop a chronic infection in these sites (21, 95).
Macrophages often serve as host cells for Salmonella during acute and chronic infections, but the fate of these cells, as well as other infected cells, is unclear. In some cases, Salmonella induce host cell death, releasing the bacteria and disseminating the infection. Our group has shown that Salmonella inhibit pyroptosis in B cells because they abrogate IL-1β production by impairing NLRC4 transcription; thus, B cell death is not induced (87, 96). This mechanism could allow Salmonella survival within these cells during an innate immune response. Using the B cell line A20, we discovered the vacuolar compartment in which Salmonella reside is different from that in macrophages (28). Interestingly, fluorescent dilution analysis revealed that the SCV environment and nutritional deprivation of infected macrophages activate Salmonella virulence genes, leading to the presence of non-replicating, persistent bacteria (31). In B cells, primary infection is followed by the production of reactive oxygen species, iNOS, and pro-inflammatory cytokines IL-1β, TNF-α, and IL-6, which often control the bacteria (87). Salmonella replication rates within infected B cells are likely low, as we and other groups have found very few bacteria in these cells. For example, Souwer et al. used in vitro infection assays to determine that only 4% of human B cells phagocytose the bacteria via their BCR (73). Similarly, we have observed approximately 0.1–1% of mouse splenic primary B cells, bone marrow B cell precursors, and plasma cells get infected with Salmonella. However, we also discovered that after 2 months post-infection, Salmonella can still be isolated from infected bone marrow B cell precursors and infected plasma cells (97). Our experiments involving susceptible BALB/c mice infected with a single dose of 50 virulent Salmonella bacteria showed that after 1 month post-infection, bacterial CFUs could be isolated from infected splenic B1a and B1b, MZ-B, and FO-B cells (unpublished data). Thus, Salmonella likely exploit B cell populations to persist long-term in the host. Interestingly, if Salmonella infect and persist within all splenic B2 cells, infected FO-B cells, which possess migratory properties, could as act as carriers for further dissemination of Salmonella. Moreover, plasma cells migrate to the bone marrow and eventually undergo apoptosis (98), but these cells could also be involved in spreading the bacteria. However, it is unknown if infected splenic B cells could also differentiate into antibody-secreting cells.
The microbes’ level of persistence depends on a balance between the immune response of the host and the bacteria’s ability to survive within the cell. Certain viral pathogens (LCMV, HIV, HCV, HBV) (99–102), parasites (Trypanosoma cruzi, Schistosoma mansoni, Tenia crassiceps) (103–105), and some bacteria (M. tuberculosis, Helicobacter pylori, Chlamydia trachomatis) (106–108) can render T cell responses ineffective by benefiting from inhibitory stimuli such as PD-1:PD-L (PD-L1 and PD-L2) interactions. Some of the experiments described above showed B cells can process and present Salmonella antigens in vitro and in vivo. In addition, we have found that B cells remain infected long-term, suggesting they may avoid elimination by CTLs. In this context, PD-L1 and PD-L2 expression in B cells infected in vitro and in vivo with Salmonella was observed (unpublished data). These results suggest Salmonella infection provides signals that trigger the transcription of PD-L1 and PD-L2 genes. Furthermore, infected B cells likely produce both positive and inhibitory signals. These inhibitory signals may be more dominant during infection because they allow the bacteria to avoid effector CD8+ T cell responses. Therefore, expression of PD-L1 and PD-L2 by infected B cells could be one possible mechanism employed by the bacteria to survive within these cells and evade cell-mediated immunity. However, no current evidence indicates the PD-1:PD1-Ls axis directly terminates or attenuates CD8+ T cell responses during chronic Salmonella infection. Our current studies simply show that Salmonella-infected B cells express PD1-Ls during acute and chronic infections. We have also found that PD-1 is expressed on antigen-specific CD8+ T cells (unpublished data), so the participation of this axis during infection could explain why previous studies reported no significant CD8+ T cell involvement during Salmonella infections. Furthermore, our group has also found B1 cells can produce IL-10 when infected in vitro with virulent Salmonella (unpublished data), which, along with IL-35 production, can inhibit both innate and acquired immune responses against Salmonella (76, 109).
Concluding Remarks
Many studies have sought to elucidate how Salmonella achieves a balance between avoiding immune responses and surviving long-term in its host. Previous research indicates Salmonella exploits several types of immune cells to persist during chronic infections. Here, we presented evidence that B cells are an amenable bacterial reservoir, promoting their persistence, dissemination, and evasion of CD8+ T cell-mediated responses. Identifying the mechanisms employed by Salmonella-infected B cells to avoid cell-mediated immunity is clinically significant for understanding the chronic, asymptomatic carrier stage of Salmonella infection that occurs in human beings following typhoid fever.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by a Conacyt-SEP 132310 grant to Vianney Ortiz-Navarrete and a Conacyt-CVU 228852 grant to Marcela Lopez-Medina.
References
1. Coburn B, Grassl GA, Finlay BB. Salmonella, the host and disease: a brief review. Immunol Cell Biol (2007) 85:112–8. doi:10.1038/sj.icb.7100007
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
2. Knodler LA, Steele-Mortimer O. Taking possession: biogenesis of the Salmonella-containing vacuole. Traffic (2003) 4:587–99. doi:10.1034/j.1600-0854.2003.00118.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
3. Alpuche-Aranda CM, Racoosin EL, Swanson JA, Miller SI. Salmonella stimulate macrophage macropinocytosis and persist within spacious phagosomes. J Exp Med (1994) 179:601–8. doi:10.1084/jem.179.2.601
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
4. Ruby T, McLaughlin L, Gopinath S, Monack D. Salmonella’s long-term relationship with its host. FEMS Microbiol Rev (2012) 36:600–15. doi:10.1111/j.1574-6976.2012.00332.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
5. Haraga A, Ohlson MB, Miller SI. Salmonellae interplay with host cells. Nat Rev Microbiol (2008) 6:53–66. doi:10.1038/nrmicro1788
6. Finlay BB, Brumell JH. Salmonella interactions with host cells: in vitro to in vivo. Philos Trans R Soc Lond B Biol Sci (2000) 355:623–31. doi:10.1098/rstb.2000.0603
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
7. Prost LR, Sanowar S, Miller SI. Salmonella sensing of anti-microbial mechanisms to promote survival within macrophages. Immunol Rev (2007) 219:55–65. doi:10.1111/j.1600-065X.2007.00557.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
8. Kirby AC, Yrlid U, Svensson M, Wick MJ. Differential involvement of dendritic cell subsets during acute Salmonella infection. J Immunol (2001) 166:6802–11. doi:10.4049/jimmunol.166.11.6802
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
9. Sundquist M, Rydstrm A, Wick MJ. Immunity to Salmonella from a dendritic point of view. Cell Microbiol (2004) 6:1–11. doi:10.1046/j.1462-5822.2003.00336.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
10. Yrlid U, Svensson M, Kirby A, Wick MJ. Antigen-presenting cells and anti-Salmonella immunity. Microbes Infect (2001) 3:1239–48. doi:10.1016/S1286-4579(01)01484-8
11. Watts C. Capture and processing of exogenous antigens for presentation on MHC molecules. Annu Rev Immunol (1997) 15:821–50. doi:10.1146/annurev.immunol.15.1.821
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
12. Vyas JM, Van der Veen AG, Ploegh HL. The known unknowns of antigen processing and presentation. Nat Rev Immunol (2008) 8:607–18. doi:10.1038/nri2368
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
13. Tsomides TJ, Eisen HN. Antigenic structures recognized by cytotoxic T lymphocytes. J Biol Chem (1991) 266:3357–60.
14. Kovacsovics-Bankowski M, Clark K, Benacerraf B, Rock KL. Efficient major histocompatibility complex class I presentation of exogenous antigen upon phagocytosis by macrophages. Proc Natl Acad Sci U S A (1993) 90:4942–6. doi:10.1073/pnas.90.11.4942
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
15. Ackerman AL, Cresswell P. Cellular mechanisms governing cross-presentation of exogenous antigens. Nat Immunol (2004) 5:678–84. doi:10.1038/ni1082
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
16. Kovacsovics-Bankowski M, Rock KL. A phagosome-to-cytosol pathway for exogenous antigens presented on MHC class I molecules. Science (1995) 267:243–6. doi:10.1126/science.7809629
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
17. Pfeifer JD, Wick MJ, Roberts RL, Findlay K, Normark SJ, Harding CV. Phagocytic processing of bacterial antigens for class I MHC presentation to T cells. Nature (1993) 361:359–62. doi:10.1038/361359a0
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
18. Ramirez MC, Sigal LJ. The multiple routes of MHC-I cross-presentation. Trends Microbiol (2004) 12:204–7. doi:10.1016/j.tim.2004.03.008
19. Harding CV, Song R. Phagocytic processing of exogenous particulate antigens by macrophages for presentation by class I MHC molecules. J Immunol (1994) 153:4925–33.
20. Martín-Orozco N, Isibasi A, Ortiz-Navarrete V. Macrophages present exogenous antigens by class I major histocompatibility complex molecules via a secretory pathway as a consequence of interferon-gamma activation. Immunology (2001) 103:41–8. doi:10.1046/j.0019-2805.2001.01226.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
21. Crawford RW, Rosales-Reyes R, Ramírez-Aguilar M, Chapa-Azuela O, Alpuche-Aranda C, Gunn JS. Gallstones play a significant role in Salmonella spp. gallbladder colonization and carriage. Proc Natl Acad Sci U S A (2010) 107:4353–8. doi:10.1073/pnas.1000862107
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
22. Tobar JA, Carreño LJ, Bueno SM, Gonzalez PA, Mora JE, Quezada SA, et al. Virulent Salmonella enterica serovar typhimurium evades adaptive immunity by preventing dendritic cells from activating T cells. Infect Immun (2006) 74:6438–48. doi:10.1128/IAI.00063-06
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
23. Buchmeier NA, Heffron F. Inhibition of macrophage phagosome-lysosome fusion by Salmonella typhimurium. Infect Immun (1991) 59:2232–8.
24. Uchiya K, Barbieri MA, Funato K, Shah AH, Stahl PD, Groisman EA. A Salmonella virulence protein that inhibits cellular trafficking. EMBO J (1999) 18:3924–33. doi:10.1093/emboj/18.14.3924
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
25. Bueno SM, Gonzalez PA, Carreño LJ, Tobar JA, Mora GC, Pereda CJ, et al. The capacity of Salmonella to survive inside dendritic cells and prevent antigen presentation to T cells is host specific. Immunology (2008) 124:522–33. doi:10.1111/j.1365-2567.2008.02805.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
26. Hernandez LD, Hueffer K, Wenk MR, Galán JE. Salmonella modulates vesicular traffic by altering phosphoinositide metabolism. Science (2004) 304:1805–7. doi:10.1126/science.1098188
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
27. Bakowski MA, Braun V, Lam GY, Yeung T, Heo WD, Meyer T, et al. The phosphoinositide phosphatase SopB manipulates membrane surface charge and trafficking of the Salmonella-containing vacuole. Cell Host Microbe (2010) 7:453–62. doi:10.1016/j.chom.2010.05.011
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
28. Rosales-Reyes R, Alpuche-Aranda C, Ramírez-Aguilar M, Castro-Eguiluz AD, Ortiz-Navarrete V. Survival of Salmonella enterica serovar Typhimurium within late endosomal-lysosomal compartments of B lymphocytes is associated with the inability to use the vacuolar alternative major histocompatibility complex class I antigen-processing pathway. Infect Immun (2005) 73:3937–44. doi:10.1128/IAI.73.7.3937-3944.2005
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
29. Luu RA, Gurnani K, Dudani R, Kammara R, van Faassen H, Sirard JC, et al. Delayed expansion and contraction of CD8+ T cell response during infection with virulent Salmonella typhimurium. J Immunol (2006) 177:1516–25. doi:10.4049/jimmunol.177.3.1516
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
30. Sad S, Dudani R, Gurnani K, Russell M, van Faassen H, Finlay B, et al. Pathogen proliferation governs the magnitude but compromises the function of CD8 T cells. J Immunol (2008) 180:5853–61. doi:10.4049/jimmunol.180.9.5853
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
31. Helaine S, Cheverton AM, Watson KG, Faure LM, Matthews SA, Holden DW. Internalization of Salmonella by macrophages induces formation of nonreplicating persisters. Science (2014) 343:204–8. doi:10.1126/science.1244705
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
32. Lapaque N, Hutchinson JL, Jones DC, Meresse S, Holden DW, Trowsdale J, et al. Salmonella regulates polyubiquitination and surface expression of MHC class II antigens. Proc Natl Acad Sci U S A (2009) 106:14052–7. doi:10.1073/pnas.0906735106
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
33. Al-Ramadi BK, Brodkin MA, Mosser DM, Eisenstein TK. Immunosuppression induced by attenuated Salmonella. Evidence for mediation by macrophage precursors. J Immunol (1991) 146:2737–46.
34. Cheminay C, Mohlenbrink A, Hensel M. Intracellular Salmonella inhibit antigen presentation by dendritic cells. J Immunol (2005) 174:2892–9. doi:10.4049/jimmunol.174.5.2892
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
35. MacFarlane AS, Schwacha MG, Eisenstein TK. In vivo blockage of nitric oxide with aminoguanidine inhibits immunosuppression induced by an attenuated strain of Salmonella typhimurium, potentiates Salmonella infection, and inhibits macrophage and polymorphonuclear leukocyte influx into the spleen. Infect Immun (1999) 67:891–8.
36. Santhanam SK, Dutta D, Parween F, Qadri A. The virulence polysaccharide vi released by Salmonella typhi targets membrane prohibitin to inhibit T-cell activation. J Infect Dis (2014) 210:79–88. doi:10.1093/infdis/jiu064
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
37. Van der Velden AWM, Copass MK, Starnbach MN. Salmonella inhibit T cell proliferation by a direct, contact-dependent immunosuppressive effect. Proc Natl Acad Sci U S A (2005) 102:17769–74. doi:10.1073/pnas.0504382102
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
38. Srinivasan A, McSorley SJ. Pivotal advance: exposure to LPS suppresses CD4+ T cell cytokine production in Salmonella-infected mice and exacerbates murine typhoid. J Leukoc Biol (2007) 81:403–11. doi:10.1189/jlb.0306194
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
39. Ertelt JM, Johanns TM, Mysz MA, Nanton MR, Rowe JH, Aguilera MN, et al. Selective culling of high avidity antigen-specific CD4+ T cells after virulent Salmonella infection. Immunology (2011) 134:487–97. doi:10.1111/j.1365-2567.2011.03510.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
40. Srinivasan A, Nanton M, Griffin A, McSorley SJ. Culling of activated CD4 T cells during typhoid is driven by Salmonella virulence genes. J Immunol (2009) 182:7838–45. doi:10.4049/jimmunol.0900382
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
41. Deobagkar-Lele M, Chacko SK, Victor ES, Kadthur JC, Nandi D. Interferon gamma- and glucocorticoid-mediated pathways synergize to enhance death of CD4(+) CD8(+) thymocytes during Salmonella enterica serovar Typhimurium infection. Immunology (2013) 138:307–21. doi:10.1111/imm.12047
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
42. Ross EA, Coughlan RE, Flores-Langarica A, Lax S, Nicholson J, Desanti GE, et al. Thymic function is maintained during Salmonella-induced atrophy and recovery. J Immunol (2012) 189:4266–74. doi:10.4049/jimmunol.1200070
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
43. Van der Velden AWM, Dougherty JT, Starnbach MN. Down-modulation of TCR expression by Salmonella enterica serovar Typhimurium. J Immunol (2008) 180:5569–74. doi:10.4049/jimmunol.180.8.5569
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
44. Johanns TM, Ertelt JM, Rowe JH, Way SS. Regulatory T cell suppressive potency dictates the balance between bacterial proliferation and clearance during persistent Salmonella infection. PLoS Pathog (2010) 6:e1001043. doi:10.1371/journal.ppat.1001043
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
45. Naiki Y, Nishimura H, Kawano T, Tanaka Y, Itohara S, Taniguchi M, et al. Regulatory role of peritoneal NK1.1+ alpha beta T cells in IL-12 production during Salmonella infection. J Immunol (1999) 163:2057–63.
46. Nakashima M, Kinoshita M, Nakashima H, Habu Y, Miyazaki H, Shono S, et al. Pivotal advance: characterization of mouse liver phagocytic B cells in innate immunity. J Leukoc Biol (2012) 91:537–46. doi:10.1189/jlb.0411214
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
47. Vidard L, Kovacsovics-Bankowski M, Kraeft SK, Chen LB, Benacerraf B, Rock KL. Analysis of MHC class II presentation of particulate antigens of B lymphocytes. J Immunol (1996) 156:2809–18.
48. Kraal G, Mebius R. New insights into the cell biology of the marginal zone of the spleen. Int Rev Cytol (2006) 250:175–215. doi:10.1016/S0074-7696(06)50005-1
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
49. Pillai S, Cariappa A, Moran ST. Marginal zone B cells. Annu Rev Immunol (2005) 23:161–96. doi:10.1146/annurev.immunol.23.021704.115728
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
50. Cinamon G, Zachariah MA, Lam OM, Foss FW, Cyster JG. Follicular shuttling of marginal zone B cells facilitates antigen transport. Nat Immunol (2008) 9:54–62. doi:10.1038/ni1542
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
51. Oliver AM, Martin F, Kearney JF. IgMhighCD21high lymphocytes enriched in the splenic marginal zone generate effector cells more rapidly than the bulk of follicular B cells. J Immunol (1999) 162:7198–207.
52. Attanavanich K, Kearney JF. Marginal zone, but not follicular B cells, are potent activators of naive CD4 T cells. J Immunol (2004) 172:803–11. doi:10.4049/jimmunol.172.2.803
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
53. Martin F, Kearney JF. B1 cells: similarities and differences with other B cell subsets. Curr Opin Immunol (2001) 13:195–201. doi:10.1016/S0952-7915(00)00204-1
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
54. Parra D, Rieger AM, Li J, Zhang Y-A, Randall LM, Hunter CA, et al. Pivotal advance: peritoneal cavity B-1 B cells have phagocytic and microbicidal capacities and present phagocytosed antigen to CD4+ T cells. J Leukoc Biol (2012) 91:525–36. doi:10.1189/jlb.0711372
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
55. Martin F, Oliver AM, Kearney JF. Marginal Zone and B1 B Cells Unite in the Early Response against T-Independent Blood-Borne Particulate Antigens. Immunity (2001) 14:617–29. doi:10.1016/S1074-7613(01)00129-7
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
56. Cariappa A, Mazo IB, Chase C, Shi HN, Liu H, Li Q, et al. Perisinusoidal B cells in the bone marrow participate in T-independent responses to blood-borne microbes. Immunity (2005) 23:397–407. doi:10.1016/j.immuni.2005.09.004
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
57. Li J, Barreda DR, Zhang Y-A, Boshra H, Gelman AE, Lapatra S, et al. B lymphocytes from early vertebrates have potent phagocytic and microbicidal abilities. Nat Immunol (2006) 7:1116–24. doi:10.1038/ni1389
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
58. Gitlin AD, Shulman Z, Nussenzweig MC. Clonal selection in the germinal centre by regulated proliferation and hypermutation. Nature (2014) 509:637–40. doi:10.1038/nature13300
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
59. Choi YS, Kageyama R, Eto D, Escobar TC, Johnston RJ, Monticelli L, et al. ICOS receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor Bcl6. Immunity (2011) 34:932–46. doi:10.1016/j.immuni.2011.03.023
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
60. Barnett LG, Simkins HMA, Barnett BE, Korn LL, Johnson AL, Wherry EJ, et al. B cell antigen presentation in the initiation of follicular helper T cell and germinal center differentiation. J Immunol (2014) 192:3607–17. doi:10.4049/jimmunol.1301284
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
61. Crotty S. Follicular helper CD4 T cells (Tfh). Annu Rev Immunol (2011) 29:621–63. doi:10.1146/annurev-immunol-031210-101400
62. Barr TA, Brown S, Mastroeni P, Gray D. B cell intrinsic MyD88 signals drive IFN-gamma production from T cells and control switching to IgG2c. J Immunol (2009) 183:1005–12. doi:10.4049/jimmunol.0803706
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
63. Barr TA, Brown S, Mastroeni P, Gray D. TLR and B cell receptor signals to B cells differentially program primary and memory Th1 responses to Salmonella enterica. J Immunol (2010) 185:2783–9. doi:10.4049/jimmunol.1001431
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
64. Ciavarra RP, Burgess DH. Antigen-presenting B cells: efficient uptake and presentation by activated B cells for induction of cytotoxic T lymphocytes against vesicular stomatitis virus. Cell Immunol (1988) 114:27–40. doi:10.1016/0008-8749(88)90252-3
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
65. Battegay M, Moskophidis D, Waldner H, Brundler MA, Fung-Leung WP, Mak TW, et al. Impairment and delay of neutralizing antiviral antibody responses by virus-specific cytotoxic T cells. J Immunol (1993) 151:5408–15.
66. Planz O, Seiler P, Hengartner H, Zinkernagel RM. Specific cytotoxic T cells eliminate B cells producing virus-neutralizing antibodies [corrected]. Nature (1996) 382:726–9. doi:10.1038/382726a0
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
67. Bonilla WV, Fröhlich A, Senn K, Kallert S, Fernandez M, Johnson S, et al. The alarmin interleukin-33 drives protective antiviral CD8? T cell responses. Science (2012) 335:984–9. doi:10.1126/science.1215418
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
68. Zhang N, Bevan MJ. CD8(+) T cells: foot soldiers of the immune system. Immunity (2011) 35:161–8. doi:10.1016/j.immuni.2011.07.010
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
69. Liu Y, Mullbacher A. Activated B cells can deliver help for the in vitro generation of antiviral cytotoxic T cells. Proc Natl Acad Sci (1989) 86:4629–33. doi:10.1073/pnas.86.12.4629
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
70. Tobian AAR, Harding CV, Canaday DH. Mycobacterium tuberculosis heat shock fusion protein enhances class I MHC cross-processing and -presentation by B lymphocytes. J Immunol (2005) 174:5209–14. doi:10.4049/jimmunol.174.9.5209
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
71. Robson NC, Donachie AM, Mowat AM. Simultaneous presentation and cross-presentation of immune-stimulating complex-associated cognate antigen by antigen-specific B cells. Eur J Immunol (2008) 38:1238–46. doi:10.1002/eji.200737758
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
72. De Wit J, Souwer Y, Jorritsma T, Klaasse Bos H, ten Brinke A, Neefjes J, et al. Antigen-specific B cells reactivate an effective cytotoxic T cell response against phagocytosed Salmonella through cross-presentation. PLoS One (2010) 5:e13016. doi:10.1371/journal.pone.0013016
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
73. Souwer Y, Griekspoor A, Jorritsma T, de Wit J, Janssen H, Neefjes J, et al. B cell receptor-mediated internalization of Salmonella: a novel pathway for autonomous B cell activation and antibody production. J Immunol (2009) 182:7473–81. doi:10.4049/jimmunol.0802831
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
74. Mauri C, Bosma A. Immune regulatory function of B cells. Annu Rev Immunol (2012) 30:221–41. doi:10.1146/annurev-immunol-020711-074934
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
75. Wang R-X, Yu C-R, Dambuza IM, Mahdi RM, Dolinska MB, Sergeev YV, et al. Interleukin-35 induces regulatory B cells that suppress autoimmune disease. Nat Med (2014) 20:633–41. doi:10.1038/nm.3554
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
76. Shen P, Roch T, Lampropoulou V, O’Connor RA, Stervbo U, Hilgenberg E, et al. IL-35-producing B cells are critical regulators of immunity during autoimmune and infectious diseases. Nature (2014) 507:366–70. doi:10.1038/nature12979
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
77. Eckmann L, Kagnoff MF. Cytokines in host defense against Salmonella. Microbes Infect (2001) 3:1191–200. doi:10.1016/S1286-4579(01)01479-4
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
78. Pie S, Truffa-Bachi P, Pla M, Nauciel C. Th1 response in Salmonella typhimurium-infected mice with a high or low rate of bacterial clearance. Infect Immun (1997) 65:4509–14.
79. Mittrücker HW, Köhler A, Kaufmann SH. Characterization of the murine T-lymphocyte response to Salmonella enterica serovar Typhimurium infection. Infect Immun (2002) 70:199–203. doi:10.1128/IAI.70.1.199-203.2002
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
80. Hess J, Ladel C, Miko D, Kaufmann SH. Salmonella typhimurium aroA-infection in gene-targeted immunodeficient mice: major role of CD4+ TCR-alpha beta cells and IFN-gamma in bacterial clearance independent of intracellular location. J Immunol (1996) 156:3321–6.
81. Monack DM, Bouley DM, Falkow S. Salmonella typhimurium persists within macrophages in the mesenteric lymph nodes of chronically infected Nramp1+/+ mice and can be reactivated by IFN-gamma neutralization. J Exp Med (2004) 199:231–41. doi:10.1084/jem.20031319
82. Mastroeni P, Simmons C, Fowler R, Hormaeche CE, Dougan G. Igh-6(-/-) (B-cell-deficient) mice fail to mount solid acquired resistance to oral challenge with virulent Salmonella enterica serovar Typhimurium and show impaired Th1 T-cell responses to Salmonella antigens. Infect Immun (2000) 68:46–53. doi:10.1128/IAI.68.1.46-53.2000
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
83. Mittrücker HW, Raupach B, Köhler A, Kaufmann SH. Cutting edge: role of B lymphocytes in protective immunity against Salmonella typhimurium infection. J Immunol (2000) 164:1648–52. doi:10.4049/jimmunol.164.4.1648
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
84. McSorley SJ, Jenkins MK. Antibody is required for protection against virulent but not attenuated Salmonella enterica serovar typhimurium. Infect Immun (2000) 68:3344–8. doi:10.1128/IAI.68.6.3344-3348.2000
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
85. Ugrinovic S, Menager N, Goh N, Mastroeni P. Characterization and development of T-Cell immune responses in B-cell-deficient (Igh-6(-/-)) mice with Salmonella enterica serovar Typhimurium infection. Infect Immun (2003) 71:6808–19. doi:10.1128/IAI.71.12.6808-6819.2003
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
86. Nanton MR, Way SS, Shlomchik MJ, McSorley SJ. Cutting edge: B cells are essential for protective immunity against Salmonella independent of antibody secretion. J Immunol (2012) 189:5503–7. doi:10.4049/jimmunol.1201413
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
87. Perez-Lopez A, Rosales-Reyes R, Alpuche-Aranda CM, Ortiz-Navarrete V. Salmonella downregulates Nod-like receptor family CARD domain containing protein 4 expression to promote its survival in B cells by preventing inflammasome activation and cell death. J Immunol (2013) 190:1201–9. doi:10.4049/jimmunol.1200415
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
88. Lee S, Dunmire S, McSorley SJ. MHC class-I-restricted CD8 T cells play a protective role during primary Salmonella infection. Immunol Lett (2012) 148:138–43. doi:10.1016/j.imlet.2012.10.009
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
89. Nauciel C. Role of CD4+ T cells and T-independent mechanisms in acquired resistance to Salmonella typhimurium infection. J Immunol (1990) 145:1265–9.
90. Soloski MJ, Metcalf ES. The involvement of class Ib molecules in the host response to infection with Salmonella and its relevance to autoimmunity. Microbes Infect (2001) 3:1249–59. doi:10.1016/S1286-4579(01)01485-X
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
91. Ugrinovic S, Brooks CG, Robson J, Blacklaws BA, Hormaeche CE, Robinson JH. H2-M3 major histocompatibility complex class Ib-restricted CD8 T cells induced by Salmonella enterica serovar Typhimurium infection recognize proteins released by Salmonella serovar Typhimurium. Infect Immun (2005) 73:8002–8. doi:10.1128/IAI.73.12.8002-8008.2005
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
92. Mariño E, Tan B, Binge L, Mackay CR, Grey ST. B-cell cross-presentation of autologous antigen precipitates diabetes. Diabetes (2012) 61:2893–905. doi:10.2337/db12-0006
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
93. Wain J, Diep TS, Ho VA, Walsh AM, Nguyen TT, Parry CM, et al. Quantitation of bacteria in blood of typhoid fever patients and relationship between counts and clinical features, transmissibility, and antibiotic resistance. J Clin Microbiol (1998) 36:1683–7.
94. Gonzalez-Escobedo G, Marshall JM, Gunn JS. Chronic and acute infection of the gall bladder by Salmonella Typhi: understanding the carrier state. Nat Rev Microbiol (2011) 9:9–14. doi:10.1038/nrmicro2490
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
95. Gonzalez-Escobedo G, Gunn JS. Gallbladder epithelium as a niche for chronic Salmonella carriage. Infect Immun (2013) 81:2920–30. doi:10.1128/IAI.00258-13
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
96. Rosales-Reyes R, Perez-Lopez A, Sanchez-Gomez C, Hernandez-Mote RR, Castro-Eguiluz D, Ortiz-Navarrete V, et al. Salmonella infects B cells by macropinocytosis and formation of spacious phagosomes but does not induce pyroptosis in favor of its survival. Microb Pathog (2012) 52:367–74. doi:10.1016/j.micpath.2012.03.007
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
97. Castro-Eguiluz D, Pelayo R, Rosales-Garcia V, Rosales-Reyes R, Alpuche-Aranda C, Ortiz-Navarrete V. B cell precursors are targets for Salmonella infection. Microb Pathog (2009) 47:52–6. doi:10.1016/j.micpath.2009.04.005
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
98. Radbruch A, Muehlinghaus G, Luger EO, Inamine A, Smith KGC, Dorner T, et al. Competence and competition: the challenge of becoming a long-lived plasma cell. Nat Rev Immunol (2006) 6:741–50. doi:10.1038/nri1886
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
99. Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature (2006) 439:682–7. doi:10.1038/nature04444
100. Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med (2006) 12:1198–202. doi:10.1038/nm1106-1329b
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
101. Golden-Mason L, Palmer B, Klarquist J, Mengshol JA, Castelblanco N, Rosen HR. Upregulation of PD-1 expression on circulating and intrahepatic hepatitis C virus-specific CD8+ T cells associated with reversible immune dysfunction. J Virol (2007) 81:9249–58. doi:10.1128/JVI.00409-07
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
102. Peng G, Li S, Wu W, Tan X, Chen Y, Chen Z. PD-1 upregulation is associated with HBV-specific T cell dysfunction in chronic hepatitis B patients. Mol Immunol (2008) 45:963–70. doi:10.1016/j.molimm.2007.07.038
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
103. Dulgerian LR, Garrido VV, Stempin CC, Cerbán FM. Programmed death ligand 2 regulates arginase induction and modifies Trypanosoma cruzi survival in macrophages during murine experimental infection. Immunology (2011) 133:29–40. doi:10.1111/j.1365-2567.2011.03406.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
104. Smith P, Walsh CM, Mangan NE, Fallon RE, Sayers JR, McKenzie ANJ, et al. Schistosoma mansoni worms induce anergy of T cells via selective up-regulation of programmed death ligand 1 on macrophages. J Immunol (2004) 173:1240–8. doi:10.4049/jimmunol.173.2.1240
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
105. Terrazas LI, Montero D, Terrazas CA, Reyes JL, Rodriguez-Sosa M. Role of the programmed Death-1 pathway in the suppressive activity of alternatively activated macrophages in experimental cysticercosis. Int J Parasitol (2005) 35:1349–58. doi:10.1016/j.ijpara.2005.06.003
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
106. Jurado JO, Alvarez IB, Pasquinelli V, Martínez GJ, Quiroga MF, Abbate E, et al. Programmed death (PD)-1:PD-ligand 1/PD-ligand 2 pathway inhibits T cell effector functions during human tuberculosis. J Immunol (2008) 181:116–25. doi:10.4049/jimmunol.181.1.116
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
107. Das S, Suarez G, Beswick EJ, Sierra JC, Graham DY, Reyes VE. Expression of B7-H1 on gastric epithelial cells: its potential role in regulating T cells during Helicobacter pylori infection. J Immunol (2006) 176:3000–9. doi:10.4049/jimmunol.176.5.3000
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
108. Fankhauser SC, Starnbach MN. PD-L1 limits the mucosal CD8+ T cell response to Chlamydia trachomatis. J Immunol (2014) 192:1079–90. doi:10.4049/jimmunol.1301657
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
109. Neves P, Lampropoulou V, Calderon-Gomez E, Roch T, Stervbo U, Shen P, et al. Signaling via the MyD88 adaptor protein in B cells suppresses protective immunity during Salmonella typhimurium infection. Immunity (2010) 33:777–90. doi:10.1016/j.immuni.2010.10.016
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Keywords: B cells, Salmonella, CD8 T cells, cross-presentation, PD-L1
Citation: Lopez-Medina M, Perez-Lopez A, Alpuche-Aranda C and Ortiz-Navarrete V (2014) Salmonella modulates B cell biology to evade CD8+ T cell-mediated immune responses. Front. Immunol. 5:586. doi: 10.3389/fimmu.2014.00586
Received: 14 July 2014; Accepted: 03 November 2014;
Published online: 21 November 2014.
Edited by:
Adam Cunningham, University of Birmingham, UKReviewed by:
June Round, University of Utah, USAHeinrich Korner, Menzies Research Institute Tasmania, Australia
Copyright: © 2014 Lopez-Medina, Perez-Lopez, Alpuche-Aranda and Ortiz-Navarrete. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vianney Ortiz-Navarrete, Departamento de Biomedicina Molecular, Centro de Investigación y Estudios Avanzados del IPN, Av. IPN 2508, México City, DF 07360, México e-mail: vortiz@cinvestav.mx
 Marcela Lopez-Medina
Marcela Lopez-Medina Araceli Perez-Lopez2
Araceli Perez-Lopez2 Vianney Ortiz-Navarrete
Vianney Ortiz-Navarrete