Malettinin E, an antibacterial and antifungal tropolone produced by a marine Cladosporium strain
- 1Kieler Wirkstoff-Zentrum KiWiZ, GEOMAR Helmholtz Centre for Ocean Research Kiel, Kiel, Germany
- 2Institut für Anorganische Chemie, Christian-Albrechts-Universität zu Kiel, Kiel, Germany
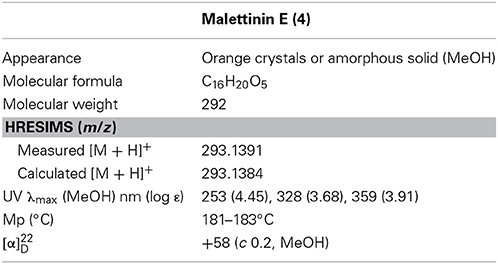
The isolation and structure elucidation of malettinins A–C (1–3) along with the new malettinin E (4) are described. The compounds were produced by the fungus Cladosporium sp. strain KF501, which was isolated from the German Wadden Sea. The malettinins are built up of tropolone/dihydropyran ring structures linked to a furan ring. The structure elucidation of the isolated compounds was achieved by means of one- and two-dimensional NMR spectroscopy supported by mass and UV data. The relative configuration of 4 was determined on the basis of single-crystal X-ray diffraction analysis. 1–4 exhibited antibacterial and antifungal activities when profiled against Xanthomonas campestris and Trichophyton rubrum. The influence of the chemical structure of the furan ring and of configurational changes on biological activities was observed.
Introduction
Marine fungi harbor an untapped potential for the production of secondary metabolites which are valuable for humankind, owing to their manifold bioactivities. In comparison to terrestrial strains, information on marine fungi is still little. Hence, fungi isolated from marine habitats are promising study objects for the search of new bioactive natural products. In our ongoing search for new bioactive compounds a Cladosporium strain isolated from the Wadden Sea showed antibacterial and antifungal activities and was therefore investigated in detail with regard to its natural products. The genus Cladosporium represents one of the largest and most heterogeneous genera of the Hyphomycetes (Bensch et al., 2012), showing ubiquitous occurrence. Species of the genus often have pathogenic or saprophytic lifestyles and are frequently found in air, soil, on foods, on plant materials or as endophytes (Stevens, 1974; Samson et al., 2000). Occurring as airborne fungi, some species of Cladosporium are of clinical importance as they can cause allergies such as allergic asthma (Simon-Nobbe et al., 2008). Concerning marine habitats, a minimum of three Cladosporium species were described from marine sources (Kirk and Clipson, 2013). Cladosporium species have been shown to possess the ability to produce a variety of natural products, among them the melanins which are pigments giving the fungal colonies their typical dark colored appearance. Other natural products isolated from Cladosporium species are bioactive compounds such as the antifungal cladosporides (Hosoe et al., 2000, 2001), the plant growth factors cotylenins (Sassa, 1971; Sassa et al., 1975), calphostins which specifically inhibit the protein kinase C (Kobayashi et al., 1989), and cladosporin exhibiting a broad activity spectrum including antifungal, antibacterial, insecticidal, phytotoxic and immunosuppressive properties (Scott et al., 1971; Anke et al., 1978; Grove and Pople, 1981; Springer et al., 1981; Fujimoto et al., 1999).
The study presented here, led to the isolation of the new compound malettinin E (4) along with known malettinins A–C (1–3) from a marine Cladosporium strain (Figure 1) The known malettinins were originally identified from an unidentified fungal colonist of Hypoxylon sp. stromata (Angawi et al., 2003, 2005). It is the first time that their production is reported in a Cladosporium sp. The structure elucidation of 4 and antibacterial and antifungal activities of the malettinins are described.
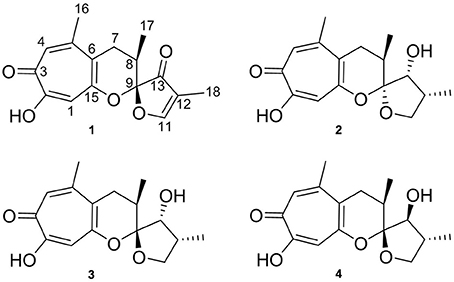
Figure 1. Structures of malettinins A–C (1–3) and malettinin E (4), isolated from Cladosporium sp. strain KF501.
Materials and Methods
General Experimental Procedures
Melting points were determined on an electrothermal melting point apparatus. Measurements of optical rotation were performed on a Perkin Elmer model 241 polarimeter. UV spectra were recorded on a Perkin Elmer Lambda 2 spectrophotometer. NMR spectra were measured on a Bruker AV 600 spectrometer (600 and 150 MHz for 1H and 13C NMR, respectively) and the residual solvent signals served as internal references (δH 3.31 and δC 49.0 for methanol-d4). High-resolution mass spectra were obtained on a Bruker micrOTOF II spectrometer using ESI ion source in negative mode. Analytical HPLC-UV/MS was conducted on a VWR-Hitachi LaChrom Elite system (pump L-2130, diode array detector L-2450, autosampler L-2200 and column oven L-2300) with a Phenomenex Onxy Monolithic column (C18, 100 × 3.00 mm) applying a gradient of 0.1% formic acid in H2O (A) and 0.1% formic acid in acetonitrile (B): 0 min 5% B, 4 min 60% B, 6 min 100% B; flow 2 ml min−1. Coupling of the HPLC system to a Bruker esquire4000 ESI-ion trap allowed mass detection.
Isolation and Taxonomy of Producing Organism
Strain KF501 was isolated from a water sample taken in the German Wadden Sea. DNA extraction, amplification of the internal transcribed spacer region (ITS) and sequencing were performed as described by Wiese et al. (2011) with slight modifications, centrifugation of the DNA at 8000 × g and 35 cycles of DNA amplification. The DNA sequence was deposited in GenBank under the accession number KF923800. Cryo-conserved stock cultures of strain KF501 were kept at −100°C using the Microbank system (Pro-Lab).
Fermentation and Compound Isolation
The fungal isolate was cultivated in the following media: casamino acids glucose medium (casein hydrolysate 0.25%, glucose × H2O 4%, MgSO4 × 7H2O 0.01%, KH2PO4 0.18%, pH 6.8) (Stevens, 1974), Czapek medium (sucrose 3%, NaNO3 0.3%, K2HPO4 0.1%, KCl 0.05%, MgSO4× 7H2O 0.05%, FeSO4× 7H2O 0.001%, pH 6.2) (Samson et al., 2000), modified malt extract medium (malt extract 3%, NaCl 1.5%, pH 5.5) (Samson et al., 2000), potato-carrot medium (potatoes 40 g, carrots 40 g, each boiled in 1 l of H2O and filtered off, 250 ml of potato extract and 250 ml of carrot extract were filled up with 500 ml of distilled H2O) (Samson et al., 2000), and modified Wickerham medium (malt extract 0.3%, yeast extract 0.3%, peptone from soymeal 0.5%, glucose × H2O 1%, NaCl 3%, agar 1.5%, pH 6.25) (Wickerham, 1951).
For isolation of the malettinins, Cladosporium sp. KF501 was cultivated in 9.75 l of casamino acids glucose medium. The cultivation experiments were performed as surface cultures at 20°C in the dark in 2-l Erlenmeyer flasks, each containing 750 ml medium. The cultures were inoculated with an agar slant (2.6 cm in diameter) of a 7 days old preculture which was grown on solid, modified Wickerham medium at room temperature in the dark. After 40 days of incubation, the mycelia of strain KF501 were harvested. For extraction, the mycelium of each flask was mixed with 150 ml of 96% EtOH, homogenized and filtered. As the obtained extract was very greasy, fats were frozen out at −20°C yielding 1.2 g of extract.
The malettinins were purified from the mycelium extract by preparative HPLC. The separations were performed on a VWR LaPrep system (pump P110, UV detector P311 UV, fraction collector Labocol Vario-2000 from LABOMATIC, autosampler Smartline 3900 from Knauer) using a Phenomenex Gemini-NX column (10 μ C18, 100A, Axia, 100 × 50.00 mm). A gradient of 0.1% formic acid in H2O (A) and 0.1% formic acid in acetonitrile (B) (0.0 min 20% B, 17.5 min 40% B, 21.0 min 60% B; flow 100 ml min−1) was applied to yield 17.5 mg of 2 (tR 8.6–9.0 min), 6.1 mg of 3 (tR 9.2–9.6 min), 4.6 mg of 4 (tR 9.9–10.3 min) and 15.1 mg of 1 (tR 12.2–18.0 min).
X-Ray Crystal Structure Determination
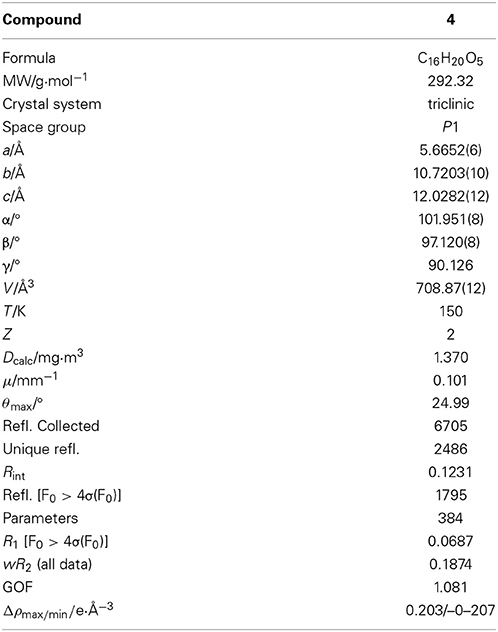
Single crystals of 4 suitable for X-ray data collection were obtained by recrystallization from a methanol solution. The data were measured using an Imaging Plate Diffraction System (IPDS-2) from STOE and CIE (Table 1). All non-hydrogen atoms were refined anisotropically. The H atoms were positioned with idealized geometry (O-H H atoms allowed to rotate but not to tip) and refined isotropically with Uiso(H) = 1.2·Ueq(C) (1.5 for methyl and O-H H atoms) using a riding model. There are two crystallographically independent molecules in the asymmetric unit, which exhibit the same relative configuration. In one of these molecules the H atoms of one methyl group are disordered and were refined using a split model with two orientations rotated by 60°. Because no strong anomalous scattering atoms are present, the absolute configuration cannot be determined. Therefore, Friedel opposites were merged in the refinement.
Antibacterial and Antifungal Assays
Antibacterial bioassays with the test strains Xanthomonas campestris and Staphylococcus epidermidis were performed as described by Silber et al. (2013). Bacillus subtilis and Candida albicans were tested as described by Ohlendorf et al. (2012). For antifungal assays using Trichophyton rubrum, cultivation was carried out in modified Sabouraud medium (peptone 1%, glucose 2%, pH 5.6). A spore solution containing 5 × 104 spores of T. rubrum per ml medium was prepared. 10 mM DMSO solutions of compounds were diluted with medium to achieve the desired test concentrations. For assaying, 200 μl of the spore solutions were mixed with 10.5 μl compound solutions in 96-well microtiter plates and incubated for 72 h at 28°C in the dark. The cell growth of T. rubrum was evaluated by measuring the optical densities with a Tecan Infinite M200 plate reader. The resulting values were compared with a positive control (0.1 and 0.5 μM clotrimazole) and a negative control (no compound). IC50 values were determined according to the inhibition of metabolic activity or cell growth in the presence of a compound as compared to the non-inhibited negative control.
Results
Isolation and Identification of the Producing Fungus
Strain KF501 was isolated from water samples taken in the German Wadden Sea. The strain grew well in casamino acids glucose, Czapek and malt extract medium, slowly in potato-carrot medium and did not grow in liquid modified Wickerham medium. In general, the growth of strain KF501 was relatively slow by comparison with other fungal isolates.
Macroscopic and microscopic morphological features, colony color and structure of conidiophores were characteristic of Cladosporium spp. (Samson et al., 2000). This classification was corroborated by the comparison of the ITS DNA sequence of strain KF501 with sequences available at GenBank employing the Basic Local Alignment Search Tool (BLAST) (Altschul et al., 1990). The search resulted in 100% similarity to Cladosporium cladosporioides, C. pseudocladosporioides, C. uredinicola, C. bruhnei and C. colombiae. Hence, strain KF501 could be identified as a member of the genus Cladosporium, but identification to the species level was not possible.
Structure Elucidation
The new malettinin E (4) along with the known malettinins A–C (1–3) were obtained from the mycelia extracts of Cladosporium sp. strain KF501 grown in casamino acids glucose medium. The compounds were isolated by preparative reversed-phase HPLC. Known compounds 1–3 were readily identified by comparison of their spectroscopic (1H NMR, UV and MS) data with literature values (Angawi et al., 2003, 2005).
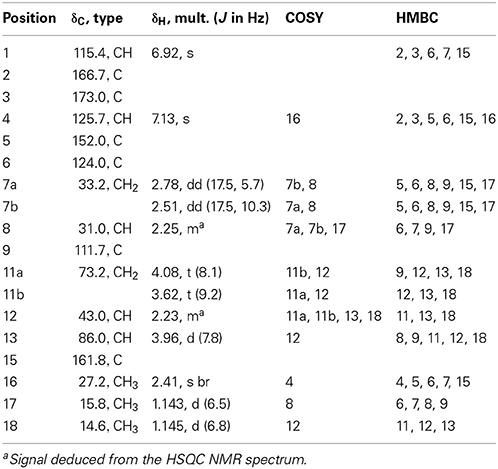
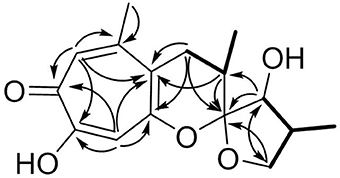
Malettinin E (4) has the molecular formula of C16H20O5 as determined by HRESIMS which requires seven degrees of unsaturation. The physico-chemical properties of 4 are summarized in Table 2. The molecular formula of 4 being the same as for 2 and 3 suggested the compounds to be isomeric. In addition, the one- and two-dimensional NMR spectra of 4 (1H, 13C, DEPT, COSY, HSQC, and HMBC; see Table 3 and Supplementary Information) revealed the presence of considerable structural similarities with 2 and 3 (Angawi et al., 2005). The 1H and 13C NMR data of 4 showed resonances for three methyl, two methylene and two aliphatic methine groups, one oxygenated methane, one ketalic carbon and one carbonyl group as well as signals for six aromatic carbons one of which appeared to be oxygenated. Thus, all structural groups and atoms present in 2 or 3 were also observed for 4. Correlations of the aromatic protons in the HMBC NMR spectrum (Figure 2) established the same tropolone moiety for 4 as found in the known malettinins. In addition to the tropolone unit, 4 contained a dihydropyran ring identical to that of 2 and 3. The proton and carbon chemical shifts of the corresponding signals were in good agreement with those reported for 2 and 3, with the proton signals agreeing particularly well to the structure of 3. HMBC couplings from H2−7 to C-5, C-6, and C-15 gave evidence for the attachment of the dihydropyran ring to the tropolone moiety. The NMR data determined the remaining structural part of 4 to be a tetrahydrofuran ring connected to the dihydropyran unit via C-9 (Figure 2). As a result, compound 4 was proven to have the same planar structure as described for 2 and 3. The vicinal JH7–H8 values of 4 (5.7 and 10.3) showed better correlations with those of 3 (5.9 and 13) than with those of 2 (6.2 and 3.8), suggesting a configuration like in 3 for the stereogenic center at C-9. However, the structures were not identical as considerable deviations in the chemical shifts of the proton and carbon signals of CH-12 (δH 2.23, δC 43.0) and CH-13 (δH 3.96, δC 86.0) in 4 were observed when compared to the reported shifts of the respective signals in 3 (δH 2.5, δC 34.7 and δH 4.32, δC 73.0, respectively) (Angawi et al., 2005). Taken the NMR data together, it seemed most likely that 4 was a stereoisomer of malettinin C (3), differing in the configuration at C-12 and/or C-13.
In order to determine the relative configuration of 4 conclusively, a single-crystal X-ray diffraction analysis was performed. It showed that the compound consisted of two crystallographically distinct molecules (4a and 4b) with identical relative configuration, exhibiting slight conformational differences between the molecules (Figure 3). The X-ray crystal structure of 4 confirmed the assumptions made on the basis of the NMR data as it was found to be identical to 3 except for the configuration at C-13. Thus, 4 was identified to be the C-13 epimer of 3 as shown in Figure 1.
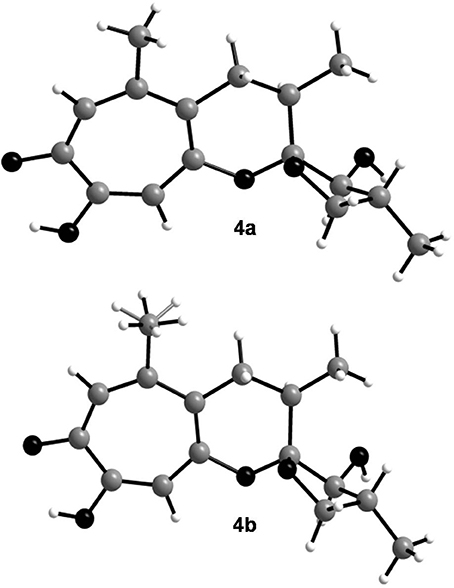
Figure 3. Crystal structures of the two crystallographically distinct molecules of malettinin E (4a and 4b). The H atoms of one of the methyl groups in 4b are disordered.
Biological Activities
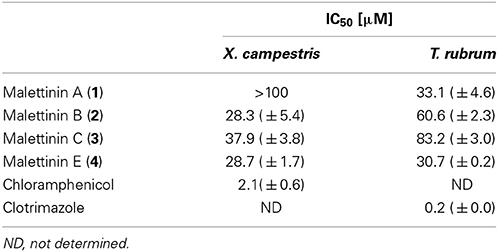
Compounds 1–4 were tested for biological activities against a set of bacterial and fungal test strains. The malettinins were found to be bioactive against the plant pathogenic bacterium Xanthomonas campestris and the human pathogenic dermatophyte Trichophyton rubrum (Table 4). Compounds 2–4 showed inhibition of X. campestris, whereas 1 did not inhibit the bacterial test strain notably. The IC50 values of 2–4 were in approximately the same concentration range (28.3–37.9 μM). With regard to T. rubrum, all tested malettinins 1–4 exhibited inhibitory activities. However, the strength of inhibition differed between the compounds with the new malettinin (4) and 1 showing highest activities (IC50 of 30.7 and 33.1), while 2 was half as active (IC50 of 60.6 μM) and 3 was even less active (IC50 of 83.2 μM).
Beside the reported antifungal and antibacterial properties in Table 4, malettinins B and C (2 and 3) showed weak inhibition (<80%) of Staphylococcus epidermidis, Bacillus subtilis and Candida albicans at a test concentration of 100 μM. At the same concentration malettinin E (4) exhibited weak inhibitory effects on the metabolic activity of S. epidermidis and C. albicans. Malettinin A did not show antibacterial effects, but also inhibited C. albicans (81% inhibition at 100 μM). As these activities were poor, IC50 values were not determined.
Discussion
For the first time malettinins A–C and E (1–4) were isolated from a fungus belonging to the genus Cladosporium. Originally, 1–3 were purified from an unidentified fungus which additionally produced a forth related metabolite, called malettinin D (Angawi et al., 2003, 2005). Malettinin D has not been detected in the culture extracts of Cladosporium sp. strain KF501, instead a new 13-epimer of 3, named malettinin E (4), was found to be produced. 1–4 are members of the family of tropolone fungal metabolites containing an α-tropolone substructure. Even though many derivatives possessing a partial structure of this tropolone type are described for plants, only few such compounds have been reported within the fungal kingdom. Representative structures of fungi are puberulic acid, puberulonic acid, stipitatic acid and related structures (Corbett et al., 1950; Iwatsuki et al., 2011). More complex known compounds are epolone A or B, pycnidione, eupenifeldin or fusariocin C (Ito et al., 1981; Mayerl et al., 1993; Cai et al., 1998), which structurally share the fused tropolone/dihydropyran ring structure with the malettinins. However, malettinins 1–4 are unique with regard to their linkage of the tropolone/dihydropyran ring to a furan.
Malettinins A–C (1–3) were described as displaying weak inhibitory properties against C. albicans, B. subtilis and S. aureus employing disk diffusion assays (Angawi et al., 2003, 2005). In addition, 1 inhibited Aspergillus flavus and Fusarium verticillioides (Angawi et al., 2003). Even though the activities against C. albicans, B. subtilis and antistaphylococcal activities against S. epidermidis were observed for 2 and 3 in this study as well, higher activities were found when profiled against the bacterium X. campestris and the fungus T. rubrum. X. campestris causes a variety of plant diseases, e.g., black rot in cabbage which is difficult to treat and therefore leads to high crop losses (Roohie and Umesha, 2012). T. rubrum is responsible for clinical pictures like tinea pedis (athlete's foot) and likewise treatments are challenging, in part due to the toxicity in long-term treatments (Ramsey et al., 2013). New bioactive compounds for better treatments in plant protection as well as in clinical aspects are hence highly desirable. Comparing activities though shows that the malettinins inhibit X. campestris with a 13-fold higher IC50 than the antibiotic chloramphenicol used as positive control. Thus, their activities can be considered as moderate, only. The inhibition of the malettinins against T. rubrum is rather poor given that the IC50 of the positive control clotrimazole is lower by a factor of greater than 170.
The activities regarding X. campestris showed that all stereoisomeric compounds (2–4) inhibited the test strain in comparable concentration ranges, while 1, possessing a very slightly varied furan ring, did not show inhibition. This observation suggests that the furan ring of the malettinins is critical for antibacterial properties against X. campestris. With regard to activities against T. rubrum, apparently configurational changes of the malettinins determine the strength of activity, since the distinct stereoisomers exhibited different IC50 values.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We gratefully thank Dr. K. Schaumann for providing strain KF501, G. Kohlmeyer-Yilmaz, M. Höftmann and Dr. F. Sönnichsen for running and processing NMR experiments and R. Schmied for help with measurements of the melting point. We also thank the Institute of Clinical Molecular Biology in Kiel for providing Sanger sequencing as supported in part by the DFG Cluster of Excellence “Inflammation at Interfaces” and “Future Ocean.” We thank the technicians S. Greve and S. Arndt for technical support. This study was part of the PhD thesis of Johanna Silber and was performed in the framework of the EU project MARINE FUNGI, which was funded within the European Union Seventh Framework Programme (FP7/2007-2013 under grant agreement number 265926).
Supplementary Material
The Supplementary Material for this article can be found online at: http://www.frontiersin.org/journal/10.3389/fmars.2014.00035/abstract
References
Altschul, S. F., Gish, W., Miller, W., Myers, E. W., and Lipman, D. J. (1990). Basic local alignment search tool. J. Mol. Biol. 215, 403–410. doi: 10.1016/S0022-2836(05)80360-2
Angawi, R. F., Swenson, D. C., Gloer, J. B., and Wicklow, D. T. (2003). Malettinin A: a new antifungal tropolone from an unidentified fungal colonist of Hypoxylon stromata (NRRL 29110). Tetrahedron Lett. 44, 7593–7596. doi: 10.1016/j.tetlet.2003.08.057
Angawi, R. F., Swenson, D. C., Gloer, J. B., and Wicklow, D. T. (2005). Malettinins B–D: new polyketide metabolites from an unidentified fungal colonist of Hypoxylon stromata (NRRL 29110). J. Nat. Prod. 68, 212–216. doi: 10.1021/np049625r
Anke, H., Zähner, H., and König, W. A. (1978). Metabolic products of microorganisms. 170. On the antibiotic activity of cladosporin. Arch. Microbiol. 116, 253–257. doi: 10.1007/BF00417848
Bensch, K., Braun, U., Groenewald, J. Z., and Crous, P. W. (2012). The genus Cladosporium. Stud. Mycol. 72, 1–401. doi: 10.3114/sim0003
Cai, P., Smith, D., Cunningham, B., Brown-Shimer, S., Katz, B., Pearce, C., et al. (1998). Epolones: novel sesquiterpene-tropolones from fungus OS-F69284 that induce erythropoietin in human cells. J. Nat. Prod. 61, 791–795. doi: 10.1021/np9800506
Corbett, R. E., Hassall, C. H., Johnson, A. W., and Todd, A. R. (1950). 1. Puberulic and puberulonic acids. Part I. The molecular formula of puberulonic acid and consideration of possible benzenoid structures for the acids. J. Chem. Soc. 1–6. doi: 10.1039/jr9500000001
Fujimoto, H., Fujimaki, T., Okuyama, E., and Yamazaki, M. (1999). Immunomodulatory constituents from an ascomycete, Microascus tardifaciens. Chem. Pharm. Bull. 47, 1426–1432. doi: 10.1248/cpb.47.1426
Grove, J. F., and Pople, M. (1981). The insecticidal activity of some fungal dihydroisocoumarins. Mycopathologia 76, 65–67. doi: 10.1007/BF00443751
Hosoe, T., Okada, H., Itabashi, T., Nozawa, K., Okada, K. de Campos Takaki, G. M., et al. (2000). A new pentanorlanostane derivative, cladosporide A, as a characteristic antifungal agent against Aspergillus fumigatus, isolated from Cladosporium sp. Chem. Pharm. Bull. 48, 1422–1426. doi: 10.1248/cpb.48.1422
Hosoe, T., Okamoto, S., Nozawa, K., Kawai, K.-I., Okada, K. de Campos Takaki, G. M., et al. (2001). New pentanorlanostane derivatives, cladosporide B–D, as characteristic antifungal agents against Aspergillus fumigatus, isolated from Cladosporium sp. J. Antibiot. 54, 747–750. doi: 10.7164/antibiotics.54.747
Ito, T., Arai, T., Ohashi, Y., and Sasada, Y. (1981). Structure of fusariocin C, a cytotoxic metabolite from Fusarium moniliforme. Agric. Biol. Chem. 45, 1689–1692. doi: 10.1271/bbb1961.45.1689
Iwatsuki, M., Takada, S., Mori, M., Ishiyama, A., Namatame, M., Nishihara-Tsukashima, A., et al. (2011). In vitro and in vivo antimalarial activity of puberulic acid and its new analogs, viticolins A–C, produced by Penicillium sp. FKI-4410. J. Antibiot. 64, 183–188. doi: 10.1038/ja.2010.124
Kirk, P., and Clipson, N. (2013). Cladosporium Link, 1816, In: Index Fungorum Partnership (2013) Index Fungorum. Accessed through: World Register of Marine Species. Available online at http://www.marinespecies.org/aphia.php?p=taxdetails&id=100218 on 2013-11-27
Kobayashi, E., Ando, K., Nakano, H., Iida, T., Ohno, H. Morimoto, et al. (1989). Calphostins (UCN-1028), novel and specific inhibitors of protein kinase C. I. Fermentation, isolation, physico-chemical properties and biological activities. J. Antibiot. 42, 1470–1474. doi: 10.7164/antibiotics.42.1470
Mayerl, F., Gao, Q., Huang, S., Klohr, S. E., Matson, J. A., Gustavson, D. R., et al. (1993). Eupenifeldin, a novel cytotoxic bistropolone from Eupenicillium brefeldianum. J. Antibiot. 46, 1082–1088. doi: 10.7164/antibiotics.46.1082
Ohlendorf, B., Schulz, D., Erhard, A., Nagel, K., and Imhoff, J. F. (2012). Geranylphenazinediol, an acetylcholinesterase inhibitor produced by a Streptomyces species. J. Nat. Prod. 75, 1400–1404. doi: 10.1021/np2009626
Ramsey, J. P., Mercurio, A., Holland, J. A., Harris, R. N., and Minbiole, K. P. C. (2013). The cutaneous bacterium Janthinobacterium lividum inhibits the growth of Trichophyton rubrum in vitro. Int. J. Dermatol. doi: 10.1111/ijd.12217. [Epub ahead of print].
Roohie, R. K., and Umesha, S. (2012). Development of multiplex PCR for the specific detection of Xanthomonas campestris pv. campestris in cabbage and correlation with disease incidence. J. Plant Pathol. Microb. 3:127. doi: 10.4172/2157-7471.1000127
Samson, R. A., Hoekstra, E. S., Frisvad, J. C., and Filtenborg, O. (2000). Introduction to Food-and Airborne Fungi. Utrecht: Centraalbureau voor Schimmelcultures (CBS).
Sassa, T. (1971). Cotylenins, leaf growth substances produced by a fungus. Agric. Biol. Chem. 35, 1415–1418. doi: 10.1271/bbb1961.35.1415
Sassa, T., Togashi, M., and Kitaguchi, T. (1975). The structures of cotylenins A, B, C, D and E. Agric. Biol. Chem. 39, 1735–1744. doi: 10.1271/bbb1961.39.1735
Scott, P. M., van Walbeek, W., and MacLean, W. M. (1971). Cladosporin, a new antifungal metabolite from Cladosporium cladosporioides. J. Antibiot. 24, 747–755. doi: 10.7164/antibiotics.24.747
Silber, J., Ohlendorf, B., Labes, A., Erhard, A., and Imhoff, J. F. (2013). Calcarides A–E, antibacterial macrocyclic and linear polyesters from a Calcarisporium strain. Mar. Drugs 11, 3309–3323. doi: 10.3390/md11093309
Simon-Nobbe, B., Denk, U., Pöll, V., Rid, R., and Breitenbach, M. (2008). The spectrum of fungal allergy. Int. Arch. Allergy Immunol. 145, 58–86. doi: 10.1159/000107578
Springer, J. P., Cutler, H. G., Crumley, F. G., Cox, R. H., Davis, E. E., and Thean, J. E. (1981). Plant growth regulatory effects and stereochemistry of cladosporin. J. Agric. Food Chem. 29, 853–855. doi: 10.1021/jf00106a044
Keywords: marine natural products, fungal natural products, antibacterial, antifungal, Cladosporium sp., malettinin, tropolone
Citation: Silber J, Ohlendorf B, Labes A, Wenzel-Storjohann A, Näther C and Imhoff JF (2014) Malettinin E, an antibacterial and antifungal tropolone produced by a marine Cladosporium strain. Front. Mar. Sci. 1:35. doi: 10.3389/fmars.2014.00035
Received: 16 June 2014; Accepted: 01 August 2014;
Published online: 19 August 2014.
Edited by:
Donatella De Pascale, National Research Council-CNR, ItalyReviewed by:
Sandra Pucciarelli, University of Camerino, ItalySimona De Marino, University of Naples “Federico II,” Italy
Copyright © 2014 Silber, Ohlendorf, Labes, Wenzel-Storjohann, Näther and Imhoff. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Johannes F. Imhoff, Kieler Wirkstoff-Zentrum KiWiZ, GEOMAR Helmholtz Centre for Ocean Research Kiel, Am Kiel-Kanal 44, 24106 Kiel, Germany e-mail: jimhoff@geomar.de
 Johanna Silber
Johanna Silber Birgit Ohlendorf1
Birgit Ohlendorf1  Antje Labes
Antje Labes Johannes F. Imhoff
Johannes F. Imhoff