A taxonomic survey of the shallow-water (<150 m) black corals (Cnidaria: Antipatharia) of the Hawaiian Islands
- National Oceanic and Atmospheric Administration, Papahānaumokuākea Marine National Monument, Honolulu, HI, USA
The shallow-water (<150 m) antipatharian fauna of the Hawaiian Archipelago is described and illustrated based on a systematic examination of skeletal spine morphology, polyp morphology, colony branching pattern and in situ photographs. A total of 172 black coral specimens were examined, including all available type material of species previously reported from shallow waters off Hawai‘i. The examined specimens were assigned to three families (Antipathidae, Aphanipathidae, and Myriopathidae), six genera (Antipathes, Cirrhipathes, Stichopathes, Aphanipathes, Acanthopathes, and Myriopathes), and eight species: Antipathes griggi Opresko, 2009, Antipathes grandis Verrill, 1928, Cirrhipathes cf. anguina (Dana, 1846), Stichopathes echinulata Brook, 1889, Stichopathes? sp., Aphanipathes verticillata mauiensis Opresko et al., 2012, Acanthopathes undulata (Van Pesch, 1914), and Myriopathes cf. ulex (Ellis and Solander, 1786). The biogeographical distribution of Hawaiian shallow-water black corals is presented and discussed.
Introduction
The taxonomic study of Hawaiian antipatharians began in 1928 when Verrill described Antipathes grandis and Antipathes irregularis. However, only the A. grandis nomen remains valid, because A.irregularis has since been identified as a gorgonian coral (Grigg and Opresko, 1977). In 1958, large aggregations of A. grandis and a second antipatharian species (see below) were discovered off Maui at depths between 30 and 90 m, a discovery that led to the establishment of a local black coral fishery (reviewed by Grigg, 2001). In 1961, this second antipatharian species was tentatively identified as Antipathes dichotoma Pallas, 1766 (Bayer, 1961), a species originally described from the Mediterranean (Opresko, 2003a). In 1977, Grigg and Opresko published a taxonomic survey of Hawaiian black corals based on colony branching pattern that included species descriptions of 14 species found in 30–570 m. Since the study of Grigg and Opresko (1977), skeletal spine morphology has become an increasingly important character in antipatharian taxonomy (Opresko, 1972, 2001, 2002, 2003b, 2004, 2005a, 2006; and references therein), because this character is thought to be largely independent of environmental factors, as compared to other more plastic morphological characters (Lapian et al., 2007; Wagner et al., 2010). In particular, the high-resolution imaging of scanning electron microscopy (SEM) has aided in the use of skeletal spine morphology as a taxonomic character for antipatharians by allowing visualization of minute spine features that may be diagnostic of individual species, genera and families (Opresko, 1972, 1998, 2001, 2002, 2003b, 2004, 2005a, 2006; Lapian et al., 2007; Wagner et al., 2010; Bo et al., 2012). As a result of this advance, type specimens of numerous antipatharian species have recently been reexamined and redescribed (Grange, 1988; Opresko and Genin, 1990; Opresko and Cairns, 1994; Opresko and Baron-Szabo, 2001; Opresko, 2003a; Molodtsova and Pasternak, 2005; Opresko and Sanchez, 2005; Ocaña et al., 2006; Bo and Opresko, 2015), including two species from Hawaiian waters (Opresko, 2009; Wagner et al., 2010).
Unlike many other parts of the world, antipatharian populations have been well documented in Hawai‘i (see Wagner et al., 2012b). This is in large part due to a black coral fishery that has operated in Hawai‘i since the late 1950's (reviewed by Grigg, 2001), and has led to many black coral surveys. However, many previous surveys in Hawai‘i did not identify black corals to species level, in large part due to the absence of detailed taxonomic studies until recently, as well as difficulties in differentiating species in situ or from video data alone (Opresko, 2009; Wagner et al., 2010; Opresko et al., 2012). The purpose of this study was to provide a taxonomic guide to the shallow-water black corals of the Hawaiian Islands using (1) skeletal spine morphology, (2) polyp morphology, (3) branching pattern, and (4) in situ photographs. For this purpose, type material of species previously reported from Hawai‘i were reexamined where available, and compared to recently collected specimens from Hawaiian waters. This study was mostly limited to those species found at depths shallower than the top of the thermocline in the Main Hawaiian Islands (~120 m; Kahng and Kelley, 2007), because of the scarcity of specimens available from deeper waters. Additionally, this depth also represents the lower limit for several Hawaiian antipatharians, and thus serves as a logical cutoff point for this study.
Materials and Methods
A total of 172 antipatharian samples were examined as part of this study, and included museum specimens deposited at (1) the Bernice P. Bishop Museum in Honolulu, Hawai‘i (BPBM), (2) the National Museum of Natural History, Smithsonian Institution in Washington, D.C. (USNM), (3) the Museum of Comparative Zoology in Cambridge, Massachusetts (MCZ), and (4) specimens recently collected using conventional SCUBA, mixed-gas technical diving and the Hawai‘i Undersea Research Laboratory (HURL) manned submersibles Pisces IV and V (Table 1). Museum samples ranged from whole colonies to colony fragments of various sizes, and included type material of Antipathes grandis, A. griggi, Stichopathes echinulata, Aphanipathes verticillata, and Acanthopathes undulata (Table 1). For recently collected specimens, entire colonies were photographed in situ, and 5–10 tissue samples were clipped from each colony and preserved in 10% formaldehyde in seawater. Morphometric measurements of polyps and spines were made from photographs of preserved specimens as described by Wagner et al. (2010). Additionally, samples were prepared for SEM analysis of skeletal spines, and viewed under a S-4800 Hitachi Field Emission SEM (Hitachi High-Technologies Corporation, Tokyo, Japan) at the University of Hawai‘i at Mānoa (Wagner et al., 2010). Literature records of Hawaiian black corals were reviewed with the purpose of synonymizing different names that have previously been used for the same species. In cases were species assignments could not be verified from literature records alone, authors were contacted and species identifications were made using previously collected specimens, in situ photographs or collection information provided by authors.
Results and Discussion
Based on the overall morphology of colonies, polyps and skeletal spines, the 172 examined samples belong to three families (Antipathidae, Aphanipathidae, and Myriopathidae), six genera (Antipathes, Cirrhipathes, Stichopathes, Aphanipathes, Acanthopathes, and Myriopathes), and eight species (Tables 1, 2). Five species were identified by directly comparing Hawaiian specimens to type material, and included (1) Antipathes griggi Opresko, 2009, (2) Antipathes grandis, Verrill, 1928, (3) Stichopathes echinulata Brook, 1889, (4) Aphanipathes verticillata mauiensis Opresko et al., 2012, and (5) Acanthopathes undulata (Van Pesch, 1914) (Table 2). Additionally, specimens that are consistent with the descriptions of Cirrhipathes anguina (Dana, 1846), and Myriopathes ulex (Ellis and Solander, 1786) were identified. However, because the type material of both C. anguina and M. ulex is lost and the original descriptions are very brief, Hawaiian specimens cannot be conclusively assigned to these species until neotypes are designated and a thorough taxonomic study is undertaken. Finally, the examined material included an undescribed wire coral species, which is tentatively assigned to the genus Stichopathes. The diagnostic characters of the Hawaiian species identified as part of this study are highlighted in Figures 1–8 and Table 2, and discussed in the systematic section and taxonomic key below.
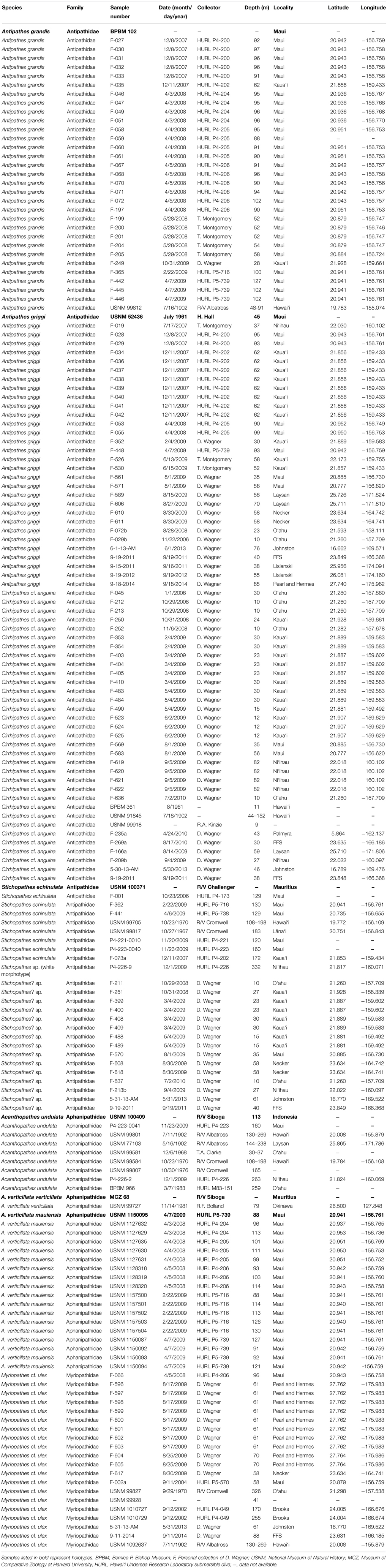
Figure 1. (A–D) Antipathes griggi holotype (USNM 52436) showing (A) entire specimen, (B) polyps on terminal branch under light microscopy (scale bar = 1 mm), and (C,D) skeletal spines on terminal branch under SEM (scale bars = 100 μm). (E–G) A. griggi colonies in situ; (H) skeletal spines under light microscopy (scale bar = 100 μm); (I) close-up of polypar spine under SEM (scale bar = 50 μm). (Photos courtesy of E,G Tony Montgomery, and F HURL).
Systematics
Family Antipathidae Ehrenberg, 1834
The Antipathidae is characterized by polyps that (1) are usually larger than 1 cm in transverse diameter (range = 1–3 mm), (2) are not elongated in the transverse plane, (3) possess 10 mesenteries (six primary and four secondary), and (4) have sagittal tentacles (the two tentacles that are perpendicular to the branch bearing the polyp) that are substantially longer than its lateral tentacles (the four tentacles that are nearly parallel to the branch bearing the polyp) when fully expanded (Opresko, 2005b; Opresko and Sanchez, 2005; Bo, 2008; Moon and Song, 2008a). However, this latter feature is frequently lost during the preservation process. The Antipathidae has historically been considered a taxonomic dumping ground, and is the oldest and most speciose antipatharian family (Daly et al., 2007; Bo, 2008). Consequently, colony and skeletal spine morphology are very heterogeneous within this family. Several taxa that formally belonged to the Antipathidae have been transferred to new families (Opresko, 2001, 2002, 2003b, 2004, 2005a, 2006); however, the family is still not monophyletic and in need of future taxonomic revisions (Daly et al., 2007).
Genus Antipathes Pallas, 1766
Antipathes is the oldest antipatharian genus and is distinguished by colonies that are all branched, with branching patterns varying from fan-shaped to bushy (Opresko, 1972; Opresko and Sanchez, 2005). Like the Antipathidae, Antipathes is also considered a taxonomic dumping ground that is morphologically heterogenous (Opresko and Baron-Szabo, 2001; Daly et al., 2007; Bo, 2008; Moon and Song, 2008a). Even though many species have been removed from Antipathes and added to new genera (Opresko and Cairns, 1994; Opresko, 2001, 2002, 2003b, 2004, 2006), the genus still groups together many uncertain species and is in need of revision (Bo, 2008).
Antipathes griggi Opresko, 2009
Antipathes dichotoma: (Bayer, 1961, p. 8); Antipathes grandis: (Grigg, 1964, pp. 1–74, Figures 2–4, 7, 9, 11–13, 17, 22, 24); Antipathes grandis: (Grigg, 1965, pp. 244–260, Figures 1, 3, 5–10); Antipathes dichotoma: (Grigg, 1974, pp. 235–240); Antipathes dichotoma: (Grigg, 1976, pp. 1–48, Figures 1, 4, 15); Antipathes dichotoma: (Grigg and Opresko, 1977, pp. 242–261, Figures 9, 10); Antipathes dichotoma: (Grigg, 1984, pp. 57–74); Antipathes dichotoma: (Grigg, 1993, pp. 50–60, Figure 5); Antipathes dichotoma: (Pyle and Chave, 1994, p. 92); Antipathes dichotoma: (Montgomery and Crow, 1998, pp. 103–108); Antipathes dichotoma: (Montgomery, 2002, pp. 157–164); Antipathes dichotoma: (Grigg, 2001, pp. 291–299, Figures 2, 3); Antipathes dichotoma: (Grigg, 2002, p. 13); Antipathes dichotoma: (Grigg et al., 2002, p. 79, Figures 6, 7); Antipathes sp.: (Opresko, 2003a, p. 491); Antipathes dichotoma: (Grigg, 2003, pp. 121–122); Antipathes dichotoma: (Grigg, 2004, pp. 1–6); Antipathes dichotoma: (Greenfield and Randall, 2004, p. 513); Antipathes dichotoma: (Boland and Parrish, 2005, pp. 411–420); Antipathes dichotoma: (Kahng and Grigg, 2005, pp. 556–562); Antipathes sp.: (Fenner, 2005, pp. 96, 99, 3 unnumbered figure on pp. 96 and 99); Antipathes sp.: (Hoover, 2006, p. 69, unnumbered figure on p. 69); Antipathes dichotoma: (Roark et al., 2006, pp. 1–14); Antipathes dichotoma: (Parrish and Baco, 2007, pp. 159, 170); Antipathes cf. dichotoma: (Parrish and Baco, 2007, p. 185); Antipathes cf. curvata: (Parrish and Baco, 2007, pp. 159, 162, 164, 170, 173, 185, Figure 4.5 right); Antipathes dichotoma: (Kahng and Kelley, 2007, pp. 684, 686); Antipathes cf. curvata: (Baco, 2007, p. 112); Antipathes griggi: (Opresko, 2009, pp. 277–291, Figures 1a,b, 2a–f, 3a–d, 4a–f); Antipathes griggi: (Wagner et al., 2010, pp. 271–290, Figures 9e–h); Antipathes griggi: (Grigg, 2010, pp. 1–9); Antipathes griggi: (Wagner et al., 2011a, pp. 249–255, Figure 2); Antipathes griggi: (Wagner et al., 2011b, pp. 1323–1328, Figures 2a,b); Antipathes griggi: (Wagner et al., 2011c, pp. 211–225, Figures 1c,d, 4a); Antipathes griggi: (Opresko et al., 2012, pp. 24, 36–38, Figures 8e–h); Antipathes griggi: (Wagner et al., 2012a, pp. 795–806, Figures 2a–j); Antipathes griggi: (Wagner et al., 2012b, pp. 67–132, Figure 2.1a); Antipathes griggi: (Wagner et al., 2013, pp. 341–345, Figure 2 right); Antipathes griggi: (Brugler et al., 2013, pp. 312–361, Figures 2–5); Antipathes griggi: (Wagner et al., 2014, pp. 4, 8).
The Hawaiian species Antipathes griggi (Figure 1) was previously identified as A. dichotoma (Bayer, 1961), a species originally described from off Marseilles in the Mediterranean (Pallas, 1766; Opresko, 2003a). Subsequent comparisons between specimens from Hawai‘i and the Mediterranean revealed considerable morphological differences (Opresko, 2003a). As a result, the Hawaiian “A. dichotoma” was assigned the new name of Antipathes griggi (Opresko, 2009). Surveys for A. griggi have been particularly frequent in Hawai‘i, because it is the main species targeted by the Hawaiian black coral fishery (Grigg, 1993, 2001, 2004; Parrish and Baco, 2007). Opresko (2009) presented a detailed taxonomic description of A. griggi, and the main diagnostic features are briefly summarized here.
A. griggi colonies can reach heights of up to 3 m, and are extensively branched with eight or more orders of branching (Figures 1E,F). Branches are arranged irregularly on all sides of the corallum on the lower part of the colony, and become more planar on the highest order branches (Figures 1E,F). Terminal branches reach up to 10 cm in length without becoming branched. Spines are conical, some have bifurcations toward their apex, and are covered with elongated tubercles over the biggest portion of their surface (Figure 1I). At midpoint, the terminal branches usually measure 0.4–1.7 mm in diameter with tissue, and contain polypar spines that are on average 181 μm tall (range = 105–382 μm), and abpolypar spines that are on average 127 μm tall (range = 68–243 μm). Smaller secondary spines, up to 40 μm tall, are present on some portions of the corallum, especially on thicker branches (Figure 1D). On branchlets and smaller branches, spines are arranged in axial rows, with adjacent rows offset in a spiral pattern around the corallum (Figures 1C,D). Polyps average 1.12 mm in transverse diameter (range = 0.58–1.75 mm), and are typically spaced 1.43 mm apart (range = 0.57–2.83 mm), resulting in 7 polyps per cm (range = 5–10). The tissues of living colonies are colored brown to bright red (Figures 1E,F).
To date, specimens identified as A. griggi have only been reported from the Hawaiian Archipelago from Hawai‘i Island to Pearl and Hermes Atoll at depths ranging between 10 and 110 m (Table 1; Opresko, 2009; Wagner et al., 2011a), as well as from Johnston Atoll at 76 m (Wagner et al., 2014). However, colonies with similar morphologies, identified as A. dichotoma, have also been reported from other locations in the Indo-West Pacific including the Philippines, Indonesia, Palau, China and Guam (Van Pesch, 1914; Grigg, 1975; Zhou and Zou, 1984, 1992; Zou and Zhou, 1984; Paulay et al., 2003; Rogers et al., 2007; Qi et al., 2009). These locations are all outside the range of A. dichotoma, which is only known from the Mediterranean and East Atlantic (Opresko, 2003a; Bo, 2008). Like the previous misidentification of A. dichotoma from Hawai‘i (see above), these misidentified A. dichotoma records may also be A. griggi. However, detailed taxonomic investigations of specimens from the Indo-West Pacific will have to be undertaken to confirm this. Currently, there are no museum specimens of A. griggi that were collected outside of Hawai‘i or Johnston Atoll.
Antipathes grandis Verrill, 1928
Antipathes grandis: (Verrill, 1928, pp. 7, 9, Figures 1i–m, pl. IIc); Antipathes grandis: (Grigg, 1974, pp. 235–240); Antipathes grandis: (Grigg, 1976, pp. 1–48); Antipathes grandis: (Grigg and Opresko, 1977, pp. 242–261, Figures 1, 11); Antipathes grandis: (Grigg, 1984, pp. 57–74); Antipathes grandis: (Grigg, 1993, pp. 50–60); Antipathes grandis: (Grigg, 2001, pp. 291–299); Antipathes grandis: (Grigg, 2002, p. 13); Antipathes grandis: (Grigg, 2003, pp. 121–122); Antipathes grandis: (Grigg, 2004, pp. 1–6); Antipathes grandis: (Fenner, 2005, p. 100, 2 unnumbered figure on p. 100); Antipathes grandis: (Boland and Parrish, 2005, pp. 411–420); Antipathes grandis: (Kahng and Grigg, 2005, pp. 556–562); Antipathes grandis: (Parrish and Baco, 2007, pp. 159, 162, 164, 170, 185, Figure 4.5 left); Antipathes grandis: (Baco, 2007, p. 112); Antipathes grandis: (Kahng and Kelley, 2007, pp. 684, 686); Antipathes grandis: (Wagner et al., 2010, pp. 271–290, Figures 2–7, 8a,b, 9a–d, 10); Antipathes grandis: (Grigg, 2010, pp. 1–9); Antipathes grandis: (Wagner et al., 2011b, pp. 1323–1328, Figure 2c); Antipathes grandis: (Wagner et al., 2012a, pp. 795–806); Antipathes grandis: (Wagner et al., 2012b, pp. 67–132); Antipathes grandis: (Brugler et al., 2013, pp. 312–361, Figures 2, 3, 5).
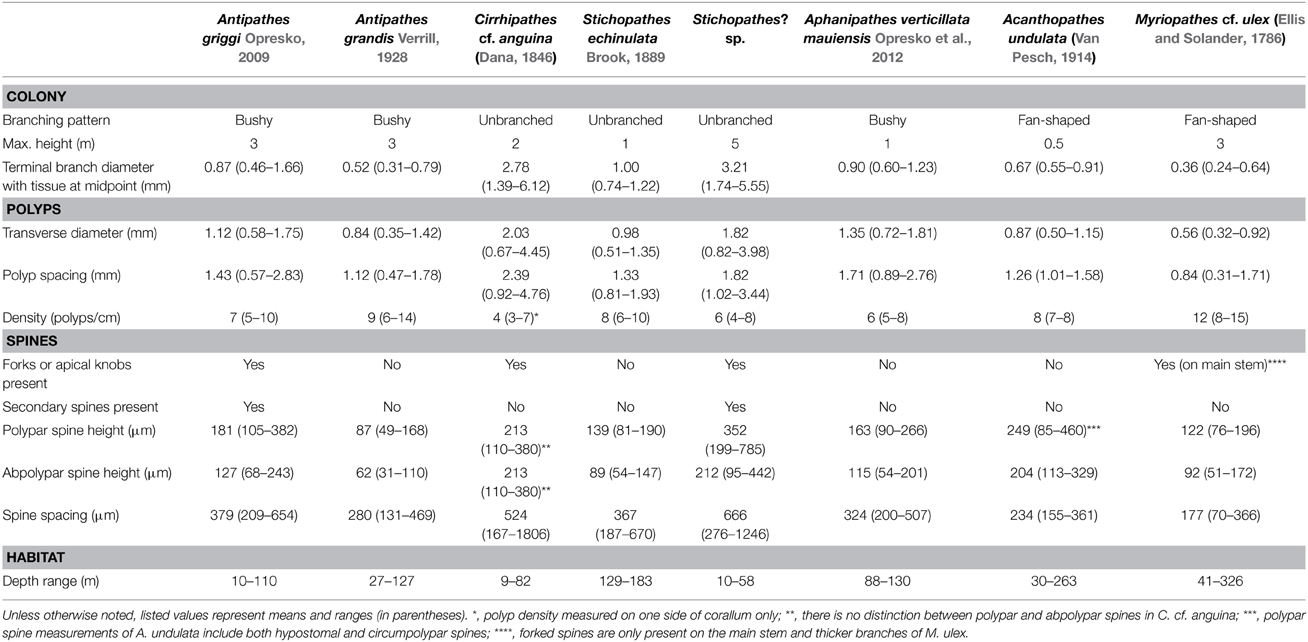
Like its sympatric congener Antipathes griggi, A. grandis (Figure 2) is also commercially harvested in Hawai‘i to supply the precious coral jewelry industry (reviewed by Grigg, 2001). A. grandis was the first antipatharian species described from Hawaiian waters (Verrill, 1928). A detailed taxonomic redescription of A. grandis is presented by Wagner et al. (2010), and briefly summarized here. Colonies can reach massive heights of over 3 m, and are extensively branched. Branches are long, distally-directed, and disposed irregularly on all sides of the corallum (Figures 2A–C). Terminal branchlets reach lengths of up to 10 cm without becoming subbranched and typically measure 0.5 mm in diameter with tissue at their midpoint (range = 0.31–0.79 mm). The spines on terminal branches are conical and never bifurcated toward their apex, and covered with circular to elongated oval-shaped tubercles over the distal half of their surface (Figures 2F,G). Polypar spines are slightly larger (range = 49–168 μm) than abpolypar spines (range = 31–110 μm) and both tend to be inclined distally (Figures 2E,F). There are no secondary spines present on any parts of the corallum. On branchlets and smaller branches spines are arranged in axial rows, with adjacent rows offset in a spiral pattern around the corallum (Figures 2E,F). On average, polyps are 0.84 mm in transverse diameter (range = 0.35–1.42 mm) and spaced 1.12 mm apart (range = 0.47–1.78 mm), resulting in 9 polyps per cm (range = 6–14). The color of living colonies ranges between red, pale-red and white (Figures 2B,C).
Figure 2. (A) Antipathes grandis holotype (BPBM 102); (B,C) colonies of A. grandis in situ; (D) preserved polyps on terminal branch under light microscopy (scale bar = 1 mm); (E) skeletal spines on terminal branch under light microscopy (scale bar = 100 μm); (F) skeletal spines on terminal branch under SEM (scale bar = 100 μm); (G) close-up of polypar spine under SEM (scale bar = 25 μm). (B,C Photos courtesy of HURL).
A. grandis was originally described from off Maui (Verrill, 1928), and subsequently reported throughout the Main Hawaiian Islands from Hawai‘i to Ni‘ihau at depths between 27 and 127 m (Wagner et al., 2010). Additionally, there are two reports of this species from China (Zhou and Zou, 1984; Zou and Zhou, 1984), however, these records cannot be confirmed until specimens from that locality are examined. Furthermore, a morphologically similar species, identified as Antipathes sp., has been been reported from Indonesia (Lapian et al., 2007; Tazioli et al., 2007).
Genus Cirrhipathes (Blainville, 1834)
The genus Cirrhipathes was originally established to differentiate antipatharian taxa with unbranched colonies from those with branched ones (Blainville, 1834). Later, Brook (1889) created Stichopathes, another genus with unbranched colonies, and used polyp arrangement as the diagnostic feature to differentiate between Cirrhipathes and Stichopathes. Polyps are arranged irregularly on all sides of the corallum in Cirrhipathes, whereas polyps are positioned in a single row on one side of the corallum in Stichopathes (Brook, 1889; Bo and Opresko, 2015). More recently, a third genus, Pseudocirrhipathes, has been established for yet another group of antipathids with unbranched colonies (Bo et al., 2009). Like Cirrhipathes, Pseudocirrhipathes also has polyps arranged irregularly on all sides of the corallum, but differs by having spines with distinct tubercles that are arranged in verticils, and tentacles that cannot completely contract (Bo et al., 2009). As a result, Cirrhipathes is now characterized by (1) unbranched colonies, (2) polyps that are arranged irregularly on all sides of the corallum, and (3) spines that are not arranged in verticils (Brook, 1889; Silberfeld, 1909; Summers, 1910; Van Pesch, 1914; Zou and Zhou, 1982, 1984; Echeverria, 2002; Moon and Song, 2008a; Bo et al., 2009).
Cirrhipathes cf. anguina (Dana, 1846)
Cirrhipathes sp.: (Davis and Cohen, 1968, pp. 749–761, Figures 1(top)–2); Cirrhipathes anguina: (Grigg and Opresko, 1977, pp. 242–261, Figure 4); Cirrhipathes anguina: (Grigg, 1993, p. 50); Antipathes anguina: (Grigg, 1993, p. 56); Cirrhipathes anguina: (Montgomery and Crow, 1998, pp. 103–108); Cirrhipathes sp.: (Coles et al., 1998, p. 24); Cirrhipathes anguina: (Greenfield and Randall, 2004, pp. 513–514, Figure 55); Cirrhipathes sp.: (Maragos et al., 2004, p. 230); Cirrhipathes anguina: (Fenner, 2005, p. 97, unnumbered figure on p. 97); Cirrhipathes anguina: (Hoover, 2006, p. 71, Figures a,b); Cirrhipathes anguina: (Parrish and Baco, 2007, pp. 159, 185); Cirrhipathes sp.: (Wagner et al., 2010, pp. 270–291, Figure 10); Cirrhipathes cf. anguina: (Wagner et al., 2011b: pp. 1323–1328, Figure 2d); Cirrhipathes cf. anguina: (Wagner et al., 2011c: pp. 211–225, Figures 1F,G); Cirrhipathes anguina TMKO-111/-113/-114: (Brugler et al., 2013, pp. 312–361, Figures 2–5); Cirrhipathes cf. anguina: (Wagner et al., 2014, p. 4).
Davis and Cohen (1968) published the first account of Cirrhipathes cf. anguina from Hawai‘i (Figure 3) as part of a description of the associated fauna of this wire coral: a gobiid fish and a palaemonid shrimp. Later descriptions of this species also highlighted these characteristic faunal associates of the wire coral (Greenfield and Randall, 2004; Fenner, 2005; Hoover, 2006). Based on previous literature accounts and specimens examined as part of this study (Table 1), the following features characterize C. cf. anguina. Colonies are unbranched and can reach vertical heights of 2 m or more. The corallum is usually straight in small colonies, and becomes irregularly sinusoidal in larger colonies (Figures 3B–E). The corallum diameter is generally 1.4–6.1 mm at midheight. Polyps are of variable size, ranging from 0.65 to 4.45 mm in transverse diameter, and are arranged irregularly on all sides of the corallum (Figures 3B–F). The spacing between adjoining polyps varies between 0.92 and 4.76 mm. The color of coenenchyme is typically brown with yellow or green tentacles, but the coloration of tentacles varies between yellow, green, red, white and pink (Figures 3B–E). Skeletal spines are conical in shape, some of which have bifurcations toward the apex, and are covered with circular to elongated oval-shaped tubercles over the distal half of their surface (Figure 3H). Spines are generally 110–380 μm tall, and arranged in regular rows, with adjoining rows offset in a spiral pattern around the corallum (Figures 3G,H). Within a row, spines spacing is highly variable and ranges between 167 and 1806 μm.
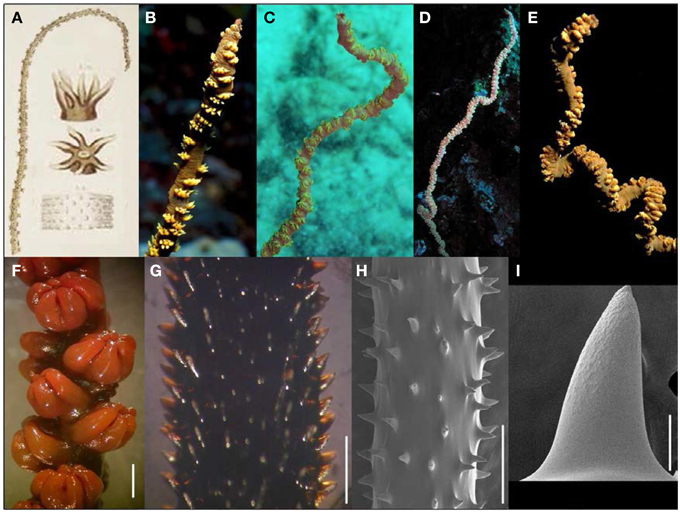
Figure 3. (A) Plate accompanying Dana's (1846) original description of Cirrhipathes anguina, a species for which the type material is now lost. (B–E) In situ photographs of Cirrhipathes cf. anguina colonies from Hawaiian waters; (F) preserved polyps of Hawaiian species under light microscopy (scale bar = 1 mm); (G) spines of Hawaiian species under light microscopy (scale bar = 1 mm); (H,I) spines of Hawaiian species under scanning electron microscopy (scale bars: H = 1 mm; I = 50 μm). (B–E Photos courtesy of Jim Maragos).
This Hawaiian wire coral species has previously been identified as Cirrhipathes anguina Dana, 1846 (Grigg and Opresko, 1977), a species originally described from the reefs off Vanua Lebu Island in Fiji (Dana, 1846). The description of C. anguina is rather brief and highlights yellowish tentacles that are brownish-gray at their base, and spines that are laterally compressed and sub-acute, features that are also evident in the Hawaiian specimens examined as part of this study (Figure 3). Unfortunately, the type material of C. anguina is lost, and therefore no further comparisons can be made until a neotype is designated. Pending such a taxonomic revision, the name Cirrhipathes cf. anguina is used to refer to the Hawaiian wire coral described here (Figure 3).
Specimens examined as part of this study were collected throughout the Hawaiian Islands including the islands of Maui, O‘ahu, Kaua‘i, Ni‘ihau, French Frigate Shoals, Necker, and Laysan at depths between 9 and 82 m, as well as off Johnston Atoll at 47 m and off Palmyra Atoll at 43 m (Table 1). C. anguina has previously been reported throughout the Indo-West Pacific at depths ranging between 2 and 158 m (Dana, 1846; Gray, 1857; Brook, 1889; Cooper, 1903, 1909; Van Pesch, 1914; Pax, 1932; Tsuda et al., 1977; Humes, 1979; Bruce, 1982; Zou and Zhou, 1982, 1984; Heard, 1986; Okiyama and Tsukamoto, 1989; Montgomery and Crow, 1998; Okuno, 1998; Jones et al., 2000; Paulay et al., 2003; Greenfield and Randall, 2004; Parrish and Baco, 2007; Rogers et al., 2007; Bo, 2008; Moon and Song, 2008a). However, a thorough taxonomic survey will be needed to determine whether these Indo-West Pacific records correspond to the same Hawaiian species described here.
Genus Stichopathes Brook, 1889
Like Cirrhipathes, the genus Stichopathes is characterized by unbranched colonies (see above). Brook (1889) established polyp arrangement as the main diagnostic feature to distinguish between these two unbranched genera. Polyps are arranged irregularly on all sides of the corallum in Cirrhipathes (see above), whereas Stichopathes colonies have polyps that are arranged in a single row on one side of the corallum. However, the validity of these two genera has been questioned by several authors (Van Pesch, 1914; Pax, 1918; Pasternak, 1977; Bo, 2008; Bo et al., 2012). Pseudocirrhipathes, a third genus with unbranched colonies, was recently established for colonies with polyps that are arranged irregularly on all sides of the corallum, and spines that are positioned in verticills (Bo et al., 2009; see above). Further taxonomic revisions among unbranched antipathids are, however, needed (Van Pesch, 1914; Pax, 1918; Pasternak, 1977; Bo, 2008). Pending such revisions, polyp arrangement is the only character that distinguishes Stichopathes from the other two unbranched antipatharian genera Cirrhipathes and Pseudocirrhipathes (Blainville, 1834; Brook, 1889; Bo et al., 2009, 2012; Bo and Opresko, 2015).
Stichopathes echinulata Brook, 1889
Stichopathes echinulata: (Brook, 1889, pp. 92, Pl. XII Figure 9); Stichopathes cf. echinulata: (Grigg and Opresko, 1977, pp. 242–261); Stichopathes cf. echinulata: (Wagner et al., 2011b, p. 1325); Stichopathes echinulata: (Wagner et al., 2011c, pp. 211–225, Figures 1h, 2b).
Stichopathes echinulata was originally described from off Mauritius (Brook, 1889). The original species description is rather brief, but emphasizes spines that are short, triangular and distally inclined, and arranged in regular rows, with nine or 10 rows visible in one aspect (Brook, 1889). Based on comparisons with the original description of S. echinulata (Brook, 1889; Grigg and Opresko, 1977) reported a morphologically similar species from Hawaiian waters (Figure 4). At the time, Grigg and Opresko (1977) did not have S. echinulata type material available for comparison, and because the original species description is rather brief (Brook, 1889), they did not conclusively assign the Hawaiian species to S. echinulata. As part of this study, a small fragment of the S. echinulata holotype (USNM 100371) was examined under SEM (Figures 4A,B). The shape, size and arrangement of the spines of the holotype is very similar to the Hawaiian species examined here (Figures 4E–G). Consequently, the Hawaiian specimens are assigned to S. echinulata, and used to emend the description of the species as follows. Colonies are up to 1 m in height or more and coiled distally forming multiple spirals. At midheight, colonies typically measure 0.74–1.22 mm in diameter with tissue. Polyps are arranged in a single row on one side of the corallum, and spaced 0.81–1.93 mm apart, resulting in 6–10 polyps per cm (Figure 4D). Skeletal spines are arranged in regular rows, with adjoining rows offset in a spiral pattern around the corallum. Within a row, spines are typically spaced 187–670 μm apart. Spines are conical, inclined distally, never bifurcated toward their apex, and covered with oval-shaped tubercles over the biggest portion of their surface (Figures 4B,G). Polypar spines are generally 81–190 μm tall and abpolypar spines are typically 54–147 μm tall. The coloration of living colonies was only noted for a few samples that were recently collected for this study (Table 1), but were all light-brown. Colonies with similar overall morphologies but with white tissues have also been reported from Hawaiian waters (Figure 4H; Chave and Malahoff, 1998). Only a single colony with white tissues was examined as part of this study (Table 1; Figure 4H). However, its skeletal spines are substantially different from S. echinulata, in that its spines are more triangular, and only covered by faint tubercles toward the very tip of spines (Figures 4I,J), whereas S. echinulata has distinct tubercles on the biggest portion of its spines (Figures 4B,G). Furthermore, the skeletal spines of the white morphotype are smaller than S. echinulata, with polypar spines ranging between 99 and 142 μm and abpolypar spines varying between 42 and 137 μm. Unfortunately only a single specimen of the white morphotype was available for comparison, however, the substantial morphological differences in spine shape, indicate that it is a different species (Figures 4I,J).
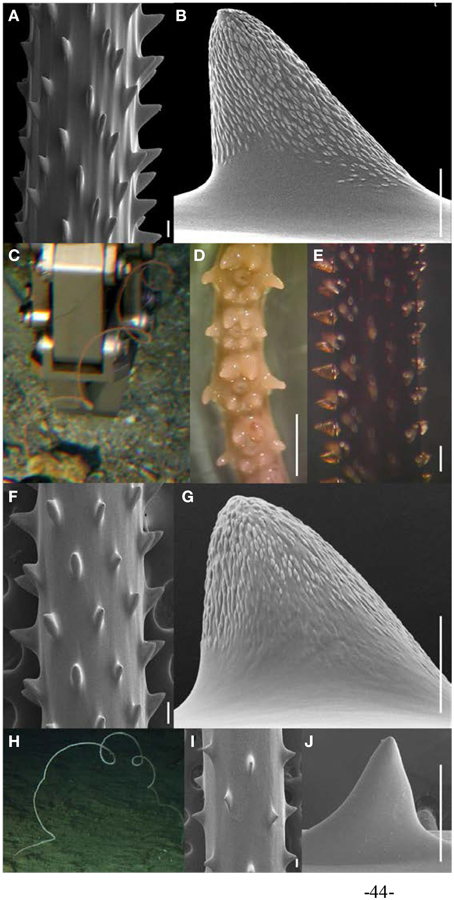
Figure 4. (A,B) Skeletal spines of Stichopathes echinulata holotype (USNM 100371) under SEM (scale bars = 100 μm). (C–G) Hawaiian S. echinulata colonies c. in situ; (D) preserved polyps under light microscopy (scale bar = 1 mm); (E) skeletal spines under light microscopy (scale bar = 200 μm); (F) skeletal spines under SEM (scale bars = 100 μm); and (G) close-up of polypar spine under SEM (scale bar = 50 μm). (H–J) Hawaiian Stichopathes sp. with white tissues (H) in situ; (I,J) Skeletal spines under SEM (scale bars = 100 μm). (C,H Photos courtesy of HURL).
S. echinulata was originally described from Mauritius (Brook, 1889), but subsequently reported from East Africa (Summers, 1910), the Seychelles (Cooper, 1909), and Madagascar (Humes, 1967). All Hawaiian specimens examined as part of this study were collected from the Main Hawaiian Islands off Hawai‘i, Lāna‘i, Maui and Kaua‘i in 108–198 m (Table 1).
Stichopathes? sp.
Unbranched species: (Grigg, 1964, p. 10); Stichopathes sp.: (Montgomery and Crow, 1998, pp. 103–108); Stichopathes cf. echinulata: (Fenner, 2005, p. 98, unnumbered figure on p. 98); Stichopathes cf. echinulata: (Hoover, 2006, p. 71, unnumbered figure on bottom of p. 71); Stichopathes sp.: (Wagner et al., 2011c, pp. 211–225, Figure 1I); Stichopathes clade D?: (Bo et al., 2012, pp. 1–13, Figures 3m,p, 5a–n); Stichopathes? sp.: (Wagner et al., 2014, pp. 3–4).
Within depths accessible through regular SCUBA diving (<40 m), two unbranched black corals can be found in Hawaiian waters in areas with high current flow and reduced light intensity: one with green or yellow polyps that are arranged irregularly on all sides of the corallum (Cirrhipathes cf. anguina; see above), and another with brown polyps that are arranged in a single row on one side of the corallum (Figure 5). The latter represents an unnamed species that has been assigned to the genus Stichopathes based on the arrangement of its polyps, which are always positioned in a single row on one side of the corallum (Montgomery and Crow, 1998; Fenner, 2005; Hoover, 2006). However, the skeletal spines of this Hawaiian wire coral are very different from other Stichopathes spp., which never have bifurcations or apical knobs toward their apex (Brook, 1889; Schultze, 1903; Roule, 1905; Thomson, 1905; Cooper, 1909; Summers, 1910; Van Pesch, 1914; Goenaga, 1977; Opresko and Genin, 1990; Opresko and Sanchez, 2005; Moon and Song, 2008a). In contrast, apical bifurcations are common on most of the spines of this Hawaiian wire coral species (Figures 5E–G). Therefore, the assignment to the genus Stichopathes is very questionable, and this Hawaiian wire coral may therefore represent both an undescribed genus and species. Colonies of this species are unbranched and can attain extreme lengths of up to 5 m (Grigg, 1964). The corallum of small colonies is relatively straight, and becomes more irregularly sinusoidal or spiraled in large colonies (Figures 5A–C). At midheight, colonies generally measure 1.74–5.55 mm in diameter with tissue. Polyps are arranged in a single row on one side of the corallum and are crowded together tightly (Figure 5D). On average, polyps measure 1.82 mm in transverse diameter (range = 0.82–3.98 mm) and are spaced 1.82 mm apart (range = 1.02–3.44 mm), resulting in 6 polyps per cm (range = 4–8). Skeletal spines are covered by elongated tubercles over the largest portion of their surface and are usually bifurcated toward their apex (Figures 5F,G). Polypar spines are distinctly larger on the polypar side, where they range between 199 and 785 μm in height, whereas abpolypar spines vary between 95 and 442 μm in height. Smaller, secondary spines (<100 μm) are present throughout the corallum (Figure 5G). The color of living colonies is greenish brown (Figures 5A–C).
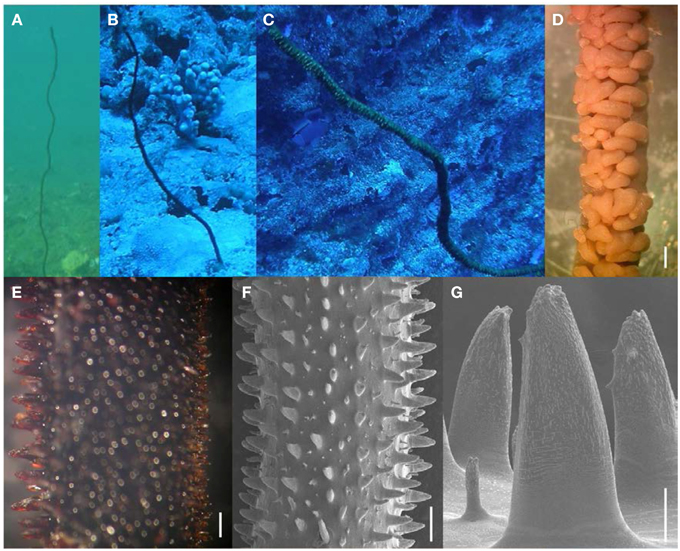
Figure 5. Stichopathes? sp. (A–C) in situ; (D) preserved polyps under light microscopy (scale bar = 1 mm); (E) skeletal spines under light microscopy (scale bar = 500 μm); (F) skeletal spines under SEM (scale bar = 500 μm); and (G) close-up of polypar spines under SEM (scale bar = 100 μm).
Specimens examined as part of this study were collected throughout the Hawaiian Islands from Maui to French Frigate Shoals at depths ranging between 10 and 58 m, as well as from Johnston Atoll at 61 m (Table 1). Based on the morphology of spines and polyps, a very similar species, referred to as Stichopathes clade D, is known from Indonesia (Bo et al., 2012), but specimens from that locality will have to examined to confirm those records.
Family Aphanipathidae Opresko, 2004
The Aphanipathidae is characterized by polyps with 10 mesenteries (six primary and four secondary), that are 0.5–1.3 mm in transverse diameter, and have short sagittal and lateral tentacles (shorted than the polyp diameter) that are nearly of the same length when fully expanded (Opresko, 2004; Opresko and Sanchez, 2005; Daly et al., 2007; Bo, 2008). Furthermore, the skeletal spines of the Aphanipathidae often penetrate through the soft tissues, are typically adorned with conical tubercles, and do not possess bifurcations toward their apex like many members of the Antipathidae (Opresko, 2004; Opresko and Sanchez, 2005; Daly et al., 2007; Bo, 2008). The family Aphanipathidae is divided into the two subfamilies Aphanipathinae and Acanthopathinae based on the relative sizes of skeletal spines in the area underneath a polyp (Opresko, 2004). The name of the family Aphanipathidae is derived from the Greek root aphano meaning invisible, in reference to its inconspicuous polyps which are often obscured through elongated spines that penetrate through the coenenchyme (Brook, 1889).
Subfamily Aphanipathinae Opresko, 2004
The subfamily Aphanipathinae is distinguished by having skeletal spines of consistently similar heights on the side of the corallum bearing the polyps (Opresko, 2004). In contrast, the subfamily Acanthopathinae (see below) has members whose skeletal spines are reduced in size in the areas directly below the oral opening (the hypostomal spines), and then give way to elongated spines in the areas underneath the outer edges of polyps (the circumpolypar spines). Spines of intermediate length are present between polyps in the Acanthopathinae (Opresko, 2004).
Genus Aphanipathes Brook, 1889
The genus Aphanipathes is characterized by colonies that are irregularly branched like a bush or broom, and skeletal spines that penetrate through the coenenchyme (Brook, 1889; Pax, 1932; Opresko and Baron-Szabo, 2001; Opresko, 2004).
Aphanipathes verticillata mauiensis Opresko et al., 2012
Undescribed Aphanipathidae: (Wagner et al., 2010, p. 274, Figure 10); Aphanipathes sp.: (Wagner et al., 2011b, p. 1325); Aphanipathes verticillata: (Wagner et al., 2011c, pp. 211–225, Figures 1j, 2c); Aphanipathes verticillata: (Wagner et al., 2012a, pp. 799, 804); Aphanipathes verticillata mauiensis: (Opresko et al., 2012, pp. 24–39, Figures 1b, 4a–d, 5a–d, 8a–d); Aphanipathes verticillata mauiensis: (Brugler et al., 2013, pp. 312–361, Figures 1–5).
During black coral surveys conducted off West Maui in 2008–2009, numerous specimens superficially resembling Antipathes griggi were collected (Opresko et al., 2012). Upon closer examination of the skeletal spines, these specimens proved to be morphologically very different from A. griggi, and similar to Aphanipathes verticillata Brook, 1889, a species never before reported from the Hawaiian Islands. Subsequent comparisons of the A. verticillata holotype to Hawaiian specimens revealed various morphological similarities, although the Hawaiian form is considered a distinct subspecies (A. verticillata mauiensis) due to unique features of its skeletal spines (Opresko et al., 2012). In particular, the tubercles on the skeletal spines of A. verticillata mauiensis occur in lower densites than on the holotype of this species, which has been assigned to the new subspecies A. verticillata verticillata Opresko et al., 2012. A detailed taxonomic description of A. verticillata mauiensis is presented by Opresko et al. (2012), and briefly summarized here. Colonies are up to 1 m in height or more, with up to 10 orders of branching. The branches are generally pointed straight upwards or slightly curved (Figures 6D–F). On terminal branchlets, polypar spines are typically 90–266 μm tall and abpolypar spines generally 54–201 μm in height. Skeletal spines are arranged in verticils with spines in the same row typically spaced 200–507 μm apart (Figures 6H–J). Spines are covered with distinct conical tubercles over the largest portion of their surface of both polypar and abpolypar spines. Polyps are on average 1.35 mm in transverse diameter (range = 0.72–1.81 mm), arranged on a single side of the corallum on terminal branches, and spaced 0.89–2.76 mm apart, resulting in 6 polyps per cm (range = 5–8) (Figure 6G).
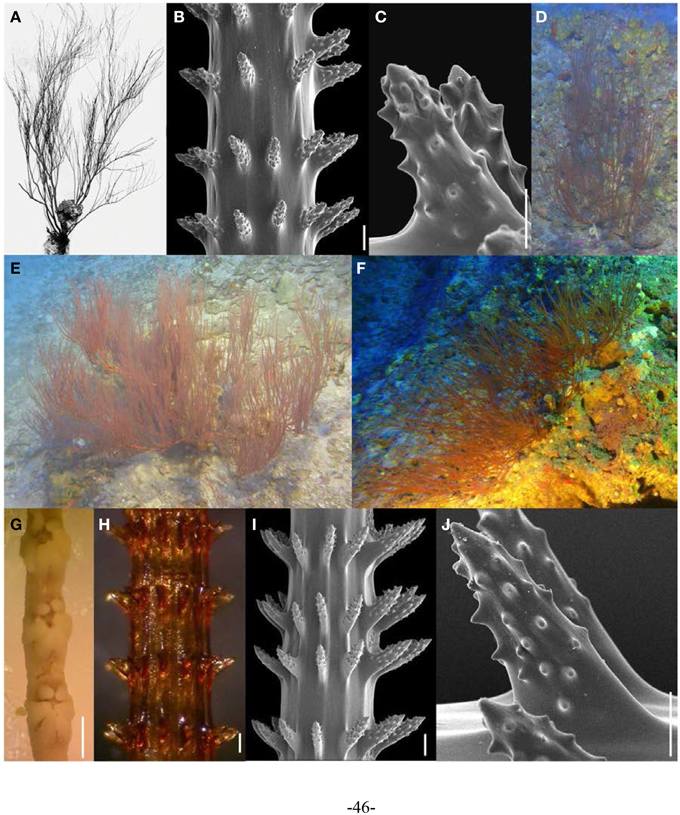
Figure 6. (A–C) Aphanipathes verticillata verticillata holotype (MCZ 68) showing (A). entire specimen, (B,C) skeletal spines under SEM (scale bars: (B) = 100; μm; C = 50 μm). (D–J) A. verticillata mauiensis specimens showing (D–F) colonies in situ, (G) preserved polyps on terminal branch under light microscopy (scale bar = 1 mm), (H) skeletal spines on terminal branch under light microscopy (scale bar = 100 μm), (I) skeletal spines on terminal branch under SEM (scale bar = 100 μm), and (J) close-up of polypar spine under SEM (scale bar = 50 μm). (D–F Photos courtesy of HURL).
A. verticillata was originally described from off Mauritius (Brook, 1889). Later, two other A. verticillata specimens were collected off Okinawa at a depth of 79 m (Table 1). All Hawaiian specimens examined as part of this study were collected at depths between 88 and 130 m in the the Au‘Au Channel, located between the islands of Maui and Lāna‘i (Table 1).
Subfamily Acanthopathinae Opresko, 2004
The subfamily Acanthopathinae is characterized by skeletal spines of different heights in the areas underneath polyps. Hypostomal spines are reduced in size, whereas circumpolypar spines are substantially elongated. Spines of intermediate length are present between polyps in the Acanthopathinae (Opresko, 2004). The name of this subfamily is derived from the Greek root acantho meaning spiny, in reference to the enlarged size of its circumpolypar spines (Opresko, 2004).
Genus Acanthopathes Opresko, 2004
The genus Acanthopathes is characterized by colonies that are branched in a single plane like a fan, and spines that are either greatly reduced or absent in the areas directly below the oral cone (Opresko, 2004).
Acanthopathes undulata (Van Pesch, 1914)
Aphanipathes undulata: (Van Pesch, 1914, pp. 87–89, Figures 74–76, Pl. VIII Figure 8); Antipathes undulata: (Grigg and Opresko, 1977, pp. 242–261, Figure 5); Acanthopathes undulata: (Opresko, 2004, p. 232); Acanthopathes undulata: (Eldredge, 2006, p. 65); Acanthopathes undulata: (Parrish and Baco, 2007, p. 186); Acanthopathes undulata: (Wagner et al., 2011b, p. 1325).
Grigg and Opresko (1977) were the first to identify Antipathes undulata (Van Pesch, 1914) from the Hawaiian Archipielago (Figure 7). In 2004, Opresko reassigned A. undulata to the newly established antipatharian family Aphanipathidae and the new genus Acanthopathes, resulting in the new name Acanthopathes undulata. Van Pesch's (1914) original description of this species emphasized (1) colonies that are fan-shaped and planar, (2) spines that are distally inclined, needle-like, covered with minute tubercles toward the distal half, generally 375–450 μm tall, spaced 150–225 μm apart, and penetrated through the tissues of the polyps, and (3) polyps that are arranged on one side of the colony and spaced 1.1 mm apart. These features are all consistent with the Hawaiian specimens examined here (Figure 7). Additionally, a fragment of the holotype was examined under SEM (USNM 100409), and its spines are also very similar to the Hawaiian specimens in terms of shape, arrangement and size (Figures 7D,J,K). Furthermore, this comparison also highlights the differences in relative sizes of the skeletal spines on the side of the branch bearing the polyp, being greatly reduced underneath the oral cone, and enlarged toward the outer edges of polyps (Figures 7I,J). Collectively, the comparison of A. undulata type material to Hawaiian specimens corroborates previous identifications of this species from Hawaiian waters (Grigg and Opresko, 1977; Chave and Malahoff, 1998; Eldredge, 2006; Parrish and Baco, 2007). Additionally, this comparison revealed several characteristic features of the species, which have previously not been described. Colonies are fan-shaped, typically smaller than 50 cm in height, and are extensively branched giving the appearance of a net (Figures 7E–H). Polyps are arranged on a single side of fan-shaped colonies, are typically 0.87 mm in transverse diameter (range = 0.50–1.15 mm) and spaced 1.26 mm apart (range = 1.01–1.58 mm), resulting in 8 polyps per cm (range = 7–8) (Figures 7C,I). The spines are needle-like in shape and penetrate through the soft tissues, except for the area underlying the oral cone (Figures 7C,I). On the side of the fan not bearing the polyps, spines are uniform in height and average 204 μm (range = 113–329 μm). In contrast, the side of the colony bearing polyps contains skeletal spines of varying lengths (Figure 7J). The tallest spines are located in the area underneath the outer edges of polyps and reach heights of 270–459 μm, whereas the shortest spines are situated in the area underneath the oral cone and reach heights of 85–257 μm. Skeletal spines are smooth or gently adorned with fine papillae toward the distal portion of the spine (Figures 7D,K). Spines are arranged in regular rows with adjoining rows being offset in a spiral pattern around the corallum (Figure 7J). Within a row, spine spacing is typically variable and ranges between 155 and 361 μm. The color of living colonies is grayish-white, and is influenced by the skeletal spines that penetrate through tissues and give colonies a brownish hint (Figures 7E,F).

Figure 7. (A,B) Plates accompanying the original description of Acanthopathes undulata (Van Pesch, 1914); (C) spines of schizoholotype (USNM 100409) under light microscopy (scale bar = 1 mm); (D) spines of schizoholotype under SEM (scale bar = 100 μm); (E,F) in situ photographs of colonies from Hawaiian waters; (G,H) specimens from Hawai‘i (scale bars = 1 cm); (I) spines and polyps on terminal branch under light microscopy (scale bar = 1 mm); (J) spines on terminal branch under SEM (scale bar = 100 μm); (K) close-up of spine under SEM (scale bar = 30 μm). (E,F Photos courtesy of HURL).
Acanthopathes undulata was originally described from specimens collected at 113 m in the Solor Strait off Indonesia (Van Pesch, 1914), and later reported from Hawai‘i (Grigg and Opresko, 1977; Chave and Malahoff, 1998; Eldredge, 2006; Parrish and Baco, 2007) and the Mariana Islands (Paulay et al., 2003). All Hawaiian specimens examined as part of this study were collected throughout the Hawaiian Islands from Hawai‘i to Laysan at depths ranging between 30 and 269 m (Table 1).
Family Myriopathidae Opresko, 2001
The Myriopathidae are characterized by polyps with 10 mesenteries (six primary and four secondary), that are 0.5–1.0 mm in transverse diameter and possess short tentacles with rounded tips. The skeletal spines of the Myriopathidae are usually blade-like or needle-like on smaller branches, and frequently forked or antler-like on the main stem and larger branches (Opresko, 2001; Opresko and Sanchez, 2005; Daly et al., 2007; Bo, 2008; Moon and Song, 2008b). The name of the Myriopathidae is derived from the Greek word myriophylla meaning many branches, in reference to the extensive branching of colonies within this family.
Genus Myriopathes Opresko, 2001
The genus Myriopathes contains colonies, whose highest order branches are pinnulated, i.e., they contain ramnifications that are arranged symmetrically in a plane like a fern. Furthermore, Myriopathes pinnules are themselves always branched giving rise to secondary and tertiary subpinnules (Opresko, 2001; Moon and Song, 2008b).
Myriopathes cf. ulex (Ellis and Solander, 1786)
Antipathella sp.: (Grigg, 1964, pp. 11, 14, Figure 5); Antipathes ulex: (Grigg and Opresko, 1977, p. 244); Antipathes ulex: (Grigg, 1993, pp. 50, 56); Antipathes ulex: (Montgomery and Crow, 1998, pp. 103–108); Antipathes ulex: (Chave and Malahoff, 1998, p. 40, Figure 93); Myriopathes ulex: (Opresko, 2001, pp. 349, 351–352); Antipathes ulex: (Montgomery, 2002, pp. 157–164); Myriopathes ulex: (Fenner, 2005, p. 101, 2 unnumbered figure on p. 101); Antipathes ulex: (Boland and Parrish, 2005, pp. 411–420); Myriopathes ulex: (Eldredge, 2006, p. 65); Myriopathes ulex: (Hoover, 2006, p. 70, unnumbered figure on p. 70); Myriopathes ulex: (Parrish and Baco, 2007, pp. 162–163, 170, 186); Myriopathes ulex: (Kahng and Kelley, 2007, p. 684); Myriopathes ulex: (Bo, 2008, unnumbered figure in app. 1); Antipathes ulex: (Grigg, 2010, p. 3); Myriopathes ulex: (Wagner et al., 2011a, pp. 249–255, Figure 3); Myriopathes ulex: (Wagner et al., 2011b, p. 1325); Myriopathes ulex: (Wagner et al., 2011c, pp. 212, 214); Myriopathes ulex: (Wagner et al., 2012a, p.796); Myriopathes ulex: (Wagner et al., 2012a, pp. 67–132, Figure 2.1b); Myriopathes ulex: (Wagner et al., 2013, pp. 341–342, Figure 1 left); Myriopathes ulex: (Wagner et al., 2014, pp. 4, 8).
Along with Antipathes griggi and A. grandis, Myriopathes cf. ulex (Figure 8) is the third antipatharian species that has been targeted by the Hawaiian black coral fishery (Grigg, 1993, 2010; Wagner et al., 2011a). However, in comparison to A. griggi and A. grandis, harvesting of M. cf. ulex is much less frequent, because the species is quite rare in the 40–75 m depth zone where black coral harvesting takes place in Hawai‘i (Grigg, 2001, 2002; Boland and Parrish, 2005). Grigg (1964) presented the first published account of M. cf. ulex from Hawai‘i (as Antipathella sp.). Since then, brief descriptions of this Hawaiian species were presented by Chave and Malahoff (1998), Fenner (2005) and Hoover (2006). The main distinguishing features of this species, are its large (up to 3 m), fan-shaped colonies that consist of small feather-like branchlets or pinnules (Figures 8A–C). This characteristic branching pattern gives rise to the common name of this species: feathery black coral. Polyps are on average 0.56 mm in transverse diameter (range = 0.32–0.92 mm), and generally spaced 0.84 mm apart (range = 0.31–1.71) resulting in 12 polyps per cm (range 8–15). On the highest order branches, spines are blade- to needle-like in shape and inclined distally (Figures 8F–H), with polypar spines generally 122 μm in height (range = 76–196 μm), and abpolypar spines typically 92 μm in height (range = 51–172 μm). Spines are smooth or covered with faint papillae, and are arranged in regular rows on the highest order branches, with spines in the same row typically spaced 177 μm apart (range = 70–366 μm). On the main stem and thicker branches, many spines are forked at their apex.
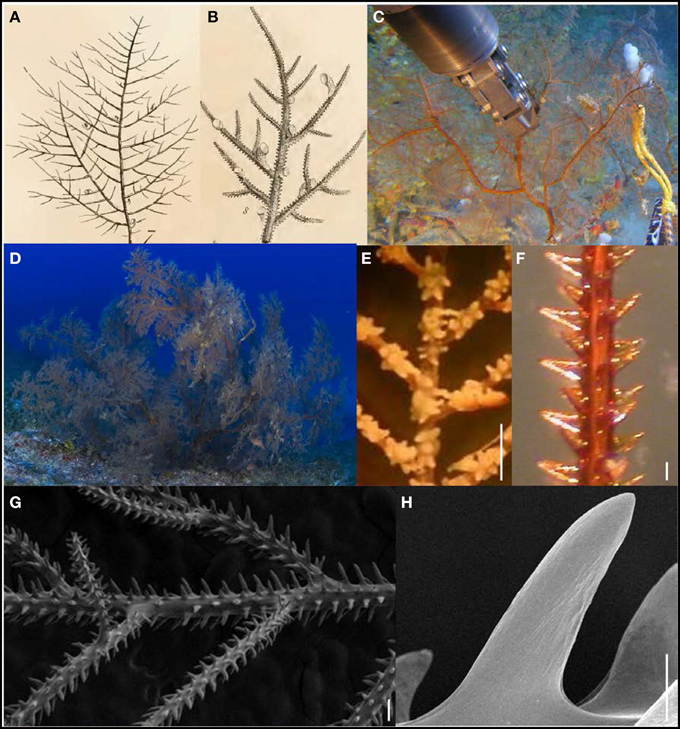
Figure 8. (A,B) Plates accompanying the original description of Myriopathes ulex (Ellis and Solander, 1786), a species for which the type material is now lost. (C,D) In situ photographs of colonies from Hawaiian waters; (E) preserved polyps of Hawaiian species under light microscopy (scale bar = 1 mm); (F) spines on terminal branch under light microscopy (scale bar = 100 μm); (G) spines on terminal branch under SEM (scale bar = 200 μm); (H) close-up of polypar spine under SEM (scale bar = 50 μm). (Photos courtesy of C HURL, and D Greg McFall).
Grigg and Opresko (1977) were the first to identify Antipathes ulex Ellis and Solander, 1786 from Hawaiian waters. In 2001, Opresko reassigned A. ulex to the newly established antipatharian family Myriopathidae and the new genus Myriopathes, resulting in the name Myriopathes ulex (Opresko, 2001; Eldredge, 2006). The original species description is rather brief and highlighted short skeletal spines and numerous epibionts including barnacles, which Ellis and Solander (1786) incorrectly identified as the ovaries of the black coral. The two plates accompanying the species description show a planar and pinnulated branching pattern (Figures 8A,B), features that are also characteristic of the Hawaiian specimens examined here (Figures 8C–E). Unfortunately, the type material of M. ulex has been lost (Opresko, 2001), and therefore no further comparisons can be made until a neotype is designated. Pending such taxonomic revisions, the name Myriopathes cf. ulex is used to refer to the Hawaiian specimens examined here, which were collected throughout the Hawaiian Archipelago from Hawai‘i Island to Pearl and Hermes Atoll at depths between 41 and 326 m, as well as off Johnston Atoll at 61 m (Table 1). Myriopathes ulex was originally described from Indonesia (Ellis and Solander, 1786), but subsequently reported throughout the Indo-West Pacific at depths ranging between 25 and 364 m (Blainville, 1834; Gray, 1857; Brook, 1889; Van Pesch, 1914; Grigg and Opresko, 1977; Colin and Arneson, 1995; Chave and Malahoff, 1998; Parrish and Baco, 2007; Rogers et al., 2007; Bo, 2008; Moon and Song, 2008b). However, a thorough taxonomic investigation is needed to verify whether all of these records correspond to the same species that is present in Hawaiian waters.
Species Key of Hawaiian Shallow-Water Antipatharians
1a. Colonies unbranched (wire like)................................................2
1b. Colonies branched.......................................................................4
2a. Polyps in multiple rows....................... Cirrhipathes cf. anguina
2b. Polyps in a single row..................................................................3
3a. Polyps ~1 mm in diameter with adjacent polyps separated by a well defined interpolypar space............................................................... Stichopathes echinulata
3b. Polyps ~1.85 mm in diameter with adjacent polyps crowded together tightly........................................ Stichopathes? sp.
4a. Colonies bushy.............................................................................5
4b. Colonies fan-shaped....................................................................7
5a. Spines arranged in verticils around thinner branches....................................Aphanipathes verticillata mauiensis
5b. Spines not arranged in verticils around thinner branches.....6
6a. Some spines with bifurcation on apex and secondary spines (≤40 μm tall) present on thicker branches................................................................... Antipathes griggi
6b. Spines without bifurcations on apex, and no secondary spines present on any parts of the corallum.....Antipathes grandis
7a. Colonies small (<0.5 m) and densely reticulated giving the appearance of a net..............................Acanthopathes undulata
7b. Colonies large (<3 m) with highest order branches forming symmetric pinnules like a fern.........Myriopathes cf. ulex
Conclusion
The use of traditional taxonomic characters, including branching pattern, polyp and skeletal spine morphology, provides a comprehensive basis for the classification of the shallow-water Hawaiian black coral fauna, an assemblage that has been previously grouped due to taxonomic constraints in ecological surveys. As a result, potential ecological differences amongst various antipatharian species have not been identified, which is critical information for the management of the Hawaiian black coral fishery. At least three different species with different habitat preferences have been targeted by the Hawaiian black coral fishery (Antipathes griggi, A. grandis, and Myriopathes cf. ulex); however, the fishery has historically been managed as a single stock, in large part due to difficulties in identifying the targeted species. In addition to fishery management applications, the combined use of various morphological characters may help in future systematic studies among the Antipatharia, a taxonomic order that has been notoriously problematic.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
I thank Dennis Opresko for taxonomic assistance, and Rob Toonen, Chris Kelley, Jeff Drazen, Les Watling, and Mark Merrifield for providing insightful reviews and comments on an earlier version of this manuscript, which was submitted as part of a Ph.D. thesis at the University of Hawai‘i. Help with sample collections was provided by the captain and crew of R/V Kaimikai-o-Kanaloa and R/V Hi‘ialakai, and by Terry Kerby, Max Cremer, Randy Kosaki, Jason Leonard, Brian Hauk, Greg McFall, Kelly Gleason, Tony Montgomery, Rich Pyle, Rob Whitton, Cori Kane, Chantel Chang, Keo Lopes, Paul Murakawa, Ken Longenecker, Linda Marsh, Joe Heacock, Scott Reed, Chris Kelley, Yannis Papastamatiou, Kyle Ryan, Ray Boland, John Rooney, Frank Parrish, Joey DiBattista, Sam Kahng, Jeff Eble, Zach Caldwell, Kydd Pollock, Robin Lee, Jill Quaintance, Dave Pence, Kevin Flanagan, Derek Smith, Keoki Stender, Kaylyn McCoy, Brian Bowen, Michelle Gaither, and Matt Iacchei. I thank the staff of the National Museum of Natural History, Smithsonian Institution, for providing help in accessing material housed in their invertebrate collections, and in particular to Stephen Cairns, Cheryl Bright, Geoff Keel, and Bill Moser for their hospitality and support. Special thanks to Holly Bolick for facilitating work with the collections housed at the Bernice P. Bishop Museum. Help with the analyses using scanning electron microscopy was provided by Tina Carvalho, Marylin Dunlap, and Scott Whittacker. This work was funded in part by the Western Pacific Regional Fisheries Management Council (NA07NMF4410114 to the University of Hawai‘i through NOAA's Coral Reef Conservation Grant Program), NOAA's Deep-Sea Coral Research and Technology Program and NOAA's Office of National Marine Sanctuaries, through the Papahānaumokuākea Marine National Monument. The scientific results and conclusions, as well as any views or opinions expressed herein, are those of the author and do not necessarily reflect the views of NOAA or the Department of Commerce.
References
Baco, A. R. (2007). Exploration for deep-sea corals on North Pacific seamounts and islands. Oceanography 20, 108–117. doi: 10.5670/oceanog.2007.11
Bo, M. (2008). Taxonomy and Ecology of Antipatharians. Ph.D. thesis. Universita Politecnica Delle, Marche: Italy.
Bo, M., Barucca, M., Biscotti, M. A., Canapa, A., Lapian, H. F. N., Olmo, E., et al. (2009). Description of Pseudocirrhipathes (Cnidaria: Anthozoa: Hexacorallia: Antipathidae), a new genus of whip black coral from the Indo-Pacific. Ital. J. Zool. 76, 392–402. doi: 10.1080/11250000802684104
Bo, M., Bavestrello, G., Barruca, M., Makapedua, D. M., Poliseno, A., Forcort, M., et al. (2012). Morphological and molecular characterization of the problematic whip coral genus Stichopathes (Hexacorallia: Antipatharia) from Indonesia (North Sulawesi, Celebes Sea). Zool. J. Linn. Soc. 166, 1–13. doi: 10.1111/j.1096-3642.2012.00834.x
Bo, M., and Opresko, D. M. (2015). Redescription of Stichopathes pourtalesi Brook, 1889 (Cnidaria: Anthozoa: Antipatharia: Antipathidae). Breviora 540, 1–18. doi: 10.3099/MCZ16.1
Boland, R. C., and Parrish, F. A. (2005). A description of fish assemblages in the black coral beds off Lahaina, Maui, Hawai‘i. Pac. Sci. 59, 411–420. doi: 10.1353/psc.2005.0032
Brook, G. (1889). Report on the Antipatharia. Report of the scientific results of the voyage of the H.M.S. Challenger. Zoology 32, 1–222.
Bruce, A. J. (1982). The pontoniine shrimp fauna of Australia. Austr. Mus. Mem. 18, 195–218. doi: 10.3853/j.0067-1967.18.1984.385
Brugler, M. R., Opresko, D. M., and France, S. C. (2013). The evolutionary history of the order Antipatharia (Cnidaria: Anthozoa: Hexacorallia) as inferred from mitochondrial and nuclear DNA: implications for black coral taxonomy and systematics. Zool. J. Linn. Soc. 169, 312–361. doi: 10.1111/zoj.12060
Chave, E. H., and Malahoff, A. (1998). In Deeper Waters: Photographic Studies of Hawaiian Deep-Sea Habitats and Life-Forms. Honolulu, HI: University of Hawai‘i Press.
Coles, S. L., DeFelice, R. C., Smith, J. E., Muir, D., and Eldredge, L. G. (1998). Determination of Baseline Conditions for Introduced Marine Species in Nearshore Water of the Island of Kaho‘olawe, Hawaii. Bishop Museum Technical Report No. 14. Bernice Pauahi Bishop Museum, Hawaii Biological Survey. Honolulu, HI.
Colin, P. L., and Arneson, C. (1995). Tropical Pacific Invertebrates -a Field Guide to the Marine Invertebrates occurring on Tropical Pacific Coralreefs, Seagrass Beds and Mangroves. Beverly Hills, CA: Coral Reef Press.
Cooper, C. F. (1903). “Antipatharia,” in The Fauna and Geography of the Maldive and Laccadive Archipelagoes, ed J. S. Gardiner (Cambridge: University Press), 791–796.
Cooper, C. F. (1909). Reports of the percy sladen trust expedition on the Indian Ocean, 1905. Antipatharia. Trans. Linn. Soc. Lon. Zool. Ser. 12, 301–321. doi: 10.1111/j.1096-3642.1909.tb00144.x
Daly, M., Brugler, M. R., Cartwright, P., Collins, A. G., Dawson, M. N., Fautin, D. G., et al. (2007). The phylum Cnidaria: a review of phylogenetic patterns and diversity 300 years after Linnaeus. Zootaxa 1668, 127–182.
Dana, J. D. (1846). Zoophytes. U.S. Exploring Expedition during the years. 1838-42. 7, 740. doi: 10.5962/bhl.title.70845
Davis, W. P., and Cohen, D. M. (1968). A gobiid fish and a paemonid shrimp living on an antipatharian sea whip in the tropical Pacific. Bull. Mar. Sci. 18, 749–761.
Echeverria, C. A. (2002). Black corals (Cnidaria: Anthozoa: Antipatharia): first records and a new species from the Brazilian coast. Rev. Biol. Trop. 50, 1067–1077.
Ehrenberg, C. G. (1834). Beiträge zur Kenntniß der Corallenthiere im allgemeinen, und besonders des rothen Meeres, nebst einem Versuche zur physiologischen Systematik derselben. Abh. K. Akad. Wiss. Berlin 1832, 225–380.
Eldredge, L. G. (2006). Numbers of Hawaiian species for 2003-2005. Bishop Mus. Occasion. Papers 88, 62–79.
Ellis, J., and Solander, D. (1786). The Natural History of Many Curious and Uncommon Zoophytes Collected by the Late John Ellis, Systematically Arranged and Described by the Late Daniel Solander. London: White and Son.
Goenaga, C. (1977). Two New Species of Stichopathes (Zoantharia; Antipatharia) with Observations on Aspects of their Biology. MS thesis, University of Puerto Rico: Puerto Rico.
Grange, K. R. (1988). Redescription of Antipathes aperta, Totton, (Coelenterata: Antipatharia), an ecological dominant in the southern fiords of New Zealand. NZ. J. Zool. 15, 55–61. doi: 10.1080/03014223.1988.10422609
Gray, J. E. (1857). Synopsis of the families and genera of axiferous zoophytes or barked corals. Pro. Zool. Soc. Lond. 25, 278–294. doi: 10.1111/j.1096-3642.1857.tb01242.x
Greenfield, D. W., and Randall, J. E. (2004). The marine gobies of the Hawaiian Islands. Proc. Calif. Acad. Sci. 55, 498–549.
Grigg, R. W. (1964). A Contribution to the Biology and Ecology of the Black Coral, Antipathes Grandis in Hawai‘i. MS thesis, University of Hawai‘i: USA.
Grigg, R. W. (1974). “Distribution and abundance of precious corals,” in Proceedings of the Second International Coral Reef Symposium, Vol. 2 (Brisbane), 235–240.
Grigg, R. W. (1975). The Commercial Potential of Precious Corals in the Western Caroline Islands, Micronesia. Sea Grant Technical Report. Honolulu, HI: UNIHI-SEAGRANT-AR-7503. 16.
Grigg, R. W. (1976). Fishery Management of Precious and Stony Corals in Hawai‘i. Sea Grant Technical Report. Honolulu, HI: UNIHI-SEAGRANT-TR-77-03. 48.
Grigg, R. W. (1984). Resource management of precious corals: a review and application to shallow water reef building corals. Mar. Ecol. 5, 57–74. doi: 10.1111/j.1439-0485.1984.tb00307.x
Grigg, R. W. (1993). Precious coral fisheries of Hawai‘i and the U.S. Pacific Islands. Mar. Fish. Rev. 55, 50–60.
Grigg, R. W. (2001). Black coral: history of a sustainable fishery in Hawai‘i. Pac. Sci. 55, 291–299. doi: 10.1353/psc.2001.0022
Grigg, R. W. (2002). Precious corals in Hawai‘i: discovery of a new bed and revised management measures for existing beds. Mar. Fish. Rev. 64, 13–20.
Grigg, R. W. (2003). Invasion of a deep coral bed by an alien species, Carijoa riisei, off Maui, Hawai‘i. Coral Reefs 22, 121–122. doi: 10.1007/s00338-003-0306-5
Grigg, R. W. (2004). Harvesting impacts and invasion by an alien species decrease estimates on black coral yield off Maui, Hawai‘i. Pac. Sci. 58, 1–6. doi: 10.1353/psc.2004.0006
Grigg, R. W. (2010). The precious corals fishery management plan of the Western Pacific Regional Fishery Management Council. Pac. Islands Fish. Monogr. 1, 1–9.
Grigg, R. W., Grossman, E. E., Earle, S. A., Gittings, S. R., Lott, D., and McDonough, J. (2002). Drowned reefs and antecedent karst topography, Au'au Channel, S. E. Hawaiian Islands. Coral Reefs 21, 73–82. doi: 10.1007/s00338-001-0203-8
Grigg, R. W., and Opresko, D. M. (1977). “Order Antipatharia, black corals,” in Reef and Shore Fauna of Hawaii Section 1: Protozoa through Ctenophora, eds D. M. Devaney and L. G. Eldredge (Honolulu, HI: Bishop Museum Press), 242–261.
Heard, R. W. (1986). Pontoniine shrimps (Decapoda: Caridea: Palaemonidae) of the Northwest Atlantic. I. The genus Neopontonides Holthuis, 1951, with the description of N. chacei, new species, and the erection of Pseudopontonides, new genus, to receive N. principis Criales, 1980. J. Crust. Biol. 6, 471–484. doi: 10.2307/1548186
Hoover, J. P. (2006). Hawai‘i’s Sea Creatures -a Guide to Hawai‘i’s Marine Invertebrates (revised edition). Honolulu, HI: Mutual Publishing.
Humes, A. G. (1967). Wahinius petax n. gen., n. sp., a cyclopoid copepod parasitic in an antipatharian coelenterate in Madagascar. Crustaceana 12, 233–242. doi: 10.1163/156854067X00198
Humes, A. G. (1979). Poecilostome copepods associated with antipatharian coelenterates in the Moluccas. Beaufortia 28, 113–120.
Jones, D. S., Hewitt, M. A., and Sampey, A. (2000). A checklist of the Cirripedia of the south China Sea. Raffles Bull. Zool. 8, 233–307.
Kahng, S. E., and Grigg, R. W. (2005). Impact of an alien octocoral, Carijoa riisei, on black corals in Hawai‘i. Coral Reefs 24, 556–562. doi: 10.1007/s00338-005-0026-0
Kahng, S. E., and Kelley, C. D. (2007). Vertical zonation of megabenthic taxa on a deep photosynthetic reef (50-140 m) in the Au'au Channel, Hawaii. Coral Reefs 26, 679–687. doi: 10.1007/s00338-007-0253-7
Lapian, H. F. N., Barucca, M., Bavestrello, G., Biscotti, M. A., Bo, M., Canapa, A., et al. (2007). A systematic study of some Black Corals species (Antipatharia, Hexacorallia) based on rDNA internal transcribed spacers sequences. Mar. Biol. 151, 785–792. doi: 10.1007/s00227-006-0525-8
Maragos, J. E., Potts, D. C., Aeby, G., Gulko, D., Kenyon, J., Siciliano, D., et al. (2004). 2000–2002 rapid ecological assessment of corals (Anthozoa) on shallow reefs of the Northwestern Hawaiian Islands. Part 1: species and distribution. Pac. Sci. 58, 211–230. doi: 10.1353/psc.2004.0020
Molodtsova, T. N., and Pasternak, F. A. (2005). Redescription of Parantipathes euantha (Pasternak, 1958) (Anthozoa: Antipatharia) from Kurile-Kamchatka Trench. Inverteb. Zool. 2, 169–179.
Montgomery, A. D. (2002). The feasibility of transplanting black coral (Order Antipatharia). Hydrobiologia 471, 157–164. doi: 10.1023/A:1016573926566
Montgomery, A. D., and Crow, G. L. (1998). “Collection and husbrandry techniques for black coral at the Waikiki Aquarium,” in American Zoo and Aquarium Association Regional Conference Proceedings (Bethesda, MD), 103–108.
Moon, H. W., and Song, J. I. (2008a). Taxonomy of the black coral family Antipathidae (Anthozoa: Antipatharia) from Korea. Kor. J. Syst. Zool. 24, 209–214. doi: 10.5635/KJSZ.2008.24.2.209
Moon, H. W., and Song, J. I. (2008b). Taxonomy of the black coral family Myriopathidae (Anthozoa: Antipatharia) from Korea. Korean J. Syst. Zool. 24, 251–263. doi: 10.5635/KJSZ.2008.24.3.251
Ocaña, O., Opresko, D. M., and Brito, A. (2006). First record of the black coral Antipathella wollastoni (Anthozoa: Antipatharia) outside of Macaronesian waters. Rev. Acad. Can. Cien. 18, 125–138.
Okiyama, M., and Tsukamoto, Y. (1989). Sea whip goby, Bryaninops yongei, collected from outer shelf off Miyakojima, East China Sea. Jap. J. Ichthyol. 36, 369–370.
Okuno, J. (1998). Miopontonia yongei Bruce, 1985 (Decapoda, Caridea, Palaemonidae): new host record and colour pattern. Crustaceana 71, 349–353. doi: 10.1163/156854098X00310
Opresko, D. M. (1972). Redescriptions and reevaluations of the antipatharians described by L. F. de Pourtales. Bull. Mar. Sci. 22, 950–1017.
Opresko, D. M. (1998). Three new species of Leiopathes (Cnidaria: Anthozoa: Antipatharia) from southern Australia. Rec. South Austr. Mus. 31, 99–111.
Opresko, D. M. (2001). Revision of the Antipatharia (Cnidaria: Anthozoa). Part I. Establishment of a new family, Myriopathidae. Zool. Med. Leiden 75, 343–370.
Opresko, D. M. (2002). Revision of the Antipatharia (Cnidaria: Anthozoa). Part II. Schizopathidae. Zool. Med. Leiden 76, 411–442.
Opresko, D. M. (2003a). Redescription of Antipathes dichotoma Pallas, 1766 (Cnidaria: Anthozoa: Antipatharia). Zool. Med. Leiden 77, 481–493.
Opresko, D. M. (2003b). Revision of the Antipatharia (Cnidaria: Anthozoa). Part III. Cladopathidae. Zool. Med. Leiden 77, 495–536.
Opresko, D. M. (2004). Revision of the Antipatharia (Cnidaria: Anthozoa). Part IV. Establishment of a new family, Aphanipathidae. Zool. Med. Leiden 78, 209–240.
Opresko, D. M. (2005a). New genera and species of antipatharian corals (Cnidaria: Anthozoa) from the North Pacific. Zool. Med. Leiden 792, 129–165.
Opresko, D. M. (2005b). A new species of antipatharian coral (Cnidaria: Anthozoa: Antipatharia) from the southern California Bight. Zootaxa 852, 1–10.
Opresko, D. M. (2006). Revision of the Antipatharia (Cnidaria: Anthozoa). Part V. Establishment of a new family, Stylopathidae. Zool. Med. Leiden 80–4, 109–138.
Opresko, D. M. (2009). A new name for the Hawaiian antipatharian coral formerly known as Antipathes dichotoma (Cnidaria: Anthozoa: Antipatharia). Pac. Sci. 63, 277–291. doi: 10.2984/049.063.0209
Opresko, D. M., and Baron-Szabo, R. C. (2001). Re-descriptions of the antipatharian corals described by E. J. C. Esper with selected English translations of the original German text (Cnidaria, Anthozoa, Antipatharia). Seckenb. Biol. 81, 1–21.
Opresko, D. M., and Cairns, S. D. (1994). Description of the new genus Allopathes (Cnidaria: Antipatharia) and its type species Cirripathes desbonni. Proc. Biol. Soc. Wash. 107, 185–192.
Opresko, D. M., and Genin, A. (1990). A new species of antipatharian (Cnidaria: Anthozoa) from seamounts in the eastern North Pacific. Bull. Mar. Sci. 46, 301–310.
Opresko, D. M., and Sanchez, J. A. (2005). Caribbean shallow-water black corals (Cnidaria: Anthozoa: Antipatharia). Caribb. J. Sci. 41, 492–507.
Opresko, D. M., Wagner, D., Montgomery, A. D., and Brugler, M. R. (2012). Discovery of Aphanipathes verticillata (Cnidaria: Anthozoa: Antipatharia) in the Hawaiian Islands. Zootaxa 3348, 24–39.
Pallas, P. S. (1766). Elenchus Zoophytorum Sistens Generum Adumbrationes Generaliores et Specierum Cognitarum Succinctas Descriptiones Cum Selectis Auctorum Synonymis. Frankfurt: Hagae-Comitum.
Parrish, F. A., and Baco, A. R. (2007). “State of deep coral ecosystems: in the U.S. Pacific Islands region: Hawaii and the U.S. Pacific territories,” in The State of Deep Coral Ecosystems in the United States, eds S. E. Lumsden, T. F. Hourigan, A. W. Bruckner, and G. Dorr (Silver Spring, MD: NOAA; Silver Spring), 159–194.
Pasternak, F. A. (1977). Antipatharia. Galathea Report Scientific Reports of the Danish Deep-Sea Expedition round the World 1950-1952, Vol. 14. Copenhagen, 157–164.
Paulay, G., Puglisi, M. P., and Starmer, J. A. (2003). The non-scleractinian Anthozoa (Cnidaria) of the Mariana Islands. Micronesica 35–36, 138–155.
Pax, F. (1932). Beitrag zur Kenntnis der japanischen Dörnchenkorallen. Zool. Jahrb. Abteil. Syst. Ökol. Geograp. Thiere 63, 407–450.
Pyle, R. L., and Chave, E. H. (1994). First record of the chaetodontid genus Prognathodes from the Hawaiian Islands. Pac. Sci. 48, 90–93.
Qi, S. H., Su, G. C., Wang, Y. F., Liu, Q. Y., and Gao, C. H. (2009). Alkaloids from the South China Sea black coral Antipathes dichotoma. Chem. Pharm. Bull. 57, 87–88.
Roark, E. B., Guilderson, T. P., Dunbar, R. B., and Ingram, B. L. (2006). Radiocarbon-based ages and growth rates of Hawaiian deep-sea corals. Mar. Ecol. Prog. Ser. 327, 1–14. doi: 10.3354/meps327001
Rogers, A. D., Baco, A., Griffiths, H., Hart, T., and Hall-Spencer, J. M. (2007). “Corals on seamounts. Supplementary material (including Appendix 8.1),” in Seamounts: Ecology, Conservation and Management Fish and Aquatic Resources Series, eds T. J. Pitcher, T. Morato, P. J. B. Hart, M. R. Clark, N. Haggan, and R. S. Santos (Oxford: Blackwell), 141–169.
Roule, L. (1905). Description des Antipathaires et Cérianthaires Recueillis par S.A.S. le Prince de Monaco dans L'Atlantique nord (1886-1902). Fascicule XXX. Monaco: Imprimerie de Monaco.
Schultze, L. S. (1903). “Die Antipatharien der deutschen Tiefsee-Expedition 1898-1899,” in Wissentschaftliche Ergebnisse der Deutschen Tiefsee-Expedition auf dem Dampfer “Valdivia” 1998-1899, ed C. Chun (Jena: Verlag von Gustav Fischer), 87–100.
Silberfeld, E. (1909). Diagnosen neuer japanischer Antipatharien aus der Sammlung von Herrn Prof. Doflein (München). Zool. Anz. 34, 760–763.
Summers, S. L. M. (1910). Antipatharians from the Indian Ocean. J. R. Microscop. Soc. 1910, 272–281.
Tazioli, S., Bo, M., Boyer, M., Rotinsulu, H., and Bavestrello, G. (2007). Ecological observations of some common antipatharian corals in the marine park of Bunaken (North Sulawesi, Indonesia). Zool. Stud. 46, 227–241.
Thomson, J. A. (1905). “Scotia collections: scottish antarctic expedition -report on the antipatharians,” in Proceedings of the Royal Physical Society of Edinburgh, Vol. 16. (Edinburgh), 76–79.
Tsuda, R. T., Amesbury, S. S., and Moras, S. C. (1977). Preliminary observations on the algae, corals, and fishes inhabiting the sunken ferry “Fujikawa Maru” in Truk lagoon. Atoll Res. Bull. 212, 1–6. doi: 10.5479/si.00775630.212.1
Verrill, A. E. (1928). Hawaiian shallow water Anthozoa. Bernice P. Bishop. Mus. Bull. 49, 3–30. doi: 10.5962/bhl.title.58574
Wagner, D., Brugler, M. R., Opresko, D. M., France, S. C., Montgomery, A. D., and Toonen, R. J. (2010). Using morphometrics, in situ observations and genetic characters to distinguish among commercially valuable Hawaiian black coral species; a redescription of Antipathes grandis Verrill, 1928 (Antipatharia: Antipathidae). Invertebr. Syst. 24, 271–290. doi: 10.1071/IS10004
Wagner, D., Kosaki, R. K., Spalding, H. L., Whitton, R. K., Pyle, R. L., Sherwood, A. R., et al. (2014). Mesophotic surveys of the flora and fauna at Johnston Atoll, Central Pacific Ocean. Mar. Biodivers. Rec. 7, e68. doi: 10.1017/S1755267214000785
Wagner, D., Luck, D. G., and Toonen, R. J. (2012b). The biology and ecology of black corals (Cnidaria: Anthozoa: Hexacorallia: Antipatharia). Adv. Mar. Biol. 63, 67–132. doi: 10.1016/B978-0-12-394282-1.00002-8
Wagner, D., Papastamatiou, Y. P., Kosaki, R. K., Gleason, K. A., McFall, G. B., Boland, R. C., et al. (2011a). New records of commercially valuable black corals (Cnidaria: Antipatharia) from the Northwestern Hawaiian Islands at mesophotic depths. Pac. Sci. 65, 249–255. doi: 10.2984/65.2.249
Wagner, D., Papastamatiou, Y. P., Kosaki, R. K., Gleason, K. A., McFall, G. B., Boland, R. C., et al. (2013). “Mesophotic surveys of the Northwestern Hawaiian Islands with new records of black coral species,” in Proceedings of the 2013 AAUS/ESDP Curaçao Joint International Scientific Diving Symposium (Dauphin Island, AL), 341–345.
Wagner, D., Pochon, X., Irwin, L., Toonen, R. J., and Gates, R. D. (2011b). Azooxanthellate? Most Hawaiian black corals contain Symbiodinium. Proc. Biol. Sci. 278, 1323–1328. doi: 10.1098/rspb.2010.1681
Wagner, D., Waller, R. G., Montgomery, A. D., Kelley, C. D., and Toonen, R. J. (2012a). Sexual reproduction of the Hawaiian black coral Antipathes griggi (Cnidaria: Antipatharia). Coral Reefs 31, 795–806. doi: 10.1007/s00338-012-0882-3
Wagner, D., Waller, R. G., and Toonen, R. J. (2011c). Sexual reproduction of Hawaiian black corals, with a review of reproduction of antipatharians (Cnidaria: Anthozoa: Hexacorallia). Inver. Biol. 130, 211–225. doi: 10.1111/j.1744-7410.2011.00233.x
Zhou, J., and Zou, R. (1984). Studies on the antipatharians of China. II. The genus Antipathes. Tropic Oceanol. 3, 56–61.
Zhou, J., and Zou, R. (1992). Studies on the antipatharians from Zhongsha Islands waters. Tropic Oceanol. 11, 45–52.
Zou, R., and Zhou, J. (1982). Studies on the antipatharians of China. I. The genus Cirrhipathes with the description of a new species. Tropic Oceanol. 1, 82–91.
Keywords: Anthozoa, Hawai‘i, precious coral, scanning electron microscopy, taxonomy
Citation: Wagner D (2015) A taxonomic survey of the shallow-water (<150 m) black corals (Cnidaria: Antipatharia) of the Hawaiian Islands. Front. Mar. Sci. 2:24. doi: 10.3389/fmars.2015.00024
Received: 24 February 2015; Accepted: 22 April 2015;
Published: 13 May 2015.
Edited by:
Tito Monteiro Da Cruz Lotufo, University of São Paulo, BrazilReviewed by:
Marzia Bo, Universitá degli studi di Genova, ItalyMercer Robert Brugler, New York City College of Technology (CUNY), USA
Copyright © 2015 Wagner. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daniel Wagner, National Oceanic and Atmospheric Administration, Papahānaumokuākea Marine National Monument, 1845 Wasp Boulevard, Building 176, Honolulu, HI 96818, USA, daniel.wagner@noaa.gov
 Daniel Wagner
Daniel Wagner