- 1Department of Pathology, Microbiology and Immunology, University of South Carolina School of Medicine, Columbia, SC, USA
- 2Clinical Laboratory, Hospital Los Patios, Los Patios, Colombia
Chronic exposure to antigens may favor the production of IgG4 antibodies over other antibody types. Recent studies have shown that up to a 30% of normal human IgG4 is bi-specific and is able to recognize two antigens of different nature. A requirement for this specificity is the presence of both eliciting antigens in the same time and at the same place where the immune response is induced. During transmission of most vector-borne diseases, the pathogen is delivered to the vertebrate host along with the arthropod saliva during blood feeding and previous studies have shown the existence of IgG4 antibodies against mosquito salivary allergens. However, there is very little ongoing research or information available regarding IgG4 bi-specificity with regard to infectious disease, particularly during immune responses to vector-borne diseases, such as malaria, filariasis, or dengue virus infection. Here, we provide background information and present our hypothesis that IgG4 may not only be a useful tool to measure exposure to infected mosquito bites, but that these bi-specific antibodies may also play an important role in modulation of the immune response against malaria and other vector-borne diseases in endemic settings.
Pathogens and Mosquito Saliva – A Brief Introduction
Infectious diseases are one of the leading causes of mortality worldwide (1, 2). From this large group, the vector-borne diseases are among the leading causes of mortality and disability in developing countries (3, 4). The majority of arthropod-borne diseases lack a specific vaccine; thus, prevention relies predominantly on vector control interventions (5, 6). In the last decade, considerable effort has been put toward the dissection of arthropod factors that modulate the transmission of pathogens (7–9). Several studies have shown that during transmission, vector saliva plays an important role in the establishment of a successful infection and favors the pathogen survival by modulating the local immune responses (7, 10–14). Most salivary proteins (SPs) are highly immunogenic, able to elicit antibody production and memory responses (15, 16). During probing and feeding, mosquitoes deposit SP in the vertebrate host to facilitate the blood meal intake (17–19). The immune response induced by arthropod saliva is mainly Th2, which favors the production of antibodies (20, 21). IgG4 antibodies are known to reflect repeated exposure to environmental antigens and allergens (22, 23). Additionally, recent research by our laboratory has found that exposure to mosquito saliva can be correlated with disease clinical presentation (15, 24, 25). Chronic exposure to mosquito bites induces higher IgG4 antibodies against SPs than other IgG subclasses (22, 26, 27), suggesting that this specific antibody subclass may be a marker of intense exposure to arthropod vectors (28, 29). Here, we present a brief review on the antibody response against SPs and the hypothetical implication of IgG4 antibodies in disease progression of several vector-borne diseases.
IgG4: A Special Molecule
As a general characteristic, IgG4 is an effective immuno-regulator (23). This is due to the fact that, although IgG4 may act as a blocking antibody, it is not efficient in forming large immune complexes (30). IgG4 antibodies are able to interact with the receptors FcγRI, FcγRIIA, FcγRIIB, FcγRIIC, and FcγRIIIA. Interestingly, the inhibitory receptor FcγRIIB has lower affinity for IgG1, IgG2, and IgG3 than the other Fc receptors (FcRs), but affinity is not lower for IgG4 (31). This interaction may be in part responsible for the anti-inflammatory properties of IgG4. In addition, IgG4 antibodies block IgE-mediated inflammatory responses by competing for antigen recognition sites, thus inhibiting Fcε-receptor cross-linking and further signaling (32, 33). IgG4 is the only IgG subclass with equal affinity for activating FcRs and for the inhibitory receptor FcγRIIB (34). If IgG4 co-interact with both inhibiting and activating receptors, the result would be inhibition of effector cell responses (34, 35).
Naïve B cells express a monomeric membrane-bound B cell receptor normally as IgM or IgD antibodies (36, 37). After activation of such B cells and aided by specific cytokines, rearrangement of the antibody heavy chain locus through class switch recombination results in the expression of a new isotype (i.e., IgG). In the case of IgG4, production relies on the Th2 cytokines IL-4 and IL-13 (38). The Th2-associated cytokine, IL-10, is a key factor in the activation of IgG4 producing B cells (39). Secretion of IL-10 will skew immune response to the production of IgG4 antibodies over other IgG subclasses. IL-10-producing B cells regulate pro-inflammatory immune responses and a lack of them can lead to exacerbated chronic inflammation (40, 41). Previous studies showed that IgG4 may be selectively produced in human B regulatory 1 (BR1) cells and that allergen-specific B cells present increased expression of both IL-10 and IgG4 (42). Distinctive from other IgG subtypes, IgG4 is the only antibody class that is able to recognize two antigens of different nature; therefore, it is often termed a bi-specific antibody (23). A requirement for this bi-specificity is that both eliciting antigens must be present at the same time and at the same place when the immune system is stimulated (43). This is the case of naturally acquired infections transmitted by arthropods where pathogens, such as parasites or viruses are delivered into human skin imbibed in saliva with SP (44).
IgG4 molecules are synthesized the same way as all the other IgG subclasses (23). However, extensive research has demonstrated important sequence differences among all IgG subclasses that confect various levels of stability between chains in the hinge region (45, 46). These studies shown that one single substitution of a proline for an serine in the light chain allows IgG4 molecules to undergo a process called the “half-molecule exchange” where one antibody molecule directed against a specific antigen has the capacity of “exchanging” a half-molecule (a heavy chain and an attached light chain) with another IgG4 molecule synthetized against another non-related antigen (45–48) (Figure 1). Normally, a single B cell will produce IgG molecules with identical copies of a heavy chain determining the immunoglobulin isotype (e.g., G) and two identical copies of light chains (κ or λ) (49, 50). During the half-molecule exchange process, hybrid IgG4 molecules could contain two heterogeneous light chains (i.e., κ/λ instead of κ/κ or λ/λ). A recent publication described that hybrid bi-specific IgG4 κ/λ antibodies represent approximately up to 30% of the total IgG4 antibodies in human sera (51). Unfortunately, the exact mechanism involved with the in vivo generation of specific IgG4 molecules is largely unknown.
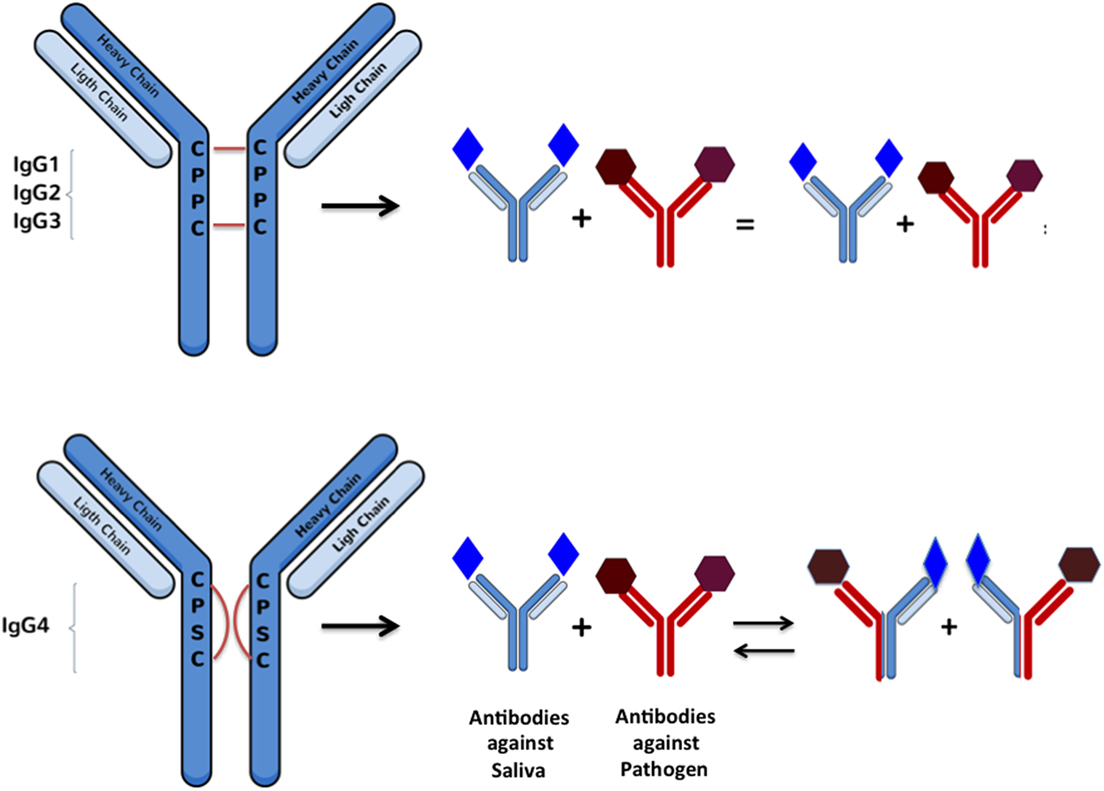
Figure 1. Schematic representation of Fab-arm exchange between IgG molecules: changes in heavy chain sequences makes the inter-chain interactions weaker and favoring intra-chain interactions in a way that allows arm disassembly and further rearrangement between molecules recognizing antigens of different nature. This reaction can be simulated in vitro under reducing conditions with glutathione.
Another special characteristic of IgG4 antibodies is their ability to interact with other IgG subclasses, particularly IgG1, in such a way as to alter/block their functionality and FcR binding (48). Specifically, previous studies have found that the Fc portion of IgG4 is able to interact with other IgG antibodies in an Fc–Fc fashion due to conformational changes that allows interaction between CH3 motifs from both molecules (45, 48). This interaction is specially favored when the target, IgG1 or another IgG4 molecule, is coupled to a solid phase (Figure 2). Fc–Fc interactions between two IgG4 may potentially be an intermediated step in the formation of bi-specific IgG4 molecules, allowing the contact and further recombination of two IgG4 molecules. A more detailed description of the Fc–IgG4 interactions with other Fc–IgG and their potential implication in vivo is explained in detail by Rispens et al. (48) and Davies et al. (45).
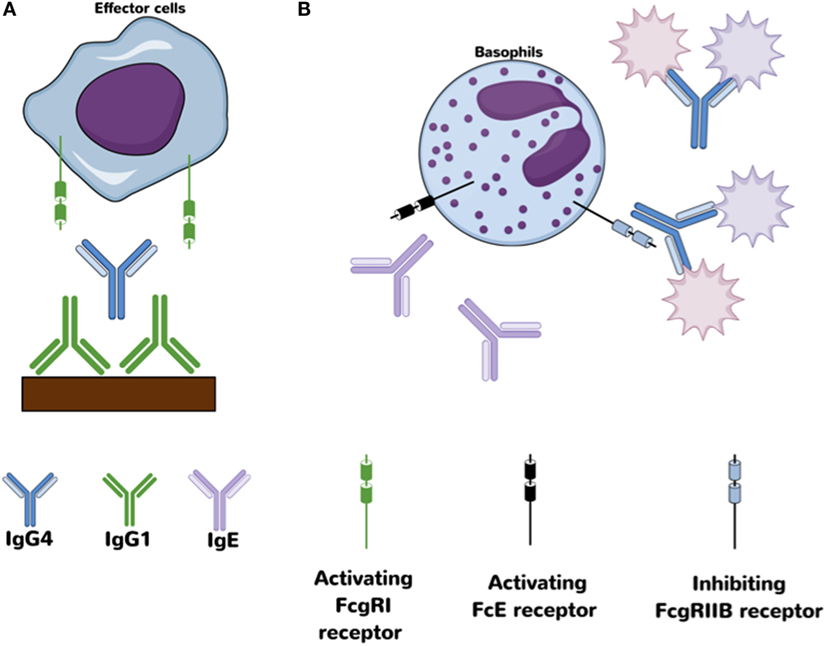
Figure 2. Proposed anti-inflammatory mechanism for IgG4 antibodies. (A) Fc–Fc interaction between IgG4 and IgG1 may block access to activating Fcγ receptors on effector cells. (B) Antigen-binding competition against IgE avoids/decreases cross-link with activating Fcε receptors.
In the case of vector-borne human diseases, anti-SP IgG4 may undergo this half-molecule exchange with IgG4 molecules against a pathogen. Since IgG4 antibodies are often present in individuals who are chronically exposed to mosquito saliva allergens, we hypothesize that some of these antibodies may form hybrid bi-specific molecules with pathogen-specific IgG4 during natural transmission of vector-borne diseases. This hypothesis is also based on the fact that mosquito saliva alone is able to increase the level of IL-10 in exposed tissues creating a favorable environment for the production of IgG4 antibodies against the antigens present. If those antibodies are against two different antigens, the half-arm exchange may be induced.
The majority of research on the characteristics of bi-specific antibodies has been focused on artificial antibodies for cancer treatment or the role of natural bi-specific antibodies in autoimmune diseases, such as rheumatoid arthritis (RA) (52–54). The mechanisms involving IgG4 in autoimmune diseases is still not understood, but findings suggest that the presence of natural bi-specific antibodies in serum from RA patients may serve as indicators for disease remission (53). Additionally, artificial bi-specific antibodies are among the most promising treatment choices for cancer and inflammatory-mediated diseases over monoclonal antibodies because they can simultaneously target two different antigens and improve therapeutic outcomes (55, 56). Two options of artificial bi-specific antibodies are already available in the market and several others are in clinical trials (55). However, there is a stark lack of research with regard to infectious diseases transmitted by arthropods and the participation, if any, of bi-specific IgG4 in the immune response against them. We believe that possible missing clues in vaccine or/and treatment development for malaria and other vector-borne diseases may be hidden within the properties of this double-handed molecule known as IgG4.
Bi-specific IgG4 molecules are stimulated in an environment of diverse antigens. For example, previous studies have shown that a single IgG4 molecule is able to bind grass/pollen antigens and house dust mite antigens at the same time (43). In the case of allergens, such as mosquito saliva, which is composed of a diverse cocktail of molecules, the IgG4 bi-specificity could be elicited against the various salivary components producing “SP–SP” bi-specific IgG4 (Figure 3A). However, in the presence of pathogen/saliva mixture (delivered via an infective insect bite), a proportion of “pathogen-SP” IgG4 would likely be produced (Figure 3B). Indeed, chronic exposure to mosquito salivary antigens has been proposed to play a role in immunity against chronic versions of several vector-borne diseases, such as filariasis, leishmaniasis, and malaria (57–59), by stimulating the production of pathogen-SP IgG4 bi-specific antibodies.
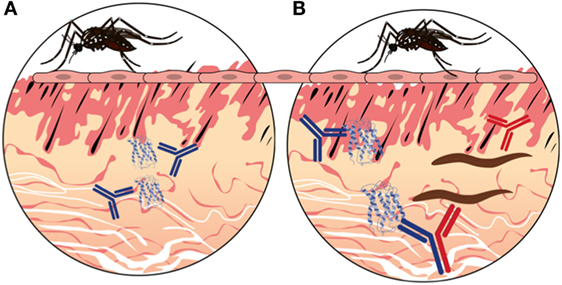
Figure 3. A hypothetical representation of the hybrid IgG4 formation in vector-borne diseases. (A) In the absence of infection in the mosquito, probing will inject saliva proteins into the vertebrate host during blood feeding. Thus, antibodies will be made against mosquito proteins only (BLUE). (B) In the presence of infection in the mosquito, the pathogen will be delivered in the skin along with the salivary proteins. Antibodies will be made that recognize mosquito proteins (BLUE) as well as pathogen proteins (RED). The chronic exposure to different antigens will stimulate the production of IgG4 molecules that will undergo fab-arm exchange when two different antigens are present, resulting in bi-specific antibody molecules (BLUE AND RED).
The human immune response against vector-borne pathogens often involves the production of IgG1 antibodies with high pro-inflammatory activities that are able to clear most pathogens. If conformational changes are induced at the Fc portion of an antigen-bound IgG1 molecule, a significant IgG4-mediated response against SPs and/or pathogen antigens may block an IgG1-mediated response, which would have a profound effect on the fate of infectious disease progression and immune response Thus, IgG4 and bi-specific IgG4 antibodies may represent attractive examples of how the immune response to environmental antigens could potentially modulate the fate of infectious diseases in humans (35).
IgG4 and Parasitic Infections
Filariasis
Human filariasis (lymphatic filariasis, Onchocerciasis, and Loiasis) is group of parasitic infections often transmitted by arthropods to humans and animals (60–62). With the exception of Dracunculus medinensis, all filarial worms use insects as intermediate hosts (63). These diseases are important causes of disability and physical deformity in developing countries (62). It is calculated that at least one-fifth of the global population is at risk and there is not any commercially available vaccine (64). However, in spite of challenges in program coverage and compliance, significant progress has been made in reducing the incidence of filarial infections by mass drug administration programs (65).
As in the rest of human helminthiasis, IgG4 antibody response is the hallmark of filarial infections. Previous studies have shown that up to 95% of IgG antibodies against filarial worms belong to the IgG4 subclass (66, 67). Filarial parasites preferentially induce the production of IgG4 antibodies to evade immune response in the infected host; thus, filarial infections are often chronic (68). Adjobimey and Hoerauf (67) suggested that the immune regulatory properties of IgG4 in filarial infection could be associated with the half-molecule exchange property of this antibody (67). Mosquitoes from the genus Aedes, Anopheles, Culex, and Mansonia can all transmit filarial parasites. Whether bi-specific antibodies are elicited against salivary/pathogen antigens in filariasis or other helminthic diseases remains to be investigated, though it seems a likely scenario.
Previous studies demonstrate that the levels of IgG antibody subclasses during infection depend on filarial transmission intensity. In higher transmission areas, antigen-specific IgG4 levels against different parasite stages (microfilaria versus adults stages) are associated with parasite carriage and asymptomatic infection (68, 69), In fact, people with asymptomatic filarial infection present significantly higher IgG4 antibody levels than people with severe disease. By contrast, lower IgG4 antibody levels, along with high titers of IgE, are present in severe cases (70–72). It is proposed that IgG4 antibody levels in filarial infected patients is associated with the blocking effect of the IgE mediated, often exacerbated, pro-inflammatory responses (61).
Malaria
Malaria is currently the most prevalent parasitic disease transmitted by mosquitoes in the world; it is caused by parasites of the genus Plasmodium (73). Several Plasmodium species can cause disease in human but it is Plasmodium falciparum that is the single species responsible for majority of human deaths (74, 75). In spite of major significant advances in the knowledge of malaria transmission and several vaccine trials in recent years, there is still not a single vaccine against Plasmodium spp. infection commercially available (76). A vaccine against malaria is desperately needed to prevent the nearly 500,000 deaths from this disease each year, most of them children under the age of 5 years (77–79). Interestingly, in endemic areas, natural “protective immunity” is developed over decades of exposure to infected mosquito bites (80). People who develop this type of immunity are partially protected against severe disease and more dangerous symptoms. This would mean that the longer someone lives in an endemic area, the more protected the individual would be against severe malaria. In fact, the association between asymptomatic malaria carriers and age has been well documented (81–85). Unfortunately, this immunity is short-lived and may wane once the person leaves the endemic, mosquito-rich area or when exposure becomes less constant (i.e., travel, emigration) (80, 86). When this happens, the body returns to a “naïve” state and the person may develop symptoms, and even severe disease, upon a new infection with Plasmodium (87).
In humans, immune response against malaria seems to be mediated by “cooperation” between cellular and antibody-mediated responses. The main antibodies associated with protective immunity against malaria are the IgG subclasses, IgG1 (88, 89). Since IgG4 may interfere with the activity of other antibody subclasses, we hypothesize that IgG1-mediated responses against Plasmodium could be influenced by an increase in IgG4 antibodies induced by the presence of saliva. These IgG4 antibodies may bind Plasmodium-bound IgG1 antibodies blocking Fc-receptor-mediated responses, opsonization and/or complement activation, consequently, enhancing parasite survival. It is also possible that, in the presence of saliva, bi-specific Plasmodium–Anopheles IgG4 antibodies are induced and play a role in developing protective/detrimental immune responses during malaria infection.
One of the main SPs in the principal malaria vector in Africa, Anopheles gambiae, is the salivary gland protein 6 (gSG6), a small proteins exclusively expressed in adult female salivary glands that plays a role blood feeding (90). Antibodies against a peptide derivative from this protein, the gSG6-P1, have been validated as a marker for Anopheles bite exposure (91, 92). The gSG6 protein is recognized by IgG4 antibodies in serum from people chronically exposed to Anopheles bites (27, 93). Although the presence of bi-specific IgG4 antibodies or a direct correlation between gSG6-IgG4 and Plasmodium IgG1 has not yet been evaluated, a recent study suggested a negative effect of mosquito bite exposure on the IgG1-mediated immune response against malaria. This study showed that children with higher levels of exposure to Anopheles bites presented lower IgG1 responses against Plasmodium antigens and parasitemia was significantly correlated with IgG1 antibody levels (94), suggesting an impact of immunity against SPs in the immune response against infection in pediatric populations. The impact of anti-salivary immunity in adults was not described in this study. It is possible that high exposure to mosquito bites in children can induce anti-SP IgG4 antibodies capable of binding Plasmodium-specific IgG1 and block its activity; therefore, resulting in more symptomatic disease. However, extensive further research is needed to evaluate whether there is a correlation between the anti-SP IgG/IgG4 ratio and symptomatic disease in children as well as whether bi-specific antibodies play a role in age-dependent protection/pathogenesis in malaria.
Leishmania
Leishmaniasis is a group of parasitic diseases [cutaneous, mucocutaneous and visceral leishmaniasis (VL)] caused by parasites of the genus Leishmania spp. Leishmaniasis is transmitted by Phlebotomus spp. (Old World) and Lutzomyia spp. (New World) sandflies (95, 96). Cutaneous leishmaniasis (CL) is often presented as chronic lesions developed from months to years. Previous studies have shown an association between disseminated CL forms of leishmaniasis and IgG4 antibody levels (97). In fact, proper healing is often associated with a Th1-mediated response while disseminated disease is associated with Th2 responses. CL and mucocutaneous leishmania (ML) infections are characterized by successively higher specific antibody titers that reflect the length of infection and parasite loads (98). High antibody titers can also be detected in VL due to a polyclonal activation of B cells (99). VL is a serious parasitic disease caused by the protozoan Leishmania donovani (Asia) and L. infantum/L. chagasi with more than 200 million people at risk globally (95, 100). The Post-kala-azar dermal leishmaniasis (PKDL) is a complication presenting in patients infected with L. donovani that have recovered from VL (101). It has been found that IgG4 is significantly elevated in PKDL compared to VL, especially in pediatric populations (102). Higher IgG4 antibody levels have also been observed in people with active VL in comparison to treated patients or those with subclinical disease (103). Additionally, a decrease in the IgG4 antibody titer has been observed in children with VL after treatment, suggesting that IgG4 may be a suitable immunological marker for the assessment of drug treatment (104).
It has been well documented that saliva from sandflies enhances Leishmania infection during the first stages of the infection. This is thought to be due to the immunosuppressive effect of several saliva components on macrophages and T cells (105–108). Interestingly, immunity against sand fly salivary molecules may confer protection against disease (108, 109). In mouse models, the protection observed in pre-exposed animals to sandfly saliva involved a strong induction of Th1 response and delayed-type hypersensitivity (DTH) response reaction against saliva that creates a detrimental environment for the parasite (110). These studies showed that the immune response against the phlebotomine sandfly’s SPs has modulatory effects on disease pathogenesis and some of these proteins are considered candidates for vaccine or drug synthesis (111, 112). It is hypothesized that the study of mosquito salivary factors with similar activity could lead to novel candidates for other arthropod transmitted diseases (11, 113). In addition, previous studies have shown that people exposed to sandfly bites present specific antibodies against the saliva of the main vectors in the area where they reside and little or no cross-reactivity to other sandfly species. People with active leishmaniasis present significantly higher IgG antibody levels against SPs of the main vector when compared with healthy individuals living in the same area (114).
One study demonstrated that IgG4 antibodies are the main antibody subclass against SPs of the Ph. papatasi sandfly (115). It has been shown that previous exposure to the American sandfly Lu. intermedia can induce a significant increase in IL-10 expression resulting in exacerbation of infection after challenge with Leishmania braziliensis (116, 117), although positive correlation between antibody response to saliva and cellular response to Leishmania has not been reported. Importantly, individuals seropositive to saliva are twice more likely to develop CL (118). Infection by L. braziliensis is responsible for the majority of ML characterized by highly destructive mucosal lesion. ML often follows skin lesions even decades before the mucosal involvement (119). In that time, it is very likely that people continue being exposed to vector bites, which may induce the production of bi-specific IgG4 antibodies against mosquito proteins and pathogen proteins.
Arboviruses: A Focus on Dengue and Antibody-Mediated Responses
Dengue is a disease caused by dengue virus (DENV), which is transmitted by Aedes mosquitoes in tropical and subtropical regions (4). While the majority of DENV infections result in little or no disease, a small proportion of cases progresses to severe forms: dengue with warning signs and severe dengue also known as dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS) (120). It is thought that cross-reactive but sub-neutralizing IgG antibodies developed during the first DENV infection may enhance a second infection with a different serotype in a phenomenon termed antibody-dependent enhancement (ADE) (121–123). During ADE, it is thought that these sub-neutralizing antibodies may increase viral load by enabling entry into immune cells via the Fc gamma receptor (FcγR) (124). ADE is a significant risk factor for severe dengue fever (125, 126). Since IgG4 molecules are able to interact with different FcγR (31), it is imperative to study whether bi-specific IgG4 antibodies play a role in ADE.
Currently, considerable research in DENV pathogenesis is focused on pathogen-induced disease severity (127–130). Although this is of relevance, there is also a need for studies on the role of arthropod vector factors involved in pathogen infectivity and disease development. Interestingly, previous studies have shown that pathogen infection of mosquito salivary gland induces changes in the composition of saliva (131, 132). Recent studies have also shown a significant correlation between the antibody levels against these upregulated mosquito SPs and exposure to disease; thus, these proteins have been proposed as potential markers of exposure to infective bites (131). We speculate that a higher proportion of bi-specific IgG4 antibodies could be elicited against these upregulated proteins along with the pathogen, since these proteins are in higher concentration in the presence of the pathogen.
Salivary Protein IgG4-Mediated Pathology
Previously, studies have demonstrated that bi-specific IgG4 antibodies are naturally produced in autoimmune diseases and other IgG4-related diseases (53, 54, 133). One autoimmune disease related to IgG4-mediated responses is the Fogo Selvagem (FS). In patients with FS, pathogenic autoantibodies of the IgG4 class react against desmoglein1 (Dsg1), a transmembrane glycoprotein component of vertebrate epithelial cells (134). It has been demonstrated that FS incidence overlaps significantly with exposure to Lu. longipalpis sandflies in Brazil (135). Interestingly, the antibody response against one specific sandfly protein, LJM11, is associated with an IgG4-mediated disease characterized by an IgG4 cross-reactivity between two antigens of different nature. It was also shown that antibodies recognizing Dsg1 during FS disease also recognize the SP LJM11, and that antibodies recognizing LJM11 can cross-react with anti-Dsg1 monoclonal autoantibodies derived from FS patients. The authors suggest that insect bites may induce a cross-reactive IgG4 antibody response, which then leads to FS disease. Their findings demonstrate a relationship between a non-infectious antigen (environmental) and the development of an autoimmune disease (136). Although the presence of bi-specific IgG4 was not evaluated, these findings suggest the potential involvement of vector saliva IgG4-mediated-pathology.
Non-Vector Borne Chronic Viral Diseases and IgG4
In general, viral infections induce the production of neutralizing IgG1 and IgG3 antibodies over IgG4 (137, 138). However, it is possible that viruses responsible for chronic infections may also induce significant levels of IgG4 antibodies. This could be the case with infections by hepatitis B or C viruses, cytomegalovirus, Epstein–Bar virus (EBV), and human immunodeficiency virus (HIV), among others. Unfortunately, the role of IgG4 in the previously mentioned diseases has been rather neglected and conflicting results are found in the literature. For instance, in the case of HIV, one study found insignificant differences in IgG4 levels between HIV-1 positive patients and healthy controls (139), while other studies have shown that IgG4 antibody levels are significantly lower in HIV-1 patients (140–142). In addition, progression to AIDS may have a negative impact on IgG4 antibody concentration (143) since rapidly progressing patients present higher antibody levels when compared to slowly progressing individuals (141). Another study described a Th2 response characterized by high IgG4 antibody levels in children with advanced HIV-1 infections, which is thought to contribute to disease progression (144). By contrast, infection with EBV has been previously associated with IgG4-related lymphadenopathy with an increase in the number of EBV-infected cells, suggesting a direct association between EBV and IgG4-related disease (145–147) The specific mechanism involving these relationships are still under investigation. Consequently, extensive further investigation is needed to characterize the role of IgG4 antibodies in viral chronic diseases as well as to determine the existence of hybrid IgG4 molecules recognizing different viral antigens or tissue-viral antigens.
The Hypothesis and Preliminary Data
In a given area, people may be exposed to hundreds of mosquito bites from different mosquito species (148). Previous studies have shown that levels of anti-SP IgG4 antibodies may increase with age and they are dependent of the transmission intensity (28, 29, 149) (Figure 4A). Chances for bi-specific IgG4 antibodies to be randomly generated, increases with time and exposure intensity (Figures 4B–D). Furthermore, arthropod saliva contains dozens of proteins, each one eliciting different responses in a given host (150). Thus, we decided to test the hypothesis that virally infected mosquito saliva is able to induce the production of bi-specific IgG4 antibodies using serum from people exposed to DENV and Aedes mosquito bites. For this pilot test, we selected 30 serum samples from DENV positive (n = 24) and control (n = 6) human subjects living in an area where Ae. aegypti is the main vector of DENV, and Ae. albopictus was recently introduced. Samples were tested for the presence of IgG4 antibodies in an ELISA-based assay (Figure 5A). Our results demonstrate that the main antibody subclass against Ae. aegypti SP is the IgG4, consistent with previous studies (22, 71). We next examined whether these IgG4 antibodies are bi-specific by testing their ability to bind both DENV antigens and Aedes SP. We found that our serum samples did contain bi-specific IgG4 antibodies (Figures 5B,C). We also found that more antibodies bound both DENV and Ae. aegypti SP than Ae. albopictus SP (Figures 5B,C). However, this was expected, as the individuals in the study had been exposed longer to Ae. aegypti. We are currently isolating the hybrid IgG4 molecules from these samples to test their effect on DENV infection.
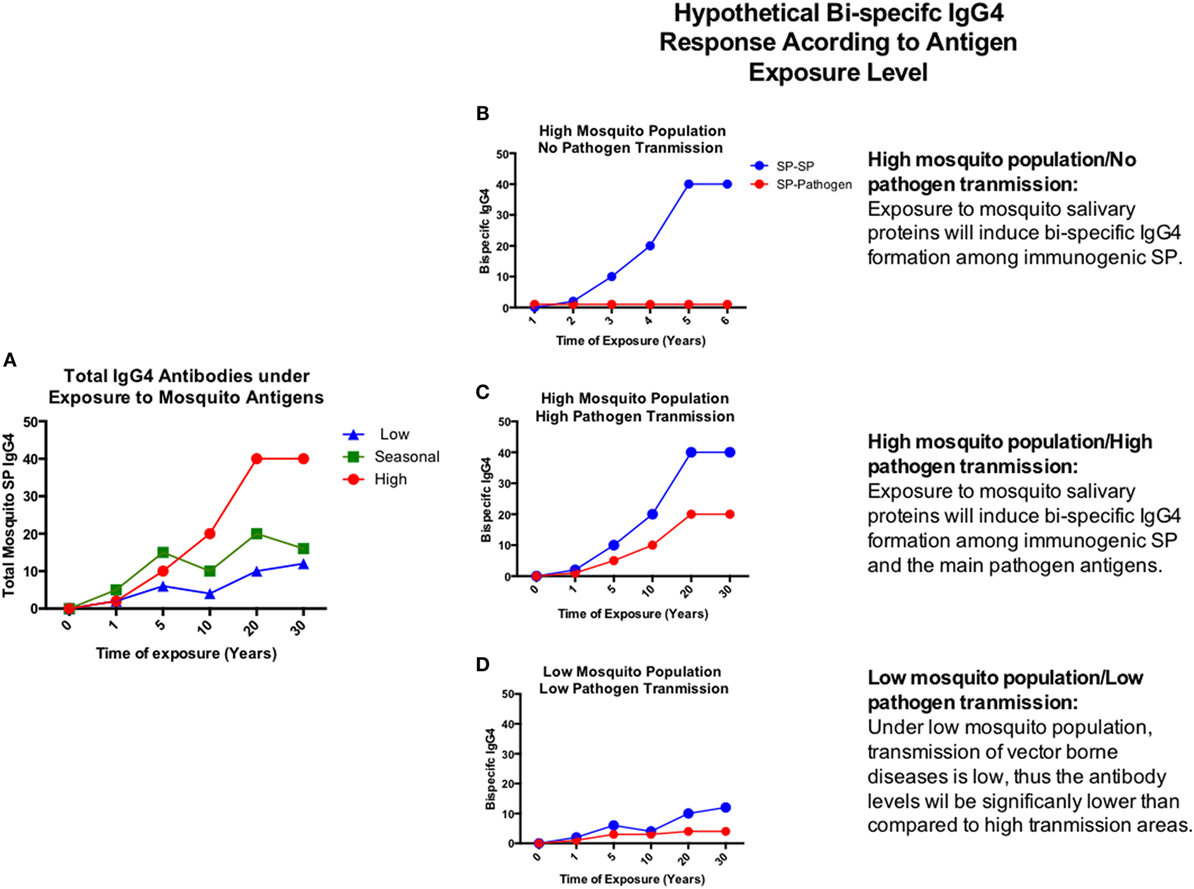
Figure 4. Hypothetical chronic progression of total and bi-specific IgG4 antibodies according to disease transmission settings. (A) Total IgG4 antibodies develop under chronic exposure to antigens. (B–D) Graphic representation of the bi-specific IgG4 antibody levels in the presence of exposure to high mosquito population/no pathogen transmission (B), high mosquito population/high pathogen transmission (C), and low mosquito population/low pathogen transmission (D). Antibody levels will depend on the time and magnitude of exposure to antigens.
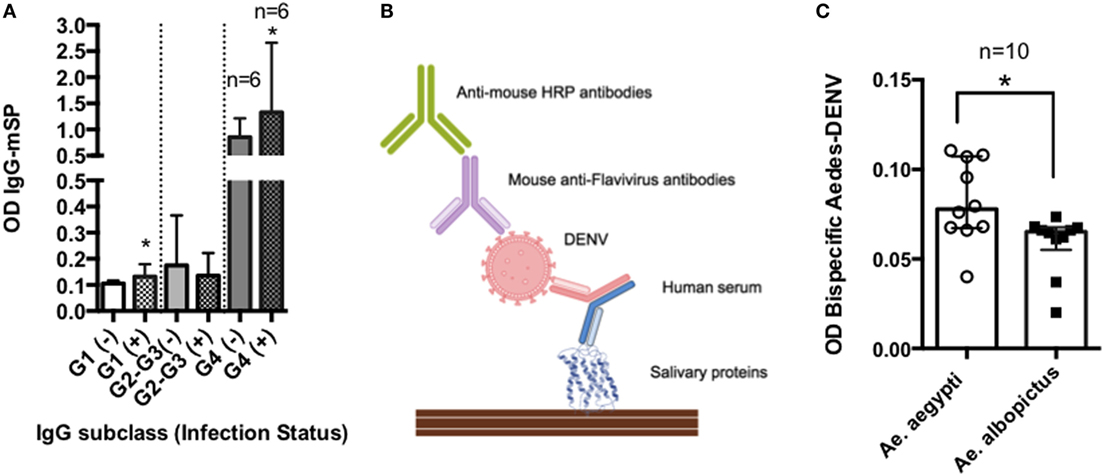
Figure 5. Bi-specific IgG4 molecules in DENV infection. Serum samples were obtained from febrile patients seeking dengue fever diagnosis. The presence of antibodies binding mosquito salivary proteins (SPs) and DENV antigen was evaluated in an ELISA-based test: plates were coated with Ae. aegypti SP and incubated with human serum. After washing, DENV particles were added to the plates followed by incubation with an anti-Flavivirus HRP labeled antibody. Optical densities (OD) were read at 450 nm. (A) IgG antibody subclass distribution in febrile individuals with (+) and without (−) active viremia. (B) Graphical representation of ELISA methods. (C) Bi-specific antibody levels against SP of the main Aedes species in the area. The inclusion of human subjects to evaluate the immune response against mosquito saliva was reviewed and approved by the IRB committees at the University of Pamplona (Colombia).
Our experiments suggest the existence of IgG4 molecules binding DENV and SP, and they are an approximation of the effect of immunity against mosquito saliva in viral infections. More specific and accurate testing must be done to evaluate single bi-specific IgG4 antibodies and demonstrate the role of hybrid IgG4 molecules in vector-transmitted diseases.
Concluding Remarks
It is of pivotal importance to determine whether there is production of saliva-pathogen IgG4 bi-specific antibodies during human infection with vector-borne pathogens, as well as the SPs that are involved in such a response. We speculate that in order to reach protective immunity against diseases, such as malaria through vaccination, treatment options should induce and resemble the natural immunity-building experience undergone by individuals living in areas with intense malaria transmission.
The study of the role of mosquito saliva immunity and antibodies against both pathogen and vector, as a tool to evaluate and monitor the exposure to infected mosquito bites, would improve control interventions and guide new strategies for protection and elimination. We have presented here evidence that IgG4-mediated responses are present in vector-borne diseases and that both arthropod saliva and the immune response against it may favor the formation of pathogen/mosquito bi-specific IgG4 antibodies. We hypothesize that bi-specific IgG4 is formed during chronic exposure to both mosquito bites and pathogen infection. Extensive additional research is necessary to characterize the nature of bi-specific antibodies and what role they may play in pathogenesis of or protection against vector-borne diseases.
Ethics Statement
A written approval for collection of human samples was approved by Universidad de Pamplona, Los Patios Hospital and the Ethics Review Board of Hospital Erasmo Meoz.
Author Notes
Dr. Londono-Renteria is a Research Associate at Dr. Colpitts’ Laboratory. This laboratory focuses on Dengue infection and pathogenesis studies as well as research on mosquito factors involved in the enhancement of transmission of vector-borne diseases.
Author Contributions
BL-R, AT, and TC were involved in the bibliography search and the manuscript writing.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank Dr. Mark A. James and Dr. Eric Calvo for their comments and suggestions during the preparation of this manuscript.
Funding
Universidad de Pamplona "50th anniversary Grant" (2010).
Abbreviations
ADE, antibody mediated enhancement; Ae, Aedes; BR1, B regulatory 1 cells; CL, cutaneous leishmaniasis; DENV, dengue virus; EBV, Epstein–Bar virus; FcR, fragment crystalizable region receptor; FS, Fogo Selvagem; gSG6-P1, salivary gland protein-6 peptide 1; HIV, human immunodeficiency virus; Ig, immunoglobulin G (IgG) or E (IgE).; L, Leishmania; Lu, Lutzomyia; ML, mucosal leishmaniasis; Ph, phlebotomus; PKDL, post Kala-Azar dermal leishmaniasis; SP, salivary protein; VL, visceral leishmaniasis.
References
1. Fauci AS. Infectious diseases: considerations for the 21st century. Clin Infect Dis (2001) 32:675–85. doi:10.1086/319235
2. Boraschi D, Abebe Alemayehu M, Aseffa A, Chiodi F, Chisi J, Del Prete G, et al. Immunity against HIV/Aids, malaria, and tuberculosis during co-infections with neglected infectious diseases: recommendations for the European Union research priorities. PLoS Negl Trop Dis (2008) 2:e255. doi:10.1371/journal.pntd.0000255
3. Gubler DJ. Resurgent vector-borne diseases as a global health problem. Emerg Infect Dis (1998) 4:442–50. doi:10.3201/eid0403.980326
4. Epelboin L, Hanf M, Dussart P, Ouar-Epelboin S, Djossou F, Nacher M, et al. Is dengue and malaria co-infection more severe than single infections? A retrospective matched-pair study in French Guiana. Malar J (2012) 11:142. doi:10.1186/1475-2875-11-142
5. Zofou D, Nyasa RB, Nsagha DS, Ntie-Kang F, Meriki HD, Assob JC, et al. Control of malaria and other vector-borne protozoan diseases in the tropics: enduring challenges despite considerable progress and achievements. Infect Dis Poverty (2014) 3:1. doi:10.1186/2049-9957-3-1
6. Hemingway J, Ranson H. Insecticide resistance in insect vectors of human disease. Annu Rev Entomol (2000) 45:371–91. doi:10.1146/annurev.ento.45.1.371
7. Ockenfels B, Michael E, Mcdowell MA. Meta-analysis of the effects of insect vector saliva on host immune responses and infection of vector-transmitted pathogens: a focus on leishmaniasis. PLoS Negl Trop Dis (2014) 8:e3197. doi:10.1371/journal.pntd.0003197
8. Colpitts TM, Barthel S, Wang P, Fikrig E. Dengue virus capsid protein binds core histones and inhibits nucleosome formation in human liver cells. PLoS One (2011) 6:e24365. doi:10.1371/journal.pone.0024365
9. Conway MJ, Watson AM, Colpitts TM, Dragovic SM, Li Z, Wang P, et al. Mosquito saliva serine protease enhances dissemination of dengue virus into the mammalian host. J Virol (2014) 88:164–75. doi:10.1128/JVI.02235-13
10. Qureshi AA, Asahina A, Ohnuma M, Tajima M, Granstein RD, Lerner EA. Immunomodulatory properties of maxadilan, the vasodilator peptide from sand fly salivary gland extracts. Am J Trop Med Hyg (1996) 54:665–71.
11. Leitner WW, Costero-Saint Denis A, Wali T. Immunological consequences of arthropod vector-derived salivary factors. Eur J Immunol (2011) 41:3396–400. doi:10.1002/eji.201190075
12. Briant L, Despres P, Choumet V, Misse D. Role of skin immune cells on the host susceptibility to mosquito-borne viruses. Virology (2014) 46(4–465):26–32. doi:10.1016/j.virol.2014.06.023
13. Schneider BS, Higgs S. The enhancement of arbovirus transmission and disease by mosquito saliva is associated with modulation of the host immune response. Trans R Soc Trop Med Hyg (2008) 102:400–8. doi:10.1016/j.trstmh.2008.01.024
14. de Moura TR, Oliveira F, Novais FO, Miranda JC, Clarencio J, Follador I, et al. Enhanced Leishmania braziliensis infection following pre-exposure to sandfly saliva. PLoS Negl Trop Dis (2007) 1:e84. doi:10.1371/journal.pntd.0000084
15. Londono-Renteria BL, Eisele TP, Keating J, James MA, Wesson DM. Antibody response against Anopheles albimanus (Diptera: Culicidae) salivary protein as a measure of mosquito bite exposure in Haiti. J Med Entomol (2010) 47:1156–63. doi:10.1603/ME09240
16. Machain-Williams C, Mammen MP Jr, Zeidner NS, Beaty BJ, Prenni JE, Nisalak A, et al. Association of human immune response to Aedes aegypti salivary proteins with dengue disease severity. Parasite Immunol (2012) 34:15–22. doi:10.1111/j.1365-3024.2011.01339.x
17. Ribeiro JM, Rossignol PA, Spielman A. Role of mosquito saliva in blood vessel location. J Exp Biol (1984) 108:1–7.
18. Hopp CS, Sinnis P. The innate and adaptive response to mosquito saliva and Plasmodium sporozoites in the skin. Ann N Y Acad Sci (2015) 1342:37–43. doi:10.1111/nyas.12661
19. Bizzarro B, Barros MS, Maciel C, Gueroni DI, Lino CN, Campopiano J, et al. Effects of Aedes aegypti salivary components on dendritic cell and lymphocyte biology. Parasit Vectors (2013) 6:329. doi:10.1186/1756-3305-6-329
20. Lawaly R, Konate L, Marrama L, Dia I, Diallo D, Diene Sarr F, et al. Impact of mosquito bites on asexual parasite density and gametocyte prevalence in asymptomatic chronic Plasmodium falciparum infections and correlation with IgE and IgG titers. Infect Immun (2012) 80:2240–6. doi:10.1128/IAI.06414-11
21. Boppana VD, Thangamani S, Adler AJ, Wikel SK. SAAG-4 is a novel mosquito salivary protein that programmes host CD4 T cells to express IL-4. Parasite Immunol (2009) 31:287–95. doi:10.1111/j.1365-3024.2009.01096.x
22. Brummer-Korvenkontio H, Lappalainen P, Reunala T, Palosuo T. Detection of mosquito saliva-specific IgE and IgG4 antibodies by immunoblotting. J Allergy Clin Immunol (1994) 93:551–5. doi:10.1016/S0091-6749(94)70066-4
23. Aalberse RC, Stapel SO, Schuurman J, Rispens T. Immunoglobulin G4: an odd antibody. Clin Exp Allergy (2009) 39:469–77. doi:10.1111/j.1365-2222.2009.03207.x
24. Londono-Renteria B. Human IgG antibody response against recombinant Ae. aegypti salivary proteins modified during DENV2 infection. Am J Trop Med Hyg (2014) 91:1.
25. Londono-Renteria B, Cardenas JC, Cardenas LD, Christofferson RC, Chisenhall DM, Wesson DM, et al. Use of anti-Aedes aegypti salivary extract antibody concentration to correlate risk of vector exposure and dengue transmission risk in Colombia. PLoS One (2013) 8:e81211. doi:10.1371/journal.pone.0081211
26. Reunala T, Brummer-Korvenkontio H, Palosuo K, Miyanij M, Ruiz-Maldonado R, Love A, et al. Frequent occurrence of IgE and IgG4 antibodies against saliva of Aedes communis and Aedes aegypti mosquitoes in children. Int Arch Allergy Immunol (1994) 104:366–71. doi:10.1159/000236693
27. Rizzo C, Ronca R, Lombardo F, Mangano V, Sirima SB, Nebie I, et al. IgG1 and IgG4 antibody responses to the Anopheles gambiae salivary protein gSG6 in the sympatric ethnic groups Mossi and Fulani in a malaria hyperhendemic area of Burkina Faso. PLoS One (2014) 9:e96130. doi:10.1371/journal.pone.0096130
28. Palosuo K, Brummer-Korvenkontio H, Mikkola J, Sahi T, Reunala T. Seasonal increase in human IgE and IgG4 antisaliva antibodies to Aedes mosquito bites. Int Arch Allergy Immunol (1997) 114:367–72. doi:10.1159/000237696
29. Remoue F, Alix E, Cornelie S, Sokhna C, Cisse B, Doucoure S, et al. IgE and IgG4 antibody responses to Aedes saliva in African children. Acta Trop (2007) 104:108–15. doi:10.1016/j.actatropica.2007.07.011
30. van der Zee JS, van Swieten P, Aalberse RC. Inhibition of complement activation by IgG4 antibodies. Clin Exp Immunol (1986) 64:415–22.
31. Bruhns P, Iannascoli B, England P, Mancardi DA, Fernandez N, Jorieux S, et al. Specificity and affinity of human Fcgamma receptors and their polymorphic variants for human IgG subclasses. Blood (2009) 113:3716–25. doi:10.1182/blood-2008-09-179754
32. Rihet P, Demeure CE, Dessein AJ, Bourgois A. Strong serum inhibition of specific IgE correlated to competing IgG4, revealed by a new methodology in subjects from a S. mansoni endemic area. Eur J Immunol (1992) 22:2063–70. doi:10.1002/eji.1830220816
33. Ozdemir C, Akdis M, Akdis CA. Nature of regulatory T cells in the context of allergic disease. Allergy Asthma Clin Immunol (2008) 4:106–10. doi:10.1186/1710-1492-4-3-106
34. Daeron M, Jaeger S, Du Pasquier L, Vivier E. Immunoreceptor tyrosine-based inhibition motifs: a quest in the past and future. Immunol Rev (2008) 224:11–43. doi:10.1111/j.1600-065X.2008.00666.x
35. James LK, Till SJ. Potential mechanisms for IgG4 inhibition of immediate hypersensitivity reactions. Curr Allergy Asthma Rep (2016) 16:23. doi:10.1007/s11882-016-0600-2
36. Wan Z, Chen X, Chen H, Ji Q, Chen Y, Wang J, et al. The activation of IgM- or isotype-switched IgG- and IgE-BCR exhibits distinct mechanical force sensitivity and threshold. Elife (2015) 4:e06925. doi:10.7554/eLife.06925
37. Geisberger R, Lamers M, Achatz G. The riddle of the dual expression of IgM and IgD. Immunology (2006) 118:429–37. doi:10.1111/j.1365-2567.2006.02386.x
38. Punnonen J, Aversa G, Cocks BG, Mckenzie AN, Menon S, Zurawski G, et al. Interleukin 13 induces interleukin 4-independent IgG4 and IgE synthesis and CD23 expression by human B cells. Proc Natl Acad Sci U S A (1993) 90:3730–4. doi:10.1073/pnas.90.8.3730
39. Kettleborough CA, Saldanha J, Ansell KH, Bendig MM. Optimization of primers for cloning libraries of mouse immunoglobulin genes using the polymerase chain reaction. Eur J Immunol (1993) 23:206–11. doi:10.1002/eji.1830230132
40. Kling JC, Korner H. Different regulatory mechanisms in protozoan parasitic infections. Int J Parasitol (2013) 43:417–25. doi:10.1016/j.ijpara.2013.02.001
41. Bhowmick S, Ravindran R, Ali N. IL-4 contributes to failure, and colludes with IL-10 to exacerbate Leishmania donovani infection following administration of a subcutaneous leishmanial antigen vaccine. BMC Microbiol (2014) 14:8. doi:10.1186/1471-2180-14-8
42. van de Veen W, Stanic B, Yaman G, Wawrzyniak M, Sollner S, Akdis DG, et al. IgG4 production is confined to human IL-10-producing regulatory B cells that suppress antigen-specific immune responses. J Allergy Clin Immunol (2013) 131:1204–12. doi:10.1016/j.jaci.2013.01.014
43. Schuurman J, Van Ree R, Perdok GJ, Van Doorn HR, Tan KY, Aalberse RC. Normal human immunoglobulin G4 is bispecific: it has two different antigen-combining sites. Immunology (1999) 97:693–8. doi:10.1046/j.1365-2567.1999.00845.x
44. Leitner WW, Wali T, Costero-Saint Denis A. Is arthropod saliva the achilles’ heel of vector-borne diseases? Front Immunol (2013) 4:255. doi:10.3389/fimmu.2013.00255
45. Davies AM, Rispens T, Ooijevaar-de Heer P, Gould HJ, Jefferis R, Aalberse RC, et al. Structural determinants of unique properties of human IgG4-Fc. J Mol Biol (2014) 426:630–44. doi:10.1016/j.jmb.2013.10.039
46. Davies AM, Rispens T, Den Bleker TH, Mcdonnell JM, Gould HJ, Aalberse RC, et al. Crystal structure of the human IgG4 C(H)3 dimer reveals the role of Arg409 in the mechanism of Fab-arm exchange. Mol Immunol (2013) 54:1–7. doi:10.1016/j.molimm.2012.10.029
47. Rispens T, Meesters J, Den Bleker TH, Ooijevaar-De Heer P, Schuurman J, Parren PW, et al. Fc-Fc interactions of human IgG4 require dissociation of heavy chains and are formed predominantly by the intra-chain hinge isomer. Mol Immunol (2013) 53:35–42. doi:10.1016/j.molimm.2012.06.012
48. Rispens T, Ooievaar-De Heer P, Vermeulen E, Schuurman J, van der Neut Kolfschoten M, Aalberse RC. Human IgG4 binds to IgG4 and conformationally altered IgG1 via Fc-Fc interactions. J Immunol (2009) 182:4275–81. doi:10.4049/jimmunol.0804338
49. Hwang JK, Alt FW, Yeap LS. Related mechanisms of antibody somatic hypermutation and class switch recombination. Microbiol Spectr (2015) 3(1):MDNA3–37. doi:10.1128/microbiolspec.MDNA3-0037-2014
50. Lievens MM. Medical and technical usefulness of measurement of kappa and lambda immunoglobulin light chains in serum with an M-component. J Clin Chem Clin Biochem (1989) 27:519–23.
51. Young E, Lock E, Ward DG, Cook A, Harding S, Wallis GL. Estimation of polyclonal IgG4 hybrids in normal human serum. Immunology (2014) 142:406–13. doi:10.1111/imm.12265
52. Riethmuller G. Symmetry breaking: bispecific antibodies, the beginnings, and 50 years on. Cancer Immun (2012) 12:12.
53. Wang W, Li J. Identification of natural bispecific antibodies against cyclic citrullinated peptide and immunoglobulin G in rheumatoid arthritis. PLoS One (2011) 6:e16527. doi:10.1371/journal.pone.0016527
54. Li W, Fan G, Chen L, Zhang R, Zhang K, Sun Y, et al. A new type of natural bispecific antibody with potential protective effect in Hashimoto thyroiditis. J Clin Endocrinol Metab (2014) 99:E1602–9. doi:10.1210/jc.2013-4108
55. Kontermann RE, Brinkmann U. Bispecific antibodies. Drug Discov Today (2015) 20:838–47. doi:10.1016/j.drudis.2015.02.008
56. Yang X, Zhang Y, Wang F, Wang LJ, Richardson D, Shameem M, et al. Analysis and purification of IgG4 bispecific antibodies by a mixed-mode chromatography. Anal Biochem (2015) 484:173–9. doi:10.1016/j.ab.2015.06.014
57. Howden BP, Vaddadi G, Manitta J, Grayson ML. Chronic falciparum malaria causing massive splenomegaly 9 years after leaving an endemic area. Med J Aust (2005) 182:186–8.
58. Douba MD, Abbas O, Wali A, Nassany J, Aouf A, Tibbi MS, et al. Chronic cutaneous leishmaniasis, a great mimicker with various clinical presentations: 12 years experience from Aleppo. J Eur Acad Dermatol Venereol (2012) 26:1224–9. doi:10.1111/j.1468-3083.2011.04266.x
59. Dandapat MC, Mohapatro SK, Dash DM. Management of chronic manifestations of filariasis. J Indian Med Assoc (1986) 84:210–5.
60. Pfarr KM, Debrah AY, Specht S, Hoerauf A. Filariasis and lymphoedema. Parasite Immunol (2009) 31:664–72. doi:10.1111/j.1365-3024.2009.01133.x
61. Hussain R, Ottesen EA. IgE responses in human filariasis. IV. Parallel antigen recognition by IgE and IgG4 subclass antibodies. J Immunol (1986) 136:1859–63.
62. Otabil KB, Tenkorang SB. Filarial hydrocele: a neglected condition of a neglected tropical disease. J Infect Dev Ctries (2015) 9:456–62. doi:10.3855/jidc.5346
63. Biswas G, Sankara DP, Agua-Agum J, Maiga A. Dracunculiasis (guinea worm disease): eradication without a drug or a vaccine. Philos Trans R Soc Lond B Biol Sci (2013) 368:20120146. doi:10.1098/rstb.2012.0146
64. Hotez PJ, Fenwick A, Savioli L, Molyneux DH. Rescuing the bottom billion through control of neglected tropical diseases. Lancet (2009) 373:1570–5. doi:10.1016/S0140-6736(09)60233-6
65. Okorie PN, de Souza DK. Prospects, drawbacks and future needs of xenomonitoring for the endpoint evaluation of lymphatic filariasis elimination programs in Africa. Trans R Soc Trop Med Hyg (2016) 110:90–7. doi:10.1093/trstmh/trv104
66. Ottesen EA, Skvaril F, Tripathy SP, Poindexter RW, Hussain R. Prominence of IgG4 in the IgG antibody response to human filariasis. J Immunol (1985) 134:2707–12.
67. Adjobimey T, Hoerauf A. Induction of immunoglobulin G4 in human filariasis: an indicator of immunoregulation. Ann Trop Med Parasitol (2010) 104:455–64. doi:10.1179/136485910X12786389891407
68. Hoerauf A, Satoguina J, Saeftel M, Specht S. Immunomodulation by filarial nematodes. Parasite Immunol (2005) 27:417–29. doi:10.1111/j.1365-3024.2005.00792.x
69. Akue JP, Devaney E, Wahl G, Moukana H. Expression of filarial-specific IgG subclasses under different transmission intensities in a region endemic for loiasis. Am J Trop Med Hyg (2002) 66:245–50.
70. Shiny C, Krushna NS, Archana B, Farzana B, Narayanan RB. Serum antibody responses to Wolbachia surface protein in patients with human lymphatic filariasis. Microbiol Immunol (2009) 53:685–93. doi:10.1111/j.1348-0421.2009.00172.x
71. Kurniawan A, Yazdanbakhsh M, Van Ree R, Aalberse R, Selkirk ME, Partono F, et al. Differential expression of IgE and IgG4 specific antibody responses in asymptomatic and chronic human filariasis. J Immunol (1993) 150:3941–50.
72. Brattig NW, Tenner-Racz K, Korten S, Hoerauf A, Buttner DW. Immunohistology of ectopic secondary lymph follicles in subcutaneous nodules from patients with hyperreactive onchocerciasis (sowda). Parasitol Res (2010) 107:657–66. doi:10.1007/s00436-010-1912-0
73. Newby G, Bennett A, Larson E, Cotter C, Shretta R, Phillips AA, et al. The path to eradication: a progress report on the malaria-eliminating countries. Lancet (2016) 387:1775–84. doi:10.1016/S0140-6736(16)00230-0
74. Cui L, Mharakurwa S, Ndiaye D, Rathod PK, Rosenthal PJ. Antimalarial drug resistance: literature review and activities and findings of the ICEMR network. Am J Trop Med Hyg (2015) 93:57–68. doi:10.4269/ajtmh.15-0007
75. Silva LS, Silva-Filho JL, Caruso-Neves C, Pinheiro AA. New concepts in malaria pathogenesis: the role of the renin-angiotensin system. Front Cell Infect Microbiol (2015) 5:103. doi:10.3389/fcimb.2015.00103
76. Beeson JG, Drew DR, Boyle MJ, Feng G, Fowkes FJ, Richards JS. Merozoite surface proteins in red blood cell invasion, immunity and vaccines against malaria. FEMS Microbiol Rev (2016) 40(3):343–72. doi:10.1093/femsre/fuw001
77. Wassmer SC, Taylor TE, Rathod PK, Mishra SK, Mohanty S, Arevalo-Herrera M, et al. Investigating the pathogenesis of severe malaria: a multidisciplinary and cross-geographical approach. Am J Trop Med Hyg (2015) 93:42–56. doi:10.4269/ajtmh.14-0841
78. Sahu PK, Satpathi S, Behera PK, Mishra SK, Mohanty S, Wassmer SC. Pathogenesis of cerebral malaria: new diagnostic tools, biomarkers, and therapeutic approaches. Front Cell Infect Microbiol (2015) 5:75. doi:10.3389/fcimb.2015.00075
80. Doolan DL, Dobano C, Baird JK. Acquired immunity to malaria. Clin Microbiol Rev (2009) 22:13–36. doi:10.1128/CMR.00025-08
81. Galatas B, Bassat Q, Mayor A. Malaria parasites in the asymptomatic: looking for the hay in the haystack. Trends Parasitol (2015) 32(4):296–308. doi:10.1016/j.pt.2015.11.015
82. Langhorne J, Ndungu FM, Sponaas AM, Marsh K. Immunity to malaria: more questions than answers. Nat Immunol (2008) 9:725–32. doi:10.1038/ni.f.205
83. Lin JT, Saunders DL, Meshnick SR. The role of submicroscopic parasitemia in malaria transmission: what is the evidence? Trends Parasitol (2014) 30:183–90. doi:10.1016/j.pt.2014.02.004
84. Lindblade KA, Steinhardt L, Samuels A, Kachur SP, Slutsker L. The silent threat: asymptomatic parasitemia and malaria transmission. Expert Rev Anti Infect Ther (2013) 11:623–39. doi:10.1586/eri.13.45
85. Pinkevych M, Petravic J, Chelimo K, Kazura JW, Moormann AM, Davenport MP. The dynamics of naturally acquired immunity to Plasmodium falciparum infection. PLoS Comput Biol (2012) 8:e1002729. doi:10.1371/journal.pcbi.1002729
86. Recker M, Nee S, Bull PC, Kinyanjui S, Marsh K, Newbold C, et al. Transient cross-reactive immune responses can orchestrate antigenic variation in malaria. Nature (2004) 429:555–8. doi:10.1038/nature02486
87. Franco-Paredes C, Santos-Preciado JI. Problem pathogens: prevention of malaria in travellers. Lancet Infect Dis (2006) 6:139–49. doi:10.1016/S1473-3099(06)70410-8
88. Tongren JE, Corran PH, Jarra W, Langhorne J, Riley EM. Epitope-specific regulation of immunoglobulin class switching in mice immunized with malarial merozoite surface proteins. Infect Immun (2005) 73:8119–29. doi:10.1128/IAI.73.12.8119-8129.2005
89. Bouharoun-Tayoun H, Druilhe P. Plasmodium falciparum malaria: evidence for an isotype imbalance which may be responsible for delayed acquisition of protective immunity. Infect Immun (1992) 60:1473–81.
90. Lombardo F, Ronca R, Rizzo C, Mestres-Simon M, Lanfrancotti A, Curra C, et al. The Anopheles gambiae salivary protein gSG6: an anopheline-specific protein with a blood-feeding role. Insect Biochem Mol Biol (2009) 39:457–66. doi:10.1016/j.ibmb.2009.04.006
91. Londono-Renteria B, Drame PM, Weitzel T, Rosas R, Gripping C, Cardenas JC, et al. An. gambiae gSG6-P1 evaluation as a proxy for human-vector contact in the Americas: a pilot study. Parasit Vectors (2015) 8:533. doi:10.1186/s13071-015-1160-3
92. Poinsignon A, Cornelie S, BA F, Boulanger D, Sow C, Rossignol M, et al. Human IgG response to a salivary peptide, gSG6-P1, as a new immuno-epidemiological tool for evaluating low-level exposure to Anopheles bites. Malar J (2009) 8:198. doi:10.1186/1475-2875-8-198
93. Rizzo C, Lombardo F, Ronca R, Mangano V, Sirima SB, Nebie I, et al. Differential antibody response to the Anopheles gambiae gSG6 and cE5 salivary proteins in individuals naturally exposed to bites of malaria vectors. Parasit Vectors (2014) 7:549. doi:10.1186/s13071-014-0549-8
94. Sarr JB, Samb B, Sagna AB, Fortin S, Doucoure S, Sow C, et al. Differential acquisition of human antibody responses to Plasmodium falciparum according to intensity of exposure to Anopheles bites. Trans R Soc Trop Med Hyg (2012) 106:460–7. doi:10.1016/j.trstmh.2012.05.006
95. Ready PD. Epidemiology of visceral leishmaniasis. Clin Epidemiol (2014) 6:147–54. doi:10.2147/CLEP.S44267
96. Ready PD. Biology of phlebotomine sand flies as vectors of disease agents. Annu Rev Entomol (2013) 58:227–50. doi:10.1146/annurev-ento-120811-153557
97. Ulrich M, Rodriguez V, Centeno M, Convit J. Differing antibody IgG isotypes in the polar forms of leprosy and cutaneous leishmaniasis characterized by antigen-specific T cell anergy. Clin Exp Immunol (1995) 100:54–8. doi:10.1111/j.1365-2249.1995.tb03603.x
98. Gutierrez Y, Salinas GH, Palma G, Valderrama LB, Santrich CV, Saravia NG. Correlation between histopathology, immune response, clinical presentation, and evolution in Leishmania braziliensis infection. Am J Trop Med Hyg (1991) 45:281–9.
99. Galvao-Castro B, Sa Ferreira JA, Marzochi KF, Marzochi MC, Coutinho SG, Lambert PH. Polyclonal B cell activation, circulating immune complexes and autoimmunity in human american visceral leishmaniasis. Clin Exp Immunol (1984) 56:58–66.
100. Alvar J, Velez ID, Bern C, Herrero M, Desjeux P, Cano J, et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS One (2012) 7:e35671. doi:10.1371/journal.pone.0035671
101. Haldar JP, Ghose S, Saha KC, Ghose AC. Cell-mediated immune response in Indian kala-azar and post-kala-azar dermal leishmaniasis. Infect Immun (1983) 42:702–7.
102. Ansari NA, Kumar R, Raj A, Salotra P. Elevated levels of IgG3 and IgG4 subclass in paediatric cases of kala azar. Parasite Immunol (2008) 30:403–9. doi:10.1111/j.1365-3024.2008.01036.x
103. Hailu A, Menon JN, Berhe N, Gedamu L, Hassard TH, Kager PA, et al. Distinct immunity in patients with visceral leishmaniasis from that in subclinically infected and drug-cured people: implications for the mechanism underlying drug cure. J Infect Dis (2001) 184:112–5. doi:10.1086/320994
104. da Matta VL, Hoshino-Shimizu S, Dietze R, Corbett CE. Detection of specific antibody isotypes and subtypes before and after treatment of American visceral leishmaniasis. J Clin Lab Anal (2000) 14:5–12. doi:10.1002/(SICI)1098-2825(2000)14:1<5::AID-JCLA2>3.0.CO;2-F
105. Hall LR, Titus RG. Sand fly vector saliva selectively modulates macrophage functions that inhibit killing of Leishmania major and nitric oxide production. J Immunol (1995) 155:3501–6.
106. Abdeladhim M, Ben Ahmed M, Marzouki S, Belhadj Hmida N, Boussoffara T, Belhaj Hamida N, et al. Human cellular immune response to the saliva of Phlebotomus papatasi is mediated by IL-10-producing CD8+ T cells and Th1-polarized CD4+ lymphocytes. PLoS Negl Trop Dis (2011) 5:e1345. doi:10.1371/journal.pntd.0001345
107. Theodos CM, Titus RG. Salivary gland material from the sand fly Lutzomyia longipalpis has an inhibitory effect on macrophage function in vitro. Parasite Immunol (1993) 15:481–7. doi:10.1111/j.1365-3024.1993.tb00634.x
108. Belkaid Y, Kamhawi S, Modi G, Valenzuela J, Noben-Trauth N, Rowton E, et al. Development of a natural model of cutaneous leishmaniasis: powerful effects of vector saliva and saliva preexposure on the long-term outcome of Leishmania major infection in the mouse ear dermis. J Exp Med (1998) 188:1941–53. doi:10.1084/jem.188.10.1941
109. Teixeira C, Gomes R, Oliveira F, Meneses C, Gilmore DC, Elnaiem DE, et al. Characterization of the early inflammatory infiltrate at the feeding site of infected sand flies in mice protected from vector-transmitted Leishmania major by exposure to uninfected bites. PLoS Negl Trop Dis (2014) 8:e2781. doi:10.1371/journal.pntd.0002781
110. Kamhawi S, Belkaid Y, Modi G, Rowton E, Sacks D. Protection against cutaneous leishmaniasis resulting from bites of uninfected sand flies. Science (2000) 290:1351–4. doi:10.1126/science.290.5495.1351
111. Tavares NM, Silva RA, Costa DJ, Pitombo MA, Fukutani KF, Miranda JC, et al. Lutzomyia longipalpis saliva or salivary protein LJM19 protects against Leishmania braziliensis and the saliva of its vector, Lutzomyia intermedia. PLoS Negl Trop Dis (2011) 5:e1169. doi:10.1371/journal.pntd.0001169
112. Morris RV, Shoemaker CB, David JR, Lanzaro GC, Titus RG. Sandfly maxadilan exacerbates infection with Leishmania major and vaccinating against it protects against L. major infection. J Immunol (2001) 167:5226–30. doi:10.4049/jimmunol.167.9.5226
113. Titus RG, Bishop JV, Mejia JS. The immunomodulatory factors of arthropod saliva and the potential for these factors to serve as vaccine targets to prevent pathogen transmission. Parasite Immunol (2006) 28:131–41. doi:10.1111/j.1365-3024.2006.00807.x
114. Rohousova I, Ozensoy S, Ozbel Y, Volf P. Detection of species-specific antibody response of humans and mice bitten by sand flies. Parasitology (2005) 130:493–9. doi:10.1017/S003118200400681X
115. Geraci NS, Mukbel RM, Kemp MT, Wadsworth MN, Lesho E, Stayback GM, et al. Profiling of human acquired immunity against the salivary proteins of Phlebotomus papatasi reveals clusters of differential immunoreactivity. Am J Trop Med Hyg (2014) 90:923–38. doi:10.4269/ajtmh.13-0130
116. de Moura TR, Oliveira F, Rodrigues GC, Carneiro MW, Fukutani KF, Novais FO, et al. Immunity to Lutzomyia intermedia saliva modulates the inflammatory environment induced by Leishmania braziliensis. PLoS Negl Trop Dis (2010) 4:e712. doi:10.1371/journal.pntd.0000712
117. de Moura TR, Oliveira F, Carneiro MW, Miranda JC, Clarencio J, Barral-Netto M, et al. Functional transcriptomics of wild-caught Lutzomyia intermedia salivary glands: identification of a protective salivary protein against Leishmania braziliensis infection. PLoS Negl Trop Dis (2013) 7:e2242. doi:10.1371/journal.pntd.0002242
118. Carvalho AM, Cristal JR, Muniz AC, Carvalho LP, Gomes R, Miranda JC, et al. Interleukin 10-dominant immune response and increased risk of cutaneous leishmaniasis after natural exposure to Lutzomyia intermedia sand flies. J Infect Dis (2015) 212:157–65. doi:10.1093/infdis/jiv020
119. Di Lella F, Vincenti V, Zennaro D, Afeltra A, Baldi A, Giordano D, et al. Mucocutaneous leishmaniasis: report of a case with massive involvement of nasal, pharyngeal and laryngeal mucosa. Int J Oral Maxillofac Surg (2006) 35:870–2. doi:10.1016/j.ijom.2006.02.015
120. WHO. Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control. Geneva: World Health Organization (2009).
121. Whitehead SS, Blaney JE, Durbin AP, Murphy BR. Prospects for a dengue virus vaccine. Nat Rev Microbiol (2007) 5:518–28. doi:10.1038/nrmicro1690
122. Pang T. Dengue haemorrhagic fever: virus or host response? Bioessays (1987) 6:141–4. doi:10.1002/bies.950060311
123. Cobra C, Rigau-Perez JG, Kuno G, Vorndam V. Symptoms of dengue fever in relation to host immunologic response and virus serotype, Puerto Rico, 1990-1991. Am J Epidemiol (1995) 142:1204–11.
124. Choffnes ER, Relman DA, Pray LA, Institute of Medicine (U.S.), Forum on Microbial Threats, Board on Global Health. The Science and Applications of Synthetic and Systems Biology: Workshop Summary. Washington, DC: National Academies Press (2011).
125. Boctor FN, Calisher CH, Peter JB. Dot-ELISA for serodiagnosis of human infections due to Western equine encephalitis virus. J Virol Methods (1989) 26:305–11. doi:10.1016/0166-0934(89)90112-2
126. Gligic A, Obradovic M, Stojanovic R, Vujosevic N, Ovcaric A, Frusic M, et al. Epidemic hemorrhagic fever with renal syndrome in Yugoslavia, 1986. Am J Trop Med Hyg (1989) 41:102–8.
127. Kuhn RJ, Dowd KA, Beth Post C, Pierson TC. Shake, rattle, and roll: impact of the dynamics of flavivirus particles on their interactions with the host. Virology (2015) 479-480C:508–17. doi:10.1016/j.virol.2015.03.025
128. Schmid MA, Diamond MS, Harris E. Dendritic cells in dengue virus infection: targets of virus replication and mediators of immunity. Front Immunol (2014) 5:647. doi:10.3389/fimmu.2014.00647
129. Green AM, Beatty PR, Hadjilaou A, Harris E. Innate immunity to dengue virus infection and subversion of antiviral responses. J Mol Biol (2014) 426:1148–60. doi:10.1016/j.jmb.2013.11.023
130. Morrison J, Aguirre S, Fernandez-Sesma A. Innate immunity evasion by Dengue virus. Viruses (2012) 4:397–413. doi:10.3390/v4030397
131. Marie A, Holzmuller P, Tchioffo MT, Rossignol M, Demettre E, Seveno M, et al. Anopheles gambiae salivary protein expression modulated by wild Plasmodium falciparum infection: highlighting of new antigenic peptides as candidates of An. gambiae bites. Parasit Vectors (2014) 7:599. doi:10.1186/s13071-014-0599-y
132. Chisenhall DM, Londono BL, Christofferson RC, Mccracken MK, Mores CN. Effect of dengue-2 virus infection on protein expression in the salivary glands of Aedes aegypti mosquitoes. Am J Trop Med Hyg (2014) 90:431–7. doi:10.4269/ajtmh.13-0412
133. Wang W, Xu R, Li J. Production of native bispecific antibodies in rabbits. PLoS One (2010) 5:e10879. doi:10.1371/journal.pone.0010879
134. Patsatsi A, Kyriakou A, Giannakou A, Pavlitou-Tsiontsi A, Lambropoulos A, Sotiriadis D. Clinical significance of anti-desmoglein-1 and -3 circulating autoantibodies in pemphigus patients measured by area index and intensity score. Acta Derm Venereol (2014) 94:203–6. doi:10.2340/00015555-1666
135. Diaz LA, Sampaio SA, Rivitti EA, Martins CR, Cunha PR, Lombardi C, et al. Endemic pemphigus foliaceus (Fogo Selvagem): II. Current and historic epidemiologic studies. J Invest Dermatol (1989) 92:4–12. doi:10.1111/1523-1747.ep13070394
136. Qian Y, Jeong JS, Maldonado M, Valenzuela JG, Gomes R, Teixeira C, et al. Cutting edge: Brazilian pemphigus foliaceus anti-desmoglein 1 autoantibodies cross-react with sand fly salivary LJM11 antigen. J Immunol (2012) 189:1535–9. doi:10.4049/jimmunol.1200842
137. Banerjee K, Klasse PJ, Sanders RW, Pereyra F, Michael E, Lu M, et al. IgG subclass profiles in infected HIV type 1 controllers and chronic progressors and in uninfected recipients of Env vaccines. AIDS Res Hum Retroviruses (2010) 26:445–58. doi:10.1089/aid.2009.0223
138. Cavacini LA, Kuhrt D, Duval M, Mayer K, Posner MR. Binding and neutralization activity of human IgG1 and IgG3 from serum of HIV-infected individuals. AIDS Res Hum Retroviruses (2003) 19:785–92. doi:10.1089/088922203769232584
139. Muller F, Froland SS, Brandtzaeg P. Altered IgG-subclass distribution in lymph node cells and serum of adults infected with human immunodeficiency virus (HIV). Clin Exp Immunol (1989) 78:153–8.
140. Wu X, Jackson S. Plasma and salivary IgG subclasses in HIV type 1 infection: evidence of both transudation and local synthesis of IgG in parotid saliva. AIDS Res Hum Retroviruses (2000) 16:1423–31. doi:10.1089/08892220050140973
141. Abbas A, Vasilescu A, Do H, Hendel H, Maachi M, Goutalier FX, et al. Analysis of IGG and IGG4 in HIV-1 seropositive patients and correlation with biological and genetic markers. Biomed Pharmacother (2005) 59:38–46. doi:10.1016/j.biopha.2004.07.001
142. Raux M, Finkielsztejn L, Salmon-Ceron D, Bouchez H, Excler JL, Dulioust E, et al. IgG subclass distribution in serum and various mucosal fluids of HIV type 1-infected subjects. AIDS Res Hum Retroviruses (2000) 16:583–94. doi:10.1089/088922200309007
143. Lyamuya EF, Maselle SY, Matre R. Serum immunoglobulin profiles in asymptomatic HIV-1 seropositive adults and in patients with AIDS in Dar es Salaam, Tanzania. East Afr Med J (1994) 71:24–8.
144. de Martino M, Rossi ME, Azzari C, Chiarelli F, Galli L, Vierucci A. Low IgG3 and high IgG4 subclass levels in children with advanced human immunodeficiency virus-type 1 infection and elevated IgE levels. Ann Allergy Asthma Immunol (1999) 83:160–4. doi:10.1016/S1081-1206(10)62629-4
145. Takeuchi M, Sato Y, Yasui H, Ozawa H, Ohno K, Takata K, et al. Epstein-Barr virus-infected cells in IgG4-related lymphadenopathy with comparison with extranodal IgG4-related disease. Am J Surg Pathol (2014) 38:946–55. doi:10.1097/PAS.0000000000000206
146. Wada Y, Kojima M, Yoshita K, Yamazaki M, Kobayashi D, Murakami S, et al. A case of Epstein-Barr virus-related lymphadenopathy mimicking the clinical features of IgG4-related disease. Mod Rheumatol (2013) 23:597–603. doi:10.1007/s10165-012-0695-9
147. Takahashi E, Kojima M, Kobayashi M, Kitamura A, Yokoi T, Hara K, et al. Primary IgG4-related lymphadenopathy with prominent granulomatous inflammation and reactivation of Epstein-Barr virus. Virchows Arch (2012) 460:225–9. doi:10.1007/s00428-011-1186-7
148. Antonio-Nkondjio C, Defo-Talom B, Tagne-Fotso R, Tene-Fossog B, Ndo C, Lehman LG, et al. High mosquito burden and malaria transmission in a district of the city of Douala, Cameroon. BMC Infect Dis (2012) 12:275. doi:10.1186/1471-2334-12-275
149. Drame PM, Poinsignon A, Besnard P, Le Mire J, Dos-Santos MA, Sow CS, et al. Human antibody response to Anopheles gambiae saliva: an immuno-epidemiological biomarker to evaluate the efficacy of insecticide-treated nets in malaria vector control. Am J Trop Med Hyg (2010) 83:115–21. doi:10.4269/ajtmh.2010.09-0684
Keywords: IgG4, bi-specific, arthropod saliva, vector-borne diseases, malaria, dengue virus, mosquito
Citation: Londono-Renteria B, Cardenas JC, Troupin A and Colpitts TM (2016) Natural Mosquito-Pathogen Hybrid IgG4 Antibodies in Vector-Borne Diseases: A Hypothesis. Front. Immunol. 7:380. doi: 10.3389/fimmu.2016.00380
Received: 31 March 2016; Accepted: 08 September 2016;
Published: 29 September 2016
Edited by:
Heinrich Korner, University of Tasmania, AustraliaReviewed by:
Dirk Alexander Mielenz, University of Erlangen-Nuremberg, GermanyAndreas Ludwig Lopata, James Cook University, Australia
Copyright: © 2016 Londono-Renteria, Cardenas, Troupin and Colpitts. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Berlin Londono-Renteria, blondono@uscmed.sc.edu
 Berlin Londono-Renteria
Berlin Londono-Renteria Jenny C. Cardenas2
Jenny C. Cardenas2 Andrea Troupin
Andrea Troupin Tonya M. Colpitts
Tonya M. Colpitts