- 1College of Veterinary Medicine, Jilin University, Changchun, Jilin, China
- 2Faculty of Veterinary Medicine, Institute of Parasitology, Justus Liebig University Giessen, Giessen, Germany
Neosporosis is considered as one of the main causes of abortion and severe economic losses in dairy industry. The Canis genus serving as one of the confirmed definitive hosts of the apicomplexan parasite Neospora caninum (N. caninum) plays a critical role in its life cycle. However, the effects of N. caninum on its definitive hosts of neutrophils extracellular traps (NETs) formation remain unclear. In the present study, N. caninum tachyzoite-induced canine NETs formation was observed by scanning electron microscopy (SEM). Visualization of DNA decorated with H3, neutrophil elastase (NE), and myeloperoxidase (MPO) within N. caninum tachyzoite-induced NETs were examined using fluorescence confocal microscopy analyses. Furthermore, the formation of canine NETs was quantified using Sytox Green staining, and the LDH levels in supernatants were examined by an LDH Cytotoxicity Assay® kit. The results clearly showed that NETs-like structures were induced by N. caninum tachyzoites, and the major components within these structures induced by N. caninum tachyzoite were further confirmed by fluorescence confocal microscopy visualization. These results suggest that N. caninum tachyzoites strongly induced NETs formation in canine polymorphonuclear neutrophils (PMN). In functional inhibition assays, the blockings of NADPH oxidase, NE, MPO, SOCE, ERK 1/2, and p38 MAPK signaling pathways significantly inhibited N. caninum tachyzoite-induced NETs formation. To our knowledge, this study is the first to report the formation of NETs in canine PMN against N. caninum infection.
Introduction
Neosporosis is caused by the apicomplexan protozoa Neospora caninum and is considered as one of the main diseases causing abortion, reproduction disorders, and thus severe economic losses in dairy industry worldwide (1, 2). N. caninum is an apicomplexan parasite closely related to Toxoplasma gondii, which infects a wide intermediate host range (3, 4). As an important veterinary pathogen, several researches have been focused on N. caninum in the past two decades. To date, its confirmed definitive hosts of N. caninum is the genus Canis, including domestic dogs (Canis familiaris) (5), dingoes (Canis lupus dingo) (6), and gray wolves (Canis lupus) (7). Neosporosis in canines principally results in a neuromuscular disease, i.e., polyradiculoneuritis-myositis, but a variety of less common lesions due to focal necrosis can also occurs in other organs (8, 9). Although several studies on cattle N. caninum infections are well reported and analyzed by serologic and/or molecular diagnostic assays, the definitive host–parasite interactions remain not well clarified.
Polymorphonuclear neutrophils (PMN) are one of the most abundant and important leukocyte population in blood, thereby considered as the first-line of defense in the host innate immune system against invasive microorganisms. Neutrophils extracellular traps (NETs) have been recognized as a novel effector mechanism of PMN in many immune processes. NETs are mainly compost of DNA, antibacterial proteins/peptides, and granule proteins, such as histones, neutrophil elastase (NE), myeloperoxidase (MPO), lactoferrin, gelatinase, pentraxin, and cathelicidin, among other molecules (10, 11). During N. caninum infection of bovine endothelial cells, PMN adhesion was increased and the expression of adhesion molecules, such as E-selectin, VCAM-1, and ICAM-1, was significantly upregulated, which revealed the critical role of PMN in the host innate immune system (12). However, whether the novel effector mechanism of PMN-NETs involved in the interactions between N. caninum and its definitive hosts has not been investigated during the acute infection of N. caninum. In this study, the influence of N. caninum on canine NETs formation was examined, and the key molecular signaling pathways were further elucidated.
Materials and Methods
Parasites
Neospora caninum tachyzoites (strain Nc-1) were maintained in VERO cells monolayer at 37°C/5% CO2. VERO cells were cultured in RPMI 1640 medium (Hyclone, USA) supplemented with 2% fetal bovine serum (FBS, Biological Industries, Israel) and 1% penicillin/streptomycin (Hyclone, USA). N. caninum tachyzoites were collected and harvested from VERO cells (3000 r/min, 10 min, room temperature), then passed through 20, 5, 1-ml syringe and a 27-gage needle in turn, purified by 40% Percoll reagent through centrifugation (3000 r/min, 30 min, room temperature), collected precipitates and washed them twice with RPMI 1640 medium (3000 r/min, 10 min, room temperature).
Isolation of Canine PMN
Adult healthy canines (n = 3) were bleeding by puncture of the femoral vein, and blood was collected in blood collection tubes containing heparin (Jun Nuo, Shandong Chengwu County Medical Products Co., China). The PMN were isolated by using the commercially available Canine PMN isolation kit® (TianJin HaoYang Biological Manufacture Co., China) according to the manufacturer’s instructions. All animal experiments were approved by the NIH Guide for the Care and Use of Laboratory Animals of the Jilin University and in accordance to the current Animal Protection Laws of China.
Scanning Electron Microscopy
Canine PMN were stimulated with N. caninum tachyzoites (ratio 1:1, 90 min, 37°C) on cover glass slides. These cover glass slides were pre-coated with poly-l-lysine (0.1 mg/ml, Sigma-Aldrich) for 12 h and washed three times with distilled water. After incubation, the cells were fixed with 4.0% glutaraldehyde (Merck) for 24 h, washed with PBS and post-fixed in 1.0% osmium tetroxide (Merck). Then, the samples were dehydrated in ascending ethanol concentrations (30, 50, 70, 80, 90, 100%), frozen in tertiary butyl alcohol at −20°C and sputtered with gold. Finally, specimens were examined using a scanning electron microscope (Hitachi S-3400N, Japan).
Fluorescence Confocal Microscopy Analyses
Canine PMN were stimulated with N. caninum tachyzoite (ratio 1:1, 90 min, 37°C) on poly-l-lysine (0.1 mg/ml, Sigma-Aldrich) pre-coated cover glass slides. After incubation, the cells were fixed with 4% (w/v) paraformaldehyde for 20 min at room temperature, washed thrice with PBS, permeabilized with 0.1% Triton X-100 for 15 min and blocked in 3% goat serum/PBS, followed by incubation with antibodies to H3, MPO, and NE at 4°C overnight. Anti-histone antibody (LS-C353149; Life Span BioSciences, Inc, 1:200, dissolved in 3% goat serum), anti-MPO antibody (Orb16003; Biorbyt, 1:200, dissolved in 3% goat serum), and anti-NE antibody (AB68672; Abcam, 1:200, dissolved in 3% goat serum) were used for the detection of H3, MPO, and NE in N. caninum tachyzoite-triggered NETs-like extracellular structures. Then, the samples were incubated with the second conjugated antibody (goat anti-rabbit IgG-FITC conjugated, Bioworld Technology Inc) and washed two times with PBS, then stained with 5-μM Sytox Orange (dissolved in PBS, Invitrogen) for 10 min at room temperature. Finally, the specimens were washed two times with PBS, mounted in anti-fading reagents (Beyotime Biotechnology, China), and examined using scanning confocal microscope (Olympus FluoView FV1000).
Quantitation of NETs
Canine PMN were stimulated with viable N. caninum tachyzoites (ratio 1:1, 1:2, 1:3, 1:6, or 1:12, 37°C) for 30, 60, and 90 min, respectively. In parallel settings, prior to stimulation with N. caninum tachyzoite (1:6), the canine PMN were pretreated with the following specific inhibitors for 30 min including the NE inhibitor (CMK, 1 mM, Sigma-Aldrich), the NADPH oxidase inhibitor diphenylene iodonium (DPI, 10 μM, Sigma-Aldrich), the MPO inhibitor (ABAH, 100 μM, Calbiochem), the inhibitors of ERK 1/2 (UO126, 50 μM, Sigma-Aldrich) and p38 MAKP (SB202190, 10 μM, Sigma-Aldrich) signaling pathway, and with the store-operated calcium entry (SOCE) inhibitor (2-amindethoxydiphery borate, 100 μM, Sigma-Aldrich) for 15 min. The formation of canine NETs was quantified using 5-μM Sytox Green (Invitrogen). The samples were examined with a fluorometric reader Infiniti M200® (TECAN, Austria) using an excitation wavelength of 488 nm and detecting at 523 nm the emission wavelength.
Detection of Reactive Oxygen Species
The reactive oxygen species (ROS) production of PMN induced by N. caninum tachyzoites (ratio 1:1, 90 min) was determined with 2,7 dichlorofluorescein diacetate (DCFH-DA, Sigma-Aldrich). PMN stimulated with zymosan (1 mg/ml, Sigma) were used as positive controls. The samples were examined with a fluorometric reader using an excitation wavelength of 485 nm and detecting at 530 nm the emission wavelength.
LDH Detection
The LDH levels in supernatant were examined by the LDH Cytotoxicity Assay kit® (Beyotime Biotechnology, China) according to the manufacturer’s protocols.
Statistical Analysis
Values were expressed as the means ± SD. Data were analyzed by the GraphPad 5.0 software. Differences among the groups were performed with one-way analysis of variance (ANOVA) with Tukey multiple comparison test. P values of <0.05 were considered as significant.
Results
Neospora caninum Tachyzoites Induced NETs Formation in Canine PMN
Neospora caninum tachyzoites strongly induced NETs formation in canine PMN and was confirmed by scanning electron microscopy (SEM) analyses (Figure 1). NETs released from canine PMN were observed in Figure 1, and N. caninum tachyzoites were captured in these thicker and thinner network extracellular structures. Furthermore, N. caninum tachyzoite-induced NETs in canine PMN were demonstrated by fluorescence confocal microscopy analyses (Figures 2B,E,H). These results confirmed that N. caninum tachyzoite surely induced NETs in canine PMN as an additional anti-parasitic effector mechanism.
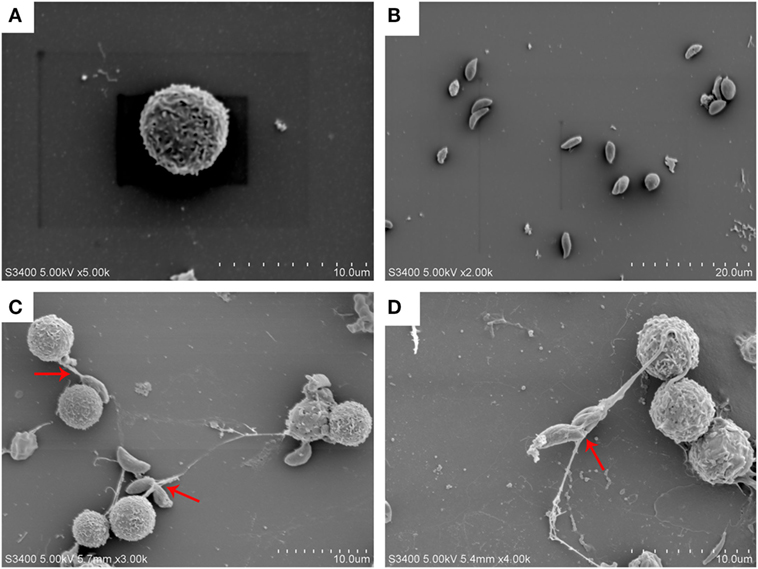
Figure 1. N. caninum tachyzoite-induced NETs formation was observed by SEM. (A) PMN. (B) N. caninum tachyzoites. (C,D) NETs were induced by N. caninum tachyzoite, and N. caninum tachyzoites were trapped in these thicker and thinner network structures.
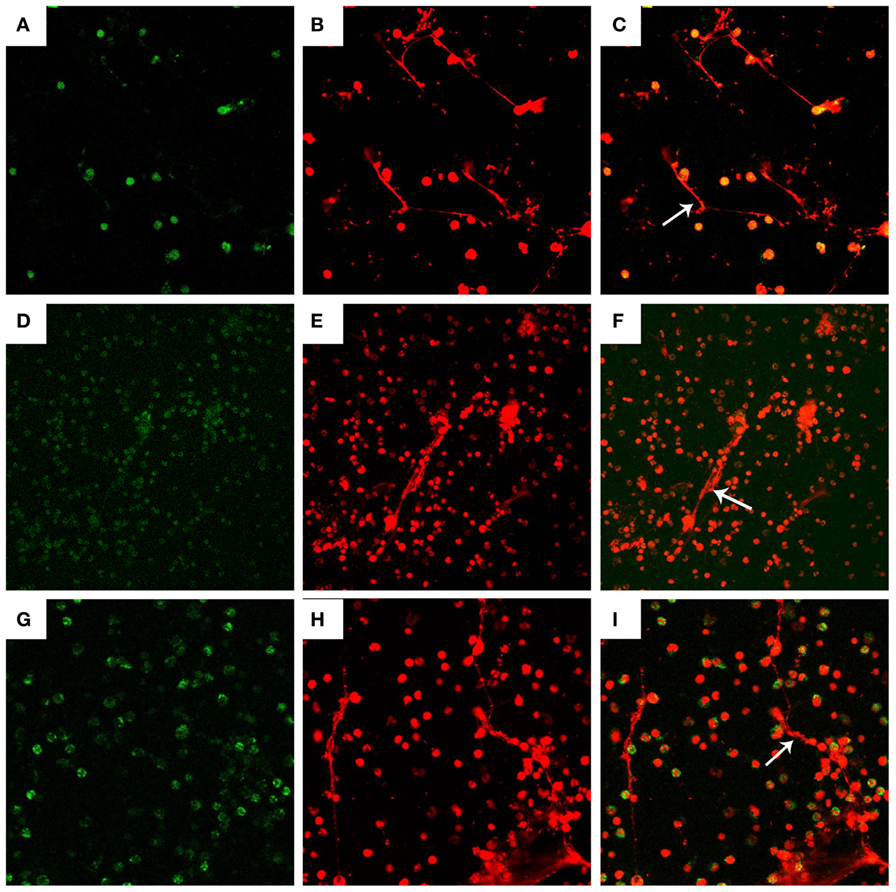
Figure 2. Visualization of DNA decorated with histones (H3), neutrophil elastase (NE), and myeloperoxidase (MPO) in N. caninum tachyzoite-induced NETs structures. PMN were stimulated with Neospora caninum (ratio: 1:1, 90 min). DNA decorated with of H3, NE, and MPO within NETs were detected using a scanning confocal microscope. (A) Histone (Green). (D) MPO (Green). (G) NE (Green). (B,E,H) DNA (Red). (C,F,I) Respective merge of DNA decorated with H3, NE, and MPO.
Visualization of DNA Decorated with Histones (H3), Neutrophil Elastase, and Myeloperoxidase in N. caninum Tachyzoite-Induced NETs Structures
Fluorescence confocal microscopy analyses revealed that NETs formation can be induced by N. caninum tachyzoites, and the nature of these structures was mainly composed by DNA (Figures 2B,E,H). Besides DNA backbone structure of N. caninum-induced NETs, H3 (Figure 2A), MPO (Figure 2D), and NE (Figure 2G) were also observed corroborating the colocalization of these molecules and DNA of these NETs. Merge image of DNA decorated with H3, MPO and NE was showed in Figures 2C,F and I. Taken together, these results confirm the main classical characteristics of NETs induced by N. caninum tachyzoites in canine PMN.
Quantitation of NETs
Canine NETs formation was quantified using Sytox Green. As shown in Figures 3 and 4, N. caninum tachyzoites significantly induced increasing quantitation of NETs when compared to negative controls but lower levels than zymosan (positive controls). These results revealed that the formation of NETs induced by N. caninum tachyzoite was a dose- and time-dependent process.
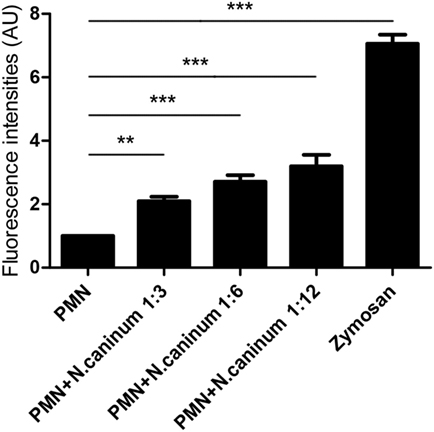
Figure 3. N. caninum tachyzoite-induced NETs formation was in a dose-dependent manner. Canine PMN were stimulated with Neospora caninum tachyzoite (PMN: tachyzoite = 1:3, 1:6, and 1:12) for 60 min. The formation of N. caninum tachyzoite-induced NETs was examined with a fluorometric reader using an excitation wavelength of 488 nm and detecting at 523 nm. Values are presented as mean ± SD (n = 5). P values of <0.05 were considered significant (**P < 0.01 and ***P < 0.001).
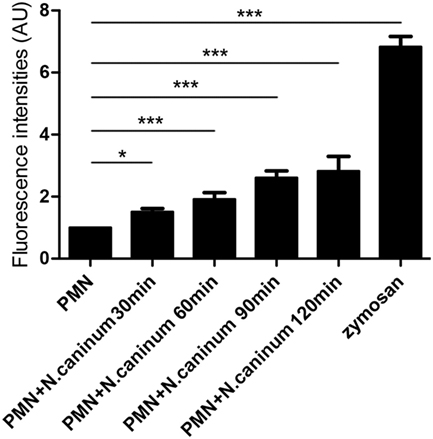
Figure 4. Kinetics of N. caninum tachyzoite-induced NETs formation. PMN were stimulated with Neospora caninum tachyzoite (PMN: tachyzoite = 1:2) for 30, 60, 90, and 120 min. The formation of N. caninum tachyzoite-induced NETs was examined with a fluorometric reader using an excitation wavelength of 488 nm and detecting at 523 nm. Values are presented as mean ± SD (n = 5). P values of <0.05 were considered significant (*P < 0.05 and ***P < 0.001).
NADPH Oxidase, NE, and MPO Are Involved in N. caninum Tachyzoite-Induced NETs Formation
To investigate the role of NADPH oxidase, NE, and MPO of whether to be involved in N. caninum tachyzoite-induced NETs, inhibitors of NADPH oxidase, NE, and MPO were here used. As shown in Figure 5, DPI, ABAH, CMK, and DNase I treatments significantly inhibited N. caninum tachyzoite-induced NETs. In addition, N. caninum tachyzoites significantly increased ROS production when compared to negative controls (Figure 6). These results suggest that NADPH oxidase, NE, and MPO are involved in N. caninum tachyzoite-induced NETs formation. Moreover, the DNase I treatment resulted in significant reduction of N. caninum-induced NET formation, thereby confirming that the primary nature of these NETs was a DNA backbone.
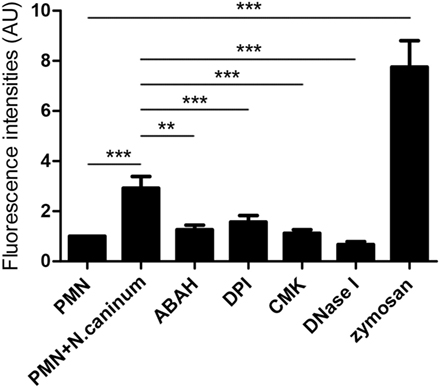
Figure 5. Inhibition assay of N. caninum tachyzoite-induced NETs in canine PMN. Prior to stimulation with N. caninum tachyzoite, the PMN were pretreated with the following inhibitors of NADPH oxidase, NE and, MPO. Zymosan was used as positive controls. The formation of canine NETs was quantified using Sytox Green (Invitrogen). The samples were examined with a fluorometric reader using an excitation wavelength of 488 nm and detecting at 523 nm. Values are presented as mean ± SD (n = 5). P values of <0.05 were considered significant (**P < 0.01 and ***P < 0.001).
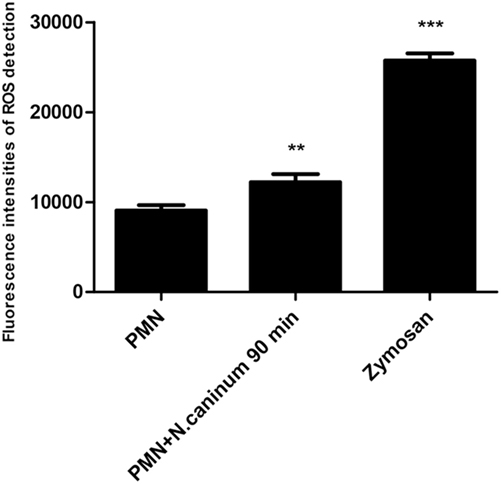
Figure 6. ROS production of canine PMN induced by N. caninum tachyzoite. The ROS production of PMN induced by N. caninum tachyzoite (ratio 1:1, 90 min) was determined with 2,7 dichlorofluorescein diacetate (DCFH-DA, Sigma). PMN stimulated with zymosan (1 mg/ml, Sigma) were used as positive controls. Values are presented as mean ± SD (n = 5). P values of <0.05 were considered significant (**P < 0.01 and ***P < 0.001).
Neospora caninum Tachyzoite-Induced NETs Formation Is an ERK 1/2-, p38 MAPK-, and SOCE-Dependent Process
In order to investigate in more detail, molecular signaling pathways of N. caninum-triggered NET formation, inhibition assays were performed. For this purpose, inhibitors of the SOCE, ERK1/2, and p38 MAPK signaling pathways were used to analyze the critical role of Ca2+ and these two kinases-dependent signaling pathways. As shown in Figure 7, 2-APB, UO126, and SB202190 significantly inhibited N. caninum tachyzoite-induced NETs. These results indicated that N. caninum tachyzoite-induced NETs formation was an ERK 1/2 and p38 MAPK signaling pathway- and SOCE-dependent process.
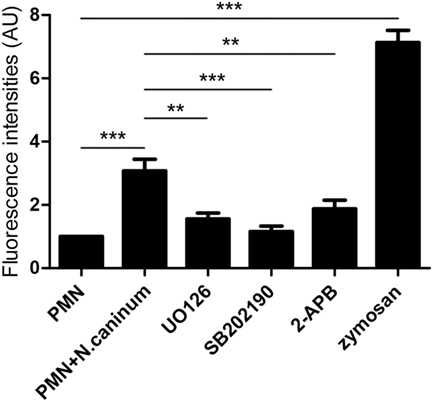
Figure 7. Effects of SOCE inhibitor, ERK, and P38 signaling pathway inhibitors on N. caninum tachyzoite-induced NETs in canine PMN. Prior to stimulation with N. caninum tachyzoite, the PMN were pretreated with the following inhibitors of SOCE, ERK, and P38 signaling pathway. Zymosan was used as positive controls. The formation of canine NETs was quantified using Sytox Green (Invitrogen). The samples were examined with a fluorometric reader using an excitation wavelength of 488 nm and detecting at 523 nm. Values are presented as mean ± SD (n = 5). P values of <0.05 were considered significant (**P < 0.01 and ***P < 0.001).
N. caninum Tachyzoite-Induced NETs Formation Is Independent of LDH Activity
To account for the novel form of cell death program-NETosis, we tested LDH activity in the progress of N. caninum tachyzoite-induced NETs formation. As shown in Figure 8, LDH activities in the supernatant were markedly induced by lysis buffer, but there were no significant changes in the progress of N. caninum tachyzoite-induced NETs formation.
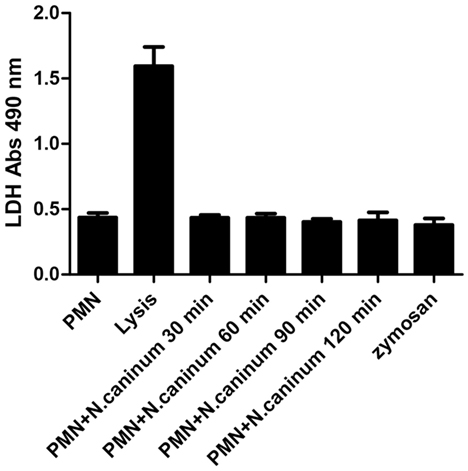
Figure 8. LDH activity in N. caninum tachyzoite-induced NETs formation. Canine PMN were stimulated with N. caninum tachyzoite (ratio 1:1) for 30, 60, 90, and 120 min. The LDH levels in supernatant were examined by an LDH Cytotoxicity Assay kit according to the manufacturer’s protocols. Values are presented as mean ± SD (n = 5). P values of <0.05 were considered significant (***P < 0.001).
Discussion
As the first-line of defense within the host innate immune system, PMN are endowed with powerful effector mechanisms, such as phagocytosis and degranulation, to resist and kill microbes (13). In 2004, another novel effector mechanism of PMN was first described and named “NETs” (10). This novel effector mechanism has been considered as a physical barrier to trap microbes and to avoid their dissemination, and even kill these entrapped microbes. It has been demonstrated that several stimuli, including bacteria, fungi, viruses, and crystal salts can induce the activation of this novel mechanism-NETs in activated PMN (14–16). While most researches concentrated on the effects of NETs on bacteria, fungi, and viral pathogens, increasing evidence on NETs formation triggered by parasites was reported recently, such as T. gondii, Eimeria bovis, and Leishmania donovani (17–19). However, the effects of N. caninum on NETs formation have not been investigated. SEM analysis revealed that NETs-like structures in canine PMN was induced by N. caninum tachyzoites in vitro, and N. caninum tachyzoites were captured by these thicker and thinner network structures. Furthermore, these NETs-like structures induced by N. caninum tachyzoites were demonstrated by fluorescence confocal microscopy analysis. Quantitative assays also revealed that N. caninum tachyzoite-induced NETs formation was a time- and dose- dependent process. These results confirmed that N. caninum tachyzoites can clearly induce NETs in vitro, which is in accordance to NETs-related data for T. gondii, E. bovis, and L. donovani (17–19). However, we do not know the effects of NETs on N. caninum infection in bovine, and whether neutrophils would also be able to recognize and attack N. caninum by NETs formation in vivo need to be further investigated.
In response to pathogens infection, the process of NETs formation has been accompanied by the concentration of several antibacterial proteins and granule proteins, including histones, MPO, NE, and cathelicidin at the site of infection (20–22). In this study, the decoration of DNA with H3, MPO, and NE in N. caninum-triggered NETs were demonstrated. These colocalization results clearly proved the nature of these NET structures after the exposure of canine PMN to vital tachyzoites. Furthermore, inhibitors of NE and MPO significantly inhibited N. caninum tachyzoite-induced NETs formation, which suggest the critical role of NE and MPO in N. caninum tachyzoite-induced NETs. These proteins were also previously observed as key molecules in T. gondii-, Besnoitia besnoiti-, and E. bovis-triggered ETs (17, 23, 24). Previous studies showed that NETs formation was a NADPH oxidase-dependent process, which resulted in the production of ROS (25). Thus, DPI was used to investigate the role of NADPH oxidase in N. caninum tachyzoite-induced NETs. As a result, pretreatment with the NADPH oxidase inhibitor significantly decreased N. caninum tachyzoite-induced NETs formation, and N. caninum tachyzoite significantly increased ROS production. Moreover, ROS production has been proved to be dependent on SOCE (26), so we next explored the role of SOCE in N. caninum tachyzoite-induced NETs formation. The results showed that N. caninum tachyzoite-induced NETs formation was significantly decreased by the SOCE inhibitor 2-APB. There is another report showing that SOCE is regulated via ERK 1/2 phosphorylation and via a ROS production-dependent activation of the Raf–MEK–ERK pathway, which has also been proved to be required for NETs formation induced by parasites (23, 27, 28). Then, we analyzed the critical role of Ca2+ influx and the role of ERK 1/2 and p38 signaling pathway in N. caninum tachyzoite-induced NETs. Our results show that 2-APB, UO126, and SB202190 significantly inhibited N. caninum tachyzoite-induced NETs formation thereby proving the potential role of these molecules in N. caninum-mediated NETosis. As reported for several pathogens, N. caninum tachyzoite-induced NETs formation may be also a NADPH oxidase-, NE-, MPO-, SOCE-, ERK 1/2-, and p38 MAPK-dependent process (23, 28). In addition, we detected LDH activity in the process of N. caninum tachyzoite-induced NET formation. These results showed that no significant necrosis occurred during N. caninum tachyzoite-induced NETs formation when compared to positive controls, which was in accordance with the characteristics of typical NETosis induction as previously demonstrated (10, 15, 29).
Taken together, N. caninum tachyzoites are strong inducers of canine NETs, which suggested a critical role of NETs in the early host innate immune response against tachyzoites. Whether other N. caninum stages, such as sporozoites or bradyzoites, might be capable to induce NETosis needs further investigations. Furthermore, several molecular mechanisms have been proved to be involved in N. caninum tachyzoite-induced NETs formation. However, the role of NETs in N. caninum infection in vivo during acute neosporosis calls for more investigations.
Author Contributions
ZY, CH, AT, and XZ designed the project and experiments. ZW, XH, and XW carried out most of the experiments. ZW and CH wrote the manuscript. ZW, PG, and JL carried out statistical analysis and prepared figures. ZY and XZ co-corresponded this paper. All authors reviewed the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
This work was funded by a project of the National Basic Science Research Program (973 program) of China (Grant No. 2015CB150300) and a grant from the Special Fund for Agro-scientific Research in the Public Interest in China (No. 20130304).
References
1. Dubey JP. Review of Neospora caninum and neosporosis in animals. Korean J Parasitol (2003) 41(1):1–16. doi:10.3347/kjp.2003.41.1.1
2. Reichel MP, Alejandra Ayanegui-Alcerreca M, Gondim LF, Ellis JT. What is the global economic impact of Neospora caninum in cattle – the billion dollar question. Int J Parasitol (2013) 43(2):133–42. doi:10.1016/j.ijpara.2012.10.022
3. Donahoe SL, Lindsay SA, Krockenberger M, Phalen D, Slapeta J. A review of neosporosis and pathologic findings of Neospora caninum infection in wildlife. Int J Parasitol Parasites Wildl (2015) 4(2):216–38. doi:10.1016/j.ijppaw.2015.04.002
4. Dubey JP, Lindsay DS. A review of Neospora caninum and neosporosis. Vet Parasitol (1996) 67(1–2):1–59. doi:10.1016/S0304-4017(96)01035-7
5. McAllister MM, Dubey JP, Lindsay DS, Jolley WR, Wills RA, McGuire AM. Dogs are definitive hosts of Neospora caninum. Int J Parasitol (1998) 28(9):1473–8. doi:10.1016/S0020-7519(98)00138-6
6. King JS, Slapeta J, Jenkins DJ, Al-Qassab SE, Ellis JT, Windsor PA. Australian dingoes are definitive hosts of Neospora caninum. Int J Parasitol (2010) 40(8):945–50. doi:10.1016/j.ijpara.2010.01.008
7. Dubey JP, Schares G. Neosporosis in animals – the last five years. Vet Parasitol (2011) 180(1–2):90–108. doi:10.1016/j.vetpar.2011.05.031
8. Reichel MP, Ellis JT, Dubey JP. Neosporosis and hammondiosis in dogs. J Small Anim Pract (2007) 48(6):308–12. doi:10.1111/j.1748-5827.2006.00236.x
9. Buxton D, McAllister MM, Dubey JP. The comparative pathogenesis of neosporosis. Trends Parasitol (2002) 18(12):546–52. doi:10.1016/S1471-4922(02)02414-5
10. Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science (2004) 303(5663):1532–5. doi:10.1126/science.1092385
11. Urban CF, Ermert D, Schmid M, Abu-Abed U, Goosmann C, Nacken W, et al. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog (2009) 5(10):e1000639. doi:10.1371/journal.ppat.1000639
12. Taubert A, Krull M, Zahner H, Hermosilla C. Toxoplasma gondii and Neospora caninum infections of bovine endothelial cells induce endothelial adhesion molecule gene transcription and subsequent PMN adhesion. Vet Immunol Immunopathol (2006) 112(3–4):272–83. doi:10.1016/j.vetimm.2006.03.017
13. Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol (2013) 13(3):159–75. doi:10.1038/nri3399
14. Nauseef WM, Borregaard N. Neutrophils at work. Nat Immunol (2014) 15(7):602–11. doi:10.1038/ni.2921
15. Pilsczek FH, Salina D, Poon KK, Fahey C, Yipp BG, Sibley CD, et al. A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. J Immunol (2010) 185(12):7413–25. doi:10.4049/jimmunol.1000675
16. Urban CF, Reichard U, Brinkmann V, Zychlinsky A. Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. Cell Microbiol (2006) 8(4):668–76. doi:10.1111/j.1462-5822.2005.00659.x
17. Reichel M, Munoz-Caro T, Sanchez Contreras G, Rubio Garcia A, Magdowski G, Gartner U, et al. Harbour seal (Phoca vitulina) PMN and monocytes release extracellular traps to capture the apicomplexan parasite Toxoplasma gondii. Dev Comp Immunol (2015) 50(2):106–15. doi:10.1016/j.dci.2015.02.002
18. Behrendt JH, Ruiz A, Zahner H, Taubert A, Hermosilla C. Neutrophil extracellular trap formation as innate immune reactions against the apicomplexan parasite Eimeria bovis. Vet Immunol Immunopathol (2010) 133(1):1–8. doi:10.1016/j.vetimm.2009.06.012
19. Guimaraes-Costa AB, Nascimento MT, Froment GS, Soares RP, Morgado FN, Conceicao-Silva F, et al. Leishmania amazonensis promastigotes induce and are killed by neutrophil extracellular traps. Proc Natl Acad Sci U S A (2009) 106(16):6748–53. doi:10.1073/pnas.0900226106
20. Wartha F, Beiter K, Normark S, Henriques-Normark B. Neutrophil extracellular traps: casting the NET over pathogenesis. Curr Opin Microbiol (2007) 10(1):52–6. doi:10.1016/j.mib.2006.12.005
21. von Kockritz-Blickwede M, Nizet V. Innate immunity turned inside-out: antimicrobial defense by phagocyte extracellular traps. J Mol Med (Berl) (2009) 87(8):775–83. doi:10.1007/s00109-009-0481-0
22. Papayannopoulos V, Zychlinsky A. NETs: a new strategy for using old weapons. Trends Immunol (2009) 30(11):513–21. doi:10.1016/j.it.2009.07.011
23. Munoz-Caro T, Mena Huertas SJ, Conejeros I, Alarcon P, Hidalgo MA, Burgos RA, et al. Eimeria bovis-triggered neutrophil extracellular trap formation is CD11b-, ERK 1/2-, p38 MAP kinase- and SOCE-dependent. Vet Res (2015) 46:23. doi:10.1186/s13567-015-0155-6
24. Munoz Caro T, Hermosilla C, Silva LM, Cortes H, Taubert A. Neutrophil extracellular traps as innate immune reaction against the emerging apicomplexan parasite Besnoitia besnoiti. PLoS One (2014) 9(3):e91415. doi:10.1371/journal.pone.0091415
25. Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, et al. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol (2007) 176(2):231–41. doi:10.1083/jcb.200606027
26. Burgos RA, Conejeros I, Hidalgo MA, Werling D, Hermosilla C. Calcium influx, a new potential therapeutic target in the control of neutrophil-dependent inflammatory diseases in bovines. Vet Immunol Immunopathol (2011) 143(1–2):1–10. doi:10.1016/j.vetimm.2011.05.037
27. Hakkim A, Fuchs TA, Martinez NE, Hess S, Prinz H, Zychlinsky A, et al. Activation of the Raf-MEK-ERK pathway is required for neutrophil extracellular trap formation. Nat Chem Biol (2011) 7(2):75–7. doi:10.1038/nchembio.496
28. Munoz-Caro T, Lendner M, Daugschies A, Hermosilla C, Taubert A. NADPH oxidase, MPO, NE, ERK1/2, p38 MAPK and Ca2+ influx are essential for Cryptosporidium parvum-induced NET formation. Dev Comp Immunol (2015) 52(2):245–54. doi:10.1016/j.dci.2015.05.007
Keywords: NETs, canine, neutrophils, Neospora caninum
Citation: Wei Z, Hermosilla C, Taubert A, He X, Wang X, Gong P, Li J, Yang Z and Zhang X (2016) Canine Neutrophil Extracellular Traps Release Induced by the Apicomplexan Parasite Neospora caninum In Vitro. Front. Immunol. 7:436. doi: 10.3389/fimmu.2016.00436
Received: 05 August 2016; Accepted: 04 October 2016;
Published: 31 October 2016
Edited by:
Wanderley De Souza, Federal University of Rio de Janeiro, BrazilReviewed by:
Arwid Daugschies, Universität Leipzig, GermanyAndrew Hemphill, University of Bern, Switzerland
Copyright: © 2016 Wei, Hermosilla, Taubert, He, Wang, Gong, Li, Yang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhengtao Yang, yangzhengtao01@sina.com;
Xichen Zhang, xczhang@jlu.edu.cn
 Zhengkai Wei
Zhengkai Wei Carlos Hermosilla
Carlos Hermosilla Anja Taubert
Anja Taubert Xuexiu He1
Xuexiu He1