- 1National Primate Research Center (NPRC), Korea Research Institute of Bioscience and Biotechnology (KRIBB), Cheongju, South Korea
- 2New Iberia Research Center, University of Louisiana Lafayette, Lafayette, LA, USA
Although the dynamics of germinal center (GC) formation, follicular helper T (TFH) cell recruitment to B cell follicles within lymphoid organs, and changes of lymphoid tissue architecture in HIV/SIV infection have been documented, the underlying immunopathology remains unclear. Here, we summarize what is known regarding the kinetics of TFH cells and GC B cells during the course of infection as well as the potential immunopathological features associated with structural changes in the lymphoid compartment. This review also explores the implications of cell dynamics in the formation and maintenance of viral reservoirs in hyperplastic follicles of secondary lymphoid organs before and after viral suppressive antiretroviral therapy.
Introduction
The ongoing human immunodeficiency virus (HIV) pandemic continues unabated with over 37 million people infected in spite of the availability of a large number of antiretroviral drugs (1). The current combination antiretroviral therapy (ART) while highly effective at controlling viral replication, is however unable to eliminate the virus, which readily rebounds upon ART cessation. Therefore, the development of a protective vaccine remains a priority, though this task is complicated by a relatively poor understanding of immune correlates of protection at this time. In addition, the persistence of HIV infection in spite of potent combinations of drugs also remains to be fully elucidated. Even more puzzling, the ongoing vigorous but inadequate antiviral immune response both during and post ART remains unable to contain chronic virus replication. Most active HIV replication occurs in CD4 T cells in secondary lymphoid organs (2, 3), and recent data also highlights these sites as important reservoirs of latent infection during ART (4, 5). Moreover, these reservoirs are seeded early postinfection (6), and early ART may decrease the size of cells harboring HIV DNA (7, 8), although an exact temporal relation between seeding magnitude of various anatomical reservoirs and specific cell lineages remains to be fully elucidated for both HIV and SIV. During the course of infection, virus has been shown to remain in the germinal center (GC) of hyperplastic follicles, while the architecture of the lymph node experiences gradual remodeling (2, 9).
Generation of Follicular Hyperplasia/Involution
Persistent immune activation is a hallmark of chronic HIV/SIV infections, which induces a progressive pathology not only in peripheral blood but also in secondary lymphoid tissues, causing a profound remodeling of the lymph node architecture throughout the course of infection. Histological patterns of structural alteration of lymphoid architecture were already well described in the 1980s, in the context of HIV infection (10–13). Specifically, lymph node morphology in HIV patients with or without AIDS is characterized initially by follicular hyperplasia, followed by involution, resulting in the lymphadenopathy and destruction of follicular architecture, which helped in the original diagnosis of AIDS (14). In SIV and SHIV infection of non-human primate models of human HIV infection, rhesus macaques that followed an accelerated disease course and died within 6 months, severe follicular involution was observed in their lymphoid tissues, while on the contrary, animals that survived longer had lymphadenopathy with confluent GCs and follicular hyperplasia (15, 16). Histologic and cellular characterization of lymph nodes during infection revealed similar structural alterations among SIV-infected rhesus macaques and HIV-infected patients. Although the mechanisms are not fully elucidated yet, gradual histological alterations such as the deposition of collagen and non-amyloid substance may be associated with follicular involution at the end stage of HIV infection (17, 18). Exposure of follicular dendritic cells to HIV may create an inflammatory environment and lead to the impaired survival of follicular B cells (19). The magnitude of GC reactions in follicular hyperplasia or involution, if any, is closely linked to the regulation of follicular helper T (TFH) cells.
Follicular Helper T Cells in the GCs of Hyperplastic Follicles During the Course of HIV Infection
Specific CD4 T helper cells termed TFH cells differentiate from precursors under the control of the transcription factor Bcl6 and are characterized by their function, which is to provide T cell help for B cells, and are distinct from other CD4 T cell subsets such as Th1, Th2, and Th17 cells (20). In lymphoid tissues, information on the location of CD4 T cells within follicles is also vital to identifying resident TFH cells (Figure 1). Although there is no single marker for distinguishing TFH cells from other CD4 subsets, they are defined by their expression of surface co-stimulatory molecules CXCR5, CD200, ICOS, and a high density of PD-1 (20, 21). These memory type cells generally express low levels of CCR7 but are able to migrate toward B cell follicles in lymphoid organs, produce IL-21, and deliver B cell help in the GC environment for the development of T cell-dependent humoral adaptive immunity (22, 23). Studies using knock-out mice for IL-6 and IL-21 have shown that both are necessary for TFH differentiation and GC development in secondary follicles. IL-6-deficient mice exhibited a marked defect in GCs formation, STAT1 and STAT3 signaling, downstream IL-21 production, and IgG production primarily due to the lack of TFH differentiation in vivo (24). IL-21 deficiency also results in a severe reduction of GCs though this signal seems downstream of the one caused by IL-6 deficiency (25). Moreover, IL-6 and IL-21 appear to regulate the generation of TFH cells in the absence of Th1, Th2, and Th17 cells (26), suggesting that conversely, increases in the expression of IL-6, IL-21, or both cytokines may lead to lymphoid hyperplasia and rapid development of GCs, as observed during HIV and SIV infection. In fact, in HIV-infected individuals, a decrease in levels of circulating IL-21 and decreased production of IL-21 by CD4 cells was noted in blood (27). Similar to HIV infection, substantial depletion of IL-21+ CD4 T cells was reported in the blood in SIV-infected macaques (28). In lymphoid tissues of HIV patients, however, a marked expansion of IL-21-secreting TFHs was noted (29). Moreover, concurrent accumulation of TFH cells and particularly within the GCs of lymph node follicles and more precisely at the periphery of the GC had significantly increased IL-21 expression during SIV infection (30), suggesting trafficking of IL-21-producing TFH cells during the chronic immune activation characteristic of chronic SIV infection.
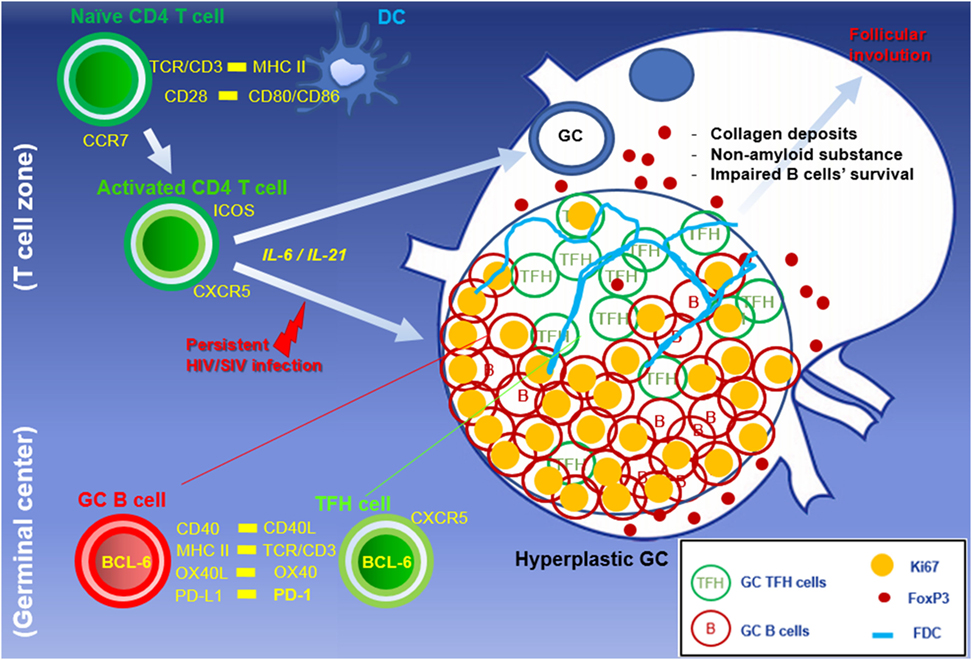
Figure 1. Abnormal accumulation of TFH cells in hyperplastic GC during HIV/SIV primary infection. Naïve mature CD4 T cells are activated through dendritic cells. The persistent viral antigens stimulate primed CD4 T cells, resulting in the formation of hyperplastic GC with the massive B cell expansion, TFH accumulation, and development of network of follicular dendritic cells. Treg and PD-L1 expressing cells within GCs are capable of modulating GC TFH cells to suppress GC-related responses at the end stage of HIV infection.
Upon HIV infection, there is a rapid infiltration of these TFH cells and formation of numerous GCs within lymphoid organs, characteristic of lymphocyte hyperplasia seen early in chronic infection. Recent studies demonstrated that HIV-infected patients displayed an aberrant accumulation of TFH cells compared to uninfected individuals (29). Similar observations were reported in lymph nodes, spleen, and gut tissues of rhesus macaques, in which the resident TFH cells (PD-1high CD4+ T cells) within GCs of hyperplastic follicles were markedly expanded, with a parallel increase and accumulation of Ki67+ GC B cells during chronic SIV infection (31, 32) (Figure 2). Of interest though, was the observation that as TFH accumulated within GCs, their expression of Ki67 decreased with up to 80% of TFH negative for this proliferation marker, suggesting that the continued input of this lineage to be contributed from cells migrating into follicles rather than local proliferation (30), and potentially, these cells have reached a terminal differentiation stage and function, which is to deliver help to local B cell differentiation and maturation. These findings are consistent with the limited proliferative capacity of human TFH cells whereby cross-linking their high level of PD-1 may dissociate continuous TCR signaling.
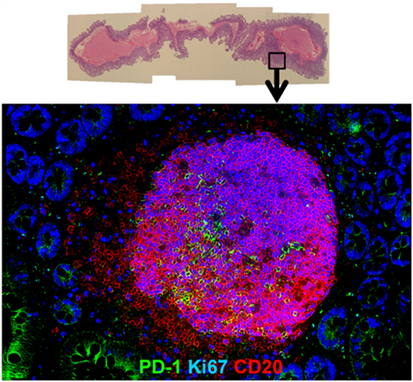
Figure 2. Hyperplastic follicle in gut tissue during chronic SIV infection. Representative H&E (upper) and immunofluorescence image (lower) of hyperplastic follicle staining with Ki67 (blue), PD-1 (green), and CD20 (red) in ileum from a chronically SIV-infected rhesus macaque.
Understanding whether GC TFH cells accumulated during HIV/SIV infection are viral antigen-specific is also important. However, this has, hitherto, rarely been addressed because of the difficulty in identifying their responses. In this respect, there is also little experimental evidence demonstrating the dynamics between antigen-specific TFH cells and hyperplastic GCs. Interestingly, two recent articles have reported a novel assay to determine the frequencies of antigen-specific TFH cells within secondary lymphoid tissues of humans and macaques using cytokine-independent activation-induced markers CD25 and OX40 (33, 34). Such new technique is expected to markedly enhance our comprehension of the role of antigen specificity in the lymphoid hyperplasia that is observed during SIV/HIV infection.
Negative Regulation of TFH Cells in Hyperplastic Follicle
Unlike GC B cells, the frequency of proliferating GC TFH cells drops once hyperplastic follicles are established during infection. There are several potential negative regulators able to suppress resident TFH cells from the persistent division in the local environment. First, a series of recent findings suggest that Foxp3+ regulatory T (Treg) cells also arise in the lymphoid compartment and may play an important role in the downregulation of TFH cell-mediated GC development. In mouse and human studies, a subset of TFH cell with a surface profile of Treg cells has been detected within GCs, which negatively regulated TFH cell-dependent B cell responses in vitro (35–37). So far, monitoring follicular Treg cells during follicular hyperplasia by HIV has been described, but few studies have focused on this issue. In both HIV and SIV infections, the density of Treg cells increase in the T cell zone but not in follicular area (38) (Figure 3). Petrovas et al. defined Foxp3+ cells among TFH cells in a SIV model and reported no expansion during the course of infection (32). In situ analyses using immunofluorescent staining for Foxp3, PD-1, CD20, and nuclei revealed that Foxp3+ cells are more abundant outside than inside follicles, and only few FoxP3+ PD-1high cells were present within GCs of hyperplastic follicles in SIV-infected macaque (Figure 1). Indeed, the frequency of Treg cells among TFH cells is decreased in chronic SIV infection (39). The abundance of IL-21 may be associated with the suppression of Treg cells in select chronic inflammatory situations (40).
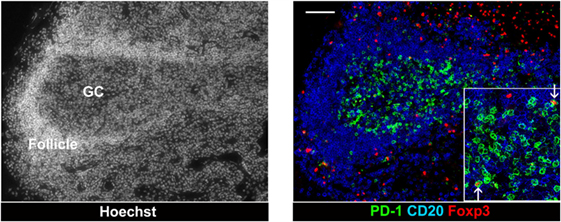
Figure 3. Follicular T regulatory cells in a hyperplastic follicle of lymph node from chronic SIV infection. Foxp3+ cells and PD-1 high cells in an expanded GC of a hyperplastic follicle. The lymph node biopsies were stained with CD20 (blue), PD-1 (green), Foxp3 (red), and Hoechst dye (white). Scale bars = 50 μm.
Another possible mechanism of down-modulation of TFH cells is signaling through PD-1, leading to decreased T cell proliferation; TFH cells are likely very sensitive to this mechanism because of their high-level expression of PD-1. In the setting of chronic HIV infection, PD-1 ligands on antigen-presenting cells including B cells were upregulated in both blood and lymph nodes (41–43), leading to functional impairment of PD-1 expressing T cells. Macaque studies have shown that PD-L1 expression was increased on dendritic cell populations in blood and lymph node post SIV infection, compared to uninfected controls (44). Of note, DC-like cells expressing PD-L1 were markedly increased locally with exaggerated GC formation, and they eventually interact with TFH cells in hyperplastic follicles during chronic SIV infection (31). PD-1/PD-L1 interaction was shown to induce a decrease in the proliferation, survival, and cytokines secretion of human TFH cells (43). These findings suggest that the suppression of proliferation of resident TFH cells may be more readily associated with the cross-linking of PD-1 with PD-L1 in hyperplastic follicles than the presence of Foxp3+ Treg cells during HIV infection.
Third, Chronic immune activation of lymphoid tissues leads to a gradual remodeling of the architecture, resulting in follicular involution. In HIV patients, collagen was gradually deposited into T cell zone of lymphoid node (45). This fibrosis inversely correlates with the presence of naïve CD4 T cells (46). TFGβ activated by inflammation induces collagen deposition, resulting in the loss of the fibroblastic reticular cells (FRCs) network that is associated with the production of growth factors such as IL-7. This loss leads to the death of T cells and then decrease in lymphotoxin-β essential for the survival of FRCs (47), suggesting the disruption of cell to cell contact between FDCs, TFH, and B cells.
TFH Serve as a Long-Lived Viral Reservoir
Resident TFH appear to not express CCR5 (48, 49), yet many are infected and replicate HIV-1 (50) and SIV (51). This leads to the question of site of infection: are they being infected before migrating into the GC, infected by cell–cell transmission from FDCs in GCs, or is there enough co-receptor expression to allow for infection even though CCR5 levels are too low to be detected by flow or in situ techniques? This aspect of TFH infection will require additional work to be resolved, though what has become readily apparent is that hyperplastic follicles with high density of resident TFH cells can serve as the latent virus reservoir during the course of infection even in elite controllers (51). Thus, the extent of infection in TFH of hyperplastic follicles in lymph nodes needs to be taken into account in any strategy aimed at reducing or eliminating latent viral reservoirs. Moreover, the analyses of antiretroviral drug penetration and conversion to their active form have only begun to be examined. Recent data suggest that treatment with ART appears to decrease the relative frequency of GC TFH cells in lymph nodes of HIV patients, perhaps secondary to partial resolution of the immune activation, though the relative frequency is still higher than in uninfected individuals (29, 52), and evidence of lower concentrations of ART in lymphatic tissues and relative to peripheral blood have been reported (53) including data showing lower conversion within GCs relative to the other lymphoid areas (personal communication). Although HIV RNA is rarely detected in the GCs of lymph node during antiretroviral therapy, viral proteins such as Gag p24 and HIV DNA remain detectable over a substantial period time (54, 55). Blood, Lymph node, and splenic TFH cells show higher level of SIV RNA compared with non-TFH cells in the presence of combined ART (51, 56). Other lymphoid tissues such as gut possess latently infected cells despite undetectable plasma HIV RNA in patients with long-term ART treatment (57), which would be conducive to reseed and rekindle of infection in blood and other secondary lymphoid organs. Importantly, hyperplastic follicles still exist in HIV patients’ after extended ART (58). It is quite possible that GC TFH cells accumulated may reactivate virus replication upon ART cessation, serving as a major source of virus rebound. Overall, the existence of hyperplastic GCs may represent an impediment to a cure for HIV-1 infection and must expressly be addressed in HIV-1 therapeutic strategies.
Conclusion
In conclusion, TFH cells not only are a critical component of the immune response but also serve an active and latent reservoir for HIV/SIV infection. A better understanding of TFH cell kinetics and their role as a latent cell reservoir is clearly needed for any ART and/or immune-based interventions to control virus replication in the absence of ART. Therefore, the recent efforts at an understanding of TFH-related GC immune responses during HIV disease will, in spite of much difficulty, likely provide major advances in the generation of therapeutic strategies to target the potential latent reservoirs of HIV and ultimately its eradication.
Author Contributions
All authors listed have made substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported in part by grants R01AI 078775 and 8R24OD010947 from the National Institute of Health, USA to FV and KGM4241642 grant to KRIBB. We thank Roger Tieu for assistance with editing the manuscript.
References
2. Pantaleo G, Graziosi C, Demarest JF, Butini L, Montroni M, Fox CH, et al. HIV infection is active and progressive in lymphoid tissue during the clinically latent stage of disease. Nature (1993) 362:355–8. doi:10.1038/362355a0
3. Embretson J, Zupancic M, Ribas JL, Burke A, Racz P, Tenner-Racz K, et al. Massive covert infection of helper T lymphocytes and macrophages by HIV during the incubation period of AIDS. Nature (1993) 362:359–62. doi:10.1038/362359a0
4. Chun TW, Carruth L, Finzi D, Shen X, DiGiuseppe JA, Taylor H, et al. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature (1997) 387:183–8. doi:10.1038/387183a0
5. Cory TJ, Schacker TW, Stevenson M, Fletcher CV. Overcoming pharmacologic sanctuaries. Curr Opin HIV AIDS (2013) 8:190–5. doi:10.1097/COH.0b013e32835fc68a
6. Whitney JB, Hill AL, Sanisetty S, Penaloza-MacMaster P, Liu J, Shetty M, et al. Rapid seeding of the viral reservoir prior to SIV viraemia in rhesus monkeys. Nature (2014) 512:74–7. doi:10.1038/nature13594
7. Ananworanich J, Chomont N, Eller LA, Kroon E, Tovanabutra S, Bose M, et al. HIV DNA set point is rapidly established in acute HIV infection and dramatically reduced by early ART. EBioMedicine (2016) 11:68–72. doi:10.1016/j.ebiom.2016.07.024
8. Cheret A, Bacchus-Souffan C, Avettand-Fenoel V, Melard A, Nembot G, Blanc C, et al. Combined ART started during acute HIV infection protects central memory CD4+ T cells and can induce remission. J Antimicrob Chemother (2015) 70:2108–20. doi:10.1093/jac/dkv084
9. Biberfeld P, Chayt KJ, Marselle LM, Biberfeld G, Gallo RC, Harper ME. HTLV-III expression in infected lymph nodes and relevance to pathogenesis of lymphadenopathy. Am J Pathol (1986) 125:436–42.
10. Biberfeld P, Porwit-Ksiazek A, Bottiger B, Morfeldt-Mansson L, Biberfeld G. Immunohistopathology of lymph nodes in HTLV-III infected homosexuals with persistent adenopathy or AIDS. Cancer Res (1985) 45:4665s–70s.
11. Chadburn A, Metroka C, Mouradian J. Progressive lymph node histology and its prognostic value in patients with acquired immunodeficiency syndrome and AIDS-related complex. Hum Pathol (1989) 20:579–87. doi:10.1016/0046-8177(89)90247-5
12. Fernandez R, Mouradian J, Metroka C, Davis J. The prognostic value of histopathology in persistent generalized lymphadenopathy in homosexual men. N Engl J Med (1983) 309:185–6. doi:10.1056/NEJM198307213090314
13. Marche C, Kernbaum S, Saimot AG, Neguesse Y, Bouton C, Diebold J, et al. Histopathological study of lymph nodes in patients with lymphadenopathy or acquired immune deficiency syndrome. Eur J Clin Microbiol (1984) 3:75–6. doi:10.1007/BF02032835
14. Paiva DD, Morais JC, Pilotto J, Veloso V, Duarte F, Lenzi HL. Spectrum of morphologic changes of lymph nodes in HIV infection. Mem Inst Oswaldo Cruz (1996) 91:371–9. doi:10.1590/S0074-02761996000300023
15. Zhang ZQ, Casimiro DR, Schleif WA, Chen M, Citron M, Davies ME, et al. Early depletion of proliferating B cells of germinal center in rapidly progressive simian immunodeficiency virus infection. Virology (2007) 361:455–64. doi:10.1016/j.virol.2006.12.006
16. Amara RR, Villinger F, Altman JD, Lydy SL, O’Neil SP, Staprans SI, et al. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science (2001) 292:69–74. doi:10.1126/science.1058915
17. Zeng M, Haase AT, Schacker TW. Lymphoid tissue structure and HIV-1 infection: life or death for T cells. Trends Immunol (2012) 33:306–14. doi:10.1016/j.it.2012.04.002
18. Hong JJ, Villinger F, Courtney CL. PAS-positive extracellular deposits within germinal centers of hyperplastic follicles during SIV infection in a rhesus macaque. J Med Primatol (2014) 43:374–7. doi:10.1111/jmp.12109
19. Sabri F, Prados A, Muñoz–Fernández R, Lantto R, Fernandez–Rubio P. Impaired B cells survival upon production of inflammatory cytokines by HIV–1 exposed follicular dendritic cells. Retrovirology (2016) 13:61–77. doi:10.1186/s12977-016-0295-4
20. Crotty S. T follicular helper cell differentiation, function, and roles in disease. Immunity (2014) 41:529–42. doi:10.1016/j.immuni.2014.10.004
21. Yu D, Vinuesa CG. The elusive identity of T follicular helper cells. Trends Immunol (2010) 31:377–83. doi:10.1016/j.it.2010.07.001
22. Breitfeld D, Ohl L, Kremmer E, Ellwart J, Sallusto F, Lipp M, et al. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J Exp Med (2000) 192:1545–52. doi:10.1084/jem.192.11.1545
23. Schaerli P, Willimann K, Lang AB, Lipp M, Loetscher P, Moser B. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J Exp Med (2000) 192:1553–62. doi:10.1084/jem.192.11.1553
24. Kopf M, Herren S, Wiles MV, Pepys MB, Kosco-Vilbois MH. Interleukin 6 influences germinal center development and antibody production via a contribution of C3 complement component. J Exp Med (1998) 188:1895–906. doi:10.1084/jem.188.10.1895
25. Vogelzang A, McGuire HM, Yu D, Sprent J, Mackay CR, King C. A fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity (2008) 29:127–37. doi:10.1016/j.immuni.2008.06.001
26. Nurieva RI, Chung Y, Hwang D, Yang XO, Kang HS, Ma L, et al. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity (2008) 29:138–49. doi:10.1016/j.immuni.2008.05.009
27. Iannello A, Boulassel MR, Samarani S, Debbeche O, Tremblay C, Toma E, et al. Dynamics and consequences of IL-21 production in HIV-infected individuals: a longitudinal and cross-sectional study. J Immunol (2010) 184:114–26. doi:10.4049/jimmunol.0901967
28. Micci L, Cervasi B, Ende ZS, Iriele RI, Reyes-Aviles E, Vinton C, et al. Paucity of interleukin (IL-21)-producing CD4+ T-cells is associated with Th17 cell depletion in SIV-infection of rhesus macaques. Blood (2012) 120:3925–35. doi:10.1182/blood-2012-04-420240
29. Lindqvist M, van Lunzen J, Soghoian DZ, Kuhl BD, Ranasinghe S, Kranias G, et al. Expansion of HIV-specific T follicular helper cells in chronic HIV infection. J Clin Invest (2012) 122:3271–80. doi:10.1172/JCI64314
30. Hong JJ, Amancha PK, Rogers KA, Courtney CL, Havenar-Daughton C, Crotty S, et al. Early lymphoid responses and germinal center formation correlate with lower viral load set points and better prognosis of simian immunodeficiency virus infection. J Immunol (2014) 193:797–806. doi:10.4049/jimmunol.1400749
31. Hong JJ, Amancha PK, Rogers K, Ansari AA, Villinger F. Spatial alterations between CD4(+) T follicular helper, B, and CD8(+) T cells during simian immunodeficiency virus infection: T/B cell homeostasis, activation, and potential mechanism for viral escape. J Immunol (2012) 188:3247–56. doi:10.4049/jimmunol.1103138
32. Petrovas C, Yamamoto T, Gerner MY, Boswell KL, Wloka K, Smith EC, et al. CD4 T follicular helper cell dynamics during SIV infection. J Clin Invest (2012) 122:3281–94. doi:10.1172/JCI63039
33. Havenar-Daughton C, Reiss SM, Carnathan DG, Wu JE, Kendric K, Torrents de la Pena A, et al. Cytokine-independent detection of antigen-specific germinal center T follicular helper cells in immunized nonhuman primates using a live cell activation-induced marker technique. J Immunol (2016) 197:994–1002. doi:10.4049/jimmunol.1600320
34. Dan JM, Lindestam Arlehamn CS, Weiskopf D, da Silva Antunes R, Havenar-Daughton C, Reiss SM, et al. A cytokine-independent approach to identify antigen-specific human germinal center T follicular helper cells and rare antigen-specific CD4+ T cells in blood. J Immunol (2016) 197:983–93. doi:10.4049/jimmunol.1600318
35. Chung Y, Tanaka S, Chu F, Nurieva RI, Martinez GJ, Rawal S, et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat Med (2011) 17:983–8. doi:10.1038/nm.2426
36. Linterman MA, Pierson W, Lee SK, Kallies A, Kawamoto S, Rayner TF, et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat Med (2011) 17:975–82. doi:10.1038/nm.2425
37. Wollenberg I, Agua-Doce A, Hernandez A, Almeida C, Oliveira VG, Faro J, et al. Regulation of the germinal center reaction by Foxp3+ follicular regulatory T cells. J Immunol (2011) 187:4553–60. doi:10.4049/jimmunol.1101328
38. Estes JD, Haase AT, Schacker TW. The role of collagen deposition in depleting CD4+ T cells and limiting reconstitution in HIV-1 and SIV infections through damage to the secondary lymphoid organ niche. Semin Immunol (2008) 20:181–6. doi:10.1016/j.smim.2008.04.002
39. Chowdhury A, Del Rio Estrada PM, Tharp GK, Trible RP, Amara RR, Chahroudi A, et al. Decreased T follicular regulatory cell/T follicular helper cell (TFH) in simian immunodeficiency virus-infected Rhesus macaques may contribute to accumulation of TFH in chronic infection. J Immunol (2015) 195:3237–47. doi:10.4049/jimmunol.1402701
40. Niu X, He D, Zhang X, Yue T, Li N, Zhang JZ, et al. IL-21 regulates Th17 cells in rheumatoid arthritis. Hum Immunol (2010) 71:334–41. doi:10.1016/j.humimm.2010.01.010
41. Trabattoni D, Saresella M, Biasin M, Boasso A, Piacentini L, Ferrante P, et al. B7-H1 is up-regulated in HIV infection and is a novel surrogate marker of disease progression. Blood (2003) 101:2514–20. doi:10.1182/blood-2002-10-3065
42. D’Souza M, Fontenot AP, Mack DG, Lozupone C, Dillon S, Meditz A, et al. Programmed death 1 expression on HIV-specific CD4+ T cells is driven by viral replication and associated with T cell dysfunction. J Immunol (2007) 179:1979–87. doi:10.4049/jimmunol.179.3.1979
43. Cubas RA, Mudd JC, Savoye AL, Perreau M, van Grevenynghe J, Metcalf T, et al. Inadequate T follicular cell help impairs B cell immunity during HIV infection. Nat Med (2013) 19:494–9. doi:10.1038/nm.3109
44. Xu H, Wang X, Pahar B, Moroney-Rasmussen T, Alvarez X, Lackner AA, et al. Increased B7-H1 expression on dendritic cells correlates with programmed death 1 expression on T cells in simian immunodeficiency virus-infected macaques and may contribute to T cell dysfunction and disease progression. J Immunol (2010) 185:7340–8. doi:10.4049/jimmunol.1001642
45. Schacker TW, Nguyen PL, Beilman GJ, Wolinsky S, Larson M, Reilly C, et al. Collagen deposition in HIV-1 infected lymphatic tissues and T cell homeostasis. J Clin Invest (2002) 110:1133–9. doi:10.1172/JCI16413
46. Schacker TW, Brenchley JM, Beilman GJ, Reilly C, Pambuccian SE, Taylor J, et al. Lymphatic tissue fibrosis is associated with reduced numbers of naive CD4+ T cells in human immunodeficiency virus type 1 infection. Clin Vaccine Immunol (2006) 13:556–60. doi:10.1128/CVI.13.5.556-560.2006
47. Zeng M, Smith AJ, Wietgrefe SW, Southern PJ, Schacker TW, Reilly CS, et al. Cumulative mechanisms of lymphoid tissue fibrosis and T cell depletion in HIV-1 and SIV infections. J Clin Invest (2011) 121:998–1008. doi:10.1172/JCI45157
48. Velu V, Mylvaganam GH, Gangadhara S, Hong JJ, Iyer SS, Gumber S, et al. Induction of Th1-biased T follicular helper (Tfh) cells in lymphoid tissues during chronic simian immunodeficiency virus infection defines functionally distinct germinal center Tfh cells. J Immunol (2016) 197:1832–42. doi:10.4049/jimmunol.1600143
49. Mylvaganam GH, Velu V, Hong JJ, Sadagopal S, Kwa S, Basu R, et al. Diminished viral control during simian immunodeficiency virus infection is associated with aberrant PD-1hi CD4 T cell enrichment in the lymphoid follicles of the rectal mucosa. J Immunol (2014) 193:4527–36. doi:10.4049/jimmunol.1401222
50. Hufert FT, van Lunzen J, Janossy G, Bertram S, Schmitz J, Haller O, et al. Germinal centre CD4+ T cells are an important site of HIV replication in vivo. AIDS (1997) 11:849–57. doi:10.1097/00002030-199707000-00003
51. Fukazawa Y, Lum R, Okoye AA, Park H, Matsuda K, Bae JY, et al. B cell follicle sanctuary permits persistent productive simian immunodeficiency virus infection in elite controllers. Nat Med (2015) 21:132–9. doi:10.1038/nm.3781
52. Perreau M, Savoye AL, De Crignis E, Corpataux JM, Cubas R, Haddad EK, et al. Follicular helper T cells serve as the major CD4 T cell compartment for HIV-1 infection, replication, and production. J Exp Med (2013) 210:143–56. doi:10.1084/jem.20121932
53. Fletcher CV, Staskus K, Wietgrefe SW, Rothenberger M, Reilly C, Chipman JG, et al. Persistent HIV-1 replication is associated with lower antiretroviral drug concentrations in lymphatic tissues. Proc Natl Acad Sci U S A (2014) 111:2307–12. doi:10.1073/pnas.1318249111
54. Stellbrink HJ, van Lunzen J, Westby M, O’Sullivan E, Schneider C, Adam A, et al. Effects of interleukin-2 plus highly active antiretroviral therapy on HIV-1 replication and proviral DNA (COSMIC trial). AIDS (2002) 16:1479–87. doi:10.1097/00002030-200207260-00004
55. Tenner-Racz K, Stellbrink HJ, van Lunzen J, Schneider C, Jacobs JP, Raschdorff B, et al. The unenlarged lymph nodes of HIV-1-infected, asymptomatic patients with high CD4 T cell counts are sites for virus replication and CD4 T cell proliferation. The impact of highly active antiretroviral therapy. J Exp Med (1998) 187:949–59.
56. Banga R, Procopio FA, Noto A, Pollakis G, Cavassini M, Ohmiti K, et al. PD-1(+) and follicular helper T cells are responsible for persistent HIV-1 transcription in treated aviremic individuals. Nat Med (2016) 22:754–61. doi:10.1038/nm.4113
57. Chun TW, Nickle DC, Justement JS, Meyers JH, Roby G, Hallahan CW, et al. Persistence of HIV in gut-associated lymphoid tissue despite long-term antiretroviral therapy. J Infect Dis (2008) 197:714–20. doi:10.1086/527324
58. Orenstein JM, Feinberg M, Yoder C, Schrager L, Mican JM, Schwartzentruber DJ, et al. Lymph node architecture preceding and following 6 months of potent antiviral therapy: follicular hyperplasia persists in parallel with p24 antigen restoration after involution and CD4 cell depletion in an AIDS patient. AIDS (1999) 13:2219–29. doi:10.1097/00002030-199911120-00004
Keywords: TFH cells, hyperplastic follicle, germinal center, HIV, SIV
Citation: Hong JJ, Chang K-T and Villinger F (2016) The Dynamics of T and B Cells in Lymph Node during Chronic HIV Infection: TFH and HIV, Unhappy Dance Partners? Front. Immunol. 7:522. doi: 10.3389/fimmu.2016.00522
Received: 08 September 2016; Accepted: 09 November 2016;
Published: 22 November 2016
Edited by:
Scott Hale, University of Utah, USAReviewed by:
Cristian Apetrei, University of Pittsburgh, USAGianfranco Pancino, French Institute of Health and Medical Research, France
Copyright: © 2016 Hong, Chang and Villinger. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Francois Villinger, fjv5939@louisiana.edu
 Jung Joo Hong
Jung Joo Hong Kyu-Tae Chang1
Kyu-Tae Chang1 Francois Villinger
Francois Villinger