- 1Zvitambo Institute for Maternal and Child Health Research, Harare, Zimbabwe
- 2Blizard Institute, Queen Mary University of London, London, UK
- 3Department of International Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA
The ZVITAMBO trial recruited 14,110 mother–infant pairs to a randomized controlled trial of vitamin A between 1997 and 2000, before the availability of antiretroviral therapy for HIV prophylaxis or treatment in Zimbabwe. The HIV status of mothers and infants was well characterized through 1–2 years of follow-up, leading to the largest cohort to date of HIV-exposed uninfected (HEU) infants (n = 3135), with a suitable comparison group of HIV-unexposed infants (n = 9510). Here, we draw on 10 years of published findings from the ZVITAMBO trial. HEU infants had increased morbidity compared to HIV-unexposed infants, with 50% more hospitalizations in the neonatal period and 30% more sick clinic visits during infancy, particularly for skin infections, lower respiratory tract infections, and oral thrush. HEU children had 3.9-fold and 2.0-fold higher mortality than HIV-unexposed children during the first and second years of life, respectively, most commonly due to acute respiratory infections, diarrhea/dysentery, malnutrition, sepsis, and meningitis. Infant morbidity and mortality were strongly related to maternal HIV disease severity, and increased morbidity remained until maternal CD4 counts were >800 cells/μL. HEU infants were more likely to be premature and small-for-gestational age than HIV-unexposed infants, and had more postnatal growth failure. Here, we propose a conceptual framework to explain the increased risk of infectious morbidity, mortality, and growth failure among HEU infants, hypothesizing that immune activation and inflammation are key drivers of both infection susceptibility and growth failure. Future studies should further dissect the causes of infection susceptibility and growth failure and determine the impact of ART and cotrimoxazole on outcomes of this vulnerable group of infants in the current era.
Introduction
Before the availability of antiretroviral therapy (ART), around a quarter of infants born to HIV-infected women in Zimbabwe acquired the infection (1) and almost two-thirds of perinatally infected children died before their second birthday (2). Coverage of effective interventions for prevention of mother-to-child transmission (PMTCT) is increasing faster than antenatal HIV prevalence is declining, meaning that fewer HIV-infected infants are born annually, but a population of HIV-exposed uninfected (HEU) infants is emerging (3). Data from several settings over the past decade suggest that HEU children have poorer health outcomes than HIV-unexposed children. However, many studies have not fully characterized maternal and infant HIV status, or have included control groups of HIV-unexposed infants who differ significantly in socioeconomic status or breast-feeding pattern, which may lead to confounding. Furthermore, modern studies are complicated by exposure to maternal and infant ART for PMTCT or cotrimoxazole prophylaxis, making the natural history of HIV exposure difficult to determine. Findings from historical cohorts with comparable control populations are necessary to understand the health outcomes of HEU infants.
Between 1997 and 2000, 14,110 mother–infant pairs were recruited to a randomized controlled trial of maternal and infant vitamin A in Zimbabwe. The Zimbabwe Vitamin A for Mothers and Babies (ZVITAMBO) trial (1) took place before the availability of ART for prophylaxis or treatment in Zimbabwe, or the recommendation to provide cotrimoxazole to HIV-exposed infants. The HIV status of mothers and infants was well characterized through 1–2 years of follow-up, leading to the largest cohort to date of HEU infants (n = 3135), with a suitable comparison group of HIV-unexposed infants (n = 9510). This Review draws on 10 years of published data from this birth cohort, which has provided some of the strongest evidence to date of the poor health outcomes of HEU infants. First, we discuss the morbidity and mortality of HEU infants and the relationship between maternal characteristics and HEU infant outcomes. Second, we discuss growth outcomes, and set these findings within the wider context of other studies that have shown heterogeneous results. Third, we discuss the need for appropriate feeding strategies in HIV-exposed infants to ensure HIV-free survival and to reduce all-cause morbidity and mortality.
We propose a conceptual framework for poor outcomes in HEU infants and discuss the potential mechanisms underlying our key findings of infection susceptibility and growth failure, drawing also on other published data.
The ZVITAMBO Trial
The ZVITAMBO trial was a randomized placebo-controlled trial of maternal and/or neonatal vitamin A to reduce HIV transmission and improve child mortality (1, 4). In brief, 14,110 postpartum mothers and their infants were enrolled within 96 h of delivery between November 1997 and January 2000 in Harare. Mothers and infants were eligible if neither had an acutely life-threatening condition; the infant was a singleton with birth weight ≥1500 g, and the mother planned to stay in Harare after delivery. A single large dose of vitamin A was given in a factorial design either to mother and infant, mother only, infant only, or neither. All but four infants started breast-feeding; at 6 months, 93% of infants were mixed breast-fed. The HIV status of mothers and infants was well characterized: at baseline, 9562 (67.8%) mothers were HIV-negative and 4495 (31.9%) HIV-positive; the remaining 53 mothers were HIV indeterminate. Of infants born to HIV-positive mothers, 381, 508, and 258 were infected in utero, intrapartum, and postnatally, respectively; 189 infants became HIV-infected, but the timing was uncertain; and 24 infants did not undergo PCR testing at any time, leaving 3135 live born infants who never had a PCR-positive test and were classified as HEU. Trial participants provided written informed consent for storage and use of data and samples for future, related studies. Overall, the trial found no effect of vitamin A on child mortality (1, 4) or on HIV transmission among HIV-exposed infants (1).
The ZVITAMBO trial offered a unique opportunity to study HEU infants for several key reasons: first, maternal and infant HIV status was well characterized throughout follow-up (see below); second, the HIV-unexposed comparison group was similar and contemporaneous; third, the majority of infants in each group had similar feeding patterns (mixed breast-feeding); and, fourth, all infants had access to a free “sick clinic,” allowing similar assessment of morbidity status between groups.
Methods for Identifying HIV Infection
Studies investigating the outcomes of HEU infants need to ensure regular HIV testing of mothers and infants. Without repeat testing of mothers, those seroconverting during follow-up will be unidentified, meaning HIV-unexposed infant groups may be contaminated with HEU or HIV-infected infants; without repeat testing of infants, HEU infant groups may be contaminated with postnatally infected infants. In the ZVITAMBO trial, mothers were first tested for HIV at baseline; those testing HIV negative were retested at every subsequent blood draw to detect seroconversion. Infants born to mothers who remained HIV uninfected throughout follow-up were classified as HIV unexposed. Children born to HIV-positive mothers had samples stored at −70°C. At the end of the follow-up period, the last available sample from each child was tested for HIV; if this was negative, the child was classified as HEU. Various methods were used to ensure that the HEU group was not contaminated with postnatally infected infants, including censoring infants at the last negative HIV test if further testing was not conducted before the end of follow-up or infant death (as described below) (2, 5).
Morbidity and Mortality of HEU Infants
Morbidity
HIV-exposed uninfected infants in the ZVITAMBO trial had clear evidence of increased infectious morbidity compared to HIV-unexposed infants (5). HEU infants had 30% more sick clinic visits in the first year of life, peaking between 1 and 3 months of age, and 50% more hospitalizations within the first 28 days of life.
Sick Clinic Visits
The incidence of sick clinic visits among HEU infants was highest in the first 3 months of life, and remained significantly higher than for HIV-unexposed infants throughout infancy. The incidence rate ratios (IRR) for sick clinic visits were 1.2 (95% CI 1.1–1.4), 1.4 (1.3–1.5), 1.1 (1.1–1.2), and 1.1 (1.1–1.2) for 0–28, 29–91, 92–182, and 182–365 days, respectively. The most common illnesses among HEU infants were skin infections, lower respiratory tract infections, and oral thrush. Lower respiratory tract infections were particularly common in the first 3 months of life, with IRR of 1.6 (95% CI 1.1–2.3) and 1.5 (1.2–1.8) for 0–28 and 29–91 days, respectively (5).
Hospitalization
All-cause hospitalization was significantly higher in the first 28 days of life (IRR 1.5, 95% CI 1.2–2.0) among HEU compared to HIV-unexposed infants, with a trend toward increased all-cause hospitalization through 6 months of age. Hospitalization for malnutrition or diarrhea was common overall, but was not increased in HEU compared to HIV-unexposed infants, which may be due to the similar breast-feeding rates between groups. Increased risk of hospitalization for lower respiratory tract infections was particularly high in the first 28 days of life (IRR 2.7, 95% CI 1.6–4.7) (5).
Mortality
HIV-exposed uninfected children had higher mortality than HIV-unexposed children through 2 years of age (2). The mortality difference between groups was twice as high during the first year (3.9-fold) compared to the second year of life (2.0-fold), highlighting infancy as a period of particularly high mortality, and suggesting an attenuation of mortality risk over time. The proportion of HEU infants who died by 30 days, 6 months, 1 and 2 years of age was 1.9% (95% CI 1.4–2.5), 6.0% (5.2–7.0), 7.4% (6.5–8.4) and 9.2% (8.1–10.5), respectively, compared to 0.7% (0.6–0.9), 1.6% (1.3–1.8), 1.9% (1.7–2.2) and 2.9% (2.5–3.5) of HIV-unexposed infants. Notably, the difference in mortality between groups was greater than the difference in morbidity; this may indicate greater severity of infections in HEU infants, as recently demonstrated in respiratory syncytial virus-associated lower respiratory tract infections in South Africa (6). The most common causes of death in HEU infants were acute respiratory infections (57.7%), diarrheal illness/dysentery (16.1%), malnutrition (13.3%), sepsis (6.0%), and meningitis (4.8%). Overall, the causes of death were similar between HIV-unexposed, HEU and HIV-infected infants.
Sensitivity Analyses
Because of the possibility that the HEU infant group was contaminated with postnatally infected infants who died prior to testing HIV-positive, several sensitivity analyses were conducted. For morbidity, HEU infants were censored 42 days before their last HIV test (taking into account the window period for the test). The original analysis may in fact have underestimated morbidity among HEU infants, because the risk of hospitalization increased in sensitivity analyses, thereby adding confidence to the initial findings (5). For mortality, the sensitivity analyses included only those infants with at least one negative HIV test after 8 weeks of age; despite this, mortality remained 2.5-fold and 2.0-fold higher among HEU compared to HIV-unexposed infants by 12 and 24 months, respectively (2).
Why Are HEU Infants at Risk of Infection and Death?
Here, we evaluate the potential underlying causes of morbidity and mortality in HEU infants, drawing on available evidence and plausible mechanisms from animal models and in vitro studies. In Figure 1, we propose a conceptual framework to explain infection susceptibility in HEU infants.
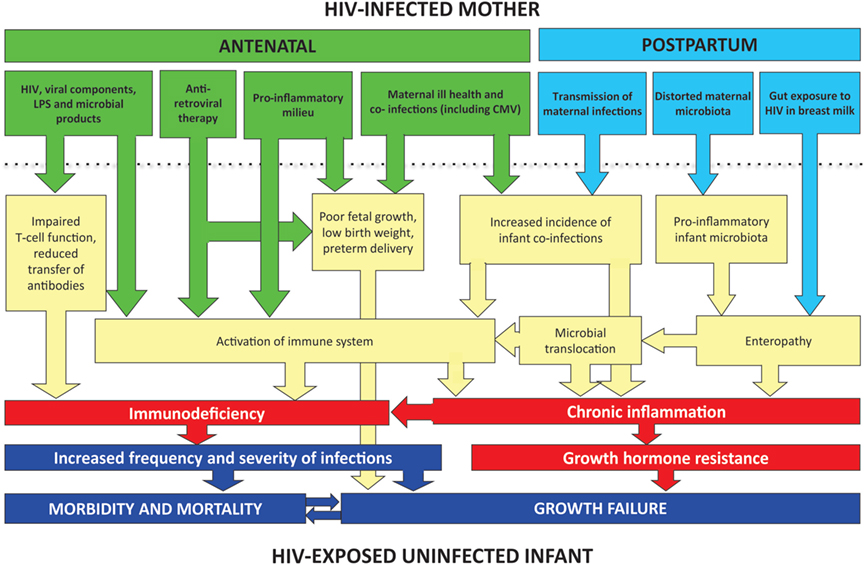
Figure 1. Conceptual framework for poor clinical outcomes of HEU infants. A combination of in utero and postnatal exposures may contribute to inflammation and immune activation in HEU infants. Immunodeficiency may be related directly to HIV exposure or may occur indirectly through reduced transplacental transfer of antibodies. Coinfections before and after birth (such as CMV and malaria) may also contribute to immune activation. Postnatally, exposure to HIV in breast milk may disrupt the intestinal barrier and lead to an enteropathy and microbial translocation. In non-breast-feeding infants, enteropathy may still be present secondary to abnormal assembly of the infant gut microbiota.
A growing body of evidence indicates that HEU infants have immunological abnormalities. First, studies show low concentrations of maternally derived antibody at birth (7–12). As newborns rely heavily on passive immunity before maturation of their own adaptive responses, this paucity of antibody may leave HEU infants at particular risk of infection. Second, there are numerous T-cell abnormalities: low CD4 count (13), high frequency of “double-negative” (CD4–/CD8–) T-cells (14, 15), and activated T-cell phenotypes (13, 14, 16–19) have all been well described. As T-cells are the primary target for HIV, it is perhaps unsurprising that they appear disproportionately affected in infants exposed to the virus. Third, HEU infants have elevated markers of immune activation and systemic inflammation (13, 14, 16–24).
Immune activation is an important cause of immune dysfunction in HIV-infected individuals, and its severity may be a better prognostic marker than HIV viral load (25). Animal models demonstrate the importance of chronic immune activation in growth failure and infection susceptibility. Transgenic mice that constitutively expressed CD70, leading to chronic T-cell stimulation, developed progressive naive T-cell depletion, weight loss, and premature death from Pneumocystis jirovecii pneumonia (26). Chronic immune activation in HEU infants may lead to infection susceptibility, and the resulting inflammation may further supress immune function. The causes of immune activation in HEU infants have not been well established; here, we speculate on plausible underlying causes (Figure 1).
Direct Exposure to the HIV Virus In Utero and the Influence of Maternal HIV Disease Severity
Fetal immune activation may result from direct exposure to HIV in utero; notably, HEU infants have evidence of HIV-specific T-cell responses (27, 28), suggestive of in utero sensitization. These responses are greater in infants born to mothers with high compared to low viral loads (29). Direct exposure to HIV or its components at a critical time of T-cell development in utero may contribute to the T-cell abnormalities described. HIV genomic material has been found in macrophages of the chorionic villus and in trophoblasts (30, 31). Components of HIV such as Nef have complex effects on the immune system, including CD4 depletion, activation, and apoptosis (32, 33). Furthermore, in a rodent model, Nef breaches placental barrier function and may enable HIV, other viral proteins and microbial products (see below) to cross the placenta (34), potentially exacerbating effects on the fetal immune system.
HIV-exposed uninfected infant morbidity and mortality outcomes in the ZVITAMBO trial were strongly influenced by maternal factors (Table 1) (2, 5). Infants born to mothers with more severe HIV disease (as assessed by maternal CD4 count) had higher rates of morbidity and mortality than those born to mothers with less severe HIV disease. Compared to HEU infants born to mothers with CD4 counts >400 cells/μL, those born to mothers with CD4 counts <200 cells/μL had 2.6-fold increased mortality by 2 years of age (95% CI 1.8–3.8). Increased morbidity risk remained until maternal CD4 counts were above 800 cells/μL. Oral candidiasis, an important indicator of immune function in the context of HIV, was particularly associated with maternal disease severity; compared to HIV-unexposed infants, HEU infants born to mothers with CD4 counts <200 cells/μL had an incidence rate ratio of oral thrush of 3.91 (95% CI 2.29–6.66), whereas those born to mothers with CD4 counts >800 cells/μL had an IRR of 1.91 (95% CI 1.02–3.58). The difference between these two HEU groups was statistically significant (P < 0.05).
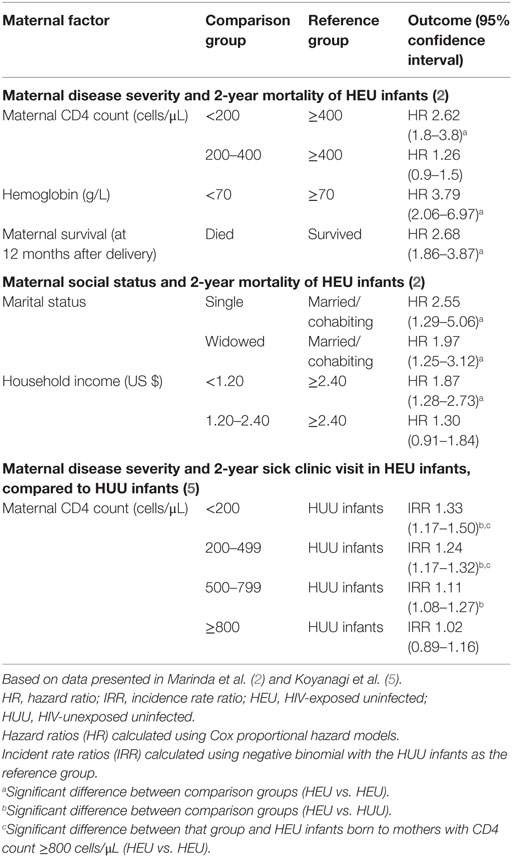
Table 1. Associations between maternal factors and HEU morbidity and mortality in the ZVITAMBO cohort.
The relative influences of maternal viremia (as assessed by viral load) and maternal immune suppression (as assessed by CD4 count) on HEU outcomes should be determined in future studies; this may help to establish the drivers of infection susceptibility, particularly in the current ART era.
Increased Exposure to Coinfections
Coinfections such as cytomegalovirus (CMV) are more prevalent in HIV-infected compared to HIV-uninfected pregnant women (35, 36), and could contribute to immune activation in their offspring both before (37) and after (38–40) birth. Infants born to HIV-infected mothers have a very high frequency of congenital CMV infection (11–29%) (36, 41, 42), and postnatal infection is almost ubiquitous during infancy in sub-Saharan Africa. There is an overlap between the effects of CMV infection and HIV exposure in infancy, including growth failure (43) and mortality (43, 44). For example, HIV-exposed infants with pneumonia in South Africa had 4.3-fold higher frequency of CMV viremia compared to asymptomatic HIV-exposed infants after adjusting for infant HIV infection (95% CI 2.6–7.0) (45).
There are plausible reasons why CMV acquisition may lead to immune dysfunction. First, in order to evade the immune system and promote latency, CMV has evolved multiple immunomodulatory properties to downregulate the human immune system (46, 47). Therefore, CMV infection in early life could be associated with increased susceptibility to other childhood infections. Second, evidence from humans and from murine models suggests that CMV causes immune activation. Studies report associations between primary and latent CMV infection and immune activation in adults; (48) a bias toward pro-inflammatory and Th17-polarized cytokines in the placenta and amniotic fluid during maternal CMV infection; (49) a lower regulatory T-cell (Treg)/Th17 ratio in CMV-infected children; and an impact of CMV coinfection on immune activation in HIV-infected individuals (50). Th17-polarized cytokines are associated with increased inflammation in response to viral infections, and it has been suggested that this may increase mortality (51). Furthermore, inflammation associated with CMV carrier status may affect responses to vaccinations (52). Third, CMV is acquired either in utero or early in infancy in sub-Saharan Africa and typically induces large magnitude immune responses. Infants with congenital CMV infection have evidence of considerable expansions in γδ T-cells (53), NK cells (54), and conventional αβ T-cells with a highly differentiated phenotype (55). Whether primary CMV infection at a critical time of immune development causes immunomodulation in HEU infants, and whether this alters infection susceptibility or mortality, has not been well addressed to date. We hypothesize that primary CMV infection in HEU infants results in inflammation and distortion of the immune system, leading to increased infection susceptibility, but further studies are required.
Pharmacological interventions to prevent CMV transmission to HEU infants have been considered a potentially important intervention but have so far reported unfavorable results. A Kenyan trial, in which women were randomized to 12 months of valaciclovir or placebo from 34 gestational weeks, did not reduce CMV transmission to HEU infants (56), and maternal nelfinavir for at least the last 4 weeks of pregnancy did not reduce congenital CMV in HEU infants in the USA (57). Formula feeding of HEU infants was associated with a lower incidence of CMV infection by 1 year of age in Kenya (58), highlighting breast-feeding as a major route of CMV transmission. However, the risks of formula feeding make it an impractical intervention to reduce CMV in developing countries.
Maternal Microbial Translocation
Microbial translocation causes immune activation in HIV infection (59), and is a key distinction between HIV infection in humans and non-pathogenic simian immunodeficiency virus (SIV) infection in sooty mangabeys (60). Lipopolysaccharide (LPS) and other bacterial components from intestinal microbes are able to cross a leaky gut barrier as a direct result of HIV exposure, activating immune cells in the systemic circulation; increased LPS, as a marker of translocation, has been directly associated with innate and adaptive immune activation in HIV infection (59). HIV-infected pregnant women have higher levels of circulating soluble CD14 (sCD14) and LPS-binding protein than HIV-uninfected women, suggesting that microbial translocation occurs throughout pregnancy; first trimester sCD14 levels were independently associated with preterm birth (61) in multivariate analyses. In an animal model (62), subclinical infection with murine gammaherpesvirus 68 sensitizes pregnant mice to a greater cytokine response to LPS, suggesting that viral infections have potential to amplify the impact of LPS exposure during pregnancy. Maternal microbial translocation in HIV-affected pregnancies may plausibly contribute to immune activation in HEU infants: LPS can cross the placental barrier in mice (63), meaning that translocated maternal LPS could potentially activate fetal immune cells, particularly in the context of Nef-mediated placental barrier dysfunction (34). Whether LPS or other microbial products cross the placental barrier in HEU infants and contribute to immune activation has yet to be confirmed.
Notably, microbial translocation, and its associated immune activation, often persists in HIV-infected individuals despite ART (64–67). If this mechanism is related to immune activation in HEU infants, HEU infants may continue to be at risk of infection susceptibility despite maternal viral suppression throughout pregnancy.
Postnatal Exposure to HIV
Postnatal contact between HIV and the gut epithelium during breast-feeding may damage the mucosal barrier, enabling infant microbial translocation (68), although we recently showed that plasma levels of intestinal fatty acid binding protein, one marker of small intestinal villous damage, were similar between HIV-exposed and HIV-unexposed infants in the ZVITAMBO trial, and between HIV-exposed infants who did and did not acquire postnatal HIV infection through breast milk.1 In South Africa, greater exclusivity of breast-feeding was associated with less gut inflammation; (69) among infants recruited to the BAN trial, plasma LPS, a marker of microbial translocation, was higher after compared to before weaning (70).
Socioeconomic Factors
Social and economic factors are also likely to be important in infection susceptibility among HEU infants, including parental health and parental care-taking capacities; socioeconomic status; and pathogen exposure in the home environment, although a detailed discussion of these remains outside the scope of this Review. In the ZVITAMBO trial, morbidity and mortality outcomes were associated with family social factors, including parental relationship stability and household income (Table 1).
Postnatal Vitamin A
Vitamin A supplementation had no effect on HIV transmission in the ZVITAMBO trial, whether it was given to mothers, infants, or both. Furthermore, maternal and/or infant vitamin A had no overall effect on child mortality. However, in subgroup analyses, it was found that vitamin A had heterogeneous effects. Maternal or neonatal vitamin A had no effect on infants who acquired HIV in utero, but neonatal vitamin A did reduce mortality in those infected around the time of birth. In infants who were uninfected at 6 weeks of age (a group that included HEU and infants who later became postnatally infected), vitamin A was associated with higher 24-month mortality compared to placebo (Table 2). On sensitivity analysis, it appeared that the majority of those who died were HIV-infected before death. It is plausible therefore that vitamin A prior to HIV infection hastened disease progression when infants were subsequently infected through breast milk transmission of HIV (1).
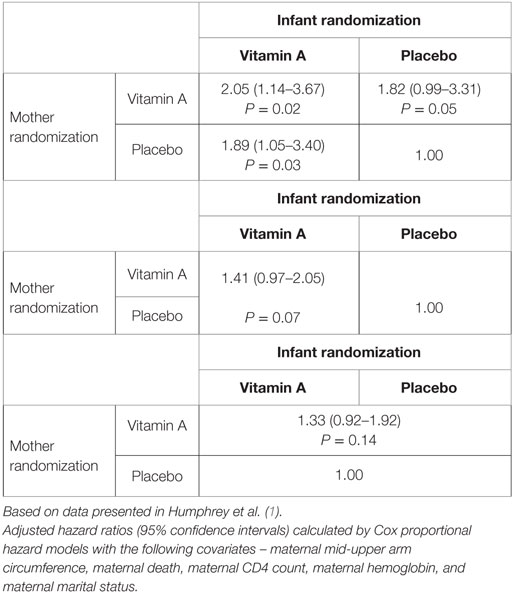
Table 2. Associations between maternal and infant vitamin A exposure and 24-month mortality in HIV-exposed infants remaining HIV PCR negative at 6 weeks (n = 2876) in ZVITAMBO.
Future Studies
Future studies should focus on better characterizing the nature and drivers of immunodeficiency in HEU infants and the relationship between immune ontogeny and clinical outcomes in this group. Whether immune dysfunction among HEU infants can be prevented or ameliorated with use of maternal ART or with other interventions remains unclear. However, immune activation in HIV-infected adults persists despite ART (64–67), and there is some evidence that ART exposure may actually worsen immune activation in HEU infants: in South Africa, T-cell activation at birth was unexpectedly higher among HEU infants exposed to antenatal and postnatal nevirapine compared to those unexposed to ART, perhaps due to an activating effect of nevirapine either on HIV-infected or bystander cells (71).
Studies of prospective cohorts are needed to evaluate the relationship between markers of immune dysfunction, such as immune activation, and infection susceptibility in HEU infants. Furthermore, studies comparing infection susceptibility and mortality outcomes in HEU and HIV-unexposed infants in context of suppressive maternal ART are required, in order to determine if the differences seen in the ZVITAMBO trial remain modern era. Although studies have been undertaken in the era of short periods of ART for PMTCT, there have so far been no studies undertaken in the setting of fully suppressive maternal ART throughout pregnancy, which is now the standard of care.
Growth of HEU Infants in the ZVITAMBO Trial
Growth failure is common in sub-Saharan Africa and is associated with childhood mortality (72). Poor growth has been well described in HIV-infected infants and children, but the effect of maternal HIV on the growth of HEU infants is less clear. Although the majority of cohorts across sub-Saharan Africa have shown trends toward poorer growth among HEU infants, many results do not reach statistical significance, potentially due to small numbers of children (3). We recently showed in the ZVITAMBO trial that HEU infants were more likely to be born premature and small-for-gestational age (SGA) than HIV-unexposed infants. HEU infants had evidence of growth failure at birth (2), and mean length-for-age and weight-for-age Z-scores remained lower among HEU compared to HIV-unexposed infants throughout 2 years of follow-up. The differences in growth between HEU and HIV-unexposed infants peaked at 6 weeks of age, when HEU infants were 25% more likely to be stunted, 55% more likely to be underweight, and 58% more likely to be wasted; these differences in stunting, underweight, and wasting persisted until 1 year of age.2 Compared to HIV-unexposed infants, HEU infants also had poorer head growth in the first year of life,3 although the influence of such a finding on neurodevelopmental outcomes remains uncertain.
To ensure robust growth outcome results, HEU infants were censored from analyses at their last negative HIV test.
Why Are HEU Infants at Risk of Growth Failure?
A number of mechanisms may underlie the association between maternal HIV infection and poor infant growth. Maternal immune activation may lead to pro-inflammatory vascular damage resulting in reduced placental blood supply (73) or increased placental inflammation and chorio-amnionitis; (74) each may lead to poor fetal growth. The high burden of coinfections in HIV-infected women may also influence growth of their offspring; for example, coinfection with HIV and malaria causes SGA (75, 76), which may be due to modifications in the placental cytokine environment (77). Congenital CMV infection causes poor fetal growth (43), and this effect may be more pronounced in infants of HIV-infected mothers.
Inflammation
Inflammation may also be a key driver of growth failure. In a recent sub-study of HIV-unexposed infants from the ZVITAMBO trial, we found linear growth failure was related both to acute illness and to chronic inflammation, with both clinical and subclinical disease associated with suppression of the growth hormone axis (78, 79). At 6 weeks of age, HEU infants in ZVITAMBO had higher C-reactive protein (CRP) than HIV-unexposed infants1; it is therefore plausible that the growth failure seen in HEU infants is related to higher levels of systemic inflammation leading to reduced insulin-like growth factor 1 (79); however, further data are needed to test this hypothesis.
Microbiota and Enteropathy
The intestinal microbiota is emerging as a key contributor to healthy postnatal growth, and a series of recent studies (80–82) has established that maturational defects in the composition and function of the microbiota underlie malnutrition in developing countries. An inflammatory pathology of the small intestine, termed environmental enteric dysfunction, is a potentially important cause of stunting among young children in developing countries (83, 84) and may be related to the configuration and function of the microbiota (85). The early life infant microbiota is founded following vertical transmission from the mother (86), so a distorted maternal microbiota in the context of HIV infection (87, 88) may lead to abnormal assembly of the microbiota in HEU infants, which could plausibly drive growth failure through subclinical intestinal damage and inflammation. However, to our knowledge, this has not been investigated to date. Greater intestinal inflammation and increased microbial translocation could drive systemic inflammation and immune activation in HEU infants, and could therefore contribute to both growth restriction and infection susceptibility. Notably, there are higher rates of stunting in HEU infants affected by diarrhea compared to those without diarrhea (89). Differences in microbiota composition and small intestinal pathology between HEU and HIV-unexposed infants warrant further exploration, as these processes are potentially amenable to gut-focused therapy.
Intrauterine Growth Restriction and Preterm Birth
It has been estimated that around 20% of stunting has fetal origins (90). In HEU infants born at term in the ZVITAMBO trial, SGA and length-for-age Z-score at birth were closely associated with stunting, highlighting intrauterine growth and development as a key contributor to future growth potential of HEU infants. Conversely, preterm birth without associated SGA was not associated with poorer growth trends across the first 12 months of life2.
Maternal Disease Severity
In contrast to morbidity and mortality, growth among HEU infants in ZVITAMBO was not associated with maternal disease severity (as determined by CD4 count at birth). This is also in contrast to studies from other countries that showed poorer fetal (91) and postnatal (92, 93) growth in HEU infants born to mothers with greater HIV disease severity. A recent study from rural Uganda highlights the importance of maternal nutritional status and HEU infant growth; HIV mothers with poor weight gain throughout pregnancy were more likely to have preterm and/or low-birth-weight HEU infants.
Antiretroviral Therapy
As infant growth was not associated with maternal disease severity in ZVITAMBO, virological suppression and immune reconstitution on ART may not necessarily improve growth outcomes. A Ugandan study undertaken in the context of maternal ART demonstrated a non-significant trend toward higher rates of stunting among HEU compared to HIV-unexposed infants [12-month adjusted odds ratio (OR) 1.55, 95% CI 0.92–2.61] (94), but this result may have been limited by a relatively small number of HEU infants included in the study. Differences in ponderal growth have not been found in most studies from the ART era (15, 94–98), although one Ugandan study reported an adjusted OR for wasting of 3.29 at median 5.2 months of age (P = 0.02) among HEU compared to HIV-unexposed infants (99). As wasting is often associated with acute illness, it is plausible that ART-related improvements in HEU infant immune function may reduce infection susceptibility and improve weight gain.
However, exposure to ART itself throughout fetal development, and choice of maternal ART regimen, may influence differences in birth outcomes and growth. It is becoming clear that maternal ART is associated with an elevated risk of adverse birth outcomes (100). In the Promoting Maternal–Infant Survival Everywhere (PROMISE) trial (101), women were randomized to lopinavir/ritonavir-based ART or zidovudine alone with intrapartum single-dose nevirapine; although mother-to-child tranmission was significantly lower in the combination ART arm, preterm delivery was significantly higher (20.5 vs. 13.1%). In some (102) studies, but not others (103), protease inhibitor-based ART has been particularly associated with risk of preterm birth (104). In most of sub-Saharan Africa, efavrienz-based ART is the standard regimen used in pregnancy; however, there is still evidence of increased preterm delivery and SGA (105, 106) following exposure to non-nucleoside reverse transcriptase inhibitor (NNRTI)-based regimens. Growth deficits associated with ART exposure that are evident at birth may persist and contribute to postnatal growth failure. For example, in Botswana, birth LAZ, WAZ, and WLZ were each significantly lower among infants exposed to triple ART compared to zidovudine monotherapy; LAZ remained significantly lower through 6 months of follow-up (107).
Socioeconomic Factors
In the ZVITAMBO trial, wasting and underweight were more frequent in HEU infants born to mothers with primary compared to A level education. Interestingly, this educational difference was apparent despite very high levels of literacy across the study population.
Taken together, there are several plausible mechanisms that could lead to growth failure in HEU infants (Figure 1), although further clinical and laboratory studies are required to dissect these mechanistic pathways further. Notably, data from the ZVITAMBO trial are unable to determine the causal association between morbidity and growth failure; poor growth may result from a higher incidence of infections, or may be itself be a cause of infection susceptibility.
Feeding HEU Infants
Targeting HIV-infected mothers and their exposed children with appropriate clinical and nutritional interventions is critical to improve survival. Until the availability of highly effective ART interventions for HIV-positive mothers and their infants, breast-feeding caused more than 200,000 new cases of pediatric HIV globally each year (108), but also prevented millions of infant deaths [one Ugandan trial reported that HEU infant mortality was over sixfold lower in infants who breast-fed for longer than 6 months compared to those who breast-fed for a shorter duration (109)]. Infant feeding therefore became one of the most profound dilemmas of the HIV epidemic.
The ZVITAMBO trial provided strong evidence for the association between exclusive breast-feeding (EBF) and reduced risk of postnatal HIV transmission (110). Among HIV-positive mothers, early EBF (feeding only breast milk) was associated with a 75% reduction in breast-feeding-associated HIV transmission by 6 months of age compared with early mixed breast-feeding (feeding both breast milk and non-breast milk liquid or solid foods). Compared to early EBF, early mixed breast-feeding was associated with 4.03-fold (95% CI 0.98–16.61), 3.79-fold (1.40–10.29), and 2.60-fold (1.21–5.55) greater risk of postnatal HIV transmission at 6, 12, and 18 months, respectively (110). This finding was due to nesting a “natural experiment” within the ZVITAMBO trial: 8 months after the trial was launched, WHO released policy that HIV testing and counseling should be available to all antenatal women, allowing mothers to make informed decisions about infant feeding (111). Importantly, the policy recommended early EBF and continued breast-feeding for women of unknown HIV status (the majority of women enrolled into the trial chose against learning their HIV status). In response, ZVITAMBO introduced an infant feeding intervention to support known HIV-positive women to make empowered choices about infant feeding, and to promote “safer breast-feeding” among HIV-negative mothers and those unwilling to learn their HIV status (early EBF; safe sex to avoid new HIV infections; prompt treatment of, and optimal techniques to reduce, breast problems) (112). Contact with this intervention was the strongest predictor of EBF in ZVITAMBO: EBF rates increased and postnatal HIV transmission rates declined with each additional exposure to the intervention (112).
Why Is Exclusive Breast-feeding Associated with Better HIV-exposed Infant Outcomes?
The underlying reasons for the protective effect of EBF on PMTCT remain uncertain (113). We hypothesize that early introduction of non-milk fluids and solid food, as is the cultural norm in Zimbabwe (112), increases intestinal inflammation due to modulation of the microbiota (114) or introduction of pathogenic bacteria (115). Intestinal inflammation may impair gut integrity and increase the pool of activated intestinal CD4 cells that are targeted by the virus. In the BAN trial, infants had higher markers of microbial translocation (LPS) after weaning than before, and pre-transmission LPS levels were a predictor of subsequent infection (70). It has alternatively been proposed that the association between EBF and reduced breast milk transmission is due to reverse causality – that women in better health, who are less likely to transmit HIV, are also more likely to exclusively breast-feed.
Breast-feeding Interventions
Although continued breast-feeding after 6 months is likely to provide the greatest chance of survival for the majority of infants living in developing countries, this policy has proved difficult to implement. However, recent experience in Zimbabwe indicates that interventions targeting specific contextual barriers may be successful in increasing rates of EBF to 6 months (116–118). HIV-exposed infants may benefit from targeted EBF promotion; contact with healthcare professionals at birth or during early infant diagnosis at 6 weeks of age could be an opportunity to empower HIV-infected mothers to exclusively breast-feed. As contact with a counseling program was the strongest predictor of EBF in the ZVITAMBO trial (112), such programs may be paramount to empower women and improve the health of HEU infants.
Summary
HIV-exposed uninfected infants in the ZVITAMBO trial had poorer health outcomes than HIV-unexposed infants. Hospitalization was 50% more frequent among HEU infants in the first month of life, and mortality rates were higher over the 24-month follow-up period. Morbidity and mortality outcomes were associated with maternal disease severity and social factors, including parental relationship stability and household income (Table 1). HEU infants were at higher risk of stunting, wasting, and underweight than HIV-unexposed infants, although maternal disease severity was not associated with growth outcomes.
We propose that immune activation and inflammation and may be key drivers of both infection susceptibility and growth failure in HEU infants. Notably, baseline CRP was higher in HEU compared to HIV-unexposed infants at 6 weeks of age, and was still elevated at 6 months of age. Our conceptual framework highlights a number of key pathways that may drive inflammation and immune activation, including maternal HIV itself (in utero and postnatally); coinfections (such as CMV and malaria); and a distorted gut microbiota (which may be acquired from the HIV-infected mother). Future work will aim to elucidate and describe pathways leading to poor health outcomes of HEU infants.
Conclusion
Infants recruited to the ZVITAMBO trial have contributed to our understanding of the HEU population. Future studies should draw on these and other results in order to determine the causes of infection susceptibility and growth failure, and determine the impact of ART and cotrimoxazole on outcomes of this vulnerable group of infants.
Author Contributions
CE wrote the first draft of the manuscript, which was critically reviewed and revised by JH, RN, and AP. JH designed and recruited to the original ZVITAMBO trial. RN was a coinvestigator on the original ZVITAMBO trial.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. CE is funded by the National Institute for Health Research. AJP is funded by the Wellcome Trust (108065/Z/15/Z).
Funding
The ZVITAMBO trial was supported by the Canadian International Development Agency (CIDA) (R/C Project 690/M3688), United States Agency for International Development (USAID) (cooperative agreement number HRN-A-00-97-00015-00 between Johns Hopkins University and the Office of Health and Nutrition – USAID) and a grant from the Bill and Melinda Gates Foundation, Seattle, WA, USA. Additional funding was received from the SARA Project, which is operated by the Academy for Educational Development, Washington DC and is funded by USAID’s Bureau for Africa, Office of Sustainable Development under the terms of Contract AOT-C-00-99-00237-00, the Rockefeller Foundation (New York, NY), and BASF (Ludwigshafen, Germany). CE is funded by the National Institute for Health Research. AJP is funded by the Wellcome Trust (108065/Z/15/Z).
Footnotes
- ^ Prendergast AJ, Chasekwa B, Rukobo S, Govha M, Mutasa K, Ntozini R, et al. Intestinal damage and immune activation in HIV-exposed and HIV-infected Zimbabwean infants (submitted 2016).
- ^Omoni AO, Ntozini R, Evans C, Prendergast AJ, Moulton LH, Christian PS, et al. Child growth according to maternal and child HIV status in Zimbabwe (submitted 2016).
- ^Evans C, Chasekwa B, Ntozini R, Humphrey JH, Prendergast AJ. Head circumferences of children born to HIV-infected and HIV-uninfected mothers in Zimbabwe during the pre-ART era (submitted 2016).
References
1. Humphrey JH, Iliff PJ, Marinda ET, Mutasa K, Moulton LH, Chidawanyika H, et al. Effects of a single large dose of vitamin A, given during the postpartum period to HIV-positive women and their infants, on child HIV infection, HIV-free survival, and mortality. J Infect Dis (2006) 193(6):860–71. doi: 10.1086/500366
2. Marinda E, Humphrey JH, Iliff PJ, Mutasa K, Nathoo KJ, Piwoz EG, et al. Child mortality according to maternal and infant HIV status in Zimbabwe. Pediatr Infect Dis J (2007) 26(6):519–26. doi:10.1097/01.inf.0000264527.69954.4c
3. Evans C, Jones CE, Prendergast AJ. HIV-exposed, uninfected infants: new global challenges in the era of paediatric HIV elimination. Lancet Infect Dis (2016) 16(6):e92–107. doi:10.1016/S1473-3099(16)00055-4
4. Malaba LC, Iliff PJ, Nathoo KJ, Marinda E, Moulton LH, Zijenah LS, et al. Effect of postpartum maternal or neonatal vitamin A supplementation on infant mortality among infants born to HIV-negative mothers in Zimbabwe. Am J Clin Nutr (2005) 81(2):454–60.
5. Koyanagi A, Humphrey JH, Ntozini R, Nathoo K, Moulton LH, Iliff P, et al. Morbidity among human immunodeficiency virus-exposed but uninfected, human immunodeficiency virus-infected, and human immunodeficiency virus-unexposed infants in Zimbabwe before availability of highly active antiretroviral therapy. Pediatr Infect Dis J (2011) 30(1):45–51. doi:10.1097/INF.0b013e3181ecbf7e
6. Cohen C, Moyes J, Tempia S, Groome M, Walaza S, Pretorius M, et al. Epidemiology of acute lower respiratory Tract infection in HIV-exposed uninfected infants. Pediatrics (2016). doi:10.1542/peds.2015-3272
7. Gaensbauer J, Rakhola JT, Onyango-Makumbi C, Mubiru M, Westcott JE, Krebs NF, et al. Impaired haemophilus influenzae type b transplacental antibody transmission and declining antibody avidity through the first year of life represent potential vulnerabilities for HIV-exposed but uninfected infants. Clin Vaccine Immunol (2014) 21(12):1661–7. doi:10.1128/CVI.00356-14
8. Jones C, Naidoo S, De Beer C, Esser M, Kampmann B, Hesseling A. Maternal HIV infection and antibody responses against vaccine-preventable diseases in uninfected infants. JAMA (2011) 305(6):576–84. doi:10.1001/jama.2011.100
9. Jones C, Pollock L, Barnett S, Battersby A, Kampmann B. Specific antibodies against vaccine-preventable infections: a mother-infant cohort study. BMJ Open (2013) 3(4). doi:10.1136/bmjopen-2012-002473
10. Madhi S, Izu A, Violari A, Cotton MF, Panchia R, Dobbels E, et al. Immunogenicity following the first and second doses of 7-valent pneumococcal conjugate vaccine in HIV-infected and uninfected infants. Vaccine (2013) 31(5):777–83. doi:10.1016/j.vaccine.2012.11.076
11. Simani O, Izu A, Violari A, Cotton MF, van Niekerk N, Adrian PV, et al. Effect of HIV-1 exposure and antiretroviral treatment strategies in HIV-infected children on immunogenicity of vaccines during infancy. AIDS (2014) 28(4):531–41. doi:10.1097/QAD.0000000000000127
12. Cumberland P, Shulman CE, Maple PA, Bulmer JN, Dorman EK, Kawuondo K, et al. HIV infection and placental malaria reduce transplacental antibody transfer and tetanus antibody levels in newborns in Kenya. J Infect Dis (2007) 196(4):550–7. doi:10.1086/519845
13. Miles D, Gadama L, Gumbi A, Nyalo F, Makanani B, Heyderman RS. Human immunodeficiency virus (HIV) infection during pregnancy induces CD4 T-cell differentiation and modulates responses to Bacille Calmette-Guérin (BCG) vaccine in HIV-uninfected infants. Immunology (2010) 129(3):446–54. doi:10.1111/j.1365-2567.2009.03186.x
14. Clerici M, Saresella M, Colombo F, Fossati S, Sala N, Bricalli D, et al. T-lymphocyte maturation abnormalities in uninfected newborns and children with vertical exposure to HIV. Blood (2000) 96(12):3866–71.
15. Moraleda C, de Deus N, Serna-Bolea C, Renom M, Quintó L, Macete E, et al. Impact of HIV exposure on health outcomes in HIV-negative infants born to HIV-positive mothers in Sub-Saharan Africa. J Acquir Immune Defic Syndr (2014) 65(2):182–9. doi:10.1097/QAI.0000000000000019
16. Kidzeru EB, Hesseling AC, Passmore JA, Myer L, Gamieldien H, Tchakoute CT, et al. In-utero exposure to maternal HIV infection alters T-cell immune responses to vaccination in HIV-uninfected infants. AIDS (2014) 28(10):1421–30. doi:10.1097/QAD.0000000000000292
17. Ono E, Nunes dos Santos AM, de Menezes Succi RC, Machado DM, de Angelis DS, Salomão R, et al. Imbalance of naive and memory T lymphocytes with sustained high cellular activation during the first year of life from uninfected children born to HIV-1-infected mothers on HAART. Braz J Med Biol Res (2008) 41(8):700–8. doi:10.1590/S0100-879X2008000800011
18. Rich K, Siegel J, Jennings C, Rydman R, Landay A. Function and phenotype of immature CD4+ lymphocytes in healthy infants and early lymphocyte activation in uninfected infants of human immunodeficiency virus-infected mothers. Clin Diagn Lab Immunol (1997) 4(3):358–61.
19. Romano MF, Buffolano W, Bisogni R, Russo R, Liuzzi R, Bunders M, et al. Increased CD154 expression in uninfected infants born to HIV-positive mothers exposed to antiretroviral prophylaxis. Viral Immunol (2006) 19(3):363–72. doi:10.1089/vim.2006.19.363
20. Bunders M, van Hamme JL, Jansen MH, Boer K, Kootstra NA, Kuijpers TW. Fetal exposure to HIV-1 alters chemokine receptor expression by CD4+T cells and increases susceptibility to HIV-1. Sci Rep (2014) 4:6690. doi:10.1038/srep06690
21. Jones C, Hesseling AC, Tena-Coki NG, Scriba TJ, Chegou NN, Kidd M, et al. The impact of HIV exposure and maternal Mycobacterium tuberculosis infection on infant immune responses to bacille Calmette-Guérin vaccination. AIDS (2015) 29(2):155–65. doi:10.1097/QAD.0000000000000536
22. Reikie BA, Adams RC, Leligdowicz A, Ho K, Naidoo S, Ruck CE, et al. Altered innate immune development in HIV-exposed uninfected infants. J Acquir Immune Defic Syndr (2014) 66(3):245–55. doi:10.1097/QAI.0000000000000161
23. Slyker J, Lohman-Payne B, John-Stewart GC, Dong T, Mbori-Ngacha D, Tapia K, et al. The impact of HIV-1 infection and exposure on natural killer (NK) cell phenotype in Kenyan infants during the first year of life. Front Immunol (2012) 3:399. doi:10.3389/fimmu.2012.00399
24. Velilla P, Montoya C, Hoyos A, Moreno M, Chougnet C, Rugeles M. Effect of intrauterine HIV-1 exposure on the frequency and function of uninfected newborns’ dendritic cells. Clin Immunol (2008) 126(3):243–50. doi:10.1016/j.clim.2007.11.004
25. Giorgi JV, Hultin LE, McKeating JA, Johnson TD, Owens B, Jacobson LP, et al. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J Infect Dis (1999) 179(4):859–70. doi:10.1086/314660
26. Tesselaar K, Arens R, van Schijndel GM, Baars PA, van der Valk MA, Borst J, et al. Lethal T cell immunodeficiency induced by chronic costimulation via CD27-CD70 interactions. Nat Immunol (2003) 4(1):49–54. doi:10.1038/ni869
27. Kuhn L, Meddows-Taylor S, Gray G, Tiemessen C. Human immunodeficiency virus (HIV)-specific cellular immune responses in newborns exposed to HIV in utero. Clin Infect Dis (2002) 34(2):267–76. doi:10.1086/338153
28. Legrand FA, Nixon DF, Loo CP, Ono E, Chapman JM, Miyamoto M, et al. Strong HIV-1-specific T cell responses in HIV-1-exposed uninfected infants and neonates revealed after regulatory T cell removal. PLoS One (2006) 1:e102. doi:10.1371/journal.pone.0000102
29. Liu A, Lohman-Payne B, Chung MH, Kiarie J, Kinuthia J, Slyker J, et al. Maternal plasma and breastmilk viral loads are associated with HIV-1-specific cellular immune responses among HIV-1-exposed, uninfected infants in Kenya. Clin Exp Immunol (2015) 180(3):509–19. doi:10.1111/cei.12599
30. Lewis SH, Reynolds-Kohler C, Fox HE, Nelson JA. HIV-1 in trophoblastic and villous Hofbauer cells, and haematological precursors in eight-week fetuses. Lancet (1990) 335(8689):565–8. doi:10.1016/0140-6736(90)90349-A
31. McGann KA, Collman R, Kolson DL, Gonzalez-Scarano F, Coukos G, Coutifaris C, et al. Human immunodeficiency virus type 1 causes productive infection of macrophages in primary placental cell cultures. J Infect Dis (1994) 169(4):746–53. doi:10.1093/infdis/169.4.746
32. Hanna Z, Priceputu E, Hu C, Vincent P, Jolicoeur P. HIV-1 Nef mutations abrogating downregulation of CD4 affect other Nef functions and show reduced pathogenicity in transgenic mice. Virology (2006) 346(1):40–52. doi:10.1016/j.virol.2005.10.010
33. Priceputu E, Hanna Z, Hu C, Simard MC, Vincent P, Wildum S, et al. Primary human immunodeficiency virus type 1 nef alleles show major differences in pathogenicity in transgenic mice. J Virol (2007) 81(9):4677–93. doi:10.1128/JVI.02691-06
34. Singh P, Agnihotri SK, Tewari MC, Kumar S, Sachdev M, Tripathi RK. HIV-1 Nef breaches placental barrier in rat model. PLoS One (2012) 7(12):e51518. doi:10.1371/journal.pone.0051518
35. Ellington SR, Clarke KE, Kourtis AP. Cytomegalovirus infection in the human immunodeficiency virus-exposed and infected infant: a systematic review. J Infect Dis (2015) 213(6):891–900. doi:10.1093/infdis/jiv549
36. Mwaanza N, Chilukutu L, Tembo J, Kabwe M, Musonda K, Kapasa M, et al. High rates of congenital cytomegalovirus infection linked with maternal HIV infection among neonatal admissions at a large referral center in sub-Saharan Africa. Clin Infect Dis (2014) 58(5):728–35. doi:10.1093/cid/cit766
37. Elbou Ould MA, Luton D, Yadini M, Pedron B, Aujard Y, Jacqz-Aigrain E, et al. Cellular immune response of fetuses to cytomegalovirus. Pediatr Res (2004) 55(2):280–6. doi:10.1203/01.PDR.0000104150.85437.FE
38. Miles DJ, Sanneh M, Holder B, Crozier S, Nyamweya S, Touray ES, et al. Cytomegalovirus infection induces T-cell differentiation without impairing antigen-specific responses in Gambian infants. Immunology (2008) 124(3):388–400. doi:10.1111/j.1365-2567.2007.02787.x
39. Miles DJ, van der Sande M, Jeffries D, Kaye S, Ojuola O, Sanneh M, et al. Maintenance of large subpopulations of differentiated CD8 T-cells two years after cytomegalovirus infection in Gambian infants. PLoS One (2008) 3(8):e2905. doi:10.1371/journal.pone.0002905
40. Miles DJ, van der Sande M, Jeffries D, Kaye S, Ismaili J, Ojuola O, et al. Cytomegalovirus infection in Gambian infants leads to profound CD8 T-cell differentiation. J Virol (2007) 81(11):5766–76. doi:10.1128/JVI.00052-07
41. Gumbo H, Chasekwa B, Church JA, Ntozini R, Mutasa K, Humphrey JH, et al. Congenital and postnatal CMV and EBV acquisition in HIV-infected Zimbabwean infants. PLoS One (2014) 9(12):e114870. doi:10.1371/journal.pone.0114870
42. Slyker JA, Lohman-Payne BL, John-Stewart GC, Maleche-Obimbo E, Emery S, Richardson B, et al. Acute cytomegalovirus infection in Kenyan HIV-infected infants. AIDS (2009) 23(16):2173–81. doi:10.1097/QAD.0b013e32833016e8
43. Gompels UA, Larke N, Sanz-Ramos M, Bates M, Musonda K, Manno D, et al. Human cytomegalovirus infant infection adversely affects growth and development in maternally HIV-exposed and unexposed infants in Zambia. Clin Infect Dis (2012) 54(3):434–42. doi:10.1093/cid/cir837
44. Slyker J, Lohman-Payne BL, Rowland-Jones SL, Otieno P, Maleche-Obimbo E, Richardson B, et al. The detection of cytomegalovirus DNA in maternal plasma is associated with mortality in HIV-1-infected women and their infants. AIDS (2009) 23(1):117–24. doi:10.1097/QAD.0b013e32831c8abd
45. Hsiao N, Zampoli M, Morrow B, Zar HJ, Hardie D. Cytomegalovirus viraemia in HIV exposed and infected infants: prevalence and clinical utility for diagnosing CMV pneumonia. J Clin Virol (2013) 58(1):74–8. doi:10.1016/j.jcv.2013.05.002
46. Mocarski EJ. Immunomodulation by cytomegaloviruses: manipulative strategies beyond evasion. Trends Microbiol (2002) 10(7):332–9. doi:10.1016/S0966-842X(02)02393-4
47. Loenen W, Bruggeman CA, Wiertz EJ. Immune evasion by human cytomegalovirus: lessons in immunology and cell biology. Semin Immunol (2001) 13(1):41–9. doi:10.1006/smim.2001.0294
48. van de Berg P, Heutinck KM, Raabe R, Minnee RC, Young SL, van Donselaar-van der Pant KA, et al. Human cytomegalovirus induces systemic immune activation characterized by a type 1 cytokine signature. J Infect Dis (2010) 202(5):690–690. doi:10.1086/655472
49. Scott G, Chow SS, Craig ME, Pang CN, Hall B, Wilkins MR, et al. Cytomegalovirus infection during pregnancy with maternofetal transmission induces a proinflammatory cytokine bias in placenta and amniotic fluid. J Infect Dis (2012) 205(8):1305–10. doi:10.1093/infdis/jis186
50. Tan Y, Yu SJ, Wang J, Li SJ. Role of Treg/Th17 balance in the pathogenesis of cytomegalovirus infection. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi (2012) 28(6):649–51.
51. Crome S, Wang AY, Levings MK. Translational mini-review series on Th17 cells: function and regulation of human T helper 17 cells in health and disease. Clin Exp Immunol (2010) 159(2):109–19. doi:10.1111/j.1365-2249.2009.04037.x
52. Khan N, Hislop A, Gudgeon N, Cobbold M, Khanna R, Nayak L, et al. Herpesvirus-specific CD8 T cell immunity in old age: cytomegalovirus impairs the response to a coresident EBV infection. J Immunol (2004) 173(12):7481–9. doi:10.4049/jimmunol.173.12.7481
53. Vermijlen D, Brouwer M, Donner C, Liesnard C, Tackoen M, Van Rysselberge M, et al. Human cytomegalovirus elicits fetal gammadelta T cell responses in utero. J Exp Med (2010) 207(4):807–21. doi:10.1084/jem.20090348
54. Noyola DE, Fortuny C, Muntasell A, Noguera-Julian A, Muñoz-Almagro C, Alarcón A, et al. Influence of congenital human cytomegalovirus infection and the NKG2C genotype on NK-cell subset distribution in children. Eur J Immunol (2012) 42(12):3256–66. doi:10.1002/eji.201242752
55. Marchant A, Appay V, Van Der Sande M, Dulphy N, Liesnard C, Kidd M, et al. Mature CD8(+) T lymphocyte response to viral infection during fetal life. J Clin Invest (2003) 111(11):1747–55. doi:10.1172/JCI200317470
56. Roxby A, Atkinson C, Asbjörnsdóttir K, Farquhar C, Kiarie JN, Drake AL, et al. Maternal valacyclovir and infant cytomegalovirus acquisition: a randomized controlled trial among HIV-infected women. PLoS One (2014) 9(2):e87855. doi:10.1371/journal.pone.0087855
57. Gantt S, Leister E, Jacobson DL, Boucoiran I, Huang ML, Jerome KR, et al. Risk of congenital cytomegalovirus infection among HIV-exposed uninfected infants is not decreased by maternal nelfinavir use during pregnancy. J Med Virol (2016) 88(6):1051–8. doi:10.1002/jmv.24420
58. Richardson B, John-Stewart G, Emery V, Atkinson C, Nduati R, Ásbjörnsdóttir K, et al. CMV Transmission from HIV-Infected Women Randomized to Formula Versus Breastfeeding. Conference on Retroviruses and Opportunistic Infections. Seattle, WA: (2015).
59. Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med (2006) 12(12):1365–71. doi:10.1038/nm1511
60. Silvestri G, Sodora DL, Koup RA, Paiardini M, O’Neil SP, McClure HM, et al. Nonpathogenic SIV infection of sooty mangabeys is characterized by limited bystander immunopathology despite chronic high-level viremia. Immunity (2003) 18(3):441–52. doi:10.1016/S1074-7613(03)00060-8
61. López M, Figueras F, Coll O, Goncé A, Hernández S, Loncá M, et al. Inflammatory markers related to microbial translocation among HIV-infected pregnant women: a risk factor of preterm delivery. J Infect Dis (2016) 213(3):343–50. doi:10.1093/infdis/jiv416
62. Cardenas I, Mor G, Aldo P, Lang SM, Stabach P, Sharp A, et al. Placental viral infection sensitizes to endotoxin-induced pre-term labor: a double hit hypothesis. Am J Reprod Immunol (2011) 65(2):110–7. doi:10.1111/j.1600-0897.2010.00908.x
63. Kohmura Y, Kirikae T, Kirikae F, Nakano M, Sato I. Lipopolysaccharide (LPS)-induced intra-uterine fetal death (IUFD) in mice is principally due to maternal cause but not fetal sensitivity to LPS. Microbiol Immunol (2000) 44(11):897–904. doi:10.1111/j.1348-0421.2000.tb02581.x
64. Hunt PW, Martin JN, Sinclair E, Bredt B, Hagos E, Lampiris H, et al. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis (2003) 187(10):1534–43. doi:10.1086/374786
65. Almeida CA, Price P, French MA. Immune activation in patients infected with HIV type 1 and maintaining suppression of viral replication by highly active antiretroviral therapy. AIDS Res Hum Retroviruses (2002) 18(18):1351–5. doi:10.1089/088922202320935429
66. Neuhaus J, Jacobs DR Jr, Baker JV, Calmy A, Duprez D, La Rosa A, et al. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis (2010) 201(12):1788–95. doi:10.1086/652749
67. Somsouk M, Estes JD, Deleage C, Dunham RM, Albright R, Inadomi JM, et al. Gut epithelial barrier and systemic inflammation during chronic HIV infection. AIDS (2015) 29(1):43–51. doi:10.1097/QAD.0000000000000511
68. Nazli A, Chan O, Dobson-Belaire WN, Ouellet M, Tremblay MJ, Gray-Owen SD, et al. Exposure to HIV-1 directly impairs mucosal epithelial barrier integrity allowing microbial translocation. PLoS Pathog (2010) 6(4):e1000852. doi:10.1371/journal.ppat.1000852
69. Moodley-Govender E, Mulol H, Stauber J, Manary M, Coutsoudis A. Increased exclusivity of breastfeeding associated with reduced gut inflammation in infants. Breastfeed Med (2015) 10:488–92. doi:10.1089/bfm.2015.0110
70. Kourtis AP, Ibegbu CC, Wiener J, King CC, Tegha G, Kamwendo D, et al. Role of intestinal mucosal integrity in HIV transmission to infants through breast-feeding: the BAN study. J Infect Dis (2013) 208(4):653–61. doi:10.1093/infdis/jit221
71. Schramm D, Kuhn L, Gray G, Tiemessen C. In vivo effects of HIV-1 exposure in the presence and absence of single-dose nevirapine on cellular plasma activation markers of infants born to HIV-1-seropositive mothers. J Acquir Immune Defic Syndr (2006) 42(5):545–53. doi:10.1097/01.qai.0000225009.30698.ce
72. Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, de Onis M, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet (2013) 382(9890):427–51. doi:10.1016/S0140-6736(13)60937-X
73. Kuzawa CW, Tallman PS, Adair LS, Lee N, McDade TW. Inflammatory profiles in the non-pregnant state predict offspring birth weight at Cebu: evidence for inter-generational effects of low grade inflammation. Ann Hum Biol (2012) 39(4):267–74. doi:10.3109/03014460.2012.692810
74. Kumar RM, Uduman SA, Khurranna AK. Impact of maternal HIV-1 infection on perinatal outcome. Int J Gynaecol Obstet (1995) 49(2):137–43. doi:10.1016/0020-7292(95)02356-H
75. Dreyfuss ML, Msamanga GI, Spiegelman D, Hunter DJ, Urassa EJ, Hertzmark E, et al. Determinants of low birth weight among HIV-infected pregnant women in Tanzania. Am J Clin Nutr (2001) 74(6):814–26.
76. Kalanda BF, van Buuren S, Verhoeff FH, Brabin BJ. Anthropometry of fetal growth in rural Malawi in relation to maternal malaria and HIV status. Arch Dis Child Fetal Neonatal Ed (2005) 90(2):F161–5. doi:10.1136/adc.2004.054650
77. Kfutwah A, Mary JY, Lemen B, Leke R, Rousset D, Barré-Sinoussi F, et al. Plasmodium falciparum infection significantly impairs placental cytokine profile in HIV infected Cameroonian women. PLoS One (2009) 4(12):e8114. doi:10.1371/journal.pone.0008114
78. Jones AD, Rukobo S, Chasekwa B, Mutasa K, Ntozini R, Mbuya MN, et al. Acute illness is associated with suppression of the growth hormone axis in Zimbabwean infants. Am J Trop Med Hyg (2015) 92(2):463–70. doi:10.4269/ajtmh.14-0448
79. Prendergast A, Rukobo S, Chasekwa B, Mutasa K, Ntozini R, Mbuya MN, et al. Stunting is characterized by chronic inflammation in Zimbabwean infants. PLoS One (2014) 9(2):e86928. doi:10.1371/journal.pone.0086928
80. Subramanian S, Huq S, Yatsunenko T, Haque R, Mahfuz M, Alam MA, et al. Persistent gut microbiota immaturity in malnourished Bangladeshi children. Nature (2014) 510(7505):417–21. doi:10.1038/nature13421
81. Kau AL, Planer JD, Liu J, Rao S, Yatsunenko T, Trehan I, et al. Functional characterization of IgA-targeted bacterial taxa from undernourished Malawian children that produce diet-dependent enteropathy. Sci Transl Med (2015) 7(276):276ra24. doi:10.1126/scitranslmed.aaa4877
82. Reyes A, Blanton LV, Cao S, Zhao G, Manary M, Trehan I, et al. Gut DNA viromes of Malawian twins discordant for severe acute malnutrition. Proc Natl Acad Sci U S A (2015) 112(38):11941–6. doi:10.1073/pnas.1514285112
83. Crane RJ, Jones KD, Berkley JA. Environmental enteric dysfunction: an overview. Food Nutr Bull (2015) 36(1 Suppl):S76–87. doi:10.1177/15648265150361S113
84. Prendergast A, Kelly P. Enteropathies in the developing world: neglected effects on global health. Am J Trop Med Hyg (2012) 86(5):756–63. doi:10.4269/ajtmh.2012.11-0743
85. Keusch G, Denno DM, Black RE, Duggan C, Guerrant RL, Lavery JV, et al. Environmental enteric dysfunction: pathogenesis, diagnosis, and clinical consequences. Clin Infect Dis (2014) 59(Suppl 4):S207–12. doi:10.1093/cid/ciu485
86. Funkhouser L, Bordenstein SR. Mom knows best: the universality of maternal microbial transmission. PLoS Biol (2013) 11(8):e1001631. doi:10.1371/journal.pbio.1001631
87. Lozupone C, Li M, Campbell TB, Flores SC, Linderman D, Gebert MJ, et al. Alterations in the gut microbiota associated with HIV-1 infection. Cell Host Microbe (2013) 14(3):329–39. doi:10.1016/j.chom.2013.08.006
88. Monaco CL, Gootenberg DB, Zhao G, Handley SA, Ghebremichael MS, Lim ES, et al. Altered virome and bacterial microbiome in human immunodeficiency virus-associated acquired immunodeficiency syndrome. Cell Host Microbe (2016) 19(3):311–22. doi:10.1016/j.chom.2016.02.011
89. Bailey RC, Kamenga MC, Nsuami MJ, Nieburg P, St Louis ME. Growth of children according to maternal and child HIV, immunological and disease characteristics: a prospective cohort study in Kinshasa, Democratic Republic of Congo. Int J Epidemiol (1999) 28(3):532–40. doi:10.1093/ije/28.3.532
90. Christian P, Lee SE, Donahue Angel M, Adair LS, Arifeen SE, Ashorn P, et al. Risk of childhood undernutrition related to small-for-gestational age and preterm birth in low- and middle-income countries. Int J Epidemiol (2013) 42(5):1340–55. doi:10.1093/ije/dyt109
91. Turner AN, Tabbah S, Mwapasa V, Rogerson SJ, Meshnick SR, Ackerman WE IV, et al. Severity of maternal HIV-1 disease is associated with adverse birth outcomes in Malawian women: a cohort study. J Acquir Immune Defic Syndr (2013) 64(4):392–9. doi:10.1097/QAI.0b013e3182a2d13c
92. McGrath CJ, Nduati R, Richardson BA, Kristal AR, Mbori-Ngacha D, Farquhar C, et al. The prevalence of stunting is high in HIV-1-exposed uninfected infants in Kenya. J Nutr (2012) 142(4):757–63. doi:10.3945/jn.111.148874
93. Jao J, Agwu A, Mhango G, Kim A, Park K, Posada R, et al. Growth patterns in the first year of life differ in infants born to perinatally vs. nonperinatally HIV-infected women. AIDS (2015) 29(1):111–6. doi:10.1097/QAD.0000000000000501
94. Muhangi L, Lule SA, Mpairwe H, Ndibazza J, Kizza M, Nampijja M, et al. Maternal HIV infection and other factors associated with growth outcomes of HIV-uninfected infants in Entebbe, Uganda. Public Health Nutr (2013) 16(9):1548–57. doi:10.1017/S1368980013000499
95. Powis KM, Quanhong L, Chinyanga Y, Tumbare E, Khan N, Sibiya J, et al. Malnutrition among HIV-Exposed Uninfected Children in Botswana. Conference on Retroviruses and Opportunistic Infections. Seattle, WA: (2015).
96. Slogrove A, Reikie B, Naidoo S, De Beer C, Ho K, Cotton M, et al. HIV-exposed uninfected infants are at increased risk for severe infections in the first year of life. J Trop Pediatr (2012) 58(6):505–8. doi:10.1093/tropej/fms019
97. Landes M, van Lettow M, Chan AK, Mayuni I, Schouten EJ, Bedell RA. Mortality and health outcomes of HIV-exposed and unexposed children in a PMTCT cohort in Malawi. PLoS One (2012) 7(10):e47337. doi:10.1371/journal.pone.0047337
98. Kerr SJ, Puthanakit T, Vibol U, Aurpibul L, Vonthanak S, Kosalaraksa P, et al. Neurodevelopmental outcomes in HIV-exposed-uninfected children versus those not exposed to HIV. AIDS Care (2014) 26(11):1327–35. doi:10.1080/09540121.2014.920949
99. Osterbauer B, Kapisi J, Bigira V, Mwangwa F, Kinara S, Kamya MR, et al. Factors associated with malaria parasitaemia, malnutrition, and anaemia among HIV-exposed and unexposed Ugandan infants: a cross-sectional survey. Malar J (2012) 11:432. doi:10.1186/1475-2875-11-432
100. Mofenson LM. Antiretroviral therapy and adverse pregnancy outcome: the elephant in the room? J Infect Dis (2016) 213(7):1051–4. doi:10.1093/infdis/jiv390
101. Fowler M, Qin M, Shapiro D, Fiscus S. PROMISE: Efficacy and Safety of Two Strategies to Prevent Perinatal HIV Transmission. Conference on Retroviruses and Opportunistic Infections. Seattle, WA: (2015).
102. Powis KM, Kitch D, Ogwu A, Hughes MD, Lockman S, Leidner J, et al. Increased risk of preterm delivery among HIV-infected women randomized to protease versus nucleoside reverse transcriptase inhibitor-based HAART during pregnancy. J Infect Dis (2011) 204(4):506–14. doi:10.1093/infdis/jir307
103. Koss CA, Natureeba P, Plenty A, Luwedde F, Mwesigwa J, Ades V, et al. Risk factors for preterm birth among HIV-infected pregnant Ugandan women randomized to lopinavir/ritonavir- or efavirenz-based antiretroviral therapy. J Acquir Immune Defic Syndr (2014) 67(2):128–35. doi:10.1097/QAI.0000000000000281
104. Kourtis AP, Fowler MG. Antiretroviral use during pregnancy and risk of preterm delivery: more questions than answers. J Infect Dis (2011) 204(4):493–4. doi:10.1093/infdis/jir318
105. Chen JY, Ribaudo HJ, Souda S, Parekh N, Ogwu A, Lockman S, et al. Highly active antiretroviral therapy and adverse birth outcomes among HIV-infected women in Botswana. J Infect Dis (2012) 206(11):1695–705. doi:10.1093/infdis/jis553
106. Li N, Sando MM, Spiegelman D, Hertzmark E, Liu E, Sando D, et al. Antiretroviral therapy in relation to birth outcomes among HIV-infected women: a cohort study. J Infect Dis (2016) 213(7):1057–64. doi:10.1093/infdis/jiv389
107. Powis KM, Smeaton L, Ogwu A, Lockman S, Dryden-Peterson S, van Widenfelt E, et al. Effects of in utero antiretroviral exposure on longitudinal growth of HIV-exposed uninfected infants in Botswana. J Acquir Immune Defic Syndr (2011) 56(2):131–8. doi:10.1097/QAI.0b013e3181ffa4f5
108. Joint United National Programme on HIV/AIDS (UNAIDS). Global Report: UNAIDS Report on the Global AIDS Epidemic. Geneva, Switzerland: WHO (2013).
109. Homsy J, Moore D, Barasa A, Were W, Likicho C, Waiswa B, et al. Breastfeeding, mother-to-child HIV transmission, and mortality among infants born to HIV-Infected women on highly active antiretroviral therapy in rural Uganda. J Acquir Immune Defic Syndr (2010) 53(1):28–35. doi:10.1097/QAI.0b013e3181bdf65a
110. Iliff PJ, Piwoz EG, Tavengwa NV, Zunguza CD, Marinda ET, Nathoo KJ, et al. Early exclusive breastfeeding reduces the risk of postnatal HIV-1 transmission and increases HIV-free survival. AIDS (2005) 19(7):699–708. doi:10.1097/01.aids.0000166093.16446.c9
111. UNAIDS/UNICEF/WHO. HIV and Infant Feeding: Guidelines for Decision-Makers. Geneva, Switzerland: WHO (1998).
112. Piwoz EG, Iliff PJ, Tavengwa N, Gavin L, Marinda E, Lunney K, et al. An education and counseling program for preventing breast-feeding-associated HIV transmission in Zimbabwe: design and impact on maternal knowledge and behavior. J Nutr (2005) 135(4):950–5.
113. Kuhn L. Milk mysteries: why are women who exclusively breast-feed less likely to transmit HIV during breast-feeding? Clin Infect Dis (2010) 50(5):770–2. doi:10.1086/650536
114. Harmsen HJ, Wildeboer-Veloo AC, Raangs GC, Wagendorp AA, Klijn N, Bindels JG, et al. Analysis of intestinal flora development in breast-fed and formula-fed infants by using molecular identification and detection methods. J Pediatr Gastroenterol Nutr (2000) 30(1):61–7. doi:10.1097/00005176-200001000-00019
115. Mackie RI, Sghir A, Gaskins HR. Developmental microbial ecology of the neonatal gastrointestinal tract. Am J Clin Nutr (1999) 69(5):1035S–45S.
116. Desai A, Mbuya MN, Chigumira A, Chasekwa B, Humphrey JH, Moulton LH, et al. Traditional oral remedies and perceived breast milk insufficiency are major barriers to exclusive breastfeeding in rural Zimbabwe. J Nutr (2014) 144(7):1113–9. doi:10.3945/jn.113.188714
117. Desai A, Smith LE, Mbuya MN, Chigumira A, Fundira D, Tavengwa NV, et al. The SHINE trial infant feeding intervention: pilot study of effects on maternal learning and infant diet quality in rural Zimbabwe. Clin Infect Dis (2015) 61(Suppl 7):S710–5. doi:10.1093/cid/civ846
118. Matare CR, Mbuya MNN, Tavengwa NV, Ntozini R, Stoltzfus RJ, Humphrey JH. A Culturally Appropriate Intervention Delivered by Village Health Workers Increases the Prevalence of Exclusive Breastfeeding in Rural Zimbabwe. Abstract Presented at the 18th International Society for Research in Human Milk and Lactation. Stellenbosch, South Africa: (2016).
Keywords: HIV exposure, infant, Zimbabwe, Africa, inflammation, immune activation, breast-feeding
Citation: Evans C, Humphrey JH, Ntozini R and Prendergast AJ (2016) HIV-Exposed Uninfected Infants in Zimbabwe: Insights into Health Outcomes in the Pre-Antiretroviral Therapy Era. Front. Immunol. 7:190. doi: 10.3389/fimmu.2016.00190
Received: 15 February 2016; Accepted: 02 May 2016;
Published: 06 June 2016
Edited by:
Tobias R. Kollmann, University of British Columbia, CanadaReviewed by:
Amy Louise Slogrove, University of Cape Town, South AfricaFatima Kakkar, University of Montreal, Canada
Copyright: © 2016 Evans, Humphrey, Ntozini and Prendergast. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ceri Evans, ceri.evans@qmul.ac.uk
 Ceri Evans
Ceri Evans Jean H. Humphrey1,3
Jean H. Humphrey1,3 Robert Ntozini
Robert Ntozini Andrew J. Prendergast
Andrew J. Prendergast